Visual Abstract
Keywords: clinical epidemiology, COVID-19, vaccination, hemodialysis
Abstract
Background and objectives
Patients receiving hemodialysis are at high risk from coronavirus disease 2019 (COVID-19) and demonstrate impaired immune responses to vaccines. There have been several descriptions of their immunologic responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination, but few studies have described the clinical efficacy of vaccination in patients on hemodialysis.
Design, setting, participants, & measurements
In a multicenter observational study of the London hemodialysis population undergoing surveillance PCR testing during the period of vaccine rollout with BNT162b2 and AZD1222, all of those positive for SARS-CoV-2 were identified. Clinical outcomes were analyzed according to predictor variables, including vaccination status, using a mixed effects logistic regression model. Risk of infection was analyzed in a subgroup of the base population using a Cox proportional hazards model with vaccination status as a time-varying covariate.
Results
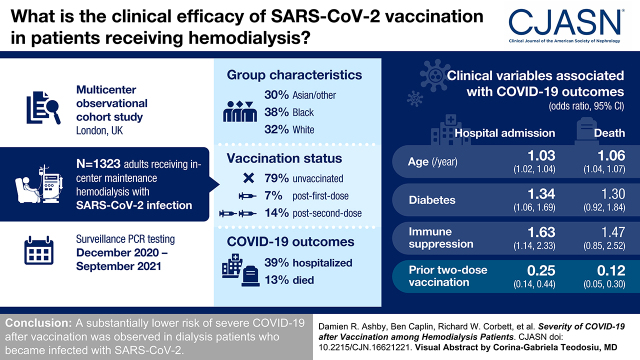
SARS-CoV-2 infection was identified in 1323 patients of different ethnicities (Asian/other, 30%; Black, 38%; and White, 32%), including 1047 (79%) unvaccinated, 86 (7%) after first-dose vaccination, and 190 (14%) after second-dose vaccination. The majority of patients had a mild course; however, 515 (39%) were hospitalized, and 172 (13%) died. Older age, diabetes, and immune suppression were associated with greater illness severity. In regression models adjusted for age, comorbidity, and time period, prior two-dose vaccination was associated with a 75% (95% confidence interval, 56 to 86) lower risk of admission and 88% (95% confidence interval, 70 to 95) fewer deaths compared with unvaccinated patients. No loss of protection was seen in patients over 65 years or with increasing time since vaccination, and no difference was seen between vaccine types.
Conclusions
These data demonstrate a substantially lower risk of severe COVID-19 after vaccination in patients on dialysis who become infected with SARS-CoV-2.
Introduction
Patients on in-center hemodialysis face a dual hazard from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). First, although the majority of the population is able to adhere to lockdown measures, the need to attend dialysis creates a greater likelihood of exposure to infection; second, as a group with comorbidity and impaired immune responses, infection is more severe once acquired (1,2). As a consequence, in these patients there is a high relative risk of death across all age groups (3).
Although vaccines have been shown to induce robust immune responses and protect individuals against infection in the general population (4,5), patients on hemodialysis have generally been excluded from these trials. Several studies have investigated either humoral (6–8) or cellular immune responses (9) to vaccination in patients on dialysis, but there has been limited evidence of clinical effectiveness.
The clinical effectiveness of vaccination remains a pressing concern in this group of vulnerable patients (10) and is vital for supporting vaccine promotion among hesitant patients. This study aims to estimate the clinical efficacy of vaccination in preventing severe disease in patients on hemodialysis developing SARS-CoV-2 infection.
Materials and Methods
This cohort study of SARS-CoV-2 infections in patients on prevalent hemodialysis included all patients with positive PCR on surveillance or otherwise indicated testing between December 1, 2020 and September 26, 2021. Dates were chosen to include as many first and second doses as possible, running from the start of the vaccination program until third doses were offered to this patient group in the United Kingdom. The study was sponsored by St. George’s Hospital and received approval from the National Research Ethics Service (Integrated Research Application System [IRAS] reference 283130).
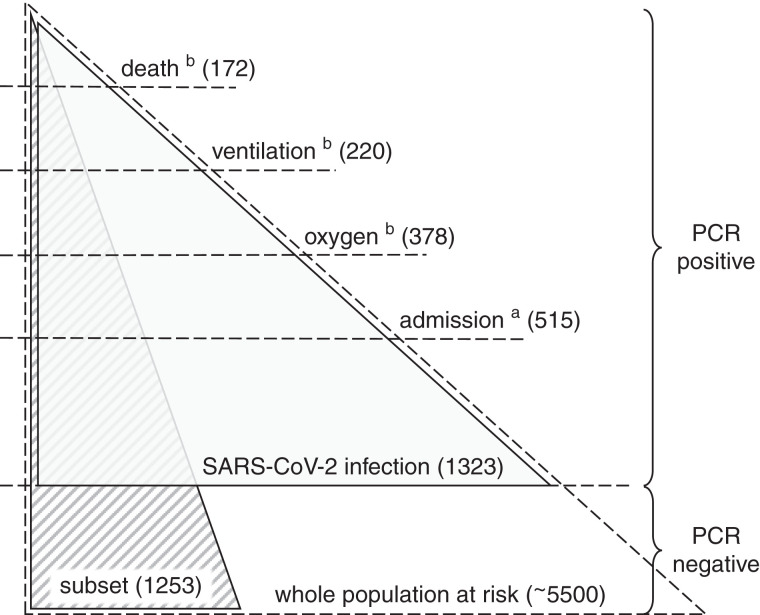
In-center hemodialysis is provided to approximately 5500 patients in London across seven nephrology centers, with enhanced infection surveillance and isolation of cases during the pandemic as described elsewhere (2). All London nephrology centers were included. The main study population included all patients on prevalent in-center hemodialysis with SARS-CoV-2 infection identified by positive PCR (Figure 1). During the study period, all centers had a policy of temperature/symptom screening at every dialysis session, SARS-CoV-2 RNA testing of all patients on a weekly basis, and additional RNA testing of contacts of cases. Cases otherwise identified with testing triggered by contact with a case or symptoms (for example, presenting to emergency services) were also included. Nephrology centers were notified about such cases by patients or their caregivers (by phone or at the next dialysis session) or by the hospital clinician if admitted, with nursing staff also routinely investigating the reason for any nonattendance at dialysis. Patients receiving home dialysis were excluded, as were those receiving short-term dialysis for recoverable kidney disease. SARS-CoV-2 infection date was defined by the date of the first positive RNA during the observation period. Prior infection was defined if there was positive RNA >90 days previously, whereas cases following prior infection within 90 days were regarded as persistent viral shedding rather than new infection and excluded.
Figure 1.
Study populations and relationship to the whole dialysis population. The whole population at risk contains all of those receiving hemodialysis (in center) during the observation period. Weekly PCR screening was carried out in this population, with additional PCR testing as indicated by symptoms or contact with a case. The main study population (gray shading) contains all severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections defined by positive PCR (in any setting) during the observation period, and it is used to assess the risk of severe disease in those with infection. The supplementary study population (striped shading) contains a subset of the whole population at risk, for which full vaccination data were available, and it is used to assess the risk of developing infection. aWithin 14 days of positive PCR. bWithin 28 days of positive PCR.
Clinical severity definitions included any hospital admission within 14 days (including a small number of infections acquired in patients already hospitalized), any period of sustained oxygen use within 28 days, any ventilatory support (including noninvasive methods) within 28 days, and death from any cause within 28 days (with or without hospital admission). These outcomes were defined hierarchically so that each category includes more severe coronavirus disease 2019 (COVID-19) outcomes. Most hospital episodes took place within nephrology centers, but cases admitted to other hospitals were reported to their nephrology center for clinical review and to arrange isolated dialysis (although it is possible that a few very brief admissions, not covering a dialysis session, could have been missed). Hospital records were reviewed to determine supportive treatment required and outcome. Deaths caused by COVID-19 were identified by excluding deaths due to an alternative pathology, to which SARS-CoV-2 was noncontributory. Immune suppression was defined if at the onset of infection, patients were receiving steroids (equivalent to prednisolone >10 mg daily), tacrolimus, mycophenolate, or azathioprine or if they had received cytotoxic chemotherapy or immunomodulating biologic agents within the last 6 months. Differences in COVID-19 outcomes have been reported, so ethnicity from electronic records was collected and grouped as Asian/other, Black, and White.
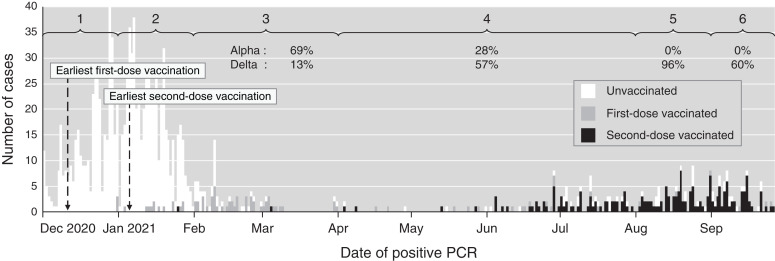
The time period of infection was included as a predictor variable to account for secular trends on the basis of month, amalgamating those with few cases and making six time periods. The dominant SARS-CoV-2 variant in London was Alpha (B.1.1.7) during periods 1–3 and Delta (B.1.617.2) during periods 4–6 (11). Only two vaccines were used in this period: BNT162b2 (Pfizer-BioNTech) or AZD1222 (Oxford AstraZeneca). Data were missing for vaccine type in six cases (omitted from vaccine type analysis) but complete for comorbidity and clinical outcome, with no missing outcomes or loss to follow-up. Patient status was not coded as vaccinated (first or second dose) until 10 days after vaccine administration. The observation period ended on September 26, with the 28-day outcome complete on October 24, 2021. Data collection took place during and after the observation period, and it was completed on December 11, 2021.
Covariates associated with clinical outcome were analyzed using mixed logistic regression models with fixed effects including age, sex, ethnicity, diabetes, immune suppression, prior SARS-CoV-2, and time period, with nephrology center as a random effect. Effect sizes were expressed as odds ratios with 95% confidence intervals (95% CIs), and estimated vaccine efficacy in preventing each outcome after SARS-CoV-2 infection was defined as 1 − odds ratio. Subgroup analyses were performed to estimate the effect of age, vaccine type, and time since vaccination, with boundaries chosen to give roughly equal group sizes. Sensitivity analyses were performed in which patients with prior SARS-CoV-2 were excluded and the analysis was restricted to individual time periods (4–6).
In a secondary analysis, a subgroup for which full vaccination data were available was defined from those at risk who were receiving in-center hemodialysis from the start of the observation period (Figure 1), with the incidence of SARS-CoV-2 infection observed during the study period defined by positive PCR. Variables associated with infection were analyzed using a Cox proportional hazards model with vaccination status as a time-varying covariate, considered to change 10 days after each dose. SPSS v27.0 (IBM, New York, NY) was used for modeling.
Results
Between December 1, 2020 and September 26, 2021, SARS-CoV-2 infection was detected by PCR in 1323 patients on hemodialysis (aged 18–95 years, 60% men, ethnicity grouped as Asian/other, 30%; Black, 38%; and White, 32%) with a bimodal epidemic time course (Figure 2). Patients began receiving first-dose vaccination from December 10 and second-dose vaccination from January 5.
Figure 2.
Epidemic time course showing the number of new SARS-CoV-2 infections by date and vaccination status. Months with fewer cases were amalgamated to make six time periods (shown at the top). The community proportions of Alpha and Delta variants (dominant during the study) are provided for time periods with adequate data as percentages (not known in periods 1 and 2).
At the time of diagnosis, 1047 patients (79%) were unvaccinated, 86 (7%) were at least 10 days beyond their first dose, and 190 (14%) were at least 10 days beyond their second dose. The majority of PCR samples were taken in the dialysis unit as part of weekly surveillance or in response to exposure or symptoms, but 6% were taken on a Sunday; therefore, at least this many were taken in an emergency health care setting. Immune-suppressing treatments were taken by 164 patients (12%), of which 44% were on tacrolimus or cyclosporin monotherapy; 20% were on monotherapy with steroids, azathioprine, or mycophenolate; 19% were on combinations of these; and 17% had been receiving cytotoxic chemotherapy or biologic agents. Further patient characteristics are given in Table 1.
Table 1.
Characteristics and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection stratified by vaccination status
| Covariate/Outcome | Unvaccinated | First Dose (>10 d After) | Second Dose (>10 d After) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Number/ Median | Percentage/ IQR | Number/ Median | Percentage/ IQR | Number/ Median | Percentage/ IQR | Number/ Median | Percentage/ IQR | |
| N | 1047 | 86 | 190 | 1323 | ||||
| Days after dose, median (IQR) | 25.5 | (18–52) | 126.5 | (95–153) | ||||
| Vaccine | ||||||||
| BNT162b2 | 46 | (53) | 79 | (42) | ||||
| AZD1222 | 38 | (44) | 107 | (56) | ||||
| Age, median (IQR) | 61 | (53–72) | 71 | (58–79) | 63 | (52–73) | 62 | (53–73) |
| Sex | ||||||||
| Men | 622 | (59) | 58 | (67) | 117 | (62) | 797 | (60) |
| Ethnicity | ||||||||
| Asian/other | 332 | (32) | 16 | (19) | 50 | (26) | 398 | (30) |
| Black | 401 | (38) | 28 | (33) | 79 | (42) | 508 | (38) |
| White | 314 | (30) | 42 | (49) | 61 | (32) | 417 | (32) |
| Diabetes | 484 | (46) | 42 | (49) | 93 | (49) | 619 | (47) |
| Immune suppressiona | 117 | (11) | 14 | (16) | 33 | (17) | 164 | (12) |
| Prior SARS-CoV-2b | 29 | (3) | 4 | (5) | 12 | (6) | 45 | (3) |
| Outcome | ||||||||
| Admissionc | 436 | (42) | 33 | (38) | 46 | (24) | 515 | (39) |
| Oxygend | 329 | (31) | 23 | (27) | 26 | (14) | 378 | (29) |
| Ventilationd | 185 | (18) | 17 | (20) | 18 | (10) | 220 | (17) |
| Deathd | 148 | (14) | 12 | (14) | 12 | (6) | 172 | (13) |
Except where stated, data are N (%). Clinical outcomes are “all cause,” not specifically due to coronavirus disease 2019. IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Any immune suppression treatment including steroids, tacrolimus, mycophenolate, azathioprine, and cytotoxic and biologic agents.
PCR positive at least 90 days prior to the current infection.
Within 14 days of positive PCR.
Within 28 days of positive PCR.
A mild course was observed in 808 patients (61%) who did not require admission; however, 378 (29%) at least required oxygen, and 172 (13%) died before 28 days. SARS-CoV-2 was thought to be incidental to the illness and death in 22 cases, and therefore, COVID-19 was the cause of death in 150 cases (11% of all cases and 87% of deaths within 28 days). The association of clinical variables with disease severity is shown in Table 2: older age, diabetes, and immune-suppressing treatment were associated with greater illness severity, as were later time periods (when Delta emerged as the dominant SARS-CoV-2 strain in London). Temporal changes in the community prevalence of variants (11) are provided in Supplemental Table 1 along with the availability of treatments for hospitalized patients.
Table 2.
Factors associated with severe coronavirus disease 2019 outcomes in patients with severe acute respiratory syndrome coronavirus 2 infection
| Covariate | Severe Coronavirus Disease 2019 Outcomes | |||||||
|---|---|---|---|---|---|---|---|---|
| Admissiona | Oxygenb | Ventilationb | Deathb | |||||
| Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | Odds Ratio | 95% Confidence Interval | |
| Age, yr | 1.03 | (1.02 to 1.04) | 1.03 | (1.02 to 1.04) | 1.04 | (1.03 to 1.05) | 1.06 | (1.04 to 1.07) |
| Sex | ||||||||
| Men | 1.09 | (0.86 to 1.39) | 0.92 | (0.71 to 1.19) | 0.89 | (0.66 to 1.21) | 0.92 | (0.65 to 1.31) |
| Ethnicityc | ||||||||
| Asian/other | 0.87 | (0.65 to 1.15) | 0.83 | (0.61 to 1.12) | 0.76 | (0.53 to 1.09) | 0.81 | (0.54 to 1.21) |
| Black | 1.00 | (0.74 to 1.35) | 0.85 | (0.61 to 1.18) | 0.76 | (0.51 to 1.12) | 0.70 | (0.45 to 1.11) |
| Diabetes | 1.34 | (1.06 to 1.69) | 1.37 | (1.06 to 1.77) | 1.32 | (0.97 to 1.79) | 1.30 | (0.92 to 1.84) |
| Immune suppressiond | 1.63 | (1.14 to 2.33) | 1.79 | (1.22 to 2.62) | 1.66 | (1.05 to 2.62) | 1.47 | (0.85 to 2.52) |
| Prior SARS-CoV-2e | 0.55 | (0.27 to 1.12) | 0.53 | (0.23 to 1.19) | 0.78 | (0.32 to 1.95) | 0.92 | (0.34 to 2.49) |
| Time period f | ||||||||
| 2 (January 2021) | 1.12 | (0.86 to 1.47) | 1.03 | (0.77 to 1.37) | 1.16 | (0.82 to 1.65) | 1.18 | (0.80 to 1.74) |
| 3 (February to March 2021) | 1.38 | (0.79 to 2.40) | 0.75 | (0.40 to 1.42) | 0.86 | (0.41 to 1.84) | 0.73 | (0.29 to 1.80) |
| 4 (April to July 2021) | 2.05 | (1.14 to 3.66) | 1.62 | (0.86 to 3.04) | 1.61 | (0.76 to 3.45) | 1.41 | (0.55 to 3.59) |
| 5 (August 2021) | 1.91 | (0.98 to 3.72) | 2.17 | (1.05 to 4.47) | 2.35 | (1.01 to 5.45) | 3.73 | (1.48 to 9.38) |
| 6 (September 2021) | 1.57 | (0.81 to 3.08) | 1.70 | (0.83 to 3.51) | 2.66 | (1.19 to 5.91) | 4.40 | (1.85 to 10.4) |
| Vaccination | ||||||||
| >10 d after first | 0.55 | (0.31 to 0.97) | 0.63 | (0.34 to 1.18) | 0.85 | (0.43 to 1.71) | 0.69 | (0.31 to 1.52) |
| >10 d after second | 0.25 | (0.14 to 0.44) | 0.18 | (0.09 to 0.34) | 0.22 | (0.10 to 0.47) | 0.12 | (0.05 to 0.30) |
Odds ratios (95% confidence intervals) by multivariable logistic regression model are adjusted for all variables shown. Clinical outcomes are “all cause,” not specifically due to coronavirus disease 2019. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Within 14 days of positive PCR.
Within 28 days of positive PCR.
Reference ethnicity White.
Any immune suppression treatment including steroids, tacrolimus, mycophenolate, azathioprine, and cytotoxic and biologic agents.
PCR positive at least 90 days prior to the current infection.
Reference time period 1 (December 2020).
Compared with unvaccinated patients, severe COVID-19 outcomes were observed less than half as often in patients testing positive for SARS-CoV-2 at least 10 days after the second dose. In logistic regression models adjusted for demographics, comorbidity, and time period, more substantial effects were seen with vaccination, associated with a 75% (95% confidence interval [95% CI], 56 to 86) lower risk of admission and 88% (95% CI, 70 to 95) fewer deaths (Table 2). Modest differences were observed after just the first dose, with a 45% (95% CI, 3 to 69) lower risk of admission.
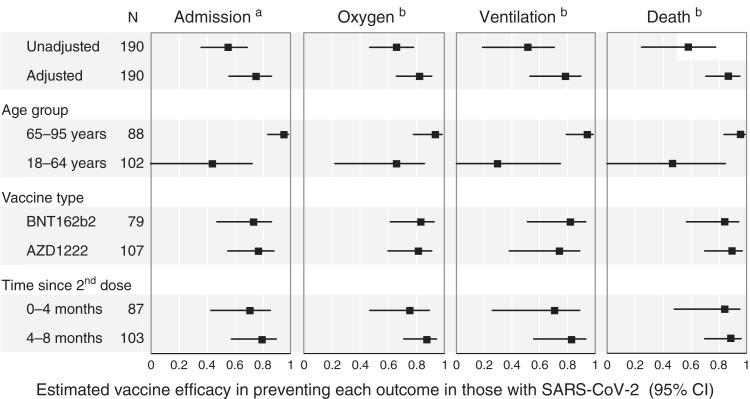
The protection from severe illness associated with vaccination was most obvious in patients over 65 years, in whom severe COVID-19 outcomes were reduced at least as much after vaccination as in their younger peers. No difference was seen in vaccine-associated protection with respect to vaccine type, and there was no waning effect observed over time, although this analysis was limited to a comparison between second doses given <4 or >4 months previously (following vaccine by median [interquartile range]: 3.0 [IQR, 2.2–3.5] or 5.0 [IQR, 4.5–5.5] months, respectively), in which a similar risk of severe COVID-19 outcomes was observed (Figure 3, Supplemental Table 2).
Figure 3.
Estimated vaccine efficacy in preventing severe illness in those with SARS-CoV-2. Severe coronavirus disease 2019 outcomes associated with second-dose vaccination, unadjusted and in the adjusted model, and the adjusted effectiveness in subgroups are shown. N is the number of SARS-CoV-2 infections at least 10 days after the second vaccine dose in each group. Efficacy is calculated as 1 – odds ratio. 95% CI, 95% confidence interval. aWithin 14 days of positive PCR. bWithin 28 days of positive PCR.
In sensitivity analyses, very similar vaccine effects were seen when those with prior SARS-CoV-2 were excluded or when the analysis was restricted to individual time periods (Supplemental Table 3).
In the secondary analysis of the subgroup of the patients at risk (Figure 1), the incidence of SARS-CoV-2 infection was observed in 1253 patients (aged 18–95, 58% men) who were on hemodialysis on December 1, 2020, with baseline characteristics given in Supplemental Table 4. During the observation period, SARS-CoV-2 infection developed in 272 (22%). In a Cox proportional hazards model censored for transplantation, death, or transfer to another center, the effect of vaccination on infection was uncertain; although lower infection risk was observed, this effect may have been due to chance (10 days after first dose: hazard ratio [HR], 0.63; 95% CI, 0.35 to 1.11; 10 days after second dose: HR, 0.59; 95% CI, 0.24 to 1.48). Robust protection was observed in the 247 (20%) with prior infection identified by positive PCR at a median of 10 (range, 4–18) months previously (HR, 0.15; 95% CI, 0.08 to 0.28) (Table 3).
Table 3.
Covariates associated with severe acute respiratory syndrome coronavirus 2 infection in a subgroup of the population at risk
| Covariate | Severe Acute Respiratory Syndrome Coronavirus 2 Infection | |
|---|---|---|
| Hazard Ratio | 95% Confidence Interval | |
| Age, yr | 1.00 | (0.99 to 1.01) |
| Sex | ||
| Men | 1.03 | (0.81 to 1.31) |
| Ethnicity a | ||
| Asian/other | 1.21 | (0.90 to 1.61) |
| Black | 1.01 | (0.77 to 1.41) |
| Diabetesb | 1.02 | (0.79 to 1.30) |
| Prior SARS-CoV-2c | 0.15 | (0.08 to 0.28) |
| Vaccination d | ||
| >10 d after first dose | 0.63 | (0.36 to 1.11) |
| >10 d after second dose | 0.59 | (0.24 to 1.48) |
Cox proportional hazards models were censored for transplantation, death, or transfer to another center. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Reference ethnicity White.
Presence of diabetes, not necessarily as the primary kidney disease.
PCR positive at least 90 days prior to the current infection.
Reference unvaccinated; vaccination status is considered to change 10 days after each dose.
Discussion
In this multicenter study of patients on hemodialysis with SARS-CoV-2 infection, significant protection from severe disease was seen after two-dose vaccination, with hospitalizations 75% lower (95% CI, 56 to 86) and risk of death 88% lower (95% CI, 70 to 95). This suggests a substantial clinical benefit from vaccination in a population that is particularly vulnerable. Some efficacy was seen after a single dose, underlining the importance of early vaccination in vulnerable groups.
Although many studies have examined immunogenicity vaccines in patients on hemodialysis, very few have attempted to estimate clinical efficacy. In a US study of over 12,000 patients on hemodialysis receiving BNT162b2, the subsequent risk of symptomatic COVID-19 was substantially lower compared with a matched unvaccinated cohort dialyzing at the same facilities (HR, 0.22; 95% CI, 0.13 to 0.35) (12). In a French hemodialysis registry study, over 1 month SARS-CoV-2 infections were identified in 1.98%, 0.65%, and 0.25% of unvaccinated, postfirst-dose, and postsecond-dose patients, respectively, although local community risk was not included (13). Additionally, in a large US dialysis provider study (not yet peer reviewed), the incidence of infection was lower with two-dose vaccination (relative risk 0.39; 95% CI, 0.32 to 0.47) (H.J. Manley et al., unpublished data). In the two former studies, case fatality remained high (4%–11%) in vaccinated patients, but among patients with a diagnosis of COVID-19, vaccination was associated with a milder clinical course, with severe illness and death around 50% lower.
In patients on dialysis, prior studies have, therefore, reported vaccine efficacy against symptomatic infection in the range 61%–87%. However, in an analysis of linked Scottish registry data that included patients on dialysis and patients with kidney transplants together, vaccine effectiveness in preventing infection was estimated at only 33% (95% CI, 0% to 52%) (14), although whether this represents a true difference in efficacy or other differences in study design is unclear. Analyses were unadjusted in these studies, with populations not screened for infection, and estimates of clinical efficacy would, therefore, be biased by diagnostic thresholds because severe events are easy to detect, whereas asymptomatic infection is often missed. This study, in which all cases came from a population screened weekly by PCR so that few infections would be missed, therefore improves on prior studies, providing reliable and fully adjusted estimates of the effect of vaccination on disease severity. Without vaccination, outcomes are poor in patients on hemodialysis (2); therefore, although substantially protected compared with their unvaccinated peers, vaccinated patients on hemodialysis remain at high risk for severe COVID-19 outcomes when compared with individuals without kidney disease.
Alongside clinical efficacy, the effect of vaccination can also be measured by immunogenicity: the ability of a vaccine to induce antibody and cellular immune responses in patients. Although one step removed from clinical outcome, immune characterization provides a more mechanistic understanding of protection, and responses can be measured at an individual level, potentially indicating individual risk. Several studies have reported reduced antibody responses in patients on dialysis, but impaired immunogenicity does not imply lower clinical efficacy, which relies on comparison with unvaccinated members of the same vulnerable group.
Following vaccination with two doses of BNT162b2 in previously uninfected patients on dialysis, neutralizing antibody levels were comparable with those of healthy controls, although this was not the case for AZD1222, after which titers were less effective in neutralizing most variants, including Delta (6). The lack of difference between vaccine types in this study does not exclude such an effect, but it is reassuring that despite poorer immunogenicity, AZD1222 was clearly associated with clinical protection from severe illness (Supplemental Table 2).
It is also somewhat reassuring to note the persistence of effect, albeit over short time frames (75% of cases in the late infection group, occurring over 4 months after their second dose, were still within 5.5 months) when there is concern that vaccine responses may wane over time (15). Antibody levels also wane after prior infection, although this does not necessarily diminish clinical protection. Clarke et al. (16) observed 129 seropositive patients on hemodialysis over 6 months, finding antibodies no longer detectable in ten of 111 patients with paired serology but robust protection from reinfection in the group. This is consistent with the effect of prior infection in this study, which was seen to provide robust protection from infection up to 18 months later. No additional effect was seen on clinical severity in those developing infection, perhaps because this group was small (n=45).
It is also reassuring that older patients appeared to benefit as much as their younger peers. Studies assessing protection from symptomatic infection in healthy individuals have reported either lower efficacy in older people (for example, from BNT162b2 in those over 70) (17) or similar efficacy (for example, from AZD1222; efficacy: 84% [54%–91%] in those over 65 versus 73% [63%–80%] in those under 65) (18). Protection from more severe COVID-19 outcomes, such as admission, appears similar; for example, first-dose vaccination was 83% effective in preventing admission in those over 80 (19).
Noticeable in this study was the higher mortality in later time periods, which may reflect the emergence of the Delta strain, which was associated with more severe COVID-19 outcomes (20), as the dominant variant in London (11). Although differences in vaccine type could be postulated, BNT162b2 and AZD1222 appear similar in their effect on Alpha and Delta variants (21). A large study of UK patients on hemodialysis found no clear difference between variants in neutralization titers after two vaccine doses (6).
This study has several important limitations; in particular, the main study only addresses clinical severity after individuals are infected, with limited focus on the likelihood of acquiring infection, assessed in the secondary analysis only. Although weekly screening allows a consistent threshold for detection, the inclusion of mild cases may impair comparison with other studies. SARS-CoV-2 variant information was not routinely available, with the dominant strain changing during the study period. Only limited comorbidity data were available, and changes in clinical practice, which evolved over the study period (in particular as new treatments became available) (Supplemental Table 1), may also have confounded the relationship between vaccination and severe COVID-19 outcomes.
These results are relevant to vaccine uptake in particular, as three doses have become standard for vaccination in many countries. Vaccine hesitancy remains a problem in this population; in a US survey, many patients on dialysis identified with the statement “I am concerned that the vaccine will not work” (22). This study may, therefore, be useful in reducing vaccine hesitancy, which has resulted in low uptake in some countries (for example, Australia, where almost a quarter of patients on dialysis declined) (23). Patients on dialysis remain vulnerable, and this study does nothing to diminish enthusiasm for third doses, which appear beneficial as two-dose protection starts to wane; in a healthy population during a Delta predominant phase, third doses of BNT162b2 were estimated to be 81% effective in preventing death compared with two doses at least 5 months earlier (24).
This study, therefore, demonstrates that vaccination is associated with a substantially lower risk of severe COVID-19 outcomes in patients on hemodialysis with SARS-CoV-2 infection. Although significant vulnerability remains, this population has much to gain from vaccination, regardless of age or vaccine type. These results support a policy of promoting and prioritizing vaccination in this vulnerable group.
Disclosures
E. Asgari reports serving as the chair of the Standards and Guidelines Committee at the British Transplant Society. D. Banerjee reports consultancy agreements with the St. George's University Hospitals NHS Foundation Trust; research funding from AstraZeneca, the British Heart Foundation, ESR AstraZeneca, and Kidney Research UK; and honoraria from AstraZeneca, Pfizer, and Viforpharma. K. Bramham reports consultancy agreements with Alexion, AstraZeneca, and Perkin Elmer; research funding from Alexion and AstraZeneca; honoraria from Alexion, AstraZeneca, and Otsuka; and serving in an advisory or leadership role for Alexion and AstraZeneca. H. Cairns reports employment with and owns shares in Airslie Ltd. software company. B. Caplin reports consultancy agreements with LifeArc; receiving research funding from AstraZeneca; and grants from the Colt Foundation, the Medical Research Council, and Royal Free Charity outside the submitted work. R.W. Corbett reports consultancy agreements with Baxter. R. Hull reports consultancy agreements with AstraZeneca, Pharmocosmos UK Ltd., and Travere Pharmaceuticals; speakers bureau for AstraZeneca and Napp Phamaceuticals; and other interests or relationships with the Renal Association, UK as an elected council member and with the Joint Specialist Committee Renal Medicine for the Royal College Physicians, London. K. McCafferty reports research funding from AstraZeneca; honoraria from Bayer, Napp, Pharmacosmos, and Vifor Fresenius; and speakers bureau for AstraZeneca. A.D. Salama reports research funding from Chiesi and Natera; honoraria from AnaptysBio, AstraZeneca, Hansa Medical, and Vifor Pharmaceuticals; and serving as Nephrology Dialysis Transplantation Editor and as a UK Renal Association executive member. A. Sarnowski reports ownership interest in Baxter and Fresenius and share portfolios on Hemogenyx, MoneyBox, Omega Diagnostics, Quantum Blockchain Technologies, and Tiziana Life Sciences. C. Sharpe reports consultancy agreements with Novartis Pharmaceuticals; honoraria from Napp Pharmaceuticals, Travere Pharmaceuticals, and Vifor Pharmaceuticals; speakers bureau for Napp Pharmaceuticals; and serving as Editor for BMC Nephrology and as a trustee and treasurer for the UK Kidney Association. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
The authors acknowledge the role of clinical nursing and medical staff who enabled this work.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: Pan-London COVID-19 Renal Audit Group, Damien R. Ashby, Ben Caplin, Richard W. Corbett, Elham Asgari, Nicola Kumar, Alexander Sarnowski, Richard Hull, David Makanjuola, Nicholas Cole, Jian Chen, Sofia Nyberg, Kieran McCafferty, Faryal Zaman, Hugh Cairns, Claire Sharpe, Kate Bramham, Reza Motallebzadeh, Kashif Jamil Anwari, Alan D. Salama, Debasish Banerjee, Omer Ali, Marilina Antonelou, Katy Bennet-Richards, Mark Blunden, John Booth, Rawya Charif, Saurabh Chaudhury, Andrea Cove-Smith, Hamish Dobbie, Phillippa Dodd, Gavin Dreyer, Neill Duncan, Suzanne Forbes, Catriona Goodlad, Megan Griffith, Sevda Hassan, Ulla Hemmilla, Heidy Hendra, Peter Hill, Ajith James, Daniel Jones, Anila Laurence, Marina Loucaidou, Gaetano Lucisano, Viyaasan Mahalingasivam, Bethia Manson, Daniel McGuiness, Adam McLean, Rosa Montero, Vasantha Muthuppalaniappan, Tom Oates, Andrew Palmer, Ravi Rajakariar, Emma Salisbury, Nasreen Samad, Eleanor Sandhu, Edward Stern, Damir Tandaric, James Tomlinson, Gisele Vajgel, Phil Webster, William White, Kate Wiles, David Wright, and Sajeda Yousef
Author Contributions
D.R. Ashby, D. Banerjee, B. Caplin, and A. Salama conceptualized the study; K.J. Anwari, E. Asgari, D.R. Ashby, D. Banerjee, K. Bramham, H. Cairns, B. Caplin, J. Chen, N. Cole, R.W. Corbett, R. Hull, N. Kumar, D. Makanjuola, K. McCafferty, R. Motallebzadeh, S. Nyberg, A.D. Salama, A. Sarnowski, C. Sharpe, and F. Zaman were responsible for data curation; D.R. Ashby, B. Caplin, and R.W. Corbett were responsible for formal analysis; D.R. Ashby and R.W. Corbett wrote the original draft; and K.J. Anwari, E. Asgari, D.R. Ashby, D. Banerjee, K. Bramham, H. Cairns, B. Caplin, J. Chen, N. Cole, R.W. Corbett, R. Hull, N. Kumar, D. Makanjuola, K. McCafferty, R. Motallebzadeh, S. Nyberg, A.D. Salama, A. Sarnowski, C. Sharpe, and F. Zaman reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.16621221/-/DCSupplemental.
Supplemental Table 1. Community prevalence of variants and available inpatient treatments by time period.
Supplemental Table 2. Association of second-dose vaccination with severe COVID-19 outcomes in subgroups with SARS-CoV-2 infection.
Supplemental Table 3. Association of second-dose vaccination with severe COVID-19 outcomes in sensitivity analyses.
Supplemental Table 4. Characteristics of subgroup patients (N=1253) stratified by SARS-CoV-2 PCR status.
References
- 1.Corbett RW, Blakey S, Nitsch D, Loucaidou M, McLean A, Duncan N, Ashby DR; West London Renal and Transplant Centre : Epidemiology of COVID-19 in an urban dialysis center. J Am Soc Nephrol 31: 1815–1823, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplin B, Ashby D, McCafferty K, Hull R, Asgari E, Ford ML, Cole N, Antonelou M, Blakey SA, Srinivasa V, Braide-Azikwe DCB, Roper T, Clark G, Cronin H, Hayes NJ, Manson B, Sarnowski A, Corbett R, Bramham K, Lioudaki E, Kumar N, Frankel A, Makanjuola D, Sharpe CC, Banerjee D, Salama AD; Pan-London COVID-19 Renal Audit Group: Risk of COVID-19 disease, dialysis unit attributes, and infection control strategy among London in-center hemodialysis patients. Clin J Am Soc Nephrol 16: 1237–1246, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savino M, Casula A, Santhakumaran S, Pitcher D, Wong E, Magadi W, Evans KM, Benoy-Deeney F, Griffin J, Plumb L, Steenkamp R, Nitsch D, Medcalf J: Sociodemographic features and mortality of individuals on haemodialysis treatment who test positive for SARS-CoV-2: A UK Renal Registry data analysis. PLoS One 15: e0241263, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, Zervos M, Rankin B, Eder F, Feldman G, Kennelly C, Han-Conrad L, Levin M, Neuzil KM, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Polakowski L, Mascola JR, Ledgerwood JE, Graham BS, August A, Clouting H, Deng W, Han S, Leav B, Manzo D, Pajon R, Schödel F, Tomassini JE, Zhou H, Miller J; COVE Study Group : Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 385: 1774–1785, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas SJ, Moreira ED Jr., Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr., Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group : Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N Engl J Med 385: 1761–1773, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr EJ, Wu M, Harvey R, Wall EC, Kelly G, Hussain S, Howell M, Kassiotis G, Swanton C, Gandhi S, Bauer DL, Billany RE, Graham-Brown MP, Beckett J, Bull K, Shankar S, Henderson S, Motallebzadeh R, Salama AD, Harper L, Mark PB, McAdoo S, Willicombe M, Beale R; Haemodialysis COVID-19 consortium; Crick COVID Immunity Pipeline : Neutralising antibodies after COVID-19 vaccination in UK haemodialysis patients. Lancet 398: 1038–1041, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia P, Anand S, Han J, Montez-Rath M, Sun S, Shang T, Parsonnet J, Chertow G, Schiller B, Abra G: COVID-19 vaccine type and humoral immune response in patients receiving dialysis. J Am Soc Nephrol 33: 33–37, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacson E Jr., Argyropoulos CP, Manley HJ, Aweh G, Chin AI, Salman LH, Hsu CM, Johnson DS, Weiner DE: Immunogenicity of SARS-CoV-2 vaccine in dialysis. J Am Soc Nephrol 32: 2735–2742, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thieme CJ, Blazquez-Navarro A, Safi L, Kaliszczyk S, Paniskaki K, Neumann IE, Schmidt K, Stockhausen M, Hörstrup J, Cinkilic O, Flitsch-Kiefner L, Meister TL, Marheinecke C, Pfaender S, Steinmann E, Seibert FS, Stervbo U, Westhoff TH, Roch T, Babel N: Impaired humoral but substantial cellular immune response to variants of concern B1.1.7 and B.1.351 in hemodialysis patients after vaccination with BNT162b2. J Am Soc Nephrol 32: 2725–2727, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliger AS, Silberzweig J: COVID-19 and dialysis patients: Unsolved problems in early 2021. J Am Soc Nephrol 32: 1018–1020, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Public Health England : SARS-CoV-2 variants of concern and variants under investigation in England. Available at.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1042688/RA_Technical_Briefing_32_DRAFT_17_December_2021_2021_12_17.pdf. Accessed December 19, 2021
- 12.Sibbel S, McKeon K, Luo J, Wendt K, Walker A, Kelley T, Lazar R, Zywno M, Connaire J, Tentori F, Young A, Brunelli S: Real-world effectiveness and immunogenicity of BNT162b2 and mRNA-1273 SARS-CoV2 vaccines in patients on hemodialysis. J Am Soc Nephrol 33: 49–57, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espi M, Charmetant X, Barba T, Mathieu C, Pelletier C, Koppe L, Chalencon E, Kalbacher E, Mathias V, Ovize A, Cart-Tanneur E, Bouz C, Pellegrina L, Morelon E, Juillard L, Fouque D, Couchoud C, Thaunat O; REIN Registry : A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int 101: 390–402, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell S, Campbell J, Lambourg E, Watters C, O’Neil M, Almond A, Buck K, Carr EJ, Clark L, Cousland Z, Findlay M, Joss N, Metcalfe W, Petrie M, Spalding E, Traynor JP, Sanu V, Thomson P, Methven S, Mark PB: The impact of vaccination on incidence and outcomes of SARS-CoV-2 infection in patients with kidney failure in Scotland. J Am Soc Nephrol 33: 677–686, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong D, Xiao S, Debes AK, Egbert ER, Caturegli P, Colantuoni E, Milstone AM: Durability of antibody levels after vaccination with mRNA SARS-CoV-2 vaccine in individuals with or without prior infection. JAMA 326: 2524–2526, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarke CL, Prendecki M, Dhutia A, Gan J, Edwards C, Prout V, Lightstone L, Parker E, Marchesin F, Griffith M, Charif R, Pickard G, Cox A, McClure M, Tedder R, Randell P, Greathead L, Guckian M, McAdoo SP, Kelleher P, Willicombe M: Longevity of SARS-CoV-2 immune responses in hemodialysis patients and protection against reinfection. Kidney Int 99: 1470–1477, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD: BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384: 1412–1423, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falsey AR, Sobieszczyk ME, Hirsch I, Sproule S, Robb ML, Corey L, Neuzil KM, Hahn W, Hunt J, Mulligan MJ, McEvoy C, DeJesus E, Hassman M, Little SJ, Pahud BA, Durbin A, Pickrell P, Daar ES, Bush L, Solis J, Carr QO, Oyedele T, Buchbinder S, Cowden J, Vargas SL, Guerreros Benavides A, Call R, Keefer MC, Kirkpatrick BD, Pullman J, Tong T, Brewinski Isaacs M, Benkeser D, Janes HE, Nason MC, Green JA, Kelly EJ, Maaske J, Mueller N, Shoemaker K, Takas T, Marshall RP, Pangalos MN, Villafana T, Gonzalez-Lopez A; AstraZeneca AZD1222 Clinical Study Group : Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine. N Engl J Med 385: 2348–2360, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasileiou E, Simpson CR, Shi T, Kerr S, Agrawal U, Akbari A, Bedston S, Beggs J, Bradley D, Chuter A, de Lusignan S, Docherty AB, Ford D, Hobbs FR, Joy M, Katikireddi SV, Marple J, McCowan C, McGagh D, McMenamin J, Moore E, Murray JL, Pan J, Ritchie L, Shah SA, Stock S, Torabi F, Tsang RS, Wood R, Woolhouse M, Robertson C, Sheikh A: Interim findings from first-dose mass COVID-19 vaccination roll-out and COVID-19 hospital admissions in Scotland: A national prospective cohort study. Lancet 397: 1646–1657, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twohig KA, Nyberg T, Zaidi A, Thelwall S, Sinnathamby MA, Aliabadi S, Seaman SR, Harris RJ, Hope R, Lopez-Bernal J, Gallagher E, Charlett A, De Angelis D, Presanis AM, Dabrera G; COVID-19 Genomics UK (COG-UK) consortium : Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect Dis 22: 35–42, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M: Effectiveness of COVID-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 385: 585–594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia P, Montez-Rath ME, Moore H, Flotte J, Fults C, Block MS, Han J, Dittrich M, Parsonnet J, Chertow GM, Block GA, Anand S: SARS-CoV-2 vaccine acceptability in patients on hemodialysis: A nationwide survey. J Am Soc Nephrol 32: 1575–1581, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace H, Mount PF: COVID-19 beliefs and vaccination uptake in dialysis patients: Lessons from an anonymous patient survey. Intern Med J 13: 287–288, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, Reis BY, Balicer RD: Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 398: 2093–2100, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.