Abstract
Objective
In this study, we aimed to compare the effects of intravenous dexamethasone and methylprednisolone on the treatment of inpatients with COVID-19.
Methods
In this randomized clinical trial, 143 patients under 80 years of age with moderate to severe COVID-19 were enrolled and randomly assigned to two groups: dexamethasone (8 mg/day) and methylprednisolone (60 mg/day in two divided doses). The primary outcome was the length of hospital stay. The secondary outcomes included: duration of oxygen therapy, absolute leukocyte and lymphocyte count, hypokalemia, hyperglycemia, intensive care unit admission, and mortality in the two groups for 28 days. Data were analyzed by SPSS version 26 using t-test, chi-square, and analysis of variance.
Results
The duration of hospitalization was significantly (P <0.001) shorter in the dexamethasone group than in the methylprednisolone group (8 [95% confidence interval [CI]:6-10] and 11 [95% CI: 7-14], respectively). In addition, the duration of oxygen therapy in the dexamethasone group (7 [95% CI: 5-9]) was significantly (P <0.001) shorter than in the methylprednisolone group (10 [95% CI: 5.5-14]). The mortality rate was 17.1% (95% CI: 8.1-26.1) in the dexamethasone group and 12.3% (95% CI: 4.6-20.0) in the methylprednisolone group, which was not statistically significant (P = 0.46).
Conclusion
Results showed better effectiveness of 8 mg/day dexamethasone compared with 60 mg/day methylprednisolone based on the shorter hospital stay, which can be considered in the therapeutic protocol of COVID-19.
Trial registration
: IRCT20210223050466N1.
Keywords: Dexamethasone, Methylprednisolone, COVID-19, Hospitalization, Hyperglycemia, Hypokalemia
Introduction
COVID-19, caused by a coronavirus, is the cause of a global pandemic that began in Wuhan, China, in December 2019. The disease progresses in approximately 19% of patients, leading to severe pneumonia, and in 5% of patients, critical pneumonia (Wu and McGoogan, 2020).
Acute respiratory distress syndrome (ARDS) may begin during the second week, not only because of the excessive replication of the virus but also because of the severe inflammatory response. The mechanism of viral entry to the human host cell is fulfilled by a cellular surface protein called angiotensin-converting enzyme 2 (ACE2) and priming of the viral spike protein by the transmembrane protease serine 2 (TMPSS2). The fusion of the virus to the host cell occurs through the cleavage step and, finally, cell entry (Matarese et al., 2020). The coronaviruses express and replicate their genomic RNA that is incorporated into new viral particles (V`kovski et al., 2021). After uncontrolled replication of the virus, which increases the number of infected epithelial cells and cellular debris, large amounts of cytokines are released, accompanied by severe inflammation, which reduces CD4+ memory T helper cells and increases the cytotoxic activity of CD8 (Guan et al., 2020; Shi et al., 2020).
In the early stages of the disease, the antiviral immune response leads to the elimination of the virus, but the inflammatory response leads to lung damage. Lung damage begins at the epithelial-interstitial-endothelial level, exudates from neutrophils, and macrophages reduce the surfactant, which leads to decreased alveoli ability and oxygen exchange. The debris of infected cells releases inflammatory cytokines such as tumor necrosis factor-α and interleukin (IL) 1 and IL 6, referred to as a cytokine storm (Sweeney and McAuley, 2016).
In the second phase, excessive virus replication leads to cytotoxicity because of the ACE2, followed by activation of the immune cycle and exacerbation of the inflammatory condition. In this phase, the patient develops lymphopenia with a decrease in CD4, CD8, T, and NK cells. Cytokine storm leads to widespread vascular inflammation, massive coagulation, shock, hypotension, and ultimately organ failure and death. Studies have shown that any factor that prevents these events can prevent lung damage and pulmonary thromboembolism (Wang et al., 2020). Given this physiopathology, it seems that corticosteroid intervention may be effective.
However, corticosteroids suppress the immune system and therefore raise fears about the spread of the virus. Severe or critical pneumonia is expected not to increase with a relatively short maintenance dose (Isidori et al., 2020). In one study, in hypoxic inpatients, methylprednisolone yielded better outcomes in terms of hospital stay length and clinical conditions (Ranjbar et al., 2021). However, two studies published in The Lancet have recommended the use of corticosteroids for the treatment of COVID-19, but these studies have been based mainly on similar viruses rather than on COVID-19 alone (Russell et al., 2020; Shang et al., 2020). In addition, the World Health Organization and the Center for Disease Control and Prevention have recommended that corticosteroids not be used to modulate immunity (National Institutes of Health, 2020; World Health Organization, 2020). However, other guidelines on sepsis in COVID-19 recommend using corticosteroids in intubated patients with ARDS to reduce the inflammatory response and treat secondary adrenal insufficiency in sepsis, especially in patients with refractory shock. However, the same guidelines in other reports have advised not to use corticosteroids in intubated patients without ARDS (Alhazzani et al., 2020). In an animal study, methylprednisolone led to a higher ratio of lung tissue-to-plasma than dexamethasone, showing that it could be more effective for lung damage (Annane et al., 2017). In a human study, treatment outcomes were significantly better in patients with severe COVID-19 who needed oxygen or mechanical ventilation and underwent treatment with dexamethasone (Horby et al., 2020). Dexamethasone has been reported to have no mineralocorticoid effect and to produce the highest anti-inflammatory effect and half-life among corticosteroids (Samuel et al., 2017).
Patients' medical history also affects the prognosis of the disease. The increased ACE2 glycation and TMPSS2 expression in cardiomyocytes in patients with diabetes mellitus (DM) versus patients without DM may favor COVID-19 entry, leading to worsening clinical outcomes and cardiovascular events in patients with COVID-19 with DM (D`Onofrio et al., 2021). The prognosis of patients with COVID-19 with elevated blood glucose may worsen because of the increased risk of severity of disease, mortality, mechanical ventilation, shock, and intensive care unit (ICU) admission because of multiple organ failure (Sardu et al., 2020a, 2020b). Moreover, there is a higher binding affinity between ACE2 and COVID-19 in patients with hypertension, leading to a higher infection rate and worsening the prognosis (Sardu et al., 2020b).
Therefore, given the inconsistency in available evidence on the effects of glucocorticoids, this study aimed to comparatively investigate the effects of dexamethasone and methylprednisolone on inpatients with COVID-19 symptoms, hospital stay length, need for ICU admission, mortality, and inflammatory markers.
Method
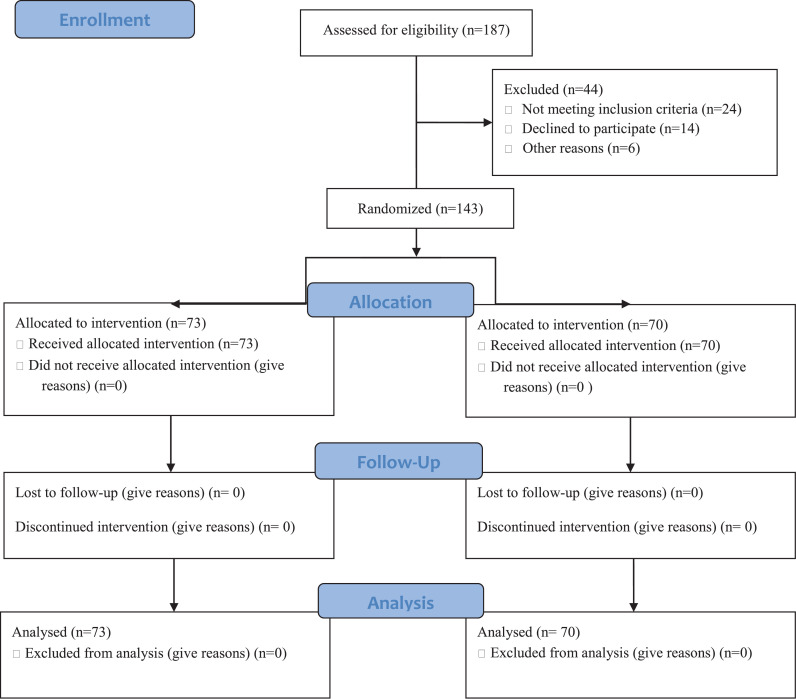
This randomized clinical was conducted between April 2021 and June 2021 in Hajar Hospital of Shahr-e-Kord province, Iran. A total of 143 patients with COVID-19 were enrolled and followed up for 28 days (Figure 1 ).
Figure 1.
Flow diagram of the study.
Study population
All patients with COVID-19 who tested positive by reverse transcription–PCR (RT-PCR) test, collected with a swab from the nasopharynx or other respiratory tract specimens, were examined. Inclusion criteria were age under 80 years, positive RT-PCR test, bilateral lung involvement in high-resolution computed tomography, oxygen saturation (SpO2) <93% and the need for supplemental oxygen, and providing consent to participate in the study. The need for oxygen in patients is fulfilled by (invasive or noninvasive) ventilation or oxygenation using a cannula or mask.
Exclusion criteria included discharge from hospital before recovery, death within the first 24 hours, acute myocardial ischemia during the hospital stay, contraindications to corticosteroids because of underlying diseases such as acute hepatitis or fungal infection, and lack of volunteering to participate in the study.
Study outcomes
Duration of hospitalization was considered the primary outcome. Secondary outcomes included absolute leukocyte and lymphocyte counts at baseline and completion of hospitalization, hypokalemia, hyperglycemia, the need for ICU admission, duration of oxygen therapy, and mortality within 28 days of hospitalization.
Treatment groups
First, the written consent form was completed by the patients. Then their demographic information, including age, gender, and medical history (DM, hypertension, ischemic heart disease, lung disease, kidney disease, autoimmune diseases, and hepatitis) was collected. All patients with estimated glomerular filtration rate >30 ml/min and liver enzyme less than five times the upper limit of normal range received remdesivir. All the patients received a proton pump inhibitor or H2-blocker to prevent a stress ulcer. Those with platelet count >50,000 and fibrinogen >50 mg/dl without active bleeding, if not previously treated with an anticoagulant, were treated with a prophylactic anticoagulant, and those with a history of a therapeutic dose of anticoagulant or any proven thrombosis, whereas hospitalized patients received anticoagulants at therapeutic doses. Hyperglycemia was treated with insulin by targeting blood glucose levels between 140 and 200 with divided insulin or according to the scale.
The patients were randomly divided into two groups by blocked randomization, and the sample size of each block was decided to be six. The randomization list was prepared using random allocation software. Implementation of the sequence was performed by a person who wasn't aware of the study design and content of the interventions.
One group received intravenous dexamethasone 8 mg/day, and the other group received intravenous methylprednisolone 60 mg/day in two divided doses (two-thirds of the dose in the morning and one-third in the afternoon). Because of the suppression of the immune system by corticosteroids, which increases the likelihood of other infections, patients were treated with corticosteroids for a maximum of 10 days. At baseline, the percentage of arterial blood oxygen was recorded using a pulse oximeter for patients needing oxygenation. The patients were followed up during hospital stay and after discharge for 28 days, and the number of days they needed to receive supplemental oxygen was recorded in the two groups. In addition, the following criteria were recorded in the two groups: absolute leukocyte and lymphocyte counts at baseline and completion of hospitalization were determined by complete blood count test, and hypokalemia (potassium<3.5 mmol/L) during the hospital stay, fasting blood sugar above 126 mg/dL during hospitalization (in duplicate and based on blood sample test), the average length of hospital stay, the need for ICU admission, mortality within 28 days of hospital stay (if the patient was discharged before 28 days, they were followed up by telephone) and the number of days since the onset of symptoms during admission (after statistical analysis, information related to this item was excluded from the study). Criteria for ICU admission were noninvasive refractory hypoxemia, unstable hemodynamics, decreased level of consciousness, hypercapnia, and respiratory distress.
Data analysis
Data were entered into SPSS version 26, and the mean (± SD) or median (interquartile range [IQR]), frequency, and percentage were used to describe the data. Data analysis was performed using a t-test, Mann-Whitney test, or chi-square.
Results
All patients completed the assigned treatments (Figure 1). The mean age of the methylprednisolone group was 61.74 ± 16.86 years and that of the dexamethasone group 64.51 ± 16.86 years, with no significant difference (P >0.05).
The study groups were not significantly different regarding gender and underlying diseases, including DM, hypertension, ischemic heart disease, lung disease, kidney disease, autoimmune disease, and hepatitis (Table 1 , P >0.05).
Table 1.
Comparison of frequency distribution of gender and underlying diseases at the admission time in the study groups.
| Variables |
Methylprednisolone Frequency (%) |
Dexamethasone Frequency (%) |
P-value | |
|---|---|---|---|---|
| Gender | Female | 34 (46.6) | 29 (41.1) | 0.54 |
| Male | 39 (53.4) | 41 (58.6) | ||
| Diabetes mellitus | 20 (27.4) | 17 (24.3) | 0.67 | |
| Hypertension | 37 (50.7) | 31 (44.3) | 0.44 | |
| Ischemic heart disease | 8 (11) | 9 (12.9) | 0.73 | |
| Lung disease | 11 (15.1) | 7 (10) | 0.36 | |
| Kidney disease | 2 (2.7) | 7 (10) | 0.09 | |
| Autoimmune disease | 4 (5.5) | 1 (1.4) | 0.37 | |
| Hepatitis | 0(0) | 0 (0) | — | |
In addition, the two groups were matched for the levels of SpO2, white blood cell (WBC), and lymphocyte count at admission (P > 0.05, Table 2 ).
Table 2.
Comparison of mean age, duration of symptoms, and oxygen saturation (SpO2), white blood cell, and lymphocyte count at the admission time in the study groups.
| Variables |
Methylprednisolone group |
Dexamethasone group |
P-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Duration of symptoms (day) | 7.05 | 3.63 | 6.50 | 3.60 | 0.361 |
| Spo2 at admission (%) | 88.14 | 9.31 | 82.7 | 10.041 | 0.79 |
| WBC at admission (absolute number) | 6578 | 3444 | 7018 | 3175 | 0.43 |
| Lymphocytes at admission (absolute number) | 1097 | 456 | 975 | 484 | 0.18 |
SD = standard deviation; SpO2 = oxygen saturation; WBC = white blood cell.
After the intervention, the comparison of hospital stay length as the main outcome and secondary outcomes, such as duration of oxygen therapy, WBC, and lymphocyte count at discharge in the study groups, are listed in Table 3 . WBC and lymphocyte count at discharge in the two groups were not significantly different (P >0.05). However, hospital stay length and oxygen therapy duration were significantly different (P <0.001), so that the durations were higher in the methylprednisolone group (11 [95% CI: 7-14] and 10 [95% CI: 5.5-14], respectively) than in the dexamethasone group (8 [95% CI: 6-10] and 7 [95% CI: 5-9], respectively). Moreover, the mortality rate was 17.1% (95% CI: 8.1-26.1) in the dexamethasone group and 12.3% (95% CI: 4.6-20.0) in the methylprednisolone group, which was not statistically significantly (P = 0.46). Furthermore, the frequency of hypokalemia and hyperglycemia were significantly different (P <0.05), so that their frequency in the methylprednisolone group was 24.7% and 78.1%, which were higher than those in the dexamethasone group (11.4% and 36.8%), respectively (Table 4 ).
Table 3.
Comparison of mean hospital stay length, oxygen therapy duration, white blood cell, and lymphocyte count after the invention in the study groups.
| Variable |
Methylprednisolone group |
Dexamethasone group |
P-value | ||
|---|---|---|---|---|---|
| Median | Interquartile range | Median | Interquartile range | ||
| Length of hospital stay | 11 | (7-14) | 8 | (6-10) | 0.001 |
| Duration of oxygen therapy | 10 | (5.5-14) | 7 | (5-9) | 0.001 |
| WBC | 6000 | (4000-7900) | 6450 | (5025-8100) | 0.28 |
| Lymphocyte count | 1000 | (814-1238) | 818.5 | (632-1239) | 0.06 |
WBC = white blood cell.
Table 4.
Comparison of the frequency distribution of mortality, intensive care unit admission, hypokalemia, and hyperglycemia at discharge time in the study groups.
| Variables | Methylprednisolone group Frequency (%) | Dexamethasone group Frequency (%) | P-value |
|---|---|---|---|
| Mortality | 9 (12.3) | 12 (17.1) | 0.42 |
| ICU admission | 20 (27.4) | 12 (17.1) | 0.14 |
| Hypokalemia | 18 (24.7) | 8 (11.4) | 0.04 |
| Hyperglycemia | 57 (78.1) | 27 (36.8) | 0.03 |
ICU = intensive care unit.
The patient's medications in the two groups were not significantly different (P >0.05) except for insulin (P <0.001), which was higher in the methylprednisolone group (Table 5 ).
Table 5.
The medications used during the hospitalization of patients in the study groups.
| Drugs | Methylprednisolone (n = 73) | Dexamethasone (n = 70) | P-value |
|---|---|---|---|
| Remdesivir | 66 (90.4) | 60 (85.7) | 0.54 |
| Anticoagulant | 70 (95.6) | 66 (94.3) | 0.95 |
| GI prophylaxis | 73 (100) | 70 (100) | 0.99 |
| Insulin | 56 (76.7) | 25 (35.7) | <0.001 |
| ACE | 2 (2.7) | 0 (0) | 0.50 |
| ARB | 27 (37.0) | 22 (31.4) | 0.60 |
| Diuretic | 9 (12.3) | 9 (12.9) | 0.88 |
| β-blocker | 9 (12.3) | 5 (7.1) | 0.45 |
| Calcium channel blocker | 11 (15.1) | 8 (11.4) | 0.69 |
| α-blocker | 0 (0) | 1 (1.4) | 0.49 |
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; GI = gastrointestinal.
No cardiac complication was observed in patients.
Discussion
In the present study, we aimed to compare the effects of intravenous dexamethasone and methylprednisolone on the treatment of inpatients with COVID-19. Two groups of patients with moderate to severe COVID-19 were treated with dexamethasone and methylprednisolone separately. The results of the present study indicated that dexamethasone was more effective in improving the complications of COVID-19 with a lower risk of hypokalemia and hyperglycemia.
There was no significant difference in mean age and gender between the two groups. In addition, there was no significant difference in medications during hospitalization (except for insulin) and the frequency of underlying diseases (DM [27.4% and 24.3%, respectively], hypertension [50.7% and 44.3%, respectively], ischemic heart disease [11% and 12.9%, respectively], lung disease [15.1% and 10%, respectively], kidney disease [2.7% and 10%, respectively], autoimmune disease [5.5% and 1.4%, respectively] and hepatitis) between the two groups. The SpO2, WBC, and lymphocyte count at admission were not significantly different between the two groups. Furthermore, the need for ICU admission, mortality, and ventilation in the two groups were not significantly different. Given that these are considered risk factors for complications in patients with COVID-19, the study groups should be homogeneous in this regard (Gattinoni et al., 2020), which was taken into account in the present study. In a study by Fatima et al. (2020) in Pakistan, 100 patients with moderate to severe COVID-19 were treated with either dexamethasone (n = 35) or methylprednisolone (n = 65) for five days. The two groups were matched for age, smoking, hypertension, lung disease, ischemic heart disease, and chronic kidney disease. However, they were not matched for DM, with the frequency of DM being higher in the dexamethasone group than in the methylprednisolone group (Fatima et al., 2020). One of the limitations of the study of Fatima et al. was the small sample size in the dexamethasone group (because of limited access to the drug) and the inequality of the number of people with DM in the two study groups. In the present study, similar to the previously cited study, there was no significant difference in mortality (12.3% and 17.1%, respectively) and ICU admission (27.4% and 17.1%, respectively) between the two groups. However, hospital stay length and oxygen therapy duration were significantly shorter in the dexamethasone group. In addition, the side effects of treatments were significantly fewer in the dexamethasone-treated group.
The results of the present study also showed that the two groups were not significantly different in mortality, ICU admission, WBC, and lymphocyte count at discharge, but hospital stay length and duration of oxygen therapy were significantly lower in the dexamethasone group than in the methylprednisolone group. In a retrospective quasi-experimental study conducted by Rana et al. (2020), the medical files of 60 patients with COVID-19 were divided into two groups of 30 each, receiving dexamethasone (8 mg twice daily) or methylprednisolone (40 mg twice daily) for 8 days, were studied. It was observed that dexamethasone was more effective than methylprednisolone in reducing C-reactive protein and increasing the arterial oxygen pressure (PaO2)/fraction of inspired oxygen (FiO2) ratio (Rana et al., 2020). In the present study, the duration of oxygen therapy was shorter in patients treated with dexamethasone, which is consistent with the study by Rana et al.
In the present study, the frequency of hypokalemia and hyperglycemia was significantly higher in the methylprednisolone group than in the dexamethasone group, indicating a lower risk of dexamethasone for hypokalemia and hyperglycemia. Hyperglycemia is a common side effect of corticosteroid therapy that affects 20-50% of patients without a history of DM. Glucose levels are often elevated in patients with DM and previously controlled DM during corticosteroid therapy. Changes in carbohydrate metabolism, including insulin resistance and decreased peripheral glucose uptake, may explain the increase in hyperglycemia in patients treated with corticosteroids (Clore and Thurby-Hay, 2009). In the study of Donihi et al. (2006), the frequency of hyperglycemia and its multiple episodes were reported in 64% and 52% of patients under methylprednisolone treatment, respectively (Donihi et al., 2006). Hyperglycemia is an independent predictor of mortality in hospitalized patients with COVID-19. Because systemic corticosteroids may exacerbate hyperglycemia, its development may neutralize the benefits of corticosteroids and worsen the prognosis in patients with COVID-19 (Sardu et al., 2020a, 2020b). Tocilizumab has been observed to be less effective as a treatment in patients with hyperglycemia (Marfella et al., 2020). In addition, oral or intravenous steroids with glucocorticoid properties, such as prednisolone and hydrocortisone, which are sometimes used to treat chronic obstructive pulmonary disease, can increase renal potassium excretion and lead to hypokalemia (Veltri and Mason, 2015). Hypokalemia is common in patients with COVID-19 pneumonia, is an independent predictor of the need for ICU admission and invasive ventilation, and appears to be a biomarker of severe COVID-19 (Moreno et al., 2020). Furthermore, the known side effects of corticosteroids, such as hyperglycemia and superimposed infection, have been reported in patients with COVID-19 (van Paassen et al., 2020). The largest meta-analysis on low-dose corticosteroids in patients with sepsis did not show an increased risk of infection or gastrointestinal bleeding. However, the risk of hyperglycemia, hypernatremia, and muscle weakness increased (Annane et al., 2019). Because of the lower frequency of hypokalemia and hyperglycemia in patients treated with dexamethasone compared with methylprednisolone in the present study, this drug can be more confidently prescribed to patients.
In our study, more hypokalemia was observed in patients treated with methylprednisolone, which can be related to its greater mineralocorticoid effect. However, because of the greater glucocorticoid effect of dexamethasone, the drug was expected to lead to more hyperglycemia, which was not observed in our patients receiving the drug. In patients with COVID-19, multiple mechanisms lead to pulmonary edema contributing to the severity of symptoms and mortality (Cui et al., 2021). Therefore it seems that methylprednisolone, with greater mineralocorticoid effect and consequently more fluid retention, may be less effective than dexamethasone.
Although we observed better efficacy of dexamethasone in improving some of the complications of moderate to severe COVID-19, one of the limitations of our study was the lack of including a control group to compare the outcomes and investigate the potentially positive efficacy of dexamethasone. Another limitation of our study was the relatively small sample size (n = 143), which necessitates conducting larger controlled studies to achieve more conclusive results. In addition, it is possible that because healthcare providers were not blinded, some may have communicated the assigned treatment to patients.
In conclusion, although the present study showed better efficacy of dexamethasone compared with methylprednisolone in improving some moderate to severe COVID-19 complications, including hospital stay length and oxygen therapy duration, the need for ICU and mortality in the two groups of patients were not significantly different. Furthermore, the frequency of hypokalemia and hyperglycemia was lower in patients receiving dexamethasone, which indicates better efficacy and lower risk of dexamethasone.
However, to achieve more conclusive results, it is recommended that similar studies be performed with a larger sample size and different doses of the two drugs.
Competing interests
The authors have no competing interests to declare.
Acknowledgments
Funding
This study was supported by Shahrekord University of Medical Sciences, Shahrekord, Iran (grant no.: 3031).
Ethical considerations
All human procedures were reviewed and approved by the Shahrekord University of Medical Sciences Ethics Committees and complied with local ethics committee regulations (IR.SKUMS.REC.1399.288).
Acknowledgment
The authors would like to thank the Clinical Research Development Unit of Hajar Hospital at Shahrekord University of Medical Sciences, Iran, for their assistance in conducting this study.
Author contributions
ZH and AB made substantial contributions to the conception and design of the study and/or the acquisition, analysis, and interpretation of data. MAS, MD, II, and HR contributed to collecting samples, performing experiments, and analyzing the data. All authors contributed to drafting and critically revising the manuscript and providing important intellectual content. MAS, AB, and ZH revised the manuscript and approved the final version of the manuscript submitted. All authors read and approved the final version of the manuscript.
Footnotes
Research implications: Given the higher efficacy of dexamethasone compared with methylprednisolone for the treatment of COVID-19, the health care system and physicians are recommended to consider dexamethasone in corticosteroid therapy for the disease.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.07.019.
Appendix. Supplementary materials
References
- Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y, et al. Corticosteroids for treating sepsis in children and adults. Cochrane Database Syst Rev. 2019;12 doi: 10.1002/14651858.CD002243.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017;43:1751–1763. doi: 10.1007/s00134-017-4919-5. [DOI] [PubMed] [Google Scholar]
- Clore JN, Thurby-Hay L. Glucocorticoid-induced hyperglycemia. Endocr Pract. 2009;15:469–474. doi: 10.4158/EP08331.RAR. [DOI] [PubMed] [Google Scholar]
- Cui X, Chen W, Zhou H, Gong Y, Zhu B, Lv X, et al. Pulmonary edema in COVID-19 patients: mechanisms and treatment potential. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.664349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio N, Scisciola L, Sardu C, Trotta MC, De Feo M, Maiello C, et al. Glycated ACE2 receptor in diabetes: open door for SARS-COV-2 entry in cardiomyocyte. Cardiovasc Diabetol. 2021;20:99. doi: 10.1186/s12933-021-01286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donihi AC, Raval D, Saul M, Korytkowski MT, DeVita MA. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr Pract. 2006;12:358–362. doi: 10.4158/EP.12.4.358. [DOI] [PubMed] [Google Scholar]
- Fatima SA, Asif M, Khan KA, Siddique N, Khan AZ. Comparison of efficacy of dexamethasone and methylprednisolone in moderate to severe COVID-19 disease. Ann Med Surg (Lond) 2020;60:413–416. doi: 10.1016/j.amsu.2020.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P, Lim W, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19 - Preliminary Report. medRxiv. 2020;10(22) https://www.medrxiv.org/content/10.1101/2020.06.22.20137273v1 22 June 2020. (accessed 22 June 2020) [Google Scholar]
- Isidori AM, Arnaldi G, Boscaro M, Falorni A, Giordano C, Giordano R, et al. COVID-19 infection and glucocorticoids: update from the Italian Society of Endocrinology Expert Opinion on steroid replacement in adrenal insufficiency. J Endocrinol Invest. 2020;43:1141–1147. doi: 10.1007/s40618-020-01266-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfella R, Paolisso P, Sardu C, Bergamaschi L, D'Angelo EC, Barbieri M, Rizzo MR, Messina V, Maggi P, Coppola N, Pizzi C, Biffi M, Viale P, Galié N, Paolisso G. Negative impact of hyperglycaemia on tocilizumab therapy in COVID-19 patients. Diabetes Metab. 2020;46:403–405. doi: 10.1016/j.diabet.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese A, Gambardella J, Sardu C, Santulli G. miR-98 regulates TMPRSS2 expression in human endothelial cells: key implications for COVID-19. Biomedicines. 2020;8:462. doi: 10.3390/biomedicines8110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno P O, Leon-Ramirez JM, Fuertes-Kenneally L, Perdiguero M, Andres M, Garcia-Navarro M, et al. Hypokalemia as a sensitive biomarker of disease severity and the requirement for invasive mechanical ventilation requirement in COVID-19 pneumonia: a case series of 306 Mediterranean patients. Int J Infect Dis. 2020;100:449–454. doi: 10.1016/j.ijid.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. COVID-19 Treatment Guidelines Panel: Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/, 2020 (accessed on 31 May 2022) [PubMed]
- Rana MA, Hashmi M, Qayyum A, Pervaiz R, Saleem M, Munir MF, et al. Comparison of efficacy of dexamethasone and methylprednisolone in improving PaO2/FiO2 ratio among COVID-19 patients. Cureus. 2020;12:e10918. doi: 10.7759/cureus.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis. 2021;21:337. doi: 10.1186/s12879-021-06045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel S, Nguyen T, Choi HA. Pharmacologic characteristics of corticosteroids. J Neurocrit Care. 2017;10:53–59. [Google Scholar]
- Sardu C, D'Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, et al. Hyperglycaemia on admission to hospital and COVID-19. Diabetologia. 2020;63:2486–2487. doi: 10.1007/s00125-020-05216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardu C, D'Onofrio N, Balestrieri ML, Barbieri M, Rizzo MR, Messina V, et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416–2430. doi: 10.1016/S0140-6736(16)00578-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24:696. doi: 10.1186/s13054-020-03400-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltri KT, Mason C. Medication-induced hypokalemia. P T. 2015;40:185–190. [PMC free article] [PubMed] [Google Scholar]
- Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J Infect Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. WHO; Geneva: 2020. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. [Google Scholar]
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.