Abstract
Susceptibility to protoxin and toxin forms of Cry1Ab and the binding of 125I-labeled Cry1Ab and Cry1Ac has been examined in three Plodia interpunctella colonies, one susceptible (688s) and two resistant (198r and Dplr) to Bacillus thuringiensis. Toxicological studies showed that the 198r colony was 11-fold more resistant to Cry1Ab protoxin than to Cry1Ab activated toxin, whereas the Dplr colony was 4-fold more resistant to protoxin versus toxin. Binding results with 125I-labeled toxins indicated the occurrence of two different binding sites for Cry1Ab in the susceptible insects, one of them shared with Cry1Ac. Cry1Ab binding was found to be altered in insects from both resistant colonies, though in different ways. Compared with the susceptible colony, insects from the Dplr colony showed a drastic reduction in binding affinity (60-fold higher Kd), although they had similar concentrations of binding sites. Insects from the 198r colony showed a slight reduction in both binding affinity and binding site concentration (five-fold-higher Kd and ca. three-fold-lower Rt compared with the 688s colony). No major difference in Cry1Ac binding was found among the three colonies. The fact that the 198r colony also has a protease-mediated mechanism of resistance (B. Oppert, R. Hammel, J. E. Throne, and K. J. Kramer, J. Biol. Chem. 272:23473–23476, 1997) is in agreement with our toxicological data in which this colony has a different susceptibility to the protoxin and toxin forms of Cry1Ab. It is noteworthy that the three colonies used in this work derived originally from ca. 100 insects, which reflects the high variability and high frequency of B. thuringiensis resistance genes occurring in natural populations.
Bacillus thuringiensis, a gram-positive entomopathogenic bacterium, produces different kinds of crystal inclusions during sporulation (22). These crystal inclusions are composed of one or various Cry proteins (also called δ-endotoxins or ICPs). Some of these proteins are highly toxic to certain insects, but they are harmless to most other organisms, including wildlife and beneficial insects.
The toxicity of B. thuringiensis crystal inclusions follows, after ingestion by the insect, a complex process including multiple steps. These include the (i) solubilization of the crystal to release the Cry proteins in their protoxin form, (ii) activation of the protoxins by midgut proteases to their active form, (iii) binding of the toxin to a midgut receptor, and (iv) pore formation. Insects that become resistant to B. thuringiensis do so by altering one or more steps of this process. Resistance to B. thuringiensis was first reported in Plodia interpunctella (11), and it was subsequently described in other insect species that have developed resistance to one or more Cry proteins. Thus, resistance to B. thuringiensis was found in field populations of Plutella xylostella and in laboratory-selected strains of Heliothis virescens, Spodoptera exigua, Trichoplusia ni, and other species (6, 25).
Knowledge of the mechanism of resistance is important in order to prolong the usefulness of B. thuringiensis commercial products, including transgenic plants expressing Cry proteins. The best-characterized mechanism of resistance is the alteration of binding of Cry proteins to their midgut receptors. Some resistant strains of P. interpunctella, P. xylostella, and H. virescens have been shown to have lost (or have reduced) the capacity of binding Cry1A-type proteins (6, 25). A different mechanism involves alterations in the gut proteinase activities that interact with B. thuringiensis toxins and has been described for P. interpunctella and in H. virescens. Absence of a major gut protease associated with Cry1Ac protoxin activation was demonstrated in the 198r colony of P. interpunctella (17, 18), which had been selected with B. thuringiensis subsp. entomocidus HD198 and became resistant to Cry1Ab and Cry1Ac (12, 13). Genetic studies in this colony revealed a linkage of the absence of this protease with resistance to B. thuringiensis (18). Strain CP73-3 from H. virescens showed that, compared to a susceptible control strain, there was slower processing of the Cry1Ac protoxin to the active toxin and faster degradation of the toxin (5); no reduction of Cry1Ac or Cry1Ab binding was detected in this strain (7). Finally, a mechanism involving faster damaged-cell repair has been proposed to be contributing to the resistance in the H. virescens CP73-3 strain (10).
In the present work, we examined the binding of Cry1Ab and Cry1Ac in three P. interpunctella colonies, one susceptible (688s) and two resistant to these two Cry proteins (selected with their protoxin form) (13). The resistant colonies (198r and Dplr) had been selected with different B. thuringiensis products (the subspecies entomocidus and kurstaki, respectively) and differed in their levels of resistance (five times higher in the Dplr colony) (19). The Dplr colony was 59 times more resistant to Cry1Ac than to Cry1Ab, whereas the 198r colony was 29 times more resistant to Cry1Ac versus Cry1Ab. Additionally, the 198r colony has a protease-mediated mechanism of resistance and the Dplr colony does not (16, 18). Our results indicate that Cry1Ab binding is altered in insects from both resistant colonies. However, this alteration is substantially different in the two colonies. Furthermore, the fact that the 198r colony possesses two mechanisms of resistance has also been supported by bioassay tests with protoxin and toxin forms of Cry1Ab.
MATERIALS AND METHODS
Description of colonies.
Colonies of P. interpunctella included the B. thuringiensis-susceptible colony 688s, collected from farm grain storage in Riley County, Kans., in 1988 (12) and reared continuously in the laboratory on a cracked-wheat diet (14). The B. thuringiensis-resistant colonies Dplr and 198r were selected from 688s with B. thuringiensis subsp. kurstaki HD-1 (Dipel; Abbott Laboratories, Chicago, Ill.) and B. thuringiensis subsp. entomocidus HD-198, respectively. Resistant colonies were reared on cracked-wheat diets containing the B. thuringiensis formulation used for selection.
Toxin preparation.
The Cry1Ab protoxin used for bioassays was an Escherichia coli recombinant protein obtained from Plant Genetic Systems (now Aventis, Ghent, Belgium). To obtain the activated form, inclusion bodies of Cry1Ab protoxin were solubilized and then incubated with α-chymotrypsin (bovine pancreas; Sigma Chemical Co., St. Louis, Mo.) 200:1 (wt/wt; protoxin-enzyme) for 2 h, 30°C. Protoxin and toxin forms were verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown). Cry1Ab and Cry1Ac toxins for binding assays were prepared from recombinant B. thuringiensis strains EG7077 and EG11070 (Ecogen, Inc.), respectively. Solubilization, activation, and purification of the Cry proteins was performed according to published protocols (21).
Bioassays.
The procedure used was a modification of the single-larva bioassay (9). The diet consisted of 2.5 g of semihydrated cereal (Grape Nuts; Post), 2.5 g of wheat germ, 0.2 g of yeast, 9 mg of sorbic acid, 9 mg of methylparaben, 1.25 g of glycerin, and 1.25 g of water. After being mixed, the diet was flattened into a flat, thin “piecrust,” and disks were removed with a 4-mm cork borer. The previous bioassay used dehydrated apple cubes and third-instar larvae (9), whereas neonate larvae will readily consume the cereal-based diet used in the present bioassay. Diet cubes were treated with either suspensions of Cry1Ab protoxin inclusions or Cry1Ab toxin solutions placed in 16-well assay trays. Eggs were added to each well. Mortality was calculated from the number of survivors from treated samples compared with untreated controls at 14 days posthatching. For each dose, 16 larvae were used. Statistical analyses were made using the program POLO-PC (20).
Binding assays.
Brush border membrane vesicles (BBMV) were prepared from whole last-instar larvae by the differential magnesium precipitation method (4, 28) and then frozen in liquid nitrogen and kept at −80°C until used. The protein concentration in the BBMV was determined by the method of Bradford (3).
Cry1Ab and Cry1Ac were 125I labeled by the chloramine-T method (26). Binding assays were performed essentially as described previously (24), in a final volume of 0.1 ml of binding buffer (8 mM Na2HPO4; 2 mM KH2PO4; 150 mM NaCl, pH 7.4; 0.1% bovine serum albumin) containing various concentrations of BBMV and a concentration of 1.25 nM 125I-labeled Cry1Ab or 0.60 nM 125I-labeled Cry1Ac. Incubations were carried out at room temperature for 60 min. An excess of unlabeled toxin was used to determine the extent of nonspecific binding, which was ca. 1% of the total radioactivity. For 125I-Cry1Ab competition experiments, the reaction mixture contained 7.5, 10, or 15 μg of BBMV proteins from the 688s, 198r, or Dplr colonies, respectively. When using 125I-Cry1Ac, competitions were performed using 5 μg of BBMV proteins from either colony. Bound toxins were separated from unbound toxins by filtration through glass-fiber filters. Cold binding buffer (5 ml/filter) was used to wash the filters, and the radioactivity retained was measured in a model 1282 Compugamma CS gamma counter (LKB Pharmacia). Binding parameters were obtained using the LIGAND computer program (15).
RESULTS
Susceptibility to Cry1Ab protoxin and toxin.
The susceptibilities of larvae from 688s, Dplr, and 198r colonies to the protoxin and chymotrypsin-activated (toxin) forms of Cry1Ab are shown in Table 1. In the Dplr colony the resistant ratio (RR; 50% lethal dose [LD50] of the resistant colony divided by the LD50 of the susceptible colony) was fourfold higher with Cry1Ab protoxin than with toxin. However, larvae from the 198r colony showed a significantly higher resistance ratio toward the protoxin (RR = 264) than toward the toxin form (RR = 25). The LD50 values were consistently higher than previously reported values (13), presumably due to the difference in assay procedures. The previously obtained LD50 used third-instar larvae, whereas the LD50 in this report were obtained with neonate larvae.
TABLE 1.
Toxicity of Cry1Ab protoxin (inclusion bodies) or chymotrypsin-activated Cry1Ab (soluble toxin) with B. thuringiensis-susceptible (688s) and B. thuringiensis-resistant (198r and Dplr) colonies of P. interpunctellaa
| Cry1Ab | LD50 (CI) and RR valuesb for colony:
|
||||
|---|---|---|---|---|---|
| 688s (LD50) | Dplr
|
198r
|
|||
| LD50 | RR | LD50 | RR | ||
| Protoxin | 0.208 (0.072–0.458) | 218 (none) | 1,049 | 54.81 (34.19–71.04) | 264 |
| Toxin | 0.005 (0.002–0.012) | 1.45 (none) | 290 | 0.123 (0.062–0.227) | 25 |
Tests were performed using a diet cube assay.
LD50 values are expressed as micrograms per larvae, with the 95% confidence interval (CI) given in parentheses. The RR value is the LD50 of the resistant colony/LD50 of the 688s colony.
Binding of labeled toxins to BBMV.
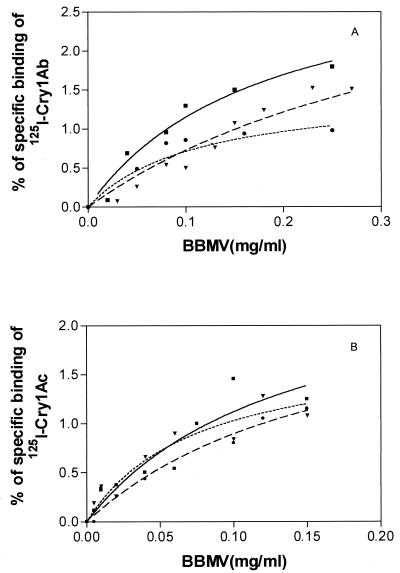
Specific binding of Cry1Ab and Cry1Ac to BBMV from insects of the 688s, 198r, and Dplr colonies was tested by incubation of 125I-labeled toxins with various concentrations of BBMV. Saturable binding was found with both toxins for all three colonies. Maximum specific binding of 125I-Cry1Ab was ca. 2, 1.5, and 1% of the total radioactivity for BBMV from the 688s, 198r, and Dplr colonies, respectively (Fig. 1A). The maximum specific binding of 125I-Cry1Ac to BBMV from all three colonies was ca. 1.3% of the total radioactivity added (Fig. 1B).
FIG. 1.
Specific binding of Cry1Ab (A) and Cry1Ac (B) as a function of P. interpunctella BBMV concentration. Nonspecific binding values were subtracted from each datum point. Lines: solid (■), 688s; dotted (●), Dplr; broken (▾), 198r.
Homologous competition experiments.
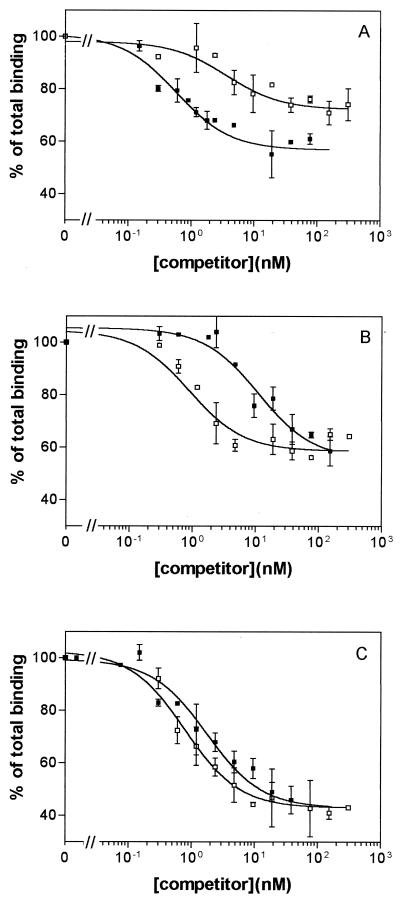
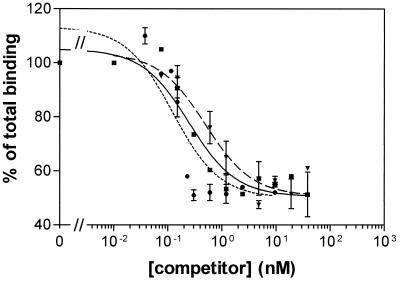
Homologous competition of 125I-Cry1Ab was performed to obtain quantitative estimates of the equilibrium dissociation constant (Kd) and the binding site concentration (Rt). Compared with BBMV from the 688s colony, Dplr showed a drastic reduction in binding affinity (60-fold-higher Kd), although a similar Rt value (Table 2 and Fig. 2). The 198r colony also showed altered Cry1Ab binding compared with the 688s colony, with a tendency for reduced binding affinity (fivefold-higher Kd, though not significantly different) and a slight significant reduction in the binding site concentration (threefold-lower Rt). The changes in the two parameters add up giving a decrease in overall binding affinity of 16-fold (Rt/Kd). In contrast, homologous competition of 125I-Cry1Ac just showed minor differences in binding among BBMV from the three colonies (Table 3 and Fig. 3). Kd and Rt values from 688s and Dplr were not significantly different, and the decrease in affinity of 198r, compared to 688s, is compensated for by the increase in binding site concentration.
TABLE 2.
Binding parameters from homologous competition experiments with 125I-Cry1Ab and BBMV from three P. interpunctella colonies
| Colony | Kd (nM) (SEM) | Rt (pmol/mg) (SEM) |
|---|---|---|
| 688s | 0.25 (0.12) | 2.11 (0.70) |
| Dplr | 14.48 (9.42) | 2.25 (0.38) |
| 198r | 1.31 (1.00) | 0.67 (0.32) |
FIG. 2.
Binding of 125I-Cry1Ab to P. interpunctella BBMV at increasing concentrations of unlabeled competitor (Cry1Ab [■] and Cry1Ac [□]). Each point represents the mean of two independent experiments. Panels: A, 688s; B, Dplr; C, 198r.
TABLE 3.
Binding parameters from homologous competition experiments with 125I-Cry1Ac and BBMV from three P. interpunctella colonies
| Colony | Kd (nM) (SEM) | Rt (pmol/mg) (SEM) |
|---|---|---|
| 688s | 0.21 (0.06) | 0.20 (0.09) |
| Dplr | 0.13 (0.02) | 0.19 (0.01) |
| 198r | 0.48 (0.01) | 0.37 (0.16) |
FIG. 3.
Binding of 125I-Cry1Ac to P. interpunctella BBMV at increasing concentrations of unlabeled Cry1Ac. Each point represents the mean of two independent experiments. Lines: solid (■), 688s; dotted (●), Dplr; broken (▾), 198r.
Heterologous competition experiments.
Incubation of a fixed amount of 125I-Cry1Ab with increasing concentrations of unlabeled Cry1Ac showed that both toxins competed for a common binding site in BBMV from the three colonies (Fig. 2). In the 688s colony, Cry1Ac competed for up to 50% of 125I-Cry1Ab specific binding (Fig. 2A), indicating the occurrence of two different binding sites for Cry1Ab, one shared with Cry1Ac and the other not shared. In contrast, total competition of specific binding was obtained when BBMV from the Dplr and 198r colonies were used (Fig. 2B and C).
DISCUSSION
The three colonies used in the present study were derived from ca. 100 adult insects (colony RC-688) collected from a farm grain storage bin with no known previous exposure to B. thuringiensis (12). The 688s colony was left unselected, Dplr was selected with Dipel (a B. thuringiensis subsp. kurstaki HD-1 formulation), and 198r was selected with B. thuringiensis subsp. entomocidus HD-198. Previous studies with these colonies revealed that 198r insects lacked a major gut proteinase that takes part in activation of Cry1Ac protoxin, whereas no difference in proteinase activity was found between Dplr and 688s (18).
Our bioassay results, using protoxin and toxin forms of Cry1Ab, show that levels of resistance in the 198r colony were different depending on the toxin form employed. Thus, the RR for Cry1Ab protoxin was 11-fold higher than the RR for activated Cry1Ab. This is in agreement with the biochemical feature previously reported for this colony and suggests that both Cry1Ab and Cry1Ac protoxins are processed by the same gut proteinase. In addition, the 198r colony was also found to be resistant to activated Cry1Ab (25-fold compared to the 688s colony). The RR of the 198r colony is 264-fold with the protoxin form and 25-fold with the toxin form of Cry1Ab, an indication that ca. 10% of the total resistance to the Cry1Ab protoxin is due to a mechanism unrelated with the toxin activation. Unexpectedly, the Cry1Ab toxin form used did somewhat influence the resistance levels in the Dplr colony. However, the effect of activated toxin was more dramatic with the 198r colony. Recently, we have found insects from the Dplr colony with proteinase patterns corresponding to insects from the 198r colony. This suggests a slight contamination of the Dplr colony with 198r insects, which would explain the difference in the RR values between protoxin and activated toxin in the Dplr colony.
In the present work we have studied differences in binding parameters as a plausible mechanism of resistance in the Dplr and 198r colonies. Although the total absence of binding was not found, quantitative differences in the binding parameters of Cry1Ab were detected. Insects from the Dplr colony showed a 60-fold reduction in Cry1Ab binding affinity compared to the 688s colony. This result indicates that the main mechanism of resistance in this colony is due to the alteration of a binding site. It is interesting to note that a similar result was previously reported in a different P. interpunctella-resistant colony that had also been selected with Dipel (27). Insects from this colony developed resistance to Cry1Ab and showed a reduction in binding affinity of this toxin of ca. 50-fold. Binding experiments of 125I-Cry1Ab with BBMV from the 198r colony showed a slightly higher Kd and a lower Rt than the susceptible colony. Compared to the susceptible insects, the 198r insects show a decrease in the overall binding affinity for Cry1Ab (estimated as the Rt/Kd ratio), which may account for the 25-fold resistance of this colony to the activated form of the Cry1Ab toxin.
Strong reduction or absence of toxin binding has been correlated with resistance to Cry1A toxins in insects from other Lepidoptera species, such as P. xylostella and H. virescens (25). Tabashnik et al. (23) have called “mode 1” of resistance to B. thuringiensis to be characterized by “extremely high resistance to at least one Cry1A toxin, recessive inheritance, little or no cross-resistance to Cry1C, and reduced binding of at least one Cry1A toxin.” The Dplr colony shows high resistance to Cry1Ab, extremely high resistance to Cry1Ac, and no cross-resistance to Cry1C (13), as well as reduced binding of Cry1Ab. Although no genetic data have been obtained for this colony, the features described above suggest that this colony could also belong to the “mode 1” resistance type.
Heterologous competition of 125I-Cry1Ab with unlabeled Cry1Ac showed that both toxins share a common binding site in P. interpunctella. However, the binding of 125I-Cry1Ac was not significantly affected in the Dplr and 198r colonies. It is possible that the same change in the Cry1A binding site had different effects on Cry1Ab and Cry1Ac binding. Whereas Cry1Ab affinity would be reduced, the change could only affect postbinding steps of the Cry1Ac mode of action, such as membrane insertion or pore formation. A similar situation has been found in two resistant strains of P. xylostella for which the Cry1A common binding site was altered in such a way that Cry1Ab binding was reduced, whereas the binding of Cry1Ac was not affected (2, 24, 29).
In the susceptible colony, Cry1Ac was unable to completely compete with 125I-Cry1Ab specific binding, indicating that not all Cry1Ab binding sites are accessible to Cry1Ac (Fig. 2A). This result means that Cry1Ab binds at least to two distinct sites in BBMV from the susceptible colony. The fact that the Cry1Ab datum points adjusted better to a one-binding-site model than to a two-binding-site model suggests similar binding parameters for the two sites. This is also in agreement with previous results with this insect species, for which one binding site for Cry1Ab was proposed from homologous competition experiments (27). In the resistant insects, Cry1Ac completely competed with 125I-Cry1Ab specific binding (Fig. 2B and C), which suggests that insects from the two resistant colonies have lost one of the Cry1Ab binding sites. Furthermore, the binding site alteration must be different in these two resistant colonies since Cry1Ab binding parameters and resistance levels differ considerably.
It is important to stress the fact that at least three different resistance alleles and/or genes occurred in the original population from which the three colonies in the present study were derived. Moreover, two of the resistance alleles and/or genes were coselected in the 198r colony. Since the original population consisted of approximately 100 insects (12), the frequency of every resistance allele in the original population should be at least 0.005 (1 in 200 copies of the gene). This frequency is in agreement with estimates obtained in other insect species for B. thuringiensis-resistant genes (1, 8, 25). However, in the case of the original population of P. interpunctella, this estimate applies to every one of the three different resistance alleles detected.
ACKNOWLEDGMENTS
We thank Luis Calzada Grau for technical assistance, Richard Hammel for developing the cereal bioassay, and Michele Zuercher for performing the bioassays. We also thank Aventis and Ecogen, Inc., for providing the recombinant strains used to prepare the toxins.
REFERENCES
- 1.Andow D A, Alstad D N. F2 screening for rare resistance alleles. J Econ Entomol. 1998;91:572–578. [Google Scholar]
- 2.Ballester V, Granero F, Tabashnik B E, Malvar T, Ferré J. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella) Appl Environ Microbiol. 1999;65:1413–1419. doi: 10.1128/aem.65.4.1413-1419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Escriche B, Silva F J, Ferré J. Testing suitability of brush border membrane vesicles prepared from whole larvae from small insects for binding studies with Bacillus thuringiensis CryIA(b) crystal protein. J Invertebr Pathol. 1995;65:318–320. [Google Scholar]
- 5.Forcada C, Alcácer E, Garcerá M D, Martinez R. Differences in the midgut proteolytic activity of two Heliothis virescens strains, one susceptible and one resistant to Bacillus thuringiensis toxins. Arch Insect Biochem Physiol. 1996;31:257–272. [Google Scholar]
- 6.Frutos R, Rang C, Royer M. Managing insect resistance to plants producing Bacillus thuringiensis toxins. Crit Rev Biotechnol. 1999;19:227–276. [Google Scholar]
- 7.Gould F, Martinez-Ramirez A, Anderson A, Ferré J, Silva F J, Moar W J. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci USA. 1992;80:7986–7990. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gould F, Anderson A, Jones A, Sumerford D, Keckel D G, Lopez J, Micinski S, Leonard R, Laster M. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens. Proc Natl Acad Sci USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson D E, McGaughey W H, Barnett B D. Small scale bioassay for the determination of Bacillus thuringiensis toxicity toward Plodia interpunctella. J Invertebr Pathol. 1991;57:159–165. [Google Scholar]
- 10.Martínez-Ramírez A C, Gould F, Ferré J. Histopathological effects and growth reduction in a susceptible and resistant strain of Heliothis virescens (Lepidoptera: Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci Technol. 1999;9:239–246. [Google Scholar]
- 11.McGaughey W H. Insect resistance to the biological insecticide Bacillus thuringiensis. Science. 1985;229:193–195. doi: 10.1126/science.229.4709.193. [DOI] [PubMed] [Google Scholar]
- 12.McGaughey W H, Johnson D E. Indianmeal moth (Lepidoptera: Pyralidae) resistance to different strains and mixture of Bacillus thuringiensis. J Econ Entomol. 1992;85:1594–1600. [Google Scholar]
- 13.McGaughey W H, Johnson D E. Influence of crystal protein composition of Bacillus thuringiensis strains on cross-resistance in Indianmeal moth (Lepidoptera: Pyralidae) J Econ Entomol. 1994;87:535–540. [Google Scholar]
- 14.McGaughey W H, Beeman R W. Resistance to Bacillus thuringiensis in colonies of Indianmeal moth and almond moth (Lepidoptera: Pyralidae) J Econ Entomol. 1988;81:28–33. [Google Scholar]
- 15.Munson P J, Rodbard D. LIGAND: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 16.Oppert B, Kramer K J, Johnson D E, MacIntosh S C, McGaughey W H. Altered protoxin activation by midgut enzymes from a Bacillus thuringiensis resistant strain of Plodia interpunctella. Biochem Biophys Res Commun. 1996;198:940–947. doi: 10.1006/bbrc.1994.1134. [DOI] [PubMed] [Google Scholar]
- 17.Oppert B, Kramer K J, Johnson D, Upton S J, McGaughey W H. Luminal proteinases from Plodia interpunctella and the hydrolysis of Bacillus thuringiensis CryIA(c) protoxin. Insect Biochem Mol Biol. 1996;26:571–583. doi: 10.1016/s0965-1748(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 18.Oppert B, Kramer K J, Beeman R W, Johnson D, McGaughey W H. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins. J Biol Chem. 1997;272:23473–23476. doi: 10.1074/jbc.272.38.23473. [DOI] [PubMed] [Google Scholar]
- 19.Oppert B, Hammel R, Throne J E, Kramer K J. Fitness costs of resistance to Bacillus thuringiensis in the Indianmeal moth, Plodia interpunctella. Entomol Exp Appl. 2000;96:281–287. [Google Scholar]
- 20.Robertson J L, Preisler H. Pesticide bioassays with arthropods. Boca Raton, Fla: CRC Press; 1992. [Google Scholar]
- 21.Sayyed A H, Haward R, Herrero S, Ferré J, Wright D J. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth. Appl Environ Microbiol. 2000;66:1509–1516. doi: 10.1128/aem.66.4.1509-1516.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabashnik B E, Liu Y-B, Malvar T, Heckel D G, Masson L, Ferré J. Insect resistance to Bacillus thuringiensis: uniform or diverse? Phil Trans R Soc Lond. 1998;353:1751–1756. [Google Scholar]
- 24.Tabashnik B E, Liu Y-B, Malvar T, Heckel D G, Masson L, Ballester V, Granero F, Ménsua J L, Ferré J. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc Natl Acad Sci USA. 1997;94:12780–12785. doi: 10.1073/pnas.94.24.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rie J, Ferré J. Insect resistance to Bacillus thuringiensis insecticidal crystal proteins. In: Charles J, Delecluse A, Nielsen-LeRoux C, editors. Entomopathogenic bacteria. Dordrecht, The Netherlands: Kluwer Academic Publications; 2000. pp. 219–237. [Google Scholar]
- 26.Van Rie J, Jansens S, Höfte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins: importance of specific receptors on the bruch border membrane of the midgut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 27.Van Rie J, McGaughey W H, Johnson D E, Barnett B D, Van Mellaert H. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science. 1990;247:72–74. doi: 10.1126/science.2294593. [DOI] [PubMed] [Google Scholar]
- 28.Wolfersberger M G, Luthy P, Maurer A, Parenti P, Sacchi V F, Giordana B, Hanozet G M. Preparation and partial characteritzation of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly (Pieris brassicae) Comp Biochem Physiol. 1987;86A:301–308. [Google Scholar]
- 29.Wright D J, Iqbal M, Granero F, Ferré J. A change in a single midgut receptor in the diamondback moth (Plutella xylostella) is only in part responsible for the field resistance to Bacillus thuringiensis subsp. kurstaki and B. thuringiensis subsp. aizawai. Appl Environ Microbiol. 1997;63:1814–1819. doi: 10.1128/aem.63.5.1814-1819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]