Abstract
People with kidney failure can experience a range of symptoms that lead to suffering and poor quality of life. Available therapies are limited, and evidence for new treatment options is sparse, often resulting in incomplete relief of symptoms. There is growing interest in the potential for cannabinoids, including cannabidiol and tetrahydrocannabinol, to treat symptoms across a wide range of chronic diseases. As legal prohibitions are withdrawn or minimized in many jurisdictions, patients are increasingly able to access these agents. Cannabinoid receptors, CB1 and CB2, are widely expressed in the body, including within the nervous and immune systems, and exogenous cannabinoids can have anxiolytic, antiemetic, analgesic, and anti-inflammatory effects. Considering their known physiologic actions and successful studies in other patient populations, cannabinoids may be viewed as potential therapies for a variety of common symptoms affecting those with kidney failure, including pruritus, nausea, insomnia, chronic neuropathic pain, anorexia, and restless legs syndrome. In this review, we summarize the pharmacology and pharmacokinetics of cannabinoids, along with what is known about the use of cannabinoids for symptom relief in those with kidney disease, and the evidence available concerning their role in management of common symptoms. Presently, although these agents show varying efficacy with a reasonable safety profile in other patient populations, evidence-based prescribing of cannabinoids for people with symptomatic kidney failure is not possible. Given the symptom burden experienced by individuals with kidney failure, there is an urgent need to understand the tolerability and safety of these agents in this population, which must ultimately be followed by robust, randomized controlled trials to determine if they are effective for symptom relief.
Keywords: chronic kidney failure, pharmacokinetics, cannabinoids
Introduction
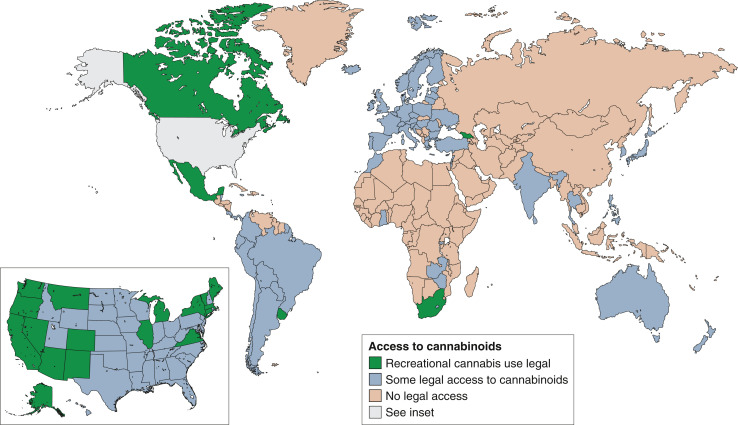
The hemp plant, Cannabis sativa, is the original source of the class of compounds known as cannabinoids. Although best known for its psychoactive properties, described by authors as diverse as Herodotus (1), Alexandre Dumas (2), and Cypress Hill (3), it has also been used therapeutically in various cultures for >2800 years (4). Now, with decriminalization or legalization spreading to many jurisdictions worldwide, there is growing access to cannabis and/or cannabinoids for recreational and medicinal use. Cannabis or cannabinoid-containing products are now potentially available to many patients in the Americas and Europe (although with important differences in permitted indications and formulations), and to some patients in Africa, Asia, and Oceania (Figure 1). Although current regulatory approved indications remain limited (Table 1), randomized studies suggest cannabinoids may improve chemotherapy-induced nausea and vomiting, reduce pain, and relieve spasticity (5). There is much interest in exploring the role of cannabinoids for a wider range of conditions and populations.
Figure 1.
Legal status of cannabis use and of cannabinoids worldwide (as of November 2021). Jurisdictions distinguished as (1) recreational cannabis use legal (green), (2) some legal access to cannabinoids (blue), and (3) no legal access (tan). Note that within category (2) there is wide variation in regulations, permitted indications, and ease of access. This category includes countries with well-established medicinal cannabis programs, but also countries where access to cannabinoids may be highly restricted and discretionary. Moreover, the status is unclear in many countries. For example, the Supreme Court of Mexico declared recreational use to be legal in June 2021, however, state and federal legislation has not yet changed to reflect this.
Table 1.
Cannabinoid products with approved indications
| Drug | Name | Indication | Date of First Approval |
|---|---|---|---|
| Nabilone | Cesamet | Refractory nausea and vomiting associated with cancer chemotherapy | 1985 |
| Dronabinol | Marinol, Syndros, | Anorexia associated with weight loss in patients with AIDS; refractory nausea and vomiting associated with cancer chemotherapy | 1985 |
| Cannabis extract (includes both THC and CBD) | Sativexa | Refractory spasticity due to multiple sclerosis | 2010 |
| CBD | Epidiolex | Seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, or tuberous sclerosis complex | 2018 |
These products have been approved for the indications listed in the United States and/or Canada, and by the European Medicines Agency. THC, tetrahydrocannabinol; CBD, cannabidiol.
Not approved by the Food and Drug Adminstration.
Kidney failure (a sustained reduction in eGFR <15 ml/min per 1.73 m2), whether treated with dialysis or conservatively, is associated with a poor quality of life and a significant and refractory symptom burden (6,7). Common symptoms include fatigue, chronic pain, pruritus, restless legs syndrome, nausea, anorexia, insomnia, and depression (7,8). These symptoms may emerge in individuals with less severe CKD, or where kidney failure has been treated with kidney transplantation, yet they are most common (and best described) in those with kidney failure. Unfortunately, such symptoms are often persistent despite current treatments, including initiating and optimizing dialysis (9). In addition to further development of clinical services, training, and resourcing dedicated to provide patients with holistic support, improved treatments for symptoms in patients with kidney failure are key priorities among both patients and clinicians (10). Recent reviews of treatments for pruritus (11), restless legs syndrome (12), sleep disorders (13), and depression (14,15) have consistently shown a range of small and heterogeneous randomized trials using a large array of interventions and a diverse set of comparators. Often these therapies are used off-label, have limited efficacy, or have substantial side effects (16,17).
A recent survey of 129 patients who are symptomatic and receiving dialysis at a Canadian dialysis unit found that only five had tried cannabis for symptom relief, including four who reported it to be efficacious for restless legs or pruritus, and 71% were interested in participating in a trial of cannabis products (18). Similarly, a survey of Canadian nephrologists revealed them to be broadly supportive of a trial of cannabinoids for refractory symptoms and of enrolling patients in clinical trials (19). This emphasizes the need for properly designed studies to determine the efficacy and safety of these agents in people with kidney failure. In this study, we review the pharmacology of cannabinoids and the evidence for their use in this patient population.
Overview of Cannabinoid Pharmacology
Cannabinoids are ligands of the two cannabinoid receptors (cannabinoid receptor 1 and 2; CB1 and CB2) and can be categorized as phytocannabinoids (derived from Cannabis sativa), endocannabinoids (endogenous ligands of CBs; e.g., anandamide), or synthetic cannabinoids (e.g., dronabinol and nabilone) (20,21). CB1 is expressed in both the central nervous system and peripherally, including the gastrointestinal tract, kidney, and skin. CB2 is predominantly expressed on cells of the immune system, including lymphocytes and other leukocytes. Tetrahydrocannabinol (THC) is the most well-known phytocannabinoid and is a partial agonist of CB1 and CB2. It has psychoactive and muscle-relaxing actions and may also have analgesic and antiemetic effects (Table 2) (22). The other primary phytocannabinoid, cannabidiol (CBD), acts as an inverse agonist at CB2 and is a noncompetitive antagonist of CB1. CBD is anticonvulsant, anti-inflammatory, and anxiolytic, but in contrast to THC, it is not psychoactive and has less analgesic, antiemetic, and abuse potential (20,23). Owing to the ability of CBD to inhibit the psychoactive effects of THC, a combination of THC and CBD may be used therapeutically. There is, however, no consensus as to whether THC or CBD dominant formulations, or some ratio of the two, should be preferred. The wide distribution of CB1 and CB2, and the complexity of cannabinoid pharmacology, makes rational selection of dosing ratios challenging.
Table 2.
Actions of tetrahydrocannabinol and cannabidiol
| Tetrahydrocannabinol | Cannabidiol |
|---|---|
| Partial agonist at CB1 and CB2 | Noncompetitive antagonist at CB1, inverse agonist at CB2 |
| Analgesic | Anticonvulsant |
| Muscle relaxation | Anti-inflammatory |
| Antiemetic | Anxiolytic |
| Appetite stimulation | Analgesia |
| Psychoactive | Neuroprotective |
| May inhibit psychoactive effects of THC |
Adapted from Davison & Davison, 2011 (22). CB, cannabinoid receptor.
Absorption, Metabolism, and Excretion.
The most common methods of administration of cannabinoids are inhalation (smoking or vaporization) and oral ingestion. In comparison with inhalation, oral ingestion of THC and/or CBD is slower, producing effects within 30–90 minutes, with peak concentrations at 2 hours, but the effects can last 4–12 hours (20). Due to first-pass metabolism, oral bioavailability is low (approximately 6% for THC and 9%–13% for CBD [24]); however, because both compounds are highly lipophilic, absorption is significantly improved when administered with fatty food (25). The metabolism of THC and CBD is primarily via the liver and intestine, with a minor contribution from other organs, including the heart, brain, and lungs (26). THC and CBD are both metabolized by the cytochrome P450 enzyme system, in particular, CYP3A4. THC is also metabolized by CYP2C9, whereas CBD is an inhibitor of 2C9, 2D6, 2B6, and 2C19 (20). CBD may also inhibit glucuronidation via uridine glucuronosyltransferases UGT1A9 and 2B7 (27). A variety of drug interactions have been reported or can be predicted with medications such as amitriptyline, warfarin, and calcineurin inhibitors (Table 3) (27). Both THC and CBD are primarily eliminated through the fecal route. Less than 35% of THC is eliminated via the urine, with low kidney excretion attributed to tubular reabsorption due to its lipophilic nature (12). A systematic review of CBD pharmacokinetics found substantial variation in t1/2, for example 1.4–10.9 hours for an oral mucosal spray, to 2–5 days after chronic oral administration (28). No studies included patients with kidney disease, and data on metabolites were sparse.
Table 3.
Important potential or reported drug interactions with tetrahydrocannabinol and cannabidiol
| Drugs Affected by Tetrahydrocannabinol and Cannabidiol | Drugs Affecting Tetrahydrocannabinol and Cannabidiol Exposure | ||||
|---|---|---|---|---|---|
| THC interacts with warfarin via an unknown mechanism | CBD inhibits CYP 2D6, 2B6, and 2C19, and UGT 1A9 and 2B7 | Both THC and CBD are metabolized by CYP 3A4. CBD is also metabolized by 2C19 | |||
| THC increases levels of: | CBD increases levels of: | CBD reduces levels of: | Increased levels of THC/CBD | Reduced levels of THC/CBD | |
| Mechanism unclear Warfarin | 2D6 substrates Amitriptyline Nortriptyline Desipramine Flecainide Haloperidol Risperidone 2C19 substrates Citalopram Diazepam Escitalopram Tacrolimus Voriconazole Warfarin Omeprazole | 2B6 substrates Methadone UGT1A9 and UGT2B7 substrates Mycophenolate Dabigatran Mechanism unclear Everolimus Sirolimus | Dependent on 2D6 for activation Codeine Tamoxifen Tramadol Partly dependent on 2C19 for activation Clopidogrel | Strong 3A4 inhibitors Clarithromycin Itraconazole Ketoconazole Posaconazole Ritonavir Voriconazole Moderate 3A4 inhibitors Amiodarone Cyclosporine Diltiazem Erythromycin Fluconazole Grapefruit juice Imatinib Verapamil | Strong 3A4 inducers Carbamazepine Phenytoin Rifampin (rifampicin)a Moderate 3A4 inducers Bosentan Dabrafenib Dexamethasone Rifabutin St. John's wort 2C19 inducersb Apalutamide |
Cannabinoid Pharmacokinetics in CKD.
Tayo et al. studied the pharmacokinetics of a single 200-mg dose of CBD in 32 participants with varying levels of kidney function, including eight individuals with a creatinine clearance <30 ml/min per 1.73 m2 (mean 21.7±6.0 ml/min per 1.73 m2). No individuals receiving dialysis were included (29). They found no differences in peak concentration or total exposure for CBD between participants with any level of kidney function. Urine CBD levels were too low to be estimated, supporting the assertion that kidney function is not required for its clearance. Few adverse events were reported, and none occurred in participants with moderate or severe impairment of kidney function. Neither the pharmacokinetics of inhaled cannabinoids, nor THC in any formulation, have been studied in people with impaired kidney function or those on dialysis. Because both THC and CBD have a very large volume of distribution (>10 L/kg) and are highly protein bound (26), these compounds are unlikely to be effectively removed by hemodialysis or peritoneal dialysis. The available data suggest that dose adjustment of cannabinoids due to kidney impairment is unnecessary, but given the reduction in albumin-binding capacity in uremia (30) and the complex physiology of this state, caution is warranted.
Cannabinoids and Symptoms Associated with Kidney Failure
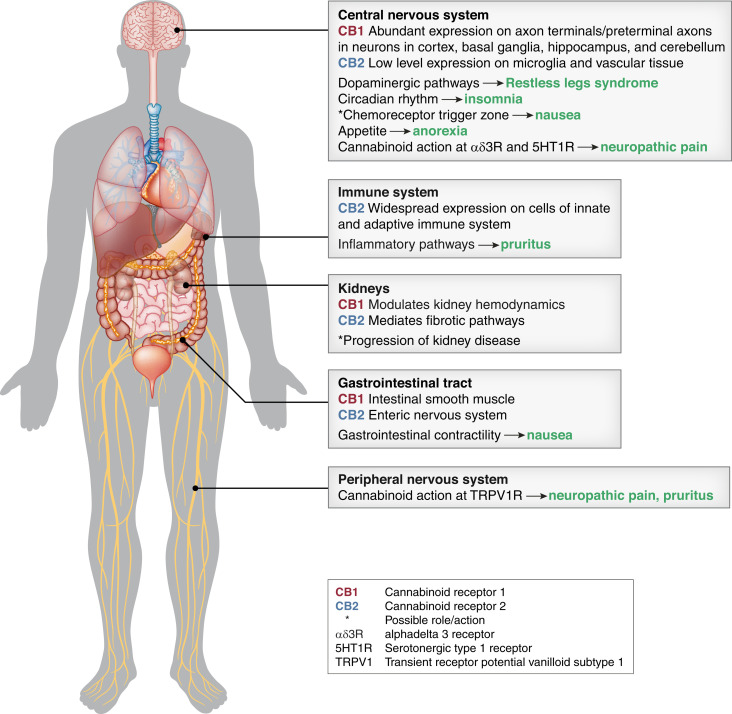
The diverse physiologic effects of cannabinoids support the hypothesis that they may be effective for at least some of the symptoms associated with kidney failure (Figure 2). In the next section, we examine the available evidence concerning the potential efficacy of cannabinoids for a number of key symptoms in this population. At present, the majority of this evidence is limited to extrapolation from experiences in other patient populations and/or biologic plausibility (Table 4).
Figure 2.
Potential mechanisms of action of cannabinoids in symptoms associated with kidney failure. Arrows indicate a putative role for cannabinoids in treating specific symptoms.
Table 4.
Summary of evidence for cannabinoid effect on symptoms associated with kidney failure
| Symptom | Potential Mechanism | Evidence in Non-CKD Population | Study Details | Evidence in CKD Population |
|---|---|---|---|---|
| Restless legs syndrome | CB1 found throughout the central nervous system Cannabinoids modulate dopaminergic and GABAergic signaling (37,39,40) | Patients with RLS: Symptom relief with smoked marijuana or sublingual CBD (41,42) | Two case series (same center), total n=18 | Nil |
| Pruritus | Inhibition of TRPV1 (46) Reduced production of inflammatory cytokines (44) | Nil | Nonrandomized study, n=21 | Hemodialysis patients: uncontrolled prospective study (n=21) found that an endocannabinoid-containing emollient was effective (51) |
| Insomnia | THC has sedating properties, possibly mitigated by CBD (54) | Patients with insomnia associated with fibromyalgia: Nabilone superior to amitriptyline (108) | Randomized, double-blinded, crossover trial, n=31 | Nil |
| Anorexia | Activation of central CB1, resulting in appetite stimulation and shift of white and brown adipocytes to anabolic state (58–60) | (a) Patients with HIV-associated cachexia and anorexia nervosa: Modest efficacy with smoked cannabis and dronabinol (61–64) (b) Patients with cancer-related anorexia: Mixed results (65,66) | (a) Four RCTs, total n=204 (b) Two RCTs, total n=290 | Nil |
| Pain | Actions on diverse range of neuronal ion-channels and receptors, including α-3 glycine receptors, 5HT1a receptors, and TRPV1 (68,74,75) | Patients with chronic non-cancer pain and neuropathic pain: Modest efficacy (69,78) | Two meta-analyses: 104 studies (47 RCTs, 57 observational), n=9958; 16 double-blind RCTs, n=1750 | Nil |
| Nausea | Modulation of CB2 in the enteric nervous system, resulting in a reduction of enterotoxin-mediated gut motility (85) Potential action via central CB1 | Patients with CINV: Nabilone, dronabinol and THC:CBD modestly effective (83,84) | Meta-analysis: 23 RCTs, n=1359; one further RCT, n=81 | Nil |
CB, cannabinoid receptor; GABA, gamma-aminobutyric acid; RLS, restless legs syndrome; CBD, cannabidiol; TRPV1, transient receptor potential cation channel subfamily V member 1; RCT, randomized controlled trial; 5HT1a, 5-hydroxytryptamine 1a; CINV, chemotherapy-induced nausea and vomiting; THC, tetrahydrocannabinol.
Restless Legs Syndrome.
Restless legs syndrome is a sensorimotor disorder defined by the unpleasant urge to move the legs. It typically occurs at rest, particularly at night, is partially or totally relieved by movement, and results in disturbed sleep and impaired quality of life (31). The etiology of restless legs syndrome is unclear, with current theories implicating disordered brain iron homeostasis and altered dopaminergic signaling (12,32). First-line pharmacological therapies are gabapentinoids, followed by dopamine agonists (33), yet many patients obtain little or incomplete relief of symptoms (34). Long-term therapy with dopamine agonists leads to the development of tolerance, and adverse effects for ≤20% of patients (32,35). Gabapentinoids may be more effective than dopamine agonists (12), but owing to kidney clearance, they must be used cautiously in patients with abnormal kidney function and treatment is frequently limited by side effects such as drowsiness (16). In patients who are refractory, clonazepam may be used (although without randomized evidence to support its efficacy [36]), which suggests cannabinoids, which modulate gamma-aminobutyric acid transmission, including via enhancing gamma-aminobutyric acid A receptor activity, might also be effective (37,38). There are also complex effects of cannabinoids on dopamine signaling, with CB1 receptors expressed abundantly within central dopaminergic pathways (39). Acute THC administration increases dopamine synthesis and dopaminergic cell activity, yet chronic cannabis users may have reduced dopamine synthesis capacity (39,40). At present, the evidence in restless legs syndrome is limited to a case series of 18 patients with refractory symptoms (without kidney disease) who demonstrated complete relief of symptoms with smoked marijuana or sublingual CBD (41,42).
Pruritus.
Uremic pruritus affects <55% of people receiving dialysis, and multiple pathways (both central and peripheral) have been implicated in its pathogenesis (43). Gabapentinoids are first-line pharmacotherapy (11). The endocannabinoid system has an important role in skin health and affects multiple cell types within the dermis, including keratinocytes, immune cells, and sensory nerves (44,45). In addition to action at CB1 and CB2, cannabinoids act at transient receptor potential (TRP) ion channels, including as inhibitors of the transient receptor potential vanilloid type 1 (TRPV1) (46), which play an important role in the pathogenesis of uremic pruritus (47). Topical capsaicin, an agonist of TRPV1, is effective for the treatment of uremic pruritus owing to subsequent downregulation of TRPV1 activity, but is frequently poorly tolerated given the initial discomfort in its application (11,48). Cannabinoids also have a diverse array of anti-inflammatory actions, mediated via the expression of CB2 receptors on immune cells (49). Activation of CB2 reduces production of inflammatory cytokines including TNF-α, IL-6, and IFN-γ (44), all of which are found in greater concentration in the skin of patients with uremic pruritus (50). A nonrandomized study of 21 people with uremic pruritus found that application of a cream containing an endogenous cannabinoid (acetylethanolamide) and a related noncannabinoid (palmitoylethanolamide) resulted in complete relief of symptoms in 38% of participants (51). No studies using THC or CBD for chronic pruritus of any cause have been published.
Insomnia.
People with kidney failure suffer poor sleep for a variety of reasons, including uncontrolled symptoms, metabolic derangements, and disturbed circadian rhythm (52). Treatment is typically multimodal, without sufficient evidence to guide clinicians (13). The role of the endocannabinoid system in sleep and circadian rhythm is complex and incompletely understood (53). THC has sedating properties, which may be mitigated by coadministration of CBD (54). The effect of CBD on sleep may be dose dependent, with low doses being stimulating and high doses sedating (53). Although habitual cannabis consumers often report that it aids in falling asleep, the effect of specific formulations of THC or CBD on sleep is not known, owing to a lack of clinical studies (55). A number of studies are underway in the non-CKD population (54,55).
Anorexia.
Anorexia and malnutrition are common in people with kidney failure and are strongly associated with mortality (56). Altered taste sensation and direct effects of uremic toxins are thought to contribute to poor appetite (57). Appetite stimulation is a common effect of recreational cannabis use and is mediated via activation of central CB1 (58,59). Cannabinoids also modulate metabolism, with activation of CB1 shifting both white and brown adipocytes to an anabolic state (60). Smoked cannabis and the synthetic cannabinoid, dronabinol, have shown modest efficacy in small studies in patients with HIV-associated cachexia (61–63) and anorexia nervosa (64). Yet, randomized, placebo-controlled studies in patients with cancer and cachexia/anorexia have found mixed results (65,66).
Chronic Pain.
Pain affects >50% of patients with CKD and has diverse etiologies (67). Treatment is often limited due to side effects, narrow therapeutic indices, and drug interactions. There has been much interest in the analgesic potential of cannabinoids. They have actions on a diverse range of neuronal ion-channels and receptors (68). Meta-analysis of randomized studies shows modest effects of cannabinoids on a range of noncancer pain syndromes and more frequent adverse effects compared with placebo (69). It is important to place these findings in the context of widespread acceptance of opioids, which are also only moderately effective and associated with important adverse events (17,70).
Neuropathy and neuropathic pain are common in patients with kidney failure and are often refractory to treatment (71). Beneficial effects of cannabinoids for neuropathic pain may be independent of CB1 and CB2, with possible alternate mediators of effect including alpha-3 glycine receptors, 5HT1a receptors, and TRPV1 (68). TRPV1 plays a key role in the transduction of neuropathic pain (72), and dysregulation of TRPV1 may be involved in the pathogenesis of uremic neuropathy (73). CBD has been shown to affect TRPV1-signaling at the level of the spinal cord (74) and centrally (75). In contrast to CBD, THC is not known to act at TRPV1, but may moderate TRPV2, TRPV3, and TRPV4 (76). A number of meta-analyses have examined the effect of cannabinoids on neuropathic pain, finding low to moderate quality evidence that cannabinoids result in modest improvement in chronic neuropathic pain, with an important degree of heterogeneity (77–79). The most recent of these meta-analyses (including 16 randomized, double-blind studies of cannabis products, and 1750 participants with a variety of causes of neuropathic pain, with no study reporting specifically on individuals with any degree of CKD) found that cannabinoids may modestly increase the proportion of people achieving ≥50% pain relief compared with placebo (21% versus 17%; risk difference 0.05, 95% confidence interval, 0.00 to 0.09); however, the quality of this evidence was rated as low (78). Another recent study suggests that topical CBD may be beneficial for peripheral neuropathic pain (80). Although cannabinoids show promise for some types of pain, further study is required to determine optimal dosing, THC:CBD ratios, and those patients likely to respond.
Nausea.
Nausea affects approximately one third of people with kidney failure (7). Standard pharmacotherapies are poorly tolerated (33) and lack evidence for efficacy in kidney failure. The cause of nausea is incompletely understood and is likely to be multifactorial (81,82). Synthetic cannabinoids (nabilone and dronabinol) are effective for chemotherapy-induced nausea and vomiting (83). A recent randomized controlled trial reported that a 1:1 THC:CBD oral formulation was efficacious for this syndrome, with more patients experiencing complete relief of symptoms with the combination of THC:CBD and standard antiemetics compared with standard antiemetics alone (25% versus 14%; relative risk 1.77, 95% confidence interval, 1.12 to 2.79) (84). Cannabinoid-related adverse events (including sedation, dizziness, and disorientation) were common, occurring in 31% of participants, but 83% of participants preferred THC:CBD to placebo. The antiemetic mechanism of cannabinoids is not clear, but may involve CB2, which is expressed in the enteric nervous system and activation of which reduces endotoxin-mediated gut motility, and potentially central action via CB1 (85).
Adverse Effects of Cannabinoids
Given the high comorbidity burden of the kidney failure population, tolerability is an important concern. Adverse effects associated with cannabinoids include dizziness, “feeling high,” hallucinations, panic attacks, slowed cognition or confusion, tachycardia, orthostatic hypotension, hypertension, and mood changes (20,65,86). Significant concern over the potential for adverse events with cannabinoids has been expressed, especially with regard to the possible neuropsychiatric effects on younger cannabis users (20). Meta-analysis of randomized studies in adults aged ≥50 years (for any indication, mostly with placebo as control) suggests adverse events are more common with both THC-only and THC:CBD preparations, but not for CBD-only preparations (87). The potential for THC-containing medications to cause disordered thinking or perception, and dizziness, in older adults has also been highlighted (88). Tolerance may develop to the effects of THC, including adverse effects, with studies in healthy volunteers showing that both psychotropic and physiologic side effects abate after a few days of consistent use (21). Fortunately, serious adverse events appear to be rare (89). Those reported with CBD, such as sedation, elevated liver enzymes, and pneumonia, appear to be largely restricted to childhood epilepsy studies that utilize high doses and where concurrent use of clobazam or valproate may have contributed to serious adverse events (90). Finally, monitoring of liver enzymes is important given multiple reports of elevations in patients treated with CBD (91,92). As yet, there are no data available to estimate the tolerability and safety of cannabinoids in those with kidney failure.
The Endocannabinoid System and CKD
Separately to their potential role in symptom management, emerging evidence supports further study into the effect of the cannabinoid system on the progression of CKD. Endocannabinoids and CB are expressed in adult and fetal kidney tissue (93,94). Predominantly via CB1, the kidney endocannabinoid system is thought to play a role in the regulation of kidney hemodynamics, sodium handling, and protein excretion (93). Human and animal studies have found upregulation of CB1 in kidney fibrosis, including in human kidney transplant recipients and patients with diabetic kidney disease (93,95,96). Animal studies (using CB1 receptor knockout or CB1 antagonists) suggest that inhibition of the CB1 pathway attenuates kidney fibrosis and albuminuria (93,96,97). Animal models also show upregulation of CB2 in injured kidneys; however, data conflict as to the role of these receptors in the development of injury and fibrosis. Activation of CB2 has been shown to be protective against ischemia-reperfusion and cisplatin-induced kidney injury (98,99). In addition, potentiation of CB2 expression has demonstrated antifibrotic action (100) and CB2 agonists have shown the ability to reduce albuminuria and fibrosis (96,101,102). Yet Zhou et al. report that activation of CB2-mediated signaling pathways directly promotes fibrosis, which was ameliorated by administration of an inverse agonist of CB2 (103,104), and a recent study found CBD worsened early diabetic kidney injury in a mouse model (105). In this context, there is interest in modulation of the endocannabinoid system as a means of modifying the course of diverse forms of CKD. Nevertheless, this research is at an early stage and the relevance of these observations to patients with established kidney failure (a state of advanced and irreversible kidney fibrosis), in whom preservation of residual kidney function must be balanced against relief of symptoms, is difficult to assess.
Although the growing body of evidence concerning the physiology of cannabinoids demonstrates diverse and complex actions, clinical studies have thus far shown, at best, modest efficacy for symptom relief. Yet, there are reasons to believe cannabinoids could prove useful for a range of common symptoms suffered by those with kidney failure. It is important to recognize that this hope is presently on the basis of biologic plausibility and on studies in other patient populations, which are of varied quality, offer mixed findings, and show clear evidence for potential harm. To date, no trials have assessed the efficacy or safety of cannabinoids in the management of symptoms in patients with kidney failure. This leaves patients and clinicians without guidance at a time when cannabinoids are becoming increasingly available. At most, one can conclude it is not unreasonable to consider a carefully monitored trial of cannabinoid therapy for refractory symptoms of kidney failure. However, this ought only to be after exhausting standard therapies for these conditions, and with the patient being thoroughly informed of the lack of evidence to support the use of these agents in people with kidney failure. In addition, although cannabinoid formulations appear generally well tolerated, adverse effects are not uncommon. A strict “start low, go slow” approach to cannabinoid dosing is imperative, particularly given the lack of understanding of the pharmacokinetics of these agents over extended dosing periods. Finally, both prescribers and patients need to be fully cognizant of the legal status of cannabinoids in their jurisdiction. For instance, limitations on driving and operating machinery may be in place, especially for products containing THC, which can leave patients at risk of criminal prosecution.
Future Directions
Although the use of cannabinoids in people with kidney failure cannot presently be recommended outside of a clinical trial, their potential effects in this population (and their increasing availability) make properly conducted studies essential. Preliminary studies are required to establish an understanding of safe dosing, tolerability, and pharmacokinetics. After this, placebo-controlled randomized trials using validated and responsive patient-reported outcome measures may be conducted to examine the effect of cannabinoids on various well-established symptoms of kidney failure. Further studies examining their use compared with, or as add-on to, current standard of care will ultimately be required to determine if these fascinating agents deserve a role in clinical practice.
Disclosures
D. Collister reports having consultancy agreements with Akebia. All remaining authors have nothing to disclose.
Funding
None.
Acknowledgments
Dr. N. Agarwal and Dr. D. O’Hara both receive support through Australian Government Research Training Program Scholarships and NHMRC Clinical Trials Centre Postgraduate Research Scholarships.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Herodotus: The history of Herodotus (translated by George Rawlinson): The Internet Classics Archive. Available at: http://classics.mit.edu//Herodotus/history.html. Accessed August 3, 2021
- 2.Dumas A: Le comte de Monte-Cristo, Tome I. Apple Books: Apple Inc. 1870. Available at: https://books.apple.com/tr/book/le-comte-de-monte-cristo-tome-i/id492180920. Accessed September 30, 2021
- 3.Muggerud L, Freese L, Reyes S: Hits from the Bong (Album: Black Sunday; Artist: Cypress Hill), Ruffhouse and Columbia Records, 1993 [Google Scholar]
- 4.Lambert Initiative for Cannabinoid Therapeutics . History of cannabis. Available at: https://www.sydney.edu.au/lambert/medicinal-cannabis/history-of-cannabis.html. Accessed August 3, 2021
- 5.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J: Cannabinoids for medical use: A systematic review and meta-analysis. JAMA 313: 2456–2473, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Saini T, Murtagh FE, Dupont PJ, McKinnon PM, Hatfield P, Saunders Y: Comparative pilot study of symptoms and quality of life in cancer patients and patients with end-stage renal disease. Palliat Med 20: 631–636, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Murtagh FE, Addington-Hall J, Higginson IJ: The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis 14: 82–99, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Kader K, Unruh ML, Weisbord SD: Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 4: 1057–1064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacson E Jr, Xu J, Lin SF, Dean SG, Lazarus JM, Hakim R: Association between achievement of hemodialysis quality-of-care indicators and quality-of-life scores. Am J Kidney Dis 54: 1098–1107, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Evangelidis N, Tong A, Manns B, Hemmelgarn B, Wheeler DC, Tugwell P, Crowe S, Harris T, Van Biesen W, Winkelmayer WC, Sautenet B, O’Donoghue D, Tam-Tham H, Youssouf S, Mandayam S, Ju A, Hawley C, Pollock C, Harris DC, Johnson DW, Rifkin DE, Tentori F, Agar J, Polkinghorne KR, Gallagher M, Kerr PG, McDonald SP, Howard K, Howell M, Craig JC; Standardized Outcomes in Nephrology– Hemodialysis (SONG-HD) Initiative : Developing a set of core outcomes for trials in hemodialysis: An international Delphi survey. Am J Kidney Dis 70: 464–475, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Hercz D, Jiang SH, Webster AC: Interventions for itch in people with advanced chronic kidney disease. Cochrane Database Syst Rev 12: CD011393, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang CW, Lee MJ, Wang LJ, Lee PT, Tu YK, Hsu CW, Lin PY: Comparative efficacy and acceptability of treatments for restless legs syndrome in end-stage renal disease: A systematic review and network meta-analysis. Nephrol Dial Transplant 35: 1609–1618, 2020 [DOI] [PubMed] [Google Scholar]
- 13.Natale P, Ruospo M, Saglimbene VM, Palmer SC, Strippoli GF: Interventions for improving sleep quality in people with chronic kidney disease. Cochrane Database Syst Rev 5: CD012625, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natale P, Palmer SC, Ruospo M, Saglimbene VM, Rabindranath KS, Strippoli GF: Psychosocial interventions for preventing and treating depression in dialysis patients. Cochrane Database Syst Rev 12: CD004542, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer SC, Natale P, Ruospo M, Saglimbene VM, Rabindranath KS, Craig JC, Strippoli GF: Antidepressants for treating depression in adults with end-stage kidney disease treated with dialysis. Cochrane Database Syst Rev (5): CD004541, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Gabapentin and pregabalin use and association with adverse outcomes among hemodialysis patients. J Am Soc Nephrol 29: 1970–1978, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida JH, McCulloch CE, Steinman MA, Grimes BA, Johansen KL: Opioid analgesics and adverse outcomes among hemodialysis patients. Clin J Am Soc Nephrol 13: 746–753, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samaha D, Kandiah T, Zimmerman D: Cannabis use for restless legs syndrome and uremic pruritus in in patients treated with maintenance dialysis: A survey. Can J Kidney Health Dis 7: 2054358120954944, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collister D, Tennankore K, Davison SN, Wald R, Rabbat C, Walsh M: Nephrologist views regarding cannabinoid use in advanced chronic kidney disease and dialysis: A survey. J Pain Symptom Manage 61: 237–245.e2, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Ho C, Martinusen D, Lo C: A review of cannabis in chronic kidney disease symptom management. Can J Kidney Health Dis 6: 2054358119828391, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotenhermen F: Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42: 327–360, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Davison SN, Davison JS: Is there a legitimate role for the therapeutic use of cannabinoids for symptom management in chronic kidney disease? J Pain Symptom Manage 41: 768–778, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Hardy J, Haywood A, Gogna G, Martin J, Yates P, Greer R, Good P: Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: A double-blind, placebo-controlled, randomised clinical trial of efficacy and safety of 1:1 delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Trials 21: 611, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heuberger JA, Guan Z, Oyetayo OO, Klumpers L, Morrison PD, Beumer TL, van Gerven JM, Cohen AF, Freijer J: Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short- and long-term pharmacokinetics. Clin Pharmacokinet 54: 209–219, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Silmore LH, Willmer AR, Capparelli EV, Rosania GR: Food effects on the formulation, dosing, and administration of cannabidiol (CBD) in humans: A systematic review of clinical studies. Pharmacotherapy 41: 405–420, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vázquez M, García-Carnelli C, Maldonado C, Fagiolino P: Clinical pharmacokinetics of cannabinoids and potential drug-drug interactions. Adv Exp Med Biol 1297: 27–42, 2021 [DOI] [PubMed] [Google Scholar]
- 27.Kocis PT, Vrana KE: Delta-9-tetrahydrocannabinol and cannabidiol drug-drug interactions. Med Cannabis Cannabinoids 3: 61–73, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millar SA, Stone NL, Yates AS, O’Sullivan SE: A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol 9: 1365, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tayo B, Taylor L, Sahebkar F, Morrison G: A phase i, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet 59: 747–755, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klammt S, Wojak HJ, Mitzner A, Koball S, Rychly J, Reisinger EC, Mitzner S: Albumin-binding capacity (ABiC) is reduced in patients with chronic kidney disease along with an accumulation of protein-bound uraemic toxins. Nephrol Dial Transplant 27: 2377–2383, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, Zucconi M, Ferri R, Trenkwalder C, Lee HB; International Restless Legs Syndrome Study Group : Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med 15: 860–873, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Ghorayeb I: Cannabis for restless legs syndrome. Adv Exp Med Biol 1297: 173–181, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Davison SN, Tupala B, Wasylynuk BA, Siu V, Sinnarajah A, Triscott J: Recommendations for the care of patients receiving conservative kidney management: Focus on management of CKD and symptoms. Clin J Am Soc Nephrol 14: 626–634, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winkelmann J, Allen RP, Högl B, Inoue Y, Oertel W, Salminen AV, Winkelman JW, Trenkwalder C, Sampaio C: Treatment of restless legs syndrome: Evidence-based review and implications for clinical practice (Revised 2017). Mov Disord 33: 1077–1091, 2018 [DOI] [PubMed] [Google Scholar]
- 35.Heim B, Djamshidian A, Heidbreder A, Stefani A, Zamarian L, Pertl MT, Brandauer E, Delazer M, Seppi K, Poewe W, Högl B: Augmentation and impulsive behaviors in restless legs syndrome: Coexistence or association? Neurology 87: 36–40, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Carlos K, Prado GF, Teixeira CD, Conti C, de Oliveira MM, Prado LB, Carvalho LB: Benzodiazepines for restless legs syndrome. Cochrane Database Syst Rev 3: CD006939, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bakas T, van Nieuwenhuijzen PS, Devenish SO, McGregor IS, Arnold JC, Chebib M: The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res 119: 358–370, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Benarroch EE: Synaptic effects of cannabinoids: Complexity, behavioral effects, and potential clinical implications. Neurology 83: 1958–1967, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Bloomfield MA, Ashok AH, Volkow ND, Howes OD: The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 539: 369–377, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD: Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry 75: 470–478, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Ghorayeb I: More evidence of cannabis efficacy in restless legs syndrome. Sleep Breath 24: 277–279, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Megelin T, Ghorayeb I: Cannabis for restless legs syndrome: A report of six patients. Sleep Med 36: 182–183, 2017 [DOI] [PubMed] [Google Scholar]
- 43.Makar M, Smyth B, Brennan F: Chronic kidney disease-associated pruritus: A review. Kidney Blood Press Res 46: 659–669, 2021 [DOI] [PubMed] [Google Scholar]
- 44.Cintosun A, Lara-Corrales I, Pope E: Mechanisms of cannabinoids and potential applicability to skin diseases. Clin Drug Investig 40: 293–304, 2020 [DOI] [PubMed] [Google Scholar]
- 45.Río CD, Millán E, García V, Appendino G, DeMesa J, Muñoz E: The endocannabinoid system of the skin. A potential approach for the treatment of skin disorders. Biochem Pharmacol 157: 122–133, 2018 [DOI] [PubMed] [Google Scholar]
- 46.Soneji ND, Paule CC, Mlynarczyk M, Nagy I: Effects of cannabinoids on capsaicin receptor activity following exposure of primary sensory neurons to inflammatory mediators. Life Sci 87: 162–168, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Andoh T, Maki T, Li S, Uta D: β2-Microglobulin elicits itch-related responses in mice through the direct activation of primary afferent neurons expressing transient receptor potential vanilloid 1. Eur J Pharmacol 810: 134–140, 2017 [DOI] [PubMed] [Google Scholar]
- 48.Tarng DC, Cho YL, Liu HN, Huang TP: Hemodialysis-related pruritus: a double-blind, placebo-controlled, crossover study of capsaicin 0.025% cream. Nephron 72: 617–622, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Turcotte C, Blanchet MR, Laviolette M, Flamand N: The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 73: 4449–4470, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, Kiefer T, Stülten C, van der Kuip H, Pauli-Magnus C, Raub U, Kuhlmann U, Mettang T: The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21: 749–755, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Szepietowski JC, Szepietowski T, Reich A: Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: A preliminary study. Acta Dermatovenerol Croat 13: 97–103, 2005 [PubMed] [Google Scholar]
- 52.Liao JL, van den Broek-Best O, Smyth B, Hong D, Vo K, Zuo L, Gray NA, Chan CT, de Zoysa J, Perkovic V, Jiang L, Jardine M: Effect of extended hours dialysis on sleep quality in a randomized trial. Nephrology (Carlton) 24: 430–437, 2019 [DOI] [PubMed] [Google Scholar]
- 53.Babson KA, Sottile J, Morabito D: Cannabis, cannabinoids, and sleep: A review of the literature. Curr Psychiatry Rep 19: 23, 2017 [DOI] [PubMed] [Google Scholar]
- 54.Suraev A, Grunstein RR, Marshall NS, D’Rozario AL, Gordon CJ, Bartlett DJ, Wong K, Yee BJ, Vandrey R, Irwin C, Arnold JC, McGregor IS, Hoyos CM: Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) for chronic insomnia disorder (‘CANSLEEP’ trial): Protocol for a randomised, placebo-controlled, double-blinded, proof-of-concept trial. BMJ Open 10: e034421, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suraev AS, Marshall NS, Vandrey R, McCartney D, Benson MJ, McGregor IS, Grunstein RR, Hoyos CM: Cannabinoid therapies in the management of sleep disorders: A systematic review of preclinical and clinical studies. Sleep Med Rev 53: 101339, 2020 [DOI] [PubMed] [Google Scholar]
- 56.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero JJ, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD, Teta D, Yee-Moon Wang A, Cuppari L: KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 76[Suppl 1]: S1–S107, 2020 [DOI] [PubMed] [Google Scholar]
- 57.Brennan F, Stevenson J, Brown M: The pathophysiology and management of taste changes in chronic kidney disease: A review. J Ren Nutr 30: 368–379, 2020 [DOI] [PubMed] [Google Scholar]
- 58.Brierley DI, Samuels J, Duncan M, Whalley BJ, Williams CM: Cannabigerol is a novel, well-tolerated appetite stimulant in pre-satiated rats. Psychopharmacology (Berl) 233: 3603–3613, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams CM, Rogers PJ, Kirkham TC: Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav 65: 343–346, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Gruden G, Barutta F, Kunos G, Pacher P: Role of the endocannabinoid system in diabetes and diabetic complications. Br J Pharmacol 173: 1116–1127, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV: Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J Pain Symptom Manage 10: 89–97, 1995 [DOI] [PubMed] [Google Scholar]
- 62.Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW: Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep. J Acquir Immune Defic Syndr 45: 545–554, 2007 [DOI] [PubMed] [Google Scholar]
- 63.Haney M, Rabkin J, Gunderson E, Foltin RW: Dronabinol and marijuana in HIV(+) marijuana smokers: Acute effects on caloric intake and mood. Psychopharmacology (Berl) 181: 170–178, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Andries A, Frystyk J, Flyvbjerg A, Støving RK: Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int J Eat Disord 47: 18–23, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko YD, Schnelle M, Reif M, Cerny T; Cannabis-In-Cachexia-Study-Group : Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the Cannabis-In-Cachexia-Study-Group. J Clin Oncol 24: 3394–3400, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Turcott JG, Del Rocío Guillen Núñez M, Flores-Estrada D, Oñate-Ocaña LF, Zatarain-Barrón ZL, Barrón F, Arrieta O: The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Support Care Cancer 26: 3029–3038, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Davison SN, Koncicki H, Brennan F: Pain in chronic kidney disease: A scoping review. Semin Dial 27: 188–204, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Mlost J, Bryk M, Starowicz K: Cannabidiol for pain treatment: Focus on pharmacology and mechanism of action. Int J Mol Sci 21: E8870, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L: Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 159: 1932–1954, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, Mulla SM, Lopes LC, Vogel N, Chen E, Kirmayr K, De Oliveira K, Olivieri L, Kaushal A, Chaparro LE, Oyberman I, Agarwal A, Couban R, Tsoi L, Lam T, Vandvik PO, Hsu S, Bala MM, Schandelmaier S, Scheidecker A, Ebrahim S, Ashoorion V, Rehman Y, Hong PJ, Ross S, Johnston BC, Kunz R, Sun X, Buckley N, Sessler DI, Guyatt GH: Opioids for chronic noncancer pain: A systematic review and meta-analysis. JAMA 320: 2448–2460, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smyth B, Krishnan AV, Gallagher M, Kiernan M, Snelling P, Hawley C, Fernando M, Hand S, Grimley K, Burman J, Heath A, Kang A, Perkovic V, Jardine MJ: Randomised controlled trial of the impact of haemodiafiltration on uraemic neuropathy: FINESSE study protocol. BMJ Open 9: e023736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malek N, Pajak A, Kolosowska N, Kucharczyk M, Starowicz K: The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol Cell Neurosci 65: 1–10, 2015 [DOI] [PubMed] [Google Scholar]
- 73.Anand U, Korchev Y, Anand P: The role of urea in neuronal degeneration and sensitization: An in vitro model of uremic neuropathy. Mol Pain 15: 1744806919881038, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anand U, Jones B, Korchev Y, Bloom SR, Pacchetti B, Anand P, Sodergren MH: CBD effects on TRPV1 signaling pathways in cultured DRG neurons. J Pain Res 13: 2269–2278, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray RA, Stott CG, Jones NA, Di Marzo V, Whalley BJ: Anticonvulsive properties of cannabidiol in a model of generalized seizure are transient receptor potential vanilloid 1 dependent. Cannabis Cannabinoid Res 5: 145–149, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muller C, Morales P, Reggio PH: Cannabinoid ligands targeting TRP channels. Front Mol Neurosci 11: 487, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreae MH, Carter GM, Shaparin N, Suslov K, Ellis RJ, Ware MA, Abrams DI, Prasad H, Wilsey B, Indyk D, Johnson M, Sacks HS: Inhaled cannabis for chronic neuropathic pain: A meta-analysis of individual patient data. J Pain 16: 1221–1232, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W: Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 3: CD012182, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nugent SM, Morasco BJ, O’Neil ME, Freeman M, Low A, Kondo K, Elven C, Zakher B, Motu’apuaka M, Paynter R, Kansagara D: The effects of cannabis among adults with chronic pain and an overview of general harms: A systematic review. Ann Intern Med 167: 319–331, 2017 [DOI] [PubMed] [Google Scholar]
- 80.Xu DH, Cullen BD, Tang M, Fang Y: The effectiveness of topical cannabidiol oil in symptomatic relief of peripheral neuropathy of the lower extremities. Curr Pharm Biotechnol 21: 390–402, 2020 [DOI] [PubMed] [Google Scholar]
- 81.Manley KJ: Saliva composition and upper gastrointestinal symptoms in chronic kidney disease. J Ren Care 40: 172–179, 2014 [DOI] [PubMed] [Google Scholar]
- 82.Van Vlem B, Schoonjans R, Vanholder R, Vandamme W, De Vos M, Lameire N: Dyspepsia and gastric emptying in chronic renal failure patients. Clin Nephrol 56: 302–307, 2001 [PubMed] [Google Scholar]
- 83.Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S: Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev 2015: CD009464, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grimison P, Mersiades A, Kirby A, Lintzeris N, Morton R, Haber P, Olver I, Walsh A, McGregor I, Cheung Y, Tognela A, Hahn C, Briscoe K, Aghmesheh M, Fox P, Abdi E, Clarke S, Della-Fiorentina S, Shannon J, Gedye C, Begbie S, Simes J, Stockler M: Oral THC:CBD cannabis extract for refractory chemotherapy-induced nausea and vomiting: A randomised, placebo-controlled, phase II crossover trial. Ann Oncol 31: 1553–1560, 2020 [DOI] [PubMed] [Google Scholar]
- 85.Duncan M, Mouihate A, Mackie K, Keenan CM, Buckley NE, Davison JS, Patel KD, Pittman QJ, Sharkey KA: Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Gastrointest Liver Physiol 295: G78–G87, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vacaflor BE, Beauchet O, Jarvis GE, Schavietto A, Rej S: Mental health and cognition in older cannabis users: A review. Can Geriatr J 23: 242–249, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Velayudhan L, McGoohan K, Bhattacharyya S: Safety and tolerability of natural and synthetic cannabinoids in adults aged over 50 years: A systematic review and meta-analysis. PLoS Med 18: e1003524, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Velayudhan L, McGoohan KL, Bhattacharyya S: Evaluation of THC-related neuropsychiatric symptoms among adults aged 50 years and older: A systematic review and metaregression analysis. JAMA Netw Open 4: e2035913, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dos Santos RG, Guimarães FS, Crippa JAS, Hallak JEC, Rossi GN, Rocha JM, Zuardi AW: Serious adverse effects of cannabidiol (CBD): A review of randomized controlled trials. Expert Opin Drug Metab Toxicol 16: 517–526, 2020 [DOI] [PubMed] [Google Scholar]
- 90.Chesney E, Oliver D, Green A, Sovi S, Wilson J, Englund A, Freeman TP, McGuire P: Adverse effects of cannabidiol: a systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 45: 1799–1806, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Silvennoinen K, Ritter LM, Nashef L, Hudgell K, Balestrini S, Sisodiya SM, Sidhu MK: Two-center experience of cannabidiol use in adults with Dravet syndrome. Seizure 91: 5–8, 2021 [DOI] [PubMed] [Google Scholar]
- 92.Crippa JAS, Zuardi AW, Guimarães FS, Campos AC, de Lima Osório F, Loureiro SR, Dos Santos RG, Souza JDS, Ushirohira JM, Pacheco JC, Ferreira RR, Mancini Costa KC, Scomparin DS, Scarante FF, Pires-Dos-Santos I, Mechoulam R, Kapczinski F, Fonseca BAL, Esposito DLA, Pereira-Lima K, Sen S, Andraus MH, Hallak JEC; Burnout and Distress Prevention With Cannabidiol in Front-line Health Care Workers Dealing With COVID-19 (BONSAI) Trial Investigators : Efficacy and safety of cannabidiol plus standard care vs standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID-19 pandemic: A randomized clinical trial. JAMA Netw Open 4: e2120603, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chua JT, Argueta DA, DiPatrizio NV, Kovesdy CP, Vaziri ND, Kalantar-Zadeh K, Moradi H: Endocannabinoid system and the kidneys: From renal physiology to injury and disease. Cannabis Cannabinoid Res 4: 10–20, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Larrinaga G, Varona A, Pérez I, Sanz B, Ugalde A, Cándenas ML, Pinto FM, Gil J, López JI: Expression of cannabinoid receptors in human kidney. Histol Histopathol 25: 1133–1138, 2010 [DOI] [PubMed] [Google Scholar]
- 95.Dao M, Lecru L, Vandermeersch S, Ferreira M, Ferlicot S, Posseme K, Dürrbach A, Hermeziu B, Mussini C, Chatziantoniou C, François H: The cannabinoid receptor 1 is involved in renal fibrosis during chronic allograft dysfunction: Proof of concept. J Cell Mol Med 23: 7279–7288, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lecru L, Desterke C, Grassin-Delyle S, Chatziantoniou C, Vandermeersch S, Devocelle A, Vernochet A, Ivanovski N, Ledent C, Ferlicot S, Dalia M, Saïd M, Beaudreuil S, Charpentier B, Vazquez A, Giron-Michel J, Azzarone B, Durrbach A, François H: Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney Int 88: 72–84, 2015 [DOI] [PubMed] [Google Scholar]
- 97.Udi S, Hinden L, Ahmad M, Drori A, Iyer MR, Cinar R, Herman-Edelstein M, Tam J: Dual inhibition of cannabinoid CB1 receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br J Pharmacol 177: 110–127, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mukhopadhyay P, Baggelaar M, Erdelyi K, Cao Z, Cinar R, Fezza F, Ignatowska-Janlowska B, Wilkerson J, van Gils N, Hansen T, Ruben M, Soethoudt M, Heitman L, Kunos G, Maccarrone M, Lichtman A, Pacher P, Van der Stelt M: The novel, orally available and peripherally restricted selective cannabinoid CB2 receptor agonist LEI-101 prevents cisplatin-induced nephrotoxicity. Br J Pharmacol 173: 446–458, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pressly JD, Mustafa SM, Adibi AH, Alghamdi S, Pandey P, Roy KK, Doerksen RJ, Moore BM Jr, Park F: Selective cannabinoid 2 receptor stimulation reduces tubular epithelial cell damage after renal ischemia-reperfusion injury. J Pharmacol Exp Ther 364: 287–299, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tang M, Cao X, Zhang K, Li Y, Zheng QY, Li GQ, He QH, Li SJ, Xu GL, Zhang KQ: Celastrol alleviates renal fibrosis by upregulating cannabinoid receptor 2 expression. Cell Death Dis 9: 601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barutta F, Bellini S, Mastrocola R, Gambino R, Piscitelli F, di Marzo V, Corbetta B, Vemuri VK, Makriyannis A, Annaratone L, Bruno G, Gruden G: Reversal of albuminuria by combined AM6545 and perindopril therapy in experimental diabetic nephropathy. Br J Pharmacol 175: 4371–4385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barutta F, Piscitelli F, Pinach S, Bruno G, Gambino R, Rastaldi MP, Salvidio G, Di Marzo V, Cavallo Perin P, Gruden G: Protective role of cannabinoid receptor type 2 in a mouse model of diabetic nephropathy. Diabetes 60: 2386–2396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou S, Wu Q, Lin X, Ling X, Miao J, Liu X, Hu C, Zhang Y, Jia N, Hou FF, Liu Y, Zhou L: Cannabinoid receptor type 2 promotes kidney fibrosis through orchestrating β-catenin signaling. Kidney Int 99: 364–381, 2021 [DOI] [PubMed] [Google Scholar]
- 104.Zhou L, Zhou S, Yang P, Tian Y, Feng Z, Xie XQ, Liu Y: Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int 94: 756–772, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carmona-Hidalgo B, García-Martín A, Muñoz E, González-Mariscal I: Detrimental effect of cannabidiol on the early onset of diabetic nephropathy in male mice. Pharmaceuticals (Basel) 14: 863, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qian Y, Gurley BJ, Markowitz JS: The potential for pharmacokinetic interactions between cannabis products and conventional medications. J Clin Psychopharmacol 39: 462–471, 2019 [DOI] [PubMed] [Google Scholar]
- 107.Kharasch ED: Current concepts in methadone metabolism and transport. Clin Pharmacol Drug Dev 6: 125–134, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ware MA, Fitzcharles MA, Joseph L, Shir Y: The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth Analg 110: 604–610, 2010 [DOI] [PubMed] [Google Scholar]