The endothelin system has been widely implicated in CKD. Binding of endothelin-1, the effector peptide of the endothelin system, to the endothelin-A receptor causes endothelial glycocalyx damage, glomerulosclerosis, and podocyte injury and activates proinflammatory and profibrotic pathways (1). Clinical studies demonstrated that endothelin receptor antagonists (ERAs) reduce albuminuria in patients with CKD, suggesting long-term clinical benefit. However, ERAs may also cause sodium retention and edema mediated via endothelin-B receptors, which may increase the risk of heart failure in high-risk patients (2,3). These studies also demonstrated that the degree of albuminuria reduction and sodium retention in response to ERAs vary between and within patients, suggesting that it is possible to identify patients with maximal potential benefit (albuminuria reduction) and minimal risk of known adverse events (sodium retention).
On the basis of strong antialbuminuric effects of the ERA atrasentan in a phase 2 study, the SONAR trial was designed to assess the long-term benefit of atrasentan on the risk of kidney failure (4). The trial design included a 6-week open label treatment period before patients were randomized, termed the enrichment period. The enrichment period was included to select patients with a large reduction (≥30%) in urinary albumin-creatinine ratio (UACR) without signs of sodium retention. The trial recruited 2648 of such responder patients. This cohort constituted the primary population to study the long-term efficacy and safety of atrasentan. However, because it was unknown if atrasentan could also benefit the nonselected patients, 1020 “nonresponders,” defined as patients with no signs of sodium retention and UACR reduction <30% during enrichment, were randomized in a separate stratum.
During the conduct of the SONAR trial, it became apparent that the rate for the primary outcome (doubling of serum creatinine, kidney failure, or death due to kidney failure) was much lower than originally anticipated. This meant that it would take much longer to complete the trial. The sponsor of the trial, therefore, decided to stop the trial after a median follow-up of 2.2 years. When the closeout visits were completed, the results showed that atrasentan significantly decreased the risk of the primary outcome compared with placebo by 35% (hazard ratio, 0.65; 95% confidence interval, 0.49 to 0.88; P=0.005) (4).
Because the SONAR trial demonstrated for the first time the long-term efficacy of an ERA for the treatment of CKD and because the trial used a design novel to diabetic kidney disease, several prespecified and post hoc analyses have been conducted to inform future trial designs in nephrology and understand the role of ERAs in kidney disease (5–7).
The early termination of the trial and the low number of end points have raised questions about the credibility of the trial results. Indeed, 184 of the 425 predefined primary outcomes had occurred at the completion of the trial, and one could thus question whether the trial results are robust enough to draw reliable conclusions. To determine the robustness of a trial result, the fragility index has been proposed. It determines the number of events that have to be added to the intervention group to reverse the P value from ≤0.05 to >0.05. Such a calculation was made for the SONAR trial by Walsh (8) and suggested that only one event had to be added to the atrasentan group to slip the observed P value of 0.005 (not 0.05!) to >0.05. This would indicate that it is difficult to draw firm conclusions on the trial. However, before we dismiss the SONAR results, it is important to consider some aspects of the fragility index in more detail. The fragility index is on the basis of reported trial results and does not use the individual patient data. It compares the numbers of events in the intervention and control group but does not take into account the time to the event of interest. It is therefore inappropriate to use the index in trials where the outcome depends on survival time, such as the time to kidney failure (9). We have recalculated the fragility index using individual patient data and the prespecified Cox proportional hazards model from SONAR taking into account the time to event. These analyses demonstrated that only when 11 events were added to the atrasentan group, the results of the trial became nonsignificant with a P value of >0.05. This fragility index is thus much larger than the previously reported index of one. Although there is no consensus about a clinically relevant fragility index threshold, a fragility index of 11 is also higher than the median fragility index of 399 high-impact clinical trials, which has been reported to be eight (9). This indicates that the results of SONAR are robust and unlikely a chance finding.
A key design aspect of the SONAR trial was the enrichment period and the enrollment of patients with and without a 30% reduction in albuminuria. Although the trial was not powered to detect a treatment effect in nonresponders, the observed hazard ratio of 0.75 (95% confidence interval, 0.55 to 1.03) in this group suggests that the benefit of atrasentan would be independent of the degree of albuminuria lowering during the 6-week enrichment period. This finding was unexpected and led to further analyses. These analyses demonstrated that within the atrasentan group, larger reductions in albuminuria were statistically significantly associated with a lower risk of kidney outcomes (7). However, in patients who were assigned to placebo treatment, the early reduction in albuminuria during the enrichment period was also associated with a lower risk of the kidney outcome. This was not expected since we had expected that the reduction in albuminuria during 6 weeks of atrasentan treatment would not predict kidney outcomes during the subsequent years when patients were treated with placebo. Nevertheless, because of this effect, the effect of atrasentan compared with placebo on kidney outcomes was similar in responders and nonresponders. The important question to answer is why the albuminuria change during enrichment predicted kidney outcomes in the placebo group. It appears that the albuminuria levels after transitioning from atrasentan to placebo at the randomization visit did not return to pre-enrichment values. As a result, the difference in albuminuria between atrasentan and placebo after randomization was similar in responders and nonresponders. We do not know why albuminuria did not return to baseline after randomization, but legacy effects, variability in albuminuria measurements, and the introduction of concomitant medications, as well as improved adherence to the standard of care treatment, may have contributed. Importantly, these results do not dismiss albuminuria as a valid surrogate because during double-blind treatment in both responders and nonresponders, the UACR levels and risk of kidney outcomes were lower in the atrasentan group compared with placebo group (Figure 1). However, they underscore careful consideration of trial design when using UACR as a response enrichment criterion in future trials.
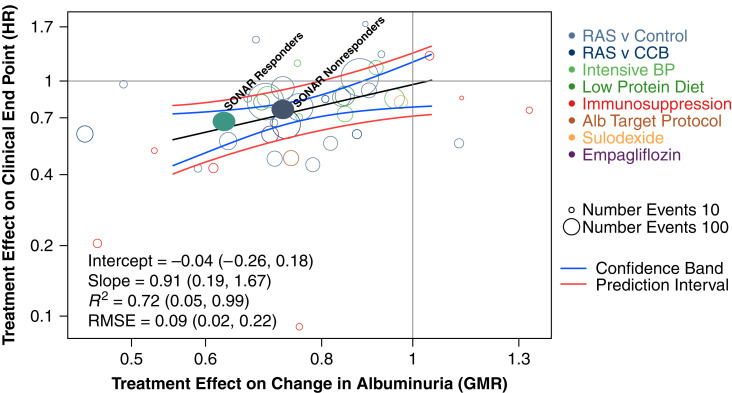
Figure 1.
The treatment effect of atrasentan compared with placebo on the early change in albuminuria is associated with the treatment effect on the clinical kidney end point. The figure shows the results of the trial-level analysis for the association between the effects of atrasentan compared with placebo on albuminuria and the effects of atrasentan compared with placebo on the clinical end point for urinary albumin-creatinine ratio (UACR) responders and nonresponders using a previously published meta-analysis of clinical trials among participants who had baseline UACR of >30 mg/g. The data from UACR responders and nonresponders are within the 95% Bayesian confidence and prediction bands, indicating that the data from the SONAR trial support albuminuria as a potential surrogate for clinical kidney outcomes. The vertical axis indicates the estimated treatment effect on a clinical end point (hazard ratio [HR]), and the horizontal axis is the estimated treatment effect on the change in UACR (geometric mean ratio [GMR] of the log-transformed albumin-creatinine ratio). The clinical end point was defined as a composite of kidney failure and doubling of serum creatinine. The different colored circles indicate intervention types; each circle is a separate clinical trial/intervention, with the size of each circle proportional to the number of events. The regression line through the studies and the Bayesian confidence and prediction bands are shown. The filled green circle represents the SONAR responder stratum, and the filled blue circle represents the SONAR nonresponder stratum. The sizes of the filled circles are not proportional to the size of the study relative to the other studies. The difference in UACR between atrasentan and placebo in UACR responders is 34% (95% confidence interval [95% CI], 29.1 to 38.2). The difference in UACR between atrasentan and placebo in UACR nonresponders is 27% (95% CI, 19.7 to 34.3). SONAR, Study of Diabetic Nephropathy with Atrasentan; RAS, renin-angiotensin system; CCB, calcium channel blocker; Alb, albumin; RMSE, root mean square error. Adapted from ref. 11, with permission.
Because previous trials demonstrated higher risk of edema and heart failure with ERA treatment, the enrichment period in SONAR also provided an opportunity to assess tolerability to atrasentan and exclude patients with signs of sodium retention in order to enhance the benefit-risk profile. During enrichment, 574 patients were excluded due to signs of sodium retention (body weight increase ≥3 kg or a BNP increase ≥300 pg/ml). Although comparisons between trials should be done with caution, the annual heart failure hospitalization rate with atrasentan in SONAR was markedly lower than the observed rate in a previous trial with the ERA avosentan (2% versus 18%) (10). This suggests that the enrichment period along with other mitigation strategies, including the use of diuretics and exclusion of patients with prior heart failure, seemed to work.
How does the future look like for ERAs? Various trials with ERAs are ongoing. These trials use different strategies to mitigate the risk of sodium retention and optimize the benefit-risk profile. A couple of trials enrolled patients at relatively low risk of heart failure, such as patients with CKD without diabetes. The efficacy and safety of atrasentan (ALIGN and AFFINITY) and sparsentan (PROTECT and DUPLEX) are being investigated in patients with IgA or FSGS nephropathy. It is noteworthy that in the PROTECT and DUPLEX trials, sparsentan significantly reduced albuminuria and was well tolerated compared with control treatment, supporting potential long-term beneficial effects in these populations. Another strategy used to mitigate the risk of heart failure is to combine an ERA with a sodium-glucose cotransporter 2 (SGLT2) inhibitor. SGLT2 inhibitors have natriuretic/diuretic effects, which may abrogate sodium retention and reduce the risk of heart failure in a similar way as thiazide or loop diuretics (10). The ZENITH trial is testing this hypothesis and randomized patients with CKD with and without type 2 diabetes to zibotentan in combination with the SGLT2 inhibitor dapagliflozin. Results of this trial are expected in 2023.
The SONAR trial has proven the efficacy of endothelin-1 blockade to reduce the risk of kidney failure in patients with type 2 diabetes and CKD. ERAs are a welcome addition to the pharmacologic armamentarium to further reduce the risk of kidney outcomes in patients already treated with RAAS and/or SGLT2 inhibitors. The degree of albuminuria lowering seems to be a valid surrogate to target ERAs, although long-term efficacy and safety data are needed from well-designed trials on the basis of the lessons from SONAR.
Disclosures
D. de Zeeuw served on advisory boards and/or was a speaker for Bayer, Boehringer Ingelheim, Fresenius, Mitsubishi-Tanabe, and Travere Pharmaceuticals; served on steering committees and/or was a speaker for AbbVie and Janssen; and served on data safety and monitoring committees for Bayer. Honoraria was paid to the institution and consultant/speaker. D. de Zeeuw reports consultancy agreements with Abbvie, Bayer, Boehringer Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals and honoraria from Bayer, Boehringer Ingelheim, Fresenius, Janssen, Mitsubishi Tanabe, and Travere Pharmaceuticals. H.J.L. Heerspink has served as a consultant for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Eli-Lilly, Gilead, Goldfinch, Janssen R&D, Merck, Mitsubishi Tanabe, Mundi Pharma, Novo Nordisk, and Travere Pharmaceuticals. H.J.L. Heerspink reports ongoing consultancy agreements with AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Fresenius, Gilead, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, Novo Nordisk, and Travere Pharmaceuticals; research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, Janssen (for research support; grant funding directed to employer), and Novo Nordisk; and speakers bureau for AstraZeneca.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
H.J.L. Heerspink wrote the original draft and D. de Zeeuw reviewed and edited the manuscript.
References
- 1.Kohan DE, Barton M: Endothelin and endothelin antagonists in chronic kidney disease. Kidney Int 86: 896–904, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wenzel RR, Littke T, Kuranoff S, Jürgens C, Bruck H, Ritz E, Philipp T, Mitchell A; SPP301 (Avosentan) Endothelin Antagonist Evaluation in Diabetic Nephropathy Study Investigators : Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol 20: 655–664, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Zeeuw D, Coll B, Andress D, Brennan JJ, Tang H, Houser M, Correa-Rotter R, Kohan D, Lambers Heerspink HJ, Makino H, Perkovic V, Pritchett Y, Remuzzi G, Tobe SW, Toto R, Viberti G, Parving HH: The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 25: 1083–1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heerspink HJL, Parving H-H, Andress DL, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Melnick JZ, Miller MG, Pergola PE, Perkovic V, Tobe S, Yi T, Wigderson M, de Zeeuw D; SONAR Committees and Investigators : Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): A double-blind, randomised, placebo-controlled trial. Lancet 393: 1937–1947, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Waijer SW, de Vries ST, Busch R, Xie D, Gansevoort RT, Hou FF, Górriz JL, Laverman GD, De Nicola L, Pascual J, Provenzano M, Pergola PE, Tang SCW, Wanner C, Zaoui P, Parving HH, de Zeeuw D, Heerspink HJL: Large between-patient variability in egfr decline before clinical trial enrollment and impact on atrasentan’s efficacy: A post hoc analysis from the SONAR trial. J Am Soc Nephrol 32: 2731–2734, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waijer SW, Gansevoort RT, Bakris GL, Correa-Rotter R, Hou FF, Kohan DE, Kitzman DW, Makino H, McMurray JJV, Perkovic V, Tobe S, Parving HH, de Zeeuw D, Heerspink HJL: The effect of atrasentan on kidney and heart failure outcomes by baseline albuminuria and kidney function: A post hoc analysis of the SONAR randomized trial. Clin J Am Soc Nephrol 16: 1824–1832, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heerspink HJL, Xie D, Bakris G, Correa-Rotter R, Hou FF, Kitzman DW, Kohan D, Makino H, McMurray JJV, Perkovic V, Rossing P, Parving HH, de Zeeuw D; on behalf on the SONAR Investigators : Early response in albuminuria and long-term kidney protection during treatment with an endothelin receptor antagonist: A prespecified analysis from the SONAR trial. J Am Soc Nephrol 32: 2900–2911, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh M: SONAR: Do a new design and statistically significant results translate to reliability? Clin J Am Soc Nephrol 15: 889–891, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh M, Srinathan SK, McAuley DF, Mrkobrada M, Levine O, Ribic C, Molnar AO, Dattani ND, Burke A, Guyatt G, Thabane L, Walter SD, Pogue J, Devereaux PJ: The statistical significance of randomized controlled trial results is frequently fragile: A case for a Fragility Index. J Clin Epidemiol 67: 622–628, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Hoekman J, Lambers Heerspink HJ, Viberti G, Green D, Mann JF, de Zeeuw D: Predictors of congestive heart failure after treatment with an endothelin receptor antagonist. Clin J Am Soc Nephrol 9: 490–498, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heerspink HJL, Greene T, Tighiouart H, Gansevoort RT, Coresh J, Simon AJ, Chan TC, Hou FF, Lewis JB, Locatelli F, Praga M, Schena FP, Levey AS, Inker LA; Chronic Kidney Disease Epidemiology Collaboration : Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol 7: 128–139, 2019 [DOI] [PubMed] [Google Scholar]