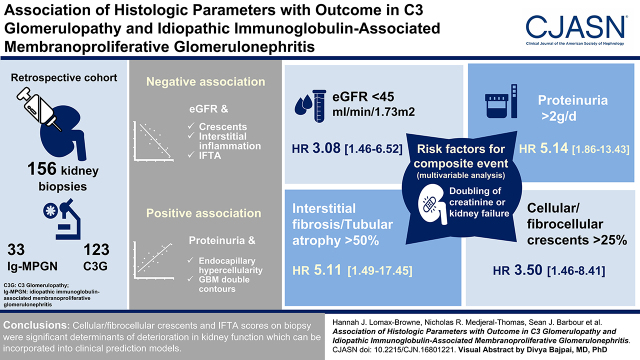
Visual Abstract
Keywords: glomerulonephritis, complement, kidney biopsy, membranoproliferative glomerulonephritis (MPGN)
Abstract
Background and objectives
C3 glomerulopathy and idiopathic Ig-associated membranoproliferative GN are kidney diseases characterized by abnormal glomerular complement C3 deposition. These conditions are heterogeneous in outcome, but approximately 50% of patients develop kidney failure within 10 years.
Design, setting, participants, & measurements
To improve identification of patients with poor prognosis, we performed a detailed analysis of percutaneous kidney biopsies in a large cohort of patients. Using a validated histologic scoring system, we analyzed 156 native diagnostic kidney biopsies from a retrospective cohort of 123 patients with C3 glomerulopathy and 33 patients with Ig-associated membranoproliferative GN. We used linear regression, survival analysis, and Cox proportional hazards models to assess the relationship between histologic and clinical parameters with outcome.
Results
Frequent biopsy features were mesangial expansion and hypercellularity, glomerular basement membrane double contours, and endocapillary hypercellularity. Multivariable analysis showed negative associations between eGFR and crescents, interstitial inflammation, and interstitial fibrosis/tubular atrophy. Proteinuria positively associated with endocapillary hypercellularity and glomerular basement membrane double contours. Analysis of second native biopsies did not demonstrate associations between immunosuppression treatment and improvement in histology. Using a composite outcome, risk of progression to kidney failure associated with eGFR and proteinuria at the time of biopsy, cellular/fibrocellular crescents, segmental sclerosis, and interstitial fibrosis/tubular atrophy scores.
Conclusions
Our detailed assessment of kidney biopsy data indicated that cellular/fibrocellular crescents and interstitial fibrosis/tubular atrophy scores were significant determinants of deterioration in kidney function.
Introduction
C3 glomerulopathy encompasses kidney diseases characterized by the presence of abnormal glomerular complement C3 dominant in intensity compared with Igs (1). C3 glomerulopathy can be subclassified into dense deposit disease (DDD), with electron-dense osmiophilic deposits in the glomerular basement membrane (GBM) and mesangium, and C3 GN, where deposits are less dense and may be mesangial, subendothelial, intramembranous, and subepithelial (2). C3 glomerulopathy is associated with uncontrolled complement activation due to impaired regulation of the complement alternative pathway (AP). Causes include mutations and autoantibodies that impair the function of the negative AP regulator, factor H (3–5), and C3 nephritic factors that promote C3 activation (6–8). C3 nephritic factors and low serum C3 are also observed in idiopathic Ig-associated membranoproliferative GN (Ig-MPGN) (8,9). In one cohort, complement gene variants and autoantibodies were identified in 24% of patients with C3 GN and 35% of patients with DDD (10).
C3 glomerulopathy and Ig-MPGN are rare and heterogeneous in presentation and outcome (8,11,12). Progression to kidney failure occurs within 10 years of diagnosis in 70% of children and 30%–50% of adults (10,13). Mycophenolate mofetil with corticosteroids may induce remission (14–18), but this is not consistent (19–21). Patients with C3 nephritic factor may be more responsive to immunosuppression than those with genetic causes (17,21). Where the cause is a monoclonal Ig, treatment of the hematologic malignancy can improve C3 glomerulopathy (22). Anticomplement C5 antibody therapy reduced glomerular inflammation in rapidly progressive disease but was of limited benefit in indolent cases (23,24). Treatments in trials include agents that inhibit AP activation and those that inhibit C3 directly.
Many studies have tried to identify clinical and histologic factors that influence outcome. In 111 patients with C3 glomerulopathy (Columbia study), there was no difference in the progression of kidney disease between C3 GN (39%) and DDD (42%) (10). Covariates associated with progression were eGFR at diagnosis and interstitial fibrosis/tubular atrophy (IFTA) (10). Biopsy activity and chronicity scores were associated with the outcome, although the chronicity score was more strongly associated. In a study of 111 patients with C3 glomerulopathy by the Spanish Group for the Study of Glomerular Diseases (GLOSEN), covariates associated with kidney failure were baseline eGFR, 24-hour proteinuria, immunosuppression, and IFTA (25). In contrast to the Columbia study (10), the chronicity (but not the activity score) associated with the outcome. Parameters associated with kidney failure were chronicity score greater than or equal to four, baseline eGFR, and 24-hour proteinuria (26). Longitudinal change in proteinuria also associated with kidney failure. A doubling of proteinuria was associated with a 2.5-fold higher risk of kidney failure. A decrease of ≥50% proteinuria was associated with a lower risk of kidney failure (27).
Because of the overlapping etiology and pathogenesis of C3 glomerulopathy and Ig-MPGN, we performed a large multicenter, centralized kidney biopsy review to identify features associated with clinical outcome that could be used to develop outcome prediction tools to enable stratification for clinical trials.
Materials and Methods
Study Cohort
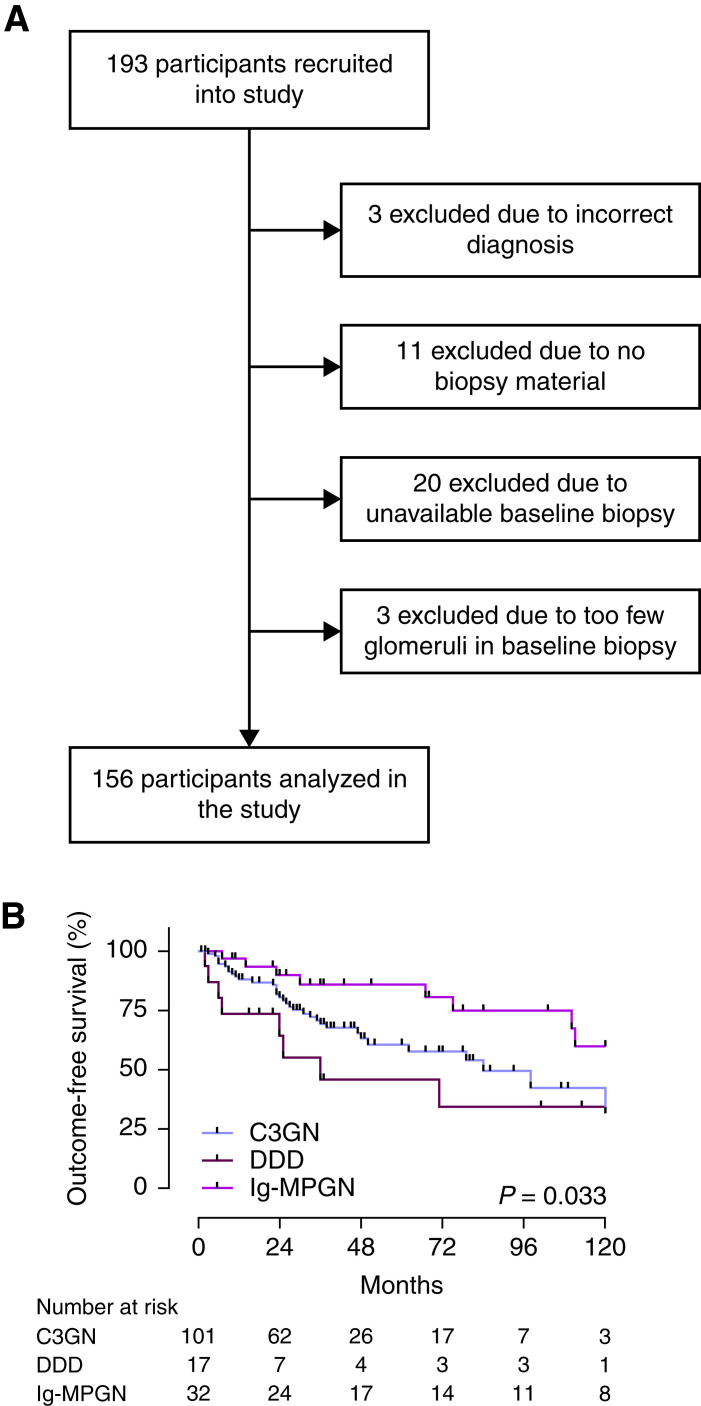
We analyzed a retrospective cohort of 193 participants with C3 glomerulopathy or Ig-MPGN approved by the UK National Research Ethics Service Committee (16/WM/0497, host site), the Columbia University Medical Center (AAAR9076), the Mayo Clinic (17–004968), and the Royal Melbourne Hospital (HREC/16/MH/203). C3 glomerulopathy was defined according to consensus guidelines (1,28). Ig-MPGN was defined as a membranoproliferative GN pattern with significant Ig deposits and absence of a secondary cause. After pathology review, 156 participants were eligible (Figure 1). Baseline clinical data collection was the time of diagnostic biopsy; data were also collected 3, 6, and 12 months postbiopsy and annually thereafter. Proteinuria was expressed in grams per 24 hours, and where urine protein-creatinine ratios were provided, was derived using equations developed by Hogan et al. (29). eGFR was estimated from serum creatinine values using the full age spectrum formula (30) and was capped at 150 ml/min per 1.73 m2.
Figure 1.
Study overview and outcome-free survival. (A) Study recruitment and inclusion. From 193 recruited participants, 156 were eligible for inclusion. Participants were excluded either due to insufficient biopsy material (n=34) or incorrect diagnosis (n=3). (B) Progression to a combined outcome event was assessed in relation to diagnosis. The C3 glomerulopathy (C3G) cohort (n=118) consisted of C3 GN (n=101) and dense deposit disease (DDD; n=17), and there were 32 patients with idiopathic Ig-associated membranoproliferative GN (Ig-MPGN).
Histology Scoring
Histologic scoring was performed on scanned (Aperio Scanscope CS2; Leica) light microscopy slides (Supplemental Table 1). Immunofluorescence staining was extracted from biopsy reports. Electron microscopy data were used for classification to Ig-MPGN, C3 GN, or DDD. Digitally annotated glomeruli were scored to generate a mean or percentage score for each glomerular feature. Ten biopsies were scored by five different pathologists to assess score reproducibility (Supplemental Table 2). Lesions with good to moderate reproducibility were analyzed in the whole cohort; 256 biopsies were scored (156 diagnostic biopsies, 60 repeat biopsies, and 40 transplant biopsies [excluded from this analysis]). Informed by the C3 glomerulopathy histologic index (10), we calculated biopsy activity and chronicity scores (Supplemental Table 3).
Outcome
The composite outcome event was defined as the time to kidney failure (eGFR <15 ml/min per 1.73 m2, dialysis, or transplantation), the first occurrence of doubling of the serum creatinine level from baseline (adults), or 30% reduction of eGFR (for pediatric patients). Note that of the five pediatric patients who reached 30% eGFR reduction, four subsequently reached 50% eGFR reduction, but data were not available for the remaining patient. The results of our outcome analyses did not differ when either 30% or 50% eGFR reduction was used. Longitudinal proteinuria was assessed across 75 months. Outcome-free survival and changes in proteinuria over the 12 months following baseline biopsy were analyzed in 75 patients, all of whom had ≥2 years of follow-up to reliably determine stable or progressive disease.
Statistical Analyses
The Kruskal–Wallis test with the Dunn multiple comparison test was used for continuous variables, and the chi-squared test was used for categorical variables. Changes in histologic features over time were assessed using the Fisher exact test. Kaplan–Meier curves were assessed using log-rank tests. The relationship between histologic scores, eGFR, and proteinuria was assessed using the Spearman rank correlation in R and by linear regression. Because mesangial hypercellularity and expansion scores were collinear, only mesangial hypercellularity was used in multivariable regression. Longitudinal changes in proteinuria across 75 months were assessed using a joint linear mixed effect model and a Cox proportional hazards model (Supplemental Figure 1). Outcome-free survival was assessed by score quartiles (Supplemental Figures 2 and 3) and subgroups: global sclerosis, segmental sclerosis, cellular/fibrocellular crescents, and fibrous crescents as 0%, 1%–25%, or >25% and interstitial inflammation and IFTA as 0%, 10%–20%, 30%–50%, or >50%. We used Cox proportional hazards models over the entire duration of follow-up to assess covariates of composite outcome. Analyses were performed using GraphPad Prism version 9.0 (GraphPad), SPSS version 26 (IBM Corp.), and R version 4.0.0.
Results
Clinical Description of the Study Cohort
From 193 recruited participants, 156 were eligible for analysis (Figure 1). Participants were excluded due to insufficient biopsy material (n=34) or incorrect diagnosis (n=3). Clinical data are summarized in Table 1. The mean age of the whole cohort was 31 years (range, 2–81 years; SD, 21.54; C3 GN, 31 years; DDD, 36 years; Ig-MPGN, 31 years), and 30% (n=47) were aged 17 years or younger. Following pathology review, the C3 glomerulopathy cohort included 106 patients with C3 GN (68%), 17 patients with DDD (11%), and 33 patients with Ig-MPGN (21%). Except for the mean duration of complete follow-up, which was longer in the Ig-MPGN group, there were no significant demographic differences between the C3 glomerulopathy and Ig-MPGN cohorts. The prevalence of low C3 was comparable across the groups (Table 1). Using our composite outcome, the outcome-free survival was significantly lower for both C3 GN and DDD compared with Ig-MPGN (Figure 1), for adult patients and for individuals with baseline eGFR <60 ml/min per 1.73 m2, and for proteinuria >2 g/d and nephrotic-range proteinuria (Supplemental Figure 4). Because a decrease in proteinuria over time has been associated with improved kidney outcome (27) and because many clinical studies utilize 12-month treatment-dependent changes in proteinuria as a surrogate marker of efficacy, we assessed the relationship between outcome-free survival and changes in proteinuria over the first 12 months following the baseline biopsy. Data were available for 75 patients with at least 2 years of follow-up from diagnostic biopsy, of whom 37 achieved a 50% decrease in proteinuria. Proteinuria was unchanged or increased in the remaining 38 patients. There was no significant difference in outcome-free survival between those who did and those who did not achieve a 50% decrease in proteinuria (Supplemental Figure 1A). We next investigated the relationship between longitudinal changes in proteinuria and progression to an outcome event by using joint linear mixed effects and Cox proportional hazards models (Supplemental Figure 1B, Supplemental Table 4). Across a period of 75 months, a doubling in the level of proteinuria was associated with a 1.98-fold higher risk of reaching an outcome event (odds ratio, 1.98; 95% confidence interval, 1.21 to 3.85; P=0.002). Although we are confident in the direction of these effects, the 95% confidence intervals are wide, so the magnitude of the relationship requires a larger study number.
Table 1.
Clinical characteristics of the cohort
| Cohort Clinical Descriptive Data | C3 GN, n=106 | Dense Deposit Disease, n=17 | Idiopathic Ig-Associated Membranoproliferative GN, n=33 |
|---|---|---|---|
| Age, yr | 25 [16–45] | 34 [13–61] | 22 [9–59] |
| Men/women, n (%) | 58 (55)/48 (45) | 11 (65)/6 (35) | 18 (55)/15 (45) |
| Adult/pediatric (<17 yr), n (%) | 79 (75)/27 (25) | 12 (71)/5 (29) | 18 (55)/15 (45) |
| Duration of complete follow-up, mo | 42 [25–80] | 25 [12–109] | 84 [36–145] |
| eGFR at baseline, ml/min per 1.73 m2 | 55 [32–102] | 49 [41–55] | 65 [32–87] |
| Proteinuria at baseline, g/24 h | 3 [1.2–5.7] | 3.04 [0.69–8.9] | 3.91 [1.86–5.81] |
| Serum C3, g/L | 0.69 [0.23–0.97] | 0.35 [0.1–0.79] | 0.59 [0.27–0.87] |
| Low serum C3a, n (%) | 37/74 (50) | 6/10 (60) | 9/17 (53) |
| Serum C4, g/L | 0.22 [0.17–0.29] | 0.27 [0.20–0.31] | 0.17 [0.10–0.29] |
| Paraproteinb, n (%) | 19/71 (27) | 4/11 (36) | 1/3 (33) |
| Outcome eventsc, n (%) | |||
| Composite | 35 (35) | 9 (53) | 8 (24) |
| Doubling creatinine (adult only) | 14 (14) | 3 (18) | 1 (3) |
| 30% loss of eGFR (pediatric only) | 1 (1) | 1 (6) | 3 (9) |
| Kidney failure | 20 (20) | 5 (29) | 4 (12) |
| Ethnicity, n (%) | |||
| White participants | 77 (73) | 14 (82) | 27 (82) |
| Asian participants | 13 (12) | 2 (12) | 3 (9) |
| Black participants | 3 (3) | 0 | 0 |
| Other | 6 (6) | 0 | 0 |
| Unknown | 7 (7) | 1 (6) | 3 (9) |
| Treatment with RAS blockade, n (%) | 86 (81) | 14 (82) | 32 (97) |
| Treatment with immunosuppression at any time, n (%) | 80 (75) | 16 (94) | 27 (82) |
| Corticosteroids | 70 (66) | 12 (71) | 26 (79) |
| Mycophenolate mofetil | 55 (52) | 7 (41) | 14 (42) |
| Cyclophosphamide | 14 (13) | 4 (24) | 3 (9) |
| Azathioprine | 3 (3) | 0 | 8 (24) |
| Tacrolimus | 21 (20) | 3 (18) | 4 (12) |
| Rituximab | 9 (8) | 1 (6) | 1 (3) |
| Eculizumab | 6 (6) | 1 (6) | 0 |
| Other complement inhibitor | 6 (6) | 0 | 0 |
| Treatment with immunosuppression in native disease, n (%) | 72 (68) | 14 (82) | 25 (76) |
| Baseline biopsies | 106 | 17 | 33 |
| Native repeat biopsies | 45 | 5 | 10 |
| Transplant biopsies | 37 | 2 | 1 |
| Total biopsies analyzed | 188 | 24 | 44 |
Values are expressed as median [interquartile range] or absolute number (percentage). The serum C3 normal range was 0.7–1.7 g/L; the serum C4 normal range was 0.16–0.54 g/L. RAS, renin-angiotensin system.
C3 was measured in 101 patients in the whole cohort, 84 patients with C3 glomerulopathy, 74 patients with C3 GN, ten patients with dense deposit disease, and 17 patients with idiopathic Ig-associated membranoproliferative GN.
Paraprotein was measured in 85 patients in the whole cohort, 82 patients with C3 glomerulopathy, 71 patients with C3 GN, 11 patients with dense deposit disease, and three patients with idiopathic Ig-associated membranoproliferative GN.
Outcome events were assessed in 150 patients in the whole cohort, 118 patients with C3 glomerulopathy, 101 patients with C3 GN, 17 patients with dense deposit disease, and 32 patients with idiopathic Ig-associated membranoproliferative GN.
Histology Scores in the Diagnostic Native Kidney Biopsies
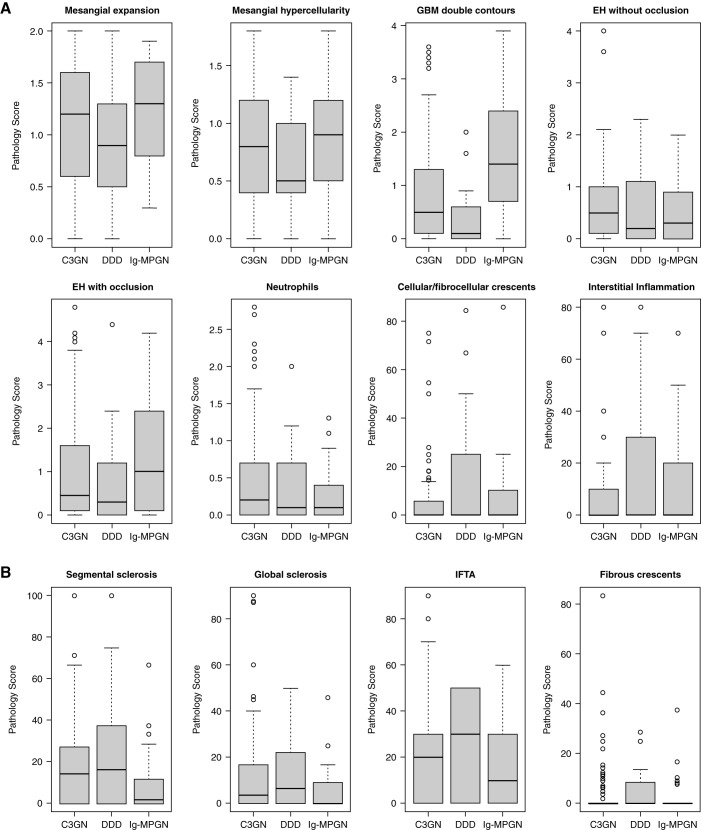
Analysis of the 156 baseline diagnostic native kidney biopsies showed that the most common features were mesangial expansion (96%), mesangial hypercellularity (94%), GBM double contours (81%), and endocapillary hypercellularity with (78%) and without (75%) capillary lumen occlusion (Table 2). The frequencies were comparable across the groups, except that GBM double contours were significantly higher in Ig-MPGN, and endocapillary hypercellularity without occlusion was higher in C3 GN (Table 2). Except for the median GBM double-contours score, which was higher in the Ig-MPGN cohort, the other histology scores were comparable across the cohorts (Figure 2, Supplemental Figures 5–7). We next looked at the correlations between the different histologic scores (Supplemental Figure 8, Supplemental Tables 5 and 6). For both the whole and C3 glomerulopathy–only cohorts, there were strong (Spearman rho =0.9, P<0.001) correlations between the mesangial expansion and mesangial hypercellularity scores, and both correlated with GBM double contours (r=0.6, P<0.001). The IFTA score correlated with global sclerosis (r=0.7, P<0.001), segmental sclerosis (r=0.5, P<0.001), and fibrous crescents (r=0.4, P<0.001).
Table 2.
Frequency of histologic features in 156 baseline biopsies
| Biopsy Feature, n (%) | C3 GN, n=106 | Dense Deposit Disease, n=17 | Idiopathic Ig-Associated Membranoproliferative GN, n=33 |
|---|---|---|---|
| Mesangial expansion | 101 (95) | 16 (94) | 33 (100) |
| Mesangial hypercellularity | 100 (94) | 16 (94) | 31 (94) |
| GBM double contours | 84 (79) | 11 (65) | 31 (94) |
| EH with occlusion | 82 (77) | 11 (65) | 29 (88) |
| EH without occlusion | 86 (81) | 9 (53) | 22 (67) |
| IFTA | 78 (74) | 11 (65) | 20 (61) |
| Segmental sclerosis | 69 (65) | 11 (65) | 17 (52) |
| Neutrophils | 64 (60) | 9 (53) | 20 (61) |
| Global sclerosis | 55 (52) | 9 (53) | 16 (49) |
| Cellular or fibrocellular crescent | 31 (29) | 8 (47) | 13 (40) |
| Interstitial inflammation | 30 (28) | 7 (41) | 13 (40) |
| Ischemic changes | 28 (26) | 8 (47) | 11 (33) |
| Normal glomeruli | 28 (26) | 7 (41) | 5 (15) |
| Fibrous crescent | 26 (25) | 5 (29) | 6 (19) |
| Visceral EC hypertrophy | 6 (0.06) | 2 (12) | 1 (3) |
| Mesangiolysis | 2 (0.02) | 0 | 2 (6) |
| Necrosis | 3 (0.03) | 1 (0.06) | 0 |
| Adhesion | 1 (0.01) | 0 | 0 |
| Tip lesion | 0 | 0 | 0 |
| Thrombosis | 0 | 0 | 0 |
GBM, glomerular basement membrane; EH, endocapillary hypercellularity; IFTA, interstitial fibrosis/tubular atrophy; EC, epithelial cell.
Figure 2.
Box plots depicting the pathology scores of (A) active and (B) chronic lesions. The cohort was divided into those with C3 GN (n=106), DDD (n=17), and Ig-MPGN (n=33). The median glomerular basement membrane (GBM) double-contours score was significantly greater in the Ig-MPGN group versus either the C3 GN (P=0.001) or DDD (P<0.001) group. Horizontal bars within boxes denote medians. The vertical size of boxes denotes the interquartile range (quartile 1, lower hinge and quartile 3, upper hinge). Whiskers denote minimum and maximum data points not exceeding 1.5 times the interquartile range from quartiles 1 and 3, respectively. Data points exceeding this range (outliers) are denoted by circles. P values were derived from the Kruskal–Wallis test with the Dunn multiple comparisons test. EH, endocapillary hypercellularity; IFTA, interstitial fibrosis/tubular atrophy.
Histology Scores Associate with Estimated Glomerular Filtration Rate and Proteinuria at Biopsy
To determine the clinical significance of the histologic scores, we next analyzed their relationship with eGFR and proteinuria at the time of biopsy. We performed correlations (Supplemental Figures 8–10, Supplemental Tables 5 and 6) and a regression analysis (Supplemental Table 7) between the histologic scores and eGFR or proteinuria. The eGFR negatively correlated with IFTA (r=−0.5, P<0.001) and global sclerosis (r=−0.4, P<0.001) in both the whole cohort and the C3 glomerulopathy cohort and negatively correlated with interstitial inflammation (r=−0.3, P<0.001) and cellular/fibrocellular crescents (r=−0.3, P<0.001) scores in the whole cohort. There was a positive correlation between eGFR and both mesangial expansion (r=0.3, P<0.001) and mesangial hypercellularity (r=0.3, P<0.001) in the whole cohort. In the multivariable regression analysis, the scores that associated with eGFR were mesangial hypercellularity, IFTA, interstitial inflammation, and cellular/fibrocellular crescents, and these were equivalent in the total and C3 glomerulopathy cohorts (Supplemental Table 7). Proteinuria at the time of biopsy correlated positively with GBM double contours (r=0.3, P<0.001 [whole cohort] and r=0.4, P<0.001 [C3 glomerulopathy cohort]), endocapillary hypercellularity with occlusion (r=0.3, P<0.001 [whole cohort] and r=0.4, P<0.001 [C3 glomerulopathy cohort]), and mesangial hypercellularity (r=0.3, P<0.001 [C3 glomerulopathy cohort]). In the multivariable regression analysis, the scores that associated with proteinuria were GBM double contours and endocapillary hypercellularity with and without occlusion. In the C3 glomerulopathy–only cohort, endocapillary hypercellularity without occlusion was no longer significant. Informed by the published C3 glomerulopathy histologic index (10), we also analyzed the relationship of scores associated with disease activity (mesangial hypercellularity, endocapillary hypercellularity, neutrophils, cellular/fibrocellular crescents, and interstitial inflammation) and chronicity (glomerular sclerosis, fibrous crescents, and IFTA) with eGFR and proteinuria (Supplemental Figure 8). The activity score positively correlated with proteinuria but not with eGFR (Supplemental Figure 8, C and D). Conversely, the chronicity score negatively correlated with eGFR but not with proteinuria (Supplemental Figure 8, E and F).
Histology Scores over Time
The relationship between Ig-MPGN and C3 glomerulopathy is complex; cases of C3 glomerulopathy can present with codominant C3 and Ig deposition, with subsequent biopsies then showing dominant C3 deposition (31). We analyzed histology scores in 39 individuals with a second native biopsy, among whom 31 had a diagnosis on that biopsy of C3 glomerulopathy and eight had a diagnosis on that biopsy of Ig-MPGN (Supplemental Table 8). In patients with an initial diagnosis of Ig-MPGN, the diagnosis changed to C3 GN on second biopsy in three of 11 individuals (Supplemental Table 9). We did not observe any cases where the diagnosis changed from C3 GN to Ig-MPGN. We compared changes in the histology scores between the baseline and second biopsy and subgrouped the patients into those who had received immunosuppression (n=26) and those who had not (n=13). There was no difference in progression of the lesions between these groups (Supplemental Figure 11). Like the diagnostic biopsy analysis, eGFR was negatively correlated with IFTA and interstitial inflammation at the second biopsy (Supplemental Table 10). However, histology scores did not correlate with proteinuria. This may reflect the small case number in this analysis, the variable timing and indication for the second biopsy, and the treatment received, which included renin-angiotensin system blockade in 25 (64%) patients.
Clinical Outcome Is Influenced by Histology Scores at Diagnostic Biopsy
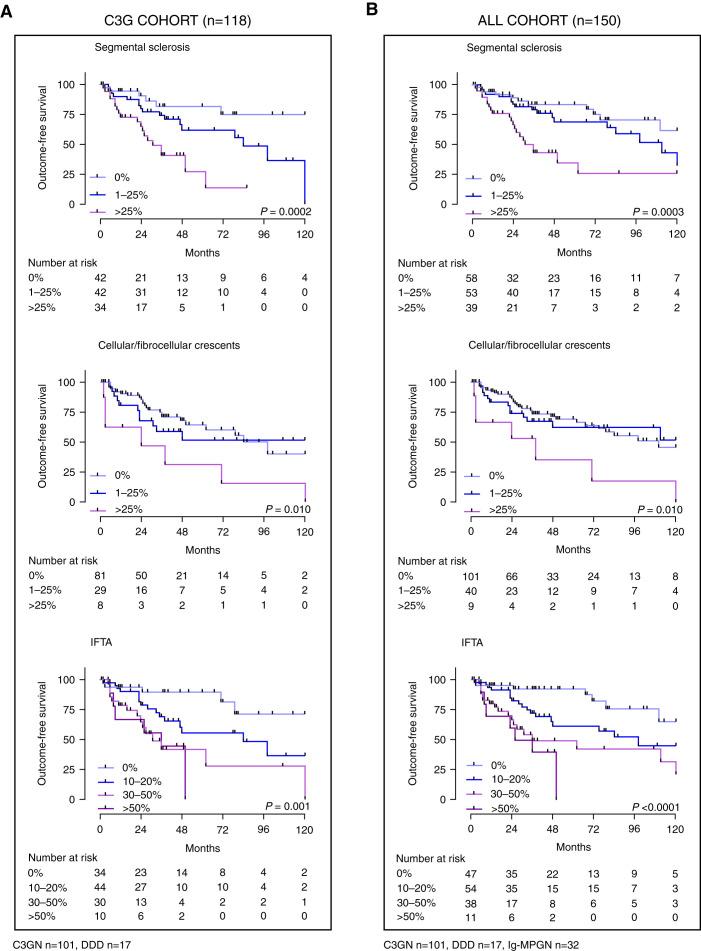
Like published cohorts, eGFR (8,10) and proteinuria (9,21) at the time of baseline biopsy were associated with outcome-free survival (Supplemental Figure 4) and higher risk of progression to the combined outcome (Table 3) in our cohort. Using our uniquely detailed histologic scores from the 156 baseline biopsies, we examined the relationship between outcome-free survival and each score. Cellular/fibrocellular crescents, segmental sclerosis, and IFTA were associated with outcome-free survival (Figure 3). Using a Cox proportional hazards model, segmental sclerosis, cellular/fibrocellular crescents, and IFTA were associated with a higher risk of progression to the combined outcome in both the whole and C3 glomerulopathy cohorts (Table 3). In the multivariable analysis of the whole cohort, the two most important histologic features associated with poor outcome were IFTA and cellular/fibrocellular crescents.
Table 3.
Covariates of the risk to progression to the combined outcome event
| Clinical Covariate, Number | C3 Glomerulopathy Cohort, n=118 | All Cohort, n=150 | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||
| B | Unadjusted Hazard Ratio (95% Confidence Interval) | B | Unadjusted Hazard Ratio (95% Confidence Interval) | B | Unadjusted Hazard Ratio (95% Confidence Interval) | B | Unadjusted Hazard Ratio (95% Confidence Interval) | |
| Age, yr | ||||||||
| <17, reference; all=47, C3G=32 | ||||||||
| ≥17, all=103, C3G=86 | 0.63 | 1.87 (0.86 to 4.08) | 0.69 | 2.00 (1.02 to 3.91)a | 0.13 | 1.14 (0.48 to 2.69) | ||
| Sex | −0.49 | 0.61 (0.33 to 1.14) | −0.27 | 0.76 (0.44 to 1.33) | ||||
| Ethnicity, White | −0.20 | 0.82 (0.43 to 1.57) | −0.14 | 0.87 (0.47 to 1.63) | ||||
| Diagnosis | ||||||||
| C3 GN, reference; all=101 | ||||||||
| DDD, all=17 | 0.58 | 1.79 (0.85 to 3.76) | 0.55 | 1.74 (0.83 to 3.66) | 0.30 | 1.34 (0.51 to 3.51) | ||
| Ig-MPGN, all=32 | −0.82 | 0.44 (0.20 to 0.97)a | −1.27 | 0.28 (0.11 to 0.70) | ||||
| eGFR, ml/min per 1.73 m2 | ||||||||
| >60, reference; all=71, C3G=53 | ||||||||
| 45–60, all=25, C3G=20 | 0.89 | 2.44 (1.04 to 5.74) | 1.20 | 3.33 (1.29 to 8.58) | 0.83 | 2.29 (1.06 to 4.93) | 0.96 | 2.62 (0.95 to 7.20) |
| <45, all=53, C3G=44 | 0.91 | 2.49 (1.24 to 4.98) | 1.25 | 3.48 (1.57 to 7.68) | 0.94 | 2.55 (1.35 to 4.81) | 1.13 | 3.08 (1.46 to 6.52) |
| Proteinuria, g/24 h | ||||||||
| <1, reference; all=30, C3G=24 | ||||||||
| 1–2, all=18, C3G=17 | 0.33 | 1.39 (0.31 to 6.22) | 0.13 | 1.14 (0.25 to 5.18) | 0.37 | 1.45 (0.34 to 6.12) | 0.09 | 1.09 (0.25 to 4.70) |
| >2, all=88, C3G=65 | 1.61 | 4.98 (1.65 to 15.09) | 1.71 | 5.53 (1.83 to 16.76) | 1.23 | 3.41 (1.32 to 8.80) | 1.61 | 5.14 (1.86 to 13.43) |
| Immunosuppression use | 0.31 | 1.37 (0.67 to 2.79) | 0.06 | 1.07 (0.58 to 1.98) | ||||
| Histologic covariate global sclerosis, % | ||||||||
| 0, reference; all=76, C3G=59 | ||||||||
| 1–25, all=51, C3G=37 | 0.38 | 1.46 (0.73 to 2.91) | 0.23 | 1.26 (0.68 to 2.33) | ||||
| >25, all=23, C3G=22 | 0.49 | 1.63 (0.73 to 3.68) | 0.62 | 1.86 (0.88 to 3.94) | ||||
| Segmental sclerosis, % | ||||||||
| 0, reference; all=58, C3G=42 | ||||||||
| 1–25, all=53, C3G=42 | 0.96 | 2.60 (1.01 to 6.71) | 0.84 | 2.32 (0.82 to 6.55) | 0.41 | 1.50 (0.72 to 3.12) | 0.10 | 1.10 (0.50 to 2.42) |
| >25, all=39, C3G=34 | 1.80 | 6.03 (2.30 to 15.83) | 1.86 | 6.42 (2.01 to 20.50) | 1.27 | 3.55 (1.73 to 7.31) | 0.66 | 1.94 (0.82 to 4.56) |
| Cellular/fibrocellular crescent, % | ||||||||
| 0, reference; all=101, C3G=81 | ||||||||
| 1–25, all=40, C3G=29 | 0.28 | 1.32 (0.66 to 2.67) | 0.89 | 2.43 (1.03 to 5.71) | 0.16 | 1.17 (0.62 to 2.22) | 0.42 | 1.52 (0.75 to 3.08) |
| >25%, all=9, C3G=8 | 1.25 | 3.49 (1.49 to 8.18) | 0.91 | 2.48 (0.88 to 7.00) | 1.22 | 3.38 (1.48 to 7.71) | 1.25 | 3.50 (1.46 to 8.41) |
| Fibrous crescent, % | ||||||||
| 0, reference; all=117, C3G=90 | ||||||||
| 1–25, all=28, C3G=24 | 0.05 | 1.05 (0.48 to 2.28) | 0.24 | 1.28 (0.63 to 2.56) | ||||
| >25, all=5, C3G=4 | 1.37 | 3.93 (0.93 to 16.73) | 0.40 | 1.49 (0.36 to 6.26) | ||||
| IFTA, % | ||||||||
| 0, reference; all=47, C3G=34 | ||||||||
| 10–20, all=54, C3G=44 | 0.89 | 2.43 (0.95 to 6.24) | 0.72 | 2.05 (0.69 to 6.09) | 0.81 | 2.25 (0.98 to 5.18) | 0.74 | 2.10 (0.85 to 5.20) |
| 30–50, all=38, C3G=30 | 1.63 | 5.09 (1.93 to 13.43) | 0.99 | 2.69 (0.89 to 8.11) | 1.43 | 4.18 (1.81 to 9.67) | 1.00 | 2.71 (1.06 to 6.94) |
| >50, all=11, C3G=10 | 1.76 | 5.81 (1.82 to 18.54) | 0.89 | 2.44 (0.64 to 9.37) | 1.93 | 6.91 (2.46 to 19.44) | 1.63 | 5.11 (1.49 to 17.45) |
| Interstitial inflammation, % | ||||||||
| 0, reference; all=103, C3G=83 | ||||||||
| 10–20, all=25, C3G=19 | −1.24 | 0.29 (0.10 to 0.85) | −0.86 | 0.42 (0.14 to 1.31) | −0.76 | 0.47 (0.20 to 1.13) | ||
| 30–50, all=17, C3G=12 | 0.79 | 2.21 (1.00 to 4.87) | 0.68 | 1.98 (0.80 to 4.90) | 0.54 | 1.72 (0.84 to 3.50) | ||
| >50, all=5, C3G=4 | 1.03 | 2.79 (0.84 to 9.27) | 1.63 | 5.13 (1.21 to 21.64) | 1.14 | 3.13 (0.95 to 10.27) | ||
| Mesangial hypercellularity | 0.41 | 1.50 (0.84 to 2.71) | 0.21 | 1.23 (0.70 to 2.16) | ||||
| EH without occlusion | −0.24 | 0.79 (0.52 to 1.20) | −0.11 | 0.90 (0.61 to 1.32) | ||||
| EH with occlusion | 0.07 | 1.08 (0.87 to 1.34) | 0.02 | 1.02 (0.83 to 1.25) | ||||
| Neutrophils | 0.05 | 1.05 (0.69 to 1.59) | 0.21 | 1.23 (0.83 to 1.82) | ||||
| GBM double contours | 0.15 | 1.16 (0.88 to 1.53) | −0.03 | 0.97 (0.75 to 1.27) | ||||
The Cox proportional hazards models utilized either clinical or histologic variables to determine predictors of risk of progression to the outcome event (defined in Table 1). C3G, C3 glomerulopathy; DDD, dense deposit disease; Ig-MPGN, idiopathic Ig-associated membranoproliferative GN; IFTA, interstitial fibrosis/tubular atrophy; EH, endocapillary hypercellularity; GBM, glomerular basement membrane.
Significant covariates of the outcome.
Figure 3.
Outcome-free survival in the whole and C3G cohorts by histologic scores. The significant histology covariates of risk to progression to the combined event in (A) the C3G cohort (n=118) and (B) the whole cohort (n=150) were segmental sclerosis, cellular/fibrocellular crescents, and IFTA. Outcome-free survival for the other histologic scores are shown in Supplemental Figure 12. P values were derived from the log-rank test.
Discussion
Our data represent the largest centralized review of C3 glomerulopathy and Ig-MPGN biopsies and enabled us to determine the relationship of histologic features with eGFR and proteinuria at biopsy and with outcome. IFTA, cellular/fibrocellular crescents, and segmental sclerosis were associated with higher risk of progression to our composite outcome. The central pathology review of all biopsies overcame previous limitations (e.g., potential inter-report variation in identifying and quantitating lesions) where biopsy data have been extracted from clinical reports (10,25,26).
We included C3 glomerulopathy and Ig-MPGN in our cohort because both have AP dysregulation to similar degrees (8,9). Both are suitable for complement inhibition trials, but enrollment is challenging due to variability in clinical outcome. Consequently, we need to identify clinical and kidney biopsy parameters of progressive disease. Our study confirms previous findings that eGFR at presentation associates with progression to kidney failure in C3 glomerulopathy (10,21,25,26) and membranoproliferative GN type 1 (8). Proteinuria at presentation and longitudinal change associated with progression in our study. This has been identified in other C3 glomerulopathy (25–27) and Ig-MPGN (9) cohorts, particularly with nephrotic-range proteinuria (21). However, in the Columbia cohort, eGFR at presentation was the only covariate associated with progression to kidney failure in a clinical multivariable model (10). These differences may relate to the prevalence of severe proteinuria. Notably, when we assessed the relationship between outcome-free survival and changes in proteinuria over the first 12 months following the baseline biopsy, there was no significant difference in outcome-free survival between those who did and those who did not achieve a 50% decrease in proteinuria. This has implications for the use of proteinuria as a surrogate marker of efficacy in trials.
Cohort studies have not consistently identified outcome differences between C3 glomerulopathy subtypes (DDD and C3 GN) (9–11,21), C3 glomerulopathy and membranoproliferative GN type 1 (8), or C3 glomerulopathy and Ig-MPGN (9,32). In our study, there was no difference in progression to the outcome between C3 GN and DDD, but progression in C3 glomerulopathy was more rapid than in Ig-MPGN. Light microscopic patterns of C3 glomerulopathy include membranoproliferative GN, mesangioproliferative lesions, diffuse endocapillary proliferative lesions, and diffuse sclerosing GN. These patterns do not associate with progression to kidney failure (10,21). Evidently, categorizing the glomerular pathology according to disease type (DDD, C3 GN, Ig-MPGN) and defining microscopic patterns of injury are not robustly associated with kidney outcome and provide limited, if any, information for risk stratification.
The most frequent glomerular changes in our cohort could be categorized as (1) active lesions (indicative of ongoing inflammation), including mesangial proliferation (expansion and hypercellularity), endocapillary hypercellularity with or without occlusion of capillary lumens, neutrophil infiltration, and cellular/fibrocellular crescents, and (2) chronic lesions, including GBM double contours, segmental and global glomerulosclerosis, and fibrous crescents. The corresponding active and chronic tubulointerstitial lesions were interstitial inflammation and IFTA, respectively. In our multivariable analysis, mesangial hypercellularity together with IFTA, interstitial inflammation, and cellular/fibrocellular crescents scores were significantly associated with eGFR at biopsy in both the whole cohort and the C3 glomerulopathy cohort. GBM double contours and endocapillary hypercellularity with occlusion of capillary lumens were associated with proteinuria at biopsy. The positive but weak (r=0.3) correlation between eGFR and mesangial hypercellularity/expansion was unexpected. GBM double contours are associated with cellular interposition where mesangial cells and leukocytes extend from mesangial areas between the layers of basement membrane. Consistent with this, GBM double contours were only seen when mesangial hypercellularity was present, whereas the converse (mesangial hypercellularity without GBM double contours) was evident in 26 of 156 biopsies. We speculate that the mesangial expansion is initially a reparative response to abnormal complement and Ig deposition when eGFR is preserved. However, eventually this leads to GBM disruption with the formation of double contours and proteinuria.
The C3 glomerulopathy histologic index has been used to assess disease activity and disease chronicity (10). Both the activity and chronicity scores associated with kidney outcome in our study. There was a negative correlation between eGFR at biopsy and chronicity score and a positive correlation between proteinuria at biopsy and activity score. All chronicity score components (glomerulosclerosis, IFTA, and arteriosclerosis) associated with outcome in a univariable analysis (10). However, cellular/fibrocellular crescent score was the only activity score component associated with outcome (10). After multivariable analysis in our study, the histologic determinants of risk of progression to kidney failure were IFTA, cellular/fibrocellular crescent, and segmental sclerosis scores. In the Mayo series, global glomerulosclerosis and arteriolar hyaline change associated with kidney outcome (21). In the GLOSEN registry, the IFTA and chronicity scores, but not activity score, associated with kidney outcome (25). One goal of our study was to provide data for the development of risk stratification tools for C3 glomerulopathy/Ig-MPGN, similar to that done in IgA nephropathy (33,34). Prediction models have the most clinical utility if they utilize readily available data. Accordingly, the histologic features identified in our study are routinely assessed at kidney biopsy.
Limitations of our study include those inherent in a retrospective cohort analysis. Treatment approaches were not uniform across recruiting sites, but this potential confounding factor is mitigated by the lack of specific disease-modifying treatments. We did not detect a difference in histology scores over time between patients treated with or without immunosuppression. We did not have comprehensive complement genetic data. However, a recent study did not find associations between rare complement gene variants, C3 glomerulopathy, and primary Ig-MPGN (35). In a large cohort of 173 patients with C3 glomerulopathy and Ig-MPGN, a clustering statistical approach that utilized genetic, clinical, and histologic data identified four groups (32). Individuals in “group 4” had the worst outcome, and relevant to our findings, had greater degrees of interstitial fibrosis and arteriosclerosis and a higher percentage of sclerosed glomeruli than the other groups.
In summary, in a large centralized review of C3 glomerulopathy and Ig-MPGN biopsies, we identified that the histologic determinants of risk of progression to kidney failure are IFTA, cellular/fibrocellular crescent, and segmental sclerosis scores. These are the histologic parameters to incorporate in risk prediction tools to stratify C3 glomerulopathy and Ig-MPGN at diagnosis.
Disclosures
C.E. Alpers reports consultancy agreements with AstraZeneca, Mantra Bio, and Travere; research funding from Boehringer-Ingelheim and Sana; and serving on the editorial boards of American Journal of Kidney Diseases, American Journal of Pathology, CJASN, Journal of Nephrology, Kidney360, and Laboratory Investigation. G.B. Appel reports consultancy agreements with Achillion, Alexion, Apellis, Aurinia, Bristol Myers Squibb, Chemocentryx, E. Lilly, EMD Serono, Genentech, Genzyme-Sanofi, GlaxoSmithKline, Mallinkrodt, Merck, Novartis, Omeros, Pfizer, Reata, Travere Therapeutics, and Vera Therapeutics; research funding from Achillion-Alexion, Apellis, Bristol Myers Squibb, Chemocentryx, EMD Serono, Genentech-Roche, Mallinkrodt, Reata, and Sanofi-Genzyme; honoraria from Aurinia and GlaxoSmithKline; royalties from UpToDate; serving in an advisory or leadership role for the UpToDate Editorial Board; serving in an advisory or leadership role on the medical advisory boards for Alexion, Alexion-Achillion, Apellis, Aurinia, BM Squib, Genentech, GlaxoSmithKline Lilly, Reata, Roche, and Sanofi; and speakers bureau for Aurinia (lectures on lupus nephritis) and GlaxoSmithKline (lectures on lupus nephritis). I. Bajema reports consultancy agreements with Aurinia, Boehringer Ingelheim, CatBio, GlaxoSmithKline, Novartis, and Toleranzia and serving as Director of the Bajema Institute of Pathology, as Vice President of the European Vasculitis Society, and as President of the Renal Pathology Society. S.J. Barbour reports consultancy agreements with Achillion, Alexion, Inception Sciences, Novartis, and Visterra; research funding from Alexion and Roche; and honoraria from Alexion and Roche. A.S. Bomback reports consultancy agreements with Catalyst, Chemocentryx, Novartis, Otsuka, Q32, Silence Therapeutics, and Visterra; honoraria from Alexion, Aurinia, Calliditas, GlaxoSmithKline, Novartis, Principio, Travere, and UpToDate; and consulting honoraria from Achillion, Alexion, Catalyst, ChemoCentryx, Novartis, Silence, and Visterra. D.C. Cattran reports consultancy agreements with Alnylam, Calliditis, Forsee, Horizon, Principia, and Reistone; research funding from Alnylam; honoraria from Alexion, Calliditis, Kyowa Hakko Kirin Co., and Principia; serving in an advisory or leadership role for Alnylam, NephCure, Standardised Outcomes in Nephrology-Glomerular Disease (SONG-GD), and UpToDate; and other interests or relationships with Aurinea, Dimerix, Novartis, and Vera Therapeutics. H.T. Cook reports consultancy agreements with Alexion Pharmaceuticals, Apellis Pharmaceuticals, Aurinia, and Novartis and research funding from Alexion Pharmaceuticals. V.D. D'Agati reports serving on the editorial board of Kidney International. F. Fakhouri reports consultancy agreements with Alexion, Alnylam, Apellis, Novartis, and Roche; honoraria from Alexion, Alnylam, Apellis, Novartis, and Roche; and serving in an advisory or leadership role for Alexion, Alnylam, Apellis, Novartis, and Roche. F.C. Fervenza reports consultancy agreements with Alexion Pharmaceuticals, Alnylam, ByoCrystal, Novartis, and Takeda; research funding from Chemocentryx, Genentech, Janssen Pharmaceutical, Questcor/Mallinckrodt, and Retrophin; honoraria from UpToDate; and serving in an advisory or leadership role for JASN, Kidney International, Nephrology, Nephrology Dialysis and Transplantation, and UpToDate. A.B. Fogo reports consultancy agreements with Novartis; research funding from Bayer, Gilead, and Novartis; honoraria from Amgen, GlaxoSmithKline, and Novartis; serving in an advisory or leadership role for an advisory committee for Bayer; serving on the editorial boards for American Journal of Pathology, American Journal of Physiology Renal Physiology, Human Pathology, and JASN; serving as an associate editor of Kidney International and Laboratory Investigation; serving as a subject editor for Current Opinion Nephrology and Hypertension (guest editor for the yearly pathology focus) and Nephrology Dialysis and Transplantation; and serving as a speaker at various national nephrology meetings and as President of the International Society of Nephrology (president as of April 19, 2021). C. Licht reports consultancy agreements with Alexion, Apellis Pharmaceuticals, Inc., AstraZeneca Rare Disease, Genentech, Inc.–Hoffmann La Roche, and Novartis; research funding from Aurin Biotech, Inc. and Pfizer Inc.; honoraria from Alexion, Apellis Pharmaceuticals, Inc., AstraZeneca Rare Disease, and Novartis; consulting honoraria and unrestricted research funding from Alexion, Aurinia, Eleva, and Novartis; patents or royalties for Factor H for the treatment of chronic nephropathies and production thereof (international patent WO 2007/038995 A1, US patent 11/992,194, CSL Behring patent 2005_M006_A105, Finnegan patent 06478.1518-00000); serving on the editorial boards of Kidney International, Nephrology Dialysis Transplantation, and Pediatric Nephrology; serving in an advisory or leadership role for Alexion, the steering committee of the Alport Syndrome Treatments and Outcomes Registry, AstraZeneca Rare Disease International atypical hemolytic uremic syndrome (aHUS) Registry scientific advisory board, the safety board of the European Treatment Trial for Alport Syndrome, and the safety advisory board of OPKO Health, Inc.; and speakers bureau for Alexion and AstraZeneca Rare Disease. S.D. Marks reports employment with Great Ormond Street Hospital for Children National Health Service (NHS) Foundation Trust; the hospital receives funding for immunosuppressive drug studies by Astellas and Novartis. S.D. Marks reports serving as an associate editor for pediatric transplantation for the following journals: British Journal for Renal Medicine, Pediatric Nephrology, Pediatric Transplantation, and Transplantation. C.C. Nast reports serving in an advisory or leadership role for BioCryst. M.C. Pickering reports consultancy agreements with Achillion, Alexion Pharmaceuticals, Apelllis Pharmaceuticals, and Gyroscope Pharmaceuticals; scientific advisory board fees from Gyroscope; consultancy fees from Alexion Pharma Consultancy and Apellis Pharma; and serving on the Gyroscope Pharmaceuticals scientific advisory board. S. Sethi reports consultancy agreements with Novartis and honorarium for teaching, grand rounds, lectures, UpToDate, and reviewing slides for a study for Novartis. S.-J. Tan reports honoraria from AstraZeneca Australia. A.M. Waters reports working as a consultant medical director for Purespring Therapeutics Ltd. All remaining authors have nothing to disclose.
Funding
This study was funded by an Achillion Pharmaceuticals unrestricted grant WIII_P63681. N.R. Medjeral-Thomas is supported by an Imperial College London and Wellcome Trust post-doctoral Research Fellowship WIII_P80067. M.C. Pickering is supported by Wellcome Trust Senior Fellow in Clinical Science grant 212252/Z/18/Z.
Supplementary Material
Acknowledgments
We thank and acknowledge David Apelian and Hetal Kocinsky for support and general advice with the study inception. We acknowledge the invaluable support for this study by Dr. Thomas Barbour. We thank all of the C3 Glomerulopathy Natural History Study patients, local site research personnel, and our site lead investigators: Dr. Kay Tan, The Royal Wolverhampton National Health Service (NHS) Trust; Dr. Mark Lambie, University Hospitals of North Midlands NHS Trust; Dr. Santhanakrishnan Balasubramanian, York Teaching Hospital NHS Foundation Trust; Dr. Arvind Ponnusamy, Lancashire Teaching Hospitals NHS Foundation Trust; Dr. Matthew Hall, Nottingham University Hospitals NHS Trust; Dr. Lucy Smyth, Royal Devon and Exeter NHS Foundation Trust; Dr. David Milford, Birmingham Women’s and Children’s NHS Foundation Trust; Dr. Bisher Kawar, Sheffield Teaching Hospitals NHS Foundation Trust; Dr. Kay Tyerman, Leeds Teaching Hospitals NHS Trust; Dr. Durga Kanigicherla, Manchester University NHS Foundation Trust; Dr. John Booth, Barts Health NHS Trust; Dr. Jo Taylor, Dorset County Hospital NHS Foundation Trust; Dr. Martin Christian, Nottingham University Hospitals NHS Trust; Dr. Jennifer Pinney, University Hospitals Birmingham NHS Foundation Trust; Dr. Nick Webb, Manchester University NHS Foundation Trust; Dr. Edwin Wong, Newcastle upon Tyne Hospitals NHS Foundation Trust; Dr. Daniel Gale, Royal Free London NHS Foundation Trust; Dr. Tarun Bansal, Bradford Teaching Hospitals NHS Foundation Trust; Dr. Colin Geddes, NHS Greater Glasgow and Clyde; Dr. Jonathan Barratt, University Hospitals of Leicester NHS Trust; Dr. Waqar Ayub, University Hospitals of Coventry and Warwickshire; and Dr. Rachel Jones, Cambridge University Hospitals NHS Foundation Trust. We acknowledge support from the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare National Health Service Trust and Imperial College London and from the NIHR Clinical Research Network.
The views expressed are those of the authors and not necessarily those of the National Health Service, NIHR, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Prognostication for C3 Glomerulopathy and Idiopathic Immunoglobulin-Associated Membranoproliferative Glomerulonephritis,” on pages 945–948.
Author Contributions
G.B. Appel, S.J. Barbour, T.H. Cairns, D.C. Cattran, H.T. Cook, F. Fakhouri, F.C. Fervenza, C. Licht, H.J. Lomax-Browne, S.D. Marks, N.R. Medjeral-Thomas, and M.C. Pickering conceptualized the study; C.E. Alpers, G.B. Appel, I. Bajema, A.S. Bomback, T.H. Cairns, H.T. Cook, V.D. D'Agati, F.C. Fervenza, A.B. Fogo, H. Han, H.J. Lomax-Browne, S.D. Marks, N.R. Medjeral-Thomas, C.C. Nast, M.C. Pickering, S. Sethi, and A.M. Waters were responsible for data curation; T.H. Cairns, H.T. Cook, F.C. Fervenza, H.J. Lomax-Browne, N.R. Medjeral-Thomas, and M.C. Pickering were responsible for investigation; C.E. Alpers, I. Bajema, S.J. Barbour, H.T. Cook, V.D. D'Agati, A.B. Fogo, J. Gisby, H.J. Lomax-Browne, N.R. Medjeral-Thomas, C.C. Nast, J.E. Peters, M.C. Pickering, S. Sethi, and R. Szydlo were responsible for formal analysis; C.E. Alpers, G.B. Appel, I. Bajema, S.J. Barbour, T.H. Cairns, D.C. Cattran, H.T. Cook, V.D. D'Agati, F. Fakhouri, A.B. Fogo, C. Licht, H.J. Lomax-Browne, S.D. Marks, N.R. Medjeral-Thomas, C.C. Nast, M.C. Pickering, and S. Sethi were responsible for methodology; H.T. Cook, H.J. Lomax-Browne, and M.C. Pickering were responsible for project administration; H.T. Cook and M.C. Pickering were responsible for resources; H.T. Cook, H.J. Lomax-Browne, N.R. Medjeral-Thomas, and M.C. Pickering were responsible for software; H.T. Cook, H.J. Lomax-Browne, N.R. Medjeral-Thomas, and M.C. Pickering were responsible for validation; H.T. Cook, H.J. Lomax-Browne, N.R. Medjeral-Thomas, and M.C. Pickering were responsible for visualization; H.T. Cook and M.C. Pickering were responsible for funding acquisition; H.T. Cook, N.R. Medjeral-Thomas, and M.C. Pickering provided supervision; H.T. Cook, H.J. Lomax-Browne, N.R. Medjeral-Thomas, and M.C. Pickering wrote the original draft; and C.E. Alpers, G.B. Appel, I. Bajema, S.J. Barbour, A.S. Bomback, T.H. Cairns, D.C. Cattran, H.T. Cook, V.D. D'Agati, F. Fakhouri, F.C. Fervenza, A.B. Fogo, J. Gisby, H. Han, C. Licht, H.J. Lomax-Browne, S.D. Marks, N.R. Medjeral-Thomas, C.C. Nast, J.E. Peters, M.C. Pickering, S. Sethi, R. Szydlo, S.-J. Tan, and A.M. Waters reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16801221/-/DCSupplemental.
Supplemental Table 1. Definitions of histologic features standardized and scored in the study.
Supplemental Table 2. Reproducibility of histology scores.
Supplemental Table 3. Definitions of activity and chronicity scores.
Supplemental Table 4. Joint model showing longitudinal change in proteinuria and the hazard of reaching an outcome event.
Supplemental Table 5. Correlations between eGFR, proteinuria, and histology features in the whole cohort
Supplemental Table 6. Correlations between eGFR, proteinuria, and histology features in C3G cohort only.
Supplemental Table 7. Regression of histologic features with eGFR and proteinuria at the time of biopsy.
Supplemental Table 8. Indications for repeat (B2) biopsy in 39 patients.
Supplemental Table 9. Indications for native kidney baseline (B1) and repeat (B2) biopsy where diagnosis changed from Ig-MPGN to C3G.
Supplemental Table 10. Regression of histologic features with eGFR and proteinuria at the time of a first repeat native biopsy.
Supplemental Figure 1. Proteinuria and outcome-free survival.
Supplemental Figure 2. Outcome-free survival in the whole cohort by individual histologic score quartiles.
Supplemental Figure 3. Outcome-free survival in the C3 glomerulopathy cohort by individual histologic score quartiles.
Supplemental Figure 4. Progression to a combined outcome event.
Supplemental Figure 5. Histograms of the histologic scores in 156 baseline kidney biopsies.
Supplemental Figure 6. Histograms of the histologic scores in 123 baseline kidney biopsies with C3G.
Supplemental Figure 7. Histograms of the histologic scores in 33 baseline kidney biopsies with Ig-MPGN.
Supplemental Figure 8. Correlation between histologic variables, eGFR, proteinuria, activity, and chronicity scores.
Supplemental Figure 9. Scatterplots of eGFR versus proteinuria and histologic features in the whole cohort.
Supplemental Figure 10. Scatterplots of proteinuria versus eGFR and histologic features in the whole cohort.
Supplemental Figure 11. Biopsy features over time.
Supplemental Figure 12. Outcome-free survival in the whole and C3G cohorts using a categorical histologic scoring system.
References
- 1.Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M, Doyle M, Fakhouri F, Fervenza FC, Fogo AB, Frémeaux-Bacchi V, Gale DP, Goicoechea de Jorge E, Griffin G, Harris CL, Holers VM, Johnson S, Lavin PJ, Medjeral-Thomas N, Paul Morgan B, Nast CC, Noel LH, Peters DK, Rodríguez de Córdoba S, Servais A, Sethi S, Song WC, Tamburini P, Thurman JM, Zavros M, Cook HT: C3 glomerulopathy: Consensus report. Kidney Int 84: 1079–1089, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook HT, Pickering MC: Histopathology of MPGN and C3 glomerulopathies. Nat Rev Nephrol 11: 14–22, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Levy M, Halbwachs-Mecarelli L, Gubler MC, Kohout G, Bensenouci A, Niaudet P, Hauptmann G, Lesavre P: H deficiency in two brothers with atypical dense intramembranous deposit disease. Kidney Int 30: 949–956, 1986 [DOI] [PubMed] [Google Scholar]
- 4.Licht C, Heinen S, Józsi M, Löschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B, Zipfel PF: Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int 70: 42–50, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Jokiranta TS, Solomon A, Pangburn MK, Zipfel PF, Meri S: Nephritogenic lambda light chain dimer: A unique human miniautoantibody against complement factor H. J Immunol 163: 4590–4596, 1999 [PubMed] [Google Scholar]
- 6.Corvillo F, Okrój M, Nozal P, Melgosa M, Sánchez-Corral P, López-Trascasa M: Nephritic factors: An overview of classification, diagnostic tools and clinical associations. Front Immunol 10: 886, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marinozzi MC, Chauvet S, Le Quintrec M, Mignotet M, Petitprez F, Legendre C, Cailliez M, Deschenes G, Fischbach M, Karras A, Nobili F, Pietrement C, Dragon-Durey MA, Fakhouri F, Roumenina LT, Fremeaux-Bacchi V: C5 nephritic factors drive the biological phenotype of C3 glomerulopathies. Kidney Int 92: 1232–1241, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grünfeld JP, Niaudet P, Lesavre P, Frémeaux-Bacchi V: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Iatropoulos P, Noris M, Mele C, Piras R, Valoti E, Bresin E, Curreri M, Mondo E, Zito A, Gamba S, Bettoni S, Murer L, Fremeaux-Bacchi V, Vivarelli M, Emma F, Daina E, Remuzzi G: Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol 71: 131–142, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Bomback AS, Santoriello D, Avasare RS, Regunathan-Shenk R, Canetta PA, Ahn W, Radhakrishnan J, Marasa M, Rosenstiel PE, Herlitz LC, Markowitz GS, D’Agati VD, Appel GB: C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int 93: 977–985, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, Teoh CW, Awan A, Waldron M, Cairns T, O’Kelly P, Dorman AM, Pickering MC, Conlon PJ, Cook HT: C3 glomerulopathy: Clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol 9: 46–53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith RJ, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Córdoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Würzner R, Zipfel PF; Dense Deposit Disease Focus Group : New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447–2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith RJH, Appel GB, Blom AM, Cook HT, D’Agati VD, Fakhouri F, Fremeaux-Bacchi V, Józsi M, Kavanagh D, Lambris JD, Noris M, Pickering MC, Remuzzi G, de Córdoba SR, Sethi S, Van der Vlag J, Zipfel PF, Nester CM: C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol 15: 129–143, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avasare RS, Canetta PA, Bomback AS, Marasa M, Caliskan Y, Ozluk Y, Li Y, Gharavi AG, Appel GB: Mycophenolate mofetil in combination with steroids for treatment of C3 glomerulopathy: A case series. Clin J Am Soc Nephrol 13: 406–413, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holle J, Berenberg-Goßler L, Wu K, Beringer O, Kropp F, Müller D, Thumfart J: Outcome of membranoproliferative glomerulonephritis and C3-glomerulopathy in children and adolescents. Pediatr Nephrol 33: 2289–2298, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Bharati J, Tiewsoh K, Kumar A, Nada R, Rathi M, Gupta KL, Kohli HS, Jha V, Ramachandran R: Usefulness of mycophenolate mofetil in Indian patients with C3 glomerulopathy. Clin Kidney J 12: 483–487, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caravaca-Fontán F, Díaz-Encarnación MM, Lucientes L, Cavero T, Cabello V, Ariceta G, Quintana LF, Marco H, Barros X, Ramos N, Rodríguez-Mendiola N, Cruz S, Fernández-Juárez G, Rodríguez A, Pérez de José A, Rabasco C, Rodado R, Fernández L, Pérez Gómez V, Ávila AI, Bravo L, Lumbreras J, Allende N, Sanchez de la Nieta MD, Rodríguez E, Olea T, Melgosa M, Huerta A, Miquel R, Mon C, Fraga G, de Lorenzo A, Draibe J, Cano-Megías M, González F, Shabaka A, López-Rubio ME, Fenollosa MA, Martín-Penagos L, Da Silva I, Alonso Titos J, Rodríguez de Córdoba S, Goicoechea de Jorge E, Praga M; Spanish Group for the Study of Glomerular Diseases GLOSEN : Mycophenolate mofetil in C3 glomerulopathy and pathogenic drivers of the disease. Clin J Am Soc Nephrol 15: 1287–1298, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabasco C, Cavero T, Román E, Rojas-Rivera J, Olea T, Espinosa M, Cabello V, Fernández-Juarez G, González F, Ávila A, Baltar JM, Díaz M, Alegre R, Elías S, Antón M, Frutos MA, Pobes A, Blasco M, Martín F, Bernis C, Macías M, Barroso S, de Lorenzo A, Ariceta G, López-Mendoza M, Rivas B, López-Revuelta K, Campistol JM, Mendizábal S, de Córdoba SR, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN) : Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int 88: 1153–1160, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Caliskan Y, Torun ES, Tiryaki TO, Oruc A, Ozluk Y, Akgul SU, Temurhan S, Oztop N, Kilicaslan I, Sever MS: Immunosuppressive treatment in C3 glomerulopathy: Is it really effective? Am J Nephrol 46: 96–107, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Yeter HH, Sütiçen E, Korucu B, Helvaci Ö, Özbaş B, Gönül İ, Derici Ü, Arinsoy T, Güz G: Evaluation of clinical, laboratory and treatment modalities in C3 glomerulopathy: Single center experience. Pril (Makedon Akad Nauk Umet Odd Med Nauki) 40: 15–23, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Ravindran A, Fervenza FC, Smith RJH, De Vriese AS, Sethi S: C3 glomerulopathy: Ten years’ experience at Mayo Clinic. Mayo Clin Proc 93: 991–1008, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chauvet S, Frémeaux-Bacchi V, Petitprez F, Karras A, Daniel L, Burtey S, Choukroun G, Delmas Y, Guerrot D, François A, Le Quintrec M, Javaugue V, Ribes D, Vrigneaud L, Arnulf B, Goujon JM, Ronco P, Touchard G, Bridoux F: Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy-associated C3 glomerulopathy. Blood 129: 1437–1447, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Le Quintrec M, Lapeyraque AL, Lionet A, Sellier-Leclerc AL, Delmas Y, Baudouin V, Daugas E, Decramer S, Tricot L, Cailliez M, Dubot P, Servais A, Mourey-Epron C, Pourcine F, Loirat C, Frémeaux-Bacchi V, Fakhouri F: Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am J Kidney Dis 72: 84–92, 2018 [DOI] [PubMed] [Google Scholar]
- 24.Medjeral-Thomas NR, Moffitt H, Lomax-Browne HJ, Constantinou N, Cairns T, Cook HT, Pickering MC: Glomerular complement factor H-related protein 5 (FHR5) is highly prevalent in C3 glomerulopathy and associated with renal impairment. Kidney Int Rep 4: 1387–1400, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caravaca-Fontán F, Trujillo H, Alonso M, Díaz-Encarnación M, Cabello V, Ariceta G, Quintana LF, Marco H, Barros X, Ramos N, Rodríguez-Mendiola N, Cruz S, Fernández-Juárez G, Rodríguez E, de la Cerda F, Pérez de José A, López I, Fernández L, Pérez Gómez V, Ávila A, Bravo L, Lumbreras J, Allende N, Sanchez de la Nieta MD, Olea T, Melgosa M, Huerta A, Miquel R, Mon C, Fraga G, de Lorenzo A, Draibe J, González F, Shabaka A, Illescas ML, Calvo C, Oviedo V, Da Silva I, Goicoechea de Jorge E, Caravaca F, Praga M; C3G Study Group of the Spanish Group for the Study of Glomerular Diseases (GLOSEN) : Validation of a histologic scoring index for C3 glomerulopathy. Am J Kidney Dis 77: 684–695.e1, 2021 [DOI] [PubMed] [Google Scholar]
- 26.Caravaca-Fontán F, Trujillo H, Caravaca F, Praga M: Contribution of a histologic index to the prognostic information of C3 glomerulopathy. Nephrol Dial Transplant 36: 2148–2150, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Caravaca-Fontan F, Diaz-Encarnacion M, Cabello V, Ariceta G, Quintana LF, Marco H, Barros X, Ramos N, Rodriguez-Mendiola N, Cruz S, Fernandez-Juarez G, Rodriguez A, de Jose AP, Rabasco C, Rodado R, Fernandez L, Gomez VP, Avila A, Bravo L, Espinosa N, Allende N, Sanchez de la Nieta MD, Rodriguez E, Olea T, Melgosa M, Huerta A, Miquel R, Mon C, Fraga G, de Lorenzo A, Draibe J, Cano-Megias M, Gonzalez F, Shabaka A, Lopez-Rubio ME, Fenollosa MA, Martin-Penagos L, Da Silva I, Titos JA, de Cordoba SR, de Jorge EG, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN) : Longitudinal change in proteinuria and kidney outcomes in C3 glomerulopathy [published online ahead of print March 29, 2021]. Nephrol Dial Transplant 10.1093/ndt/gfab075 [DOI] [PubMed] [Google Scholar]
- 28.Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis: Pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol 31: 341–348, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Hogan MC, Reich HN, Nelson PJ, Adler SG, Cattran DC, Appel GB, Gipson DS, Kretzler M, Troost JP, Lieske JC: The relatively poor correlation between random and 24-hour urine protein excretion in patients with biopsy-proven glomerular diseases. Kidney Int 90: 1080–1089, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pottel H, Hoste L, Dubourg L, Ebert N, Schaeffner E, Eriksen BO, Melsom T, Lamb EJ, Rule AD, Turner ST, Glassock RJ, De Souza V, Selistre L, Mariat C, Martens F, Delanaye P: An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant 31: 798–806, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerns E, Rozansky D, Troxell ML: Evolution of immunoglobulin deposition in C3-dominant membranoproliferative glomerulopathy. Pediatr Nephrol 28: 2227–2231, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Iatropoulos P, Daina E, Curreri M, Piras R, Valoti E, Mele C, Bresin E, Gamba S, Alberti M, Breno M, Perna A, Bettoni S, Sabadini E, Murer L, Vivarelli M, Noris M, Remuzzi G; Registry of Membranoproliferative Glomerulonephritis/C3 Glomerulopathy; Nastasi : Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J Am Soc Nephrol 29: 283–294, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki K, Katafuchi R, Er L, Espino-Hernandez G, Kim SJ, Reich HN, Feehally J, Cattran DC; International IgA Nephropathy Network : Evaluating a new international risk-prediction tool in IgA nephropathy. JAMA Intern Med 179: 942–952, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J, Herzenberg AM, Cattran DC; Oxford Derivation, North American Validation and VALIGA Consortia; Oxford Derivation North American Validation and VALIGA Consortia : The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 89: 167–175, 2016 [DOI] [PubMed] [Google Scholar]
- 35.Levine AP, Chan MMY, Sadeghi-Alavijeh O, Wong EKS, Cook HT, Ashford S, Carss K, Christian MT, Hall M, Harris CL, McAlinden P, Marchbank KJ, Marks SD, Maxwell H, Megy K, Penkett CJ, Mozere M, Stirrups KE, Tuna S, Wessels J, Whitehorn D, Johnson SA, Gale DP; MPGN/DDD/C3 Glomerulopathy Rare Disease Group; NIHR BioResource : Large-scale whole-genome sequencing reveals the genetic architecture of primary membranoproliferative GN and C3 glomerulopathy. J Am Soc Nephrol 31: 365–373, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.