Visual Abstract
Keywords: FENa, AKI, DTA, systematic review, meta-analysis, sodium
Abstract
Background and objectives
AKI is classified as prerenal, intrinsic, and postrenal. Prerenal AKI and intrinsic AKI represent the most common causes for AKI in hospitalized patients. This study aimed to examine the accuracy of the fractional excretion of sodium for distinguishing intrinsic from prerenal AKI.
Design, setting, participants, & measurements
We searched MEDLINE, Embase, Cochrane Central Register of Controlled Trials, the Cochrane Library, and Scopus for all available studies that met the criteria until December 31, 2021. We included studies that evaluated fractional excretion of sodium in differentiating AKI etiologies in adults, whereas studies that did not have sufficient data to extract a 2×2 table were excluded. We assessed the methodologic quality using the Quality Assessment of Diagnostic Accuracy Studies-2 tool and extracted the diagnostic accuracy data for all included studies. We conducted a meta-analysis using the bivariate random effects model. We performed subgroup analysis to investigate sources of heterogeneity and the effect of the relevant confounders on fractional excretion of sodium accuracy.
Results
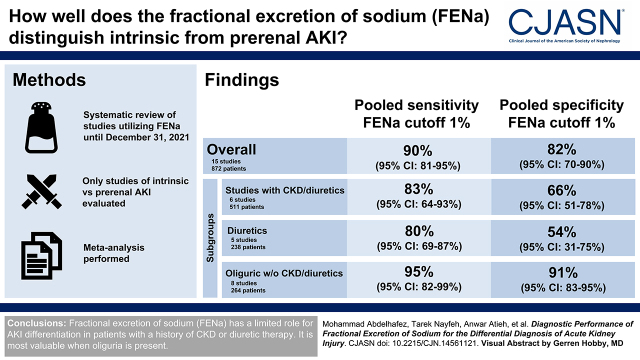
We included 19 studies with 1287 patients. In a subset of 15 studies (872 patients) that used a threshold of 1%, the pooled sensitivity and specificity for differentiating intrinsic from prerenal AKI were 90% (95% confidence interval, 81% to 95%) and 82% (95% confidence interval, 70% to 90%), respectively. In a subgroup of six studies (511 patients) that included CKD or patients on diuretics, the pooled sensitivity and specificity were 83% (95% confidence interval, 64% to 93%) and 66% (95% confidence interval, 51% to 78%), respectively. In five studies with 238 patients on diuretics, the pooled sensitivity and specificity were 80% (95% confidence interval, 69% to 87%) and 54% (95% confidence interval, 31% to 75%), respectively. In eight studies with 264 oliguric patients with no history of CKD or diuretic therapy, the pooled sensitivity and specificity were 95% (95% confidence interval, 82% to 99%) and 91% (95% confidence interval, 83% to 95%), respectively.
Conclusions
Fractional excretion of sodium has a limited role for AKI differentiation in patients with a history of CKD or those on diuretic therapy. It is most valuable when oliguria is present.
Introduction
AKI is defined as an abrupt decline in kidney function. Multiple criteria are used to define and stage AKI (1–3); it has multiple etiologies, commonly classified into prerenal, intrinsic renal, and postrenal. Prerenal AKI and intrinsic renal AKI are commonly regarded as transient and persistent AKI, respectively (4).
Identifying the cause of AKI is essential in guiding the required therapy, which highly influences the outcome of patients with AKI (5); unfortunately, there is no definitive way to differentiate intrinsic from prerenal AKI early in the disease.
Espinel (6) first introduced fractional excretion of sodium (FENa) in 1976. He showed FENa to be beneficial in differentiating prerenal and intrinsic AKI in the early course of AKI, especially in oliguric patients (7). However, FENa performance in the diagnosis of AKI has been inconsistent in the literature due to multiple reported confounders—the concurrent use of diuretics and having a history of CKD being the most important (8–11).
Given the invasive nature of kidney biopsy and the inconsistency of the literature regarding FENa usefulness, we performed a systematic review and meta-analysis encompassing different subgroups to evaluate the diagnostic performance of FENa in patients with AKI.
Materials and Methods
We performed this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies statement (12). The study protocol was not registered.
Eligibility Criteria
We included all studies that evaluated the diagnostic accuracy of the FENa for differentiating prerenal and intrinsic renal AKI. We included studies that provided information on the index test and reference standards irrespective of publication status or whether the data were collected prospectively or retrospectively. However, we excluded narrative reviews, letters, editorials, case reports, guidelines, consensus statements, trial registries, and animal studies. We also excluded studies that did not provide sufficient data to extract diagnostic test accuracy measures (i.e., true positives, false positives, false negatives, and true negatives).
The included patients were either hospitalized patients or outpatients aged ≥18 years diagnosed with AKI using any recognized criteria or equivalent. The included studies were required to categorize the patients into prerenal or intrinsic renal AKI using at least one of the following reference standards with or without urine microscopy: (1) responsiveness to volume expansion and (2) kidney biopsy. Additionally, we considered hepatorenal syndrome (HRS) and cardiorenal syndrome prerenal AKI causes. We included studies that enrolled patients with HRS if the diagnosis was consistent with recognized criteria proposed by expert panels (13–16). Moreover, for studies that comprised patients with cardiorenal syndrome, the diagnosis was to be compatible with the scientific statement from the American Heart Association (17).
Search Strategy
The search strategy was designed and conducted by an experienced librarian with input from the study’s authors for the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, The Cochrane Library (Cochrane Database of Systematic Reviews), and Scopus; all potential records published in English until December 31, 2021 were exported. Furthermore, we searched the included studies’ reference lists and any related review articles identified during the search. The actual strategy is provided in Supplemental Table 1.
Study Selection
DistillerSR was used to record the included studies. Two independent reviewers evaluated the title and abstract of each study identified from the search. We obtained the full text for references included by at least one of the two reviewers. The full-text screening was then independently carried out by two independent reviewers. A group discussion resolved disagreements.
Data Extraction
Two reviewers independently extracted the following data from each included study using a prepiloted data extraction form: first author; year of publication; publication status (abstract or full text); setting (hospitalized, outpatient, patients in the emergency room); inclusion and exclusion criteria for individual studies; the number of patients with prerenal and intrinsic renal AKI; the percentage of women; the average age of each group of study patients; comorbidities in each group of study patients; the proportion of oliguric patients in each group; the percentage of patients receiving diuretics ≤24 hours before the FENa test; mean serum creatinine of each group; the cutoff point used for the index test; reference standard; and the number of true positives, false positives, false negatives, and true negatives (i.e., 2×2 table) at each reported threshold. Any differences were resolved by discussion.
Quality Assessment
Two independent reviewers assessed the quality of the studies using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (18). We examined the bias and applicability of each study concerning four separate domains: patient selection, index test, reference standard, and the flow and timing of patients through the study. The criteria used for quality assessment were pre-established between the authors and provided in Supplemental Table 2. Any disagreement was resolved by discussion.
Statistical Analyses and Data Synthesis
We investigated how using FENa above the cutoff point performed at differentiating intrinsic AKI from prerenal AKI. We considered patients with FENa above the cutoff point who were diagnosed with intrinsic AKI true positives, and those who were diagnosed with prerenal AKI were considered false positives. We categorized patients with FENa results below the cutoff point who were diagnosed with prerenal AKI true negatives, and those diagnosed with intrinsic AKI were considered false negatives. Data from all articles were entered into Review Manager (RevMan; Version 5.4; The Cochrane Collaboration), which calculated the sensitivity, specificity, and 95% confidence interval (95% CI) for each study. For studies that applied over one cutoff point on their patients, we used 1% if it was applied, as it is the most clinically relevant. We then plotted the estimates of the perceived sensitivities and specificities together with their 95% CIs in the forest plot.
Only studies that used a cutoff of 1% were included in the quantitative analysis. To estimate the summary sensitivity and specificity and their corresponding 95% CIs for the included articles that used the 1% cutoff, we performed a meta-analysis using the bivariate random effects model on R 4.1.1 (R Core Team); we also computed the positive and negative likelihood ratios from the pooled estimates of sensitivity and specificity, as well as the diagnostic odds ratios with their corresponding 95% CIs. We also used the bivariate random effects model for the meta-analysis of the positive predictive value and the negative predictive value (19).
We identified the summary operating point and estimated average sensitivities and specificities with the 95% confidence and prediction regions on the summary receiver operating characteristics (SROC) plot. We assessed heterogeneity initially by visually inspecting the forest plot and the receiver operating characteristic space.
Finally, we performed subgroup analyses for the following: (1) patients who did not receive diuretics ≤24 hours before the FENa test; (2) patients who received diuretics; (3) studies that excluded patients with CKD; (4) studies that excluded patients with CKD and those receiving diuretics; (5) oliguric patients with AKI; (6) after combining the previously mentioned categorizations, oliguric patients with AKI who were not receiving diuretics and had no history of CKD; (7) studies that included patients with CKD or patients on diuretics; (8) studies published before 2000; and (9) studies published after 2000. Moreover, we compared subgroups when the data are independent (i.e., the participants should be part of one comparator only). We undertook comparisons of FENa accuracy between the diuretics and nondiuretics subgroups and also between the older and newer studies subgroups using meta-regression, with the subgroup added as a covariate to the bivariate model. We obtained the P value using the likelihood ratio test (Wald test).
Results
Study Selection and General Information
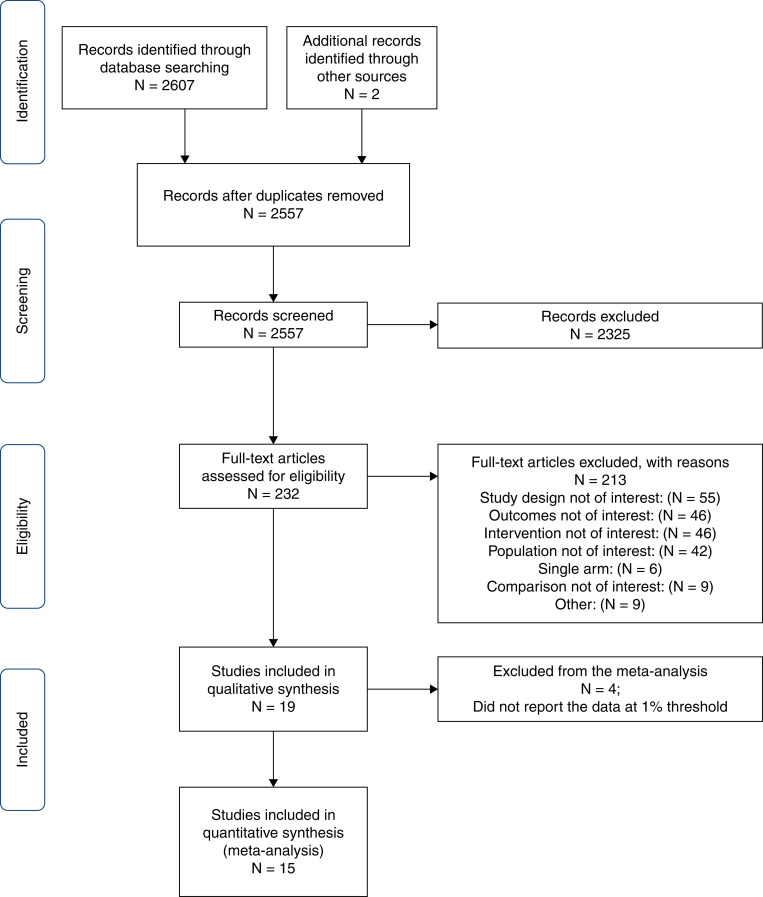
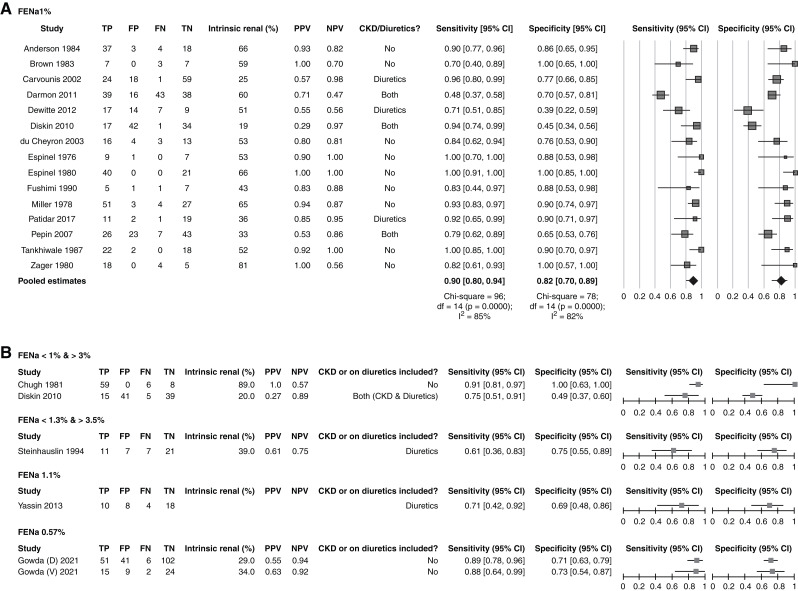
The literature search identified 2607 references. An additional two references were retrieved by checking the reference lists of the related articles. After duplicate removal and title and abstract screening, we excluded 2325 references and obtained the full text of 232 studies. Nineteen studies (1287 participants) met our inclusion criteria, of which we included 15 (872 participants) in the quantitative analysis. We present the study selection process in Figure 1. Table 1 shows the details of the 19 included studies. All of the included studies were full-text articles conducted prospectively except for one study, which was the only retrospective study (20). The majority of the intrinsic AKI in the included studies was in patients with acute tubular necrosis (ATN). All studies included hospitalized patients or patients in the intensive care unit, except for one study that did not report patients’ settings (6) and one study that enrolled patients in the emergency room (18). All but five studies (18,21–24) used the 1% threshold. In addition, we obtained the necessary data to apply the 1% threshold in one study (22). All of the studies used the clinical responsiveness to fluids as a reference standard, and two of them included patients with HRS and AKI (19,24,25). Additionally, four studies (6,7,21,26) used histopathologic examination other than response to fluid expansion, whereas nine studies (4,9,20,22–24,27–30) supported their findings using microscopic urine analysis.
Figure 1.
Flow chart of literature search and selection.
Table 1.
Studies that examined the performance of the fractional excretion of sodium in AKI
| Study, Year | Design, Settings | Intrinsic AKI, n; Age, Mean, yr; Women, %; Creatinine, Mean, mg/dl | Prerenal AKI, n; Age, Mean, yr; Women, %; Creatinine, Mean, mg/dl | Inclusion Criteria | Exclusion Criteria | Reference Standard | Fractional Excretion of Sodium Cutoff(s), % | Intrinsic AKI: Oliguric, %; Receiving Diuretics, % | Prerenal AKI: Oliguric, %; Receiving Diuretics, % | Intrinsic AKI: CKD, %; Sepsis, % | Prerenal AKI: CKD, %; Sepsis, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Espinel (6), 1976 | P, N/R | 9; N/R; N/R; 9.2±0.7 | 8; N/R; N/R; 8.3±1.1 | Acute onset of oliguria (urinary output below 20 ml/hr) | CKD, patients on diuretics, acute glomerular disease, or urinary tract obstruction | Responsiveness to fluids. ATN cases were also confirmed by histologic findings |
<1 and >3; 1 | 100; 0 | 100; 0 | 0; N/R | 0; N/R |
| Miller et al. (27), 1978 | P, hospitalized | 55; 58.1±3.5; 20; 3.9±0.26 | 30; 62±3; 34; 3±0.3 | Acute elevation of serum creatinine from normal levels (<1.4 mg/dl) to >2.0 mg/dl | CKD, patients on diuretics | Responsiveness to fluids, urine microscopy findings | 1 | 44; 0 | N/R; 0 | 0; N/R | 0; N/R |
| Espinel and Gregory (7), 1980 | P, hospitalized | 65; N/R; N/R; 5.2±1.5 | 8; N/R; N/R; 5.4±0.8 | Acute increase in creatinine level above 2 mg/dl | CKD, patients on diuretics | Response to fluids, urine microscopy, histopathology in some cases | 1 | 56; 0 | 100; 0 | 0; N/R | 0; N/R |
| Zager et al. (28), 1980 | P, hospitalized | 22; N/R; N/R; N/R | 5; N/R; N/R; N/R | Patients with AKI, defining criteria are not reported | N/R | Response to fluids, urine microscopy | 1 | 59; N/R | 100; N/R | 0; 0 | 0; 0 |
| Chugh et al. (21), 1981 | P, hospitalized | 40; N/R; N/R; N/R | 21; N/R; N/R; N/R | Oliguric AKI, defining criteria are not reported | CKD, acute GN, obstructive uropathy, diabetes mellitus, and cirrhosis | Response to fluids and pathologic findings | <1 and >3 | 100; 0 | 100; 0 | 0; N/R | 0; N/R |
| Brown et al. (29), 1983 | P, hospitalized | 10; N/R; N/R; N/R | 7; N/R; N/R; N/R | AKI defined by an acute rise in blood urea | CKD, patients on diuretics | Response to fluids, urine microscopy | 1 | 80; 0 | 100; 0 | 0; N/R | 0; N/R |
| Anderson et al. (30), 1984 | P, hospitalized | 41; N/R; N/R; 4.4±0.5 | 21; N/R; N/R; 2.6±0.2 | Acute rise in creatinine from <1.4 to >2.0 mg/dl | N/R; patients with CKD were studied separately | Responsiveness to fluids, urine microscopy findings | 1 | 53; N/R | 100; 0 | 0;a 0 | 0; 0 |
| Tankhiwale and Ungratwar (26), 1987 | P, hospitalized | 22; N/R; N/R; N/R | 20; N/R; N/R; N/R | Patients with AKI, although the defining criteria are not reported | Patients with CKD | Responsiveness to fluid expansion; histopathologic findings | 1 | 100; N/R | 100; N/R | 0; N/R | 0; N/R |
| Fushimi et al. (40), 1990 | P, hospitalized | 6; 52±16; 33; 5.2±1.3 | 8; 66.4±23; 50; 2.8±0.4 | Acute elevation of serum creatinine to >1.5 mg/dl, with a decrease in creatinine clearance | CKD, acute GN, and patients receiving diuretics | Responsiveness to fluids, urine microscopy findings | 1 | N/R; 0 | 100; 0 | 0; 0 | 0; 0 |
| Steinhäuslin et al. (18), 1994 | P, ICU and ER | 18; 39 (10th [21] to 90th [76] percentile); 64; 5.6 (10th [3.6] to 90th [9.5] percentile) | 28; 65.5 (10th [18] to 90th [88] percentile); 65; 1.8 (10th [1.2] to 90th [3.5] percentile) | A recent increase in plasma creatinine of >20% or plasma creatinine concentration above 200 μmol/L at the time of referral | Presence of acute GN, acute interstitial nephritis, and obstructive uropathy | Responsiveness to fluids, urine microscopy findings | <1.3 and >3.5 | N/R; 50 | N/R; 39 | 0; 0 | 0; 0 |
| Carvounis et al. (9), 2002 | P, hospitalized (mostly ICU) | 25; 47±3; 48; 5.9±0.5 | 77; 51±3; 51; 1.7±0.25 | Rapidly increasing BUN and creatinine (BUN >30 mg/dl and creatinine >1.5 mg/dl) with or without oliguria or serum creatinine increase in excess of 0.5 mg/dl in the preceding 2 d | Patients with acute interstitial nephritis, acute GN, and obstructive uropathy | Responsiveness to fluids, urinalysis findings | 1; 0.60; 0.80; 1.20; 1.50; 2; 3 | N/R; 0 | N/R; 35 | 0; 1 | 0; 32 |
| du Cheyron et al. (39), 2003 | P, ICU | 19; N/R; N/R; 3.3±1.6 | 17; N/R; N/R; 2.1 ± 0.5 | Sudden increase in serum creatinine level to ≥2 mg/dl or a value 50% greater than the basal concentration when CKD already existed | Patients with hepatorenal syndrome, cirrhosis | Responsiveness to fluids | 1 | N/R; N/R | N/R; N/R | 0; 0 | 0; 6 |
| Pépin et al. (4), 2007 | P, hospitalized | 33; 66.3; 55; 3.8±1.6 | 66; 66.8; 47; 2.5±1.3 | An increase in serum creatinine level of 30% or higher over the baseline with an abrupt onset (<1 wk) | Contrast examination <48 h before the onset of AKI, rhabdomyolysis, obstructive uropathy, acute GN, drug nephrotoxicity, and kidney failure | Responsiveness to fluids, urine microscopy findings | 1 | 36; 64 | 24; 65 | 45; 42 | 36; 24 |
| Diskin et al. (22), 2010 | P, hospitalized | 20; 67.5±12.7; 56; N/R | 80; 66.8±12.3; 49; N/R | AKI patients with oliguria; defined as urine output <600 ml/24 h and abrupt sustained rise in creatinine >1.9 mg/dl | All patients with possible creatinine assay–interfering drugs/conditions | Responsiveness to fluids | <1 and >3 | 100; 55 | 100; 71 | 28; 0 | 0; 7 |
| Darmon et al. (25), 2011 | P, ICU | 82; 66 (IQR, 56–74); 32; 2.5 (IQR, 1.6–4.1) | 54; 71 (IQR, 49–76); 41; 1.4 (IQR, 1.1–1.9) | AKI defined according to the AKIN or MDRD formula | Patients on dialysis, obstructive uropathy | Responsiveness to fluids | 1; 0.58 | N/R; 39 | N/R; 33 | 28; 74 | 6; 61 |
| Dewitte et al. (41), 2012 | P, ICU | 24; 69 (IQR, 54–73); 8; N/R | 23; 64 (IQR, 43–75); 35; N/R | AKI according to the consensus definition from the Acute Dialysis Quality Initiative group | Patients with CKD, obstructive uropathy | Responsiveness to fluids | 1 | 75; 58 | 74; 61 | 0; 29 | 0; 39 |
| Yassin et al. (23), 2013 | P, ICU | 14; 56.29±19.5; 42; 5.5±2.1 | 26; 60±15.15; 62; 2.2±0.6 | Patients with AKI with circulatory shock; circulatory shock was diagnosed according to the empirical criteria, AKI according to the RIFLE criteria in oliguric patients | CKD, obstructive uropathy, and patients on osmotic diuresis | Response to fluids, urinalysis | 1 | 100; 57 | 100; 46 | 0; N/R | 0; N/R |
| Patidar et al. (20), 2017 | R, hospitalized | 12; N/R; N/R; N/R | 21; N/R; N/R; N/R | Cirrhotic patients who were admitted for AKI, the defining criteria of AKI are not reported; HRS AKI was diagnosed on the basis of the International Club of Ascites definition | Patients with ascites, patients not on diuretics, advanced kidney failure | Clinical course decided by a nephrologist | 1 | N/R; 100 | N/R; 100 | 0; 0 | 0; 0 |
| Gowda et al. (D) (24), 2021 | P, hospitalized | 57; 48.47±10.8; 5; 3.2±1.78 | 143; 50.42±12.26; 6; 2.5±1.08 | Cirrhotic patients who were screened for AKI as per revised International Club of Ascites definitions at admission | CKD, history of KRT, diuretic use, exposure to potential nephrotoxic drugs, cardiovascular disease | Responsiveness to fluids; ATN AKI was diagnosed if abnormal kidney finding is present on ultrasound, proteinuria >500 mg/d, microhematuria (>50 red blood cells per high-power field), or presence of active urinary sediments; HRS AKI was diagnosed on the basis of the International Club of Ascites definition | 0.57 | N/R; 0 | N/R; 0 | 0; 0 | 0; 0 |
| Gowda et al. (V) (24), 2021 | P, hospitalized | 17; 49.24±9.5; 0; 2.5±1.11 | 33; 51.24±12.7; 15; 2.5±1.08 | Cirrhotic patients who were screened for AKI as per revised International Club of Ascites definitions at admission | CKD, history of KRT, diuretic use, exposure to potential nephrotoxic drugs, cardiovascular disease | Responsiveness to fluids; ATN AKI was diagnosed if abnormal kidney finding is present on ultrasound, proteinuria >500 mg/d, microhematuria (>50 red blood cells per high-power field), or presence of active urinary sediments; HRS AKI was diagnosed on the basis of the International Club of Ascites definition | 0.57 | N/R; 0 | N/R; 0 | 0; 0 | 0; 0 |
P, prospective; N/R, not reported; ATN, acute tubular necrosis; ICU, intensive care unit; ER, emergency room; IQR, interquartile range; AKIN, Acute Kidney Injury Network; MDRD, Modification of Diet in Renal Disease; R, retrospective; HRS, hepatorenal syndrome; D, derivation cohort; V, validation cohort.
This study included patients with CKD, but their data were not available.
Risk of Bias and Applicability
Nine studies (45%) have at least one high-risk judgment in one of the four domains. Six studies (30%) were judged to be at a high risk of bias for the patients' selection domain. Moreover, for the reference standard domain, only two studies (10%) were considered to have a low risk of bias. We considered 12 studies (60%) at low risk of applicability concerns in all three domains. Additionally, we judged all included studies to have a low-risk applicability concern in the reference standard domain. We present the QUADAS-2 results in Table 2 (Supplemental Figure 1 shows RevMan output).
Table 2.
Risk of bias and applicability concerns results
| Study Identification | Could the Selection of Patients Have Introduced Bias? | Are There Concerns that the Included Patients and Setting Do Not Match the Review Question? | Could the Conduct or Interpretation of the Index Test Have Introduced Bias? | Are There Concerns that the Index Test, Its Conduct, or Interpretation Differ from the Review Question? | Could the Reference Standard, Its Conduct, or Its Interpretation Have Introduced Bias? | Are There Concerns that the Target Condition as Defined by the Reference Standard Does Not Match the Question? | Could the Patient Flow Have Introduced Bias? |
|---|---|---|---|---|---|---|---|
| Espinel (6), 1976 | Low risk | Unclear concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Miller et al. (27), 1978 | High risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Espinel and Gregory (7), 1980 | Low risk | Low concern | Low risk | Low concern | Low risk | Low concern | Low risk |
| Zager et al. (28), 1980 | Low risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | High risk |
| Chugh et al. (21), 1981 | Low risk | Low concern | Unclear risk | High concern | Unclear risk | Low concern | Unclear risk |
| Brown et al. (29), 1983 | Low risk | Low concern | Unclear risk | Low concern | Unclear risk | Low concern | Unclear risk |
| Anderson et al. (30), 1984 | Low risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Tankhiwale and Ungratwar (26), 1987 | Low risk | Low concern | Unclear risk | Low concern | Unclear risk | Low concern | Unclear risk |
| Fushimi et al. (40), 1990 | Low risk | Low concern | Unclear risk | Low concern | Unclear risk | Low concern | Low risk |
| Steinhäuslin et al. (18), 1994 | High risk | Low concern | High risk | High concern | Unclear risk | Low concern | Low risk |
| Carvounis et al. (9), 2002 | Low risk | Low concern | Unclear risk | Low concern | High risk | Low concern | Unclear risk |
| du Cheyron et al. (39), 2003 | High risk | Low concern | Unclear risk | Low concern | High risk | Low concern | Low risk |
| Pépin et al. (4), 2007 | High risk | Low concern | Low risk | Low concern | Low risk | Low concern | Low risk |
| Diskin et al. (22), 2010 | Low risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Darmon et al. (25), 2011 | Low risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Dewitte et al. (41), 2012 | Low risk | Low concern | Low risk | Low concern | Unclear risk | Low concern | Low risk |
| Yassin et al. (23), 2013 | Low risk | Low concern | Unclear risk | Unclear concern | Unclear risk | Low concern | Unclear risk |
| Patidar et al. (20), 2017 | Low risk | Low concern | High risk | Unclear concern | Unclear risk | Low concern | Low risk |
| Gowda et al. (D) (24), 2021 | High risk | High concern | High risk | High concern | Unclear risk | Low concern | Low risk |
| Gowda et al. (V) (24), 2021 | High risk | High concern | Low risk | High concern | Unclear risk | Low concern | Low risk |
D, derivation cohort; V, validation cohort.
Synthesis of Results
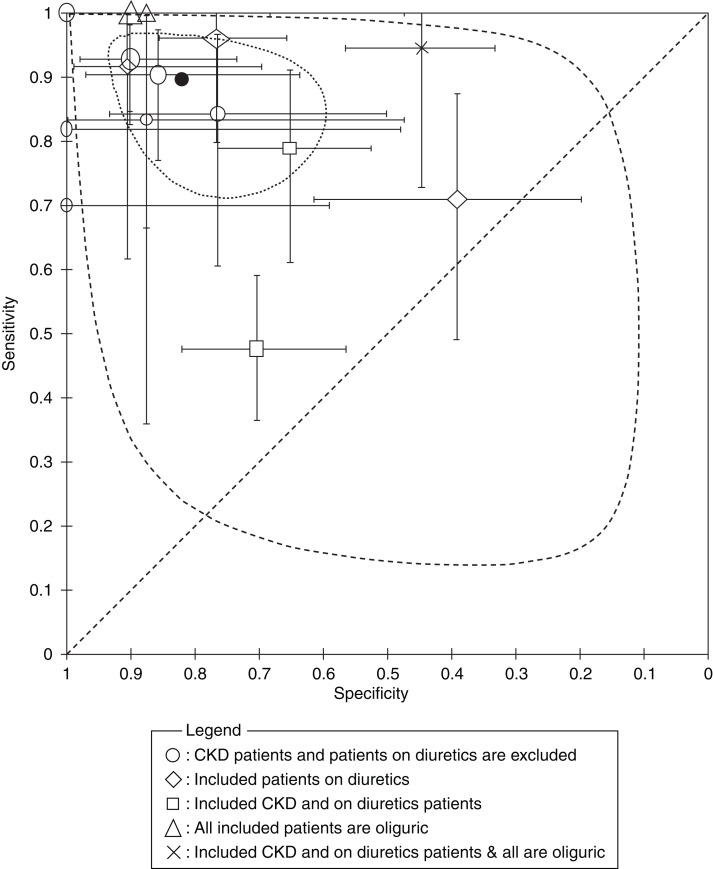
The forest plot in Figure 2 shows the true positives, false positives, false negatives, true negatives, sensitivity, and specificity of the FENa for each of the 19 studies included in the review at the principal reported threshold, with the corresponding 95% CIs. In 15 (79%) of the included studies, we extracted the data at a 1% threshold, in which the overall intrinsic AKI percentage was 48%. The overall sensitivity ranged from 48% to 100%, whereas the specificity was between 39% and 100%. The pooled sensitivity, specificity, diagnostic odds ratio, positive and negative likelihood ratios, and positive and negative predictive values of the FENa at the 1% cutoff point were 90% (95% CI, 81% to 95%), I2=85%; 82% (95% CI, 70% to 90%), I2=82%; 39 (95% CI, 13 to 122), I2=79%; 4.98 (95% CI, 2.82 to 8.78), I2=84%; 0.13 (95% CI, 0.06 to 0.26), I2=85%; 85% (95% CI, 71% to 93%), I2=90%; and 89% (95% CI, 78% to 95%), I2=87%, respectively. For the 15 studies included in the meta-analysis, we presented the summary operating point (summary sensitivity and specificity) and 95% confidence and prediction regions in the SROC plot (Figure 3).
Figure 2.
Forest plots for the included studies at the reported threshold of the fractional excretion of sodium (FENa) for intrinsic AKI versus prerenal AKI. (A) The forest plot for the 15 studies uses a 1% cutoff, including a quantitative meta-analysis of the sensitivity and specificity. The size of the square is proportional to the size of the population. The diamonds represent the pooled estimates. (B) The forest plot for studies that did not use a 1% threshold: FENa <1% and >3%, FENa <1% and >4%, FENa=1%, and FENa 0.57%. Output was from Review Manager (computer program). 95% CI, 95% confidence interval; D, derivation cohort; df, degrees of freedom; FN, false negative; FP, false positive; NPV, negative predictive value; PPV, positive predictive value; TN, true negative; TP, true positive; V, validation cohort.
Figure 3.
Summary receiver operating characteristic plot for the 15 studies used for FENa at the 1% threshold. The summary operating point (filled circle) for the 15 studies included in the meta-analysis is shown with the 95% confidence interval (dotted curve) and the 95% prediction region (dashed curve). The different legends represent the characteristics of each study population. The sizes of the ellipsoids, diamonds, squares, triangles, and crosses are proportional to the sample sizes for each study. Output was from Review Manager (computer program).
Subgroup Analyses
We conducted subgroup analyses to investigate sources of heterogeneity other than exploring the effect of the known confounders influencing FENa accuracy. We provided the results of the subgroup analysis in Table 3; forest plots and SROC plots are provided in Supplemental Figures 2 and 3, respectively. In six studies with 511 patients that included patients with CKD or patients on diuretics, the overall intrinsic AKI percentage was 38%. The pooled sensitivity, specificity, and positive and negative predictive values were 83% (95% CI, 64% to 93%), 66% (95% CI, 51% to 78%), 56% (95% CI, 43% to 69%), and 88% (95% CI, 66% to 97%), respectively. The accuracy of the FENa test was significantly higher in the nondiuretics group than in the diuretics (relative diagnostic odds ratio, 14.02; 95% CI, 2.53 to 77.74; P=0.005). The pooled sensitivity was higher in the nondiuretics group (92%; 95% CI, 85% to 96%) compared with the diuretics (80%; 95% CI, 69% to 87%) but statistically was not significant (P=0.08). Still, the pooled specificity was significantly higher in the nondiuretics group (88%; 95% CI, 83% to 92%; P<0.001) than the diuretics group (54%; 95% CI, 31% to 75%). Moreover, we were able to conduct a subgroup analysis for 264 oliguric patients with no history of CKD or diuretic therapy from eight studies. Altogether, intrinsic AKI percentage was 49%; the pooled sensitivity, specificity, and positive and negative predictive values were 95% (95% CI, 82% to 99%), 91% (95% CI, 83% to 95%), 92% (95% CI, 81% to 97%), and 96% (95% CI, 81% to 99%), respectively. The accuracy of FENa was significantly lower in studies published after 2000 than the earlier studies (relative diagnostic odds ratio, 0.06; 95% CI, 0.01 to 0.32; P=0.003), although the specificity but not the sensitivity was significantly lower in the newer studies (P<0.001).
Table 3.
Results of subgroup analysis
| Subgroup No. | Subgroup | No. of Studies | No. of Participants | Intrinsic AKI, N (%) | Pooled Sensitivity [95% Confidence Interval]; I2 | Pooled Specificity [95% Confidence Interval]; I2 | Diagnostic Odds Ratio [95% Confidence Interval]; I2 | Positive Likelihood Ratio [95% Confidence Interval]; I2 | Negative Likelihood Ratio [95% Confidence Interval]; I2 | Positive Predictive Value [95% Confidence Interval]; I2 | Negative Predictive Value [95% Confidence Interval]; I2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Patients not on diuretics | 12 | 502 | 270 (54) | 92% [85% to 96%]; 57% | 88% [83% to 92%]; 20% | 88 [34 to 226]; 24% | 7.83 [5.10 to 12.01]; 0% | 0.09 [0.05 to 0.17]; 47% | 91% [83% to 95%]; 62% | 92% [82% to 96%]; 65% |
| 2 | Patients on diuretics | 5 | 238 | 88 (37) | 80% [69% to 87%]; 0% | 54% [31% to 75%]; 86% | 5 [1 to 16]; 59% | 1.71 [0.97 to 3.02]; 77% | 0.38 [0.18 to 0.80]; 27% | 54% [31% to 75%]; 89% | 82% [61% to 93%]; 76% |
| 3 | Without patients with CKD | 13 | 632 | 294 (47) | 92% [87% to 96%]; 50% | 85% [73% to 93%]; 84% | 69 [25 to 191]; 36% | 6.22 [3.21 to 12.02]; 88% | 0.09 [0.05 to 0.16]; 38% | 89% [73% to 96%]; 92% | 92% [84% to 96%]; 69% |
| 4 | Patients without CKD and not on diuretics | 11 | 464 | 225 (49) | 93% [86% to 96%]; 54% | 89% [84% to 93%]; 14% | 107 [41 to 279]; 0% | 8.60 [5.53 to 13.38]; 0% | 0.08 [0.04 to 0.16]; 46% | 92% [85% to 96%]; 58% | 92% [82% to 97%]; 68% |
| 5 | Oliguric patients | 9 | 355 | 154 (43) | 93% [82% to 97%]; 62% | 88% [71% to 96%]; 87% | 97 [20 to 471]; 46% | 7.84 [2.91 to 21.10]; 90% | 0.08 [0.03 to 0.22]; 62% | 90% [70% to 97%]; 92% | 93% [82% to 98%]; 60% |
| 6 | Oliguric patients without CKD and not on diuretics | 8 | 264 | 130 (49) | 95% [82% to 99%]; 62% | 91% [83% to 95%]; 20% | 197 [38 to 1017]; 0% | 10.07 [5.58 to 18.15]; 0% | 0.05 [0.01 to 0.22]; 65% | 92% [81% to 97%]; 51% | 96% [81% to 99%]; 57% |
| 7 | Studies including patients with CKD or on diuretics | 6 | 511 | 194 (38) | 83% [64% to 93%]; 88% | 66% [51% to 78%]; 84% | 10 [3 to 34.0]; 78% | 2.42 [1.52 to 3.87]; 82% | 0.26 [0.10 to 0.63]; 85% | 56% [43% to 69%]; 82% | 88% [66% to 97%]; 94% |
| 8 | Studies published before 2000 | 7 | 325 | 205 (63) | 93% [84% to 97%]; 63% | 92% [85% to 95%]; 1% | 148 [43 to 508]; 0% | 10.93 [5.89 to 20.30]; 0% | 0.07 [0.03 to 0.18]; 55% | 95% [91% to 97%]; 36% | 90% [75% to 96%]; 55% |
| 9 | Studies published after 2000 | 8 | 547 | 213 (39) | 83% [67% to 92%]; 86% | 67% [53% to 78%]; 82% | 10 [3 to 30]; 76% | 2.52 [1.64 to 3.86]; 80% | 0.25 [0.12 to 0.55]; 84% | 60% [46% to 72%]; 82% | 87% [68% to 96%]; 93% |
Discussion
We conducted the first systematic review and meta-analysis to evaluate the performance of FENa in differentiating intrinsic AKI (mainly ATN) from prerenal AKI in adult patients, including controlling for the pertinent confounders. Our results showed that FENa is a good tool for differentiating intrinsic AKI from prerenal AKI in oliguric patients but performed less favorably in patients who have CKD and those on diuretics. When FENa was first introduced by Espinel (6) and examined in multiple studies later (7,27), the targeted population was patients with AKI not on diuretics, without a history of CKD, and had oliguria, in whom FENa showed robust performance. Although our findings showed a notably good performance for FENa in this subgroup of patients (Table 3, subgroup 6), considerable within-study uncertainty was observed through the 95% CI in the receiver operating characteristic plot (Supplemental Figure 3E). Additionally, the number of included studies and the number of involved patients in each study are small, which may concern the applicability of the results.
CKD and diuretics cannot be ignored when evaluating the performance of FENa; for instance, 30% and nearly 50% of patients in the intensive care unit had preadmission CKD and diuretics use, respectively (31,32). Diuretics alter urinary electrolyte concentrations; they increase the urinary sodium concentration and thus can falsely elevate the FENa in prerenal patients with AKI (i.e., higher false-positive rate) (30,33). Patients with CKD may not conserve their sodium in response to AKI because of prekidney disease or with a decrease in dietary sodium intake (11), leading to a higher false-positive rate. Also, this is consistent with our findings in the diuretics and CKD/diuretics subgroups. The accuracy of the FENa test, especially the specificity, is substantially reduced among this group of patients. Our results demonstrated better FENa performance when used in the patients not on diuretics compared with patients on diuretics, and this was particularly true for test specificity (P<0.001). This finding is concurrent with the effect of diuretics on sodium reabsorption (30,33). Moreover, excluding patients on diuretics and patients with CKD provided a prominently high pooled sensitivity and specificity (Table 3, subgroup 4) and explained much of the observed heterogeneity in Figure 3 (Supplemental Figure 3C).
FENa can have higher false negatives when used in nonoliguric patients with AKI; nonoliguric ATN can falsely decrease the result of FENa below 1%. The large volume of solvent in nonoliguric ATN may lower the concentration of sodium in the urine when there is neither salt nor water conservation (34). However, we could not extract the nonoliguric subgroup to test the accuracy of FENa in patients with AKI with no oliguria. Most of the studies were not clear about the urine output state or did not provide enough data to extract the required 2 × 2 table. Nevertheless, we were able to perform a subgroup analysis for oliguric patients (Table 3, subgroup 5). Additionally, we observed a higher performance for the FENa test in most of the earlier studies (before 2000) compared with more recent studies, and this was statistically significant for specificity (P<0.001) but not for sensitivity (P=0.12). However, this variation can be explained by including patients with CKD or patients on diuretics in most of the recent studies (Supplemental Figure 3F).
Perazella et al. (35) recently reported on using urine microscopy as an estimable diagnostic tool for enhancing ATN diagnosis. They showed that it strongly supports the differentiation between the etiologies of AKI. They developed a scoring system that solely relies on the number of granular casts and renal tubular epithelial cells in the microscopic analysis of urine. Their approach provided a sensitivity of 76% and a specificity of 86% in distinguishing ATN from prerenal AKI (35). Still, when clinical suspicion contradicts urine microscopy, FENa would be beneficial to support the diagnosis, especially in oliguric patients without CKD and not on diuretics. A scoring system that combines urine microscopy and FENa would be of interest but has not been developed. Given the findings in our review, future studies combining the FENa and urine microscopy could improve the sensitivity and specificity of the scoring system. Additionally, Carvounis et al. (9) introduced fractional excretion of urea (FEUrea) for distinguishing prerenal and intrinsic AKI and showed that it has better accuracy in patients with AKI using diuretics. Their results revealed that 48% of prerenal patients on diuretics had FENa <1%. By contrast, 89% had FEUrea <35% (9). Moreover, their findings were confirmed by Diskin et al. (22) as 96% of prerenal patients receiving diuretics had FEUrea <40%, whereas 29% of them had FENa <1%. Yet, FENa was reported to be superior in patients not on diuretics (4).
Ultimately, there are multiple known causes of intrinsic AKI with FENa below 1%. Early in the course of sepsis-induced ATN (36), in patients with chronic prekidney disease overlays by ATN, such as liver cirrhosis and heart failure (37), and patients with less severe early assessed ischemic ATN, as these patients might be in the transition state from prekidney disease to postischemic ATN (38).
This study has some limitations. Some variations are present in defining AKI in the included studies, as many were conducted before the introduction of the recent definitions of AKI (1–3). Although we are reasonably confident that the reference standard used in our review is robust and clinically appropriate, it should be mentioned that in two (9,39) of the included studies, the reference standard was carried out with the knowledge of the index test results; only two studies (4,7) of the remaining 17 were clear about interpreting the reference standard apart from the index test. This may overestimate the accuracy of the index test. Although the FENa test should be done early in the disease before the final diagnosis, six studies (21,23,26,29,39,40) were unclear if the index test was interpreted separately from the reference standard. In five (9,21,23,26,29) of the included studies, we were uncertain whether the interval between the index test and the reference standard was appropriate. Finally, most of the studies' patients were hospitalized, which may affect the external validity of our findings.
FENa can be used to distinguish intrinsic from prerenal AKI in oliguric patients, where we found it to perform the best. Its ability to distinguish intrinsic from prerenal AKI and, hence, its clinical utility are significantly diminished in patients with CKD and those receiving diuretics. On the basis of the results of this meta-analysis, obtaining an FENa value for every patient with AKI, as commonly practiced, should be discouraged, and a thorough clinical review of existing comorbidities, including the presence of CKD and current use of diuretics, is needed to help guide its clinical use and interpretation. A large prospective study that considers patients' history, comorbidities, medications, and urine output is much needed to further define the clinical role of FENa in the diagnosis of AKI.
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
We thank Dr. Charles Diskin for sharing the requested data with us.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Fractional Excretion of Sodium (FENa): An Imperfect Tool for a Flawed Question,” on pages 777–778.
Author Contributions
M. Abdelhafez, O. AbuShamma, A. Atieh, B. Babaa, M. Baniowda, K. Gharaibeh, and A. Hamadah conceptualized the study; M. Abdelhafez, O. AbuShamma, A. Atieh, B. Babaa, M. Baniowda, K. Gharaibeh, A. Hamadah, B. Hasan, A. Hrizat, and T. Nayfeh were responsible for data curation; M. Abdelhafez, O. AbuShamma, A. Atieh, B. Babaa, M. Baniowda, K. Gharaibeh, A. Hamadah, B. Hasan, A. Hrizat, and T. Nayfeh were responsible for investigation; M. Abdelhafez and T. Nayfeh were responsible for formal analysis; M. Abdelhafez, O. AbuShamma, A. Atieh, B. Babaa, M. Baniowda, K. Gharaibeh, A. Hamadah, B. Hasan, A. Hrizat, and T. Nayfeh were responsible for methodology; M. Abdelhafez, K. Gharaibeh, A. Hamadah, B. Hasan, and T. Nayfeh were responsible for project administration; B. Hasan, L. Hassett, and T. Nayfeh were responsible for resources; M. Abdelhafez, O. AbuShamma, A. Atieh, and T. Nayfeh were responsible for software; K. Gharaibeh, A. Hamadah, B. Hasan, and T. Nayfeh were responsible for validation; M. Abdelhafez, K. Gharaibeh, A. Hamadah, and B. Hasan were responsible for visualization; K. Gharaibeh, A. Hamadah, B. Hasan, and T. Nayfeh provided supervision; M. Abdelhafez wrote the original draft; and M. Abdelhafez, O. AbuShamma, A. Atieh, K. Gharaibeh, A. Hamadah, B. Hasan, A. Hrizat, and T. Nayfeh reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14561121/-/DCSupplemental.
Supplemental Figure 1. Risk of bias and applicability concerns.
Supplemental Figure 2. Forest plots for the subgroup analysis.
Supplemental Figure 3. The summary ROC plots for the subgroup analysis.
Supplemental Table 1. Actual search strategies.
Supplemental Table 2. QUADAS-2 classification.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup : Acute renal failure - Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A; Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kidney Disease Improving Global Outcomes (KDIGO) : Acute Kidney Injury (AKI). Available at: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed August 28, 2021
- 4.Pépin MN, Bouchard J, Legault L, Ethier J: Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis 50: 566–573, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Schrier RW: Need to intervene in established acute renal failure. J Am Soc Nephrol 15: 2756–2758, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Espinel CH: The FENa test. Use in the differential diagnosis of acute renal failure. JAMA 236: 579–581, 1976 [DOI] [PubMed] [Google Scholar]
- 7.Espinel CH, Gregory AW: Differential diagnosis of acute renal failure. Clin Nephrol 13: 73–77, 1980 [PubMed] [Google Scholar]
- 8.Pru C, Kjellstrand CM: The FENa test is of no prognostic value in acute renal failure. Nephron 36: 20–23, 1984 [DOI] [PubMed] [Google Scholar]
- 9.Carvounis CP, Nisar S, Guro-Razuman S: Significance of the fractional excretion of urea in the differential diagnosis of acute renal failure. Kidney Int 62: 2223–2229, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Danovitch GM, Bourgoignie J, Bricker NS: Reversibility of the “salt-losing” tendency of chronic renal failure. N Engl J Med 296: 14–19, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Coleman AJ, Arias M, Carter NW, Rector FC, Seldin DW: The mechanism of salt wastage in chronic renal disease. J Clin Invest 45: 1116–1125, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salameh JP, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P, Deeks JJ, Leeflang M, Korevaar DA, Whiting P, Takwoingi Y, Reitsma JB, Cohen JF, Frank RA, Hunt HA, Hooft L, Rutjes AWS, Willis BH, Gatsonis C, Levis B, Moher D, McInnes MDF: Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies (PRISMA-DTA): Explanation, elaboration, and checklist. BMJ 370: m2632, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J: Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology 23: 164–176, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V: Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Postgrad Med J 84: 662–670, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Angeli P, Ginès P, Wong F, Bernardi M, Boyer TD, Gerbes A, Moreau R, Jalan R, Sarin SK, Piano S, Moore K, Lee SS, Durand F, Salerno F, Caraceni P, Kim WR, Arroyo V, Garcia-Tsao G: Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol 62: 968–974, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR: Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 74: 1014–1048, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, Lerma EV, Mezue K, Molitch M, Mullens W, Ronco C, Tang WHW, McCullough PA; American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Clinical Cardiology : Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 139: e840–e878, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Steinhäuslin F, Burnier M, Magnin JL, Munafo A, Buclin T, Diezi J, Biollaz J: Fractional excretion of trace lithium and uric acid in acute renal failure. J Am Soc Nephrol 4: 1429–1437, 1994 [DOI] [PubMed] [Google Scholar]
- 19.Leeflang MMG, Deeks JJ, Rutjes AWS, Reitsma JB, Bossuyt PMM: Bivariate meta-analysis of predictive values of diagnostic tests can be an alternative to bivariate meta-analysis of sensitivity and specificity. J Clin Epidemiol 65: 1088–1097, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Patidar KR, Kang L, Siddiqui MS, Carl D, Henry G, Bajaj JS, Sanyal AJ: The utility of fractional excretion of urea for the differential diagnosis of acute kidney injury in decompensated cirrhotics on diuretic therapy. Gastroenterology 152: S1149, 2017 [Google Scholar]
- 21.Chugh KS, Bhoopal R, Sakhuja V, Malik GH, Choudhry N, Chhabra S, Mehta RL, Singhal PC: Diagnostic indices in acute renal failure. Indian J Med Res 73: 101–105, 1981 [PubMed] [Google Scholar]
- 22.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB: The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron Clin Pract 114: c145–c150, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Yassin AR, Sherif HM, Mousa AY, Esmat A: Comparison between fractional excretion of sodium and fractional excretion of urea in differentiating prerenal from renal azotemia in circulatory shock. Egyptian J Crit Care Med 1: 69–77, 2013 [Google Scholar]
- 24.Gowda YHS, Jagtap N, Karyampudi A, Rao NP, Deepika G, Sharma M, Gupta R, Tandan M, Ramchandani M, John P, Kulkarni A, Kumar P, Bhaware B, Turpati MV, Reddy ND: Fractional excretion of sodium and urea in differentiating acute kidney injury phenotypes in decompensated cirrhosis [published online ahead of September 28, 2021]. J Clin Exp Hepatol 10.1016/J.JCEH.2021.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darmon M, Vincent F, Dellamonica J, Schortgen F, Gonzalez F, Das V, Zeni F, Brochard L, Bernardin G, Cohen Y, Schlemmer B: Diagnostic performance of fractional excretion of urea in the evaluation of critically ill patients with acute kidney injury: A multicenter cohort study. Crit Care 15: R178, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tankhiwale SR, Ungratwar KP: Diagnostic evaluation of urinary indices in acute renal failure. J Assoc Physicians India 35: 557–559, 1987 [PubMed] [Google Scholar]
- 27.Miller TR, Anderson RJ, Linas SL, Henrich WL, Berns AS, Gabow PA, Schrier RW: Urinary diagnostic indices in acute renal failure: A prospective study. Ann Intern Med 89: 47–50, 1978 [DOI] [PubMed] [Google Scholar]
- 28.Zager RA, Rubin NT, Ebert T, Maslov N: Rapid radioimmunoassay for diagnosing acute tubular necrosis. Nephron 26: 7–12, 1980. 10.1159/000181942 [DOI] [PubMed] [Google Scholar]
- 29.Brown MA, Trew PA, Smart RC: A simple aid to the differential diagnosis of oliguria. Aust N Z J Med 13: 608–612, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Anderson RJ, Gabow PA, Gross PA: Urinary chloride concentration in acute renal failure. Miner Electrolyte Metab 10: 92–97, 1984 [PubMed] [Google Scholar]
- 31.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 32.McCoy IE, Chertow GM, Chang TIH: Patterns of diuretic use in the intensive care unit. PLoS One 14: e0217911, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner RW: Interpreting the fractional excretion of sodium. Am J Med 77: 699–702, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Diskin CJ, Stokes TJ, Dansby LM, Radcliff L, Carter TB: Toward the optimal clinical use of the fraction excretion of solutes in oliguric azotemia. Ren Fail 32: 1245–1254, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Perazella MA, Coca SG, Kanbay M, Brewster UC, Parikh CR: Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol 3: 1615–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vanmassenhove J, Glorieux G, Hoste E, Dhondt A, Vanholder R, Van Biesen W: Urinary output and fractional excretion of sodium and urea as indicators of transient versus intrinsic acute kidney injury during early sepsis. Crit Care 17: R234, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diamond JR, Yoburn DC: Nonoliguric acute renal failure associated with a low fractional excretion of sodium. Ann Intern Med 96: 597–600, 1982 [DOI] [PubMed] [Google Scholar]
- 38.Brosius FC, Lau K: Low fractional excretion of sodium in acute renal failure: Role of timing of the test and ischemia. Am J Nephrol 6: 450–457, 1986 [DOI] [PubMed] [Google Scholar]
- 39.du Cheyron D, Daubin C, Poggioli J, Ramakers M, Houillier P, Charbonneau P, Paillard M: Urinary measurement of Na+/H+ exchanger isoform 3 (NHE3) protein as new marker of tubule injury in critically ill patients with ARF. Am J Kidney Dis 42: 497–506, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Fushimi K, Shichiri M, Marumo F: Decreased fractional excretion of urate as an indicator of prerenal azotemia. Am J Nephrol 10: 489–494, 1990 [DOI] [PubMed] [Google Scholar]
- 41.Dewitte A, Biais M, Petit L, Cochard JF, Hilbert G, Combe C, Sztark F: Fractional excretion of urea as a diagnostic index in acute kidney injury in intensive care patients. J Crit Care 27: 505–510, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.