Visual Abstract
Keywords: diabetes mellitus, renal osteodystrophy, epidemiology and outcomes, sodium-glucose transporter 2 inhibitors, chronic kidney disease
Abstract
Background and objectives
Sodium-glucose cotransporter-2 (SGLT2) inhibitors have been associated with a higher risk of skeletal fractures in some randomized, placebo-controlled trials. Secondary hyperparathyroidism and increased bone turnover (also common in CKD) may contribute to the observed fracture risk. We aimed to determine if SGLT2 inhibitor use associates with a higher risk of fractures compared with dipeptidyl peptidase-4 (DPP-4) inhibitors, which have no known association with fracture risk. We hypothesized that this risk, if present, would be greatest in patients with lower eGFR.
Design, setting, participants, & measurements
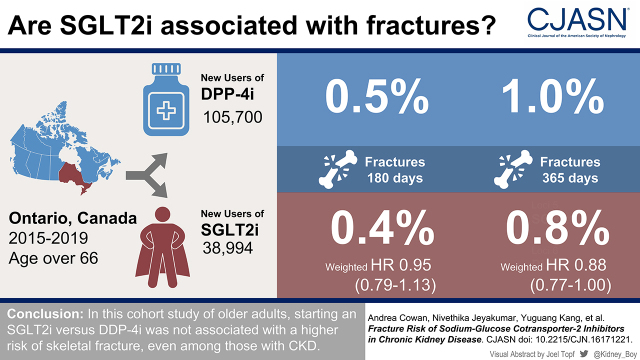
We conducted a population-based cohort study in Ontario, Canada between 2015 and 2019 using linked provincial administrative data to compare the incidence of fracture between new users of SGLT2 inhibitors and DPP-4 inhibitors. We used inverse probability of treatment weighting on the basis of propensity scores to balance the two groups of older adults (≥66 years of age) on indicators of baseline health. We compared the 180- and 365-day cumulative incidence rates of fracture between groups. Prespecified subgroup analyses were conducted by eGFR category (≥90, 60 to <90, 45 to <60, and 30 to <45 ml/min per 1.73 m2). Weighted hazard ratios were obtained using Cox proportional hazard regression.
Results
After weighting, we identified a total of 38,994 new users of a SGLT2 inhibitor and 37,449 new users of a DPP-4 inhibitor and observed a total of 342 fractures at 180 days and 689 fractures at 365 days. The weighted 180- and 365-day risks of a fragility fracture did not significantly differ between new users of a SGLT2 inhibitor versus a DPP-4 inhibitor: weighted hazard ratio, 0.95 (95% confidence interval, 0.79 to 1.13) and weighted hazard ratio, 0.88 (95% confidence interval, 0.88 to 1.00), respectively. There was no observed interaction between fracture risk and eGFR category (P=0.53).
Conclusions
In this cohort study of older adults, starting a SGLT2 inhibitor versus DPP-4 inhibitor was not associated with a higher risk of skeletal fracture, regardless of eGFR.
Introduction
Because of their proven cardio- and renoprotective benefits, sodium-glucose cotransporter-2 inhibitors (SGLT2is) are now recommended in all patients with diabetic kidney disease who have an estimated glomerular filtration rate (eGFR) ≥30/min per 1.73 m2 (1–4). However, in some large trials, their use has been reported to increase the risk of skeletal fracture. For example, the CANVAS trial (n=10,142) found that canagliflozin was associated with a higher risk of fractures than placebo (15.4 versus 11.9 fractures per 1000 patient-years, respectively; P=0.02) (1). This led the US Food and Drug Administration and Health Canada to issue a “warning and precaution” about fracture risk on canagliflozin’s product monograph (5,6). A subsequent meta-analysis of nine randomized controlled trials also found a higher risk of fracture with SGLT2is compared with placebo or active control, although CANVAS participants made up over half of the included individuals and drove this positive finding (7).
There are two proposed mechanisms for SGLT2i-induced fractures: a higher risk of falls through volume depletion or hypoglycemia and a decrease in bone quality through weight loss, increased bone turnover, and disturbed calcium phosphate balance (8–13). Patients with CKD might be particularly susceptible to changes in bone quality due to a predisposition to the metabolic derangements of CKD mineral bone disorder (14). A unique feature of the CANVAS population was that participants had a lower baseline eGFR when compared with other SGLT2i studies (16% of CANVAS participants had an eGFR <60 ml/min per 1.73 m2 compared with 9% of other study participants included in the meta-analysis) (15). This raises the possibility that patients with CKD may be at a greater risk of SGLT2i-associated fractures (7). Supporting this finding was a small study of dapagliflozin in patients with CKD (all with eGFR <60 ml/min per 1.73 m2), which also found a higher risk of fracture over placebo (9). Subsequent studies that concluded that SGLT2is do not alter fracture risk did not specifically examine patients with CKD (16–23). Skeletal fractures are of particular importance in the CKD population as they are associated with a higher risk of mortality compared with those with normal kidney function, even in those with an eGFR <45 ml/min per 1.73 m2 (24).
We conducted this population-based study of older adults to determine the 180- and 365-day risks of fracture associated with starting a SGLT2i versus a dipeptidyl peptidase-4 inhibitor (DPP-4i), with a special focus on heterogeneity by eGFR. We selected DPP-4i as a comparator drug to reduce confounding by indication because, like SGLT2is, DPP-4is are also frequently used in addition to insulin or metformin for diabetes management. Unlike SGLT2is, they have no known risk of fracture (25,26). We hypothesized that if a higher risk of fracture was observed with SGLT2i versus DDP-4i, the risk would be greatest in patients with advanced CKD.
Materials and Methods
Study Design and Setting
We conducted a population-based cohort study of older adults aged 66 years or older in Ontario, Canada, using linked administrative health data. All Ontario residents (∼14 million) have universal access to insured hospital and physician services. Residents over 65 years of age (∼2.2 million) also receive universal prescription drug coverage (27). The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, and the Research Ethics Board did not require additional review. In this study, we followed the Reporting of Studies Conducted Using Observational Routinely Collected Health Data Guidelines for Pharmacoepidemiology studies using health care databases (28) (Supplemental Appendix).
Data Sources
Patient characteristics, prescription drug use, covariate information, and outcome data were obtained from eight health databases at ICES (ices.on.ca). Detailed information on these datasets and variables used in this study can be found in Supplemental Appendix. These datasets were linked using unique encoded identifiers and analyzed at ICES Western. Less than 0.2% of patients in this study would be expected to emigrate from the province each year, which was the only reason for lost to follow-up (27).
Population
We created a cohort of older adults (≥66 years) in Ontario who were new outpatient users of a SGLT2i (canagliflozin, empagliflozin, or dapagliflozin) or DPP-4i (saxagliptin, sitagliptin, or linagliptin) between July 1, 2015 (the earliest date of universal provincial coverage of SGLT2i) (29) and September 30, 2019.
New use was defined as having no evidence of a prescription for either medication class in the preceding 180 days. The dispensing date of the first eligible prescription was considered the cohort entry or index date. Drug identification numbers used to identify SGLT2i and DPP-4i prescriptions are listed in Supplemental Appendix.
After standard data cleaning, we excluded the following patients: those <66 years of age (to allow a full 1-year look back for baseline medication use), patients prescribed concurrent SGLT2i and DPP-4i (to ensure mutually exclusive groups), patients with more than one prescription for the same medication class on the index date, patients with unusual study drug doses (to exclude atypical prescription patterns), and patients discharged from the hospital in the 2 days prior to filling the prescription (as those patients who start treatment in hospital will typically fill prescriptions shortly after discharge). We also excluded individuals with no evidence of serum creatinine measurement in the year prior and those with an eGFR <30 ml/min per 1.73 m2 or receiving dialysis (as SGLT2is were contraindicated in this group over the study period).
Patient Characteristics
We captured demographics and comorbidities in the preceding 5 years and health care utilization, medication use, and laboratory testing in the preceding 1 year. Codes used to capture baseline characteristics are presented in Supplemental Appendix.
We determined kidney function on the basis of the most recent eGFR in the year prior to index date. Serum creatinine values were used to calculate the eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation (30–33). As per recent Ontario Renal Network guidelines, race was not included in the eGFR calculation (34).
Continuous use of SGLT2is and DPP-4is was defined as consecutive prescription claims for the same drug within a period equivalent to 150% of the days supplied for the previous prescription (35). For example, if an individual was given a 30-day prescription and renewed it within 45 days, this would be counted as continuous use.
Outcomes
Our primary outcome was a hospital encounter (hospitalization or emergency department visit) for a fragility fracture (hip, spine, shoulder/upper arm, forearm/wrist, and pelvis) within 180 days of a new prescription for a SGLT2i or DPP-4i. We used an algorithm for fracture that has been used in several previous research studies from our region (Supplemental Appendix) (36,37). We chose 180 days as our window of interest to align with the time frame of higher fracture risk observed in the CANVAS trial and to avoid crossovers that could occur in SGLT2i exposure with longer periods of follow-up (1). We kept patients in their initially assigned group for the entire follow-up period, irrespective of if they had their initial prescription renewed in follow-up.
We examined hospital encounters for fragility fracture at 365 days and site of fracture as secondary outcomes. To explore possible mechanisms for a short-term higher fracture risk, we also specified hospital encounter with fall, hypotension, or severe hypoglycemia as secondary outcomes. These outcomes were evaluated at 180 days as patients can experience them shortly after starting SGLT2is (1).
Statistical Analyses
Continuous variables were summarized using mean (SD) or median (25th to 75th percentile), and categorical measures were summarized as frequency (proportion). We used the inverse probability of treatment weighting on the propensity score to balance comparison groups on baseline health indicators (38,39). Datasets were complete for all variables except hemoglobin A1c (3799 values or 3% missing, recoded using simple imputation using regression to replace missing values) and urine albumin-creatinine ratio (41,185 values or 29% missing, coded as “missing”). Missing values of income quintile were recoded as quintile 3 (389 values or 0.3% missing), and missing values of rurality were recoded as urban area (312 values or 0.2% missing). We used multivariable logistic regression to estimate propensity scores using 71 covariates (Supplemental Appendix) chosen a priori; these variables are known to be associated with both antihyperglycemic medication prescribing and fracture risk (38,39). We weighted patients in the reference group (DPP-4i) using average treatment effects for the treated weights defined as [propensity score/(1−propensity score)], with patients receiving a SGLT2i receiving a weight of one (39). To avoid instability in our models due to extreme weights, we trimmed weights larger than the 99th percentile and weights smaller than the 1st percentile. This resulted in a pseudosample of patients in the DPP-4i group who had the same distribution of covariates as those in the SGLT2i group. We compared baseline differences in both the weighted and unweighted groups using standardized differences, with ≥10% being considered clinically meaningful (40).
We then obtained weighted hazard ratios (HRs) using a weighted Cox proportional hazards regression analysis with the variance and 95% confidence intervals (95% CIs) estimated using bootstrap sampling (39). A total of 200 bootstrap samples with an unrestricted random sampling scheme were drawn from the study samples. The 95% CI widths and P values were not adjusted for multiple testing (41). Patients were followed until the development of the outcome of interest, death, or end of study follow-up (March 31, 2020).
To assess whether the association between SGLT2i use and fracture differed by eGFR category, an interaction term was included in our model. We determined whether there was treatment heterogeneity using the overall Wald chi-squared test (not adjusted for multiple testing). We further compared the risks of fracture at 180 and 365 days between SGLT2i and DPP-4i users by eGFR category (eGFR ≥90, 60 to <90, 45 to <60, and 30 to <45 ml/min per 1.73 m2). We reweighted within those categories using the propensity scoring method detailed above (42). All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
We identified 38,994 new users of SGLT2i and 105,700 new users of DPP-4i. Details of the cohort build and the number of patients excluded at each step can be found in Supplemental Appendix.
Selected unweighted and weighted baseline characteristics for each group can be found in Table 1 (full characteristics are in Supplemental Appendix). In the unweighted groups, SGLT2i users were younger (mean age 72 versus 74 years), more likely to be men (60% versus 53% men), and less likely to have dementia (3% versus 7%) or live in long-term care (1% versus 3%) than new DPP-4i users. There were higher rates of concomitant bisphosphonate use in the DPP-4i group (9% versus 5%). Coronary artery disease was more common in the SGLT2i users (31% versus 23%), consistent with indications for use. In the unweighted cohort, we also found that a larger proportion of DPP-4i users had lower eGFR levels: 13% of DPP-4i users had an eGFR of 30 to <45 ml/min per 1.73 m2 compared with 6% of the SGLT2i users.
Table 1.
Selected characteristics of older adults in Ontario, Canada, upon initiation of a sodium-glucose cotransporter-2 inhibitor or dipeptidyl peptidase-4 inhibitor
| Baseline Characteristics | Unweighted, n=144,694 | Weighted, n=76,443 | ||||
|---|---|---|---|---|---|---|
| Dipeptidyl Peptidase-4 Users, n=105,700 | Sodium-Glucose Cotransporter-2 Inhibitor Users, n=38,994 | Standardized Difference | Dipeptidyl Peptidase-4 Users, n=37,449 | Sodium-Glucose Cotransporter-2 Inhibitor Users, n=38,994 | Standardized Difference | |
| Demographics | ||||||
| Age, mean, yr (SD) | 74 (7) | 72 (5) | 0.38 | 72 (3) | 72 (5) | 0.00 |
| Women, no. (%) | 49,289 (47) | 15,457 (40) | 0.14 | 15,258 (41) | 15,457 (40) | 0.02 |
| Long-term care, no. (%) | 3560 (3) | 283 (1) | 0.19 | 302 (1) | 283 (1) | 0.01 |
| Prescriber specialty, no. (%) | ||||||
| Cardiology | 440 (0) | 1580 (4) | 0.25 | 636 (2) | 1580 (4) | 0.14 |
| Endocrinology | 8743 (8) | 5480 (14) | 0.18 | 5068 (14) | 5480 (14) | 0.02 |
| General practitioner | 85,858 (81) | 26,190 (67) | 0.32 | 26,398 (71) | 26,190 (67) | 0.07 |
| Internal medicine | 3579 (3) | 2652 (7) | 0.16 | 2421 (7) | 2652 (7) | 0.01 |
| Nephrology | 803 (1) | 758 (2) | 0.10 | 612 (2) | 758 (2) | 0.02 |
| Other | 6277 (6) | 2334 (6) | 0.00 | 2313 (6) | 2334 (6) | 0.01 |
| Comorbidities, no.(%) | ||||||
| Mean duration of diabetes, yr (SD) | 11.5 (7.4) | 12.4 (7.6) | 0.11 | 12.2 (4.4) | 12.4 (7.6) | 0.03 |
| Fragility fracture | 4,012 (4) | 1204 (3) | 0.04 | 1197 (3) | 1204 (3) | 0.01 |
| Previous fall | 17,225 (16) | 5572 (14) | 0.06 | 5439 (15) | 5572 (14) | 0.01 |
| Dementia | 7636 (7) | 1094 (3) | 0.20 | 1111 (3) | 1094 (3) | 0.01 |
| Rheumatoid arthritis | 2398 (2) | 848 (2) | 0.01 | 815 (2) | 848 (2) | 0.00 |
| Osteoporosis | 7839 (7) | 1969 (5) | 0.10 | 1926 (5) | 1969 (5) | 0.00 |
| Coronary artery disease | 24,571 (23) | 12,258 (31) | 0.18 | 10,961 (29) | 12,258 (31) | 0.05 |
| Diabetic retinopathy | 750 (1) | 338 (1) | 0.02 | 314 (1) | 338 (1) | 0.01 |
| Diabetic neuropathy | 1431 (1) | 604 (2) | 0.01 | 577 (2) | 604 (2) | 0.00 |
| Medication use, no. (%) | ||||||
| Bisphosphonates | 9199 (9) | 1952 (5) | 0.15 | 1939 (5) | 1952 (5) | 0.01 |
| Denosumab | 2053 (2) | 486 (1) | 0.06 | 479 (1) | 486 (1) | 0.01 |
| Oral steroid | 8038 (8) | 2732 (7) | 0.02 | 2641 (7) | 2732 (7) | 0.00 |
| Diabetes and kidney function, no. (%) | ||||||
| No. of diabetes medications | ||||||
| 0 | 37,006 (35) | 10,916 (28) | 0.15 | 10,454 (28) | 10,916 (28) | 0.00 |
| 1 | 51,484 (49) | 20,902 (54) | 0.10 | 19,976 (53) | 20,902 (54) | 0.01 |
| 2+ | 17,210 (16) | 7176 (18) | 0.06 | 7019 (19) | 7176 (18) | 0.01 |
| Metformin | 61,485 (58) | 25,896 (66) | 0.17 | 24,803 (66) | 25,896 (66) | 0.00 |
| Mean hemoglobin A1C, % (SD) | 8.1 (1.6) | 8.0 (1.5) | 0.03 | 8.1 (0.9) | 8.0 (1.5) | 0.02 |
| Diabetes management | 54,022 (51) | 22,108 (57) | 0.11 | 21,383 (57) | 22,108 (57) | 0.01 |
| Mean no. of general practitioner visits (SD) | 14.2 (19.2) | 12.37 (15.0) | 0.11 | 12.4 (8.2) | 12.37 (14.96) | 0.01 |
| Mean no. of endocrinology visits (SD) | 0.5 (2.0) | 0.8 (2.2) | 0.13 | 0.7 (1.2) | 0.8 (2.2) | 0.06 |
| Mean eGFR, ml/min per 1.73 m2 (SD) | 69 (19) | 73 (17) | 0.23 | 73 (10) | 73 (17) | 0.01 |
| eGFR category, ml/min per 1.73 m2 | ||||||
| ≥90 | 14,853 (14) | 6485 (17) | 0.07 | 6319 (17) | 6485 (17) | 0.01 |
| 60 to <90 | 55,500 (53) | 23,520 (60) | 0.16 | 22,547 (60) | 23,520 (60) | 0.00 |
| 45 to <60 | 20,617 (20) | 6577 (17) | 0.07 | 6250 (17) | 6577 (17) | 0.01 |
| 30 to <45 | 14,730 (14) | 2412 (6) | 0.26 | 2332 (6) | 2412 (6) | 0.00 |
eGFR is measured in milliliters per minute per 1.73 m2. The most recent eGFR measurement in the 365-day period was before the cohort entry date (including the cohort entry date). eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation: 141×min([serum creatinine concentration in micromoles per liter per 88.4]/ĸ, 1)α×max([serum creatinine concentration in micromoles per liter per 88.4]/ĸ, 1)−1.209×0.993Age×1.018 [if a woman]. ĸ=0.7 if a woman and 0.9 if a man; α=−0.329 if a woman and −0.411 if a man. Min is the minimum of serum creatinine concentration/ĸ or one; max is the maximum of serum creatinine concentration/ĸ or one. On the basis of recent Ontario Renal Network guidelines, race was not factored into the calculation of eGFR.
After weighting, baseline characteristics were well balanced between groups except for the proportion of prescriptions written by cardiologists, which remained higher in the SGLT2i group. When stratified by eGFR, the baseline characteristics of SGLT2i users and DPP-4i users were well balanced within each eGFR category (Supplemental Appendix).
Prescription Characteristics
Prescription characteristics are provided in Table 2. The mean continuous usage of DPP-4i was slightly longer than that of SGLT2i (428 versus 501 days in SGLT2i versus DPP-4i, respectively). Empagliflozin and sitagliptin were the most prescribed SGLT2i and DPP-4i, respectively.
Table 2.
Prescription characteristics of sodium-glucose cotransporter-2 inhibitors and dipeptidyl peptidase-4 inhibitors
| Medication | Mean Continuous Use, d (SD) | Median Continuous Use, d (Interquartile Range) |
|---|---|---|
| SGLT2i | ||
| All, n=38,994 | 428 (414) | 287 (89–645) |
| Empagliflozin, n=22,095 | 379 (342) | 283 (85–566) |
| Canagliflozin, n=11,939 | 464 (477) | 272 (78–711) |
| Dapagliflozin, n=4960 | 414 (368) | 291 (91–664) |
| DPP-4i | ||
| All, n=105,700 | 501 (454) | 348 (135–778) |
| Sitagliptin, n=78,633 | 480 (443) | 329 (125–733) |
| Linagliptin, n=22,415 | 504 (452) | 358 (125–794) |
| Saxagliptin, n=4652 | 470 (462) | 283 (104–723) |
SGLT2i, sodium-glucose cotransporter-2 inhibitor; DPP-4i, dipeptidyl peptidase-4 inhibitor.
Outcomes
After weighting, we observed 342 fractures within 180 days and 689 fractures within 365 days. New SGLT2i use was not associated with a higher risk of fracture at 180 days compared with new DPP-4i use (weighted HR, 0.95; 95% CI, 0.79 to 1.13) (Table 3). When examined by fracture site, there was also no difference between groups (Supplemental Appendix). We found no substantial difference in the 180-day risk of hospital encounter with falls, hypoglycemia, or hypotension (Table 3).
Table 3.
Primary and secondary outcomes in the sodium-glucose cotransporter-2 inhibitor or dipeptidyl peptidase-4 inhibitor cohorts
| Outcome at 180 d | Dipeptidyl Peptidase-4, n=37,449, n (%) | Sodium-Glucose Cotransporter-2 Inhibitor, n=38,994, n (%) | Weighted Hazard Ratio (95% Confidence Interval) |
|---|---|---|---|
| All fracture | 172 (0.5) | 170 (0.4) | 0.95 (0.79 to 1.13) |
| Falls | 880 (2.4) | 897 (2.3) | 0.98 (0.91 to 1.05) |
| Hypotension | 40 (0.1) | 41 (0.1) | 0.98 (0.65 to 1.47) |
| Hypoglycemia | 81 (0.2) | 77 (0.2) | 0.91 (0.68 to 1.22) |
When fracture risk was assessed at 365 days, there was a modestly significant lower risk of fracture in new SGLT2i users compared with DPP-4i users (HR, 0.88; 95% CI, 0.77 to 1.00).
In subgroup analysis, eGFR did not appear to modify the association between SGLT2i versus DDP-4i use and fracture outcome at 180 or 365 days (the P values for interaction: 0.37 and 0.53, respectively) (Table 4). In all eGFR categories, there did not appear to be evidence of a higher risk of fracture with SGLT2i versus DDP-4i.
Table 4.
Fractures at 180 and 365 days assessed by eGFR group
| eGFR Group, ml/min per 1.73 m2 and Medication | Fracture at 180 d, N (%) | Weighted Hazard Ratio (95% Confidence Interval) | P Value for Subgroup Interaction | Fracture at 365 d, N (%) | Weighted Hazard Ratio (95% Confidence Interval) | P Value for Subgroup Interaction |
|---|---|---|---|---|---|---|
| All | ||||||
| DPP-4i, n=37,449 | 172 (0.46) | 0.95 (0.79 to 1.13) | N/A | 360 (0.96) | 0.88 (0.77 to 1.00) | N/A |
| SGLT2i, n=38,994 | 170 (0.44) | 329 (0.84) | ||||
| eGFR ≥90 | ||||||
| DPP-4i, n=6330 | 28 (0.45) | 0.79 (0.46 to 1.38) | 0.37 | 61 (0.96) | 0.90 (0.63 to 1.28) | 0.53 |
| SGLT2I, n=6485 | 23 (0.35) | 56 (0.86) | ||||
| eGFR 60 to <90 | ||||||
| DPP-4i, n=22,625 | 95 (0.42) | 1.10 (0.81 to 1.36) | 194 (0.86) | 0.94 (0.78 to 1.13) | ||
| SGLT2i, n=23,520 | 104 (0.44) | 189 (0.80) | ||||
| eGFR 45 to <60 | ||||||
| DPP-4i, n=6198 | 28 (0.46) | 1.00 (0.70 to 1.50) | 68 (1.10) | 0.82 (0.61 to 1.10) | ||
| SGLT2i, n=6577 | 31 (0.47) | 59 (0.90) | ||||
| eGFR 30 to <45 | ||||||
| DPP-4i, n=2206 | 19 (0.88) | 0.56 (0.30 to 1.06) | 36 (1.64) | 0.64 (0.43 to 0.95) | ||
| SGLT2i, n=2412 | 12 (0.50) | 25 (1.04) | ||||
DPP-4i, dipeptidyl peptidase-4 inhibitor; SGLT2i, sodium-glucose cotransporter-2 inhibitor, eGFR, estimated glomerular filtration rate.
Discussion
Patients with CKD have a two- to five-fold higher risk of fracture compared with the general population (43,44). Recent guidelines recommend starting SGLT2is in all patients with diabetic kidney disease and eGFR >30 ml/min per 1.73 m2 (2,45), but in short-term studies, dapagliflozin and canagliflozin (but not empagliflozin) have been associated with hyperphosphatemia, hyperparathyroidism, and increased bone turnover (8–10,12,46). As such, it is increasingly important to ensure that SGLT2is do not increase the fracture risk in patients with CKD.
In this large Canadian cohort of older adults, we found that new use of SGLT2i was not associated with a higher risk of fracture at 180 or 365 days compared with new DPP-4i use. This was also true when results were stratified by eGFR category. This provides further real-world assurance that these medications can be safely prescribed without a higher risk of fracture.
Our results are consistent with a previously published meta-analyses and population-based studies of SGLT2is versus placebo or active comparator in the general diabetes populations (16–23,47). Although the CREDENCE and DAPA-CKD trials of SGLT2i included patients with CKD, the trials’ ability to detect a potential fracture risk was limited by low numbers of events and study of a relatively healthy population (1,48).
In our study conducted in the real world (older and higher proportion of women), we observed more fracture events than in previous randomized controlled trials but still did not find a higher fracture risk. We did observe a signal of lower risk of fracture in new SGLT2i users versus new DPP-4i users in the lowest level of eGFR (30 to <45 ml/min per 1.73 m2) at 365 days. However, the significance of this finding is limited by small sample size and a lack of adjustment for multiple comparisons. As such, this result may not be reproducible and should be used to generate hypotheses rather than draw conclusions.
Although a similar protective effect by eGFR category was seen in one large meta-analysis of SGLT2is in the general diabetes population (HR for fracture, 0.55; 95% CI, 0.37 to 0.81) versus placebo, the finding did not persist in trials where patients were followed for over 52 weeks (20).
Our study has several strengths. To our knowledge, this is the first study of its kind to specifically examine fracture risk in patients with CKD. We used outpatient laboratory values that are more accurate in identifying individuals with CKD than administrative data codes, which are more sensitive in identifying these patients (49). We also stratified our analysis on the basis of eGFR categories and were able to achieve well-balanced groups within each eGFR strata.
Our cohort was also composed of individuals prescribed SGLT2is in usual clinical care, making it more generalizable to real-world older adult populations.
However, our study has limitations. To preserve statistical power, we were unable to stratify the analysis by SGLT2i type. Given that empagliflozin is associated with the lowest number of metabolic derangements (hyperphosphatemia, hyperparathyroidism) and made up the most prescriptions, we may have observed a biased result toward a null effect. Although we adjusted for 71 baseline characteristics and were able to achieve well-balanced groups, we cannot rule out residual confounding on unmeasured factors (i.e., smoking, severity of diabetes). There were other drugs/factors that we could have included in our analysis (e.g., insulin use, DKA), but we adjusted for a multitude of other measures related to diabetes severity, including duration of diabetes, complication rates, care utilization (endocrinologist visits), and number of oral hypoglycemic medications (all were well balanced between the two groups). Finally, we limited our secondary analyses to 1-year follow-up given the typical duration of continuous use. It is possible that if there is a change in bone density or quality caused by SGLT2i (such as increased bone turnover and secondary hyperparathyroidism), an associated change in fracture risk may take longer to become apparent.
In this cohort study of over 140,000 patients in Ontario, Canada, new use of SGLT2is was not associated with a higher risk of fracture compared with new use of DPP-4i. This also held true in patients with an eGFR of 30–90 ml/min per 1.73 m2. This finding should be reassuring to clinicians and patients.
Disclosures
K.K. Clemens reports employment with Western University; has received a research award sponsored in part by AstraZeneca; and has received honoraria for delivering Certified Medical Education talks from the Canadian Medical and Surgical Knowledge Translation Group, the Continuing Professional Development Network, and Sutherland Global Services Canada. A. Cowan reports employment with the University of Western Ontario and was supported by the Division of Nephrology, the Lawson Health Research Institute, Physicians Services Incorporated, and the Schulich School of Medicine and Dentistry. S.N. Dixon reports employment with Lawson Health Research Institute and the Institute for Clinical Evaluative Sciences. A.X. Garg reports employment with London Health Sciences Centre; reports research funding from Astellas and Baxter; reports serving on the editorial boards of American Journal of Kidney Diseases and Kidney International; reports serving on the data safety and monitoring board for an anemia trial program funded by GlaxoSmithKline (activity now complete) and in the medical lead role to improve access to kidney transplantation and living kidney donation for the Ontario Renal Network (government-funded agency located within Ontario Health); and was supported by the Dr. Adam Linton Chair in Kidney Health Analytics. N. Jeyakumar reports employment with Institute for Clinical and Evaluative Sciences (ICES) Western. Y. Kang reports employment with ICES Western and Curry's Artists' Materials. K. Naylor reports employment with London Health Sciences Centre. M.A. Weir reports employment with Western University.
Funding
This study was supported by the Institue for Clinical and Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care. The research was done at the ICES Western facility with partners, which include the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute. The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team at the ICES Western facility who are supported by a grant from the Canadian Institutes of Health Research (FRN 148377).
Supplementary Material
Acknowledgments
Parts of this material are based on data and information compiled and provided by Ontario Ministry of Health (MOH). The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
We thank IQVIA Solutions Canada Inc. for use of their Drug Information File.
Parts of this material are on the basis of data and information compiled and provided by the Canadian Institutes of Health Information. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
No endorsement by Institute of Clinical and Evaluative Sciences, the Ontario Ministry of Health and Long-Term Care, the Academic Medical Organization of Southwestern Ontario, the School of Medicine and Dentistry, Western University, the Lawson Health Research Institute, Canadian Institutes of Health Information, or Canadian Institutes of Health Research is intended or should be inferred. The study sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Safety of SGLT2 Inhibitors in CKD: Walking the Fine Line,” on pages 774–776.
Author Contributions
K.K. Clemens, A. Cowan, S.N. Dixon, A.X. Garg, N. Jeyakumar, Y. Kang, K. Naylor, and M.A. Weir conceptualized the study; K.K. Clemens, A. Cowan, S.N. Dixon, N. Jeyakumar, and Y. Kang were responsible for data curation; K.K. Clemens, A. Cowan, S.N. Dixon, A.X. Garg, N. Jeyakumar, and Y. Kang were responsible for investigation; A. Cowan, S.N. Dixon, N. Jeyakumar, and Y. Kang were responsible for formal analysis; K.K. Clemens, A. Cowan, S.N. Dixon, A.X. Garg, N. Jeyakumar, Y. Kang, K. Naylor, and M.A. Weir were responsible for methodology; N. Jeyakumar was responsible for project administration; A.X. Garg was responsible for resources; A.X. Garg was responsible for funding acquisition; K.K. Clemens and A.X. Garg provided supervision; A. Cowan wrote the original draft; and K.K. Clemens, A. Cowan, S.N. Dixon, A.X. Garg, N. Jeyakumar, Y. Kang, K. Naylor, and M.A. Weir reviewed and edited the manuscript.
Data Sharing Statement
The analysis was conducted by members of the Institute of Clinical and Evaluative Sciences Kidney, Dialysis and Transplantation team at the ICES Western facility (London, Ontario). The protocol can be obtained by emailing K.K. Clemens at kristin.clemens@sjhc.london.on.ca.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16171221/-/DCSupplemental.
Supplemental Appendix A. Record PE Checklist of Recommendations.
Supplemental Appendix B. Descriptions of databases used to obtain demographic, comorbid condition, and outcome data.
Supplemental Appendix C. Codes used to obtain information about baseline measures and the databases used to obtain the information.
Supplemental Appendix D. Drug Identification Numbers used to identify study drugs.
Supplemental Appendix E. Codes used to define outcomes and references for validation.
Supplemental Appendix F. Covariates used to create the propensity score.
Supplemental Appendix G. Number of patients included at each step of the cohort build.
Supplemental Appendix H. Full baseline characteristics for the weighted and unweighted cohorts.
Supplemental Appendix I. Full baseline characteristics, stratified by eGFR category, after weighting.
Supplemental Appendix J. Fracture at 180 days by fracture site.
References
- 1.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators : Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 380: 347–357, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators : Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 373: 2117–2128, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Lipscombe L, Butalia S, Dasgupta K, Eurich DT, MacCallum L, Shah BR, Simpson S, Senior PA; Diabetes Canada Clinical Practice Guidelines Expert Committee : Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 44: 575–591, 2020 [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration : Highlights of Prescribing Information: Invokana, 2018. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet. Accessed August 10, 2021
- 6.Health Canada : Invokana. Product monograph. Available at: https://pdf.hres.ca/dpd_pm/00038913.PDF. Accessed August 10, 2021
- 7.Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G: Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 101: 157–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weir MR, Kline I, Xie J, Edwards R, Usiskin K: Effect of canagliflozin on serum electrolytes in patients with type 2 diabetes in relation to estimated glomerular filtration rate (eGFR). Curr Med Res Opin 30: 1759–1768, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Kohan DE, Fioretto P, Tang W, List JF: Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85: 962–971, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jong MA, Petrykiv SI, Laverman GD, van Herwaarden AE, de Zeeuw D, Bakker SJL, Heerspink HJL, de Borst MH: Effects of dapagliflozin on circulating markers of phosphate homeostasis. Clin J Am Soc Nephrol 14: 66–73, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau JE, Taylor SI: Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol 14: 473–474, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N: Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab 101: 44–51, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye Y, Zhao C, Liang J, Yang Y, Yu M, Qu X: Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol 9: 1517, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB: Executive summary of the 2017 KDIGO Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update: What’s changed and why it matters. Kidney Int 92: 26–36, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Watts NB, Roux C, Modlin JF, Brown JP, Daniels A, Jackson S, Smith S, Zack DJ, Zhou L, Grauer A, Ferrari S: Infections in postmenopausal women with osteoporosis treated with denosumab or placebo: Coincidence or causal association? Osteoporos Int 23: 327–337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmedt N, Andersohn F, Walker J, Garbe E: Sodium-glucose co-transporter-2 inhibitors and the risk of fractures of the upper or lower limbs in patients with type 2 diabetes: A nested case-control study. Diabetes Obes Metab 21: 52–60, 2019 [DOI] [PubMed] [Google Scholar]
- 17.Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E: Fracture risk after initiation of use of canagliflozin: A cohort study. Ann Intern Med 170: 155–163, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrahami D, Douros A, Yin H, Yu OHY, Azoulay L: Sodium-glucose cotransporter 2 inhibitors and the risk of fractures among patients with type 2 diabetes. Diabetes Care 42: e150–e152, 2019 [DOI] [PubMed] [Google Scholar]
- 19.Ueda P, Svanström H, Melbye M, Eliasson B, Svensson AM, Franzén S, Gudbjörnsdottir S, Hveem K, Jonasson C, Pasternak B: Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: Nationwide register based cohort study. BMJ 363: k4365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng L, Li YY, Hu W, Bai F, Hao HR, Yu WN, Mao XM: Risk of bone fracture associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials. Diabetes Metab 45: 436–445, 2019 [DOI] [PubMed] [Google Scholar]
- 21.Li X, Li T, Cheng Y, Lu Y, Xue M, Xu L, Liu X, Yu X, Sun B, Chen L: Effects of SGLT2 inhibitors on fractures and bone mineral density in type 2 diabetes: An updated meta-analysis. Diabetes Metab Res Rev 35: e3170, 2019 [DOI] [PubMed] [Google Scholar]
- 22.Ruanpeng D, Ungprasert P, Sangtian J, Harindhanavudhi T: Sodium-glucose cotransporter 2 (SGLT2) inhibitors and fracture risk in patients with type 2 diabetes mellitus: A meta-analysis [published online ahead of print June 16, 2017]. Diabetes Metab Res Rev 10.1002/dmrr.2903 [DOI] [PubMed] [Google Scholar]
- 23.Zhuo M, Hawley CE, Paik JM, Bessette LG, Wexler DJ, Kim DH, Tong AY, Kim SC, Patorno E: Association of sodium-glucose cotransporter-2 inhibitors with fracture risk in older adults with type 2 diabetes. JAMA Netw Open 4: e2130762, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitsch D, Mylne A, Roderick PJ, Smeeth L, Hubbard R, Fletcher A: Chronic kidney disease and hip fracture-related mortality in older people in the UK. Nephrol Dial Transplant 24: 1539–1544, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Ting QC, Haonan L, Huawei Z, Yan PC: Risk of fractures associated with dipeptidyl peptidase-4 inhibitor treatment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Ther 10: 1879–1892, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu J, Zhu J, Hao Y, Guo C, Zhou Z: Dipeptidyl peptidase-4 inhibitors and fracture risk: An updated meta-analysis of randomized clinical trials. Sci Rep 6: 29104, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of Ontario : Ontario Population Projections, 2018–2046, 2019. Available at: https://www.fin.gov.on.ca/en/economy/demographics/projections/#s3b. Accessed February 12, 2020
- 28.Langan SM, Schmidt SA, Wing K, Ehrenstein V, Nicholls SG, Filion KB, Klungel O, Petersen I, Sorensen HT, Dixon WG, Guttmann A, Harron K, Hemkens LG, Moher D, Schneeweiss S, Smeeth L, Sturkenboom M, von Elm E, Wang SV, Benchimol EI: The reporting of studies conducted using observational routinely collected health data statement for pharmacoepidemiology (RECORD-PE). BMJ 363: k3532, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ontario Drug Benefit Formulary : Search Results. Available at: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?q=flozin&type=1. Accessed April 3, 2019
- 30.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institute of Diabetes and Digestive and Kidney Diseases : CKD & Drug Dosing: Information for Providers, 2015. Available at: https://www.niddk.nih.gov/research-funding/research-programs/kidney-clinical-research-epidemiology/laboratory/ckd-drug-dosing-providers. Accessed July 8, 2021
- 32.Garg AX, Mamdani M, Juurlink DN, van Walraven C; Network of Eastern Ontario Medical Laboratories (NEO-MeL) : Identifying individuals with a reduced GFR using ambulatory laboratory database surveillance. J Am Soc Nephrol 16: 1433–1439, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Inal B, Oguz O, Emre T, Usta M, Inal H, Altunoglu E, Topkaya C: Evaluation of MDRD, Cockcroft-Gault, and CKD-EPI formulas in the estimated glomerular filtration rate. Clin Lab 60: 1685–1694, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Brimble KS, Treleaven D, Cooper R, Blake PG: Removal of eGFR adjustment for race in Ontario. CMAJ 193: 2021 [Google Scholar]
- 35.Iksander C, Cherney DZ, Clemens KK, Dixon SN, Harel Z, Jeyakumar N, McArthur E, Muanda FT, Parikh CR, Paterson JM, Tangri N, Udell JA, Wald R, Garg AX: Use of sodium–glucose cotransporter-2 inhibitors and risk of acute kidney injury in older adults with diabetes: A population-based cohort study. CMAJ 192: E351–E360, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Donnell S; Canadian Chronic Disease Surveillance System (CCDSS) Osteoporosis Working Group : Use of administrative data for national surveillance of osteoporosis and related fractures in Canada: Results from a feasibility study. Arch Osteoporos 8: 143, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papaioannou A, Kennedy CC, Ioannidis G, Cameron C, Croxford R, Adachi JD, Mursleen S, Jaglal S: Comparative trends in incident fracture rates for all long-term care and community-dwelling seniors in Ontario, Canada, 2002-2012. Osteoporos Int 27: 887–897, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Austin PC: An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 46: 399–424, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austin PC: Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 35: 5642–5655, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Austin PC: Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38: 1228–1234, 2009 [Google Scholar]
- 41.Harrington D, D’Agostino RB Sr., Gatsonis C, Hogan JW, Hunter DJ, Normand ST, Drazen JM, Hamel MB: New guidelines for statistical reporting in the journal. N Engl J Med 381: 285–286, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Izem R, Liao J, Hu M, Wei Y, Akhtar S, Wernecke M, MaCurdy TE, Kelman J, Graham DJ: Comparison of propensity score methods for pre-specified subgroup analysis with survival data. J Biopharm Stat 30: 734–751, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, Pouget JG, Lok CE, Hodsman AB, Adachi JD, Garg AX: The three-year incidence of fracture in chronic kidney disease. Kidney Int 86: 810–818, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group : KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 98[4S]: S1–S115, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Kohler S, Zeller C, Iliev H, Kaspers S: Safety and tolerability of empagliflozin in patients with type 2 diabetes: Pooled analysis of phase I-III clinical trials. Adv Ther 34: 1707–1726, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian BB, Chen Q, Li L, Yan CF: Association between combined treatment with SGLT2 inhibitors and metformin for type 2 diabetes mellitus on fracture risk: A meta-analysis of randomized controlled trials. Osteoporos Int 31: 2313–2320, 2020 [DOI] [PubMed] [Google Scholar]
- 48.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 49.Fleet JL, Dixon SN, Shariff SZ, Quinn RR, Nash DM, Harel Z, Garg AX: Detecting chronic kidney disease in population-based administrative databases using an algorithm of hospital encounter and physician claim codes. BMC Nephrol 14: 81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.