Visual Abstract
Keywords: polycystic kidney disease, kidney volume, ultrasonography, ADPKD
Abstract
Background and objectives
Total kidney volume is a validated prognostic biomarker for autosomal dominant polycystic kidney disease. Total kidney volume by magnetic resonance imaging (MRI) and manual segmentation is considered the “reference standard,” but it is time consuming and not readily accessible. By contrast, three-dimensional (3D) ultrasound provides a promising technology for total kidney volume measurements with unknown potential. Here, we report a comparative study of total kidney volume measurements by 3D ultrasound versus the conventional methods by ultrasound ellipsoid and MRI ellipsoid.
Design, setting, participants, & measurements
This single-center prospective study included 142 patients who completed a standardized 3D ultrasound and MRI. Total kidney volumes by 3D ultrasound and ultrasound ellipsoid were compared with those by MRI. We assessed the agreement of total kidney volume measurements by Bland–Altman plots and misclassification of the Mayo Clinic imaging classes between the different imaging methods, and we assessed prediction of Mayo Clinic imaging classes 1C–1E by average ultrasound kidney length >16.5 cm.
Results
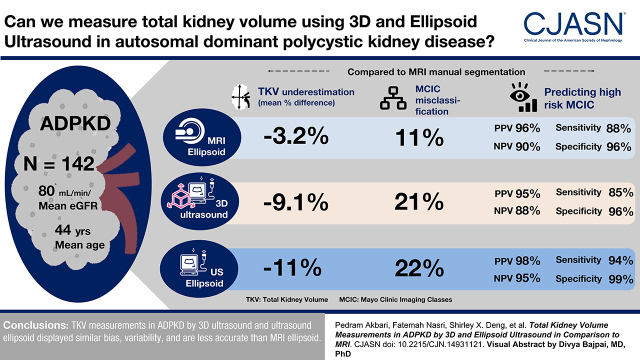
Compared with MRI manual segmentation, MRI ellipsoid, 3D ultrasound, and ultrasound ellipsoid underestimated total kidney volume (mean difference: −3%, −9%, and −11%, respectively), with Mayo Clinic imaging classes misclassified in 11%, 21%, and 22% of patients, respectively; most misclassified cases by MRI ellipsoid (11 of 16), 3D ultrasound (23 of 30), and ultrasound ellipsoid (26 of 31) were placed into a lower Mayo Clinic imaging class. Predictions of the high-risk Mayo Clinic imaging classes (1C–1E) by MRI ellipsoid, 3D ultrasound, and ultrasound ellipsoid all yielded high positive predictive value (96%, 95%, and 98%, respectively) and specificity (96%, 96%, and 99%, respectively). However, both negative predictive value (90%, 88%, and 95%, respectively) and sensitivity (88%, 85%, and 94%, respectively) were lower for 3D ultrasound and ultrasound ellipsoid compared with MRI ellipsoid. An average ultrasound kidney length >16.5 cm was highly predictive of Mayo Clinic imaging classes 1C–1E only in patients aged ≤45 years.
Conclusions
Total kidney volume measurements in autosomal dominant polycystic kidney disease by 3D ultrasound and ultrasound ellipsoid displayed similar bias and variability and are less accurate than MRI ellipsoid. Prediction of high-risk Mayo Clinic imaging classes (1C–1E) by all three methods provides high positive predictive value, but ultrasound ellipsoid is simpler to use and more readily available.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary kidney disease worldwide and the fourth leading cause of advanced kidney failure in North America (1,2). Mutations of two genes, PKD1 and PKD2, account for most of the genetically resolved cases (3). Progressive increase in cyst number and size with age in ADPKD leads to distortion of normal kidney architecture and, ultimately, advanced kidney failure in at least 50% of patients (2). Kidney function tests, such as serum creatinine and eGFR, are poor biomarkers for disease progression as they typically remain within or close to the normal range until late in the clinical course (4,5). By contrast, the Consortium for Radiologic Imaging Study of Polycystic Kidney Disease (CRISP) has shown that total kidney volume (TKV) in ADPKD expands quasiexponentially during adult life at an average rate of 5% per year and is a sensitive biomarker predicting CKD progression (5,6). Both the Food and Drug Administration and the European Medicines Agency have recently accepted TKV as a prognostic biomarker for ADPKD (7). Using age- and height-adjusted TKV, the Mayo Clinic imaging class (MCIC) provides a validated risk assessment tool for selecting “high-risk” patients, defined as classes 1C–1E, for clinical trials. TKV measurements for MCIC are currently on the basis of magnetic resonance imaging (MRI) using an ellipsoid formula (MRI ellipsoid), which is simpler to implement (8,9). With the approval of tolvaptan as the first disease-modifier treatment for high-risk patients with ADPKD in multiple countries, TKV-based risk assessment assumes an increasingly important role for patient management (10,11).
TKV can be captured using MRI, computed tomography (CT), or ultrasonography, and it can be measured using a variety of techniques, including stereology and the ellipsoid method (12). Both MRI and CT can provide a highly accurate measurement of TKV; however, MRI is preferred over CT because the latter requires exposure to radiation and nephrotoxic contrast agents (13). The reference standard for calculating TKV is manual segmentation of MRI-derived images, wherein each kidney is divided into slices of equal thickness and each slice is manually traced to quantify its area (8,14). However, MRI is costly and may not be readily available. Moreover, manual segmentation requires special radiologic expertise and is labor intensive (15). By contrast, ultrasound is widely available and inexpensive, from which the volume of each kidney can be approximated by measuring its three orthogonal axes using an ellipsoid formula (ultrasound ellipsoid), providing a simplified and more rapid but less accurate alternative (16). The availability of three-dimensional (3D) ultrasound using specialized transducers in most tertiary care radiology departments provides a promising tool for clinical volumetric measurements and has been evaluated for TKV quantification in patients without ADPKD (17,18). The accuracy of TKV measurements by 3D ultrasound with manual segmentation versus ultrasound ellipsoid was also assessed in the pediatric ADPKD population (19). Here, we report a single-center prospective study to assess the agreement of TKV measurements and misclassification of the MCIC between 3D ultrasound, ultrasound ellipsoid, MRI ellipsoid, and MRI manual segmentation (reference standard).
Materials and Methods
Study Population
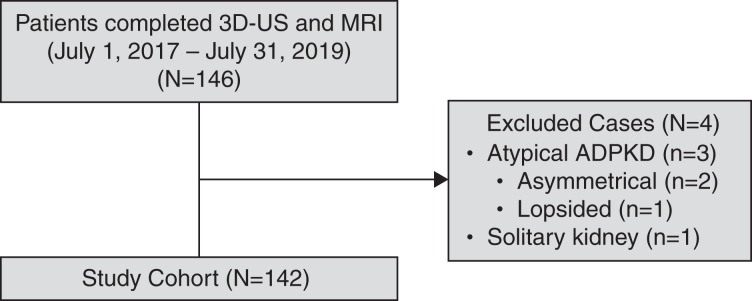
We prospectively enrolled 146 patients into our study from the Center for Innovative Management of Polycystic Kidney Disease (https://www.cimpkd.ca/) at the University Health Network in Toronto, Ontario, Canada, between July 1, 2017 and July 31, 2019. All study participants were aged 18 years or older, were diagnosed with ADPKD by ultrasound or MRI-based criteria (20,21), and had no contraindications to undergo MRI. Kidney failure and prior cyst reduction were not exclusionary criteria; however, none of the study patients had advanced kidney failure (i.e., CKD stage 5) at the time of their scans. All participants provided informed consent according to a prespecified study protocol approved by the institutional ethics review board at the University Health Network. Four study participants (i.e., three with atypical kidney patterns and one with a solitary polycystic kidney) were subsequently excluded upon review of their MRI (Figure 1); 142 patients were analyzed for this report.
Figure 1.
Assembly of the study cohort. Three patients with atypical kidney imaging (i.e., Mayo Clinic imaging class 2) and one patient with a solitary polycystic kidney were excluded from analysis. ADPKD, autosomal dominant polycystic kidney disease; MRI, magnetic resonance imaging; 3D-US, three-dimensional ultrasound.
Exposure
Study patients underwent standardized clinical evaluation including serum creatinine and eGFR (using the Chronic Kidney Disease Epidemiology Collaboration equation) within 3 months of their kidney imaging (22). They also underwent a standardized 3D ultrasound and MRI; 134 of 142 patients had both 3D ultrasound and MRI performed on the same day, with eight other patients completing the two scans within a 3-week period.
Ultrasounds were performed using a Canon Aplio 500 scanner (Canon Medical Systems Corporation, Otawara, Japan) equipped with a 3.5-MHz mechanical convex 3D transducer. If the ends of the kidney did not fit within the image display, the lengths were measured using a panoramic function (i.e., extended field of view ultrasound). The transducer is placed in a longitudinal plane along the upper pole and moved in a linear direction along the length of the kidney to reach the lower pole; the software then dynamically “stitches” the images obtained as the transducer is displaced. All scans were performed by five different technicians who were individually trained by an experienced instructor to acquire kidney dimensions by 3D ultrasound (Supplemental Material). The five technicians scanned 48, 33, 28, 24, and nine patients, respectively, and they were blinded to the clinical information. The patient distribution per technologist solely relied on his or her schedule.
MRI was performed using 1.5-T GE MRI scanners with the following parameters: coronal T2-weighted single-shot fast spin-echo MR images with fat saturation and 0.59- to 1.41-mm per pixel resolution in plane, 3-mm slice thickness, 90° flip angle, and 500–1491 ms/82–101 ms repetition time/echo time. All DICOM files from MRI were retrieved to a workstation and inspected to confirm complete coverage of both kidneys and image quality. Manual segmentation was reviewed by an experienced radiologist (F.N.) blinded to the clinical information. From the abdominal MR images, the boundary of each kidney was manually delineated slice by slice using commercially available software (Analyze 12.0; Mayo Clinic, Rochester, MN). Kidney volumes were calculated from the set of contiguous images by summing the products of the area measurements within the kidney boundaries and slice thickness. Tissues outside the kidneys (e.g., the kidney hilum) were excluded from measurement. MRI ellipsoid was calculated using three measured orthogonal axes of the kidney in an ellipsoid equation (8,14).
Statistical Analyses
Continuous variables were expressed as means and SDs, non-normally distributed variables were expressed as medians and interquartile ranges, and categorical variables were expressed as percentages and 95% confidence intervals (95% CIs). To compare TKV measurements, box and whisker and Bland–Altman plots, normality tests, and Friedman tests with post hoc analyses comparing mean ranks were generated and calculated using GraphPad Prism v.8.4.3 (San Diego, CA). From the Bland–Altman plots, biases were calculated as the mean percentage differences from zero; a bias of zero indicated no difference in the mean value of two measurement methods. All tests were two sided, and a P value of 0.05 was considered statistically significant. We tested the normality of our data using the Anderson–Darling, D’Agostino and Pearson, Shapiro–Wilk, and Kolmogorov–Smirnov tests, and we classified the data as normally distributed if three or more of the tests were passed (α value set at 0.05). Intraclass correlation from a two-way mixed effect model (to control for multiple raters) was used to evaluate the agreement of pairwise comparisons of TKV measurements between the different methods (IBM SPSS Statistics v.27, Armonk, NY); intraclass correlation values <0.5, 0.5–0.7, 0.75–0.9, and >0.9 were indicative of poor, moderate, good, and excellent agreeability, respectively (23). To investigate TKV measurement variation in ultrasound methods introduced by ultrasound technologists, a linear mixed model was used in R statistical software (v3.6.2; R Foundation for Statistical Computing, Vienna, Austria). Technologists were considered as random effects, and age, sex, body mass index, TKV (by MRI manual segmentation), and eGFR were considered as fixed effects to adjust for potential confounding. The likelihood ratio test with boundary correction was used to test if the “technologist effect” was statistically significant (24). Height-adjusted TKV and age were used to generate the MCIC using an online calculator (https://www.mayo.edu/research/documents/pkd-center-adpkd-classification/doc-20094754). The accuracy of MCIC classification by each imaging method was determined as the number of correctly assigned MCICs (on the basis of MRI manual segmentation) divided by the total patient number (n=142).
Results
Patient Characteristics
The clinical characteristics of our study cohort are shown in Table 1. The mean (SD) age of the patients was 44 (15) years, with a slight predominance of women (1.1: 1), and the mean (SD) eGFR was 79 (26) ml/min per 1.73 m2. Their median height-adjusted TKV was 506 (interquartile range, 336–818) ml/m. Their MCIC as determined by MRI manual segmentation covered a full range of disease severity (i.e., 1A–1E) with approximately two thirds of the cases assigned to the prognostic borderline zone between the “low-risk” class 1B (n=52; 37%) and the “high-risk” class 1C (n=40; 28%).
Table 1.
Clinical characteristics of study cohort
| Study Patients, n=142 | Value |
|---|---|
| Age, yr | 44±15 |
| Sex, men:women | 1:1.1 |
| Height, m | 1.7±0.1 |
| BMI, kg/m2 | 26±5 |
| eGFR, ml/min per 1.73 m2a | 79±26 |
| TKV, ml | 877 [559–1424] |
| Ht-TKV, ml/m | 506 [336–818] |
| Mayo Clinic imaging class | |
| 1A | 23 (16%) |
| 1B | 52 (37%) |
| 1C | 40 (28%) |
| 1D | 21 (15%) |
| 1E | 6 (4%) |
TKV and Ht-TKV are presented as median [interquartile range], all of the other variables are presented as mean ± SD or n (percentage). BMI, body mass index; TKV, total kidney volume; Ht-TKV, height-adjusted total kidney volume.
Value reported for 140 patients.
Comparison of Total Kidney Volume Measurements by Different Imaging Methods
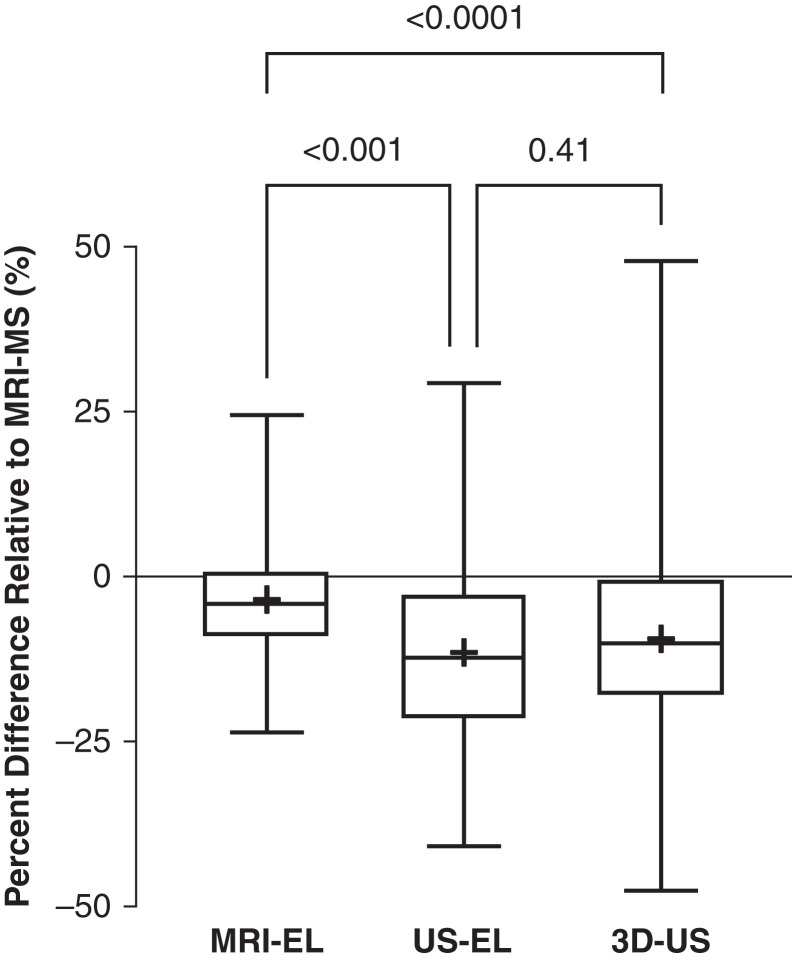
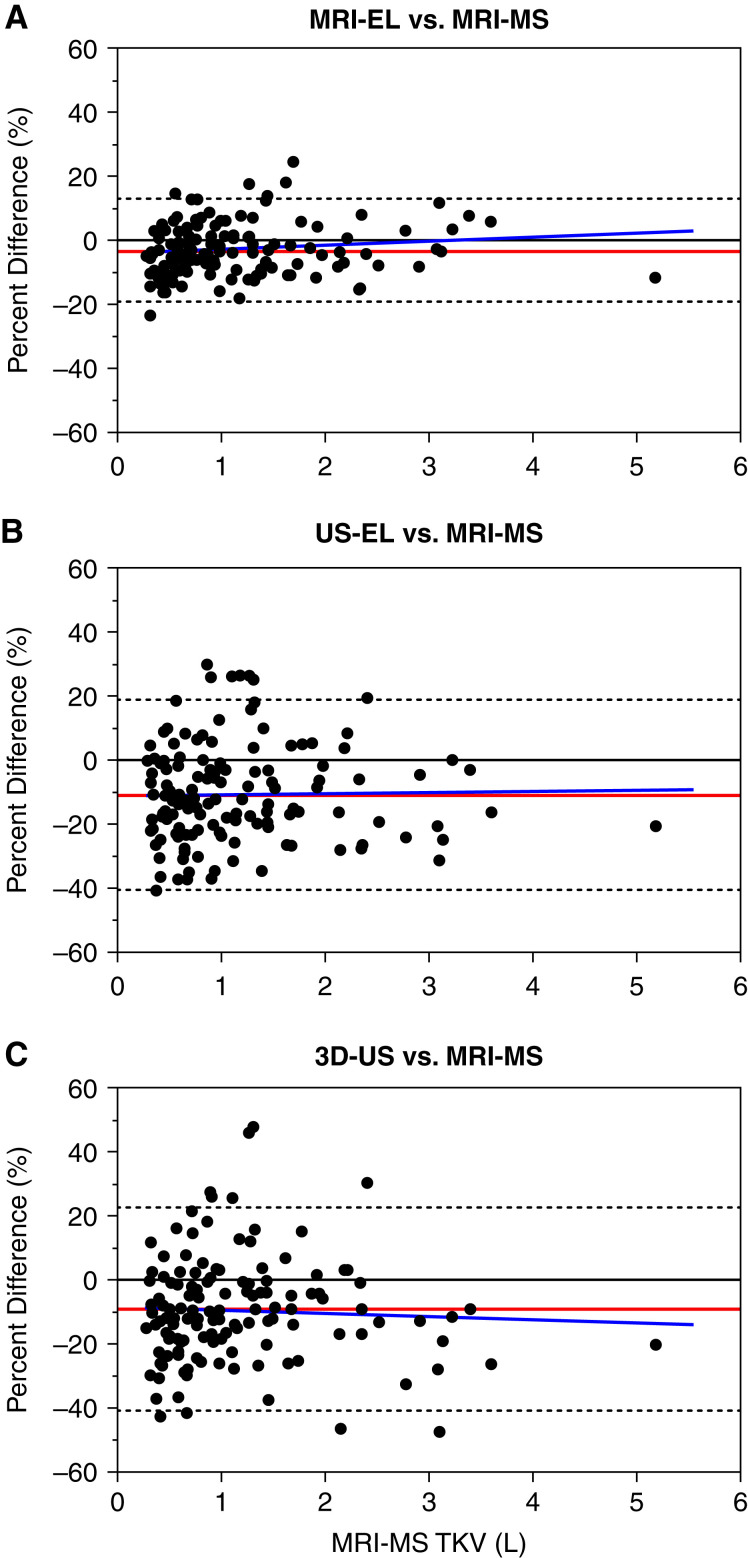
Compared with MRI manual segmentation (reference standard), MRI ellipsoid, ultrasound ellipsoid, and 3D ultrasound all displayed a systematic bias for underestimating TKV (mean within-patient percentage difference: −3.2, −9.1, and −11.0, respectively) (Figure 2, Table 2). This bias (mean ± SD) was more pronounced for ultrasound ellipsoid (−11±15.2) and 3D ultrasound (−9.1±16.2) than MRI ellipsoid (−3.2±8.2; P<0.001), but it was not significantly different between the two ultrasound methods (P=0.41); the mean and median values of these three methods were similar (Figure 2). Both ultrasound ellipsoid and 3D ultrasound also displayed more variability in TKV measurements compared with MRI ellipsoid (SDs of 15%, 16%, and 8%, respectively) but no difference between themselves (Figure 3, Table 2). Analyses of within-patient TKV percentage difference as a function of TKV indicated that bias for all methods is relatively constant throughout all measured TKVs, as the mean slopes were not statistically different from zero (MRI ellipsoid: 1.41; 95% CI, −0.28 to 3.09; ultrasound ellipsoid: 0.33; 95% CI, −2.82 to 3.49; 3D ultrasound: −1.08; 95% CI, −4.43 to 2.28; they were also not statistically different from one another [P=0.47]). Intraclass correlation coefficients of TKV measurement between all six possible pairwise comparisons of four different imaging methods ranged from 0.93 (MRI manual segmentation versus 3D ultrasound) to 0.99 (MRI manual segmentation versus MRI ellipsoid), indicating excellent agreement between these methods (Supplemental Table 1).
Figure 2.
Systematic bias for underestimating total kidney volume by magnetic resonance imaging ellipsoid (MRI-EL), ultrasound ellipsoid (US-EL), and 3D-US compared with magnetic resonance imaging manual segmentation (MRI-MS). This bias is more pronounced for 3D-US and US-EL compared with MRI-EL. Pairwise comparisons of within-patient total kidney volume differences by different imaging methods are expressed as medians, interquartile ranges, and ranges.
Table 2.
Systematic bias of three different imaging methods of total kidney volume measurements in comparison with magnetic resonance imaging manual segmentation (reference standard)
| Total Kidney Volume Method | Bias (%) | SD of Bias (%) | 95% Limits of Agreement (%) |
|---|---|---|---|
| MRI ellipsoid | −3.2 | 8.2 | −19.2–12.8 |
| Ultrasound ellipsoid | −11.0 | 15.2 | −40.8–18.8 |
| 3D ultrasound | −9.1 | 16.2 | −40.9–23.6 |
MRI, magnetic resonance imaging; 3D, three-dimensional.
Figure 3.
Bland–Altman plots showing within-patient differences of total kidney volume (TKV) by (A) MRI-EL, (B) US-EL, and (C) 3D-US in comparison with MRI-MS (reference standard). The red line represents the mean percentage difference (bias). The gray dotted lines are the 95% limits of agreement. The blue line is the slope of the bias.
Effect of Total Kidney Volume Measurement Errors on Mayo Clinic Imaging Classification
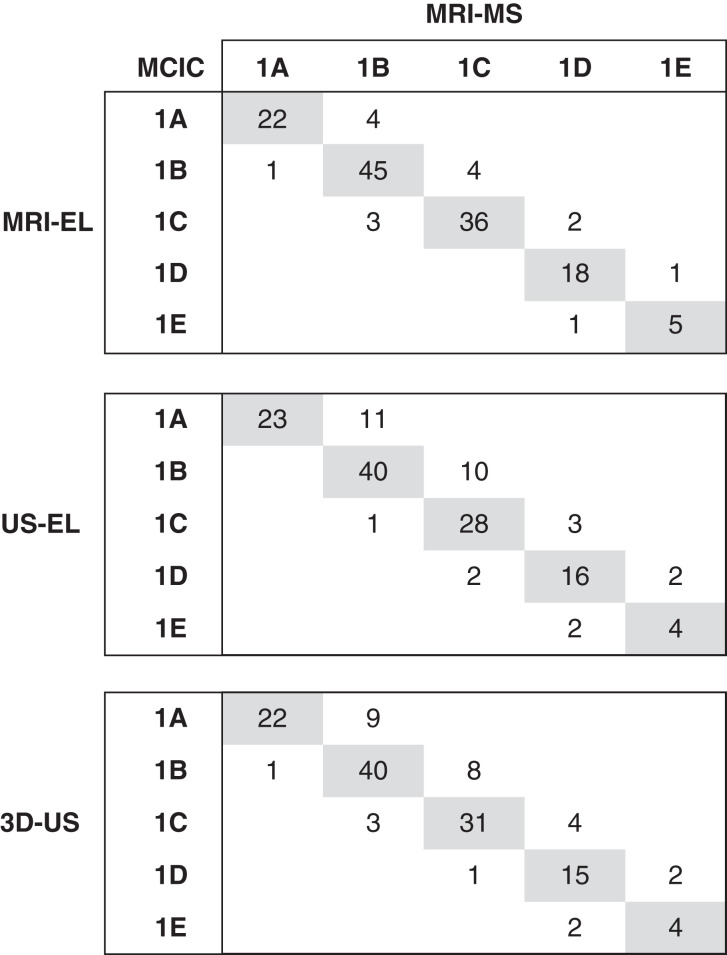
We next assessed how TKV measurement errors might affect the correct assignment of MCIC by comparing each method with MRI manual segmentation as the reference standard. We found that of the 142 study patients, MRI ellipsoid misclassified 16 patients, ultrasound ellipsoid misclassified 31, and 3D ultrasound misclassified 30, yielding accuracies of 89%, 78%, and 79%, respectively (Figure 4). For all three methods, there was no misclassification spanning more than one class. Furthermore, most misclassified cases by MRI ellipsoid (11 of 16), ultrasound ellipsoid (26 of 31), and 3D ultrasound (23 of 30) were placed into a lower MCIC. Currently, patients with MCIC 1A and 1B are considered as low risk, whereas those with MCIC 1C–1E are considered as high risk for progression to advanced kidney failure. We found that predictions of the high-risk MCIC (i.e., 1C–1E) by MRI ellipsoid, 3D ultrasound, and ultrasound ellipsoid all yielded high positive predictive value (96%, 95%, and 98%, respectively) and specificity (96%, 96%, and 99%, respectively). However, both negative predictive value (90%, 88%, and 95%, respectively) and sensitivity (88%, 85%, and 94%, respectively) were lower for 3D ultrasound and ultrasound ellipsoid compared with MRI ellipsoid, although with overlapping 95% CIs (Table 3). We found that eight of 40 (20%) and ten of 40 (25%) of the cases with MCIC 1C by MRI manual segmentation were misclassified as MCIC 1B by 3D ultrasound and ultrasound ellipsoid. By contrast, three of 52 (6%) and one of 52 (2%) of the cases with MCIC 1B by MRI manual segmentation were misclassified as MCIC 1C by 3D ultrasound and ultrasound ellipsoid.
Figure 4.
Misassignment of Mayo Clinic imaging classes (MCICs) by MRI-EL, US-EL, and 3D-US in comparison with MRI-MS (reference standard). The shaded boxes denote the number of patients who were correctly assigned by the three different TKV measurement methods compared with MRI-MS. The number of patients misassigned to a specific MCIC are shown outside the shaded boxes.
Table 3.
Performance of prediction for the high-risk Mayo Clinic imaging classes (1C–1E) by three different imaging methods in comparison with magnetic resonance imaging manual segmentation (reference standard)
| Method | Sensitivity, % (95% Confidence Interval) | Specificity, % (95% Confidence Interval) | PPV, % (95% Confidence Interval) | NPV, % (95% Confidence Interval) | Accuracy, % (95% Confidence Interval) |
|---|---|---|---|---|---|
| MRI ellipsoid | 94 (85 to 98) | 96 (89 to 99) | 96 (87 to 99) | 95 (87 to 98) | 95 (90 to 98) |
| Ultrasound ellipsoid | 85 (74 to 93) | 99 (93 to 100) | 98 (89 to 100) | 88 (81 to 93) | 92 (87 to 96) |
| 3D ultrasound | 88 (78 to 95) | 96 (89 to 99) | 95 (87 to 98) | 90 (82 to 95) | 92 (87 to 961) |
PPV, positive predictive value; NPV, negative predictive value; MRI, magnetic resonance imaging; 3D, three-dimensional.
Total Kidney Volume Measurement Variability by Ultrasound Technologists
Whereas a single radiologist performed MRI manual segmentation and MRI ellipsoid, five ultrasound technologists were involved in measuring ultrasound ellipsoid and 3D ultrasound (48, 33, 28, 24, and nine patients); the patient distribution per technologist was solely on the basis of the schedules of the technologists. The study patient characteristics by each technologist were not all uniform as evidenced by differences in their mean age, sex, body mass index, TKV, and eGFR (Supplemental Table 2). Using a linear mixed model to adjust for age, sex, body mass index, TKV, and eGFR of the study patients, we found that the ultrasound technologists were a significant source of variation in TKV measurements by ultrasound, accounting for 15% and 27% of the overall variation for ultrasound ellipsoid and 3D ultrasound, respectively (P<0.001 for both).
Predicting Mayo Clinic Imaging Class by Average Kidney Length
Using baseline data from the CRISP cohort, an average kidney length of >16.5 cm by ultrasound was found to be highly predictive of CKD progression in ADPKD in patients aged ≤45 years (25). We examined the performance of this ultrasound kidney length cutoff in predicting the high-risk MCIC 1C–1E and found a positive predictive value of 100% and a sensitivity of 25% in our patients aged <45 years (n=79). However, this same cutoff yielded a positive predictive value of 85% and a sensitivity of 51% when applied to the complete study cohort (n=142), which included older patients (Table 4).
Table 4.
Prediction of Mayo Clinic imaging classes 1C–1E by ultrasound average kidney length >16.5 cm
PPV, positive predictive value; NPV, negative predictive value.
Denotes patient number from the entire study cohort.
Denotes patient number from a subgroup of the study cohort ≤45 years of age.
Discussion
Currently, MCIC by MRI ellipsoid is widely used in North America for clinical risk assessment in ADPKD (8–10). However, MRI may not be readily available and is conservatively at least twice as expensive as that of ultrasound in Canada (scan and radiologist fees on the basis of 2022 Medicare physician fee schedules and Ontario interprovincial billing rates are CAN $480 and $1020, respectively). By contrast, although less expensive and widely available, ultrasound-derived TKV is widely regarded as less accurate than MRI ellipsoid, as confirmed by this study. Compared with MRI manual segmentation (reference standard), we found that MRI ellipsoid, ultrasound ellipsoid, and 3D ultrasound all displayed a systematic bias for underestimating TKV (average biases of −3%, −11%, and −9%, respectively); however, this bias was more pronounced for both ultrasound methods, which also displayed more variability than MRI ellipsoid. Underestimation of TKV by ultrasound methods has been previously reported (19,26–29). Our finding agrees with a recent study that found an underestimation of kidney volume by both two-dimensional ultrasound and 3D ultrasound compared with MRI in 30 pediatric patients with ADPKD (19). We speculate that some of the benefits of 3D ultrasound may be offset by its limited range of volumetric acquisition for very large kidneys; however, substantial bias is evident even for smaller kidney volumes (Figure 3C). Ultrasound is known to be susceptible to inter-rater variability (29). In this study, we found that the ultrasound technologists are an important source that may contribute to 15% and 27% TKV measurement variability by ultrasound ellipsoid and 3D ultrasound, respectively.
We next assessed how TKV measurement errors might affect the assignment of MCIC and clinical decision making. We found accuracies for correct MCIC assignment of 88%, 78%, and 79% by MRI ellipsoid, ultrasound ellipsoid, and 3D ultrasound, respectively (Figure 4). There was no misclassification spanning more than one class for all three methods, and most misclassified cases by 3D ultrasound (77%) and ultrasound ellipsoid (84%) were placed into a lower MCIC. Currently, patients with MCIC 1A and 1B are considered low risk for advanced kidney failure, and they require only routine care to optimize their body mass index, BP, and metabolic profile and maintain a high water intake for urinary dilution. By contrast, patients with the high-risk MCIC 1C–1E are generally recommended for tolvaptan treatment or participation in clinical trials (8–10). On the basis of these two major risk categories, only misassignment of MCIC 1B or 1C due to TKV measurement errors affects clinical decision making. We found that predictions of the MCIC 1C–1E categories by MRI ellipsoid, 3D ultrasound, and ultrasound ellipsoid all yielded very high positive predictive values (96%, 95%, and 98%, respectively). Because of the TKV underestimation bias, the two ultrasound methods are highly unlikely to make false-positive assignments of MCIC 1C–1E, thereby allowing them to perform as well as MRI ellipsoid to rule in (i.e., positive predictive values ≥95%) high-risk cases. However, they were not as good as MRI ellipsoid (i.e., negative predictive values approximately 5% lower) to rule out low-risk cases, and the assignment of MCIC 1B by both ultrasound methods may not be reliable. This is evident by the findings that 20%–25% of the cases with MCIC 1C by MRI manual segmentation were misclassified as MCIC 1B but that only 2%–6% of the cases with MCIC 1B by MRI manual segmentation were misclassified as MCIC 1C by the ultrasound methods (Figure 4). Taken together, ultrasound ellipsoid, which is simpler to use and more readily available than 3D ultrasound, may be a reasonable tool for TKV-based risk assessment in ADPKD, provided that all cases classified as MCIC 1B are further validated by MRI ellipsoid or MRI manual segmentation. Consistent with the report from the CRISP cohort, we also found that an average ultrasound kidney length >16.5 cm predicted MCIC 1C–1E with high certainty in patients aged ≤45 years but not in older patients. This is not a surprise as the prognostic value of the average kidney length, like TKV, is age dependent.
There are limitations to our study. First, our study focused on TKV measurement errors by different methods in patients with typical imaging patterns and did not include any cases with atypical patterns (MCIC 2). Should ultrasound be used for TKV-based risk assessment in the clinical setting, it would be important to exclude patients with atypical imaging patterns (i.e., unilateral, segmental, asymmetric, or lopsided pattern or bilateral cysts with unilateral or bilateral kidney atrophy) who have generally an excellent kidney function prognosis and may account for approximately 9% of cases suspected to have ADPKD (8). However, specific ultrasound criteria used to recognize these atypical polycystic kidney disease patterns have not been developed, and thus, the means to categorize an undifferentiated patient with suspected polycystic kidney disease as typical versus atypical via ultrasound is not addressed. Second, the findings of our study are limited to patients with mostly mild to moderately severe ADPKD. However, misassignment of MCIC 1B as 1C or vice versa carries the most serious clinical consequence, whereas misassignment within the high-risk category (MCIC 1C–1E) would not alter clinical management. With >60% of our patients in MCIC 1B and 1C, our study should provide a robust assessment of this important prognostic zone. Third, we did not assess intra- and interobserver variability of MRI raters and intraobserver variability of ultrasound raters. However, excellent inter-rater agreement (i.e., reliability coefficients of 0.994) had been reported for MRI-based whole-kidney volume measurement in CRISP (14).
In conclusion, TKV measurements in ADPKD by 3D ultrasound and ultrasound ellipsoid displayed similar bias and variability to one another, and they are both less accurate than MRI ellipsoid. Prediction of high-risk MCIC (1C–1E) by all three methods provides high positive predictive value, but ultrasound ellipsoid is simpler to use and more readily available. Because of a systematic bias in underestimating TKV, the classification of MCIC 1B by the ultrasound methods may not be reliable and should be confirmed by MRI ellipsoid or manual segmentation. Additionally, ultrasound-specific criteria need to be developed for the recognition and exclusion of patients with atypical polycystic kidney disease imaging patterns (MCIC 2). TKV-based risk assessment strategy using ultrasound ellipsoid may provide an alternative to MRI ellipsoid to reduce the need for MRI, thereby saving costs and resources.
Disclosures
K. Khalili reports research funding from MedoAI. Y. Pei reports consultancy agreements with Maze Therapeutics, Otsuka, Reata, and Sanofi; reports honoraria from Maze Therapeutics, Otsuka, and Sanofi; received compensation for participation in advisory boards from Maze Therapeutics, Otsuka, Reata Pharmaceuticals, and Sanofi-Genzyme; and serves in an advisory role for Maze Therapeutics, Otsuka, Reata, and Sanofi. All remaining authors have nothing to disclose.
Funding
This work was supported in part by Canadian Institutes of Health Research grants PJT-376307 (to Y. Pei) and CAN-Solve-CKD SCA-145103 (to Y. Pei).
Supplementary Material
Acknowledgments
The authors thank all of the study patients as well as the research staff of the Center for Innovative Management of Polycystic Kidney Disease at the Toronto General Hospital.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
M. Atri, K. Khalili, and Y. Pei conceptualized the study; P. Akbari, S.X. Deng, S. Khowaja, S.H. Lee, F. Nasri, and Y. Pei were responsible for data curation; M. Atri, K. Khalili, and Y. Pei were responsible for investigation; P. Akbari, H. Lu, Y. Pei, and W. Warnica were responsible for formal analysis; M. Atri, K. Khalili, A. Rattansingh, and Y. Pei were responsible for methodology; P. Akbari, S.X. Deng, S.H. Lee, and Y. Pei were responsible for project administration; Y. Pei was responsible for resources; P. Akbari and Y. Pei were responsible for visualization; Y. Pei was responsible for funding acquisition; M. Atri, K. Khalili, and Y. Pei provided supervision; P. Akbari and Y. Pei wrote the original draft; and P. Akbari, M. Atri, S.X. Deng, K. Khalili, F. Nasri, Y. Pei, and W. Warnica reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.14931121/-/DCSupplemental.
Supplemental Material. Details of ultrasound kidney imaging.
Supplemental Table 1. Intraclass correlations of TKV measurements between six possible pairwise comparisons of four different imaging methods.
Supplemental Table 2. Characteristics of patient subgroups scanned by different ultrasound technologists.
References
- 1.Lanktree MB, Haghighi A, Guiard E, Iliuta I-A, Song X, Harris PC, Paterson AD, Pei Y: Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 29: 2593–2600, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Lanktree MB, Haghighi A, di Bari I, Song X, Pei Y: Insights into autosomal dominant polycystic kidney disease from genetic studies. Clin J Am Soc Nephrol 16: 790–799, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grantham JJ, Mulamalla S, Swenson-Fields KI: Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7: 556–566, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr., Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP; CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Yu ASL, Shen C, Landsittel DP, Grantham JJ, Cook LT, Torres VE, Chapman AB, Bae KT, Mrug M, Harris PC, Rahbari-Oskoui FF, Shi T, Bennett WM; Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) : Long-term trajectory of kidney function in autosomal-dominant polycystic kidney disease. Kidney Int 95: 1253–1261, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrone RD, Mouksassi MS, Romero K, Czerwiec FS, Chapman AB, Gitomer BY, Torres VE, Miskulin DC, Broadbent S, Marier JF: Total kidney volume Is a prognostic biomarker of renal function decline and progression to end-stage renal disease in patients with autosomal dominant polycystic kidney disease. Kidney Int Rep 2: 442–450, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irazabal MV, Abebe KZ, Bae KT, Perrone RD, Chapman AB, Schrier RW, Yu AS, Braun WE, Steinman TI, Harris PC, Flessner MF, Torres VE; HALT Investigators : Prognostic enrichment design in clinical trials for autosomal dominant polycystic kidney disease: The HALT-PKD clinical trial. Nephrol Dial Transplant 32: 1857–1865, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE: A practical guide for treatment of rapidly progressive ADPKD with tolvaptan. J Am Soc Nephrol 29: 2458–2470, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soroka S, Alam A, Bevilacqua M, Girard LP, Komenda P, Loertscher R, McFarlane P, Pandeya S, Tam P, Bichet DG: Assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease: A Canadian expert consensus. Can J Kidney Health Dis 4: 2054358117695784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tangri N, Hougen I, Alam A, Perrone R, McFarlane P, Pei Y: Total kidney volume as a biomarker of disease progression in autosomal dominant polycystic kidney disease. Can J Kidney Health Dis 4: 2054358117693355, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevilacqua MU, Hague CJ, Romann A, Sheitt H, Vasilescu DM, Yi TW, Levin A: CT of kidney volume in autosomal dominant polycystic kidney disease: Accuracy, reproducibility, and radiation dose. Radiology 291: 660–667, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Shi B, Akbari P, Pourafkari M, Iliuta IA, Guiard E, Quist CF, Song X, Hillier D, Khalili K, Pei Y: Prognostic performance of kidney volume measurement for polycystic kidney disease: A comparative study of ellipsoid vs. manual segmentation. Sci Rep 9: 10996, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alam A, Dahl NK, Lipschutz JH, Rossetti S, Smith P, Sapir D, Weinstein J, McFarlane P, Bichet DG: Total kidney volume in autosomal dominant polycystic kidney disease: A biomarker of disease progression and therapeutic efficacy. Am J Kidney Dis 66: 564–576, 2015 [DOI] [PubMed] [Google Scholar]
- 16.O’Neill WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, Torres VE, King BF, Wetzel LH, Thompson PA, Miller JP: Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: The Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis 46: 1058–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Brancaforte A, Serantoni S, Silva Barbosa F, Di Leo G, Sardanelli F, Cornalba GP: Renal volume assessment with 3D ultrasound. Radiol Med (Torino) 116: 1095–1104, 2011 [DOI] [PubMed] [Google Scholar]
- 18.de Amorim Paiva CC, de Mello Junior CF, Guimarães Filho HA, de Brito Gomes CA, Silva Junior LR, Junior GMB, Paiva CSM: Reproducibility of renal volume measurement in adults using 3-dimensional sonography. J Ultrasound Med 33: 431–435, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Breysem L, De Rechter S, De Keyzer F, Smet MH, Bammens B, Van Dyck M, Hofmans M, Oyen R, Levtchenko E, Mekahli D: 3DUS as an alternative to MRI for measuring renal volume in children with autosomal dominant polycystic kidney disease. Pediatr Nephrol 33: 827–835, 2018 [DOI] [PubMed] [Google Scholar]
- 20.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei Y, Hwang YH, Conklin J, Sundsbak JL, Heyer CM, Chan W, Wang K, He N, Rattansingh A, Atri M, Harris PC, Haider MA: Imaging-based diagnosis of autosomal dominant polycystic kidney disease. J Am Soc Nephrol 26: 746–753, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koo TK, Li MY: A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15: 155–163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Self SG, Liang K-Y: Asymptotic properties of maximum likelihood estimators and likelihood ratio tests under nonstandard conditions. J Am Stat Assoc 82: 605–610, 1987 [Google Scholar]
- 25.Bhutani H, Smith V, Rahbari-Oskoui F, Mittal A, Grantham JJ, Torres VE, Mrug M, Bae KT, Wu Z, Ge Y, Landslittel D, Gibbs P, O’Neill WC, Chapman AB; CRISP Investigators : A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 88: 146–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, Beek FJ: Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Turco D, Busutti M, Mignani R, Magistroni R, Corsi C: Comparison of total kidney volume quantification methods in autosomal dominant polycystic disease for a comprehensive disease assessment. Am J Nephrol 45: 373–379, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Bakker J, Olree M, Kaatee R, de Lange EE, Beek FJ: In vitro measurement of kidney size: Comparison of ultrasonography and MRI. Ultrasound Med Biol 24: 683–688, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Emamian SA, Nielsen MB, Pedersen JF: Intraobserver and interobserver variations in sonographic measurements of kidney size in adult volunteers. A comparison of linear measurements and volumetric estimates. Acta Radiol 36: 399–401, 1995 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.