To the Editor:
Hemophagocytic lymphohistiocytosis (HLH) is an aggressive syndrome of immune dysregulation driven by abnormal lymphocyte and macrophage activation and proliferation. CD8+ T lymphocytes secrete interferon-gamma (IFN-γ) on activation, which then causes macrophages to produce inflammatory cytokines, including chemokine motif ligand (CXCL)-9. Many HLH cases are related to an inability to control a viral infection, such as Epstein–Barr virus (EBV). Some cases of EBV-driven HLH are due to chronic active EBV (CA-EBV), wherein EBV may infect T and NK with progression to lymphoproliferative neoplasms.
We report a case of CA-EBV-associated HLH, with central nervous system (CNS) involvement, that was refractory to HLH-2004 treatment and interleukin-1 blockade (anakinra). He achieved remission with the novel combination of emapalumab and ruxolitinib. This is the first report to our knowledge combining these drugs. This combination therapy was well tolerated, resulted in a complete disease remission, and allowed a successful hematopoietic stem cell transplant (HSCT) with 100% donor chimerism.
The patient was diagnosed with HLH at 26 years of age when he presented with recurrent fevers, hemophagocytosis on BM biopsy, hypogammaglobulinemia (109 mg/dl), and EBV viremia (362,318 IU/ml). He met additional criteria for HLH. Bone marrow biopsy was notable for absence of B cells.
Fourteen months prior he had been diagnosed with nephrotic syndrome (NS), including proteinuria, hypogammaglobulinemia (234 mg/dl), and kidney biopsy consistent with minimal change disease. EBV viremia (43,006 IU/ml) had been observed 7 months prior to HLH diagnosis. NS was treated with steroids and cyclosporin A (CSA).
Based on his duration of EBV viremia and presence of EBV in non-B cells (T and NK cells), he was diagnosed with CA-EBV. Genetic testing for causes of HLH (Cincinnati Children’s Molecular Genetic Lab) was negative. Whole exome sequencing was performed at the Children’s Hospital of Philadelphia and did not identify a variant in genes known to be associated with inborn errors of immunity.1 He was of Afro-Carribean and mixed Northern European ancestry, had no full siblings, and no family history of HLH or immunodeficiency. His history was remarkable for recurrent ear infections, including during childhood and adolescence.
He had an HLH flare after 8 weeks of dexamethasone therapy, which necessitated addition of etoposide. There was no sign of CNS involvement. He developed prolonged severe cytopenias with etoposide, sepsis secondary to Escherichia coli bacteremia, and nonhealing pressure ulcers. He developed steroid-induced delirium while hospitalized. This improved with weaning steroids; CSA and anakinra were added. He developed significant steroid-induced myopathy and depression; Karnofsky performance score fell to 50.
One month later, he had a second HLH flare while off therapeutic steroids, but with subtherapeutic CSA. Blood CXCL9 was 12,260 pg/ml (upper limit of normal [ULN]:121). Relapse featured significant CNS involvement complicated by status epilepticus. Cerebrospinal fluid neopterin was 283.5 nmol/L (ULN:16.5). Etoposide and dexamethasone were restarted. The treatment with intrathecal methotrexate was held given the refractory seizures and altered mental status. His Karnofsky performance status declined to 40.
Because of CNS disease and medication intolerance, emapalumab was started twice a week for 2 months (17 doses). His CXCL9 rapidly decreased (Figure 1) to 5185 pg/ml, 6 days after starting therapy. His mental health declined with appearance of suicidal ideation. Given refractory HLH, inadequate performance status for HSCT eligibility, and suicidal ideation, ruxolitinib was started 2 weeks after emapalumab. Ruxolitinib was up-titrated to 25 mg/m2/day (50% dose reduction based on posaconazole interaction).
FIGURE 1.
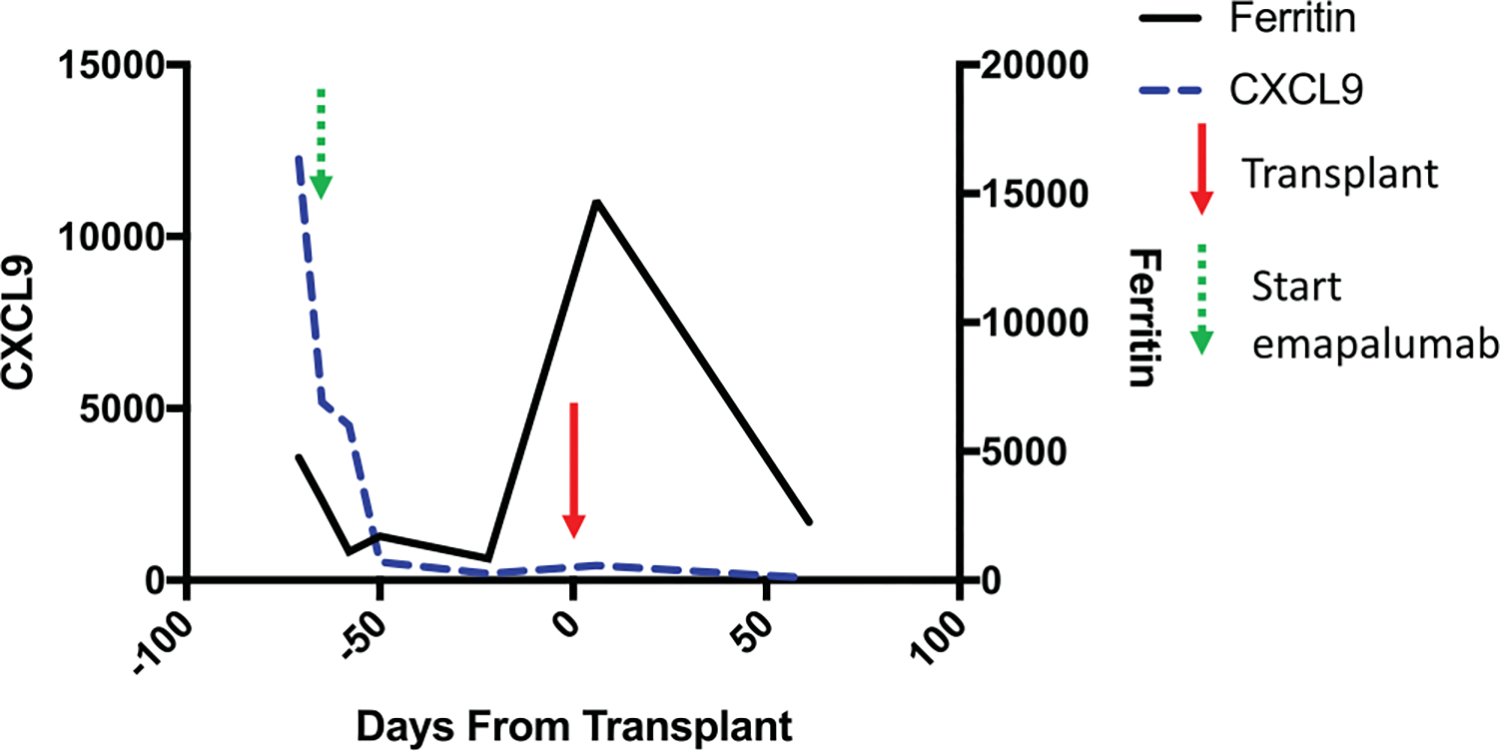
CXCL9 (blue dashed line) with units along left y-axis (pg/ml). Ferritin (black solid line) with units along right y-axis (ng/ml). Transplant day 0 marked with red arrow. Start of emapalumab (green arrow), 3 days after initial CXCL9 measurement
He tolerated the combination well; his only infection was severe Herpes simplex virus (HSV) stomatitis, treated with acyclovir/valacyclovir. His Karnofsky performance score improved to 60 after physical rehabilitation. He received a mismatched (9/10) unrelated donor peripheral stem cell transplant with TCRαβ/CD19-depletion (NCT03145545) after conditioning with thymoglobulin, busulfan, fludarabine, thiotepa, and posttransplant rituximab. Emapalumab was last given day −16 from transplant and ruxolitinib day −12, two days prior to conditioning. He had persistent fevers starting on day −3, with hyperferritinemia (19,076 mg/dl), and increased CXCL9 (434 pg/ml); presumed HLH flare was treated with methylprednisolone.
He engrafted at day 13 (absolute neutrophil count > 500/mm3 × 3 days). Day 28 chimerism studies showed 99% donor in nonenriched peripheral blood cells and 98% T cell, 100% myeloid cell, and 100% NK donor cells. Peripheral blood at day 60 and 120 showed 100% chimerism (B cells were insufficient). EBV DNA by PCR decreased from 169,787 IU/ml prior to transplant to <5700 IU/ml 1 week after transplant. EBV PCR was negative 2 months after HSCT. He developed mild acute graft-versus-host disease of the skin (stage 2, grade 1) that was treated with oral steroids and tacrolimus. Unfortunately, 5 months post-HSCT he developed self-derived EBV+ peripheral T-cell lymphoma featuring an acquired TET2 mutation. T-cell receptor clonality studies (T-cell gamma gene rearrangement PCR, Hematologics) were performed retrospectively on paraffin sections from the T-cell lymphoma that arose after HSCT and two bone marrow aspirate specimens that were banked before HSCT. Monoclonal amplicons of identical sizes were detected in all three specimens.
This is the first report of a patient receiving emapalumab and ruxolitinib. Despite the potential for profound immune-suppression, dual therapy was safe and well tolerated. Emapalumab is the first approved therapy for HLH2 and blocks IFN-γ signaling directly. Ruxolitinib blocks Jak1/2, working downstream of IFN-γ and other cytokines, and has similar efficacy in HLH.3,4 Although use of either agent alone could cause significant immunosuppression, the only complication was HSV stomatitis and viremia. When used together, this therapy induced a deep and durable remission allowing 2 months for physical rehabilitation and successful HSCT. We posit engraftment may have been improved by the IFN-γ blockade given known toxicity of IFN-γ to HSC5 and association with graft loss. IFN-γ blockade should be studied prospectively in patients with high risk of graft loss.6,7
ACKNOWLEDGMENTS
This work was supported by the Immune Dysregulation Frontier Program at the Children’s Hospital of Philadelphia. This publication was made possible by an NHLBI-funded postdoctoral fellowship to MPT (T32 HL007150). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI or NIH.
Footnotes
CONFLICT OF INTEREST
DTT serves on advisory boards for Janssen, Amgen, La Roche and Sobi. MPL is an advisory board member for Octapharma and Shionogi and a consultant for Amgen, Novartis, Shionogi, Dova, Bayer, and Astra Zeneca. DM is consultant for, receives royalties from, and owns options at Omixon.
REFERENCES
- 1.Bousfiha A, Jeddane L, Picard C, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40(1):66–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Blood. 2018;132(Suppl 1). 10.1182/blood-2018-120810. [DOI] [Google Scholar]
- 3.Wang J, Wang Y, Wu L, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2019;105:e210–e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed A, Merrill SA, Alsawah F, et al. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6(12):e630–e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L, Dybedal I, Bryder D, et al. IFN-gamma negatively modulates self-renewal of repopulating human hemopoietic stem cells. J Immunol. 2005;174(2):752–757. [DOI] [PubMed] [Google Scholar]
- 6.Rottman M, Soudais C, Vogt G, et al. IFN-gamma mediates the rejection of haematopoietic stem cells in IFN-gamma R1-deficient hosts. PLoS Med. 2008;5(1):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merli P, Caruana I, De Vito R, et al. Role of interferon-gamma in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. Haematologica. 2019;104(11):2314–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]


