Abstract
A pseudomonad (CRB5) isolated from a decommissioned wood preservation site reduced toxic chromate [Cr(VI)] to an insoluble Cr(III) precipitate under aerobic and anaerobic conditions. CRB5 tolerated up to 520 mg of Cr(VI) liter−1 and reduced chromate in the presence of copper and arsenate. Under anaerobic conditions it also reduced Co(III) and U(VI), partially internalizing each metal. Metal precipitates were also found on the surface of the outer membrane and (sometimes) on a capsule. The results showed that chromate reduction by CRB5 was mediated by a soluble enzyme that was largely contained in the cytoplasm but also found outside of the cells. The crude reductase activity in the soluble fraction showed a Km of 23 mg liter−1 (437 μM) and a Vmax of 0.98 mg of Cr h−1 mg of protein−1 (317 nmol min−1 mg of protein−1). Minor membrane-associated Cr(VI) reduction under anaerobiosis may account for anaerobic reduction of chromate under nongrowth conditions with an organic electron donor present. Chromate reduction under both aerobic and anaerobic conditions may be a detoxification strategy for the bacterium which could be exploited to bioremediate chromate-contaminated or other toxic heavy metal-contaminated environments.
Soluble hexavalent chromium species [Cr(VI)] are extremely toxic and exhibit mutagenic and carcinogenic effects on biological systems due to their strong oxidizing nature (40). Chromate (CrO42−) is the dominant Cr(VI) species in aqueous environments at pH 6.5 to 9. Cr(III) is less toxic and bioavailable than Cr(VI), as it readily forms insoluble oxides and hydroxides above pH 5 (31). Previous research into microbe-mediated Cr(VI) reduction has identified two enzymatic mechanisms (12). Aerobic reduction is thought to be a detoxification where cells use a soluble enzyme to reduce Cr(VI) to Cr(III) internal or external to the plasma membrane. Reduction may also proceed through the use of CrO42− as a terminal electron acceptor during anaerobic respiration (22, 38). As such, this activity is associated with the membrane where cytochromes have been implicated (38). It is possible that not enough energy is generated from anaerobic chromate reduction to sustain cell growth (22, 23, 32). However, a sulfate-reducing anaerobe that can reduce Cr(VI) and other metals to support growth has been isolated (35).
A soluble enzyme from Pseudomonas putida MK1 has been characterized for genetic engineering to enhance the aerobic chromate remediation potential of this strain (30). Pseudomonas ambigua G-1 (18) and P. putida PRS2000 (19) also possess a soluble reductase which can reduce chromate both aerobically and anaerobically, with higher reduction rates under aerobic conditions. In contrast, the reduction activity of Pseudomonas fluorescens LB300 is membrane associated (9). However, this strain could not reduce chromate anaerobically on solid media unless acetate was added as an electron donor. Accordingly, two separate reduction systems (one membrane-bound and the other soluble) and mechanisms (one aerobic and one anaerobic) must exist within the Pseudomonas genus. It is not clear how bacteria such as P. ambigua and P. putida PRS2000 can reduce chromate under both aerobic and anaerobic conditions. The fact that a soluble enzyme is responsible for the catalytic reduction of Cr(VI) in these two species suggests that it is a detoxification and that no energy is generated from reduction. It has also been suggested that the soluble chromate reductases found in chromate-reducing bacteria may have different primary roles (19, 30).
Our Cr(VI)-reducing pseudomonad, designated CRB5, was recently isolated from a decommissioned wood preservation site. It can reduce chromate under both aerobic and anaerobic conditions and accumulates Cr(III) internal and external to its plasma membrane (25). We have identified a soluble chromate reductase and a complex strategy for chromate resistance. Using both intact cells and their extracts, we located most reductase activity in the cytoplasm and determined the enzyme's kinetics. Since there is an interest in the bioremediation potential of such bacteria (37), we have also characterized CRB5 for its ability to tolerate and reduce Cr(VI) as well as additional toxic metal species under various conditions through batch bioreactor experiments.
MATERIALS AND METHODS
Media.
Vogel-Bonner (VB) broth was modified as described by Bopp et al. (8) and was used in most of the experiments as a minimal salts medium. VB broth was chosen because it did not contain Fe, which can reduce Cr(VI), and to ensure that our results would be comparable to those of Bopp et al. (8). VB broth was prepared by aseptically mixing 10 ml of VB concentrate, 10 ml of 25% (wt/vol) d-glucose solution, and 480 ml of deionized water (all previously sterilized). VB contained the following (in grams per liter of deionized water): K2HPO4, 10.0; Na(NH4)HPO4 · 4H2O, 3.5; citric acid, 2.0; and MgSO4, 0.2. The medium designated VBA had additions of amino acids (MEM 50×, an amino acid supplement; Sigma product no. M5550), which were added in the bioreactor experiments to stimulate growth, and CaCl2 (0.08 g liter−1), which was added to simulate the high Ca concentration in the groundwater at the contaminated field site.
Batch reactors.
In order to monitor the chemical changes that occurred during chromate reduction in the growth media, 3-liter batch reactor systems were used. VB medium was added to the reactor and autoclaved, followed by the addition of filter-sterilized MEM 50×, CaCl2, and Cr(VI) to a total of 3 liters. Cells were first grown in Trypticase soy broth (TSB) (Sigma) without chromate at 30°C, harvested after 18 h, and washed three times with 0.01 M HEPES. The reactor was inoculated with CRB5 to approximately 6 × 108 cells ml−1, and samples were immediately taken for measurement of initial parameters. Optical density at 600 nm (OD600), pH, Cr(VI), total Cr and Eh were monitored over the experimental time course in the unstirred vessel at 20°C. The values obtained for the OD600 measurements include the light-scattering capacity of cell-sized precipitates formed during the experiments in addition to the increasing cell numbers. Eh was a measure of the relative reduction potential of the bulk solution, which can be due to a combination of several redox couples. Samples for metal, chemical, and transmission electron microscopy (TEM) analyses were withdrawn aseptically through a tube inserted to a depth of 15 cm (the total medium depth was 35 cm) into the reactor, and an Eh probe was immersed at a depth of 5 cm. For more exact sampling details, see reference 26.
Reduction experiments.
Since CRB5 was isolated from a site containing copper and arsenate in addition to chromium, the effect of Cu and As on Cr(VI) reduction was determined under growth conditions. Aerobic reduction tests were conducted in crimp-sealed or screw-cap Balch tubes (Bellco Glass, Inc.), leaving a headspace filled with air, and these were sampled using sterile needles and syringes. Cell suspensions were prepared as described above and were added to a final concentration of 7 × 107 cells ml−1 to Balch tubes containing VB media and glucose with various concentrations of chromate, copper and arsenic. Cultures were incubated at 30°C without shaking. Samples were taken for Cr(VI) analyses and TEM preparations at various times during chromate reduction. Aliquots were plated on Trypticase soy agar (Sigma) without Cr(VI) for cell viability testing. Chromate reduction in the tubes containing Cu and As was monitored over time by measuring the disappearance of Cr(VI). For each treatment, cell-free controls were prepared to monitor whether abiotic chromate reduction occurred. All experiments, including abiotic controls, were done in duplicate. Chromate tolerance was assessed by incubating CRB5 for 24 h at ∼7 × 107 cells ml−1 in VB broth at chromate concentrations of 52, 520, 2,600, and 5,200 mg liter−1, and the cells were spread by plating 100 μl of the culture on Trypticase soy agar.
Metal reduction by resting cells.
Tests were conducted to determine if CRB5 could reduce several toxic metals under nongrowth or resting conditions. Anaerobic conditions were obtained by bubbling bicarbonate medium (2.5 g of NaHCO3, 0.1 g of KCl) for 15 min in Balch tubes with CO2 and N2 (20:80), which lowered the dissolved oxygen concentration and gave a pH of 6.8. The headspace was purged for 5 min with the gas mixture before being crimp sealed. Anaerobic additions of 100 μl of 1 M lactate (as an electron donor) to a final concentration of 10 mM plus 200 μl of metal solution [either K2CrO4, Fe(III)NTA, Co(III)EDTA, or U(VI) acetate] to a final concentration of 15 mg liter−1 were combined with 8.7 ml of the anaerobic medium. Harvested cells were washed three times with the anaerobic bicarbonate buffer solution before resuspension in 25 ml of the same buffer. Balch tubes containing 9 ml of medium were subsequently anaerobically inoculated with 1 ml of culture to a concentration of ∼1.5 × 107 cells ml−1. Cultures were incubated at 30°C with shaking. Each treatment was conducted in duplicate, with the initial sample taken anaerobically after the cells were inoculated. Cell-free controls were not included due to the known stability of these metals under the conditions of this experiment. Samples were taken using disposable syringes, which were purged with N2 to avoid introducing any O2 into the culture tube.
Cell fractionation.
Cells were grown in TSB at 22°C and harvested at mid-exponential phase by centrifugation at 4°C and 4,000 × g. They were washed three times by centrifugation in HEPES buffer (pH 7), resuspended in 10 ml of the same buffer, and kept in an ice bath. Cells were mechanically ruptured using a French pressure cell at 17,000 lb/in2. After cell breakage, the suspension was centrifuged at 12,000 × g for 15 min to pellet unbroken cells. Five milliliters of the supernatant (S12) was then set aside and diluted to 10 ml for further assay. The supernatant was then spun at 150,000 × g for 2 h at 4°C. This high-speed supernatant (S150) was retained, and the pellet obtained from this step was used as the membrane fraction, which was resuspended in 10 ml of buffer for analysis. Soluble fractions (S12 and S150) were filtered using a 0.2-μm-pore-size filter, and the fractions were stored at 4°C for less than 1 h before use. Cr(VI) reduction assays using these fractions were conducted for each cell fraction at 22°C in shaken, sealed 50-ml serum vials (anaerobic) and screw-capped bottles with rubber seals (aerobic). Each treatment was done in duplicate, with the initial sample taken immediately after Cr(VI) was added.
Extraction of periplasmic components was accomplished using the osmotic shock procedure (29). Harvested cells from a 2-liter TSB culture were washed twice with cold 10 mM Tris-HCl (pH 7.1) with 30 mM NaCl and resuspended in 33 mM Tris-HCl (pH 7.1). Cells were rapidly mixed into 40% (wt/vol) sucrose in 33 mM Tris-HCl, followed by the addition of 0.1 M EDTA to a concentration of 0.1 mM EDTA. The suspension was placed on a rotary shaker at 180 rpm for 10 min before centrifugation to recover whole cells. The cell pellet was rapidly dispersed in cold 0.5 mM MgCl2 for 10 min. After centrifugation, the supernatant containing the osmotic shock proteins (periplasmic contents) was assayed for Cr(VI) reduction activity. Total protein in the supernatant fraction was measured before and after treatment with EDTA to ensure that the outer membrane was permeabilized.
Analytical methods.
Cell concentrations were determined by microscopic counting of acridine orange-stained cells. Chromate [Cr(VI)] analyses of the liquid fraction of each treatment were performed at various time points by aseptic removal of liquid followed by centrifugation at 16,000 × g for 5 min in 1.5-ml metal-free microcentrifuge tubes. Cr(VI) in the supernatant was then measured colorimetrically at 542 nm using the diphenylcarbazide method (2), which had a detection limit of 0.05 mg of Cr(VI) liter−1. Total Cr in solution after filtration (0.22-μm-pore-size filter) was analyzed using graphite furnace- or flame-atomic absorption spectroscopy after acidification. Fe(II) production was assayed by colorimentric reaction with ferrozine (33). Co(II) was determined on a Dionex LC-50 liquid chromatograph (20). Uranium concentrations were determined by kinetic phosphorescence analysis (36). An increase in total cellular protein was used as an indication of cell growth, and these samples were solubilized in 1 M NaOH before analysis. Protein was measured using the Bio-Rad (Richmond, Calif.) protein assay kit. Eh was measured using a Corning redox electrode. Where possible, all materials (glassware, plasticware, and centrifuge tubes, etc.) were acid leached in 50% HNO3 for at least 24 h to remove residual metals and rinsed using deionized water.
TEM.
Whole cells and fractions from reduction experiments were collected and processed for TEM observation without fixation and staining. Since no TEM stains were used, the contrast seen in the micrographs is due to the binding of metals during treatments. Thin sections were prepared on cells enrobed in Noble agar, passed through an ethanol dehydration series, and infiltrated with LR White resin. Electron micrographs were taken using a Philips EM400T instrument operating at 100 kV. Energy dispersive X-ray spectroscopy (EDS) and selected-area electron diffraction (SAED) were carried out using the EM400T, which was coupled to a Link X-ray detector. Spectra were collected over 100 s (live count time) using a spot size of 400 nm and a beam current of 0.1 μA. SAED used a camera length of 640 mm.
RESULTS
Batch bioreactor experiments.
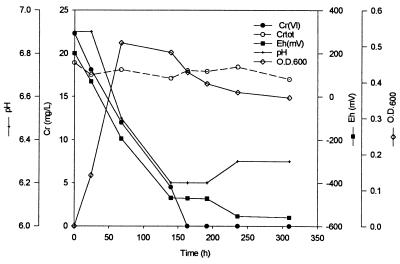
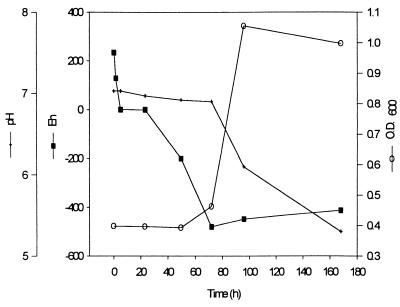
In order to monitor changes in chemistry during Cr(VI) reduction, measurements of Eh, pH, Cr(VI), total Cr (in solution), and OD600 were taken over time in unstirred batch reactors containing 3 liters of VB medium. Samples were also taken for TEM to monitor CRB5 cells for the accumulation of reduced Cr precipitates. In VB medium with amino acids and CaCl2, as the initial biomass increased (as monitored by OD600), several changes in chromate concentration and chemistry were observed (Fig. 1). Although the Cr(VI) was reduced over time, the total chromium in solution fluctuated but did not decrease significantly. The pH dropped (6.9 to 6.3), and the Eh decreased to below −470 mV from initial oxic conditions. Similar changes in solution chemistry were observed in the control bioreactor with no Cr(VI) present (Fig. 2). There was no increase in cell number (OD600 = 0.45), however, until the Eh was below −400 mV, at which point the pH decreased. The Eh values reflect relative decreases in redox potential and should not be taken as absolute values.
FIG. 1.
Batch bioreactor results in VBA medium (VB with amino acids and CaCl2) at an initial cell density of 6 × 108 cells ml−1. Cr(VI) reduction, total Cr [Cr(III)] plus Cr(VI)] in solution after filtration (Crtot), pH, Eh, and OD600 are shown.
FIG. 2.
Bioreactor control in VBA medium with no Cr(VI) added.
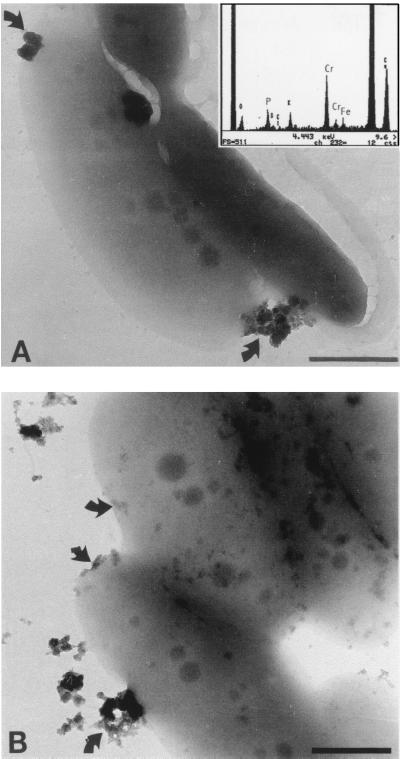
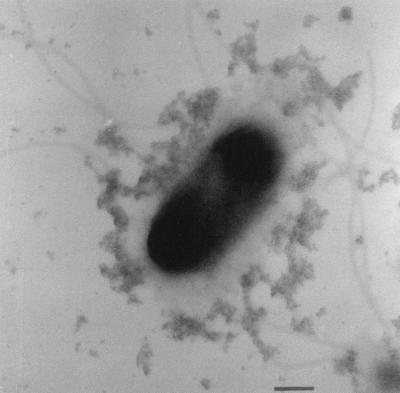
As the culture in the Cr-containing reactors grew, its yellow color [due to soluble Cr(VI)] was replaced by a turbid milky white to gray color. Whole mounts of bacteria for TEM were prepared to determine if this turbidity was in part due to the precipitation of reduced Cr(III) and not just because of increased cell numbers. Even though these cells were not conventionally stained for TEM, they possessed higher electron density than control cells. Elemental analysis of the cells by EDS revealed that Cr was uniformly bound to bacterial surfaces, allowing them to be readily imaged by TEM. The absorbed chromium was assumed to be Cr(III), since chromate anions should not bind to electronegative surface functional groups found on gram-negative envelopes. Figure 3 reveals that Cr(III) precipitates of various sizes were bound to the cell as well as distributed throughout the bulk solution. The largest precipitates were slightly rounded, did not produce regular SAED patterns, and were therefore amorphous mineral phases. Given the chemistry of the solution at sampling time (i.e., pH = ∼6.8 and Eh = −200 mV), the EDS spectra of the precipitates, and visual information of the electron micrographs, the predicted mineral phase was amorphous Cr(III) hydroxide [i.e., Cr(OH)3]. Formation of these precipitates at random sites on the cells was observed in all samples. Figure 4 shows a cell which was incubated in the Cr(VI)-contaminated groundwater from the site [∼20 mg of Cr(VI) liter−1. Electron-dense cells with particulate Cr(III) coatings similar to those seen in Figure 3 were commonly seen in this preparation. Many cells also possessed polymeric capsules (Fig. 4) when CRB5 was incubated in the groundwater, and their capsules were uniformly coated with Cr(III) precipitates. These precipitates were not as dense as those seen on the cell surface and were (presumably) more highly hydrated. Even the numerous flagella of CRB5 were often visible due to the accumulation of metal ions (Fig. 4).
FIG. 3.
Whole mounts of CRB5 from VB medium with 20 mg of Cr(VI) liter−1. (A) Cr(III) precipitates were found as discrete particles bound to the cell surface (arrows). One cell has pulled away from the support film after interaction with the electron beam. An EDS spectrum from the dense particles generated a large Cr peak and a small Fe peak, indicating that it is most likely an amorphous Cr(III) hydroxide or mixed Fe-Cr hydroxide. (B) Amorphous chromium precipitates (arrows) forming on cells. Bars, 500 nm.
FIG. 4.
Unstained whole mount of the peritrichous flagellated CRB5 with precipitates on its capsule. Cr has also accumulated on the outer membrane of the cell, which appears dark in the electron micrograph. Bar, 500 nm.
Metal reduction and tolerance.
Since the potential of this organism for bioremediation of chromated-copper arsenate-contaminated sites (such as those found in the wood preservation industry) may also rely on CRB5's ability to tolerate other toxic metals besides chromium, experiments were designed to explore tolerance to Cr, Cu, and As. At our contaminated site, Cu and As were bound to the soil constituents and immobilized, but it is possible that an on-site remediation process would remobilize these toxic metals. When CRB5 was incubated with increasing Cr concentrations, no viable cells were recovered at concentrations above 520 mg liter−1 Cr(VI) reduction in cultures containing Cr(VI) alone or mixed with Cu or As was monitored. There was no significant difference in the extent of aerobic Cr(VI) reduction after 24 h with 60 mg of As(V) liter−1 or 20 and 40 mg of Cu(II) liter−1 (Table 1). Arsenic at a concentration of 120 mg liter−1 (most likely as AsO42−) had a significant effect on initial reduction. All treatments showed complete Cr(VI) reduction after 120 h.
TABLE 1.
Percent reduction of 20 mg of Cr(VI) liter−1 with copper and arsenic in VB medium
| Metal(s) added | Initial concn (mg/liter)a | % of initial Cr(VI) reduced at 24 h |
|---|---|---|
| Cr(VI) | 20 | 23.4 |
| As(V) + Cr(VI) | 60 (20) | 18.7 |
| As(V) + Cr(VI) | 120 (20) | 7.8 |
| Cu(II) + Cr(VI) | 20 (20) | 27.2 |
| Cu(II) + Cr(VI) | 40 (20) | 23.0 |
The Cr(VI) concentration is in parentheses.
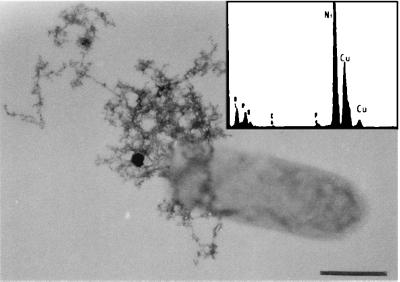
The high tolerance of CRB5 to copper in solution was investigated through the use of TEM. In whole-mount preparations, a proportion of cells had extensive accumulations of copper bound to the extracellular capsules seen in the groundwater experiments (cf. Fig. 4 and 5). EDS showed high copper peaks, and the mineral phases were not highly hydrated. It is possible that some of the copper tolerance of CRB5 may be attributed to the production of these extracellular polymers, which can bind copper on electronegatively charged functional groups in their structure (16).
FIG. 5.
Whole mount of a cell grown in VB medium with 40 mg of Cu liter−1. A number of cells were found to have extensive accumulations of Cu in a matrix of capsular polymers. Bar, 500 nm. An EDS spectrum generated from the precipitates generated a large Cu peak. The Ni peak is from the nickel support grid.
Anaerobic reduction of other metals.
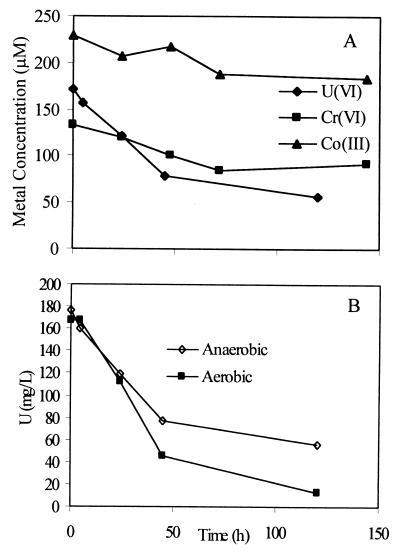
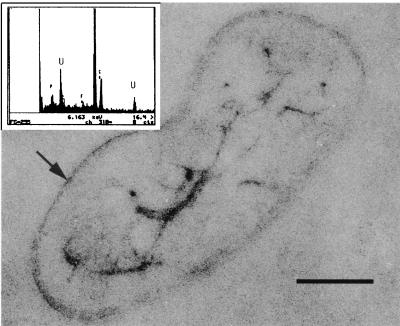
Alternate electron acceptors such as Fe(III), Co(III), and U(VI), which can be present in groundwaters, were added separately to a bicarbonate buffer containing KCl with lactate or dextrose added as the sole electron donor. Nongrowth conditions were chosen so as to separate metal reduction from growth-related processes. Truex et al. (36) suggested that metal reduction under nongrowth conditions might simulate competition for limited substrates in natural environments. CRB5 reduced Cr(VI), U(VI), and Co(III), whereas there was no significant reduction of Fe(III) (Fig. 6A). The average concentration of the reduced form of each metal in replicate tubes was plotted in Fig. 6A, with standard deviations of less than 5% (n = 2). The results show that reduction of Co(III), as Co(III)EDTA, was low. Surprisingly, U(VI) at a concentration of 13.7 mg liter−1 was reduced to a higher degree than Cr(VI) under these conditions. Uranium reduction was further investigated in tubes that were not degassed but were crimp sealed with air in the headspace and dextrose added to the medium as the electron donor. The resulting reduction rates compared to those for the anaerobic treatments were higher, with complete reduction occurring after 120 h (Fig. 6B). TEM and EDS of thin sections and whole mounts revealed that U was located internally as well as being highly concentrated on the cell envelope (Fig. 7).
FIG. 6.
Reduction of alternate electron acceptors. (A) Anaerobic reduction of Cr(VI), Co(III), and U(VI) in bicarbonate buffer with lactate as an electron donor. (B) U(VI) reduction under anaerobic and aerobic conditions.
FIG. 7.
Internal accumulation of uranium confirmed by thin section. Uranium was also bound on the cell envelope (small arrow). Bar, 500 nm. An EDS spectrum of internal uranium precipitates is also shown. The P peak is from the polyphosphate granules; the Cu peak is from the copper support grid.
Cellular chromate reduction.
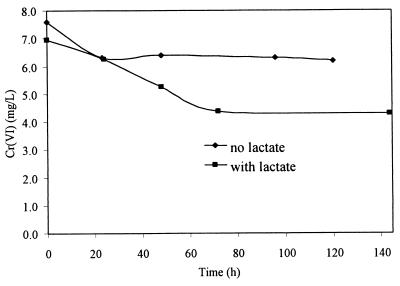
Washed cell suspensions incubated anaerobically under nongrowth (resting) conditions in bicarbonate buffer with lactate as an electron donor reduced Cr(VI) from 7 to 4 mg liter−1 (standard deviation, <5%; n = 2) (Fig. 8). The initial reduction rates with no electron donor (control) were similar to those in tubes with lactate, but reduction ceased in control tubes after 24 h. There was significantly more reduction occurring in the presence of an electron donor than without a donor under resting conditions.
FIG. 8.
Anaerobic chromate reduction by washed cells in bicarbonate buffer with and without lactate as an electron donor.
Chromate reduction by cell extracts.
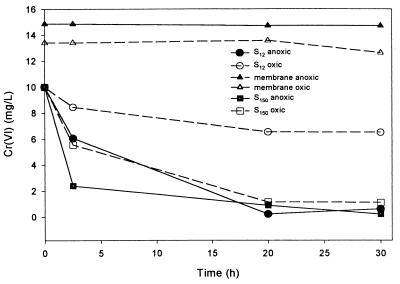
Cr(VI) reduction activities was evident in the soluble fractions of cell extracts S12 and S150 under aerobic and anaerobic conditions. Figure 9 shows the cell extracts prepared from a 24-h culture grown at 22°C with no shaking (stationary culture). Under anaerobic conditions, approximately 80% of a solution of 10 mg of Cr(VI) liter−1 was reduced within 2.5 h by the S150 fraction. Under aerobic conditions, only 55% was reduced during the same time period. Since reduction is associated with the soluble fraction, the enzyme responsible could be either cytoplasmic or periplasmic in origin. The cellular distribution of the soluble enzyme was investigated by monitoring the Cr(VI)-reducing activity in isolated periplasmic contents and cell filtrates of the culture medium. After EDTA treatment to permeabilize the outer membrane there was a threefold increase in the amount of protein in solution (from 0.5 to 1.5 mg liter−1) outside the cells, indicating that periplasmic proteins were released. There was only a slight reduction of Cr(VI) using the periplasmic fraction under anaerobic or aerobic conditions when the total protein concentration was approximately the same as the concentration in the S150 fraction (Fig. 10A).
FIG. 9.
Cr(VI) reduction by cellular fractions isolated after differential centrifugation and mechanical breakage. Cells were harvested from a stationary (unshaken) culture incubated at 22°C. Anaerobic reduction (solid lines with closed symbols) and aerobic reduction (dashed lines with open symbols) of cell fractions are shown.
FIG. 10.
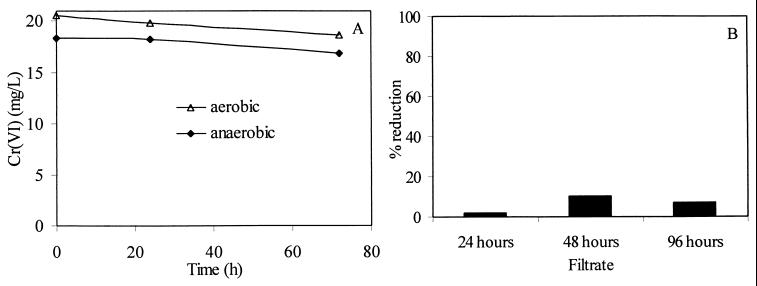
(A) Cr(VI) reduction in the isolated periplasmic fraction under aerobic and anaerobic conditions. (B) Percent chromate reduction in cell-free culture filtrates obtained at different incubation times. The initial Cr(VI) concentration was 20 mg liter−1.
Cell filtrates were obtained from a culture grown in VB medium with no Cr(VI) present to determine if active enzyme was released from the cell into the culture medium. This could be through secretion or through cell lysis. Culture medium was withdrawn at 24, 48, and 96 h; cells were separated from the bulk solution by centrifugation, and the resulting supernatant was filtered (0.22-μm-pore-size filter before being assayed. There was slight reduction in Cr(VI) by the filtrate samples taken at 24 and 96 h (Fig. 10B). Approximately 20% of a solution of 20 mg of Cr(VI) liter−1 was reduced over 72 h in the 48-h filtrate sample.
Kinetic analysis.
Km and Vmax values for the crude reductase from cell extracts and whole cells were determined. The kinetic constants were calculated by fitting the initial rate data to a double-reciprocal Lineweaver-Burk plot of 1/V [milligrams of Cr(VI) hour−1 milligram of protein−1] versus 1/[Cr(VI)] (milligrams liter−1) derived from a linear transformation of the Michaelis-Menten equation. This allowed the estimation of the specific Km and Vmax for the crude enzyme and the non-specific rate constants for whole cell reduction.
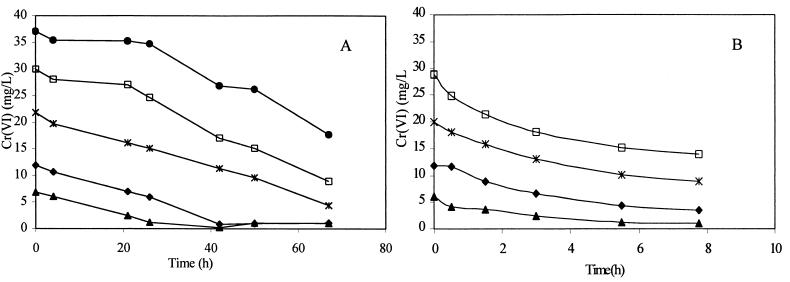
Washed cells were added to fresh VB medium containing various concentrations of Cr(VI), and the reaction was monitored in replicate samples for the loss of Cr(VI) over time (Fig. 11A). The cultures were incubated aerobically at 30°C in sealed Balch tubes. Initial reduction rates were estimated from the data in Fig. 11A. The nonspecific half-saturation or Michaelis-Menten constant, Km, was estimated to be 1.16 mg liter−1 (22.3 μM) and the maximum nonspecific reduction rate or maximum velocity, Vmax, was estimated to be 0.25 mg of Cr h−1 (4.8 μM h−1). Fraction S150, prepared from cell extracts as described in Materials and Methods, was used to determine the apparent Km and the maximum specific reduction rate, Vmax, of the crude reductase. S150 was assayed anaerobically in 0.01 M HEPES buffer (pH 7.1) at 30°C for the rate of reduction from 5 to 30 mg of chromate liter−1. No additional electron donors (other than found in this soluble cell extract fraction) were added to the solution. The total protein in the S150 fraction was measured and then added at a concentration of 6.4 mg liter−1. It was assumed that with excess substrate the reaction would be pseudo first order and the initial reaction rate would be independent of the substrate concentration. Cr(VI) reduction rates in the S150 fraction for 10 to 30 mg liter−1 are shown in Fig. 11B. Anaerobic reduction by S150 resulted in an apparent Km of 23 mg liter−1 (437μM) and a maximum specific velocity, Vmax, of 0.98 mg of Cr h−1 mg of protein−1 (19 μM h−1 mg of protein−1).
FIG. 11.
(A) Cr(VI) reduction of intact cells in VB medium at five different initial Cr(VI) concentrations. (B) Chromate reduction in the soluble fraction of cell extracts (S150). The supernatant fraction was assayed anaerobically at various initial chromate concentrations in 0.1 M HEPES buffer at 30°C.
DISCUSSION
Chromate was readily reduced by CRB5 under aerobic and anaerobic conditions as cell density increased, with concomitant decreases in redox potential and pH. The changes in solution chemistry were not simply a result of reactions involving chromate reduction; they were the ultimate result of cellular metabolism as CRB5 grew. Production of acidic metabolic byproducts from aerobic or anaerobic respiration may account for the observed pH decrease. The Eh decrease was a result of using oxygen as a terminal electron acceptor during aerobic respiration as well as Cr(VI) reduction. Similar changes in chemistry were observed with the groundwater from the site. At neutral pH, chemical thermodynamics predict that Cr(III) will readily form insoluble oxides and hydroxides. The low Eh generated by CRB5 actually favors the precipitation of Cr(III) hydroxides (31). TEM and EDS revealed that Cr(III) was uniformly adsorbed to the surface of the cells and that precipitates resulted (Fig. 3). Precipitates were also dispersed in the bulk solution. Bacteria are excellent nucleation sites for fine-grained mineral formation due to their high surface area-to-volume ratio (3) and the presence of electronegative surface functional groups (e.g., carboxyl), phosphoryl, and hydroxyl groups [6, 14, 15]). As Cr(VI) is reduced, Cr(III) is free to bind stoichiometrically to these sites and, once bound, will act as a template for further heterogeneous nucleation and crystal growth (5). The presence of measurable Cr in solution also suggests that a proportion of the reduced Cr is complexed with soluble organic compounds, perhaps electronegative exopolymers liberated from capsules (Fig. 4 and 5) (16). DeLeo and Ehrlich reported that formation of Cr(III) precipitates was not evident in batch and continuous cultures of P. fluorescens LB300 (13), whereas Pseudomonas chromatophilia, P. ambigua, and Pseodomonas maltophilia all induce the formation of precipitates (7, 18, 21).
The toxic effect of Cu (40 mg liter−1) and As (20 mg liter−1) on the rate of reduction of Cr(VI) was insignificant compared to that for controls containing Cr(VI) only. At high As levels, the low reduction rate may be due to the toxicity of As(V) on CRB5, which would decrease the number of viable cells. It is also possible that arsenate could compete as an electron acceptor. Using TEM and EDS, copper was adsorbed on extracellular polymeric fibers produced by the cells (Fig. 5), which should lower the metal's toxicity. The potential bioremediation use of CRB5 at site is enhanced by its ability to tolerate high levels of Cr(VI), Cu(II), and As(V); these metals will not inhibit reduction.
Resting cells were able to anaerobically reduce Cr(VI), Co(III), and U(VI) but not Fe(III) with lactate as an electron donor. Slightly higher U(VI) reduction rates were observed under aerobic conditions with glucose rather than lactate as the electron donor. Complete reduction of 170 mg of U(VI) liter−1 was observed after 125 h, with cells accumulating the reduced uranium both on the surface and internally (Fig. 7). We believe surface precipitation was via exposed reactive groups (5, 6), although it could also be due to surface phosphatase activity (24). The internalization of U was not predicted, as it is most likely toxic to the cell once in the cytoplasm. CRB5, however, contains large polyphosphate granules (26) which bind metals (4). Uranium forms strong complexes with phosphate and could be bound to these internal granules as well as to other components within the cytoplasm (Fig. 7). Although CRB5 can reduce Cr(VI), Co(III), and U(VI), it is not clear if the metal ions are reduced so as to derive energy (as terminal electron acceptors) during anaerobic respiration or to detoxify them.
CRB5, although closely related to P. fluorescens (26), uses a soluble reductase to reduce chromate. After separation of the cell membranes from soluble cellular components, aerobic and anaerobic chromate reduction activity was found in the soluble fractions (S12 and S150). The membrane fraction showed no activity; the location of the enzyme is restricted to the cytoplasm or, possibly, the periplasm. All soluble fractions, including periplasmic and cell-free filtrates, were obtained from cultures grown without Cr(VI), therefore, the presence of Cr(VI) is not required for enzyme production. Although some reductase may be present in the periplasm, there was not a significant amount of reduction by this periplasmic fraction over 72 h compared to that observed in the S150 fraction. Total protein concentrations in the S150 and periplasmic fractions were 1.4 and 1.5 mg liter−1, respectively. Since reductase activity was also observed in cell filtrates from 48-h cultures, the enzyme could be secreted or its presence could be due to cell lysis. There are obvious advantages to the bacterium if it transports the enzyme into its environment, such as our toxic metal-contaminated site, since it would act as a detoxifying agent. And, since a proportion of reductase is also available in the periplasm, it would further inhibit the entry of toxic metal into cells. The majority of the enzyme resides in the cytoplasm. Accordingly, we suggest that CRB5 could have three lines of defense against Cr(VI) and other toxic heavy metals. The cytoplasm is where the reductase is synthesized so as to develop a cellular pool [which could detoxify Cr(VI) if necessary and be the last line of defense]. This pool would supply the periplasm with a small but active concentration of the enzyme so that incoming metal ions could be detoxified (second line of defense). The pool could also act as a resevoir for secretion or be liberated by cell lysis (first line of defense).
Other bacteria possess different resistance mechanisms. For example, a plasmid confers Cr resistance in P. fluorescens LB300, Pseudomonas aeruginosa, P. putida, and Alcaligenes eutrophus via decreased uptake of the metal (8, 11, 27, 28), whereas sensitive strains do not contain the plasmid (10). A membrane protein, ChrA, confers tolerance by extrusion of chromate ions in P. aeruginosa and A. eutrophus (1), but P. ambigua G-1 has a capsule thought to inhibit chromate from entering (18). It is interesting, then, that in the groundwater and Cu situations, CRB5 produced an exoplymeric capsule in which Cr(III) precipitates were found (Fig. 4 and 5). This could be an additional line of defense.
One result, however, does not fully support the detoxification model of chromate reduction. Anaerobic reduction by whole-cell suspensions under resting conditions with lactate as an electron donor was significantly greater than that by controls without lactate (Fig. 8). Chromate reduction ceased after 70 h and was not complete over the 140 h it was monitored. Increased reduction in lactate-containing cultures indicated that it was an active process. Cells may be reducing chromate by using it as a terminal electron acceptor in anaerobic respiration. Another alternative is that the soluble reductase may be enhanced by the addition of an electron donor, which has been reported previously with glucose in P. fluorescens (9). If cells are using chromate to derive energy, membrane-associated reductive activity would be expected, since the electron transport chain is in the plasma membrane.
Reduction by Escherichia coli ATCC 33456, under anaerobic and aerobic conditions, has linked a soluble Cr(VI) reductase to the membrane electron transport chain (32). Although Cr(VI) reduction was largely due to soluble reductase activity, the results from those authors indicate that there was minor Cr(VI) reductase activity associated with this respiratory chain. These cytochromes required the presence of the soluble reductase for electron transport to Cr(VI). H2-reduced cytochromes b and d were reoxidized by Cr(VI) and oxygen in a fraction containing both the soluble reductase and the cytochromes, whereas the isolated cytochrome membrane fraction was not reoxidized by Cr(VI). The authors proposed that only one enzyme was responsible for electron transport to Cr(VI) and that an active site in the reductase became membrane associated. They also suggested that there was not enough energy generated from this reduction at the cell surface by a soluble reductase to support growth. However, fermentation with an eventual Cr(VI) electron acceptor via an abbreviated electron transport chain is energetically favorable and may be a possible mechanism by which cells can derive energy from chromate reduction (32). This may also explain the minor reductive activity of CRB5 observed during resting when an electron donor such as glucose is present.
Characterization of the chromate reduction activity by CRB5 revealed certain similarities to the E. coli system. Both E. coli and CRB5 can use endogenous electron donor reserves and do not require the presence of an external electron donor such as NADH or glucose. Furthermore, both E. coli and CRB5 have an increased rate of reduction with increased cell density and no significant influence on the rate of Cr(VI) reduction with redox change. It is possible that CRB5 can reduce Cr(VI) in the same manner as E. coli under aerobic and anaerobic conditions. In comparison, the reduction by the soluble fractions of both P. ambigua and P. putida PRS2000 and MK1 required NADH or NADPH as an electron donor for activity. Kinetic analysis of our crude reductase activity in the soluble fraction (S150), conducted under anaerobic conditions, resulted in a Michaelis-Menten constant (Km) of 23 mg liter−1 (437 μM) and a maximum specific reduction rate (Vmax) of 0.98 mg of Cr h−1 mg of protein−1 (317 nmol min−1 mg of protein−1) These Km values are 10 times higher than those reported for P. putida PRS2000 (19) and 34 times higher than those for P. ambigua G-1. With the addition of NADH, the soluble reductase activity from P. putida MK1 was 374 μM and 1.72 μmol min−1 mg of protein−1 (30).
CRB5 appears to have developed a highly complex set of strategies to deal with toxic metals at high concentrations through enzymatic and nonenzymatic processes. By producing a soluble enzyme that can catalyze the reduction of Cr(VI) to Cr(III), it can detoxify its environment. Cell envelope and capsule exopolymer chromium complexation also inhibit the metal from entering the cytoplasm. Once reduced, Cr(III) is then free to bind to the electronegatively charged surface functional groups on the cell surface, including those on the outer membrane and capsule, which serve as nucleation sites for further precipitation (5, 6). By employing this battery of processes, chromate can be effectively detoxified by reduction and removed from solution. These particular traits for Cr(VI) and other metal reductions, along with the bacterium's ability to survive in the stressful on-site environmental conditions from which it was isolated (25, 26), may make CRB5 a suitable candidate for a bioremediation strategy.
ACKNOWLEDGMENTS
The assistance of R. Harris and D. Moyles with TEM and EDS is gratefully acknowledged.
This work was funded by a Natural Sciences and Engineering Research Council (NSERC) of Canada research grant to T.J.B. J.M. was funded by an NSERC industrial scholarship combined with partial funding through Total Forest Industries Ltd., Guelph, Ontario, Canada. The electron microscopy was performed in the NSERC Guelph Regional Scanning Transmission Electron Microscopy Facility (GRSF), which is partially funded by an NSERC Major Facilities Access grant to T.J.B.
REFERENCES
- 1.Alvarez A H, Moreno-Sanchez R, Cervantes C. Chromate efflux by means of the ChrA chromate resistance protein from Pseudomonas aeruginosa. Appl Microbiol Biotechnol. 1999;181:7398–7400. doi: 10.1128/jb.181.23.7398-7400.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Public Health Association. Standard methods for the examination of water and wastewater. 17th ed. Washington, D.C.: American Public Health Association; 1989. [Google Scholar]
- 3.Beveridge T J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988;34:363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge T J. The structure of bacteria. In: Leadbetter E R, Poindexter J S, editors. Bacteria in nature: a treatise on the interaction of bacteria and their habitats. Vol. 3. New York, N.Y: Plenum Publishing Co.; 1989. pp. 1–65. [Google Scholar]
- 5.Beveridge T J, Murray R G E. Uptake and retention of metals by cell walls of Bacillus subtilis. J Bacteriol. 1976;127:1502–1518. doi: 10.1128/jb.127.3.1502-1518.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beveridge T J, Murray R G E. Sites of metal deposition in the cell wall of Bacillus subtilis. J Bacteriol. 1980;141:876–887. doi: 10.1128/jb.141.2.876-887.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake R C, II, Choate D M, Bardhan S, Revis N, Barton L L, Zocco T G. Chemical transformation of toxic metals by a Psuedomonas strain from a toxic waste site. Environ Toxicol Chem. 1993;12:1365–1376. [Google Scholar]
- 8.Bopp L H, Chakrabarty A M, Ehrlich H L. Chromate resistance plasmid in Pseudomonas fluorescens. J Bacteriol. 1983;155:1105–1109. doi: 10.1128/jb.155.3.1105-1109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bopp L H, Ehrlich H L. Chromate resistance and reduction in Pseudomonas fluorescence strain LB300. Arch Microbiol. 1988;150:426–431. [Google Scholar]
- 10.Cervantes C. Bacterial interactions with chromate. Antonie Leeuwenhoek. 1991;59:229–233. doi: 10.1007/BF00583675. [DOI] [PubMed] [Google Scholar]
- 11.Cervantes C, Ohtake H. Plasmid-determined resistance to chromate in Pseudomonas aeruginosa. FEMS Microbiol Lett. 1988;56:173–176. [Google Scholar]
- 12.Chen J M, Hao O J. Environmental factors and modeling in microbial chromium(VI) reduction. Water Environ Chem. 1996;68:1156–1164. [Google Scholar]
- 13.De Leo P C, Ehrlich H L. Reduction of hexavalent chromium by Pseudomonas fluorescens LB300 in batch and continuous cultures. Appl Microbiol Biotechnol. 1994;40:756–759. [Google Scholar]
- 14.Doyle R J, Matthews T H, Streips U N. Chemical basis for selectivity of metal ions by the Bacillus subtilis cell wall. J Bacteriol. 1980;143:471–480. doi: 10.1128/jb.143.1.471-480.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin D, Ferris F G, Beveridge T J. Surface-mediated mineral development by bacteria. Rev Mineral. 1997;35:161–180. [Google Scholar]
- 16.Geesey G G, Jang L. Interactions between metal ions and capsular polymers. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley and Sons; 1989. pp. 325–358. [Google Scholar]
- 17.Gopalan R, Veeramani H. Studies on microbial chromate reduction by Pseudomonas sp. in aerobic continuous suspended growth cultures. Biotechnol Bioeng. 1994;43:471–476. doi: 10.1002/bit.260430606. [DOI] [PubMed] [Google Scholar]
- 18.Horitsu H, Futo S, Miyazawa Y, Ogai S, Kawai K. Enzymatic reduction of hexavalent chromium by hexavalent chromium tolerant Pseudomonas ambigua G-1. Agric Biol Chem. 1987;51:2417–2420. [Google Scholar]
- 19.Ishibashi Y, Cervantes C, Silver S. Chromium reduction in Pseudomonas putida. Appl Environ Microbiol. 1990;56:2268–2270. doi: 10.1128/aem.56.7.2268-2270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jardine P M, Taylor D L. Kinetics and mechanisms of Co(II)EDTA oxidation by pyrolusite. Geochim Cosmochim Acta. 1995;59:4193–4203. [Google Scholar]
- 21.Lebedeva E V, Lyalikova N N. Reduction of crocoite by Pseudomonas chromatophila species nova. Mikrobiologiya. 1979;48:517–522. [PubMed] [Google Scholar]
- 22.Lovley D R. Dissimilatory metal reduction. Annu Rev Microbiol. 1993;47:263–290. doi: 10.1146/annurev.mi.47.100193.001403. [DOI] [PubMed] [Google Scholar]
- 23.Lovley D R, Coates J D. Bioremediation of metal contamination. Curr Opin Biotechnol. 1997;8:285–289. doi: 10.1016/s0958-1669(97)80005-5. [DOI] [PubMed] [Google Scholar]
- 24.Macaskie L E, Blackmore J D, Empson R M. Phosphatase overproduction and enhanced uranium accumulation by a stable mutant of a Citrobacter sp. isolated by a novel method. FEMS Microbiol Lett. 1988;55:157–162. [Google Scholar]
- 25.McLean J, Beveridge T J, Phipps D. Chromate removal from contaminated groundwater using indigenous bacteria. In: Alleman B C, Leeson A, editors. Proceedings of the In-Situ and On-Site Bioremediation Fifth International Symposium. Columbus, Ohio: Battelle Press; 1999. pp. 121–126. [Google Scholar]
- 26.McLean J, Beveridge T J. Isolation and characterization of a chromium-reducing bacterium from a chromated-copper arsenate contaminated site. Environ Microbiol. 2000;1:1–10. doi: 10.1046/j.1462-2920.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 27.Mondaca M A, Gonzalez C L, Zaror C A. Isolation, characterization and expression of a plasmid encoding chromate resistance in Pseudomonas putida KT2441. Lett Appl Microbiol. 1998;26:367–371. [Google Scholar]
- 28.Nies D H, Silver S. Plasmid-determined inducible efflux is responsible for resistance to cadmium, zinc, and cobalt in Alcaligenes eutrophus. J Bacteriol. 1989;171:896–900. doi: 10.1128/jb.171.2.896-900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nossel N G, Hessel L A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966;241:3055–3062. [PubMed] [Google Scholar]
- 30.Park C H, Keyhan M, Wielinga B, Fendorf S, Matin A. Characterization of a novel Pseudomonas putida chromate reductase. Appl Environ Microbiol. 2000;66:1788–1795. doi: 10.1128/aem.66.5.1788-1795.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rai D, Sass B M, Moore D A. Chromium(III) hydrolysis constants and solubility of chromium(III) hydroxide. Inorg Chem. 1987;26:345–349. [Google Scholar]
- 32.Shen H, Wang Y T. Characterization of enzymatic reduction of hexavalent chromium by Escherichia coli ATCC 33456. Appl Environ Microbiol. 1993;59:3771–3777. doi: 10.1128/aem.59.11.3771-3777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 34.Suzuki T, Miyata N, Horitsu H, Kawai K, Takamizawa K, Tai Y, Okazaki M. NAD(P)H dependent chromium reductase of Pseudomonas ambigua G-1: a Cr(V) intermediate is formed during the reduction of Cr(VI) to Cr(III) J Bacteriol. 1992;174:5340–5345. doi: 10.1128/jb.174.16.5340-5345.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tebo B M, Obraztsova A Y. Sulfate-reducing bacterium grows with Cr(VI), U(VI), Mn(IV) and Fe(III) as electron acceptors. FEMS Microbiol Lett. 1998;162:193–198. [Google Scholar]
- 36.Truex M J, Peyton B M, Valentine N B, Gorby Y A. Kinetics of U(VI) reduction by a dissimilatory Fe(III)-reducing bacterium under non-growth conditions. Biotechnol Bioeng. 1997;55:490–496. doi: 10.1002/(SICI)1097-0290(19970805)55:3<490::AID-BIT4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Turick C E, Graves C, Apel W A. Bioremediation potential of Cr(VI)-contaminated soil using indigenous microoganisms. Bioremediation J. 1998;2:1–6. [Google Scholar]
- 38.Wang P C, Mori T, Komori K, Sasatsu M, Toda K, Ohtake H. Isolation and characterization of an Enterobacter cloacae strain that reduces hexavalent chromium under anaerobic conditions. Appl Microbiol Biotechnol. 1989;55:1665–1669. doi: 10.1128/aem.55.7.1665-1669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto K, Kato J, Yano T, Ohtake H. Kinetics and modeling of hexavalent chromium reduction in Enterobacter cloacae. Biotechnol Bioeng. 1993;41:129–133. doi: 10.1002/bit.260410117. [DOI] [PubMed] [Google Scholar]
- 40.Yassi A, Nieboer E. Carcinogenicity of chromium compounds. In: Nriagu J O, Nieboer E, editors. Chromium in the natural and human environments. New York, N.Y: John Wiley and Sons; 1988. pp. 514–530. [Google Scholar]