Abstract
The bioemulsifier of Acinetobacter radioresistens KA53, referred to as alasan, is a high-molecular-weight complex of polysaccharide and protein. The emulsifying activity of the purified polysaccharide (apo-alasan) is very low. Three of the alasan proteins were purified by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis and had apparent molecular masses of 16, 31, and 45 kDa. Emulsification assays using the isolated alasan proteins demonstrated that the active components of the alasan complex are the proteins. The 45-kDa protein had the highest specific emulsifying activity, 11% higher than the intact alasan complex. The 16- and 31-kDa proteins gave relatively low emulsifying activities, but they were significantly higher than that of apo-alasan. The addition of the purified 16- and 31-kDa proteins to the 45-kDa protein resulted in a 1.8-fold increase in the specific emulsifying activity and increased stability of the oil-in-water emulsion. Fast-performance liquid chromatography analysis indicated that the 45-kDa protein forms a dimer in nondenaturing conditions and interacts with the 16- and 31-kDa proteins to form a high-molecular-mass complex. The 45-kDa protein and the three-protein complex had substrate specificities for emulsification and a range of pH activities similar to that of alasan. The fact that the purified proteins are active emulsifiers should simplify structure-function studies and advance our understanding of their biological roles.
Microorganisms synthesize a wide variety of high- and low-molecular-mass bioemulsifiers (16). The low-molecular-mass bioemulsifiers are generally glycolipids, such as trehalose lipids, sophorolipids, and rhamnolipids, or lipopeptides, such as surfactin, gramicidin S, and polymyxin. The high-molecular-mass bioemulsifiers are amphipathic polysaccharides, proteins, lipopolysaccharides, lipoproteins, or complex mixtures of these biopolymers. Bioemulsifiers have several important advantages over chemical surfactants, which should allow them to become prominent in industrial and environmental applications. The potential commercial applications of bioemulsifiers include bioremediation of oil-polluted soil and water (25), enhanced oil recovery (1), replacement of chlorinated solvents used in cleaning-up oil-contaminated pipes, vessels and machinery (15), use in the detergent industry (17), formulations of herbicides and pesticides (14), and the formation of stable oil-in-water emulsions for the food (23) and cosmetic industries (6).
The majority of Acinetobacter strains, including both hospital and environmental isolates, produce high-molecular-mass bioemulsifiers (22). The best studied are the bioemulsans of Acinetobacter calcoaceticus RAG-1 and A. calcoaceticus BD4 (17). RAG-1 emulsan is a complex of an anionic heteropolysaccharide and protein (19). Its surface activity is largely due to the presence of fatty acids, comprising 15% of the emulsan dry weight, which are attached to the polysaccharide backbone via O-ester and N-acyl linkages (3). The protein component of RAG-1 emulsan stimulates the emulsifying activity (27). A. calcoaceticus BD4, initially isolated by Taylor and Juni (24), produces a large polysaccharide capsule. Under certain growth conditions, the capsule is released together with the bound protein, producing a highly active emulsifier complex (4). The purified polysaccharide and protein components have no emulsifying activity by themselves. However, mixing the polysaccharide and protein led to the reconstitution of the emulsifying activity (5). Other Acinetobacter surfactants that have been reported include biodispersan from A. calcoaceticus A2 (20), an emulsifier effective on heating oil (8), and whole cells of A. calcoaceticus 2CA2 (12).
The present study deals with the purification and characterization of protein components of alasan, the bioemulsifier complex of A. radioresistens KA53. Alasan is composed of a polysaccharide (apo-alasan) containing covalently bound alanine and proteins (9). The proteins of alasan appeared to play an essential role in both the structure and surface activity of the complex, because apo-alasan had no emulsifying activity and did not show the large temperature-induced hydrodynamic shape changes that were characteristic of alasan (10). Furthermore, treatment of alasan with specific proteases inactivated the emulsifying activity. Purification of the alasan proteins, described in this report, provide the basis for demonstrating that the proteins by themselves and synergistically are active emulsifiers. Using the potent tools of protein structure analysis, it should now be possible to examine the structure-function properties of bioemulsans with regard to both their ability to stabilize oil-in-water emulsions and their role in the interaction of bacteria with interfaces (11).
MATERIALS AND METHODS
Bacterial strain and production of alasan and apo-alasan.
A. radioresistens KA53 (NCIMB 40692), isolated previously on acetate medium (9), was maintained on brain heart infusion agar (Difco Laboratories, Detroit, Mich.). After incubation for 3 days, the plates were stored at 4°C. For emulsifier production, inocula were prepared in ethanol medium (EM) containing (per liter of deionized water) 5 ml of ethanol, 1.8 g of urea, 13.7 g of Na2HPO4, 7.26 g of KH2PO4, 0.4 g of MgSO4 · 7H2O, and 1 ml of trace elements. Alasan production was carried out at 30°C in a 2.5-liter fermentor (Multigen; New Brunswick Scientific Co., Inc.) containing 1.4 liters of EM. Bacterial growth was initiated by introducing a 0.1% inoculum. After 80 h of batch-fed fermentation, as described previously (9), the culture reached a turbidity of 30 at 600 nm. The cell-free culture broth was then obtained by centrifugation and filtration through a 0.45-μm-pore-size membrane filter. After the addition of ammonium sulfate to 65% saturation and standing overnight at 4°C, the turbid suspension was centrifuged at 10,000 × g for 20 min. The pellets were dissolved in water, dialyzed extensively against deionized water, and lyophilized, yielding 3.5 g of alasan. Apo-alasan (deproteinized alasan) was obtained by the hot-phenol method (26). After two successive hot phenol treatments, the apo-alasan contained less than 1% protein. Stock solutions of alasan and apo-alasan were prepared fresh by hydration at 0°C in 20 mM Tris HCl (pH 8.5).
Determination of emulsifying activity.
A micromodification of the standard emulsification assay (21) was used to measure emulsifying activity. Samples to be tested were introduced into 10-ml glass tubes containing TM buffer (20 mM Tris-HCl buffer, pH 7.0; 10 mM MgSO4) to a final volume of 1.5 ml, and then 0.02 ml of a 1:1 (vol/vol) mixture of hexadecane and 2-methylnaphthalene was added. The tubes were then vortexed at room temperature for 30 min. The turbidity was determined after standing for 30 s, using a Gilford spectrophotometer (model 240). One unit of emulsifying activity was defined as the amount of biopolymer that yielded an A600 of 0.1 in the assay. The average and standard error of triplicate samples are reported.
Protein electrophoresis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed for (i) estimating the molecular weight and determining the composition of various alasan protein fractions and (ii) preparation of milligram quantities of purified alasan proteins. The former was carried out by the method of Laemmli (7). Samples were dissolved in 2% SDS, 4% β-mercaptoethanol, 8% glycerol, 50 mM Tris-HCl (pH 6.8), and 0.02% bromphenyl blue and then heated at 100°C for 10 min. Prestained broad-range SDS-PAGE standards (Bio-Rad Co., Hercules, Calif.) were used as molecular mass markers. The running buffer was 0.1% SDS, 192 mM glycine, and 25 mM Tris-HCl (pH 8.3). Gels were stained with Coomassie brilliant blue.
Preparative SDS-PAGE was performed with the model 491 Prep Cell (Bio-Rad), using continuous elution electrophoresis. The same sample treatment and buffers were used as described above. After up to 100 mg of the treated alasan was loaded, the proteins were run at 12 W of constant power for ca. 11 h on a 12.5% gel, until the ion front reached the lower part of the gel. At this point the peristaltic pump was activated, and 5-ml fractions were collected. After we determined the protein content of the fractions spectrophotometrically (A280) and by the Bradford reaction (Bio-Rad), we precipitated the alasan proteins with cold trichloroacetic acid (TCA) and then centrifuged them. The pellets were washed twice with cold acetone, air dried, and dissolved in water. The protein solutions were adjusted to pH 8.0 with saturated Tris. For further purification, pooled fractions (5 to 10 mg of protein) were rerun as described above on the Bio-Rad Mini Prep Cell column.
Size exclusion chromatography of alasan proteins.
Purified protein fractions (ca. 0.5 mg) obtained by preparative SDS-PAGE were applied to a Hi Load 16/60 Superdex 200 Prepgrade fast-performance liquid chromatography (FPLC) column (Pharmacia Biotech, Inc.) and developed with 50 mM Tris (pH 11.0) containing 0.17 M NaCl. Flow rates were adjusted to 1 ml/min; 2.5-ml fractions were then collected and analyzed for protein and emulsifying activity as described above. The column was standardized with molecular mass markers of from 12 to 200 kDa (Sigma Chemical Co.).
RESULTS
Isolation of alasan proteins.
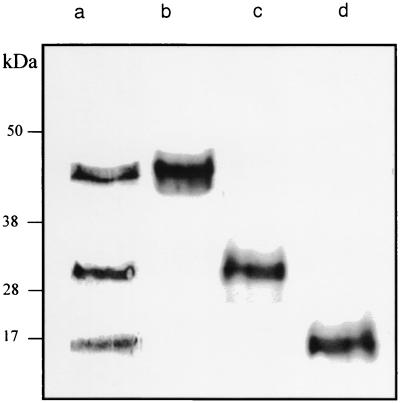
SDS-PAGE analysis of alasan indicated the existence of three major proteins with apparent molecular masses of 16, 31, and 45 kDa (Fig. 1, lane a). In addition to the three major proteins, a small amount of Coomassie blue-staining remained near the origin (not shown in Fig. 1). Purification of these proteins from 100 mg of alasan, containing 20 mg of total protein, yielded ca. 1.5, 1.75, and 1.9 mg of the 16-, 31-, and 45-kDa proteins, respectively, after two sequential SDS-PAGE preparative column runs. After the first preparative run, 59% of the input protein was recovered in the 16-, 31-, and 45-kDa peaks. Another 38% of the input protein were recovered as mixtures of the 31- and 45-kDa proteins and of the 16- and 31-kDa proteins. The relatively low recovery of the final purified proteins was due to the fact that only the peak fractions were pooled from the first column (to enhance purity) and that TCA precipitation may not have been quantitative. The purity of the three alasan proteins are shown in Fig. 1, lanes b to d.
FIG. 1.
SDS-PAGE of alasan and purified alasan proteins. Numbers to the left indicate molecular mass markers in kilodaltons. Samples were treated at 100°C for 10 min in Laemmli buffer (7), resolved by SDS-PAGE, and stained with Coomassie blue. Lane a is alasan; lanes b, c, and d are the purified 45-, 31-, and 16-kDa proteins.
Emulsifying activity of the purified alasan proteins.
Table 1 summarizes the emulsifying activities of the alasan proteins compared to alasan, apo-alasan, and bovine serum albumin (control). The 45-kDa protein was the most active, yielding a higher specific emulsifying activity (792 U/mg) than alasan (712 U/mg). The 16- and 31-kDa proteins gave relatively low emulsifying activities, but they were significantly higher than those obtained with apo-alasan and bovine serum albumin. The emulsion induced by the 45-kDa protein was considerably less stable than that produced by alasan. The emulsifying activity of alasan and each of the alasan proteins was proportional to concentrations up to 10 μg per assay (data not shown).
TABLE 1.
Emulsifying activities of alasan, apo-alasan, and purified alasan proteins
| Test materiala | Emulsifying activityb
|
|
|---|---|---|
| U ± SE | % Stability (%)c | |
| 16-kDa protein | 0.65 ± 0.05 | |
| 31-kDa protein | 0.63 ± 0.01 | |
| 45-kDa protein | 3.96 ± 0.09 | 35 |
| Alasan | 3.56 ± 0.03 | 100 |
| Apo-alasan | 0.10 ± 0.02 | |
| Bovine serum albumin | 0.32 ± 0.03 | |
A total of 5 μg of each material was used in the assay.
The microemulsifying assay was used as described in Materials and Methods.
The A600 of the emulsion after 24 h of standing undisturbed compared to the A600 after 30 min of vortexing. The emulsifying activity of the 16- and 31-kDa proteins, apo-alasan, and bovine serum albumin were too low to measure stability.
The synergistic effect of the alasan proteins on emulsifying activity and stability is shown in Table 2. The mixture of the 16- and 31-kDa proteins gave the emulsifying activity predicted from the data in Table 1. However, all of the mixtures that contained the 45-kDa protein showed a synergistic effect, with values higher than those predicted from the sum of the individual proteins. This was observed both for emulsifying activity and emulsion stability. The highest activity was obtained with equal molar concentrations of the three alasan proteins (1,300 U/mg and 96% stability). Addition of the alasan polysaccharide, apo-alasan, to the three alasan proteins had no effect.
TABLE 2.
Emulsifying activity of mixtures of alasan proteins.
| Test materialsa | Emulsifying activityb
|
|||
|---|---|---|---|---|
| U ± SE (determined) | U (predicted) | % Stability (determined) | % Stability (predicted) | |
| 16- plus 31-kDa proteins | 0.65 ± 0.07 | 0.64 | ||
| 16- plus 45-kDa proteins | 3.21 ± 0.16 | 3.09 | 89 | 38 |
| 31- plus 45-kDa proteins | 5.78 ± 0.21 | 2.60 | 74 | 40 |
| 16- plus 31- plus 45-kDa proteins | 6.51 ± 0.15 | 2.37 | 96 | 43 |
| 16- plus 31- plus 45-kDa proteins plus apo-alasanc | 6.22 ± 0.13 | 2.77 | 96 | 43 |
A total of 5 μg of protein was used in the assay, with each protein in the mixture being present in an equal molar quantity.
The emulsifying units and percent stability values were determined as described in Table 1. The predicted units and stability were calculated from the data in Table 1.
A total of 20 μg of apo-alasan was added to the 5-μg mixture of the three proteins.
Emulsifying properties of the 45-kDa protein.
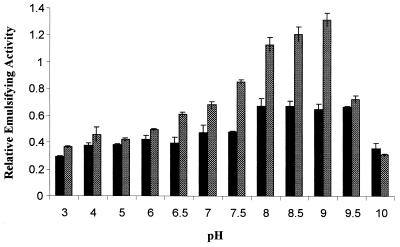
The effect of pH on the emulsifying activities of alasan and the 45-kDa protein are summarized in Fig. 2. Both emulsifiers were active from pH 3 to 10. Alasan showed a peak at pH 9.0, whereas the 45-kDa protein had maximum activity from pH 8.0 to 9.5.
FIG. 2.
Effect of pH on the emulsifying activity of the 45-kDa protein (■) compared to alasan ( ). The standard microemulsion assay was used except that the pH was varied. Values ± the standard error (SE) relative to pH 7.0 are presented.
). The standard microemulsion assay was used except that the pH was varied. Values ± the standard error (SE) relative to pH 7.0 are presented.
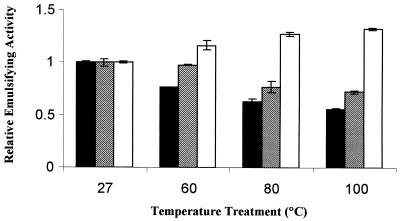
One of the unique features of alasan is its heat stability. As seen in Fig. 3, the alasan emulsifying activity increased 30% after heating at 100°C. The emulsifying activity of the 45-kDa protein was less stable to heat, losing ca. 40% of its activity at 100°C. A mixture of the three alasan proteins retained 90% of its activity after treatment at 100°C.
FIG. 3.
Effect of heat treatment on the emulsifying activity of alasan (□), the 45-kDa protein (■), and a mixture of the three alasan proteins ( ). Each of the samples was heated at the indicated temperature for 10 min prior to the standard microemulsion assay. Average values ± the SE are presented.
). Each of the samples was heated at the indicated temperature for 10 min prior to the standard microemulsion assay. Average values ± the SE are presented.
The substrate specificity for emulsification of the 45-kDa protein compared to alasan is summarized in Table 3. Similar to alasan, the 45-kDa protein was more effective in emulsifying hexylbenzene, hexadecane, and crude oil than in emulsifying low-molecular-weight aliphatic and aromatic hydrocarbons. It should be noted that emulsion turbidity using different substrates may not be a direct measure of emulsification. However, this property is useful for comparative studies.
TABLE 3.
Hydrocarbon substrate specificity of alasan and the 45-kDa proteina
| Hydrocarbon substrate | Emulsion turbidity (A600) of:
|
|
|---|---|---|
| Alasan | 45-kDa protein | |
| n-Heptane | 0.36 | 0.45 |
| Toluene | 1.21 | 0.62 |
| Hexylbenzene | 4.20 | 3.06 |
| Hexadecane | 3.65 | 2.95 |
| Crude oil | 16.52 | 9.25 |
The standard emulsification assay (21) was used with 0.1 mg of alasan or the 45-kDa protein and 0.1 ml of the indicated hydrocarbon substrate.
Physical interactions between the alasan proteins.
The individual SDS-PAGE-purified 45-, 31-, and 16-kDa alasan proteins eluted from an FPLC column as single peaks, with apparent molecular masses of ca. 100, 31, and 16 kDa, respectively (data not presented). Thus, in the absence of SDS, the 45-kDa protein appears to be a dimer, while the 31- and 16-kDa proteins elute as monomers.
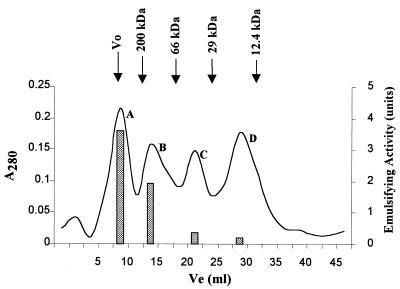
When the three purified alasan proteins were mixed and then run on the FPLC column, four protein peaks were observed (Fig. 4). Peak A, eluting in the void volume, had most of the emulsifying activity and the highest specific activity. SDS-PAGE analysis of peak A indicated the presence of the 16-, 31-, and 45-kDa proteins. Peak B eluted with an apparent molecular mass of 100 kDa and is probably a residual 45-kDa dimer. It showed ca. 50% of the emulsifying activity of peak A. Peaks C and D eluted at the positions of the 31- and 16-kDa proteins and showed little emulsifying activity.
FIG. 4.
FPLC analysis of a mixture of the three alasan proteins. The 45-, 31-, and 16-kDa proteins (0.1 mg of each) were mixed in the elution buffer and then run on the column as described in Materials and Methods. An aliquot (0.5 ml) of each of the peak tubes was assayed for emulsifying activity by the microemulsion assay. The elution volumes (Ve) of the molecular weight markers are indicated by the vertical arrows.
DISCUSSION
To our knowledge this study represents the first detailed investigation of the role of proteins of a polymeric bioemulsifier complex. Most Acinetobacter bioemulsans are complexes of polysaccharides and proteins (17). In the case of alasan, the active component of the emulsifier complex is protein. The specific emulsifying activity of the 45-kDa protein was 11% higher than the intact alasan complex. However, 45-kDa-protein-induced oil-in-water emulsions were less stable than alasan-induced emulsions, suggesting that the polysaccharide (apo-alasan) and/or the other alasan proteins played a role in emulsion stability. Addition of the purified 16- and 31-kDa proteins to the 45-kDa protein resulted in a large increase in specific emulsifying activity and stability of the emulsion, whereas addition of apo-alasan to the 45-kDa protein or the mixture of the three proteins had no effect on its emulsifying activity. The 45-kDa protein and the three-protein complex had substrate specificities and a range of pH activities similar to that of alasan. Protein-protein interactions may play an important role in producing the surface active complex. The role of the polysaccharide is not clear. It may play a role in the release of the proteins into the medium and in protecting the protein complex against proteolytic activities. In this regard it is interesting that the purified 45-kDa protein was readily hydrolyzed by trypsin, whereas the protein in the alasan complex was resistant.
What are the special structural properties of the 45-kDa protein that allow it to be such an effective emulsifier? To begin with, it is an extremely stable molecule. It retained its activity after successive treatments with hot SDS, TCA precipitation, and 10 min at 100°C. At present, the only information we have on the structure of the 45-kDa protein is that it forms a dimer in nondenaturing conditions and interacts with the 16- and 31-kDa proteins to form a complex with a molecular mass greater than 400 kDa. The stoichiometry and optimum conditions for producing the complex have not been determined. Each of the three alasan proteins contains a unique N-terminal amino acid sequence (data not presented). The N-terminal amino acid sequence of the 45-kDa showed high similarity to the OmpA protein of Acinetobacter spp. (13) and other gram-negative bacteria (2).
The finding that the active emulsifiers of the alasan complex are proteins will simplify the structural analysis of alasan. It should be possible to perform a series of defined mutations in the genes coding for the alasan proteins. The effect of these mutations on the activity of alasan will be important in structure-function studies. Such studies will contribute to our understanding of the activity and functions of alasan and can be extended to the study of additional high-molecular-weight microbial emulsifiers.
ACKNOWLEDGMENTS
This investigation was supported by the Ministry of Science, Israel, the Pasha Gol Chair for Applied Microbiology, and the Manja and Moris Chair for Biophysics and Biotechnology.
REFERENCES
- 1.Banata I M. Biosurfactants production and possible use in microbial enhanced oil recovery and oil pollution remediation. A review. Biosource Technol. 1995;51:1–12. [Google Scholar]
- 2.Beher M, Schnaitman C A, Pugsley A P. Major-modifiable outer membrane protein in gram-negative bacteria: comparison with the OmpA protein of Escherichia coli. J Bacteriol. 1980;143:906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belsky I, Gutnick D L, Rosenberg E. Emulsifier of Arthrobacter RAG-1: determination of emulsifier-bound fatty acids. FEBS Lett. 1979;101:175–178. doi: 10.1016/0014-5793(79)81320-4. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan N, Rosenberg E. Exopolysaccharide distribution and bioemulsifier production in Acinetobacter calcoaceticus BD4 and BD413. Appl Environ Microbiol. 1982;44:1335–1341. doi: 10.1128/aem.44.6.1335-1341.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan N, Zosim Z, Rosenberg E. Acinetobacter calcoaceticus BD4 emulsan: reconstitution of emulsifying activity with pure polysaccharide and protein. Appl Environ Microbiol. 1987;53:440–446. doi: 10.1128/aem.53.2.440-446.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klekner V, Kosaric N. Biosurfactants for cosmetics. In: Kosaric N, editor. Biosurfactants: production, properties, applications. Surfactant Science Series. Vol. 48. New York, N.Y: Marcel Dekker; 1993. pp. 329–372. [Google Scholar]
- 7.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Marin M, Pedregosa A, Laborda F. Emulsifier production and microscopical study of emulsions and biofilms formed by the hydrocarbon-utilizing bacteria Acinetobacter calcoaceticus MM5. Appl Microbiol Biotechnol. 1996;44:660–66. [Google Scholar]
- 9.Navon-Venezia S, Zosim Z, Gottlieb A, Legmann R, Carmeli S, Ron E Z, Rosenberg E. Alasan, a new bioemulsifier from Acinetobacter radioresistens. Appl Environ Microbiol. 1995;61:3240–3244. doi: 10.1128/aem.61.9.3240-3244.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navon-Venezia S, Banin E, Ron E Z, Rosenberg E. The bioemulsifier alasan: role of protein in maintaining structure and activity. Appl Microbiol Biotechnol. 1998;49:382–384. [Google Scholar]
- 11.Neu T R. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol Rev. 1996;60:151–166. doi: 10.1128/mr.60.1.151-166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neufeld R J, Zajic J E. The surface activity of Acinetobacter calcoaceticus sp. 2CA2. Biotechnol Bioeng. 1984;26:1108–1114. doi: 10.1002/bit.260260914. [DOI] [PubMed] [Google Scholar]
- 13.Ofori-Darko E, Zavros Y, Rieder G, Tarle S A, Van Antwerp M, Merchant J L. An OmpA-like protein from Acinetobacter spp. stimulates gastrin and interlukin-8 promoters. Infect Immun. 2000;68:3657–3666. doi: 10.1128/iai.68.6.3657-3666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel M N, Gopinathan K P. Lysozyme-sensitive bioemulsifier for immiscible organophosphorus pesticides. Appl Environ Microbiol. 1986;52:1224–1226. doi: 10.1128/aem.52.5.1224-1226.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson K, Gosh M, Shu Z. Mineralization enhancement iof non-aqueous phase and soil-bound PCB using biosurfactant. Water Sci Technol. 1996;34:303–309. [Google Scholar]
- 16.Rosenberg E, Ron E Z. Bioemulsans: microbial polymeric emulsifiers. Curr Opin Biotechnol. 1997;8:313–316. doi: 10.1016/s0958-1669(97)80009-2. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg E, Ron E Z. Surface active polymers from the genus Acinetobacter. In: Kaplan D L, editor. Biopolymers from renewable resources. New York, N.Y: Springer; 1998. pp. 281–289. [Google Scholar]
- 18.Rosenberg E, Ron E Z. High- and low-molecular-mass microbial surfactants. Appl Microbiol Biotechnol. 1999;52:154–162. doi: 10.1007/s002530051502. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg E, Zuckerberg A, Rubinovitz C, Gutnick D L. Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl Environ Microbiol. 1979;37:402–408. doi: 10.1128/aem.37.3.402-408.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg E, Rubinovitz C, Gottlieb A, Rosenhak S, Ron E Z. Production of biodispersan by Acinetobacter calcoaceticus A2. Appl Environ Microbiol. 1988;54:317–322. doi: 10.1128/aem.54.2.317-322.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinovitz C, Gutnick D L, Rosenberg E. Emulsan production by Acinetobacter calcoaceticus in the presence of chloramphenicol. J Bacteriol. 1982;152:126–132. doi: 10.1128/jb.152.1.126-132.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sar N, Rosenberg E. Emulsifier production by Acinetobacter calcoaceticus strains. Curr Microbiol. 1983;9:309–314. [Google Scholar]
- 23.Shepherd R, Rockey J, Sutherland I W, Roller S. Novel bioemulsifiers from microorganisms for use in foods. J Biotechnol. 1995;40:207–217. doi: 10.1016/0168-1656(95)00053-s. [DOI] [PubMed] [Google Scholar]
- 24.Taylor W H, Juni E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Volkering F, Breure A, Rulkens W. Microbiological aspects of surfactant use for biological soil remediation. Biodegradation. 1997;8:401–417. doi: 10.1023/a:1008291130109. [DOI] [PubMed] [Google Scholar]
- 26.Westphal O, Jann K. Bacterial lipopolysaccharides: extraction with phenol-water and further applications of the procedure. In: Whistler R L, editor. Carbohydrate chemistry. New York, N.Y: Academic Press, Inc.; 1965. pp. 83–91. [Google Scholar]
- 27.Zosim Z, Fleminger G, Gutnick D L, Rosenberg E. Effect of protein on the emulsifying activity of emulsan. J Dispersion Sci Technol. 1989;10:307–317. [Google Scholar]