Abstract
Mechanical life support therapies exist in many forms to temporarily replace the function of vital organs. Generally speaking, these tools are supportive therapy to allow for organ recovery but, at times, require transition to long-term mechanical support. This review will examine nonrenal extracorporeal life support for cardiac and pulmonary support as well as other mechanical circulatory support options. This is intended as a general primer and overview to assist nephrologist consultants participating in the care of these critically ill patients who often experience acute renal injury as a result of cardiopulmonary shock and from their exposure to mechanical circulatory support.
Keywords: critical care nephrology and acute kidney injury series, cardiovascular, cardiovascular disease, heart failure
Introduction
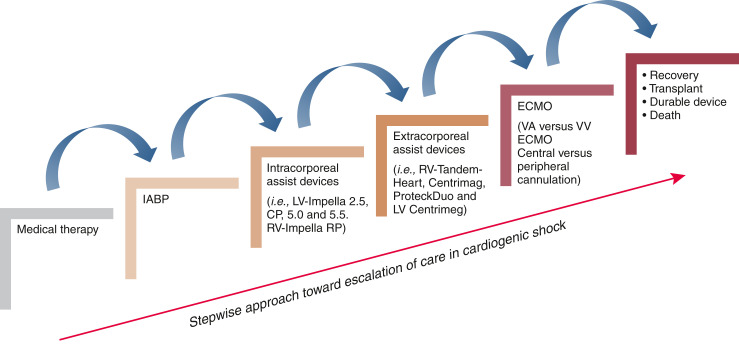
Cardiogenic shock is a state of low cardiac output that, if left untreated, will result in end organ hypoperfusion and death. The causes of cardiogenic shock are numerous, including vascular disorders, arrhythmias, and nonischemic or ischemic cardiomyopathy. Landmark studies, including the Early Revascularization in Acute Myocardial Infarction Complicate by Cardiogenic Shock trial and the Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock trial, as well as the European Society Cardiology Heart Failure guidelines define cardiogenic shock as a systolic BP <90 mm Hg with clinical signs of hypoperfusion, including cold extremities, altered mental status, low urine output, elevated serum lactate, and requirement of catecholamines to sustain BP (1–3). Initially, medical therapy is the mainstay treatment; however, as cardiogenic shock progresses, escalation of care with mechanical circulatory support may be warranted (Figure 1).
Figure 1.
Stepwise escalation of care in cardiogenic shock. ECMO, extracorporeal membrane oxygenation; IABP, intra-aortic balloon pump; LV, left ventricular; RV, right ventricular; VA ECMO, venoarterial extracorporeal membrane oxygenation; VV ECMO, venovenous extracorporeal membrane oxygenation.
Left Ventricular Failure
There are two main categories of left ventricular failure: heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction. HFrEF was classically described as systolic heart failure, characterized by impaired left ventricular contractility and reduced stroke volume followed by a cascade of maladaptive remodeling involving neurohormonal pathways, leading to dilation and thinning of the left ventricular walls (4,5). In contrast, heart failure with preserved ejection fraction, historically referred to as diastolic heart failure, results from chronic myocardial changes as a response to pressure overload, most commonly in chronic, uncontrolled hypertension.
In acute or decompensated chronic left heart failure, patients present with dyspnea and orthopnea from pulmonary edema and venous congestion leading to cardiorenal syndrome or congestive hepatopathy. In HFrEF, inotropic medications are used as first-line therapy to augment cardiac myocyte contractility (i.e., epinephrine, dobutamine, milrinone, and dopamine) (6). Patients with advanced heart failure or cardiogenic shock that fails medical therapy should be considered for mechanical circulatory support.
The intra-aortic balloon pump (IABP) was the first and is still the most used mechanical circulatory support device. IABPs are frequently inserted in patients with acute myocardial infarction complicated by cardiogenic shock, as well as used for postcardiotomy shock or high-risk percutaneous coronary intervention (PCI) (7). It is a temporary mechanical circulatory assist device that is meant for short-term duration of use (hours to days). The device is typically inserted percutaneously through the common femoral artery, threading a vascular catheter up to the upper descending aorta roughly 2 cm from the left subclavian artery. The catheter is surrounded by a long cylindrical balloon at the distal tip and contains one lumen for arterial pressure transduction and another lumen to provide balloon inflation or deflation (8). The balloon inflates with helium during ventricular diastole and deflates during systole. Assuming a competent aortic valve, this “counterpulsation” increases the pressure from the ascending aorta to the aortic root, thereby increasing the diastolic pressure in the coronaries during inflation. The balloon deflates immediately before systole, which creates a venturi effect to lower the afterload and facilitate ejection from the left ventricle (9). The IABP only produces a modest, direct increase in cardiac output (0.5–1 L/min), but the increase in coronary perfusion pressure can lead to enhanced stroke volume and improved cardiac output (10). Although some recent trials have called into question the use of IABP in patients with acute myocardial infarction, studies in subsets of patients have shown efficacy (1,7,11). A meta-analysis involving four randomized controlled trials including 148 patients demonstrated that IABP is effective in reducing all-cause mortality in patients who had an IABP while undergoing high-risk PCI (12). Furthermore, IABP may also be useful in patients with cardiogenic shock and postcardiotomy syndrome placed on venoarterial extracorporeal membrane oxygenation (VA ECMO) on the basis of several studies that demonstrated lower in-hospital mortality and more successful wean from VA ECMO (12,13). The benefit may be an improvement in end organ perfusion, improved coronary perfusion, reduction in left ventricular afterload, and improved left ventricular decompression in patients who have both VA ECMO and IABP therapies (12,13).
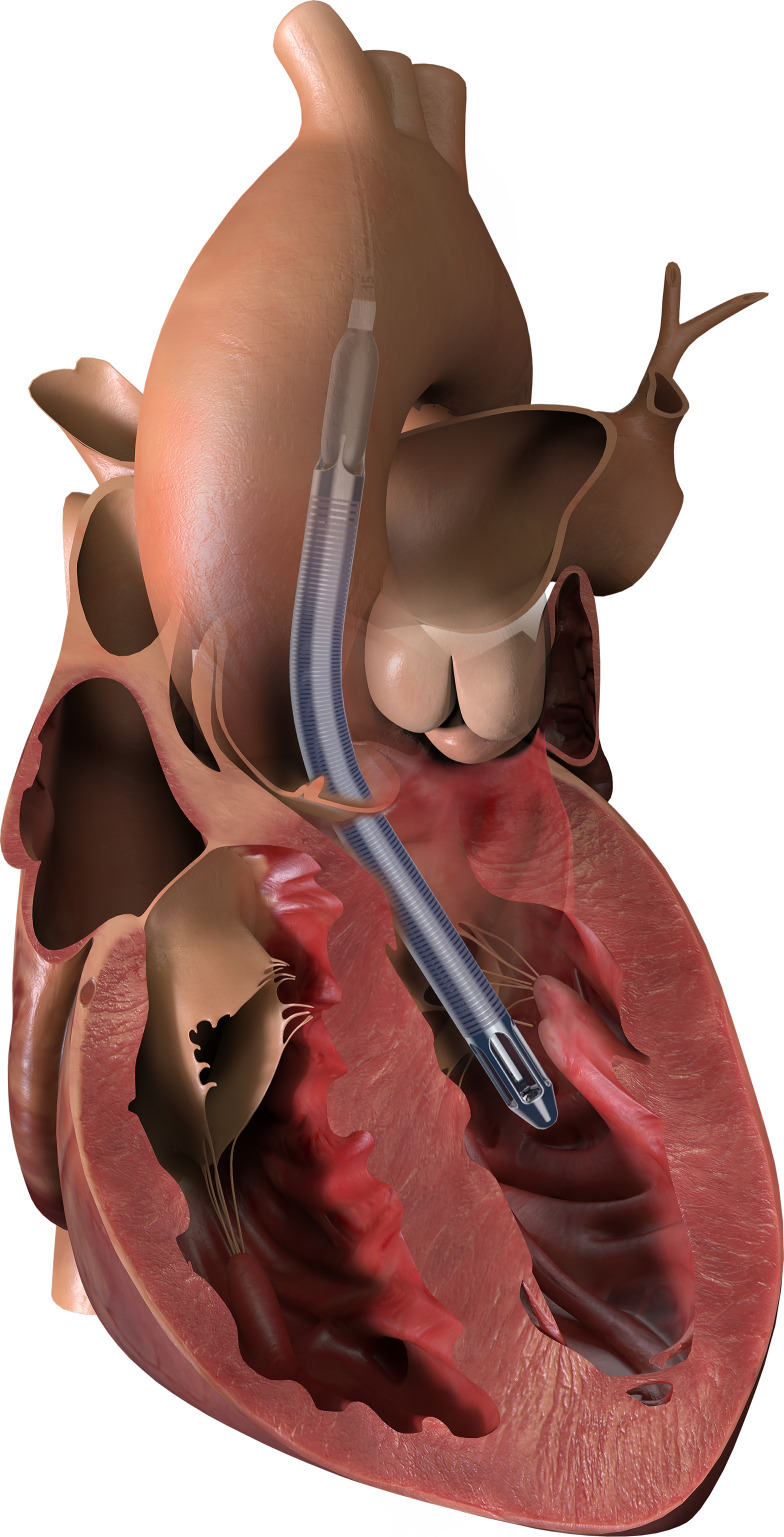
To provide a higher level of support, an intracorporeal heart pump can be used. For short-term (up to 14 days depending on model) support, a percutaneous, intracorporeal pump, such as the Abiomed Impella (Abiomed, Inc., Danvers, MA) device, provides temporary left ventricular assistance (Figure 2). It is a miniaturized, continuous flow, axial pump, which is inserted from the axillary or femoral arteries, retrograde up the aorta and across the aortic valve. The pump draws blood from the left ventricular chamber and directs it out of an orifice in the ascending aorta, thus providing left ventricular decompression and increasing cardiac output (7). Different versions of the device can provide differing levels of cardiac output support. The Impella 2.5 can generate up to 2.5 L/min of blood flow, and the Impella CP can provide 3–4 L/min. Impella 5.0 and 5.5 can provide 5 and 6.2 L/min of cardiac output, respectively, but require a surgical cut down to insert (10,14).
Figure 2.
Diagram of an Impella 5.5 for LV support. Adapted from Abiomed, Inc., Danvers, MA, with permission.
Long-term mechanical support for patients with end-stage heart failure is accomplished by implantation of a durable left ventricular assist device (LVAD). The general LVAD configuration includes an outflow cannula placed into the left ventricular apex connected to an axial or centrifugal pump, which removes blood from the ventricular chamber and delivers it into the ascending aorta via a tube graft (15). The two indications for LVAD implantation were traditionally termed “bridge to transplant” for patients listed for a heart transplant and “destination therapy” for those deemed ineligible for transplant who ostensibly would be relying on the LVAD for rest of their lives. Designs of first-generation LVADs attempted to replicate the pulsatile action of native hearts, but this was found to be inferior to the continuous flow, nonpulsatile devices used in second- and third-generation devices (15).
The first continuous flow LVAD to come to market was the Heartmate II (Thoratec, Pleasanton, CA), originally approved for bridge to transplant (16,17). This axial flow device is smaller than the devices that preceded it and expanded the opportunity for smaller patients, but it still required implantation into a preperitoneal pocket. Patients with the Heartmate II were found to have significantly greater chance of survival without disabling stroke or device repair or replacement at 2 years compared with the previous generation.
Third-generation LVADs use centrifugal flow and partial or full magnetic levitation of the pump to avoid blood contact with bearings. The noncontact environment reduces heat production and decreases the shearing forces on the blood elements, reducing hemolysis and lowering the propensity for thrombosis (18). Differences in stroke risk have most heavily influenced the current choice of device (19,20). The most recent annual report by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) suggests that Heartmate 3 (Thoratec, Pleasanton, CA) has become the most commonly implanted LVAD (Table 1) (21–23). Modern LVADs have excellent durability, and the overall 2-year survival even in patients with destination therapy is over 70% (24).
Table 1.
Interagency Registry for Mechanically Assisted Circulatory Support scale for classifying patients with advanced heart failure
| Profile | Description |
|---|---|
| INTERMACS 1 | Hemodynamic instability in spite of increasing doses of catecholamines and/or mechanical circulatory support with critical hypoperfusion of end organs (severe cardiogenic shock) |
| INTERMACS 2 | Intravenous inotropic support with acceptable BP but rapid deterioration of kidney function, nutritional status, or signs of congestion |
| INTERMACS 3 | Hemodynamic stability with low or intermediate (but necessary due to hypotension) doses of inotropic support, worsening of symptoms, or progressive kidney failure |
| INTERMACS 4 | Temporary cessation of inotropic support is possible, but patient presents frequently with symptom recurrence and typically with fluid overload |
| INTERMACS 5 | Complete cessation of physical activity; stable at rest but frequently with moderate water retention and some level of kidney dysfunction |
| INTERMACS 6 | Minor limitation of physical activity and absence of congestion while at rest; easily fatigued by light activity |
| INTERMACS 7 | Patient in NYHA functional class IIa or IIIb with no current or recent unstable water balance |
Adapted from INTERMACS. INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association.
NYHA class II: slight limitation of physical activity; comfortable at rest but ordinary physical activity results in fatigue, palpitation, or dyspnea.
NYHA class III: marked limitation of physical activity; comfortable at rest but less than ordinary physical activity results in fatigue, palpitation, or dyspnea.
Patients with continuous flow LVADs may have minimal pulsatility on arterial tracing. The mean arterial pressure (MAP) is the only reliable indication of BP and may not be measurable with a noninvasive, oscillometric BP cuff. Noninvasive BPs are most accurately measured by inflation of a sphygmomanometer cuff, which is progressively released until Doppler signal is detected in the brachial artery, revealing the MAP. In addition to BP, the principal determinant of cardiac performance is right ventricular function.
Right Ventricular Failure
As with left ventricular failure, mechanical circulatory support is considered for acute right ventricular failure when a patient fails medical therapy. Long-term right ventricular assist device (RVAD) support is limited due to the geometric shape of the right ventricle. The majority of long-term mechanical circulatory support devices are designed for the left ventricle (25). The use of durable devices for long-term right ventricular support is on the basis of an off-label use of axial flow pump devices (25). Small observational studies demonstrated some success in long-term use of this device, but it is not widely used. The outcomes of patients with mechanical circulatory support for right ventricular failure are widely variable in the literature; 42%–75% of patients reportedly recover (25). The type of mechanical circulatory support is particular for the patient depending on the etiology of the right ventricular failure.
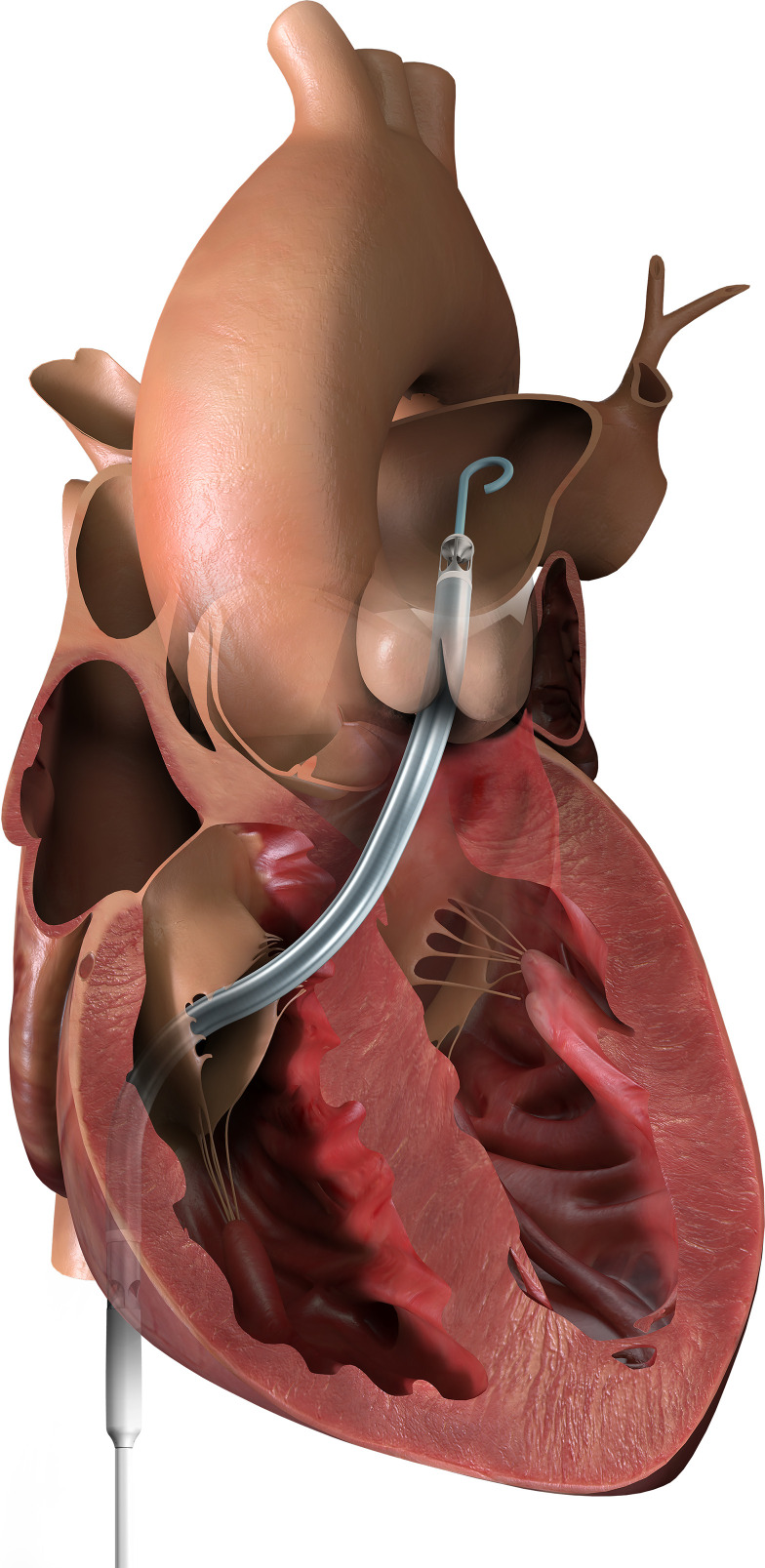
An Impella RP is a microaxial pump that provides temporary percutaneous right ventricular support device (for up to 14 days) (Figure 3). It is implanted via the femoral vein across the tricuspid and pulmonic valves, with the distal tip terminating in the pulmonary artery (26,27). Blood is drained from the right atrium and returned into the pulmonary artery, bypassing the right ventricle. This pump provides up to 4 L/min of flow and is the only Food and Drug Administration (FDA)–approved percutaneous pump for the right ventricle. Impella RP is a bridge to recovery and can remain in place for approximately 14 days on the basis of FDA approval (26,27). The Impella RP is not without complications, including hemolysis, bleeding secondary to anticoagulation therapy, limb ischemia, thrombus in the system, pulmonary edema, worsened pulmonic insufficiency, and tricuspid regurgitation (26). Vascular injury can also be encountered during initial placement of the device, requiring vascular surgery repair. Impella RP is contraindicated in patients with pulmonic insufficiency because the increased blood flow in the pulmonary artery will make it worse (26). It is also a relative contraindication in patients following tricuspid valve replacement and repair due to the position of the device across the operative site (26).
Figure 3.
Diagram of an Impella RP for RV support. Adapted from Abiomed, Inc., Danvers, MA, with permission.
Higher levels of right ventricular support can be achieved with extracorporeal pumps, although they are more invasive. These devices are inserted via cannulation of the right atrium or right ventricle for venous drainage by an extracorporeal centrifugal pump, with the outflow cannula positioned in the pulmonary artery. The cannulas exit the body via subcostal stab incisions, and as with other central cannulation strategies, re-entry into the chest is necessary for removal. The use of an RVAD is most commonly reported in the literature following LVAD implantation. The Tandem Heart in Right Ventricular Support study demonstrated some success with RVAD implantation for multiple indications (28). A total of 46 patients were included into this retrospective study at eight centers (28). The patients either had percutaneous cannulation or had central surgical implantation of the RVAD (28). The authors found that in both cannulation strategies, acute hemodynamic improvement, including higher cardiac output and lower filling pressures, was achieved (28). Patients who had right ventricular failure due to an acute myocardial infarction or post-LVAD enjoyed the greatest reduction in mortality in this study (28).
Total Artificial Heart
The total artificial heart has been shown to be effective for circulatory support as a bridge to transplant in select patients who are otherwise poor candidates for LVAD/biventricular assist device(s) (29). Current clinical indications for total artificial heart include (1) irreversible severe biventricular failure, (2) decompensated right heart failure on LVAD support, (3) heart allograft failure or rejection, (4) recurrent/recalcitrant ventricular tachycardia/fibrillation, (5) intracardiac thrombus or tumor, (6) postinfarction ventricular septal defect, (7) end-stage congenital heart disease, (8) aortic insufficiency or other valve issues with left and/or right ventricular failure, and (9) ventricular failure with small ventricles in patients with restrictive cardiomyopathies (29). In the past 10 years, over 75% of patients receiving total artificial heart were categorized as INTERMACS profiles 1 or 2 as defined in Table 1 (30).
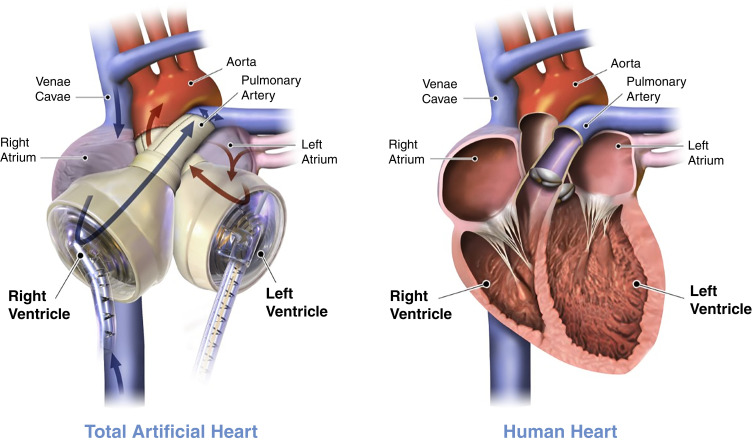
The Syncardia total artificial heart (SynCardia Systems, Tucson, AZ) is the only FDA-approved system for use at the present time, although there are several novel total artificial heart systems currently under investigation (Figure 4). The Syncardia total artificial heart is solely used as a bridge to transplant; however, it is actively undergoing clinical trial for use as destination therapy as well (31). This pulsatile total artificial heart consists of two artificial polyurethane ventricles with polyurethane diaphragms that separate blood from air (29). Each ventricle has inflow and outflow valves to control the flow of blood and, on the basis of the size chosen, can generate a stroke volume of either 50 ml (for those with a body surface area <1.85 m2) or 70 ml (for those with a body surface area at least 1.7 m2) (29). The ventricles are connected to the native atria with outflow tracts sewn to the aorta and pulmonary artery. A percutaneous pneumatic driveline from each ventricle (which is connected to an external console) provides diagnostic monitoring information. More recently, the Freedom Driver (which is a portable, battery-powered, lighter pneumatic pump) has been approved for use and allows patients to be discharged from the hospital (29).
Figure 4.
Syncardia total artificial heart (TAH) device. The left panel represents a depiction of postplacement TAH with anastomosis to both the right and left atria as well as the main pulmonary artery and aorta. The right panel represents a depiction of a human heart preimplantation of TAH. Adapted from ref. 31, with permission.
Postoperative concerns for those undergoing total artificial heart placement include kidney failure, pump thrombosis, bleeding, hemodynamic control, and infection. Interestingly, patients with total artificial hearts are uniquely subject to an abrupt reduction of B-type natriuretic peptide, which has been hypothesized to lead to kidney dysfunction and failure, even in those without prior kidney injury (32). To mitigate some of these postoperative complications, patients are typically anticoagulated initially with unfractionated heparin and later with coumadin; targeting INR 2.0–3.0, BP is maintained with a MAP goal between 65 and 85, and meticulous care of the driveline site is encouraged (33).
There have been multiple studies looking at outcomes for patients who undergo total artificial heart as a bridge to transplant. The Virginia Commonwealth University Medical Center published data for 66 patients; mean time of support was 87.5 days, 76% were successfully bridged to transplant, 15% were discharged home on Freedom Driver, 11% were still awaiting transplantation, and 14% died on the device (34). Similar results were found by Copeland et al. (35); 101 patients were supported for a mean of 87 days, 68% successfully bridged to transplant, and 32 patients died.
Extracorporeal Membranous Oxygenation
At times, complete cardiopulmonary bypass is required. Combined cardiac and pulmonary support can be achieved with VA ECMO, which is also the treatment of choice for full right ventricular support as well as a consideration for short-term biventricular support. The absolute contraindication to VA ECMO is no expectations of recovery without a pathway to durable support. If that cannot be determined, VA ECMO may be considered as a “bridge to candidacy.” Ideally, a reasonable timeline for recovery should be set before implantation to guide withdrawal of support when neither recovery nor alternative treatments exist. Other relative contraindications include (1) age, (2) obesity, (3) preexisting conditions that affect quality of life (neurologic status or advanced malignancy), and (4) risk of uncontrollable hemorrhage (36).
There are two types of cannulation strategies: central and peripheral. Central VA ECMO is most often used in patients with postcardiotomy shock to provide maximal cardiac decompression and optimal hemodynamic support (Figure 5A). Cannulation usually entails a venous drainage cannula into the right atrium as well as a return cannula into the ascending aorta. This configuration requires re-entry through the sternotomy incision for exploration. Central cannulation can also be achieved by arterial cannulation of the axillary artery via a cut-down incision.
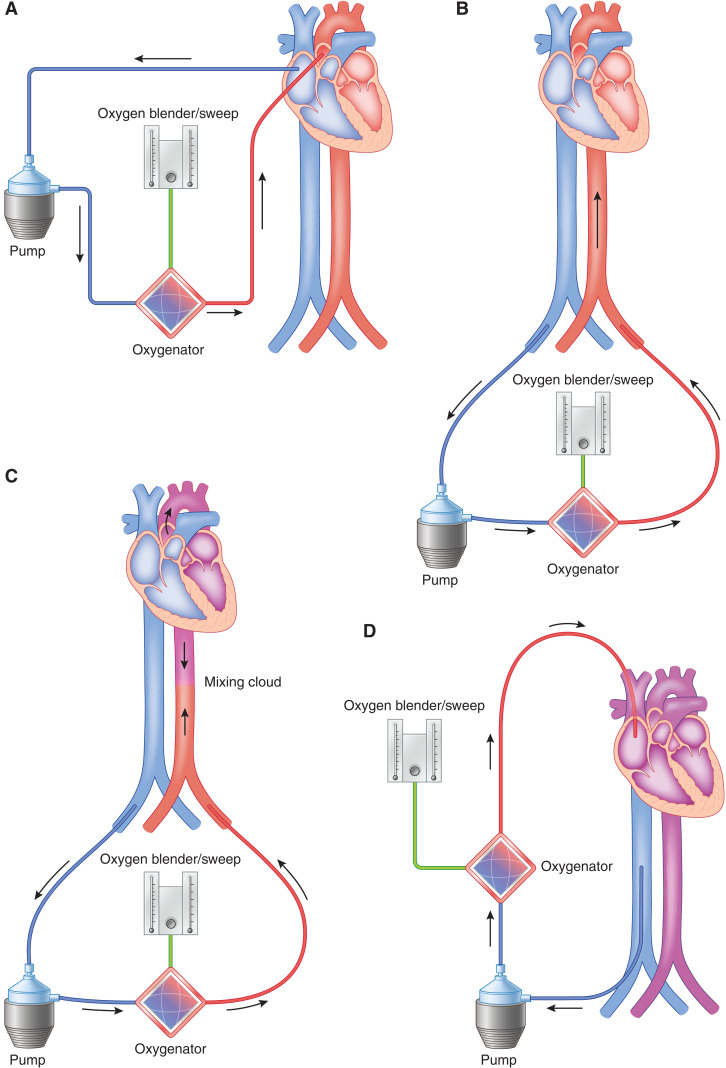
Figure 5.
ECMO may be configured either peripherally or centrally with different risks and benefits of either choice. (A) Diagram demonstrating central cannulation for VA ECMO. Right atrium/Inferior Vena Cava (IVC) is cannulated, and blood is flowing to the ECMO pump into the oxygenator and flowing back into the patient in an antegrade fashion directly into the ascending aorta. (B) Diagram demonstrating peripheral cannulation for VA ECMO. The femoral vein is cannulated, and blood is flowing from the patient to the ECMO pump into the oxygenator and flowing back into the patient in a retrograde fashion via the femoral artery. For (A) and (B), the oxygen blender and sweep as shown can be titrated accordingly on the basis of the partial pressure of oxygen in arterial blood (PaO2) and the partial pressure of carbon dioxide in arterial blood (PaCO2). This is the most invasive type of cannulation for ECMO. (C) Diagram demonstrating North-South syndrome or Harlequinn syndrome as seen in patients with peripheral VA ECMO. The mixing cloud occurs at the junction of retrograde oxygenation blood from the ECMO with that of the patient’s native LV cardiac output. Blood proximal to the mixing cloud is often deoxygenated, hence the importance of a right upper extremity arterial line in peripheral VA ECMO. (D) Diagram demonstrating VV ECMO with a right internal jugular vein and right femoral vein cannulation. Blood is flowing from the right femoral vein to the ECMO pump into the oxygenator and returning back into the patient in the right internal jugular vein.
Peripheral extracorporeal membranous oxygenation is a less invasive approach that typically involves percutaneous placement of cannulas in the femoral vein and artery; however, the axillary artery and internal jugular vein can sometimes be used as well (Figure 5B). The venous cannula is multiorifice and extends all of the way up the venal cava, terminating in the right atrium. Blood is drained from the venous system, passes through an extracorporeal centrifugal pump and oxygenator, and is returned into the arterial system, which provides retrograde perfusion of the upper half of the body. Anticoagulation is usually accomplished with heparin, which is initiated at cannulation and continued with a goal of partial thromboplastin time approximately 1.5 times the normal value. Distal ischemia of the ipsilateral leg due to occlusion by the cannula is a significant concern, and a distal perfusion catheter is frequently inserted at the time of cannulation to provide antegrade flow past the point of cannulation (37). In average-size adults, VA ECMO flows of 3–4 L/min allow for adequate right heart drainage, decreased peripheral venous congestion, and adequate blood flow to meet metabolic demands. The retrograde arterial flow during peripheral VA ECMO represents an afterload stress on the left ventricle. Therefore, limited left ventricular ejection by a weakly functioning left ventricle or the presence of aortic insufficiency may lead to left ventricular distention. In patients with rising pulmonary wedge pressures, inotropes or mechanical venting strategies (i.e., addition of an IABP or Impella) should be used to decompress the left ventricle. Increased left ventricle (left ventricular) distension leads to stasis and thrombosis formation, reduced coronary perfusion, and pulmonary edema and decreases the likelihood of recovery.
Another complication of retrograde oxygenated arterial flow is “Harlequin” or “North-South” syndrome (Figure 5C). When the lung function is severely impaired, deoxygenated blood is ejected by the left ventricle and mixes with the retrograde oxygenated blood. If the left ventricular ejection is significant, the area of mixing may be pushed toward the descending aorta, leaving the great vessels to be perfused by deoxygenated blood. To monitor for this condition, an arterial line should be placed in the right upper extremity to approximate the oxygen saturation of the brain and upper spinal cord arteries most closely. Other common complications include (1) hemorrhage, (2) neurologic injury, (3) limb ischemia, (4) hemolysis, and (5) air embolism (38). Anticoagulation is recommended throughout the extracorporeal membrane oxygenation run, but there are many anecdotal experiences in the literature in which anticoagulation was safely withheld due to bleeding complications (39).
VA ECMO is progressively weaned when improvement in left ventricular function is identified on echocardiography or improved pulsatility on the arterial or pulmonary artery tracings. Ultimately, a “turndown test” should be done. Typically, therapeutic levels of anticoagulation are first administered and then ECMO pump flows are slowly reduced to approximately 1 L/min or less. If the patient is able to maintain adequate signs of perfusion and oxygenation during this period (central venous oxygenation, peripheral arterial oxygenation, echocardiographic function, and pulmonary catheter hemodynamic changes), explantation of the device may be considered (36).
Mechanical Circulatory Support for Pulmonary Failure
The use of extracorporeal oxygenation was first described for respiratory failure (40). In venovenous extracorporeal membranous oxygenation (VV ECMO), deoxygenated blood is removed from the vena cava and passed through an extracorporeal membrane lung to return oxygenated blood to the right atrium (Figure 5D). Indications for VV ECMO include (1) acute respiratory distress syndrome (hypoxemic and hypercarbic respiratory failure), (2) bridge to lung transplant, (3) pulmonary hemorrhage, and (4) air leak syndromes (41). In hypoxic respiratory failure, it is recommended to consider VV ECMO in patients with PaO2/FiO2 <150 on FiO2 >90%. VV ECMO is indicated for patient with a PaO2/FiO2 <100 on FiO2 >90% for 6 hours or less, despite optimal medical and mechanical ventilator support (42). VV ECMO is contraindicated for acute respiratory failure if there is minimal chance of lung recovery (42). Patients with end-stage lung disease with severe ARDS have been successfully bridged to lung transplantation as seen in a case series of patients with coronavirus disease 2019 (COVID-19) ARDS (43). However, the recommendations and outcomes of VV ECMO in the setting of COVID-19 ARDS remain controversial at this time (44,45). Reports in the literature suggest that the immunologic inflammatory response seen in patients with COVID-19 is unique to this virus and may further be exacerbated by exposure to extracorporeal membrane oxygenation (44). Additional research and data are necessary to further ascertain mechanical circulatory support in this patient population. The relative contraindications, which are associated with poor outcomes in the Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure and the ECMO to Rescue Lung Injury in Severe ARDS trials, include (1) mechanical ventilation at high settings (FiO2 >90% or Pplat >30 cm H2) for >7 days, (2) CNS hemorrhage, (3) terminal malignancy, (4) nonrecoverable CNS injury, (5) major pharmacologic immunosuppression (ANS <400 mm3), and (6) uncontrollable hemorrhage (42,46).
Cannulation for VV ECMO may be a single double-lumen canula into the right internal jugular vein or two separate venous inflow and outflow cannulas. In the case of the dual-lumen catheter, the smaller lumen is oriented toward the tricuspid valve to reinfuse oxygenated blood, and the larger drainage lumen is oriented toward the IVC and requires fluoroscopy or transesophageal echocardiography for placement. The single dual-lumen cannula comes with the benefit of improved mobilization but at the risk of cannula movement and recirculation (oxygenated blood being drained by drainage cannula).
Two-cannula access, using the femoral vein and an internal jugular vein, is often the fastest approach. Typically, the drainage cannula is placed via the femoral vein into the IVC to the level of the renal veins, and the return cannula is positioned in the SVC/right atrial junction. Keeping the tips of the two cannulas at least 10 cm apart avoids recirculation of oxygenated blood that is reinfused to be drawn into the drainage cannula. A bolus of heparin (50–100 units/kg) is dosed at the time of cannulation, and partial thromboplastin time should be maintained at approximately 1.5 times the normal if there are no bleeding complications. The main objective of VV ECMO is to allow for lung-protective ventilation and time for lung recovery. Therefore, the goal is to reduce the ventilator setting to the lowest possible pressure and volume to minimize ventilator-induced lung injury (47).
Impella/Intra-Aortic Balloon Pump and Kidney Injury
Percutaneous ventricular assist devices, such as Impella or IABP, are commonly used in patients with cardiogenic shock to promote perfusion to vital end organs or in patients with significant coronary artery disease to decrease myocardial oxygen consumption. Impella support in patients undergoing high-risk PCI has been shown to decrease the incidence of AKI; however, data regarding IABP are inconclusive (48). The effects of percutaneous mechanical circulatory support in patients with cardiogenic shock on kidney injury are variable in the literature as there are few studies comparing kidney injury outcomes among different devices. A meta-analysis in 2018 compared four randomized controlled trials that included IABP and Impella use in patients during high-risk PCI or cardiogenic shock and showed no difference in short- or long-term mortality outcome between the two devices (49). Device-associated complications, including AKI, limb ischemia, infection, major bleeding, and vascular injury, were higher in patients where Impella was used compared with IABP (fixed effect relative risk, 1.65; 95% confidence interval, 1.14 to 2.39; P=0.008) (49). However, in a retrospective study comparing kidney function outcomes among patients in cardiogenic shock with or without mechanical circulatory support, patients with an Impella were shown to have an improvement in kidney function among patients with AKI, and it was superior to an IABP (50).
Left Ventricular Assist Device and Kidney Injury
Kidney dysfunction is a common comorbidity in patients with left ventricular heart failure as a result of cardiorenal syndrome. As the number of LVAD implantations increases, the number of patients with concurrent acute kidney disease and CKD will also invariably increase (51). INTERMACS reported a 12% incidence of patients who required dialysis or had a significant depreciation in kidney function measured by serum creatinine following LVAD surgery (52). Although data are sparse on the long-term outcome of kidney failure following LVAD implantation, on the basis of observational studies, kidney function due to cardiorenal syndrome may improve with LVAD hemodynamic optimization after several months (51). However, it is important to note a 1-year mortality rate of 29% among patients who develop AKI and require KRT postoperatively (53). Dialysis in patients with an LVAD poses significant challenges in the acute hospital setting as well as in those who require outpatient dialysis. Hemodialysis with a central venous catheter is the preferred option in patients with an LVAD (51). However, these patients are at risk for bloodstream infections associated with the dialysis catheter, which can subsequently infect the LVAD, leading to chronic infections that can affect candidacy for transplantation (51). Arteriovenous fistula creation and use as access for hemodialysis in patients with an LVAD has been reported in case reports that demonstrated success in fistula maturity and maintenance dialysis (51). Further investigation with larger trials is necessary to determine the ramifications of nonpulsatile flow on fistula maturity and outcomes in patients with an LVAD. Peritoneal dialysis is also a possibility and has been reported in the literature as case reports. Peritoneal dialysis may be beneficial as it has a lesser risk for bloodstream infections and is more hemodynamically stable as fluid is removed gradually over an extended period of time (51). However, the potential infectious complications associated with the driveline or with earlier LVAD models, if the peritoneum is violated, must be considered and further evaluated with research trials (51).
Extracorporeal Membrane Oxygenation and Kidney Injury
Extracorporeal membrane oxygenation is a maximally invasive therapy offering life support in patients with refractory cardiac, pulmonary, or cardiopulmonary failure. However, VA ECMO and VV ECMO are not benign therapeutic modalities; end organ dysfunction, especially AKI, is commonly reported. Incidence of AKI is variable in the literature, ranging from 30% to 80% of patients on extracorporeal membrane oxygenation mainly due to differing criteria to define AKI (54,55). About 50% of patients with AKI on extracorporeal membrane oxygenation require KRT, with continuous KRT (CKRT) being the most common on the basis of meta-analyses (56). Multifactorial causative factors prelude these patients developing AKI, aside from the primary premorbid causes of cardiogenic shock or refractory hypoxia. Extracorporeal membrane oxygenation stimulates inflammatory mediators, ischemia-reperfusion injury, nephrotoxins, and extracorporeal membrane oxygenation–related hemolysis as well as platelet and coagulation abnormalities that synergistically increase the risk of AKI (57–59).
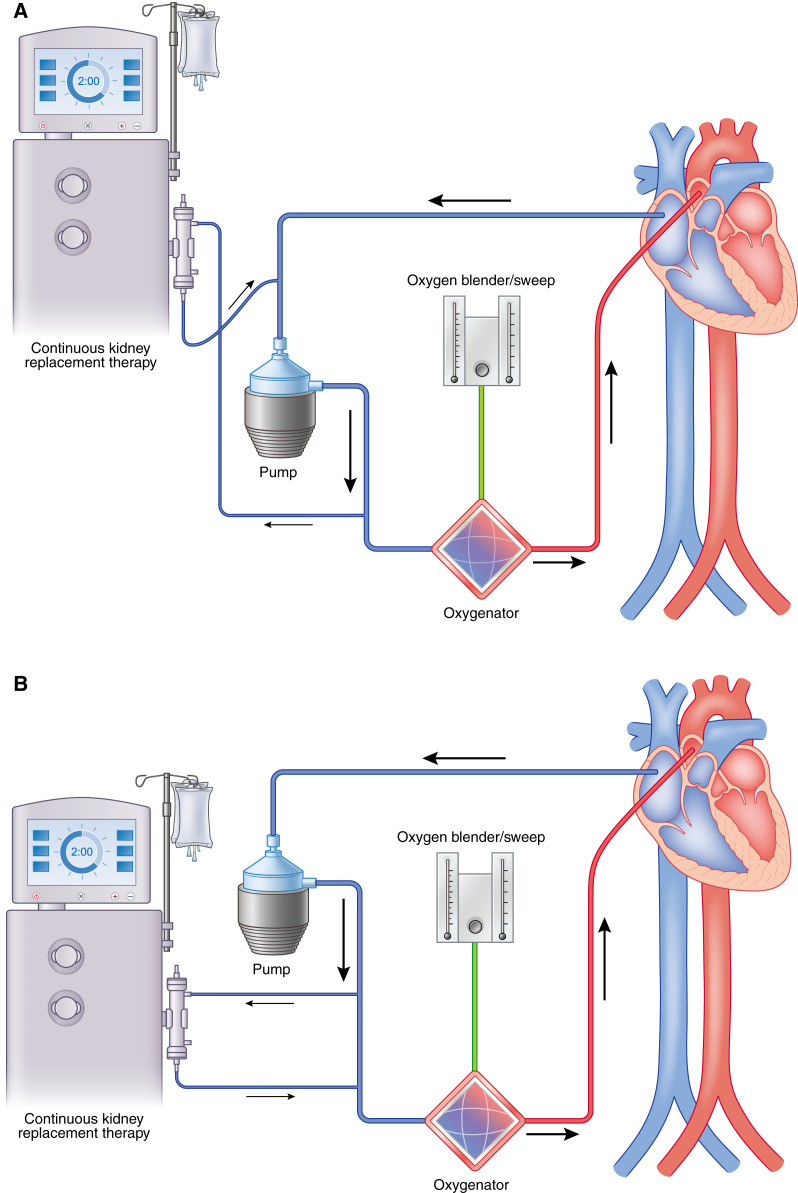
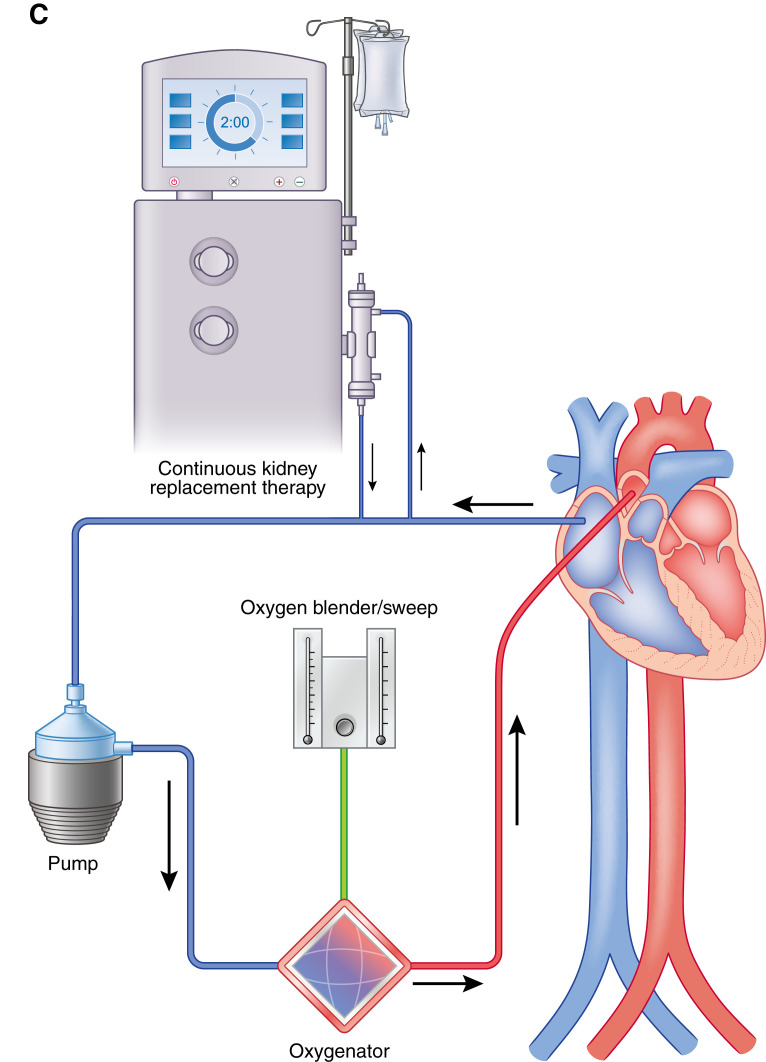
CKRT can be initiated as either an integrated or a parallel system approach (60). An integrated system involves connecting the dialysis machine to the extracorporeal membrane oxygenation cannula circuit, whereas a parallel system involves a separate dialysis circuit from extracorporeal membrane oxygenation (Figure 6). Both approaches have advantages and disadvantages, which is beyond the breadth of discussion in this review article. Patients who develop AKI and subsequently require CKRT while on extracorporeal membrane oxygenation may be at a higher risk of 90-day mortality; however, data in the literature are variable (55,61,62). The overall long-term effects of CKRT while on extracorporeal membrane oxygenation are not known, and further research is needed to elucidate this outcome measure.
Figure 6.
Continuous kidney replacement therapy may be incorporated into the ECMO circuit in place of using a dialysis catheter. (A) Continuous KRT (CKRT) machine with venous limb connected post-ECMO pump and the arterial limb in the pre-ECMO pump but before the oxygenator. (B) The CKRT machine with venous and arterial limb post-ECMO pump but before the oxygenator. (C) The CKRT machine with venous and arterial lines pre-ECMO pump and before the oxygenator. It is important to maintain the CKRT circuit, especially the return limb before the oxygenator, to prevent air embolization, venous admixture, and pressure elevations in the ECMO circuit (58).
Even with improvements in clinical diagnosis and medical management of patients with cardiogenic shock and severe respiratory failure, the prognosis is still poor. Mechanical circulatory support continues to evolve and play an increasing role in the management of these patients with advanced life support needs. It is useful for all physicians managing critically ill patients to have a familiarity with these devices, including their indication and potential complications.
Disclosures
All authors have nothing to disclose.
Funding
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
N.S. Ivascu, L. Shen, and C.W. Tam conceptualized the study; N.S. Ivascu provided supervision; L. Shen, A. Srivastava, C.W. Tam, and A.D. Zeidman wrote the original draft; and N.S. Ivascu and C.W. Tam reviewed and edited the manuscript.
References
- 1.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K; IABP-SHOCK II Trial Investigators : Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 367: 1287–1296, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH: Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 341: 625–634, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; ESC Scientific Document Group : 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [published correction appears in Eur Heart J 2016;38:ehw383]. Eur Heart J 37: 2129–2200, 2016. 27206819 [Google Scholar]
- 4.McMurray JJ, Pfeffer MA: Heart failure. Lancet 365: 1877–1889, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Tanai E, Frantz S: Pathophysiology of heart failure. Compr Physiol 6: 187–214, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, Mebazaa A: Acute heart failure. Nat Rev Dis Primers 6: 16, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiritano F, Lo Coco V, Matteucci M, Fina D, Willers A, Lorusso R: Temporary mechanical circulatory support in acute heart failure. Card Fail Rev 6: e01, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC) : 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol 65: e7–e26, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Mullins CB, Sugg WL, Kennelly BM, Jones DC, Mitchell JH: Effect of arterial counterpulsation on left ventricular volume and pressure. Am J Physiol 220: 694–698, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Wong ASK, Sin SWC: Short-term mechanical circulatory support (intra-aortic balloon pump, Impella, extracorporeal membrane oxygenation, TandemHeart): A review. Ann Transl Med 8: 829, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Böhm M, Ebelt H, Schneider S, Werdan K, Schuler G; Intraaortic Balloon Pump in cardiogenic shock II (IABP-SHOCK II) trial investigators : Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): Final 12 month results of a randomised, open-label trial. Lancet 382: 1638–1645, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Thiele H, Jobs A, Ouweneel DM, Henriques JPS, Seyfarth M, Desch S, Eitel I, Pöss J, Fuernau G, de Waha S: Percutaneous short-term active mechanical support devices in cardiogenic shock: A systematic review and collaborative meta-analysis of randomized trials. Eur Heart J 38: 3523–3531, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Smedira NG, Moazami N, Golding CM, McCarthy PM, Apperson-Hansen C, Blackstone EH, Cosgrove DM 3rd: Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: Survival at five years. J Thorac Cardiovasc Surg 122: 92–102, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Shishehbor MH, Moazami N, Tong MZ, Unai S, Tang WH, Soltesz EG: Cardiogenic shock: From ECMO to Impella and beyond. Cleve Clin J Med 84: 287–295, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Miller LW, Rogers JG: Evolution of left ventricular assist device therapy for advanced heart failure: A review. JAMA Cardiol 3: 650–658, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH; HeartMate II Clinical Investigators : Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 357: 885–896, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH; HeartMate II Investigators : Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol 54: 312–321, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA: Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 376: 451–460, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr., Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C; MOMENTUM 3 Investigators : A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med 376: 440–450, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Mehra MR, Goldstein DJ, Uriel N, Cleveland JC Jr., Yuzefpolskaya M, Salerno C, Walsh MN, Milano CA, Patel CB, Ewald GA, Itoh A, Dean D, Krishnamoorthy A, Cotts WG, Tatooles AJ, Jorde UP, Bruckner BA, Estep JD, Jeevanandam V, Sayer G, Horstmanshof D, Long JW, Gulati S, Skipper ER, O’Connell JB, Heatley G, Sood P, Naka Y; MOMENTUM 3 Investigators : Two-year outcomes with a magnetically levitated cardiac pump in heart failure. N Engl J Med 378: 1386–1395, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D, Kirklin JK, Pagani FD, Cowger JA: The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann Thorac Surg 111: 778–792, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, Jeevanandam V, Anderson AS, Kormos RL, Teuteberg JJ, Levy WC, Naftel DC, Bittman RM, Pagani FD, Hathaway DR, Boyce SW; HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators : Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 125: 3191–3200, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Slaughter MS, Pagani FD, McGee EC, Birks EJ, Cotts WG, Gregoric I, Howard Frazier O, Icenogle T, Najjar SS, Boyce SW, Acker MA, John R, Hathaway DR, Najarian KB, Aaronson KD; HeartWare Bridge to Transplant ADVANCE Trial Investigators : HeartWare ventricular assist system for bridge to transplant: Combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant 32: 675–683, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Mehra MR, Uriel N, Naka Y, Cleveland JC Jr., Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ; MOMENTUM 3 Investigators : A fully magnetically levitated left ventricular assist device - Final report. N Engl J Med 380: 1618–1627, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Konstam MA, Kiernan MS, Bernstein D, Bozkurt B, Jacob M, Kapur NK, Kociol RD, Lewis EF, Mehra MR, Pagani FD, Raval AN, Ward C; American Heart Association Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Cardiovascular Surgery and Anesthesia : Evaluation and management of right-sided heart failure: A scientific statement from the American Heart Association. Circulation 137: e578–e622, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Pieri M, Pappalardo F: Impella RP in the treatment of right ventricular failure: What we know and where we go. J Cardiothorac Vasc Anesth 32: 2339–2343, 2018 [DOI] [PubMed] [Google Scholar]
- 27.Anderson MB, Goldstein J, Milano C, Morris LD, Kormos RL, Bhama J, Kapur NK, Bansal A, Garcia J, Baker JN, Silvestry S, Holman WL, Douglas PS, O’Neill W: Benefits of a novel percutaneous ventricular assist device for right heart failure: The prospective RECOVER RIGHT study of the Impella RP device. J Heart Lung Transplant 34: 1549–1560, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Cheung AW, White CW, Davis MK, Freed DH: Short-term mechanical circulatory support for recovery from acute right ventricular failure: Clinical outcomes. J Heart Lung Transplant 33: 794–799, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Melton N, Soleimani B, Dowling R: Current role of the total artificial heart in the management of advanced heart failure. Curr Cardiol Rep 21: 142, 2019 [DOI] [PubMed] [Google Scholar]
- 30.Yaung J, Arabia FA, Nurok M: Perioperative care of the patient with the total artificial heart. Anesth Analg 124: 1412–1422, 2017 [DOI] [PubMed] [Google Scholar]
- 31.SynCardia Systems, LLC .: SynCardia 70cc TAH-t for Destination Therapy (DT) (RA-540), 2014. Available at: https://clinicaltrials.gov/ct2/show/NCT02232659. Accessed March 20, 2022
- 32.Maynes EJ, O’Malley TJ, Luc JGY, Weber MP, Horan DP, Choi JH, Patel S, Abbas Rizvi SS, Morris RJ, Entwistle JW, Massey HT, Tchantchaleishvili V: Comparison of SynCardia total artificial heart and HeartWare HVAD biventricular support for management of biventricular heart failure: A systematic review and meta-analysis. Ann Cardiothorac Surg 9: 69–80, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Presti CR, Crenshaw NA: Overview of ventricular assist devices and the total artificial heart. Dimens Crit Care Nurs 40: 3–13, 2021 [DOI] [PubMed] [Google Scholar]
- 34.Thanavaro KL, Tang DG, Kasirajan V, Shah KB: Clinical indications for implantation of the total artificial heart. ASAIO J 60: 594–596, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Copeland JG, Copeland H, Gustafson M, Mineburg N, Covington D, Smith RG, Friedman M: Experience with more than 100 total artificial heart implants. J Thorac Cardiovasc Surg 143: 727–734, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Extracorporeal Life Support Organization (ELSO) : General Guidelines for all ECLS Cases, 2017. Available at: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.118.004905. Accessed March 20, 2022
- 37.Rao P, Khalpey Z, Smith R, Burkhoff D, Kociol RD: Venoarterial extracorporeal membrane oxygenation for cardiogenic shock and cardiac arrest. Circ Heart Fail 11: e004905, 2018 [DOI] [PubMed] [Google Scholar]
- 38.Allen S, Holena D, McCunn M, Kohl B, Sarani B: A review of the fundamental principles and evidence base in the use of extracorporeal membrane oxygenation (ECMO) in critically ill adult patients. J Intensive Care Med 26: 13–26, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Wood KL, Ayers B, Gosev I, Kumar N, Melvin AL, Barrus B, Prasad S: Venoarterial-extracorporeal membrane oxygenation without routine systemic anticoagulation decreases adverse events. Ann Thorac Surg 109: 1458–1466, 2020 [DOI] [PubMed] [Google Scholar]
- 40.Hill JD, O’Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, Gerbode F: Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 286: 629–634, 1972 [DOI] [PubMed] [Google Scholar]
- 41.Extracorporeal Life Support Organization (ELSO) : Guidelines for Adult Respiratory Failure, 2017. Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1800385. Accessed March 19, 2022
- 42.Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A; EOLIA Trial Group, REVA, and ECMONet : Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med 378: 1965–1975, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Bharat A, Machuca TN, Querrey M, Kurihara C, Garza-Castillon R Jr., Kim S, Manerikar A, Pelaez A, Pipkin M, Shahmohammadi A, Rackauskas M, Kg SR, Balakrishnan KR, Jindal A, Schaheen L, Hashimi S, Buddhdev B, Arjuna A, Rosso L, Palleschi A, Lang C, Jaksch P, Budinger GRS, Nosotti M, Hoetzenecker K: Early outcomes after lung transplantation for severe COVID-19: A series of the first consecutive cases from four countries. Lancet Respir Med 9: 487–497, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowalewski M, Fina D, Słomka A, Raffa GM, Martucci G, Lo Coco V, De Piero ME, Ranucci M, Suwalski P, Lorusso R: COVID-19 and ECMO: The interplay between coagulation and inflammation-a narrative review. Crit Care 24: 205, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D; CESAR trial collaboration : Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet 374: 1351–1363, 2009. 19762075 [Google Scholar]
- 47.Combes A, Schmidt M, Hodgson CL, Fan E, Ferguson ND, Fraser JF, Jaber S, Pesenti A, Ranieri M, Rowan K, Shekar K, Slutsky AS, Brodie D: Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med 46: 2464–2476, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flaherty MP, Pant S, Patel SV, Kilgore T, Dassanayaka S, Loughran JH, Rawasia W, Dawn B, Cheng A, Bartoli CR: Hemodynamic support with a microaxial percutaneous left ventricular assist device (Impella) protects against acute kidney injury in patients undergoing high-risk percutaneous coronary intervention. Circ Res 120: 692–700, 2017 [DOI] [PubMed] [Google Scholar]
- 49.Rios SA, Bravo CA, Weinreich M, Olmedo W, Villablanca P, Villela MA, Ramakrishna H, Hirji S, Robles OA, Mahato P, Gluud C, Bhatt DL, Jorde UP: Meta-analysis and trial sequential analysis comparing percutaneous ventricular assist devices versus intra-aortic balloon pump during high-risk percutaneous coronary intervention or cardiogenic shock. Am J Cardiol 122: 1330–1338, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Upadhyaya VD, Alshami A, Patel I, Douedi S, Quinlan A, Thomas T, Prentice J, Calderon D, Asif A, Sen S, Mehra A, Hossain MA: Outcomes of renal function in cardiogenic shock patients with or without mechanical circulatory support. J Clin Med Res 13: 283–292, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roehm B, Vest AR, Weiner DE: Left ventricular assist devices, kidney disease, and dialysis. Am J Kidney Dis 71: 257–266, 2018 [DOI] [PubMed] [Google Scholar]
- 52.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB: Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 34: 1495–1504, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Borgi J, Tsiouris A, Hodari A, Cogan CM, Paone G, Morgan JA: Significance of postoperative acute renal failure after continuous-flow left ventricular assist device implantation. Ann Thorac Surg 95: 163–169, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, Scheinkestel C, Pellegrino V: Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: A 5-year cohort study. Crit Care 17: R73, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin CY, Chen YC, Tsai FC, Tian YC, Jenq CC, Fang JT, Yang CW: RIFLE classification is predictive of short-term prognosis in critically ill patients with acute renal failure supported by extracorporeal membrane oxygenation. Nephrol Dial Transplant 21: 2867–2873, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, Esmailian F, Azarbal B: Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1,866 adult patients. Ann Thorac Surg 97: 610–616, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Kurundkar AR, Killingsworth CR, McIlwain RB, Timpa JG, Hartman YE, He D, Karnatak RK, Neel ML, Clancy JP, Anantharamaiah GM, Maheshwari A: Extracorporeal membrane oxygenation causes loss of intestinal epithelial barrier in the newborn piglet. Pediatr Res 68: 128–133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mildner RJ, Taub N, Vyas JR, Killer HM, Firmin RK, Field DJ, Kotecha S: Cytokine imbalance in infants receiving extracorporeal membrane oxygenation for respiratory failure. Biol Neonate 88: 321–327, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Reed RC, Rutledge JC: Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol 13: 385–392, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Ostermann M, Connor M Jr., Kashani K: Continuous renal replacement therapy during extracorporeal membrane oxygenation: Why, when and how? Curr Opin Crit Care 24: 493–503, 2018 [DOI] [PubMed] [Google Scholar]
- 61.Schmidt M, Bailey M, Kelly J, Hodgson C, Cooper DJ, Scheinkestel C, Pellegrino V, Bellomo R, Pilcher D: Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med 40: 1256–1266, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Antonucci E, Lamanna I, Fagnoul D, Vincent JL, De Backer D, Silvio Taccone F: The impact of renal failure and renal replacement therapy on outcome during extracorporeal membrane oxygenation therapy. Artif Organs 40: 746–754, 2016 [DOI] [PubMed] [Google Scholar]