Abstract
The definition of sepsis has evolved significantly over the past three decades. Today, sepsis is defined as a dysregulated host immune response to microbial invasion leading to end organ dysfunction. Septic shock is characterized by hypotension requiring vasopressors after adequate fluid resuscitation with elevated lactate. Early recognition and intervention remain hallmarks for sepsis management. We addressed the current literature and assimilated thought regarding optimum initial resuscitation of the patient with sepsis. A nuanced understanding of the physiology of lactate is provided in our review. Physiologic and practical knowledge of steroid and vasopressor therapy for sepsis is crucial and addressed. As blood purification may interest the nephrologist treating sepsis, we have also added a brief discussion of its status.
Keywords: critical care nephrology and acute kidney injury series, sepsis, acute kidney injury, septic shock
Introduction
Sepsis accounts for high morbidity, mortality, and economic burden. Sepsis incidence has been rising, but mortality has decreased worldwide (1,2). AKI is common in sepsis, with mortality rates as high as 60% (3). We will focus on modern sepsis management methods most relevant to nephrologists.
Definitions
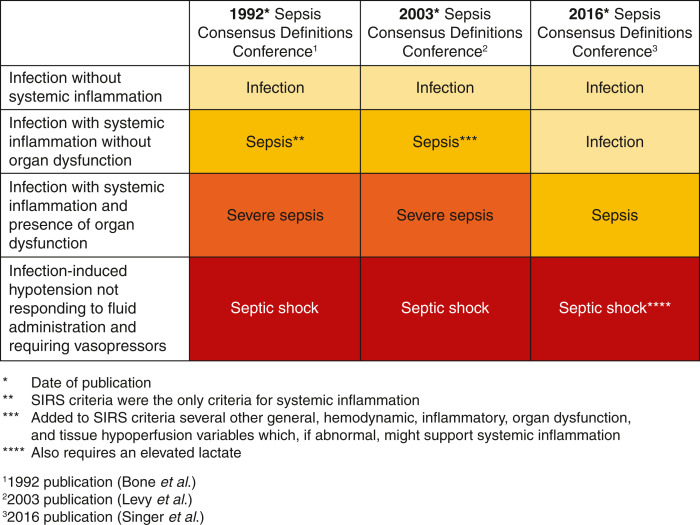
The definition of sepsis has evolved over the past three decades. Figure 1 shows how sepsis definitions have changed over the three international consensus conferences (4–6). In 1991, sepsis was defined as two of four systemic inflammatory response (SIRS) criteria with suspected or confirmed infection. These SIRS criteria included fever, tachycardia, tachypnea, and altered WBC count. The recent consensus conference de-emphasizes the SIRS criteria and defines sepsis as a dysregulated host immune response to infection leading to organ dysfunction. A suspected sepsis source and a SOFA score increase of greater than or equal to two due to infection were correlated with higher mortality. The SOFA score awards points on the basis of the degree of organ dysfunction. Scoring is on the basis of oxygenation status, hematologic (platelets), central nervous system, bilirubin, cardiovascular, and renal. Sepsis 3 also introduced quick SOFA as a bedside tool requiring no laboratory results that might alert the provider to the presence of sepsis if two of the following three were present: systolic BP ≤100, altered mental status, and respiratory rate 22 or greater. However, the 2021 Surviving Sepsis Campaign (SSC) guidelines recommend against using quick SOFA as a singular screening tool. Sepsis 3 definitions have had mixed reviews, and adoption has been slow. In line with the proposed 2016 Sepsis 3 definitions, we will use the term “sepsis” in this paper to describe infection-induced organ dysfunction/tissue hypoperfusion.
Figure 1.
The evolution of sepsis definitions. SIRS, systemic inflammatory response.
As the definition of sepsis evolves, so does the epidemiology of sepsis-related AKI. As the definition of sepsis becomes more or less sensitive, the incidence of sepsis and sepsis-related AKI changes. It is estimated that about 47%–51% of patients diagnosed with sepsis in the intensive care unit (ICU) will develop AKI, but these numbers should be interpreted with caution (7). Both conditions are clinical diagnoses with the criteria that are not consistent, further clouding the true incidence of sepsis-related AKI. In addition, inaccurate urine output measurements outside of the ICU, reduced creatinine production during sepsis, and the dilution of serum creatinine with fluid resuscitation can confound the diagnosis of AKI (8).
Antibiotics
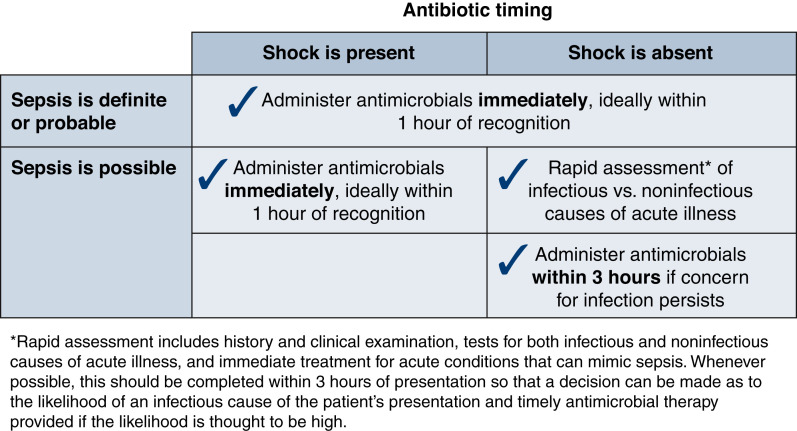
The SSC guidelines, before 2021, recommended that broad-spectrum antibiotics be given within the first hour of recognition of sepsis. Evidence suggests that delays in antibiotic administration worsen outcomes (9). The 2021 SSC guidelines, recognizing that the strongest literature support for early antibiotics is in septic shock, recommend taking a two-tier approach for the timing of antibiotics when considering the presence of sepsis (Figure 2). New organ dysfunction with confirmed or suspected infection and undifferentiated shock warrants antibiotics within an hour. A 3-hour window is acceptable in the absence of shock and when infection risk is not high. However, some experts disagree with this approach, favoring a best practice goal within 1 hour for all patients with potential for sepsis (10).
Figure 2.
Antibiotic timing. The timing of antibiotics depends on the presence of shock and the probability of sepsis. Patients with a high probability of sepsis should receive antibiotics within 1 hour. In patients with possible sepsis, antibiotics should be initiated within an hour if shock is present and within 3 hours if they are hemodynamically stable. Reprinted from ref. 4, with permission.
Cultures and source control are priorities but should not delay antibiotics. Antibiotic initiation can be divided into empirical or culture-directed treatment. Empirical antibiotics should reflect local antibiotic resistance, prior cultures, comorbidities, immunosuppression, shock, and infection site. Antimicrobial de-escalation is a daily priority. Prolonged antibiotics administration increases antibiotic exposure and associated risk for resistance and drug toxicity.
Pathophysiology
In sepsis, microorganisms have specific pathogen-associated molecular patterns, which bind to pattern recognition receptors (PRRs) to stimulate immune cells to produce proinflammatory cytokines (11). A second mechanism of PRR stimulation is the damage-associated pathogen pattern, which occurs due to molecular particle release from damaged cells secondary to the invasive microbe. Once the PRR is bound, both proinflammatory and anti-inflammatory mediators are released. These pathways lead to tissue damage and a decreased ability to mount an immune response to ensuing infections in the case of the anti-inflammatory mediators. In addition, proinflammatory cytokines stimulate endothelial cells, leading to procoagulant factors, complement activation, vascular permeability, vasodilation, and organ dysfunction (12).
Traditionally, the mechanism for sepsis-associated AKI was thought to be related to ischemic injury secondary to reduced oxygen delivery (DO2), leading to acute tubular necrosis (13). However, animal models indicate an increase in renal blood flow during induced early septic shock, and biopsies performed do not appear to have a significant degree of acute tubular necrosis (14). The primary mechanisms for sepsis-related AKI appear to be inflammatory, microvascular derangements, and adaptive cellular programming (15,16).
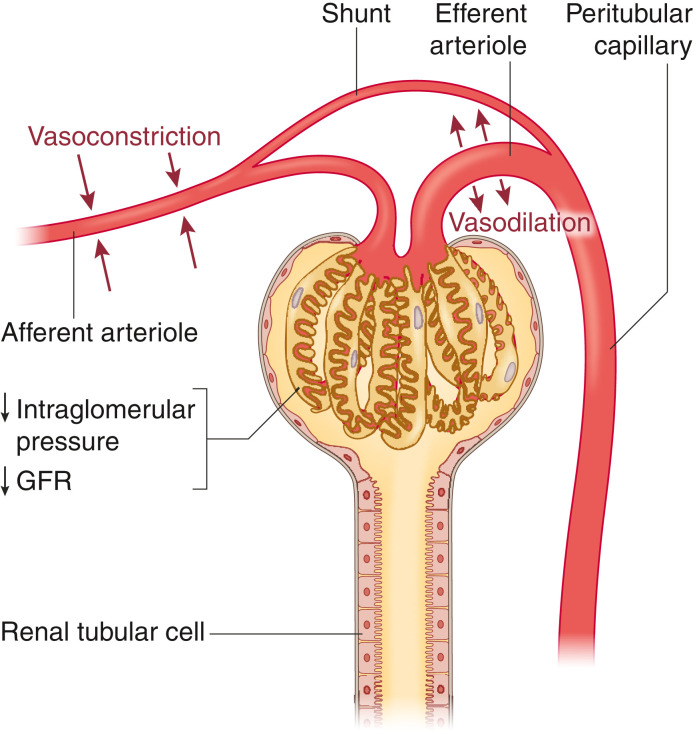
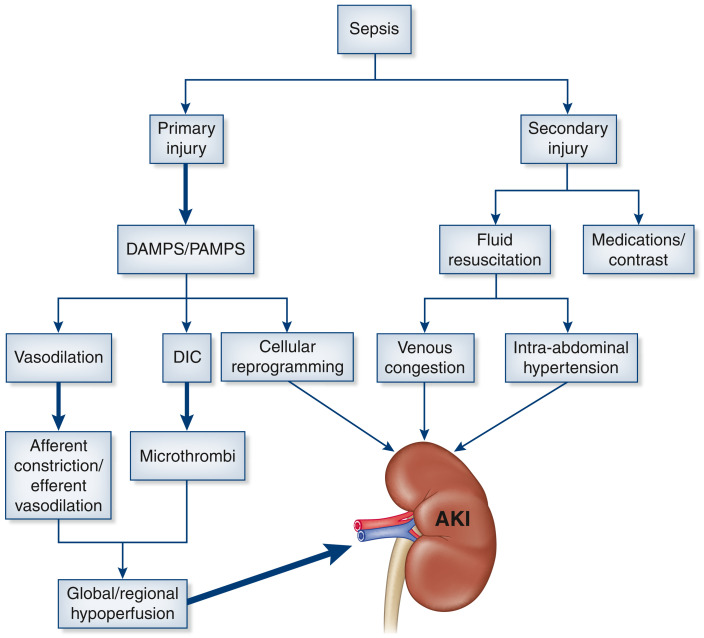
In sepsis-related AKI, there is a decoupling of acro- and microcirculation. The systemic vasodilation that accompanies sepsis leads to an increase in renin-angiotensin-aldosterone and sympathetic tone, predisposing to afferent arteriolar vasoconstriction. The gradient for filtration decreases due to constriction of the afferent arteriole and concurrent vasodilation of the efferent arteriole (Figure 3). In addition, there may be low-resistance shunting of blood from the glomerulus via direct connections between the afferent and efferent arteriole (17). In sepsis-related AKI, secondary insults contribute to and maintain AKI (Figure 4). Aggressive fluid resuscitation contributes to interstitial kidney edema and intra-abdominal hypertension (18). The kidney is an encapsulated organ; therefore, positive fluid balances predispose to interstitial edema, reducing the filtration gradient between the glomerular capillaries and Bowman's space. Medications can contribute to kidney injury, including antibiotics, calcineurin inhibitors, and proton pump inhibitors. Finally, source control in sepsis is important, and this often requires CT scans with contrast, which further increase the risk for secondary kidney injury.
Figure 3.
In sepsis, the afferent arteriole undergoes vasoconstriction, whereas the efferent arteriole vasodilates, leading to a reduction in glomerular filtration pressure. The differences in resistance between the two arterioles predispose to glomerular shunting via periglomerular shunting pathways. Both mechanisms lead to a reduction in GFR, despite an increase in renal blood flow early in sepsis.
Figure 4.
Sepsis-related kidney injury can be divided into primary and secondary mechanisms. The primary mechanisms include damage-associated molecular patterns (DAMPS) and pathogen-associated molecular patterns (PAMPS), leading to an innate immune response with consequential systemic vasodilation, disseminated intravascular coagulopathy (DIC), and cellular reprogramming of renal tubular epithelial cells. The innate immune response further contributes to microvascular changes and glomerular microthrombi, resulting a net reduction in GFR. Secondary injury occurs due to fluid resuscitation that can lead to venous congestion and intra-abdominal hypertension, which further contributes to AKI.
Resuscitation and Kidney Function
As mentioned previously, sepsis is characterized by vasodilation and capillary leak, which reduces effective circulating blood volume. Therefore, the 2021 SSC guidelines have a downgraded (from 2016) weak recommendation for initial volume expansion with 30 ml/kg crystalloid within 3 hours of identification of sepsis-induced tissue hypoperfusion (hypotension or lactate ≥4 mEq/L) (19). Although an initial 30 ml/kg crystalloid is the standard used by the Centers for Medicare & Medicaid Services as part of the reporting for treating sepsis-induced tissue hypoperfusion (SEP-1), the optimal amount of fluid remains controversial (20).
Some literature has demonstrated an association between poor outcomes and the magnitude of positive fluid balances in patients with septic shock (21,22). A 2019 meta-analysis noted no difference in outcomes when comparing fluid administration approaches (23). Three randomized controlled septic shock trials published in 2014 and 2015 compared resuscitation approaches utilizing various combinations of (1) protocolized resuscitation guided by central venous pressure and oxygenation monitoring, (2) protocolized resuscitation without the use of central venous monitoring, and (3) usual care as determined by the treating physician without central venous monitoring (24–26). The predetermined combined analysis (PRISM) concluded no difference in outcome with these approaches (27). The mortality throughout all treatment arms of these three trials was unexpectedly low at approximately 25%. Of note, the mean fluid administered at the time of randomization across all enrolled patients was 27.5 ml/kg, close to the current SSC recommendation and CMS metric target for initial fluid administration. Initial fluid bolus and continued fluid administration remain controversial in septic shock.
A single-center study evaluating a liberal versus restrictive approach for fluid resuscitation in sepsis found no statistically significant difference in outcomes but a trend toward reduced AKI in the restrictive group (28). The Crystalloid Liberal or Vasopressors Early Resuscitation of Sepsis trial offers some guidance for dosing fluids in septic shock (29). This trial randomized patients with septic shock to a liberal versus a conservative approach for fluids in sepsis. The trial was stopped early due to a strong signal denoting no difference between the two strategies. Fluid administration warrants a probabilistic approach. Unfortunately, only 50% of critical patients are volume responsive, defined as a 10%–15% increase in cardiac output with a bolus (30). Dynamic measurements take precedence over static measurements to determine the probability of volume responsiveness.
The perfusion pressure of the kidney is the gradient between renal artery pressure and renal vein pressure (with mean arterial pressure [MAP] and CVP as surrogates). With constant MAP, any increase in CVP with fluid administration would decrease renal perfusion. The MAP goal in the patient with septic AKI remains unclear. A recent study compared high and low MAP goals in early sepsis AKI and noted increased creatinine clearance in the higher MAP group (31). A prior study compared high and low MAP goals in sepsis-related AKI. No statistically significant difference was noted; however, in the chronic arterial hypertension subgroup, there was a trend toward the reduced need for KRT (32). Therefore, we recommend that MAP goals be at least >65 in patients with sepsis AKI, with consideration for higher goals in patients with a history of hypertension.
CVP is not predictive of volume responsiveness but remains valuable for volume tolerance. A recent meta-analysis notes the association of elevated CVP and AKI, noting that every 1–mm Hg increase in CVP leads to an AKI odds increase of 6% (33). This finding is replicated in patients with sepsis-related AKI (34). Patients that are not volume tolerant, oliguric, and hypotensive should preferentially receive early pressors over fluids.
Fluid Type
In years past, normal saline was the standard for fluid expansion in sepsis. However, normal saline has a higher chloride concentration than plasma, which can worsen acidemia and possibly contribute to AKI. Study results comparing saline with more balanced solutions have been mixed. The SMART trial showed a statistically significant improvement in mortality, creatinine, and KRT using balanced crystalloids (35). Similarly, the SALT-ED trial demonstrated a reduction in kidney dysfunction using balanced crystalloids (36). Conversely, multiple recent trials have not shown a statistically significant increase in adverse events with the use of saline (37–39). There is a significant amount of heterogeneity among the trials, including in fluid volume, patient type, degree of hyperchloremia, and fluid administration type crossover. Extrapolation of these findings to patients with sepsis should be done with the knowledge that the SPLIT, BASIC, and PLUS trials had <50% of patients with sepsis. A recent meta-analysis found that the risk ratio for 90-day mortality for balanced crystalloid solutions compared with 0.9% saline was 0.96 (95% confidence interval, 0.91 to 1.01) (40). Furthermore, treatment with balanced solutions compared with saline demonstrated a 0.96 RR for developing AKI (95% confidence interval, 0.89 to 1.02). Overall, using a frequentist approach, the trial did not show a statistically significant difference, but using a Bayesian method, they found a 90% probability that balanced crystalloids reduce mortality. Given the possibility of harm from using saline and the low inertia needed to use balanced fluids instead, we recommend that balanced fluids be considered the first line for fluid expansion in sepsis. Hetastarch should not be used as a means of volume expansion given the increased risk for AKI (41). Finally, the 2021 SSC guidelines suggest reserving albumin for patients who have received large volumes of crystalloids and are judged to need additional fluid. Sepsis induces endothelial glycocalyx injury, leading to a higher capillary leak, and albumin might provide stabilization of this layer (42,43).
Lactate/Oxygen Delivery/Oxygen Consumption
Lactate rises in sepsis can indicate hypoperfusion and associated anaerobic glycolysis, but they can also be due to a wide variety of other causes, including aerobic glycolysis, cytokine-driven inflammation, catecholamine-driven accelerated glycolytic flux, stimulation of sodium-potassium ATPase pump activity, decreased lactate clearance, and inhibition of pyruvate dehydrogenase.
Lactate levels ≥4 mmol/L are associated with a marked worsened outcome in sepsis (44–46). Lactate clearance >10% during the initial hours of resuscitation correlated with improved sepsis outcomes and was shown to be comparable with targeting normalization of ScVO2, a target in other studies as well (47). The meaning of this is less clear now that more recent trials have shown usual care to be as effective as therapy, which targeted normalization of ScVO2 (27). Currently, there is no strong evidence to guide the frequency of lactate checks for patients with sepsis. Our recommendation would be to repeat every 2 hours until there is a declining trend; then, the frequency can be de-escalated. Given the many mechanisms of lactate rise, it is important to identify patients in whom the rise indicates hypoperfusion, as these patients may benefit from methods to increase DO2.
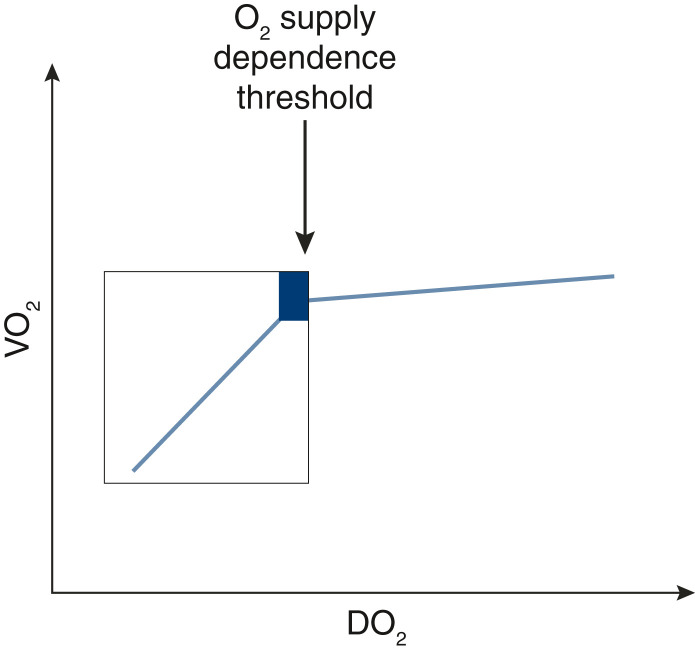
Lactate should be understood within the relationship between DO2 and oxygen consumption (VO2). Early shock is typically a low effective circulating volume state due to capillary leak and associates with low DO2—the shock state after fluid expansion transitions to a normal to high cardiac output state. If cardiac output falls, VO2 remains constant until a critical threshold is reached, at which time VO2 starts to decrease (Figure 5). This elbow on the DO2/VO2 curve represents the supply-dependent transition for VO2. Logically, one can surmise that patients operating below the critical DO2 level are the patients who would benefit most from inotropes and fluids. Such a patient would benefit from established methods to increase DO2 via increased cardiac output (fluids, inotropes), as an increase in VO2 would be anticipated. VO2 increase that follows a rise in DO2 is called VO2 responsiveness (48,49).
Figure 5.
The figure illustrates the relationship between oxygen delivery (DO2) and oxygen consumption (VO2). As DO2 decreases, VO2 remains relatively constant until it reaches a critical O2 supply dependence threshold, at which point there will be a significant drop in VO2. Lactate rises prior to the O2 supply dependence threshold are independent of the DO2-VO2 relationship, whereas below the threshold, they become DO2-VO2 dependent.
Sepsis performance improvement bundles typically recommend serial lactates to guide resuscitation, but an alternate approach is available for low-resource settings. In this approach, a lactate-driven protocol was compared with a peripheral perfusion approach using capillary refill time (CRT) (50). The CRT group was noninferior, with less resource use. Therefore, we recommend the CRT approach for prognostication and ongoing resuscitation assessment in low-resource settings for patients with septic shock.
De-Resuscitation
Malbrain et al. (22) performed a systematic review to evaluate positive fluid balances in sepsis and noted higher intra-abdominal hypertension with increases in the magnitude of positive fluid balance and association with mortality. As sepsis resolves in patients with septic shock who have undergone fluid resuscitation and are in positive fluid balance, endothelial healing occurs, fluid is mobilized, urine output increases, and a negative fluid balance ensues. This might be called “natural” de-resuscitation. However, there are circumstances when “physician-driven” de-resuscitation might be appropriate. De-resuscitation should be on the basis of the patient's shock state and evidence of volume overload. Malbrain et al. (22) proposed that de-resuscitation should occur when volume overload contributes to end organ damage.
The gradient between MAP and intra-abdominal pressure is called the abdominal perfusion pressure. It is unclear whether targeting abdominal perfusion pressure in septic shock can modify the AKI trajectory, but with the advent of continuous bladder pressure monitoring, the inertia to study this question should be lower (51). Elevated intra-abdominal pressure and reduced abdominal perfusion pressure warrant the avoidance of additional fluid administration, and pressors should be prioritized in the setting of shock. Care should be taken for accurate intra-abdominal pressure measurements, as technique errors can occur (52). Additionally, fluid removal is a sensible approach, which has been shown to improve intra-abdominal pressure (53). Bedside ultrasound and extravascular lung water index can help quantify volume overload. Protocols, such as the venous excess ultrasonography score (VEXUS), can grade the severity of venous congestion (54). The VEXUS protocol is a four-step ultrasound protocol that may be a better predictor of AKI than traditional CVP estimates. However, this protocol requires skill with bedside ultrasound.
Venous congestion, renal interstitial edema, and intra-abdominal hypertension can contribute to a vicious cycle of sodium retention and worsening volume-related pathology through neurohormonal mechanisms (55,56). The method of volume removal for fluid overload is on the basis of local resources, hemodynamics, and the presence of diuretics resistance. To expedite de-resuscitation, we recommend the use of the furosemide stress test (FST). In the setting of AKI, the FST can quickly identify patients likely to progress to advanced AKI and require KRT. The timing of KRT remains controversial and beyond the scope of this review, but the data remain mixed (57–60). The FST has been shown to identify those at low risk for requiring KRT, which reduces the risk of initiating therapy on patients who may not have required escalation beyond diuretics (60). Given the mixed data, early versus late KRT questions should be relegated to FST nonresponders and individualized on the basis of the degree of volume overload.
Volume removal in the ICU can be challenging in the shock setting. We recommend a physiology-framed approach. If the patient demonstrates volume responsiveness, then a cardiac output drop associated with BP decreases is likely to occur with volume removal. If the patient is not volume responsive, there is little likelihood of a hemodynamically significant drop in cardiac output and BP with fluid removal. A study performed by Monnet et al. (61) on patients in shock demonstrated that a passive leg raise–induced increase in cardiac index >9% was associated with worsening hypotension with ultrafiltration with an AUC of 0.89.
Vasopressors
Vasopressors should be initiated if the patient with sepsis remains hypotensive despite initial fluid administration. Early vasopressors can be considered given that protocol-based peripheral vasopressor use is safe during initial resuscitation (62). The first-line vasopressor for septic shock is norepinephrine (NE). NE is a venous and arterial vasoconstrictor via α-agonism and provides positive inotropy via B1 receptor activation. NE should be initiated if the MAP goal of 65 mm Hg is not achieved with initial volume resuscitation. Earlier vasopressor initiation may allow for a more fluid-restrictive approach. The CENSER trial demonstrated that earlier use of NE led to a reduction in cardiogenic pulmonary edema (63).
The two second-line agents recommended to achieve MAP targets are low-dose vasopressin and epinephrine. The SSC recommends vasopressin use first. Vasopressin-induced vasoconstriction occurs through stimulation of V1a receptors. Patients with septic shock may have a relative deficiency in vasopressin. Vasopressin preferentially vasoconstricts the renal efferent arteriole, which can maintain GFR in sepsis, but the evidence for kidney benefit is mixed. Two trials have now demonstrated a reduction in the need for dialysis using vasopressin (64,65).
Epinephrine works via α- and β-receptors, increasing vascular resistance and cardiac output; when compared with NE, there is greater inotropy. Patients with poorly filled, hyperdynamic, and hypertrophic left ventricles can risk dynamic outflow tract obstruction with epinephrine. No head-to-head trials compare NE with epinephrine. A randomized, protocolized comparison of epinephrine versus dobutamine plus NE guided by pulmonary artery catheterization did not differ in outcomes (66). Lactate levels may rise with epinephrine, but this is a marker of higher adrenergic activity and is not thought to be harmful. In a randomized controlled septic shock trial of NE versus dopamine, dopamine caused more tachyarrhythmias and mortality and is no longer considered first-line therapy. However, septic shock with bradycardia is still a consideration for the use of dopamine, although epinephrine is also appropriate. Phenylephrine, a pure α-agonist, is not for first-line vasopressor use, but it can be considered in patients who are hyperdynamic and tachycardic with profound vasodilation. It would be the vasopressor of choice when serious tachyarrhythmias occur with NE. Fortunately, NE does not usually produce tachycardia or tachyarrhythmias as the β1-chronotropic stimulation is countered by intense venoconstriction and stimulation of right atrium baroreceptors.
Angiotensin II is a recently Food and Drug Administration–approved vasopressor that acts on the peripheral vasculature angiotensin II receptor to vasoconstrict and raise BP (67). It was shown in a clinical trial to raise BP in distributive shock and be safe in the population that met the criteria for study enrollment. Because it is a pure vasoconstrictor without inotropic activity, we do not recommend its use in septic shock in the presence of a known or potential low–cardiac output state. It may be considered as an adjunctive vasopressor in refractory septic shock where cardiac output is not suspected to be low. More study is needed with angiotensin II in septic shock, emphasizing safety and risk for thromboembolism (68).
The SSC and SEP 3 recommend titrating vasopressors to a MAP goal >65 mm Hg. A clinical trial in septic shock evaluated higher versus lower MAP goals and found no significant difference in mortality. However, patients with a hypertension history had less KRT with the higher MAP goal. In addition, lower MAP goals have been evaluated in patients older than 75, suggesting improved outcomes with a goal of 60–65 mm Hg.
Steroids
Glucocorticoid steroids are the body's primary method for ameliorating an overzealous toxin/mediator response to infection, thought to be a significant driver of the organ dysfunction in sepsis. Earlier large randomized trials evaluated steroids for septic shock in the early 2000s and found inconsistent results. However, two large recent trials (ADRENAL and APROCCHS) collectively support steroids in patients with moderate to severe degrees of septic shock (69,70). Although only one of the two trials had a statistically significant reduction in mortality, both trials showed reduced vasopressor and ICU days. Therefore, the recommendation from the 2021 SSC guidelines is for administering steroids in moderate to severe septic shock. The APROCCHS trial showed a mortality benefit and added 50 μg PO fludrocortisone to the standard hydrocortisone dosing for additional mineralocorticoid activity (hydrocortisone does have both glucocorticoid and mineralocorticoid activity). In addition, a beneficial effect of mineralocorticoids (aldosterone) on survival, BP, and vascular reactivity in an endotoxin model of septic shock has been demonstrated and was associated with a restoration of α1-adrenoceptor expression.
Blood Purification
Sepsis involves an exaggerated response to circulating pathogen-associated molecular patterns and endotoxins, leading to alterations of the inflammatory cascade. Proposed sepsis treatments include removal of elevated toxins and cytokines using hemoperfusion and high-volume hemofiltration. Hemoperfusion filters bind LPS, cytokines, or both. Polymyxin B hemoperfusion was approved for treatment in 1991 in some countries and has been demonstrated to remove LPS. The evidence for polymyxin-based hemoperfusion remains mixed, with an early trial demonstrating survival benefit in abdominal sepsis, but larger subsequent trials have not replicated this finding (71,72). CytoSorb is an extracorporeal hemadsorption device that works to remove middle molecules, such as cytokines. CytoSorb studies have been small and contradictory, with no collective evidence of improved sepsis outcomes (73–77). High-volume hemofiltration has also been studied in sepsis as a route to remove toxins and mediators, but the preponderance of the literature, including several meta-analyses, indicates no evidence of clinical benefit (78,79). Oxiris (enhanced hemoadsorption for endotoxin and cytokine removal) has been studied in septic shock with mixed evidence as to effectiveness of endotoxin/cytokine removal and a suggestion of hemodynamic improvement in retrospective uncontrolled cohort data (80–82). Prospective randomized studies are needed to judge the clinical potential of this technology. The coronavirus disease 2019 pandemic and the proposed link between critically ill coronavirus disease 2019 and inflammatory mediators have led to heightened interest in blood purification.
Blood purification techniques are resource laden and are not without side effects. Further research with the above technologies is needed to support use in sepsis. The evidence is insufficient to recommend use outside of research studies to date. Research gaps need to be identified and addressed.
Conclusion
As our understanding of sepsis evolves, so have our means for management. Early recognition and treatment with antibiotics and judicious fluid expansion are essential. Early administration of an appropriate antibiotic regimen is critical and should be individualized to the patient on the basis of the site of infection and risks for resistant organisms. Vasopressors should be initiated promptly for septic shock with NE as the first-line agent. Lactate can guide resuscitation, but care must be taken to avoid a reductionist approach by administering fluids for any lactate elevation in the absence of other parameters supporting tissue hypoperfusion. Steroids should be in the sepsis algorithm for patients with moderate to severe shock. De-resuscitation should be considered for select patients as persistent volume overload may be worsening instead of facilitating better outcomes.
Disclosures
R.P. Dellinger reports consultancy agreements with Merck and Phillips and honoraria from Baxter. All remaining authors have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
R.P. Dellinger, S. Patel, and N. Puri wrote the original draft and reviewed and edited the manuscript.
References
- 1.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R: Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 311: 1308–1316, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group : Acute kidney injury in septic shock: Clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35: 871–881, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Joost Wiersinga W, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Yataco AC, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M: Executive summary: Surviving Sepsis Campaign: International guidelines for the management of sepsis and septic shock 2021. Crit Care Med 49: 1974–1982, 2021 [DOI] [PubMed] [Google Scholar]
- 5.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ; The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644–1655, 1992 [DOI] [PubMed] [Google Scholar]
- 6.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; SCCM/ESICM/ACCP/ATS/SIS : 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA: Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 96: 1083–1099, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO Clinical Practice Guidelines for Acute Kidney Injury, 2012. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf. Accessed April 24, 2022 [Google Scholar]
- 9.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L, Gurka D, Kumar A, Cheang M: Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34: 1589–1596, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Townsend SR: Antibiotic administration and timing: Risks, delay, zombies. Crit Care Med 49: 1818–1821, 2021 [DOI] [PubMed] [Google Scholar]
- 11.Gyawali B, Ramakrishna K, Dhamoon AS: Sepsis: The evolution in definition, pathophysiology, and management. AGE Open Med 7: 2050312119835043, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito S, Uchino S, Hayakawa M, Yamakawa K, Kudo D, Iizuka Y, Sanui M, Takimoto K, Mayumi T, Sasabuchi Y; Japan Septic Disseminated Intravascular Coagulation (JSEPTIC DIC) study group : Epidemiology of disseminated intravascular coagulation in sepsis and validation of scoring systems. J Crit Care 50: 23–30, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Langenberg C, Gobe G, Hood S, May CN, Bellomo R: Renal histopathology during experimental septic acute kidney injury and recovery. Crit Care Med 42: e58–e67, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE: Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Gómez H, Kellum JA: Sepsis-induced acute kidney injury. Curr Opin Crit Care 22: 546–553, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calzavacca P, May CN, Bellomo R: Glomerular haemodynamics, the renal sympathetic nervous system and sepsis-induced acute kidney injury. Nephrol Dial Transplant 29: 2178–2184, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Perner A, Prowle J, Joannidis M, Young P, Hjortrup PB, Pettilä V: Fluid management in acute kidney injury. Intensive Care Med 43: 807–815, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Howell MD, Davis AM: Management of sepsis and septic shock. JAMA 317: 847–848, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Rhee C, Filbin MR, Massaro AF, Bulger AL, McEachern D, Tobin KA, Kitch BT, Thurlo-Walsh B, Kadar A, Koffman A, Pande A, Hamad Y, Warren DK, Jones TM, O’Brien C, Anderson DJ, Wang R, Klompas M; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program : Compliance with the national SEP-1 quality measure and association with sepsis outcomes: A multicenter retrospective cohort study. Crit Care Med 46: 1585–1591, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL; Program to Improve Care in Acute Renal Disease (PICARD) Study Group : Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, Van Regenmortel N: Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther 46: 361–380, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Pepper DJ, Sun J, Cui X, Welsh J, Natanson C, Eichacker PQ: Antibiotic- and fluid-focused bundles potentially improve sepsis management, but high-quality evidence is lacking for the specificity required in the Centers for Medicare and Medicaid Service’s Sepsis Bundle (SEP-1). Crit Care Med 47: 1290–1300, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC; ProCESS Investigators : A randomized trial of protocol-based care for early septic shock. N Engl J Med 370: 1683–1693, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P; ARISE Investigators; ANZICS Clinical Trials Group : Goal-directed resuscitation for patients with early septic shock. N Engl J Med 371: 1496–1506, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM; ProMISe Trial Investigators : Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 372: 1301–1311, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Rowan KM, Angus DC, Bailey M, Barnato AE, Bellomo R, Canter RR, Coats TJ, Delaney A, Gimbel E, Grieve RD, Harrison DA, Higgins AM, Howe B, Huang DT, Kellum JA, Mouncey PR, Music E, Peake SL, Pike F, Reade MC, Sadique MZ, Singer M, Yealy DM; PRISM Investigators : Early, Goal-directed therapy for septic shock—A patient-level meta-analysis. N Engl J Med 376: 2223–2234, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Meyhoff TS, Hjortrup PB, Møller MH, Wetterslev J, Lange T, Kjaer MN, Jonsson AB, Hjortsø CJS, Cronhjort M, Laake JH, Jakob SM, Nalos M, Pettilä V, van der Horst I, Ostermann M, Mouncey P, Rowan K, Cecconi M, Ferrer R, Malbrain MLNG, Ahlstedt C, Hoffmann S, Bestle MH, Nebrich L, Russell L, Vang M, Rasmussen ML, Sølling C, Rasmussen BS, Brøchner AC, Perner A: Conservative vs liberal fluid therapy in septic shock (CLASSIC) trial: Protocol and statistical analysis plan. Acta Anaesthesiol Scand 63: 1262–1271, 2019 [DOI] [PubMed] [Google Scholar]
- 29.Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, Ginde AA, Grissom CK, Janz DR, Jones AE, Liu KD, Macdonald SPJ, Miller CD, Park PK, Reineck LA, Rice TW, Steingrub JS, Talmor D, Yealy DM, Douglas IS, Shapiro NI; CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators : Liberal versus restrictive intravenous fluid therapy for early septic shock: Rationale for a randomized trial. Ann Emerg Med 72: 457–466, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarado Sánchez JI, Caicedo Ruiz JD, Diaztagle Fernández JJ, Amaya Zuñiga WF, Ospina-Tascón GA, Cruz Martínez LE: Predictors of fluid responsiveness in critically ill patients mechanically ventilated at low tidal volumes: Systematic review and meta-analysis. Ann Intensive Care 11: 28, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewitte A, Labat A, Duvignaud PA, Bouche G, Joannes-Boyau O, Ripoche J, Hilbert G, Gruson D, Rubin S, Ouattara A, Boyer A, Combe C: High mean arterial pressure target to improve sepsis-associated acute kidney injury in patients with prior hypertension: A feasibility study. Ann Intensive Care 11: 139, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Dequin PF, Gergaud S, Weiss N, Legay F, Le Tulzo Y, Conrad M, Robert R, Gonzalez F, Guitton C, Tamion F, Tonnelier JM, Guezennec P, Van Der Linden T, Vieillard-Baron A, Mariotte E, Pradel G, Lesieur O, Ricard JD, Hervé F, du Cheyron D, Guerin C, Mercat A, Teboul JL, Radermacher P; SEPSISPAM Investigators : High versus low blood-pressure target in patients with septic shock. N Engl J Med 370: 1583–1593, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Chen CY, Zhou Y, Wang P, Qi EY, Gu WJ: Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: A meta-analysis. Crit Care 24: 80, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J, Wu W, He Y, Lin S, Zhu D, Zhong M: Value of the combination of renal resistance index and central venous pressure in the early prediction of sepsis-induced acute kidney injury. J Crit Care 45: 204–208, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, Stollings JL, Kumar AB, Hughes CG, Hernandez A, Guillamondegui OD, May AK, Weavind L, Casey JD, Siew ED, Shaw AD, Bernard GR, Rice TW; SMART Investigators and the Pragmatic Critical Care Research Group : Balanced crystalloids versus saline in critically ill adults. N Engl J Med 378: 829–839, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Self WH, Semler MW, Wanderer JP, Wang L, Byrne DW, Collins SP, Slovis CM, Lindsell CJ, Ehrenfeld JM, Siew ED, Shaw AD, Bernard GR, Rice TW; SALT-ED Investigators : Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med 378: 819–828, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, McGuinness S, Mehrtens J, Myburgh J, Psirides A, Reddy S, Bellomo R; SPLIT Investigators; ANZICS CTG : Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: The SPLIT randomized clinical trial. JAMA 314: 1701–1710, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Zampieri FG, Machado FR, Biondi RS, Freitas FG, Veiga VC, Figueiredo RC, Lovato WJ, Amêndola CP, Serpa-Neto A, Paranhos JL, Guedes MA, Lúcio AE, Oliveira-Júnior LC, Lisboa TC, Lacerda FH, Maia IS, Grion CMC, Assunção MSC, Manoel ALO, Silva-Junior JM, Duarte P, Soares RM, Miranda TA, de Lima LM, Gurgel RM, Paisani DM, Corrêa TD, Azevedo LCP, Kellum JA, Damiani LP, da Silvada NB, Cavalcanti AB; BaSICS investigators and the BRICNet members : Effect of intravenous fluid treatment with a balanced solution vs 0.9% saline solution on mortality in critically ill patients: The BaSICS randomized clinical trial. JAMA 326: 818–829, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammond NE, Bellomo R, Gallagher M, Gattas D, Glass P, Mackle D, Micallef S, Myburgh J, Saxena M, Taylor C, Young P, Finfer S: The Plasma-Lyte 148 v Saline (PLUS) study protocol: A multicentre, randomised controlled trial of the effect of intensive care fluid therapy on mortality. Crit Care Resusc 19: 239–246, 2017 [PubMed] [Google Scholar]
- 40.Hammond DA, Lam SW, Rech MA, Smith MN, Westrick J, Trivedi AP, Balk RA: Balanced crystalloids versus saline in critically ill adults—A systematic review with meta-analysis. Ann Pharmacother 54: 5–13, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, Fergusson DA: Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: A systematic review and meta-analysis. JAMA 309: 678–688, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Patel A, Laffan MA, Waheed U, Brett SJ: Randomised trials of human albumin for adults with sepsis: Systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ 349: g4561, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldecoa C, Llau JV, Nuvials X, Artigas A: Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: A review. Ann Intensive Care 10: 85, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, Rodríguez-Seijas J: Comprehensive review on lactate metabolism in human health. Mitochondrion 17: 76–100, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R: Bench-to-bedside review: Lactate and the kidney. Crit Care 6: 322–326, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luft FC: Lactic acidosis update for critical care clinicians. J Am Soc Nephrol 12[Suppl 17]: S15–S19, 2001 [PubMed] [Google Scholar]
- 47.Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators : Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: A randomized clinical trial. JAMA 303: 739–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mallat J, Lemyze M, Tronchon L, Vallet B, Thevenin D: Use of venous-to-arterial carbon dioxide tension difference to guide resuscitation therapy in septic shock. World J Crit Care Med 5: 47–56, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL: Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med 41: 1412–1420, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Granda-Luna V, Cavalcanti AB, Bakker J, Hernández G, Ospina-Tascón G, Petri Damiani L, Estenssoro E, Dubin A, Hurtado J, Friedman G, Castro R, Alegría L, Teboul JL, Cecconi M, Cecconi M, Ferri G, Jibaja M, Pairumani R, Fernández P, Barahona D, Cavalcanti AB, Bakker J, Hernández G, Alegría L, Ferri G, Rodriguez N, Holger P, Soto N, Pozo M, Bakker J, Cook D, Vincent JL, Rhodes A, Kavanagh BP, Dellinger P, Rietdijk W, Carpio D, Pavéz N, Henriquez E, Bravo S, Valenzuela ED, Vera M, Dreyse J, Oviedo V, Cid MA, Larroulet M, Petruska E, Sarabia C, Gallardo D, Sanchez JE, González H, Arancibia JM, Muñoz A, Ramirez G, Aravena F, Aquevedo A, Zambrano F, Bozinovic M, Valle F, Ramirez M, Rossel V, Muñoz P, Ceballos C, Esveile C, Carmona C, Candia E, Mendoza D, Sanchez A, Ponce D, Ponce D, Lastra J, Nahuelpán B, Fasce F, Luengo C, Medel N, Cortés C, Campassi L, Rubatto P, Horna N, Furche M, Pendino JC, Bettini L, Lovesio C, González MC, Rodruguez J, Canales H, Caminos F, Galletti C, Minoldo E, Aramburu MJ, Olmos D, Nin N, Tenzi J, Quiroga C, Lacuesta P, Gaudín A, Pais R, Silvestre A, Olivera G, Rieppi G, Berrutti D, Ochoa M, Cobos P, Vintimilla F, Ramirez V, Tobar M, García F, Picoita F, Remache N, Granda V, Paredes F, Barzallo E, Garcés P, Guerrero F, Salazar S, Torres G, Tana C, Calahorrano J, Solis F, Torres P, Herrera L, Ornes A, Peréz V, Delgado G, López A, Espinosa E, Moreira J, Salcedo B, Villacres I, Suing J, Lopez M, Gomez L, Toctaquiza G, Cadena Zapata M, Orazabal MA, Pardo Espejo R, Jimenez J, Calderón A, Paredes G, Barberán JL, Moya T, Atehortua H, Sabogal R, Ortiz G, Lara A, Sanchez F, Hernán Portilla A, Dávila H, Mora JA, Calderón LE, Alvarez I, Escobar E, Bejarano A, Bustamante LA, Aldana JL; The ANDROMEDA SHOCK Investigators and the Latin America Intensive Care Network (LIVEN) : Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: The ANDROMEDA-SHOCK randomized clinical trial. JAMA 321: 654–664, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balogh Z, De Waele JJ, Malbrain MLNG: Continuous intra-abdominal pressure monitoring. Acta Clin Belg 62[Suppl 1]: 26–32, 2007 [PubMed] [Google Scholar]
- 52.Malbrain MLNG, Deeren DH: Effect of bladder volume on measured intravesical pressure: A prospective cohort study. Crit Care 10: R98, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regli A, De Keulenaer B, De Laet I, Roberts D, Dabrowski W, Malbrain MLNG: Fluid therapy and perfusional considerations during resuscitation in critically ill patients with intra-abdominal hypertension. Anaesthesiol Intensive Ther 47: 45–53, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Rola P, Miralles-Aguiar F, Argaiz E, Beaubien-Souligny W, Haycock K, Karimov T, Dinh VA, Spiegel R: Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J 13: 32, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patil VP, Salunke BG: Fluid overload and acute kidney injury. Indian J Crit Care Med 24[Suppl 3]: S94–S97, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Firth JD, Raine AE, Ledingham JG: Raised venous pressure: A direct cause of renal sodium retention in oedema? Lancet 1: 1033–1035, 1988 [DOI] [PubMed] [Google Scholar]
- 57.Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, Du B, Gallagher MP, Gaudry S, Hoste EA, Lamontagne F, Joannidis M, Landoni G, Liu KD, McAuley DF, McGuinness SP, Neyra JA, Nichol AD, Ostermann M, Palevsky PM, Pettilä V, Quenot J-P, Qiu H, Rochwerg B, Schneider AG, Smith OM, Thomé F, Thorpe KE, Vaara S, Weir M, Wang AY, Young P, Zarbock A; STARRT-AKI Investigators; Canadian Critical Care Trials Group; Australian and New Zealand Intensive Care Society Clinical Trials Group; United Kingdom Critical Care Research Group; Canadian Nephrology Trials Network; Irish Critical Care Trials Group : Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med 383: 240–251, 2020 [DOI] [PubMed] [Google Scholar]
- 58.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, Gerß J, Meersch M: Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: The ELAIN randomized clinical trial. JAMA 315: 2190–2199, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Barbar SD, Clere-Jehl R, Bourredjem A, Hernu R, Montini F, Bruyère R, Lebert C, Bohé J, Badie J, Eraldi JP, Rigaud JP, Levy B, Siami S, Louis G, Bouadma L, Constantin JM, Mercier E, Klouche K, du Cheyron D, Piton G, Annane D, Jaber S, van der Linden T, Blasco G, Mira JP, Schwebel C, Chimot L, Guiot P, Nay MA, Meziani F, Helms J, Roger C, Louart B, Trusson R, Dargent A, Binquet C, Quenot JP; IDEAL-ICU Trial Investigators and the CRICS TRIGGERSEP Network : Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 379: 1431–1442, 2018 [DOI] [PubMed] [Google Scholar]
- 60.Lumlertgul N, Peerapornratana S, Trakarnvanich T, Pongsittisak W, Surasit K, Chuasuwan A, Tankee P, Tiranathanagul K, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, Kellum JA, Srisawat N; FST Study Group : Early versus standard initiation of renal replacement therapy in furosemide stress test non-responsive acute kidney injury patients (the FST trial). Crit Care 22: 101, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monnet X, Cipriani F, Camous L, Sentenac P, Dres M, Krastinova E, Anguel N, Richard C, Teboul JL: The passive leg raising test to guide fluid removal in critically ill patients. Ann Intensive Care 6: 46, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian DH, Smyth C, Keijzers G, Macdonald SP, Peake S, Udy A, Delaney A: Safety of peripheral administration of vasopressor medications: A systematic review. Emerg Med Australas 32: 220–227, 2020 [DOI] [PubMed] [Google Scholar]
- 63.Permpikul C, Tongyoo S, Viarasilpa T, Trainarongsakul T, Chakorn T, Udompanturak S: Early use of norepinephrine in septic shock resuscitation (CENSER). A randomized trial. Am J Respir Crit Care Med 199: 1097–1105, 2019 [DOI] [PubMed] [Google Scholar]
- 64.Gordon AC, Russell JA, Walley KR, Singer J, Ayers D, Storms MM, Holmes CL, Hébert PC, Cooper DJ, Mehta S, Granton JT, Cook DJ, Presneill JJ: The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med 36: 83–91, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Gordon AC, Mason AJ, Thirunavukkarasu N, Perkins GD, Cecconi M, Cepkova M, Pogson DG, Aya HD, Anjum A, Frazier GJ, Santhakumaran S, Ashby D, Brett SJ; VANISH Investigators : Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH randomized clinical trial. JAMA 316: 509–518, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Annane D, Vignon P, Renault A, Bollaert PE, Charpentier C, Martin C, Troché G, Ricard JD, Nitenberg G, Papazian L, Azoulay E, Bellissant E; CATS Study Group : Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: A randomised trial. Lancet 370: 676–684, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Khanna A, English SW, Wang XS, Ham K, Tumlin J, Szerlip H, Busse LW, Altaweel L, Albertson TE, Mackey C, McCurdy MT, Boldt DW, Chock S, Young PJ, Krell K, Wunderink RG, Ostermann M, Murugan R, Gong MN, Panwar R, Hästbacka J, Favory R, Venkatesh B, Thompson BT, Bellomo R, Jensen J, Kroll S, Chawla LS, Tidmarsh GF, Deane AM; ATHOS-3 Investigators : Angiotensin II for the treatment of vasodilatory shock. N Engl J Med 377: 419–430, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Jadhav AP, Sadaka FG: Angiotensin II in septic shock. Am J Emerg Med 37: 1169–1174, 2019 [DOI] [PubMed] [Google Scholar]
- 69.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, Billot L, Correa M, Glass P, Harward M, Joyce C, Li Q, McArthur C, Perner A, Rhodes A, Thompson K, Webb S, Myburgh J; ADRENAL Trial Investigators and the Australian–New Zealand Intensive Care Society Clinical Trials Group : Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med 378: 797–808, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Annane D, Renault A, Brun-Buisson C, Megarbane B, Quenot JP, Siami S, Cariou A, Forceville X, Schwebel C, Martin C, Timsit JF, Misset B, Ali Benali M, Colin G, Souweine B, Asehnoune K, Mercier E, Chimot L, Charpentier C, François B, Boulain T, Petitpas F, Constantin JM, Dhonneur G, Baudin F, Combes A, Bohé J, Loriferne JF, Amathieu R, Cook F, Slama M, Leroy O, Capellier G, Dargent A, Hissem T, Maxime V, Bellissant E; CRICS-TRIGGERSEP Network : Hydrocortisone plus fludrocortisone for adults with septic shock. N Engl J Med 378: 809–818, 2018 [DOI] [PubMed] [Google Scholar]
- 71.Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S, Walker PM; EUPHRATES Trial Investigators : Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: The EUPHRATES randomized clinical trial. JAMA 320: 1455–1463, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C: Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 301: 2445–2452, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Poli EC, Alberio L, Bauer-Doerries A, Marcucci C, Roumy A, Kirsch M, De Stefano E, Liaudet L, Schneider AG: Cytokine clearance with CytoSorb® during cardiac surgery: A pilot randomized controlled trial. Crit Care 23: 108, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sathe P, Sakhavalkar P, Kumar S, Choudhary S: Clinical experience of using a novel extracorporeal cytokine adsorption column for treatment of septic shock with multiorgan failure. Crit Care 19: 130, 2015. 25887027 [Google Scholar]
- 75.Ankawi G, Neri M, Zhang J, Breglia A, Ricci Z, Ronco C: Extracorporeal techniques for the treatment of critically ill patients with sepsis beyond conventional blood purification therapy: The promises and the pitfalls. Crit Care 22: 262, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornejo R, Downey P, Castro R, Romero C, Regueira T, Vega J, Castillo L, Andresen M, Dougnac A, Bugedo G, Hernandez G: High-volume hemofiltration as salvage therapy in severe hyperdynamic septic shock. Intensive Care Med 32: 713–722, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Boussekey N, Chiche A, Faure K, Devos P, Guery B, d’Escrivan T, Georges H, Leroy O: A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med 34: 1646–1653, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Joannes-Boyau O, Honoré PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Rozé H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A: High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): A multicentre randomized controlled trial. Intensive Care Med 39: 1535–1546, 2013 [DOI] [PubMed] [Google Scholar]
- 79.Borthwick EM, Hill CJ, Rabindranath KS, Maxwell AP, McAuley DF, Blackwood B: High-volume haemofiltration for sepsis. Cochrane Database Syst Rev 1: CD008075, 2013 [DOI] [PubMed] [Google Scholar]
- 80.Broman ME, Hansson F, Vincent JL, Bodelsson M: Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS One 14: e0220444, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang K, Luo Y, Gao Y, Zhang J, Wang C, Fei D, Yang W, Meng X, Ye M, Gao Y, Liu H, Du X, Ji Y, Wei J, Xie W, Wang J, Zhao M, Yu K: Continuous renal replacement therapy with oXiris filter may not be an effective resolution to alleviate cytokine release syndrome in non-AKI patients with severe and critical COVID-19. Front Pharmacol 13: 817793, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lumlertgul N, Srisawat N: The haemodynamic effects of oXiris haemofilter in septic shock patients requiring renal support: A single-centre experience. Int J Artif Organs 44: 17–24, 2021 [DOI] [PubMed] [Google Scholar]