Abstract
Novel immunotherapy drugs have changed the landscape of cancer medicine. Immune checkpoint inhibitors and chimeric antigen receptor T cells are being used and investigated in almost all types of cancers. Immune-related adverse events have been associated with immunotherapies. AKI has been the most commonly associated kidney adverse event. In this review, we showcase the several associated electrolyte disorders seen with immunotherapy. Immune checkpoint inhibitors can lead to hyponatremia by several mechanisms, with the syndrome of inappropriate antidiuresis being the most common. Endocrine causes of hyponatremia are rare. Hypokalemia is not uncommon and is associated with both proximal and distal renal tubular acidosis. Hypercalcemia associated with immune checkpoint inhibitors has led to some interesting observations, including immune checkpoint inhibitor–induced parathyroid hormone–related peptide production, sarcoid-like granulomas, and hyperprogression of the disease. Hypocalcemia and hyperphosphatemia may be seen with immune checkpoint inhibitor–induced tumor lysis syndrome. Chimeric antigen receptor T cell therapy–associated electrolyte disorders are also common. This is associated chiefly with hyponatremia, although other electrolyte abnormalities can occur. Early recognition and prompt diagnosis may help providers manage the mechanistically varied and novel electrolyte disorders associated with immunotherapy.
Keywords: immunotherapy, hyponatremia, hypokalemia, hypercalcemia, cancer
Overview of Cancer Immunotherapy for the Nephrologist
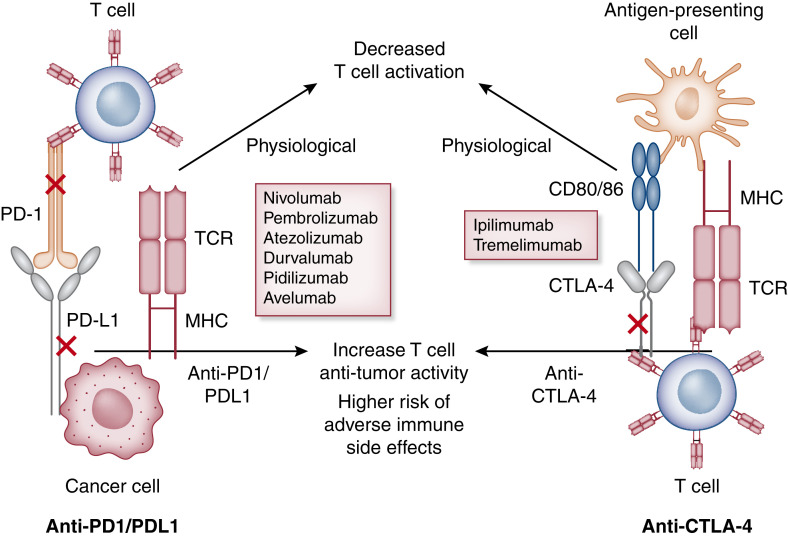
Novel immunotherapies have become synonymous with immune checkpoint inhibitors (ICIs) and chimeric antigen receptor–T (CAR-T) cell therapy, which have revolutionized the field of oncology (1). Several cancers are able to evade destruction by attenuating activated cancer-specific T cells, which allows tumor cells to proliferate unchecked and metastasize. Immune checkpoints also regulate activated T cells at later stages by either allowing continued anticancer activity or deactivating T cells to avoid overstimulation and autoimmunity (2) (Figure 1). ICIs are now considered standard of care in the management of many advanced cancers (3). Three main classes of these agents are used in clinical practice, namely cytotoxic T lymphocyte–associated protein 4 (CTLA-4) inhibitors, programmed cell death protein 1 (PD-1), and programmed cell death ligand 1 (PD-L1) inhibitors. The immune-related adverse events are inflammatory in nature with the potential to affect multiple organ systems. Common ICI-associated immune-related adverse events include dermatitis, colitis, and endocrinopathies, and they can be life threatening. AKI after ICI was noted in early case reports with acute tubulointerstitial nephritis (AIN) as the most common pattern of injury, and AIN can frequently be accompanied by glomerular lesions (4–6). However, electrolyte disorders have been described in association with ICI use since 2017 (7).
Figure 1.
Mechanism of action of immune checkpoint inhibitors. Cytotoxic T lymphocyte–associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD-1) signaling networks at homeostasis. Integration of both positive and negative costimulatory signals during and after the initial T cell activation will determine the fate and intensity of the alloimmune response. For CTLA-4 (right panel), the first step in antigen recognition is the binding of the antigen to MHC molecules on the antigen-presenting cell and creating a complex with the T cell receptor (TCR) located on the T cell. This is followed by the interaction of the CD28 molecule with B7 (CD 80/86) initiating a costimulatory signal, leading to further T cell stimulation (this is in addition to other costimulatory molecules not depicted here). As a negative feedback process to prevent overstimulation, T cell activation leads to the upregulation of the CTLA-4 molecule, which competes with the B7-CD28 ligand and, in turn, leads to T cell arrest, thus providing brakes to the immune system. CTLA-4 antagonist binds to the CTLA-4 molecule and prevents it from binding to B7, leading to the sustained activation of the T cell (lifting the foot off the brakes). Similarly, binding of the PD-1 (left panel) molecule with programmed cell death ligand 1 (PD-L1) and PD-L2 leads to an inhibitory signal with decreased effector T cell function, suppressing immune surveillance and permitting neoplastic growth. PD-1 inhibitors bind to the PD-1 molecule, preventing its interaction with PD-L1/L2 and thus leading to continued T cell stimulation (pressing on the accelerator). Majority of the data support the role of increased PD-L1 expression in human tumors and serve as the biomarker to consider PD-1 inhibitors for treatment.
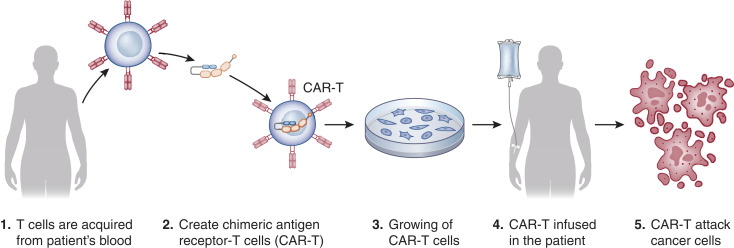
Another novel immunotherapy agent uses genetically engineered host T cells, known as CAR-T cells, which directly bind/destroy cancer cells, thereby overcoming immune roadblocks exploited by cancer cells (8) (Figure 2). Tumor-infiltrating lymphocytes are isolated from the patient, clonally expanded, and infused into the patient. The data on various electrolyte disorders with ICI and CAR-T have been better characterized, and in this review, we will describe the known electrolyte disorders associated with immunotherapies. Figure 3 summarizes the various mechanisms associated with electrolyte disorders seen with these therapies.
Figure 2.
Mechanism of action of chimeric antigen receptor–T (CAR-T) therapy.
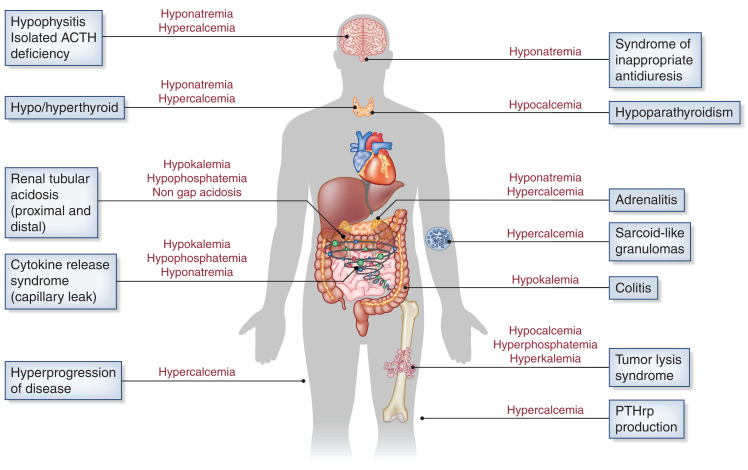
Figure 3.
Various mechanisms associated with immunotherapy-associated electrolyte disorders. ACTH, adrenocorticotropic hormone; PTHrp, parathyroid hormone–related peptide.
Hyponatremia
Hyponatremia is the most common electrolyte disorder in patients with cancer (9), with cancer immunotherapy being a novel and important etiology causing hyponatremia by a variety of mechanisms (Table 1). The toxicities associated with ICI are distinct from cytotoxic chemotherapy because the mechanism of organ injury is inflammatory and can be diffuse or isolated. The most common kidney toxicity is the development of electrolyte disorders, of which hyponatremia is the most commonly reported (7). Manohar et al. (10) conducted a meta-analysis that included 39 randomized controlled trials (RCTs) for a total of approximately 4300 patients receiving PD-1 inhibitors for various malignancies, and they found that the pooled incidence rate for hyponatremia was only 1% (95% confidence interval [95% CI], 0.7% to 2.1%). Cantini et al. (11) performed a meta-analysis of six RCTs comparing ICI with standard chemotherapy in patients with advanced nonsmall cell lung cancer that revealed an incidence of hyponatremia of 9%. In contrast, Seethapathy et al. (12) conducted a retrospective study that included 2459 patients from a single cancer center receiving ICI and observed an exceptionally high rate; 62% experienced hyponatremia (serum sodium ≤134 mEq/L), whereas 6% experienced severe hyponatremia (serum sodium ≤124 mEq/L). Moreover, a recent query of the Food and Drug Administration Adverse Event Reporting System (FAERS) on ICI from 2011 to 2021 uncovered 2556 reported cases of electrolyte disorders, with hyponatremia being the most commonly reported electrolyte abnormality (54%) (12,13). These “real-world data” suggest that hyponatremia associated with ICI is much more common than what the RCTs suggested.
Table 1.
Mechanisms of hyponatremia associated with cancer immunotherapy according to agent class
| Immune Checkpoint Inhibitors | T Cell Transfer Therapy | mAbs | Immunomodulators | ||||
|---|---|---|---|---|---|---|---|
| Programmed Cell Death Protein 1/Programmed Cell Death Ligand 1 Inhibitors/Anticytotoxic T Lymphocyte–Associated Protein 4 | Tumor-Infiltrating Lymphocyte Therapy | Chimeric Antigen Receptor T Cell Therapy | IL-2 | IFNα | |||
| SIAD | Cortisol deficiency (1) Hypophysitis: panhypopituitarism,a isolated ACTH deficiencyb (2) Adrenalitis,b primary adrenal insufficiency | Hypothyroidism, thyroiditisb | SIAD | Hypovolemia, capillary leak syndrome | SIAD | Hypovolemia, capillary leak syndrome | SIAD |
SIAD, syndrome of inappropriate antidiuresis; ACTH, adrenocorticotropic hormone.
More common with anticytotoxic T lymphocyte–associated protein 4.
More common with programmed cell death protein 1/programmed cell death ligand 1 inhibitors.
Several mechanisms by which ICI could cause hyponatremia have been proposed. Several case reports have described ICI-induced hyponatremia in association with hypophysitis, and there are reports that have described hyponatremia in association with adrenalitis (14–16). No cases of ICI-induced thyroiditis resulting in hyponatremia have been reported, but it remains a theoretical concern.
Barroso-Sousa et al. (17) conducted a meta-analysis of 38 clinical trials of patients with advanced solid tumors treated with ICI. The observed incidence of hypophysitis was 6% for combination therapy, 3% for anti–CTLA-4 inhibitors, 0.4% for PD-1 inhibitors, and <0.1% for PD-L1 inhibitors. The great majority of cases of hypophysitis occurred in patients with melanoma. Patients who received PD-1 inhibitors were significantly less likely to experience hypophysitis (odds ratio [OR], 0.29; 95% CI, 0.18 to 0.49; P<0.001); in contrast, those who received combination therapy with anti–CTLA-4 plus PD-1/PD-L1 inhibitors were significantly more likely to develop this complication (OR, 2.2; 95% CI, 1.39 to 3.60; P=0.001). The overall incidence of primary adrenal insufficiency was 0.7%, but among patients treated with combination therapy, the incidence was 4%. The overall incidence of hypothyroidism in this cohort was estimated to be 7%. Patients who received PD-1 inhibitors (OR, 1.89; 95% CI, 1.17 to 3.05; P=0.03) or combination therapy (OR, 3.81; 95% CI, 2.10 to 6.91; P<0.001) were significantly more likely to develop hypothyroidism.
In the study by Seethapathy et al. (12), the most common etiologies of severe hyponatremia encountered were the syndrome of inappropriate antidiuresis (SIAD; 36%), terminal illness (34%), hemodynamic (20%), endocrinopathies (7%), and other (4%). Endocrinopathies were responsible for only nine patients (0.3%) of hyponatremia in the overall cohort (12). All of these cases of endocrinopathy were due to secondary adrenal insufficiency caused by hypophysitis with panhypopituitarism (n=8) and hypophysitis with isolated adrenocorticotropic hormone (ACTH) deficiency (n=1). Of these nine patients, five also developed thyroiditis. Most of these cases occurred in patients with melanoma. The use of anti–CTLA-4 agents was associated with a high risk of severe hyponatremia in this cohort (adjusted OR, 2.69; 95% CI, 1.42 to 5.09; P=0.01).
Overall, anti–CTLA-4 is more commonly associated with hypophysitis, less commonly associated with thyroiditis, and rarely associated with adrenalitis. Moreover, an autopsy series revealed that among six patients with cancer who received anti–CTLA-4 therapy, CTLA-4 antigen was expressed by pituitary endocrine cells in all patients but at different levels, with the highest levels encountered in the patient with clinical and pathologic evidence of more severe disease (18). PD-1/PD-L1 inhibitors have been predominantly associated with thyroiditis.
Altogether, the studies suggest that ICI-induced endocrinopathy is a relatively rare cause of hyponatremia. Mechanistically, endocrinopathy leading to ICI-associated hyponatremia can be explained by two mechanisms: cortisol deficiency and hypothyroidism. ICI can result in cortisol deficiency by causing hypophysitis, leading to either panhypopituitarism or ACTH-isolated deficiency. ICI can also affect the adrenal gland, causing adrenalitis, which can lead to primary adrenal insufficiency. Cortisol deficiency results in hyponatremia by loss of inhibitory effects over arginine vasopressin (AVP), leading to AVP hypersecretion. Cortisol inhibits corticotropin-releasing hormone, which, in turn, stimulates AVP release (19). Cortisol can also directly suppress AVP gene transcription in parvocellular neurons (20–22). Cortisol may also regulate vascular reactivity by increasing sensitivity of vascular smooth muscle to circulating catecholamines (23,24), with cortisol deficiency leading to reduced effective arterial blood volume, which also stimulates AVP release. In addition, in patients with primary adrenal insufficiency, aldosterone deficiency contributes to AVP hypersecretion by causing kidney salt wasting and subsequent hypovolemia (25).
ICI can also lead to hypothyroidism by causing thyroiditis. Thyroiditis can present initially as thyrotoxicosis due to the release of preformed thyroid hormone from the inflamed gland followed by a hypothyroid state from inflammatory damage to the gland. The mechanism of hyponatremia in hypothyroidism is less clear, but proposed mechanisms include decreased cardiac output with reduced effective arterial blood volume and AVP hypersecretion as well as decreased GFR (25,26). Hyponatremia seems to occur more commonly in patients with severe forms of hypothyroidism (i.e., myxedema).
The treatment of ICI-induced SIAD follows the same principles as the treatment of SIAD in the general population: fluid restriction with or without oral urea or vasopressin antagonists (9). ICI-induced endocrinopathy is rarely reversible, and patients typically require long-term hormone replacement therapy (e.g., glucocorticoids or thyroid hormone) (9,27,28). ICI therapy can often proceed uninterrupted after the hormonal replacement is initiated.
CAR-T cell therapy has been associated with various electrolyte disorders, including hyponatremia. Lee et al. (29) conducted a phase 1 trial of 19 children and young adults with acute lymphoblastic leukemia who received CD19–CAR-T cell therapy and observed an incidence of hyponatremia of 5%. In another multicenter phase 1 study, Locke et al. (30) enrolled seven patients with diffuse large B cell lymphoma who received CD3ζ/CD28–CAR-T cell therapy and observed an incidence of hyponatremia of 14% (29). Gupta et al. (31) conducted a case series of 78 patients with diffuse large B cell lymphoma who received CAR-T cell therapy in two major cancer centers; hyponatremia (serum sodium <130 mEq/L) occurred in 15% of patients, with 1% of patients experiencing serum sodium <125 mEq/L within the first 30 days of therapy.
The mechanism of hyponatremia associated with CAR-T cell therapy is unclear, but it might be related to the occurrence of cytokine release syndrome, an inflammatory response that occurs secondary to cytokine release by infused CAR-T cells, occurring in over 40% of patients (32–36). The release of high concentrations of cytokines, predominantly IL-6, can lead to vasodilation, decreased cardiac output, and decreased effective arterial blood volume due to increased vascular permeability and third spacing of fluids, resulting in AVP hypersecretion (37). In addition, there is mounting evidence for a key role of IL-6 in AVP secretion in various inflammatory states (38). Dixon et al. (39) conducted a single-center retrospective study of patients who received CD19 + CAR-T cell therapy and found an inverse correlation between IL-6 levels and lowest serum sodium in patients who developed hyponatremia (P=0.001). IL-2 therapy has been associated with hyponatremia as a result of capillary leak syndrome, with subsequent decreased effective arterial blood volume and AVP hypersecretion (39,40).
Hypokalemia
Hypokalemia has been reported using ICIs, but it is unknown what proportion of these reported adverse events was therapy related, and there is a nascent understanding of the specific mechanism of injury. In a recent evaluation of the FAERS database, hypokalemia was the second most common electrolyte abnormality reported (19%) after hyponatremia (13). Nevertheless, it is important to be aware that severe hypokalemia can develop with serious consequences. Several reported cases illustrate the potential for severe hypokalemia related to immunotherapy resulting from gastrointestinal or kidney losses. Immune-mediated gastritis and colitis have been described with associated hypokalemia (41,42). Hypokalemia from kidney losses can also be induced by ICIs (43–46). Mechanisms and a summary of distal and proximal renal tubular acidosis (RTA) cases are discussed later in this review under the acidosis section.
In a report examining 78 patients who received CAR-T cell therapy, no patient experienced severe hypokalemia (serum potassium <3.0 mEq/L) (31). However, 43 (54%) patients developed serum potassium <3.5 mEq/L; there was no protocol for replacement, but generally, patients were closely monitored. Electrolyte abnormalities, in general, occurred 5–6 days from the time of CAR-T administration. The authors hypothesized that hypokalemia could be related to cortisol release or a global renal tubular defect, but not much is known about more specific mechanisms.
Hypercalcemia
Parathyroid hormone–related peptide (PTHrP) from tumor cells, osteolytic lesions, and 1,25-dihydroxy vitamin D3 production are common mechanisms in hypercalcemia associated with cancer (47,48). ICI-related hypercalcemia is the third most common electrolyte abnormality noted in a recent query of FAERS (13). No specific published cases or case reports have been reported with other forms of immunotherapy.
In phase 2 and 3 trials with ICI therapy, hypercalcemia has been rarely mentioned (49–51). In the expansion cohorts of the phase 1 study of cemiplimab for patients with locally advanced or metastatic cutaneous squamous cell carcinoma, hypercalcemia occurred in 15% (n=4) and 8% (n=2), respectively (50,51). Another retrospective review of endocrine side effects related to anti–CTLA-4 therapy documented two patients with cases of incidental hypercalcemia of 256 patients (52). Additional case reports have been published that suggest an association of hypercalcemia with ICI therapy (53,54).
Several mechanisms are thought to be responsible for the pathophysiology of ICI-associated hypercalcemia (Figure 3). Endocrinopathies reported with ICI therapy are the most likely mechanism (55). Thyroid disorders are not uncommon following ICI therapy (55,56). Hypercalcemia secondary to hyperthyroidism is nonparathyroid hormone dependent, with enhanced bone resorption and calcium mobilization. Hypothyroidism can affect calcium homeostasis by decreasing bone turnover and by increasing parathyroid hormone and 1,25-dihydroxyvitamin D3 concentrations (57). ICI-related adrenal disorders, including primary adrenal insufficiency or hypophysitis, and isolated ACTH deficiency can be associated with hypercalcemia (58,59). Although hypercalcemia is reported in 5%–6% of ICI-unrelated primary adrenal insufficiency, its incidence is unknown in ICI-related primary adrenal insufficiency and/or isolated ACTH deficiency (60). It is possible that lack of cortisol may be related to the increase in calcium reabsorption from renal tubules and release from bone. Deficient adrenal hormone and a decreased level of stanniocalcin (a paracrine hormone secreted by the adrenal gland) may affect skeletal calcium efflux into circulation and result in hypercalcemia (60). Activity of 1α-hydroxylase may be increased in adrenal insufficiency, leading to increased intestinal absorption of calcium. Sarcoidosis-like granulomas, which are noncaseating epithelioid granulomas found in the absence of systemic sarcoidosis, have been reported following ICI therapy (61–67). They have also been reported with other immunomodulatory agents, such as IFNα (68), and B-RAF inhibitors, such as vemurafenib (69). Sarcoidosis-like granulomas are likely induced by a shift toward T helper-1 and T helper-17 immune pathway activation. Combination or sequential therapy with these ICIs may synergistically increase the risk for sarcoidosis-like granulomas as reported by Rambhia et al. (70). Sarcoidosis-related hypercalcemia is reported in 11% of patients (71). Prompt investigation of potential sarcoidosis-like granulomas in patients receiving ICI is necessary to distinguish these from malignancy progression (71,72). Rarely, immune-related PTHrP-mediated hypercalcemia can occur in patients receiving ICI therapy, as reported by Deligiorgi et al. (73) in two patients who developed hypercalcemia concurrent with immune-related adverse events following administration of nivolumab. In both patients, hypercalcemia arose while the cancer was in remission, suggesting that the cause of the hypercalcemia was ICI-related PTHrP production (73). Further studies are needed to unravel the source of PTHrP attributed to immunotherapy. A final mechanism that can lead to hypercalcemia in patients getting ICI therapy is hyperprogression of disease. This occurs when the tumor initially increases in size after ICI therapy with a subsequent decrease of the tumor burden (53,74). Kobari et al. (49) discuss three patients with hyperprogression of disease after nivolumab therapy in metastatic renal cell carcinoma, one of whom developed hypercalcemia 3 days after the first dose of nivolumab.
Treatment of ICI-associated hypercalcemia should be tailored to lower the serum calcium to treat the patient’s symptoms and target the underlying cause. If the patient is not symptomatic, mild hypercalcemia and moderate hypercalcemia do not require immediate therapy, and management of the underlying cause is required. Most commonly, treating endocrinopathies will help treat hypercalcemia. The initial treatment for sarcoidosis is prednisone at a dose of 20–40 mg/d for 6–12 weeks, followed by a taper over 3 months. This is a different dose and taper plan than what is normally used for immune-related adverse events management as a result of ICI therapy, which tends to utilize higher initial doses with a faster taper (4,75). Furthermore, hypercalcemia related to sarcoidosis usually responds promptly to glucocorticoid therapy (49,72). ICI-induced PTHrP-related hypercalcemia could also respond to systemic steroid therapy (49).
Hypocalcemia
Hypocalcemia, as compared with hypercalcemia, is less common with the use of cancer immunotherapy. Although the data on hypocalcemia with the use of ICIs are limited to case reports, it has been described in association with both CTLA-4 and PD-1 inhibitors as well as with their combined use for the treatment of cancer.
In the meta-analysis performed by Manohar et al. (10), hypocalcemia was found to be prevalent in patients on PD-1 inhibitors (pembrolizumab or nivolumab). The pooled incidence rate of hypocalcemia was reported to be 1%, with a severe degree of hypocalcemia (grade 3 or higher) seen in 13% of cases (10). However, the study performed by Seethapathy et al. (12) did not delineate an association between ICIs and hypocalcemia and found grade 3 or 4 hypocalcemia to be rare (incidence rate of 0.2%) in these individuals. The more recent review of the FAERS database on electrolyte disorders associated with ICIs noted hypocalcemia in 5% of the reported events (13).
Most of the cases of hypocalcemia associated with ICIs have proposed autoimmune hypoparathyroidism as the underlying mechanism for the onset of hypocalcemia (76–82). It is postulated that hypoparathyroidism can develop either due to inflammation of the parathyroid gland related to immune-mediated damage (77,80,82) or in response to calcium-sensing receptor–activating antibodies. The presence of calcium-sensing receptor antibodies has been seen in several reported cases (78,79,81). Dadu et al. (81) demonstrated that these antibodies were negative prior to initiation of ICI therapy, and they were later detected at multiple occasions corresponding to the patient's clinical course of hypoparathyroidism after introduction of immunotherapy. Few patients who developed autoimmune hypoparathyroidism were also noted to have other immune-related adverse events (77,82), suggesting that the presence of an immune-related adverse event may predict the development of another immune-related adverse event (82).
Hypocalcemia can occur as a manifestation of tumor lysis syndrome as well, which has been described with the use of both ICI and CAR-T cell therapies. Additionally, the use of denosumab (humanized mAb to RANK ligand) in combination with ICIs can increase the likelihood of hypocalcemia (83).
Treatment of ICI-associated hypocalcemia includes intravenous and oral calcium and vitamin D supplementation. Although the persistence of hypoparathyroidism despite discontinuation of ICI therapy has been recognized (81), it remains unclear whether hypocalcemia and hypoparathyroidism may resolve after the discontinuation of these agents. ICIs can be continued with close monitoring of calcium level and repletion as needed.
Hypophosphatemia
The review of the FAERS database showed that hypophosphatemia was reported in 3% of patients receiving a CTLA-4 inhibitor, 1% of patients receiving a PD-1 inhibitor, and 3% of patients receiving a PD-L1 inhibitor (13). In a meta-analysis of 27 clinical trials using nivolumab, the most frequent severe (grade ≥3) adverse event requiring hospitalization or invasive intervention was hypophosphatemia (13,84). Hypophosphatemia in patients receiving immunotherapy can develop by several proposed mechanisms. We describe below in the acidosis section the association of ICI with proximal tubulopathy and Fanconi syndrome. Urinary phosphate wasting can occur in association, resulting in hypophosphatemia. Also, if patients have gastrointestinal- and immune-related adverse events, hypophosphatemia can occur as a result of diarrhea or prolonged decreased nutrient intake.
In a study examining 78 patients receiving CAR-T therapy, hypophosphatemia was the most common electrolyte abnormality, with 51% of patients experiencing severe hypophosphatemia with serum phosphates <2 mg/dl and 18% with serum phosphate <1.5 mg/dl (31). There are no published data on the fractional excretion of phosphate and other methods to characterize mechanistically how hypophosphatemia develops. Hypotheses include intracellular shifts in phosphate or gastrointestinal or kidney losses. Interestingly, CAR-T therapy induces high levels of IL-6, which has been shown to increase fibroblast growth factor 23 levels in other settings and may contribute to the hypophosphatemia observed in AKI and CKD; therefore, elevations in IL-6 levels in cytokine release syndrome may contribute to phosphaturia and hypophosphatemia (31,85,86).
Hyperphosphatemia
Hyperphosphatemia in patients receiving ICIs is not common across all classes (13). Hyperphosphatemia can occur as a result of tumor lysis syndrome, but the syndrome is not as frequent in solid tumors, where ICIs are commonly used. However, the use of immunotherapy in solid tumors has resulted in a higher incidence of tumor lysis syndrome. Tumor lysis syndrome, marked by hyperphosphatemia and other elements in the Cairo–Bishop criteria, has been described in multiple reports with atezolizumab, nivolumab, and pembrolizumab (76,77). Shah et al. (87) examined several published cases of ICI-related tumor lysis syndrome. They note that the risk factors that need to be taken into consideration for tumor lysis syndrome include chemosensitivity of the tumor, the burden of the disease includes size >10 cm, bone marrow involvement, and pretreatment hyperuricemia and hyperphosphatemia (76,77). Although infrequently reported, hypoparathyroidism can occur as an immune-related adverse event of checkpoint inhibitors (76,77,87).
Tumor lysis syndrome can also occur after CAR-T cell therapy, resulting in hyperphosphatemia from the destruction of tumor cells, especially in patients with large tumor burden. Cellular contents from nontumor cells also occur because of on-target off-tumor toxicity (85). There is a narrow set of tumor-specific antigens that are recognized by CAR-T cells, but in addition, there are tumor-associated antigens, which are weakly expressed in normal tissues and can be damaged as a result of the therapy contributing to hyperphosphatemia (85,88).
Metabolic Acidosis
The most frequent form of acidosis noted in association with the use of ICI is distal RTA. It is a common occurrence with hypokalemia (as described above). Table 2 summarizes the several published cases of both proximal and distal RTA seen with ICIs. Over 90% of the patients required steroid and alkali therapy. Kidney biopsies described in patients exposed to ICI with distal RTA even without AKI showed mild to moderate forms of interstitial nephritis. This suggests that distal RTA may be an early sign of ICI kidney toxicity (89). Additionally, Herrmann et al. (90) describe a case series investigating the potential mechanism for developing distal RTA using special staining for transporters in α-intercalated cells. They found decreased expression of V-ATPase pump and anion-exchanger 1 in kidney biopsy specimens, a finding also observed in patients with Sjogren syndrome. Although phenotypically, the cases were consistent with distal RTA, they also observed a reduction in electrogenic sodium bicarbonate cotransporter 1 staining in the proximal tubule.
Table 2.
Reported cases of renal tubular acidosis with use of immune checkpoint inhibitors
| Reported Case | Renal Tubular Acidosis Type | AKI | Cancer | Immune Checkpoint Inhibitor | Serum pH | Serum Potassium, mEq/L | Serum Phosphate, mg/dl | Urine pH | Urine Anion Gap | Kidney Biopsy | Treatment | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Proximal | No | HCC | N | 7.39 | 1.7 | 1.2 | 7.0 | NR | Not performed | Electrolyte replacement of potassium phosphate | ICI held, acidosis improved | 44 |
| 2 | Proximal | Yes | Lung | P | NR | 2.5 | 1.5 | NR | NR | Not performed | Electrolyte replacement of potassium, phosphate, magnesium, and iv steroids | ICI was held, resolution of electrolyte abnormalities | 45 |
| 3 | Proximal | Yes | Lung | N/P | 7.35 | 4.5 | 2.3 | NR | NR | Not performed | iv bicarbonate therapy and prednisone | ICI held, resolution of electrolyte abnormalities | 46 |
| 4 | Proximal | Yes | Melanoma | I/N | NR | 1.7 | NR | NR | NR | Not performed | iv bicarbonate, steroids and electrolyte replacement | Presented with hypokalemia and paralysis that resolved, rechallenged with P | 43 |
| 5 | Distal | Yes | Lung | N | 7.21 | 2.4 | NR | 6.5 | 22 | Not performed | iv bicarbonate therapy, and dexamethasone 4 mg every 8 h | ICI held | 94 |
| 6 | Distal | Yes | Lung | P | 7.26 | 2.8 | NR | 6.5 | 10 | Tubulointerstitial nephritis | Oral sodium bicarbonate, prednisone | AKI, acidosis improved | 90 |
| 7 | Distal | Yes | Melanoma | N | 7.25 | 4.2 | NR | 6.3 | 48 | Acute and chronic tubulointerstitial nephritis | iv normal saline, oral potassium citrate, prednisone | AKI, acidosis improved | 90 |
| 8 | Distal | Yes | RCC | N | 7.23 | 3.9 | NR | 6.7 | NR | Not performed | Prednisone, oral sodium bicarbonate | ICI held, AKI, acidosis improved | 90 |
| 9 | Distal | No | Lung | N/P | 7.29 | 3.3 | NR | 6.0 | 36 | Interstitial edema, tubulitis of DCT and collecting ducts | Initially oral bicarbonate for a week, then initiated on steroids 1mg/kg per d | ICI held, acidosis improved | 89 |
| 10 | Distal | Yes | Melanoma | P | 7.05 | 3.2 | NR | 6.0 | 49 | Not performed | iv and oral bicarbonate supplementation, prednisone ×15 d | ICI held, acidosis improved | 95 |
| 11 | Distal | No | Melanoma | P | NR | 3.6 | 2.6 | 6.0 | 8 | Not performed | iv and oral steroids | Acidosis improved | 96 |
HCC, hepatocellular carcinoma; N, nivolumab; NR, not reported; ICI, immune checkpoint inhibitor; P, pembrolizumab; iv, intravenous; RCC, renal cell cancer; I, ipilimumab; DCT, distal convoluted tubule.
Proximal RTA has also been described as a result of immunotherapy in four recent cases (43–46). All cases required aggressive potassium and phosphate replacement, steroid therapy, and temporary discontinuation of ICI. Most cases also had AKI associated with the acidosis. Although AIN is a frequent feature in patients who develop RTA, to date there are no detailed examinations of kidney pathology to describe the specific lesions associated with proximal and diffuse tubulopathy. It has been shown that PD-L1 is expressed on renal epithelial cells, which may protect the kidney against ischemia-reperfusion injury, but it is unknown to what degree exposure to PD-L1 blockade may be inducing an autoimmune response (91). Interestingly, a single case of type B lactic acidosis after the first dose of nivolumab has also been reported (92). Recently, another case report has described severe diabetic ketoacidosis in the setting of acute development of autoimmune diabetes mellitus secondary to ICI therapy. This patient had no known history of diabetes; however, the patient presented with severe diabetic ketoacidosis (arterial pH 6.9, serum glucose 1123 mg/dl, serum anion gap 34 with positive serum ketones) after the second cycle of pembrolizumab. C-peptide levels were undetectable, and antiglutamic acid decarboxylase antibodies were positive, suggestive of autoimmune diabetes. The patient was successfully treated with insulin and volume expansion (93).
Summary
Several types of electrolyte disorders have been described with the use of immunotherapy. Early recognition and prompt diagnosis may help oncologists better manage the various novel electrolyte disorders associated with immunotherapy. Presently, there are no known patient-specific risk factors that may predispose a given patient to a particular ICI- or CAR-T–related electrolyte/acid-base abnormality. We have summarized what is known about these agents from descriptions in case reports and case series and have extrapolated tissue pathologic findings, but the reader should be aware that early summaries may be prone to bias and confounding factors. Further research is needed to better understand the true incidence and pathophysiology associated with various forms of electrolyte disturbances with immunotherapy and how to anticipate them.
Disclosures
K.D. Jhaveri reports consultancy agreements with Astex Pharmaceuticals, ChemoCentryx, ChinookGSK, GlaxoSmithKline, Natera, George Clinicals and Travere Therapeutics; reports honoraria from the American Society of Nephrology and the International Society of Nephrology; is a paid contributor to UpToDate.com and is section editor for onconephrology for Nephrology Dialysis Transplantation; serves on the editorial boards of American Journal of Kidney Diseases, CJASN, Clinical Kidney Journal, Frontiers in Nephrology, Journal of Onco-Nephrology, and Kidney International; serves as the Editor-in-Chief of ASN Kidney News; and serves as Co-President of the American Society of Onco-Nephrology. H. Rondon-Berrios reports honoraria from Memorial Sloan Kettering Cancer; serving as an associate editor for Frontiers in Medicine/Nephrology; and serving as an editorial board member for CJASN. B.T. Workeneh reports serving on speakers bureau for AstraZeneca. The remaining author has nothing to disclose.
Funding
H. Rondon-Berrios is funded by National Institute of Diabetes and Digestive and Kidney Diseases exploratory/developmental research grant R21DK122023.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Schreiber RD, Old LJ, Smyth MJ: Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 331: 1565–1570, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Weber J: Immune checkpoint proteins: A new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 37: 430–439, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Twomey JD, Zhang B: Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J 23: 39, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta S, Short SAP, Sise ME, Prosek JM, Madhavan SM, Soler MJ, Ostermann M, Herrmann SM, Abudayyeh A, Anand S, Glezerman I, Motwani SS, Murakami N, Wanchoo R, Ortiz-Melo DI, Rashidi A, Sprangers B, Aggarwal V, Malik AB, Loew S, Carlos CA, Chang W-T, Beckerman P, Mithani Z, Shah CV, Renaghan AD, Seigneux S, Campedel L, Kitchlu A, Shin DS, Rangarajan S, Deshpande P, Coppock G, Eijgelsheim M, Seethapathy H, Lee MD, Strohbehn IA, Owen DH, Husain M, Garcia-Carro C, Bermejo S, Lumlertgul N, Seylanova N, Flanders L, Isik B, Mamlouk O, Lin JS, Garcia P, Kaghazchi A, Khanin Y, Kansal SK, Wauters E, Chandra S, Schmidt-Ott KM, Hsu RK, Tio MC, Sarvode Mothi S, Singh H, Schrag D, Jhaveri KD, Reynolds KL, Cortazar FB, Leaf DE; ICPi-AKI Consortium Investigators : Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer 9: e003467, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, Gaber L, Lahoti A, Workeneh B, Chen S, Lin J, Abdel-Wahab N, Tayar J, Lu H, Suarez-Almazor M, Tannir N, Yee C, Diab A, Abudayyeh A: Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J Immunother Cancer 7: 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitchlu A, Jhaveri KD, Wadhwani S, Deshpande P, Harel Z, Kishibe T, Henriksen K, Wanchoo R: A systematic review of immune checkpoint inhibitor-associated glomerular disease. Kidney Int Rep 6: 66–77, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanchoo R, Karam S, Uppal NN, Barta VS, Deray G, Devoe C, Launay-Vacher V, Jhaveri KD; Cancer and Kidney International Network Workgroup on Immune Checkpoint Inhibitors : Adverse renal effects of immune checkpoint inhibitors: A narrative review. Am J Nephrol 45: 160–169, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD: The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13: 394, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workeneh BT, Jhaveri KD, Rondon-Berrios H: Hyponatremia in the cancer patient. Kidney Int 98: 870–882, 2020 [DOI] [PubMed] [Google Scholar]
- 10.Manohar S, Kompotiatis P, Thongprayoon C, Cheungpasitporn W, Herrmann J, Herrmann SM: Programmed cell death protein 1 inhibitor treatment is associated with acute kidney injury and hypocalcemia: Meta-analysis. Nephrol Dial Transplant 34: 108–117, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Cantini L, Merloni F, Rinaldi S, Lenci E, Marcantognini G, Meletani T, Fiordoliva I, Morgese F, Torniai M, Ricci G, Giampieri R, Berardi R: Electrolyte disorders in advanced non-small cell lung cancer patients treated with immune check-point inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol 151: 102974, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Seethapathy H, Rusibamayila N, Chute DF, Lee M, Strohbehn I, Zubiri L, Faje AT, Reynolds KL, Jhaveri KD, Sise ME: Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol Dial Transplant 36: 2241–2247, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanchoo R, Sakhiya V, Jhaveri KD: Immune checkpoint inhibitor-associated electrolyte disorders: Query of the Food and Drug Administration Adverse Event Reporting System. Kidney Int 100: 945–947, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Bacanovic S, Burger IA, Stolzmann P, Hafner J, Huellner MW: Ipilimumab-induced adrenalitis: A possible pitfall in 18F-FDG-PET/CT. Clin Nucl Med 40: e518–e519, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Min L, Ibrahim N: Ipilimumab-induced autoimmune adrenalitis. Lancet Diabetes Endocrinol 1: e15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uppal NN, Wanchoo R, Barnett R, Sinha A, Jhaveri KD: Hyponatremia in a patient with cancer. Am J Kidney Dis 75: A15–A18, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, Tolaney SM: Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: A systematic review and meta-analysis. JAMA Oncol 4: 173–182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caturegli P, Di Dalmazi G, Lombardi M, Grosso F, Larman HB, Larman T, Taverna G, Cosottini M, Lupi I: Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: Insights into pathogenesis from an autopsy series. Am J Pathol 186: 3225–3235, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edgell RC, Savitz SI, Dalfino J, editors: Neurointervention in the Medical Specialties: A Comprehensive Guide, New York, Springer, 2015 [Google Scholar]
- 20.Kovács KJ, Földes A, Sawchenko PE: Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci 20: 3843–3852, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma X-M, Aguilera G: Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology 140: 5642–5650, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kim JK, Summer SN, Wood WM, Schrier RW: Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochem Biophys Res Commun 289: 1252–1256, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Christianson A, Bender H: The Complete Idiot’s Guide to Thyroid Disease: Clear Information on Causes, Symptoms, and Treatments, New York, Penguin, 2011 [Google Scholar]
- 24.Walker BR, Connacher AA, Webb DJ, Edwards CRW: Glucocorticoids and blood pressure: A role for the cortisol/cortisone shuttle in the control of vascular tone in man. Clin Sci (Lond) 83: 171–178, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Rondon-Berrios H, Berl T: Physiology and pathophysiology of water homeostasis. Front Horm Res 52: 8–23, 2019 [DOI] [PubMed] [Google Scholar]
- 26.Mariani LH, Berns JS: The renal manifestations of thyroid disease. J Am Soc Nephrol 23: 22–26, 2012 [DOI] [PubMed] [Google Scholar]
- 27.de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B: Incidence of thyroid-related adverse events in melanoma patients treated with pembrolizumab. J Clin Endocrinol Metab 101: 4431–4439, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min L, Hodi FS, Giobbie-Hurder A, Ott PA, Luke JJ, Donahue H, Davis M, Carroll RS, Kaiser UB: Systemic high-dose corticosteroid treatment does not improve the outcome of ipilimumab-related hypophysitis: A retrospective cohort study. Clin Cancer Res 21: 749–755, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL: T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385: 517–528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locke FL, Neelapu SS, Bartlett NL, Siddiqi T, Chavez JC, Hosing CM, Ghobadi A, Budde LE, Bot A, Rossi JM, Jiang Y, Xue AX, Elias M, Aycock J, Wiezorek J, Go WY: Phase 1 results of ZUMA-1: A multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 25: 285–295, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta S, Seethapathy H, Strohbehn IA, Frigault MJ, O’Donnell EK, Jacobson CA, Motwani SS, Parikh SM, Curhan GC, Reynolds KL, Leaf DE, Sise ME: Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 76: 63–71, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter RO, Evbuomwan MO, Pittaluga S, Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT, Kochenderfer JN: B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 19: 2048–2060, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL, Frey NV, Grupp SA, Teachey DT: Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 45: e124–e131, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferri FF: 2021 Ferri’s Clinical Advisor 2021 E-Book: 5 Books in 1, Philadelphia, Elsevier Health Sciences, 2021 [Google Scholar]
- 35.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA: Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371: 1507–1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter DL, Levine BL, Kalos M, Bagg A, June CH: Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365: 725–733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jhaveri KD, Rosner MH: Chimeric antigen receptor T cell therapy and the kidney: What the nephrologist needs to know. Clin J Am Soc Nephrol 13: 796–798, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swart RM, Hoorn EJ, Betjes MG, Zietse R: Hyponatremia and inflammation: The emerging role of interleukin-6 in osmoregulation. Nephron, Physiol 118: 45–51, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Dixon BN, Daley RJ, Buie LW, Hsu M, Park JH, Brentjens RJ, Purdon TJ, Latcha S: Correlation of IL-6 secretion and hyponatremia with the use of CD19+ chimeric antigen receptor T-cells. Clin Nephrol 93: 42–46, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shulman KL, Stadler WM, Vogelzang NJ: High-dose continuous intravenous infusion of interleukin-2 therapy for metastatic renal cell carcinoma: The University of Chicago experience. Urology 47: 194–197, 1996 [DOI] [PubMed] [Google Scholar]
- 41.Cheung VTF, Fryer E, Payne MJ, Brain O: Anorexia, vomiting and weight loss in a 22-year-old woman. Gut 68: 803–927, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Anson D, Norton J, Chaucer B, Bansal S: Ipilimumab- and nivolumab-induced colitis causing severe hypokalemia and QTc prolongation. Case Rep Oncol Med 2019: 7896749, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balakrishna P, Villegas A: Hypokalemic paralysis secondary to immune checkpoint inhibitor therapy. Case Rep Oncol Med 2017: 5063405, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinawi M, Bastani B: A case of Fanconi syndrome as a complication of treatment with a checkpoint inhibitor in a patient with hepatocellular carcinoma. J Nephropathol 9: e19, 2019 [Google Scholar]
- 45.Tseng P-J, Yan M-T: Acute diffuse renal tubulopathy in a patient with lung cancer: A case report. Front Med (Lausanne) 8: 742489, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farid S, Latif H, Nilubol C, Kim C: Immune checkpoint inhibitor-induced Fanconi syndrome. Cureus 12: e7686, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stewart AF: Clinical practice. Hypercalcemia associated with cancer. N Engl J Med 352: 373–379, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Rosner MH, Dalkin AC: Onco-nephrology: The pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol 7: 1722–1729, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Kobari Y, Kondo T, Takagi T, Omae K, Nakazawa H, Tanabe K: Rapid progressive disease after nivolumab therapy in three patients with metastatic renal cell carcinoma. In Vivo 31: 769–771, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML: Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375: 1856–1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, Chung CH, Hernandez-Aya L, Lim AM, Chang ALS, Rabinowits G, Thai AA, Dunn LA, Hughes BGM, Khushalani NI, Modi B, Schadendorf D, Gao B, Seebach F, Li S, Li J, Mathias M, Booth J, Mohan K, Stankevich E, Babiker HM, Brana I, Gil-Martin M, Homsi J, Johnson ML, Moreno V, Niu J, Owonikoko TK, Papadopoulos KP, Yancopoulos GD, Lowy I, Fury MG: PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 379: 341–351, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA: Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: A comprehensive retrospective review from a single institution. Endocr Relat Cancer 21: 371–381, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mills TA, Orloff M, Domingo-Vidal M, Cotzia P, Birbe RC, Draganova-Tacheva R, Martinez Cantarin MP, Tuluc M, Martinez-Outschoorn U: Parathyroid hormone-related peptide-linked hypercalcemia in a melanoma patient treated with ipilimumab: Hormone source and clinical and metabolic correlates. Semin Oncol 42: 909–914, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takebayashi K, Ujiie A, Kubo M, Furukawa S, Yamauchi M, Shinozaki H, Suzuki T, Naruse R, Hara K, Tsuchiya T, Inukai T: Isolated adrenocorticotropic hormone deficiency and severe hypercalcemia after destructive thyroiditis in a patient on nivolumab therapy with a malignant melanoma. J Clin Med Res 10: 358–362, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdel-Rahman O, ElHalawani H, Fouad M: Risk of endocrine complications in cancer patients treated with immune check point inhibitors: A meta-analysis. Future Oncol 12: 413–425, 2016 [DOI] [PubMed] [Google Scholar]
- 56.Chalan P, Di Dalmazi G, Pani F, De Remigis A, Corsello A, Caturegli P: Thyroid dysfunctions secondary to cancer immunotherapy. J Endocrinol Invest 41: 625–638, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reddy PA, Harinarayan CV, Sachan A, Suresh V, Rajagopal G: Bone disease in thyrotoxicosis. Indian J Med Res 135: 277–286, 2012 [PMC free article] [PubMed] [Google Scholar]
- 58.Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE, Bachelot A: Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO VigiBase report analysis. Oncologist 25: 696–701, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Percik R, Shlomai G, Tirosh A, Tirosh A, Leibowitz-Amit R, Eshet Y, Greenberg G, Merlinsky A, Barhod E, Steinberg-Silman Y, Sella T: Isolated autoimmune adrenocorticotropic hormone deficiency: From a rare disease to the dominant cause of adrenal insufficiency related to check point inhibitors. Autoimmun Rev 19: 102454, 2020 [DOI] [PubMed] [Google Scholar]
- 60.Ahn SW, Kim TY, Lee S, Jeong JY, Shim H, Han YM, Choi KE, Shin SJ, Yoon HE: Adrenal insufficiency presenting as hypercalcemia and acute kidney injury. Int Med Case Rep J 9: 223–226, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel WV, Guislain A, Kvistborg P, Schumacher TN, Haanen JB, Blank CU: Ipilimumab-induced sarcoidosis in a patient with metastatic melanoma undergoing complete remission. J Clin Oncol 30: e7–e10, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Eckert A, Schoeffler A, Dalle S, Phan A, Kiakouama L, Thomas L: Anti-CTLA4 monoclonal antibody induced sarcoidosis in a metastatic melanoma patient. Dermatology 218: 69–70, 2009 [DOI] [PubMed] [Google Scholar]
- 63.Berthod G, Lazor R, Letovanec I, Romano E, Noirez L, Mazza Stalder J, Speiser DE, Peters S, Michielin O: Pulmonary sarcoid-like granulomatosis induced by ipilimumab. J Clin Oncol 30: e156–e159, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Wilgenhof S, Morlion V, Seghers AC, Du Four S, Vanderlinden E, Hanon S, Vandenbroucke F, Everaert H, Neyns B: Sarcoidosis in a patient with metastatic melanoma sequentially treated with anti-CTLA-4 monoclonal antibody and selective BRAF inhibitor. Anticancer Res 32: 1355–1359, 2012 [PubMed] [Google Scholar]
- 65.Andersen R, Nørgaard P, Al-Jailawi MK, Svane IM: Late development of splenic sarcoidosis-like lesions in a patient with metastatic melanoma and long-lasting clinical response to ipilimumab. OncoImmunology 3: e954506, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Firwana B, Ravilla R, Raval M, Hutchins L, Mahmoud F: Sarcoidosis-like syndrome and lymphadenopathy due to checkpoint inhibitors. J Oncol Pharm Pract 23: 620–624, 2017 [DOI] [PubMed] [Google Scholar]
- 67.Tetzlaff MT, Nelson KC, Diab A, Staerkel GA, Nagarajan P, Torres-Cabala CA, Chasen BA, Wargo JA, Prieto VG, Amaria RN, Curry JL: Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. J Immunother Cancer 6: 14, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heinzerling LM, Anliker MD, Müller J, Schlaeppi M, von Moos R: Sarcoidosis induced by interferon-α in melanoma patients: Incidence, clinical manifestations, and management strategies. J Immunother 33: 834–839, 2010 [DOI] [PubMed] [Google Scholar]
- 69.Lheure C, Kramkimel N, Franck N, Laurent-Roussel S, Carlotti A, Queant A, Goldwasser F, Avril MF, Dupin N: Sarcoidosis in patients treated with vemurafenib for metastatic melanoma: A paradoxical autoimmune activation. Dermatology 231: 378–384, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Rambhia PH, Reichert B, Scott JF, Feneran AN, Kazakov JA, Honda K, Koon H, Gerstenblith MR: Immune checkpoint inhibitor-induced sarcoidosis-like granulomas. Int J Clin Oncol 24: 1171–1181, 2019 [DOI] [PubMed] [Google Scholar]
- 71.Iannuzzi MC, Rybicki BA, Teirstein AS: Sarcoidosis. N Engl J Med 357: 2153–2165, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Izzedine H, Chazal T, Wanchoo R, Jhaveri KD: Immune checkpoint inhibitor-associated hypercalcaemia [published online ahead of print December 29, 2020]. Nephrol Dial Transplant 10.1093/ndt/gfaa326 [DOI] [PubMed] [Google Scholar]
- 73.Deligiorgi MV, Panayiotidis MI, Trafalis DT: Parathyroid hormone related protein (PTHrP)-mediated hypercalcemia in malignancy associated with anti-PD-1 immune checkpoint inhibitor treatment and related inflammatory reactions. Int Immunopharmacol 77: 105942, 2019 [DOI] [PubMed] [Google Scholar]
- 74.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferté C: Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23: 1920–1928, 2017 [DOI] [PubMed] [Google Scholar]
- 75.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network : Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36: 1714–1768, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Win MA, Thein KZ, Qdaisat A, Yeung SJ: Acute symptomatic hypocalcemia from immune checkpoint therapy-induced hypoparathyroidism. Am J Emerg Med 35: 1039.e5–1039.e7, 2017 [DOI] [PubMed] [Google Scholar]
- 77.Trinh B, Sanchez GO, Herzig P, Läubli H: Inflammation-induced hypoparathyroidism triggered by combination immune checkpoint blockade for melanoma. J Immunother Cancer 7: 52, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piranavan P, Li Y, Brown E, Kemp EH, Trivedi N: Immune checkpoint inhibitor-induced hypoparathyroidism associated with calcium-sensing receptor-activating autoantibodies. J Clin Endocrinol Metab 104: 550–556, 2019 [DOI] [PubMed] [Google Scholar]
- 79.Lupi I, Brancatella A, Cetani F, Latrofa F, Kemp EH, Marcocci C: Activating antibodies to the calcium-sensing receptor in immunotherapy-induced hypoparathyroidism. J Clin Endocrinol Metab 105: dgaa092, 2020 [DOI] [PubMed] [Google Scholar]
- 80.Mahmood I, Kuhadiya ND, Gonzalaes M: Pembrolizumab-associated hypoparathyroidism: A single case report. AACE Clin Case Rep 7: 23–25, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dadu R, Rodgers TE, Trinh VA, Kemp EH, Cubb TD, Patel S, Simon JM, Burton EM, Tawbi H: Calcium-sensing receptor autoantibody-mediated hypoparathyroidism associated with immune checkpoint inhibitor therapy: Diagnosis and long-term follow-up. J Immunother Cancer 8: e000687, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El Kawkgi OM, Li D, Kotwal A, Wermers RA: Hypoparathyroidism: An uncommon complication associated with immune checkpoint inhibitor therapy. Mayo Clin Proc Innov Qual Outcomes 4: 821–825, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao Y, Afzal MZ, Shirai K: Does denosumab offer survival benefits? -Our experience with denosumab in metastatic non-small cell lung cancer patients treated with immune-checkpoint inhibitors. J Thorac Dis 13: 4668–4677, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tie Y, Ma X, Zhu C, Mao Y, Shen K, Wei X, Chen Y, Zheng H: Safety and efficacy of nivolumab in the treatment of cancers: A meta-analysis of 27 prospective clinical trials. Int J Cancer 140: 948–958, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Zhou H, Yang M, Cui L, Jiang J: Chimeric antigen receptor T cell therapy and nephrotoxicity: From diagnosis to treatment strategies. Int Immunopharmacol 89[Pt B]: 107072, 2020 [DOI] [PubMed] [Google Scholar]
- 86.Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T: Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int 94: 315–325, 2018 [DOI] [PubMed] [Google Scholar]
- 87.Shah M, Jain S, Abe T, Surapaneni PK, Bhatia K: Pembrolizumab-axitinib-induced tumor lysis syndrome in a patient with metastatic renal cancer. Clin Case Rep 8: 704–708, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu S, Yi M, Qin S, Wu K: Next generation chimeric antigen receptor T cells: Safety strategies to overcome toxicity. Mol Cancer 18: 125, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Charmetant X, Teuma C, Lake J, Dijoud F, Frochot V, Deeb A: A new expression of immune checkpoint inhibitors’ renal toxicity: When distal tubular acidosis precedes creatinine elevation. Clin Kidney J 13: 42–45, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herrmann SM, Alexander MP, Romero MF, Zand L: Renal tubular acidosis and immune checkpoint inhibitor therapy: An immune-related adverse event of PD-1 inhibitor—A report of 3 cases. Kidney Med 2: 657–662, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Perazella MA, Shirali AC: Nephrotoxicity of cancer immunotherapies: Past, present and future. J Am Soc Nephrol 29: 2039–2052, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakajima E, Leger P, Mayer IA, Neuss MN, Chism DD, Rathmell WK: A case report of severe type B lactic acidosis following first dose of nivolumab in a VHL-mutated metastatic renal cell carcinoma. Kidney Cancer 1: 83–88, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kedzior SK, Jacknin G, Hudler A, Mueller SW, Kiser TH: A severe case of diabetic ketoacidosis and new-onset type 1 diabetes mellitus associated with anti-glutamic acid decarboxylase antibodies following immunotherapy with pembrolizumab. Am J Case Rep 22: e931702, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El Bitar S, Weerasinghe C, El-Charabaty E, Odaimi M: Renal tubular acidosis an adverse effect of PD-1 inhibitor immunotherapy. Case Rep Oncol Med 2018: 8408015, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Atiq SO, Gokhale T, Atiq Z, Holmes R, Sparks MA: A case of pembrolizumab induced distal renal tubular acidosis. J Onco-Nephrol 5: 23–26, 2021 [Google Scholar]
- 96.Adoor D, Tariq H, Rashidi A: Metabolic acidosis and hyponatremia in a patient with metastatic melanoma. Am J Kidney Dis 78: A16–A18, 2021 [DOI] [PubMed] [Google Scholar]