Abstract
In the same way that cry genes, coding for larvicidal delta endotoxins, constitute a large and diverse gene family, the cyt genes for hemolytic toxins seem to compose another set of highly related genes in Bacillus thuringiensis. Although the occurrence of Cyt hemolytic factors in B. thuringiensis has been typically associated with mosquitocidal strains, we have recently shown that cyt genes are also present in strains with different pathotypes; this is the case for the morrisoni subspecies, which includes strains biologically active against dipteran, lepidopteran, and coleopteran larvae. In addition, while one Cyt type of protein has been described in all of the mosquitocidal strains studied so far, the present study confirms that at least two Cyt toxins coexist in the more toxic antidipteran strains, such as B. thuringiensis subsp. israelensis and subsp. morrisoni PG14, and that this could also be the case for many others. In fact, PCR screening and Western blot analysis of 50 B. thuringiensis strains revealed that cyt2-related genes are present in all strains with known antidipteran activity, as well as in some others with different or unknown host ranges. Partial DNA sequences for several of these genes were determined, and protein sequence alignments revealed a high degree of conservation of the structural domains. These findings point to an important biological role for Cyt toxins in the final in vivo toxic activity of many B. thuringiensis strains.
Bacillus thuringiensis constitutes a large family of strains found in different habitats (2, 6) and highly specialized as insect pathogens. The main insecticidal factors displayed by these bacilli are the parasporal crystalline inclusions synthesized during the sporulation process (1). Other insecticidal factors also contribute to the final biological effects: vegetative insecticidal proteins, proteases, chitinases, exotoxins, and lipases have all been described (17, 30).
The delta endotoxins known so far fall into two categories, Cry and Cyt, that do not share significant sequence homology, although both types of toxins seem to work through pore formation that leads to cell lysis and irreversible damage of the insect midgut (24, 25, 26). While Cry toxins act via specific receptor recognition and binding (20), no specific receptors have been described for Cyt toxins, although they show specificity of action in vivo (13, 27).
More than 100 genes coding for Cry proteins have been isolated to date; they constitute a biodiverse family with different insect and noninsect targets including nematodes and protozoans (8, 19, 37). Antidipteran B. thuringiensis strains commonly feature the presence of Cyt proteins with cytolytic and hemolytic activities. Cyt1Aa, according to the new nomenclature (8), is a major component of the toxic crystal of B. thuringiensis subsp. israelensis (16) and was the first cytolytic toxin to be isolated and thoroughly characterized (4). Since then, others have been detected in different antidipteran B. thuringiensis strains: some related to Cyt1, like the one reported for the medellin subspecies (38), and some that have been classified into other groups based on immunological criteria (34, 44).
We have recently detected the presence of a second cyt gene in B. thuringiensis subsp. israelensis, named cyt2Ba1, which is functional and expresses a 27- to 28-kDa polypeptide (21); its predicted protein product is 67% similar to Cyt2Aa1, the 29-kDa cytolytic toxin from B. thuringiensis subsp. kyushuensis (28).
According to these results, the B. thuringiensis subsp. israelensis crystal would comprise three types of Cry toxins (Cry4A, Cry4B, and Cry11A), two types of Cyt toxins (Cyt1A and Cyt2B), and other components yet to be defined, all working altogether to give the final biological activity (12).
PCR amplification and Southern blot analysis confirmed the presence of homologues of cyt2Ba from B. thuringiensis subsp. israelensis in other mosquitocidal strains and, interestingly, also in the anticoleopteran subspecies morrisoni serovar Tenebrionis and others belonging to the morrisoni subspecies.
We report a broader screening for cyt2 genes and Cyt2-related proteins. Partial DNA sequences corresponding to the central portions of the predicted protein products were determined for several of these genes and aligned with other known Cyt toxins.
The positive correlation found between the coexistence of Cyt1-Cyt2 proteins and high mosquitocidal activity, in addition to the high conservation found among the sequences determined so far, point to an important biological role for Cyt proteins in the overall toxicity of the crystals. There is growing evidence that Cyt and Cry proteins interact in specific, synergistic combinations in the insect gut to exert their final biological effects (7, 33, 42). Our results indicate that cyt genes coding for hemolytic toxins are widely distributed among a range of B. thuringiensis subspecies and constitute another family of highly related genes.
MATERIALS AND METHODS
Strains, plasmids, and media.
The B. thuringiensis strains used in this work are listed in Tables 1 and 2. Escherichia coli DH5α (D. Hanahan) was used for plasmid propagation (22). B. thuringiensis subsp. kyushuensis, B. thuringiensis subsp. morrisoni serovar Tenebrionis, B. thuringiensis subsp. kurstaki, B. thuringiensis subsp. fukuokaensis, B. thuringiensis subsp. darmstadiensis, B. thuringiensis subsp. morrisoni HD518, B. thuringiensis subsp. morrisoni HD12, and B. thuringiensis subsp. israelensis 4Q2-72 were obtained from the Bacillus Genetic Stock Center (Ohio State University, Columbus). All other strains are from the IEBC collection held by the Laboratoire des Bactéries and Champignons Entomopathogènes (Pasteur Institute, Paris, France). Strains were maintained in Schaeffer's sporulation agar medium (36) and grown in Luria-Bertani medium (32) for DNA isolation. Liquid cultures were grown with aeration (shaking) at 37°C (E. coli) or 30°C (B. thuringiensis). Ampicillin was added to autoclaved media at 100 μg/ml.
TABLE 1.
cyt2-positive strains: host ranges and the presence of IS240
| Strain | Subspecies | Serotype | Host rangea | PCR bandsb | IS240c |
|---|---|---|---|---|---|
| 1884 | israelensis | H14 | 1 | 469 | + |
| 4Q2-72 | israelensis | H14 | 1 | 469 | + |
| PG14 | morrisoni | H8a, H8b | 1, 3 | 469 | + |
| B51 | AAT021 | ND | 1 | 469 | + |
| 11S2-1 | canadensis | H5a, H5c | 1 | 469 | + |
| B175 | thompsoni | H12 | 1 | 469 | + |
| IMR 81-1 | malaysiensis | H36 | 1 | 469 | + |
| CIB 163-131 | medellin | H30 | 1 | 469 | + |
| 367 | jegathesan | H28a, H28c | 1 | 469 | + |
| 74-F-6-18 | kyushuensis | H11a, H11c | 1 | 469 | + |
| 84-I-1-13 | kukuokaensis | H3a, H3d, H3e | 1 | 469 | + |
| 73-E-10-2 | darmstadiensis | H10a, H10b | 1 | 469 | + |
| B.006 | ostriniae | H8a, H8c | ND | 469 | + |
| 78-FS-29-17 | tohokuensis | H17 | ND | 469 | + |
| Serovar Tenebrionis | morrisoni | H8a, H8b | 2 | 469, 600 | − |
| HD12 | morrisoni | H8a, H8b | 1, 3 | 469 | + |
| HD518 | morrisoni | H8a, H8b | 1, 3 | 469 | + |
| EA10192 | andalousiensis | H37 | 4 | 469 | + |
Host range was scored as follows: 1, toxic for dipteran larvae; 2, toxic for coleopteran larvae; 3, toxic for lepidopteran larvae; and 4, nontoxic for dipteran larvae (Culex or Aedes sp.).
Occurrence of cyt2-related genes based on PCR amplification with cyt2B-specific primers (in base pairs).
Occurrence of IS240 sequences based on hybridization with a IS240A probe (35).
ND, not determined.
TABLE 2.
cyt2-negative strains: host ranges and the presence of IS240-related sequences
| Strain | Subspecies | Serotype | Host rangea | IS240b |
|---|---|---|---|---|
| T08001 | morrisoni | H8a, H8b | 4 | + |
| DD960 | thompsoni | H12 | 4 | + |
| T03C001 | fukuokaensis | H3a, H3d, H3e | 1 | − |
| GM33 | monterrey | H28a, H28b | 4 | + |
| 271 | colmeri | H21 | 4 | + |
| Indiana | indiana | H16 | ND | + |
| Yunnanensis | yunnanensis | H20a, H20b | ND | + |
| 273B | cameroun | H32 | 4 | + |
| Finitimus | finitimus | H2 | 4 | + |
| HL51 | leesis | H33 | 1* | + |
| Pak 94 | pakistani | H13 | 4 | + |
| 3-71 | kumamotoensis | H18a, H18b | 4* | + |
| Ygd22-03 | jinghongiensis | H42 | 4* | + |
| HL47 | konkukian | H34 | 4* | + |
| 19-105 | novosibirsk | H24a, H24c | ND | + |
| GM18 | neoleonensis | H24a, H24b | ND | + |
| Berliner | thuringiensis | H1 | 3 | − |
| SLM 5.A | silo | H26 | 4* | + |
| 92-KU-137-4 | higo | H44 | 4** | + |
| Sotto G | sotto | H4a, H4b | 3 | + |
| HL1 | coreanensis | H25 | 4 | + |
| GM43 | mexicanensis | H27 | 4* | − |
| LFB 855 | oswaldocruzi | H38 | 4* | + |
| ABr33 | londrina | H10a, H10c | 4 | + |
| H11 | toumanoffi | H11a, H11b | 1* | + |
| B23 | pondicheriensis | H20a, H20c | ND | + |
| Toguchini | toguchini | H31 | 4* | + |
| DMU-38 | roskildiensis | H45 | 4 | + |
| KK31-01 | guiyangiensis | H43 | 4 | + |
| T2 | shandongiensis | H22 | ND | + |
| 84-F-58.20 | amagiensis | H29 | 4* | + |
Host range was scored as follows: 1, toxic for dipteran larvae (*, weakly mosquitocidal for Culex sp.); 2, toxic for coleopteran larvae; 3, toxic for lepidopteran larvae; 4, nontoxic for dipteran larvae (*, Culex or Aedes sp.; **, moderate antidipteran activity). ND, not determined.
Occurrence of IS240 sequences based on hybridization with a IS240A probe (35).
DNA manipulations.
Restriction enzymes and T4 DNA ligase (Gibco-BRL) were used as recommended by the manufacturers. DNA fragments were purified from gels with a Gene Clean kit (Bio 101). Plasmids from E. coli were prepared as described by Birnboim and Doly (3). Plasmid DNA was isolated from B. thuringiensis strains as described previously (5) and further purified by using Qiagen columns (Diagen GmbH; Qiagen, Inc.).
DNA sequencing.
cyt2B-like genes were amplified by using the upper and lower primers described below. PCR products were cloned into pGEM-T Easy Vector (Promega) and sequenced.
PCR reaction conditions.
A total of 20 to 50 ng of purified plasmid DNA was added to the PCR mixtures (2.2 mM concentrations of deoxynucleoside triphosphates, 2 mM MgCl2, 0.5 U of Taq polymerase [Promega], and 100 ng of PCR primers) in a final volume of 50 μl. The oligonucleotide primers were as follows: upper, 5′-AATACATTTCAAGGAGCTA-3′, and lower, 5′-TTTCATTTTAACTTCATATC-3′. Amplification was performed in a thermal cycler (M. J. Research Minicycler PTC100) by using a single denaturation step (3 min at 94°C), followed by a 35-cycle program, with each cycle consisting of the following: denaturation at 94°C for 45 s, annealing at 45°C for 45 s, and extension at 72°C for 1 min. A final extension step (72°C for 5 min) was also included. Next, 20-μl samples from each PCR reaction were electrophoresed on 1.5% agarose gels in 0.5× Tris-borate-EDTA buffer at 100 V for 30 to 35 min and stained with ethidium bromide.
Computer analysis.
DNA sequences were analyzed with the National Center for Biotechnology Information's BLAST WWW server and with the MegAlign program (Macintosh, v3.03; DNASTAR, Inc.).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported here have been submitted to the GenBank-EMBL database and were assigned accession numbers as follows (gene, number, B. thuringiensis subspecies, strain designation [where available]): cyt2Ba2, AF020789, morrisoni, PG14; cyt2Ba3, AF022884, fukuokaensis; cyt2Ba4, AF022885, morrisoni, HD12; cyt2Ba5, AF022886, morrisoni, HD518; cyt2B–, pending, medellin, 163-130; and cyt2Ba6, AF034926, morrisoni (serovar Tenebrionis).
Protein analysis.
B. thuringiensis strains were grown in Schaeffer's liquid sporulation medium (36) until lysis. Spore-crystal mixtures from 10-ml samples were harvested by centrifugation at 12,000 × g and then washed once in 1 M NaCl–2 mM phenylmethylsulfonyl fluoride–10 mM EDTA. Pellets were resuspended in sample buffer (32) supplemented with phenylmethylsulfonyl fluoride and EDTA as described earlier, boiled for 10 min, and subjected to sodium dodecyl sulfate (SDS)–15% polyacrylamide gel electrophoresis (PAGE). Protein concentrations were determined by the Bradford assay (Bio-Rad) on samples solubilized as previously described (10). Proteins were electrotransferred to nitrocellulose membranes and detected immunologically by the following method: the membrane was treated with 3% low-fat milk in a 1× Tris-buffered saline (TBS) solution at room temperature with gentle shaking for 1 h. Incubations with anti-Cyt2Aa1 (kindly provided by David Ellar, University of Cambridge) or anti-Cyt2Ba recombinant protein from B. thuringiensis subsp. israelensis were performed at room temperature for 1 h and then overnight at 4°C. Anti-Cyt2Aa1 was used at a 1:500 dilution, and anti-Cyt2Ba was added at a 1:1,000 dilution in a solution containing 3% low-fat milk.
Membranes were washed with gentle shaking at room temperature with three changes of 1× TBS for 10 min before being incubated with the secondary antibody with gentle shaking for 1 h. The Gibco-BRL detection system (biotinylated second antibody, streptavidin-alkaline phosphatase, and nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate toluidinium) was used as recommended by the manufacturer. Secondary antibody bound to the filter was visualized after three washes in 1× TBS for 5 min, one wash with NP-40 at 0.05%, and then one final wash in 1× TBS for 5 min.
RESULTS
PCR analysis.
A pair of oligonucleotide primers was designed from two highly conserved regions shared by the cyt2 genes from B. thuringiensis subsp. israelensis and B. thuringiensis subsp. kyushuensis (21). This primer set was used in PCR amplification experiments in order to search for the presence of this gene in a wider range of B. thuringiensis strains. As shown in Table 1, amplification products of the expected size (469 bp) were observed in all of the strains with known antidipteran activities. Other strains showing this fragment were either nonmosquitocidal (i.e., B. thuringiensis subsp. morrisoni serovar Tenebrionis and strain HD518) or have been described to have both antidipteran and antilepidopteran activities (HD12). Finally, the PCR-positive B. thuringiensis subsp. andalousiensis and subsp. ostriniae have not yet been fully characterized regarding their host ranges.
On the other hand, the PCR-negative group shown in Table 2 is composed mostly of nonmosquitocidal strains and the strain B. thuringiensis subsp. fukuokaensis T03C001 which, interestingly, was also found to be negative for IS240 by hybridization standards.
These findings suggest that the known correlation between a mosquitocidal pathotype and the presence of cyt genes may be particularly strong for cyt2-related genes. The morrisoni subspecies seems to be a special case, since all of the strains belonging to this group show cyt2 genes, although not all are known to display mosquitocidal activities.
Sequence alignments.
PCR amplification products from most of the positive strains were cloned and sequenced. DNA sequence comparisons made with the BLASTN and MegAlign programs revealed a high degree of homology (>90%). Interestingly, this high homology was also observed in the nonmosquitocidal strains B. thuringiensis subsp. morrisoni serovar Tenebrionis and HD518.
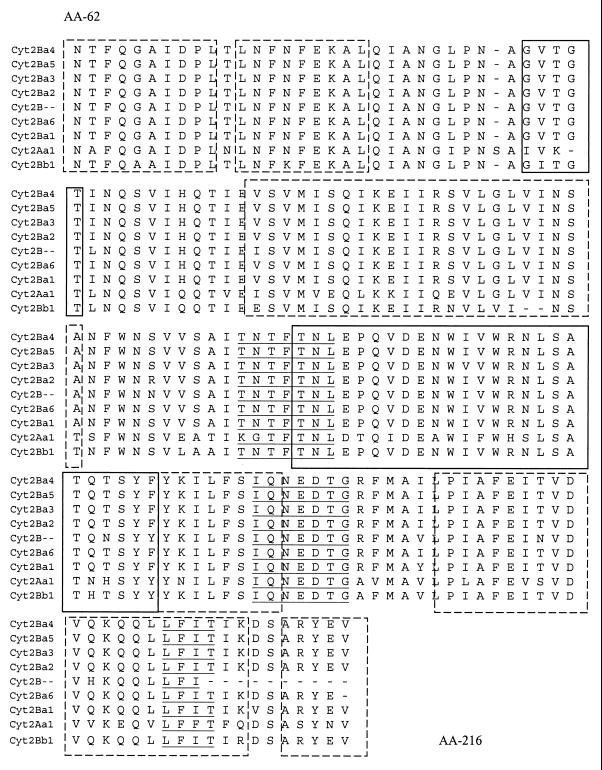
Sequence alignments of the central portions of the predicted protein products (154 amino acids from residues 62 to 216) representing 58% of the whole proteins, confirmed as shown in Fig. 1, that predicted α helices and β sheets are highly conserved throughout the different gene versions compared to the previously described Cyt2 toxins (see Discussion).
FIG. 1.
Amino acid sequence alignment of the different Cyt2 gene versions. Cyt2Ba4, B. thuringiensis subsp. morrisoni HD-12; Cyt2Ba5, B. thuringiensis subsp. morrisoni HD-518; Cyt2Ba3, B. thuringiensis subsp. fukuokaensis; Cyt2Ba2, B. thuringiensis subsp. morrisoni PG14; Cyt2B, B. thuringiensis subsp. medellin; Cyt2Ba6, B. thuringiensis subsp. morrisoni serovar Tenebrionis; Cyt2Ba1, B. thuringiensis subsp. israelensis; Cyt2Aa1, B. thuringiensis subsp. kyushuensis; Cyt2Bb1, B. thuringiensis subsp. jegathesan. Solid and broken boxes show predicted β strands and α helices, respectively. Underlining indicates amino acid motifs (see Discussion).
Expression of cyt2B-related genes.
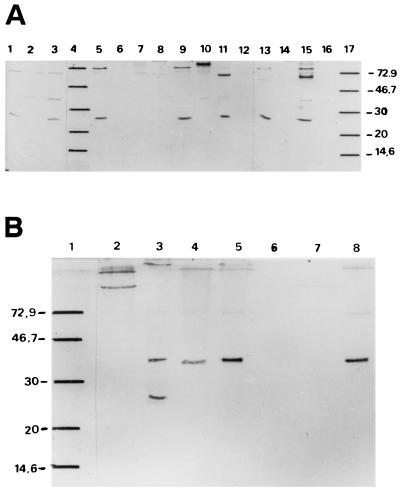
Western blot experiments were performed on spore-crystal extracts from cyt2-positive and some negative strains. Three groups could be defined according to their immunoreactivity with an antiserum raised against the B. thuringiensis subsp. israelensis Cyt2Ba1 recombinant protein (see Materials and Methods) (Fig. 2): group 1 includes strains that showed one reactive band of ca. 26 to 27 kDa (B. thuringiensis subsp. israelensis 1884, B. thuringiensis subsp. israelensis 4Q2-72, and B. thuringiensis subspp. canadensis, tohokuensis, and thompsoni 12007 and B51 [AAT021]); group 2 includes strains that showed one reactive band of ca. 33 kDa (B. thuringiensis subspp. higo, ostriniae, and morrisoni HD12 and B. thuringiensis subsp. morrisoni serovar Tenebrionis); and group 3, which includes strains B. thuringiensis subsp. morrisoni PG14 and B. thuringiensis subsp. morrisoni HD518, which showed both bands with similar intensities.
FIG. 2.
Immunological cross-reactivity analysis with antibody against Cyt2Ba1. A total of 2 μg of B. thuringiensis spore-crystal preparations were loaded into each lane, together with the molecular mass standard. (A) Lanes: 1, B. thuringiensis subsp. israelensis 4Q2-72; 2, B. thuringiensis subsp. kurstaki; 3, B. thuringiensis subsp. morrisoni HD12; 4, molecular mass standards; 5, B. thuringiensis subsp. tohokuensis; 6, B. thuringiensis subsp. guiyangiensis; 7, B. thuringiensis subsp. israelensis acrystalliferous strain; 8, B. thuringiensis subsp. roskildiensis; 9, B. thuringiensis AAT021; 10, B. thuringiensis subsp. higo; 11, B. thuringiensis subsp. thompsoni 12007; 12, B. thuringiensis subsp. ostriniae; 13, B. thuringiensis subsp. israelensis 1884; 14, B. thuringiensis subsp. medellin; 15, B. thuringiensis subsp. canadensis; 16, B. thuringiensis subsp. malaysiensis; 17, molecular mass standard. (B) Lanes: 1, molecular mass standard; 2, B. thuringiensis subsp. kurstaki; 3, B. thuringiensis subsp. morrisoni PG14; 4, B. thuringiensis subsp. ostriniae; 5, B. thuringiensis subsp. morrisoni HD518; 6, B. thuringiensis subsp. malaysiensis; 7, B. thuringiensis subsp. medellin; 8, B. thuringiensis subsp. morrisoni serovar Tenebrionis. Molecular mass markers are indicated on the left in kilodaltons.
Aggregation of these polypeptides might account for the higher bands observed in some cases, since this has also been observed upon SDS-PAGE of cloned Cyt proteins (A. Delécluse, unpublished results). Therefore, the bands of a similar size observed for B. thuringiensis subsp. kurstaki (negative for cyt2-related genes) could be attributable to nonspecific binding. When less protein was loaded into the gels, these higher bands were less intense, except for the group 3 strains.
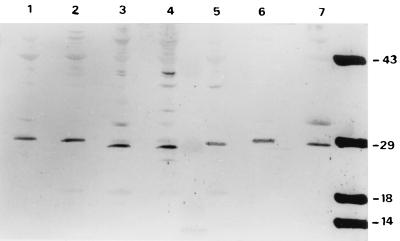
This expression study was also performed with an anti-Cyt2Aa from B. thuringiensis subsp. kyushuensis antiserum (27). As shown in Fig. 3, bands of between 27 and 29 kDa were revealed in B. thuringiensis subsp. kyushuensis, B. thuringiensis subsp. israelensis 1884 and 4Q2-72, B. thuringiensis subsp. morrisoni PG14, and B. thuringiensis subsp. morrisoni serovar Tenebrionis and strains HD12 and HD518. In this experiment, the 33-kDa band was also observed in some strain samples, but with less intensity.
FIG. 3.
Immunological cross-reactivity analysis with antibody against Cyt2Aa1. A total of 2 μg of B. thuringiensis spore-crystal preparations were loaded into each lane. Lanes: 1, B. thuringiensis subsp. morrisoni PG14; 2, B. thuringiensis subsp. tenebrionis; 3, B. thuringiensis subsp. kyushuensis; 4, B. thuringiensis subsp. israelensis 1884; 5, B. thuringiensis subsp. israelensis 4Q2-72; 6, B. thuringiensis subsp. morrisoni HD12; 7, B. thuringiensis subsp. morrisoni HD518. Molecular mass markers are indicated on the right in kilodaltons.
In general terms, all strains showed Cyt2B-related polypeptides that cross-reacted with both antisera. However, B. thuringiensis subspp. medellin and malayensis did not show reactive bands.
Only a faintly reactive 27-kDa band was observed in the B. thuringiensis subsp. malayensis extract when it was revealed with the anti-Cyt2Aa antibody from subsp. kyushuensis for an extended period of time, suggesting that this variant may be closer to Cyt2Aa than to Cyt2Ba (data not shown).
DISCUSSION
Fifty B. thuringiensis strains belonging to various serotypes and host ranges were screened for the presence of cyt2-related genes using PCR amplification. This analysis revealed a wide distribution for these genes coding for cytolytic factors, especially but not only, among strains that display antidipteran activities.
The presence of a cytolytic factor has been always associated with the antidipteran host ranges. In fact, Cyt1 toxins are the first ones described in the most active B. thuringiensis subspecies, israelensis and morrisoni PG14 (15). Although the exact role of these factors in the final biological activity of the crystals has not been fully defined (7, 10, 23) it is clear that they are able to synergize with some Cry toxins (33, 43). It has been recently shown that Cyt1 can lower resistance to Cry toxins in a number of target insects, as well as enhance the activity of B. sphaericus strains (40, 41). Positive interactions have been observed for Cry4-Cyt1 combinations (42, 43), whereas antagonistic effects were found with the Cry1Ac-Cyt1Aa combination (9). Also, sensitivity to Cyt1 was reported in Cry3-resistant B. thuringiensis subsp. morrisoni serovar Tenebrionis (18). This could be consistent with the fact that while cyt genes have been found to naturally occur in cry3- and cry4-bearing strains, it has not been detected in strains displaying exclusively antilepidopteran activities due to Cry1 toxins (reference 21 and this work).
The presence of insertion sequences in B. thuringiensis is very broad (see reference 31 for a review), and many of them are structurally associated with cry genes (37). Although IS240 insertion sequences have invariably been found in antidipteran strains, IS240-related sequences have been found to occur in a wide range of B. thuringiensis strains (35). These sequences have been found in the 5′ region of the cyt1Ab1 gene from B. thuringiensis subsp. medellin (38) and upstream of the cry11B gene from B. thuringiensis subsp. jegathesan (11). Similar observations were made for a plasmid in B. thuringiensis subsp. fukuokaensis (14).
The strong correlation observed between the presence of cyt2 genes, IS240 sequences, and antidipteran activity in the best-studied mosquitocidal strains (Tables 1 and 2) could reflect structural associations that might have promoted cyt dispersion among them. Therefore, the presence of cyt2-related genes could be considered a strong indicator of antidipteran host range, even when these genes may be present in other pathotypes as well.
The application of primers based on both cyt2 and IS240 sequences to PCR screening programs could help in the detection of new antidipteran strains with high predictability. According to this approach, B. thuringiensis subsp. ostriniae and subsp. andalousiensis should prove to be dipteran active once their biological activities are characterized.
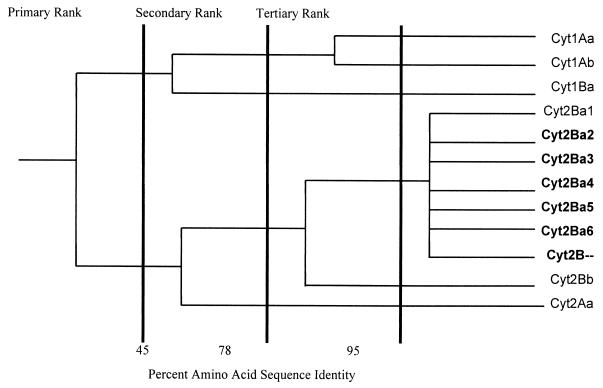
Figure 4 integrates the cyt2 gene family into the Cyt branch of the B. thuringiensis toxin dendrogram (8). Although this tree has been constructed based on partial sequences for many cyt2 genes and therefore may change once the full sequences are determined, it reflects the high degree of relatedness of the Cyt family of proteins. In fact, analysis of the predicted amino acid sequences shows that the different Cyt2 versions show a high degree of conservation in α-helices and β-sheets known to be involved in the formation of the lytic pore and in the structural integrity of the toxin molecule. The conservation of specific motifs (highlighted in Fig. 1, in the loops between α and β domains) that have been associated with cytolytic activity, interaction with the insect gut membrane, or pore formation indicate an important biological role for these toxins (29, 39).
FIG. 4.
Phylogram showing relationships between Cyt family components. This phylogenetic tree was modified from a TREEVIEW visualization of NEIGHBOR treatment of a CLUSTAL multiple alignment and distance matrix for the full-length toxin sequences. Thicker vertical lines demarcate the four levels of nomenclature ranks according to the results of Crickmore et al. (8). Protein names in boldface indicate that the central regions of the genes have been used for comparison.
Further work on the biological activity of isolated Cyt2 toxins alone and in combination with other Cyt and Cry toxins, as well as the construction of cyt2− mutants, is under way and will help in our understanding of the mechanisms operating in the toxicity of the native crystals. This knowledge may have great impact on the application of toxin combinations to insect resistance management programs.
ACKNOWLEDGMENTS
This work was supported by research grants from the Conservation, Food, and Health Foundation (United States) and CONICET (Argentine National Research and Technology Council).
We are grateful to Carmen Sanchez Rivas in whose laboratory this work was performed and for helpful discussion.
REFERENCES
- 1.Agaisse H, Lereclus D. How does Bacillus thuringiensis produce so much insecticidal crystal protein? J Bacteriol. 1995;177:6027–6032. doi: 10.1128/jb.177.21.6027-6032.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhard K, Jarrett P, Meadows M, Butt J, Ellis D J, Roberts G M, Pauli S, Rodgers P, Buerges H D. Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization, and activity against insect pests. J Invertbrate Pathol. 1997;70:59–68. [Google Scholar]
- 3.Birnboim H, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourgouin C, Delécluse A, Rapoport G. Characterization of the genes encoding the haemolytic toxin and the mosquitocidal delta-endotoxin of Bacillus thuringiensis israelensis. Mol Gen Genet. 1986;205:390–397. doi: 10.1007/BF00338072. [DOI] [PubMed] [Google Scholar]
- 5.Bourgouin C, Klier A, Rapoport G. Characterization of the genes encoding the haemolytic toxin and the mosquitocidal δ-endotoxins of Bacillus thuringiensis var. israelensis. Mol Gen Genet. 1986;205:390–397. doi: 10.1007/BF00338072. [DOI] [PubMed] [Google Scholar]
- 6.Chaufaux J, Marchal, Gilois N, Jehanno I, Buisson C. Investigation of natural strains of Bacillus thuringiensis in different biotopes throughout the world. Can J Microbiol. 1997;43:337–343. [Google Scholar]
- 7.Crickmore N, Bone E J, Williams J A, Ellar D J. Contribution of the individual components of the δ-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol Lett. 1995;131:249–254. [Google Scholar]
- 8.Crickmore N, Zeigler D R, Feitelson J, Schnepf E, Van Rie J, Lereclus D, Baum J, Dean D H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:808–813. doi: 10.1128/mmbr.62.3.807-813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Rincón Castro M C, Barajas-Huerta J, Ibarra J. Antagonism between Cry1Ac1 and Cyt1A1 toxins of Bacillus thuringiensis. Appl Environ Microbiol. 1999;65:2049–2053. doi: 10.1128/aem.65.5.2049-2053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delécluse A, Charles J-F, Klier A, Rapoport G. Deletion by in vivo recombination shows that the 28-kilodalton cytolytic polypeptide from Bacillus thuringiensis subsp. israelensis is not essential for mosquitocidal activity. J Bacteriol. 1991;173:3374–3381. doi: 10.1128/jb.173.11.3374-3381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delécluse A, Rosso M-L, Ragni A. Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl Environ Microbiol. 1995;61:4230–4235. doi: 10.1128/aem.61.12.4230-4235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delécluse A, Juárez-Pérez V, Berry C. Vector-active toxins: structure and diversity. In: Charles J-F, Delécluse A, Nielsen-Le Roux X, editors. Entomopathogenic bacteria: from laboratory to field application. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 101–125. [Google Scholar]
- 13.Drobniewski F A, Kowles B H, Ellar D J. Nonspecific ionic effects on the cytolytic properties of Bacillus thuringiensis δ-endotoxin. Curr Microbiol. 1987;15:295–299. [Google Scholar]
- 14.Dunn M G, Ellar D J. Identification of two sequence elements associated with the gene encoding the 24-kDa crystalline component in Bacillus thuringiensis subspecies fukuokaensis: an example of transposable element archaelogy. Plasmid. 1997;37:205–215. doi: 10.1006/plas.1997.1283. [DOI] [PubMed] [Google Scholar]
- 15.Earp D J, Ellar D J. Bacillus thuringiensis var. morrisoni strain PG14: nucleotide sequence of a gene encoding a 27 kDa crystal protein. Nucleic Acids Res. 1987;15:3619. doi: 10.1093/nar/15.8.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Earp D J, Ward E S, Ellar D J. Investigation of possible homologies between crystal proteins of three mosquitocidal strains of Bacillus thuringiensis. FEMS Microbiol Lett. 1987;42:195–199. [Google Scholar]
- 17.Estruch J J, Warren G W, Mullinis M A, Nye G J, Craig J A, Koziel M G. Vip3A, a novel Bacillus thuringiensis vegetative insecticidal protein with a wide spectrum of activities against lepidopteran insects. Proc Natl Acad Sci USA. 1996;93:5389–5394. doi: 10.1073/pnas.93.11.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federici B A, Bauer L S. Cyt1Aa protein of Bacillus thuringiensis is toxic to the cottonwood leaf beetle, Chrysomela scripta, and suppresses high levels of resistance to Cry3A. Appl Environ Microbiol. 1999;64:4368–4371. doi: 10.1128/aem.64.11.4368-4371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feitelson J S. The Bacillus thuringiensis family tree. In: Kim L, editor. Advanced engineered pesticides. New York, N.Y: Marcel Dekker, Inc; 1993. pp. 63–71. [Google Scholar]
- 20.Gill S S, Cowles E A, Pietrantonio P P. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 21.Guerchicoff A, Ugalde R, Rubinstein C. Identification and characterization of a previously undescribed cyt gene in Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 1997;63:2716–2721. doi: 10.1128/aem.63.7.2716-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D. Studies on the transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 23.Held G A, Huang Y-S, Kawanishi C Y. Effect of removal of the cytolytic factor of Bacillus thuringiensis subsp israelensis on mosquito toxicity. Biochem Biophys Res Commun. 1986;141:937–941. doi: 10.1016/s0006-291x(86)80133-4. [DOI] [PubMed] [Google Scholar]
- 24.Knowles B H, Ellar D J. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different specificity. Biochim Biophys Acta. 1987;924:509–518. [Google Scholar]
- 25.Knowles B H, Ellar D J. Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different specificity. Biochim Biophys Acta. 1987;924:509–518. [Google Scholar]
- 26.Knowles B H, Blatt M R, Tester M, Horsnell J M, Carroll J, Menestrina G, Ellar D J. A cytolytic δ-endotoxin from Bacillus thuringiensis var. israelensis forms cation-selective channels in planar lipid bilayers. FEBS Lett. 1989;244:259–262. doi: 10.1016/0014-5793(89)80540-x. [DOI] [PubMed] [Google Scholar]
- 27.Koni P A, Ellar D J. Biochemical characterization of Bacillus thuringiensis cytolytic delta-endotoxins. Microbiology. 1994;140:1869–1880. doi: 10.1099/13500872-140-8-1869. [DOI] [PubMed] [Google Scholar]
- 28.Koni P A, Ellar D J. Cloning and characterization of a novel Bacillus thuringiensis cytolytic delta-endotoxin. J Mol Biol. 1993;229:319–327. doi: 10.1006/jmbi.1993.1037. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Koni P A, Ellar D J. Structure of the mosquitocidal δ-endotoxin CytB from Bacillus thuringiensis sp. kyushuensis and implications for membrane pore formation. J Mol Biol. 1996;257:129–152. doi: 10.1006/jmbi.1996.0152. [DOI] [PubMed] [Google Scholar]
- 30.Lövgren A, Shang M-Y, Engström A, Dalhammmar G, Landén R. Molecular characterization of immune inhibitor A, a secreted virulence protease from Bacillus thuringiensis. Mol Microbiol. 1990;4:2137–2146. doi: 10.1111/j.1365-2958.1990.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 31.Mahillon J, Rezsöhazy R, Hallet B, Delcour J. IS231 and other Bacillus thuringiensis transposable elements: a review. Genetica. 1994;93:13–26. doi: 10.1007/BF01435236. [DOI] [PubMed] [Google Scholar]
- 32.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 33.Poncet S, Delécluse A, Kilier A, Rapoport G. Evaluation of synergistic interactions between the CryIVA, CryIVB and CryIVD toxic components of Bacillus thuringiensis subsp. israelensis crystals. J Invertebrate Pathol. 1995;66:131–135. [Google Scholar]
- 34.Ragni A, Thiéry I, Delécluse A. Characterization of six highly mosquitocidal Bacillus thuringiensis strains that do not belong to H-14 serotype. Curr Microbiol. 1996;32:48–54. doi: 10.1007/s002849900009. [DOI] [PubMed] [Google Scholar]
- 35.Rosso M-L, Delécluse A. Distribution of the insertion sequence IS240 among Bacillus thuringiensis strains. Curr Microbiol. 1997;34:348–353. doi: 10.1007/s002849900194. [DOI] [PubMed] [Google Scholar]
- 36.Schaeffer P, Millet J, Aubert J. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;554:701–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler R D, Dean H D. Bacillus thuringiensis and its pesticidal crystal proteins. Appl Environ Microbiol. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thiery I, Delecluse A, Tamayo M C, Orduz S. Identification of a gene for Cyt1A-like hemolysin from Bacillus thuringiensis subsp. medellin and expression in a crystal-negative Bacillus thuringiensis strain. Appl Environ Microbiol. 1996;63:468–473. doi: 10.1128/aem.63.2.468-473.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward E S, Ellar D J, Chilcott C N. Single amino acid changes in the Bacillus thuringiensis var. israelensis delta-endotoxin affect the toxicity and expression of the protein. J Mol Biol. 1988;202:527–535. doi: 10.1016/0022-2836(88)90283-5. [DOI] [PubMed] [Google Scholar]
- 40.Wirth M C, Federici B A, Walton W E. Cyt1A from Bacillus thuringiensis synergizes activity of Bacillus sphaericus against Aedes aegypti (Diptera: Culicidae) Appl Environ Microbiol. 2000;66:1093–1097. doi: 10.1128/aem.66.3.1093-1097.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wirth M C, Walton W E, Federici B A. Cyt1A from Bacillus thuringiensis restores toxicity of Bacillus sphaericus against resistant Culex quinquefasciatus (Diptera: Culicidae) J Med Entomol. 2000;37:401–407. [PubMed] [Google Scholar]
- 42.Wu D, Chang F N. Synergism in mosquitocidal activity of 26 and 65 kDa proteins from Bacillus thuringiensis subsp. israelensis crystal. FEBS Lett. 1985;190:232–236. [Google Scholar]
- 43.Wu D, Johnson J J, Federici B A. Synergism of mosquitocidal toxicity between CytA and CryIVD proteins using inclusions produced from cloned genes of Bacillus thuringiensis. Mol Microbiol. 1994;13:965–972. doi: 10.1111/j.1365-2958.1994.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, Ohba M, Gill S S. Characterization of mosquitocidal activity of Bacillus thuringiensis subsp. fukuokaensis crystal proteins. Appl Environ Microbiol. 1991;57:1075–1081. doi: 10.1128/aem.57.4.1075-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]