Abstract
We examined the diversity of the plasmids and of the gene tdnQ, involved in the oxidative deamination of aniline, in five bacterial strains that are able to metabolize both aniline and 3-chloroaniline (3-CA). Three strains have been described and identified previously, i.e., Comamonas testosteroni I2 and Delftia acidovorans CA28 and BN3.1. Strains LME1 and B8c were isolated in this study from linuron-treated soil and from a wastewater treatment plant, respectively, and were both identified as D. acidovorans. Both Delftia and Comamonas belong to the family Comamonadaceae. All five strains possess a large plasmid of ca. 100 kb, but the plasmids from only four strains could be transferred to a recipient strain by selection on aniline or 3-CA as a sole source of carbon and/or nitrogen. Plasmid transfer experiments and Southern hybridization revealed that the plasmid of strain I2 was responsible for total aniline but not 3-CA degradation, while the plasmids of strains LME1 and B8c were responsible only for the oxidative deamination of aniline. Several transconjugant clones that had received the plasmid from strain CA28 showed different degradative capacities: all transconjugants could use aniline as a nitrogen source, while only some of the transconjugants could deaminate 3-CA. For all four plasmids, the IS1071 insertion sequence of Tn5271 was found to be located on a 1.4-kb restriction fragment, which also hybridized with the tdnQ probe. This result suggests the involvement of this insertion sequence element in the dissemination of aniline degradation genes in the environment. By use of specific primers for the tdnQ gene from Pseudomonas putida UCC22, the diversity of the PCR-amplified fragments in the five strains was examined by denaturing gradient gel electrophoresis (DGGE). With DGGE, three different clusters of the tdnQ fragment could be distinguished. Sequencing data showed that the tdnQ sequences of I2, LME1, B8c, and CA28 were very closely related, while the tdnQ sequences of BN3.1 and P. putida UCC22 were only about 83% identical to the other sequences. Northern hybridization revealed that the tdnQ gene is transcribed only in the presence of aniline and not when only 3-CA is present.
For many years, anilines and chloroanilines have been among the most important industrially produced amines. They are used widely in the production of polyurethanes, rubber, azo dyes, drugs, photographic chemicals, varnishes, and pesticides (20, 29). As a consequence of this widespread use, they are detected in wastewaters (31, 49). Moreover, chloroanilines have been found in waters as a consequence of the transformation of frequently used acetamide and urea herbicides (31). These toxic and recalcitrant compounds are considered important environmental pollutants (37) and are subject to legislative control by the 76/464/EEC Directive (13) and by the Priority Pollutant List of the U.S. Environmental Protection Agency (15).
In aquatic environments, the major way to remove aniline is through biodegradation (20, 35). The first step of the aerobic degradation pathway is oxidative deamination, which results in the formation of catechol, which is then further degraded by an ortho-cleavage pathway (3, 34) or a meta-cleavage pathway (30). Recently, the different genes of Pseudomonas putida UCC22(pTDN1) involved in the transformation of aniline to catechol were sequenced as tdnA1, tdnA2, tdnB, tdnR, tdnQ, and tdnT (18); that from Acinetobacter sp. strain YAA was sequenced as aniline oxygenase gene atdA (17). Based on sequence similarities with other aniline degradation pathways and on a gene expression study, Fukumori and Saint (18) tentatively concluded that tdnA1, tdnA2, tdnB, and tdnT are structural genes and that tdnR is a positive regulatory gene. tdnQ could be a structural gene, and its product, TdnQ, shows ca. 30% amino acid sequence similarity with glutamine synthetases.
The hypothetical pathway for aniline conversion is as follows. Both atoms of molecular oxygen are incorporated into the 1 and 2 positions of aniline by the oxygenase (TdnA1 and TdnA2) to form a diol, and then the amino group is transferred to TdnQ. TdnT may further transfer the amino group to an unknown substance or release ammonium. All the tdn genes are essential for the conversion of aniline to catechol. A number of catabolic plasmids, such as pCIT1, pTDN1, and pYA1, that can degrade aniline have been described previously (2, 17, 38, 48).
In contrast to aniline, which is rapidly metabolized, chloroaniline is more persistent in the environment (27, 55). Therefore, many efforts have been undertaken to isolate bacteria capable of degrading chlorinated anilines. Moraxella sp. strain G (64) was the first strain isolated that could use 4-chloroaniline as a sole source of carbon, nitrogen, and energy. Later, more chloroaniline-metabolizing strains were isolated, such as Pseudomonas sp. strain JL2 (32), Brevundimonas (previously Pseudomonas) diminuta INMI KS-7 (54), Delftia (previously Pseudomonas) acidovorans CA28 (34), D. acidovorans BN3.1 (6), Comamonas testosteroni I2 (4), Aquaspirillum sp. strain 2C, and Paracoccus denitrificans 3CA (53). (For the current taxonomic situation of B. diminuta and D. acidovorans, see Segers et al. [51] and Wen et al. [63], respectively.) The pathway of monochloroaniline degradation in some of these strains was found to lead directly to a modified ortho- or meta-cleavage pathway after oxidation of the monochloroaniline to the corresponding chlorocatechol (4, 26, 32, 34, 65). The involvement of plasmids in chloroaniline degradation was not clear from these studies.
In contrast to the situation for the aniline degradation pathway, no specific genes for the transformation of chloroaniline have yet been described. The present study was designed to investigate the genetic diversity of five different aniline- and 3-chloroaniline (3-CA)-degrading strains. We compared the involvement of the plasmids in these strains in the degradation of aniline and 3-CA as well as the diversity of the tdnQ gene, one of the genes involved in the oxidative deamination of aniline.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. Strains I2, B8c, and LME1 were deposited in the BCCM/LMG Bacterium Collection (Ghent, Belgium) under the numbers LMG 19554, LMG 19553, and LMG 19555, respectively. Escherichia coli S17–1 λpir (24) was transformed with plasmid pUTgfp (58) as described by Chung et al. (7). This plasmid contains a mini-Tn5 transposon with the nptII (Kmr) and gfp genes and was used to insert the latter two genes into the chromosome of rifampin-resistant Ralstonia eutropha JMP228 (60). This procedure was done by means of biparental mating between E. coli S17–1 λpir(pUTgfp) (24, 58) and R. eutropha JMP228, with selection on Luria broth (LB) agar plates containing rifampin (100 mg/liter) and kanamycin (50 mg/liter). The new strain, JMP228gfp, is rifampin and kanamycin resistant and shows green fluorescence under UV light.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| Comamonas testosteroni I2 | AniNC 3-CANC Rifr, Hgr | 4 |
| Delftia acidovorans LME1 | AniNC 3-CANC Rifr | This study |
| Delftia acidovorans B8c | AniNC 3-CAN | This study |
| Delftia acidovorans CA28 | AniNC 3-CANC Rifr | 34 |
| Delftia acidovorans BN3.1 | AniNC 3-CANC Rifr | 6 |
| Ralstonia eutropha JMP228 | Rifr | 60 |
| Ralstonia eutropha JMP228gfp | Rifr Kmr GFP | This study |
| Escherichia coli S17-1 λpir | 24 | |
| Escherichia coli S17-1 λpir(pUTgfp) | Ampr Kmr GFP | This study |
| Plasmids | ||
| pNB2 (from strain I2) | AniNC Hgr | This study |
| pNB1 (from strain LME1) | AniN | This study |
| pNB8c (from strain B8c) | AniN | This study |
| pC1-1 (from strain CA28) | AniN 3-CAN | This study |
| pC1-2 (from strain CA28) | AniN | This study |
| pC1-3 (from strain CA28) | AniN 3-CAN | This study |
| pB1 (from strain BN3.1) | This study | |
| pUTgfp | Ampr Kmr; expresses GFP | 58 |
| pTDN1-3112 | pUC19 + HindIII (2.59 kb)-EcoRI (4.15 kb) fragment containing tdnQ of P. putida UCC22; Ampr | F. Fukumori |
| pBRH4 | Ampr IS1071 | 42 |
Ani, aniline; GFP, green fluorescent protein. Superscript “N” and “C” indicate sole nitrogen source and sole carbon and energy source, respectively.
Media and culture conditions.
Mineral medium MMN (mineral medium without nitrogen and carbon) is derived from mineral medium MMO (52) by elimination of all nitrogen. MMN medium contained 1,419.6 mg of Na2HPO4, 1,360.9 mg of KH2PO4, 98.5 mg of MgSO4, 5.88 mg of CaCl2 · 2H2O, 1.16 mg of H3BO4, 2.78 mg of FeSO4 · 7H2O, 1.15 mg of ZnSO4 · 7H2O, 1.69 mg of MnSO4 · H2O, 0.38 mg of CuSO4 · 5H2O, 0.24 mg of CoCl2 · 6H2O, 0.10 mg of MoO3, and 3.2 mg of EDTA in 1 liter of distilled water (4). The liquid mineral medium was supplemented with 200 mg of aniline (Sigma-Aldrich Chemie, Steinheim, Germany) (MMN-A) or 3-CA (Fluka AG Chemische Fabrik, Buchs, Switzerland) (MMN-CA) per liter; for the solidified mineral medium, aniline and 3-CA were each used at a concentration of 500 mg/liter. Sodium pyruvate (1,000 mg/liter of MMN medium) was added as an additional carbon source to MMN-A and MMN-CA in order to select for bacteria by utilizing aniline or 3-CA as a sole source of nitrogen (MMN-AP and MMN-CAP, respectively). LB medium containing 10 g of Bacto Peptone (Difco, Detroit, Mich.), 5 g of Bacto Yeast Extract (Difco), and 5 g of NaCl in 1 liter of demineralized water was used as a rich medium. These media were solidified with 2% agar for plate growth.
Isolation of 3-CA-degrading microorganisms.
C. testosteroni I2 (4), D. acidovorans CA28 (34), and D. acidovorans BN3.1 (6) were isolated previously. In this study, strain B8c was isolated from a wastewater treatment plant of a potato-processing company (in Waregem, Belgium), and strain LME1 was isolated from soil that has been treated annually with 3 kg of linuron/ha for at least 10 years (Royal Research Station of Gorsem, Sint-Truiden, Belgium) (see also reference 12). Erlenmeyer flasks (0.25-liter capacity) containing 100 ml of activated sludge (4 g [dry weight]/liter) or soil (5 g in 95 ml of MMN-CA medium) were used to select for 3-CA-degrading microorganisms over a 6-week period by adding 200 mg of 3-CA/liter at the beginning and once a week when less than 5 ml of 3-CA/liter was left in the flasks. The dry-weight determination was performed by incubating a 50-ml sample at 105°C for 24 h and measuring the loss of weight after incubation (22). Subsequently, a 0.5-liter Erlenmeyer flask containing 200 ml of MMN-CA medium (200 mg/liter) was inoculated with 2 ml of the enrichment culture. After 6 days, the second generation of the enrichment culture was transferred to fresh MMN-CA medium (1% inoculum) in a 0.5-liter Erlenmeyer flask. After 6 days of incubation, 100 μl of the culture was spread onto MMN-CA and MMN-CAP agar plates, which were incubated aerobically at 28°C for 1 week. Bacteria that were able to form colonies and that grew in liquid MMN-CA or MMN-CAP medium were regarded as 3-CA-assimilating bacteria.
Cultivation of the isolated microorganisms.
Overnight cultures in 5 ml of LB were used as inocula for degradation experiments. After 1 ml of culture was centrifuged for 5 min at 7,000 × g, washed, and resuspended in 1 ml of saline (0.85% NaCl), an inoculum (1% the final volume) was transferred to liquid MMN medium with the previously described concentrations of 3-CA and/or sodium pyruvate. All cultures were incubated aerobically at 28°C in the dark on a shaker (140 rpm).
Identification of the isolates.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis of whole-cell proteins was performed as previously described (44). Briefly, cells were harvested from tryptic soy agar plates (BBL) after 48 h of incubation at 37°C. Protein extracts were prepared in an SDS- and beta-mercaptoethanol-containing buffer and separated on a discontinuous SDS-polyacrylamide gel. The gel was then stained with Coomassie blue and scanned with an LKB 2202 Ultroscan laser densitometer (LKB, Bromma, Sweden). The protein extract of Psychrobacter immobilis LMG 1125 was used as a standard for normalization (45). Numerical interpretation of the data was completed with the GelCompar 4.1 software package (Applied Maths, Kortrijk, Belgium).
DNA preparation and determination of the moles percent content of guanine plus cytosine via high-pressure liquid chromatography (HPLC) were done as described by Logan et al. (33); nonmethylated phage lambda DNA (Sigma) was used as the calibration standard. Total genomic DNA-DNA hybridizations were performed by the microplate method of Ezaki et al. (14) using black MaxiSorp (Nunc, Roskilde, Denmark) microplates and an HTS7000 bioassay reader (Perkin-Elmer, Norwalk, Conn.). The hybridization temperature was 55°C.
Methods for plasmid DNA extraction, restriction analysis, and Southern hybridization.
Plasmid DNA was isolated by a modified version (59) of the alkaline extraction procedure for large plasmids (28). Restriction endonuclease digestion was done according to the instructions of the enzyme supplier (Hoffmann-La Roche, Basel, Switzerland). Southern hybridizations were performed at high stringency as described by Top et al. (60). Digested plasmid DNA was separated by electrophoresis on a 0.7% agarose gel and blotted onto Hybond-N nylon membranes (Amersham International, Little Chalfont, Buckinghamshire, England). The tdnQ probe was prepared by using the PCR digoxigenin (DIG) labeling mix (Hoffmann-La Roche) according to the instructions of the supplier and using two primers designed in this study (see description of PCR amplification below) and vector pTDN1–3112 (Table 1) as a template. The IS1071 probe was removed from vector pBRH4 (42) with HindIII and subsequently labeled with a DIG DNA random labeling kit. The korA probes for the IncP-1α and IncP-1β groups were prepared by PCR labeling as previously described using plasmids RP4 (8) and pJP4 (11) as templates, respectively.
Plate matings.
Biparental matings were performed by using LB agar plates with the donor and the recipient, R. eutropha JMP228gfp, grown separately overnight in LB. The first selection step for aniline- or 3-CA-degrading transconjugants was done with liquid MMN-CA, MMN-CAP, MMN-A, and MMN-AP media (5 ml in 20-ml tubes) supplemented with kanamycin (50 μg/ml). After the transconjugants showed growth in the liquid media (as observed by turbidity measurements), they were plated on the corresponding solid MMN media. Green fluorescence under UV light confirmed that potential transconjugant colonies were indeed JMP228gfp. Selection for transfer of Hgr was performed directly with LB agar supplemented with HgCl2 (20 mg/liter).
Northern hybridization.
C. testosteroni I2 and D. acidovorans CA28 were grown overnight at 28°C in LB, LB-aniline (200 mg/liter), and LB–3-CA (200 mg/liter). Total RNA was extracted as described by Reddy et al. (46). In brief, 10 ml of culture was centrifuged for 10 min at 12,000 × g and 4°C. The pellet was resuspended in 10 ml of protoplasting buffer (15 mM Tris, 0.45 M sucrose, 8 mM EDTA, 0.1% diethylpyrocarbonate [DEPC] [pH 8.0]) with the addition of 80 μl of 50-mg/ml lysozyme and incubated on ice for 15 min. Subsequently, the protoplasts were centrifuged for 5 min at 5,900 × g, and the pellet was resuspended in 0.5 ml of gram-negative bacterium lysing buffer (10 mM Tris, 10 mM NaCl, 1 mM sodium citrate, 1.5% SDS, 0.1% DEPC [pH 8.0]), incubated for 5 min at 37°C, and chilled on ice. A 250-ml quantity of saturated NaCl (40 g of NaCl/100 ml of H2O) was added, and the solution was incubated on ice for 10 min and centrifuged at 12,000 × g for 10 min. The supernatant was removed to a clean tube, 1 ml of ice-cold 100% ethanol was added, and the RNA was precipitated on dry ice (30 min). Afterward, the tube was centrifuged at 12,000 × g for 15 min, and the pellet was rinsed in 70% ethanol, air dried, and dissolved in 100 μl of DEPC-treated water. Equal amounts of total RNA were loaded on a denaturing agarose gel with formaldehyde, and the gel was Northern blotted onto a Hybond-N nylon membrane. Northern hybridization was done as described by Thomas (57).
Chemical analysis.
Supernatants of bacterial cultures were analyzed by reverse-phase HPLC after the cells were removed by centrifugation (10 min at 5,000 × g). The HPLC system consisted of a Kontron liquid chromatograph with a DEGASYS DG-1310 system to degas the mobile phase, three Kontron 325 high-pressure pumps, a Kontron MSI 660 injector with a 20-μl loop, a Kontron DAD 495 diode-array detector, and a 450 MT2/DAD software system. An Alltima C18 column (250- by 8-mm inner diameter, 5-μm particle size; Alltech, Deerfield, Ill.) was used. The mobile phase consisted of CH3OH–0.1% H3PO4 (60:40), the flow rate was 0.75 ml/min, and the UV detector was set to 210 nm. Quantitative determinations of aniline and 3-CA were done using an external standard ranging from 1 to 250 mg/liter. The detection limit was ca. 0.5 mg/liter.
Gas chromatography (GC)-mass spectrometry (MS) analyses were carried out with a model 2700 GC (Varian, Palo Alto, Calif.)-MAT112S (Finnigan, San Jose, Calif.) gas chromatograph-mass spectrometer equipped with a DB-1 capillary column (100% dimethylsiloxane; length, 30 m; internal diameter, 0.53 mm; film thickness, 5 μm). The temperature of the injector was 200°C, and that of the detector was 250°C. The oven temperature was programmed to increase from 40 to 220°C at a rate of 2°C/min. Helium was used as the carrier gas at a flow rate of 3.5 ml/min.
PCR amplification.
For pure cultures, the template for PCR amplification was obtained by extracting total genomic DNA by the procedure of Bron and Venema (5). One microliter of genomic DNA solution was used in a PCR. The PCR mixture contained 0.5 μM (each) primers, 100 μM (each) deoxynucleoside triphosphates, 10 μl of 10× Expand High Fidelity PCR buffer and 2 U of Expand High Fidelity DNA polymerase (both from Hoffmann-La Roche), 400 ng of bovine serum albumin (Hoffmann-La Roche)/μl, and sterile water (Sigma) to a final volume of 50 μl. The tdnQ gene was amplified with primers tdnQ1F (5′-TCC-CTG-CCT-GGA-GCC-CGA-AAC-3′) and tdnQ1R (5′-TCC-CGC-GCC-GTG-AGT-GAC-TG-3′). The latter were designed in this study on the basis of specific regions of the tdnQ sequence (DDBJ-EMBL-GenBank accession number D85415). The length of the expected amplified fragment was 384 bp. A GC clamp (5′-CGCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGG-3′) (39) was attached to the 5′ end of the tdnQ1F primer. PCR was performed with a Perkin-Elmer 9600 thermal cycler as follows: 94°C for 5 min and then 30 cycles of 92°C for 1 min, 53°C for 1 min, and 72°C for 2 min. A final extension was carried out at 72°C for 10 min. For incompatibility group determinations, korA primers specific for incompatibility group IncP-1 were used, and PCR amplification was performed as described previously (21).
DGGE.
Denaturing gradient gel electrophoresis (DGGE) based on the protocol of Muyzer et al. (39) was performed with a D Gene System (Bio-Rad, Hercules, Calif.). PCR samples were loaded onto 8% (wt/vol) polyacrylamide gels in 1× TAE (20 mM Tris, 10 mM acetate, 0.5 mM EDTA [pH 7.4]). The polyacrylamide gels were made with a denaturing gradient ranging from 50 to 80% (where 100% denaturant contains 7 M urea and 40% formamide). Electrophoresis was carried out for 5 h at 60°C and 180 V. Then, the gels were stained with SYBR GreenI nucleic acid gel stain (1:10,000 dilution; FMC BioProducts, Rockland, Maine) and photographed (4).
DNA cloning and sequencing.
Putative tdnQ gene fragments were cloned by using a TOPO TA cloning kit (Invitrogen, Carlsbad, Calif.) according to the manufacturer's instructions. DNA sequencing was carried out at Eurogentec (Liège, Belgium). Analysis of DNA sequences and homology searches were completed with standard DNA sequencing programs and the BLAST server of the National Center for Biotechnology Information using the BLAST algorithm (1) and using the BLASTN and BLASTX programs for the comparison of a nucleotide query sequence against a nucleotide sequence database and a nucleotide query sequence translated in all reading frames against a protein sequence database, respectively.
Nucleotide sequence accession numbers.
Nucleotide sequences for fragments tdnQ-I2, tdnQ-LME1, tdnQ-B8c, tdnQ-CA28, and tdnQ-BN3.1 have been deposited in the GenBank database under accession numbers AF315641, AF315640, AF315643, AF315639, and AF315642, respectively.
RESULTS
Isolation and identification of 3-CA-metabolizing bacteria.
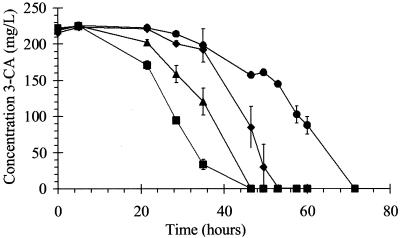
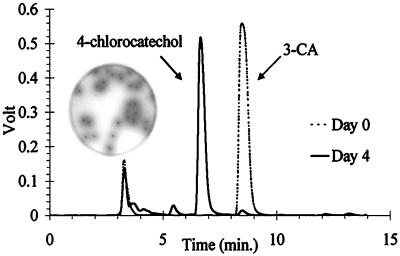
Strains B8c and LME1 were isolated as new 3-CA-metabolizing bacteria from activated sludge and from linuron-treated soil, respectively. One of these two strains, strain LME1, and strains I2, CA28, and BN3.1 are able to use aniline and 3-CA as sole sources of carbon and nitrogen. When aniline and 3-CA were used as sole carbon sources, all strains could degrade the compounds between 40 and 75 h (Fig. 1). When aniline and 3-CA were used as sole nitrogen sources and sodium pyruvate was used as an additional carbon source, the degradation of both compounds was already completed between 14 and 24 h. No aromatic intermediates were observed by HPLC analysis. Strain B8c, on the contrary, grew with aniline as a sole carbon source but not with 3-CA (data not shown). However, it grew in MMN-CAP medium with 3-CA as a sole N source and formed a brown intermediate (Fig. 2). This result was corroborated by the detection of an aromatic intermediate by HPLC analysis (Fig. 2). The mass spectrum of this product, analyzed by GC-MS, was consistent with the structure of 4-chlorocatechol (25). The molecular ion (M) at m/z 144 showed the characteristic 3:1 M/M + 2 isotope ratio of a single Cl atom. Major fragment ions had m/z ratios of 126, 98, and 63. The accumulation of 4-chlorocatechol in the culture of strain B8c is consistent with the inability of this strain to use 3-CA as a carbon source and indicates that this strain can only transform 3-CA into 4-chlorocatechol.
FIG. 1.
Degradation of 3-CA in MMN-CA as a sole source of carbon, nitrogen, and energy in C. testosteroni I2 (♦) and D. acidovorans CA28 (▴), LME1 (●), and BN3.1 (▪). Data points are averages for duplicate cultures, and error bars represent standard deviations.
FIG. 2.
HPLC chromatogram for MMN-CAP medium incubated with D. acidovorans B8c at day 0 and at day 4. The inset shows strain B8c grown on MMN-CAP plates. The colonies are surrounded by a brown color.
Strain B8c has a nucleotide composition of 66.6 mol % G+C and showed 92% DNA reassociation when hybridized with LMG 1226T (56), the type strain of Comamonas acidovorans, which was recently accommodated in the new genus Delftia as Delftia acidovorans (63). Much lower DNA reassociation values of 28 and 32% were found when strain B8c was hybridized with C. testosteroni LMG 1800T (36) and Comamonas terrigena LMG 1253T (9), respectively. Since strains B8c and LME1 showed identical SDS-PAGE patterns for whole-cell proteins, both strains were unambiguously identified as D. acidovorans.
Involvement of plasmids in degradation.
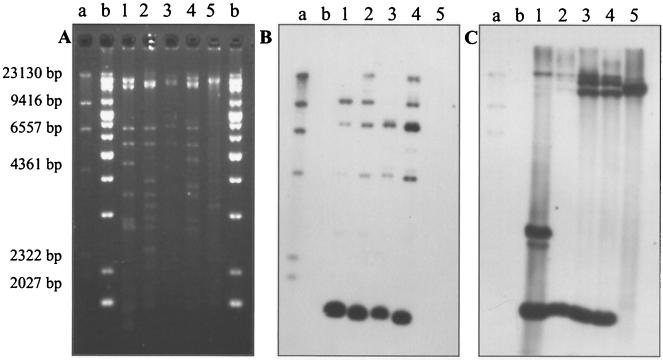
Extraction of plasmids from strains LME1, I2, B8c, CA28, and BN3.1 revealed that they all contained a plasmid with a size of ca. 100 kb, designated pNB1, pNB2, pNB8c, pC1, and pB1, respectively. An EcoRI-PstI digest of the plasmids revealed different restriction patterns (Fig. 3A). All plasmids yielded an amplification product after PCR with the korA primers, which are specific for the IncP-1 group of broad-host-range plasmids. Southern hybridization of these korA PCR products with RP4 (IncP-1α)- and pJP4 (IncP-1β)-generated probes revealed that plasmids pNB1, pNB2, pNB8c, and pC1 hybridized with the IncP-1β-derived probe; pNB8c also hybridized with the IncP-1α-derived probe; and pB1 did not hybridize with either of the two probes, although an amplification product had been obtained (data not shown). Four of the five plasmids clearly belong to the IncP-1 group; three of them appear to be IncP-1β plasmids.
FIG. 3.
Restriction digestion analysis and Southern hybridization. (A) Analysis on a 0.7% agarose gel of EcoRI-PstI-digested plasmids. (B and C) Hybridization with tdnQ (B) and IS1071 (C). Lane 1, C. testosteroni I2 (plasmid pNB2); lane 2, D. acidovorans LME1 (plasmid pNB1); lane 3, D. acidovorans B8c (plasmid pB8c); lane 4, D. acidovorans CA28 (plasmid pC1); lane 5, D. acidovorans BN3.1 (plasmid pB1); lane a, DIG-labeled Marker II (Hoffmann-La Roche); lane b, 1-kb extended marker. The Southern blot in panel C was obtained from a gel different from that shown in panel A but containing the same DNA samples.
To determine if some of the catabolic genes were plasmid encoded, conjugative transfer of the aniline or 3-CA degradation phenotype from strains I2, LME1, B8c, CA28, and BN3.1 to the recipient strain R. eutropha JMP228gfp was examined. From these mating experiments, different plasmid-encoded functions could be derived, as summarized in Table 1. All transconjugants, except for that resulting from the mating with D. acidovorans BN3.1, used aniline as a nitrogen source; however, only JMP228gfp(pNB2) degraded aniline completely in MMN-A medium and thus used it as a sole carbon source as well. As determined by HPLC, the other transconjugants, which can use aniline only as a nitrogen source and not as a carbon source, transformed only 50% of the added aniline in MMN-A medium. This result could have been due to the toxic effect of accumulated intermediates, such as catechol. A brown color, usually caused by polymerization products of catechol, was indeed observed in these cultures. Some of the transconjugants obtained from matings between CA28 and JMP228gfp showed different degradation capacities, and their plasmids were named pC1–1, pC1–2, and pC1–3. Plasmids pC1–1 and pC1–3 contain the genes necessary for the oxidative deamination of 3-CA, while transconjugants with pC1–2 could not use 3-CA as a nitrogen source. When the three types of transconjugants were grown in MMN-AP medium, the medium became brown, probably due to the formation and accumulation of catechol and its polymerization products. This result was not observed when transconjugants JMP228gfp(pC1–1) and JMP228gfp(pC1–3) were grown in MMN-CAP medium. Plasmid extraction with EcoRI-PstI digestion and PCR with the korA primers for IncP-1 plasmids revealed the presence of the different plasmids in the JMP228gfp transconjugants. The plasmids in the donors and the respective transconjugants had identical restriction patterns (data not shown). Conjugation between D. acidovorans BN3.1 and R. eutropha JMP228gfp did not yield any transconjugants that were able to metabolize aniline or 3-CA. C. testosteroni I2, which was the only mercury-resistant strain, could transfer this mercury resistance to JMP228gfp, indicating that the resistance gene is also located on plasmid pNB2 (Table 1).
If the aniline- and/or 3-CA-degradative genes located on the plasmids have enough similarity with some of the cloned and sequenced tdn genes of P. putida UCC22, involved in the oxidative deamination of aniline (18), we should be able to confirm their localization on the plasmids by hybridization. Therefore, primers were designed and a probe was developed for one of the genes, tdnQ. Restriction digestion of plasmids pNB2, pNB1, pNB8c, and pC1 with EcoRI and PstI showed a clear hybridization signal of a 1.4-kb fragment after Southern hybridization with the tdnQ probe (Fig. 3B). Only with plasmid pB1 was no hybridization signal obtained. The probe derived from IS1071 also hybridized with a 1.4-kb fragment (Fig. 3C). This probe also hybridized to an additional, 2.7-kb fragment of plasmid pNB2 as well as to a large fragment (ca. 17 kb) of plasmid pB1. The large fragments of plasmids pNB8c and pC1 are due to incomplete digestion (Fig. 3C). The data show that tdnQ-like genes, very similar to tdnQ of P. putida UCC22, are located on all four plasmids that could transfer the capacity to deaminate aniline and/or 3-CA by conjugation.
Comparison of partial tdnQ sequences
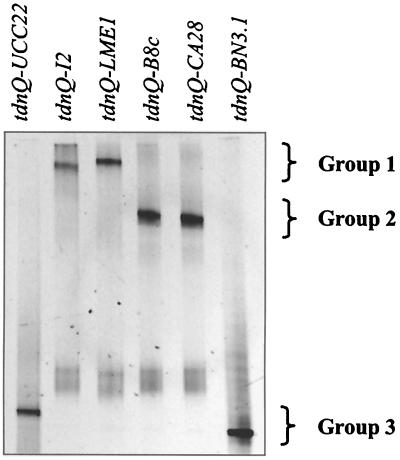
To investigate the diversity of the tdnQ-like genes in the five strains, PCR amplification with tdnQ primers, including one GC clamp, was performed. All the aniline- and 3-CA-metabolizing strains, I2, LME1, B8c, CA28, and BN3.1, as well as the positive control (vector pTDN1–3112) yielded a PCR amplification product of the expected length of 384 bp. An initial comparison of the sequences of the amplified fragments was done via DGGE analysis. A 50 to 80% gradient of denaturing agents resulted in the best separation of the fragments of the different strains. The tdnQ fragments were clearly not identical and could be classified in three groups (Fig. 4). The first group, tdnQ from C. testosteroni I2 (tdnQ-I2) and from D. acidovorans LME1 (tdnQ-LME1), was denatured at rather low denaturant concentrations (upper part of the gel), and a very small difference in migration between the two fragments was observed. The second group, tdnQ from D. acidovorans B8c (tdnQ-B8c) and from D. acidovorans CA28 (tdnQ-CA28), was localized at higher concentrations of denaturing agents (middle of the gel) and seemed to migrate at the same rates. The fragments of the original tdnQ gene of P. putida (tdnQ-UCC22) and of D. acidovorans BN3.1 (tdnQ-BN3.1) both migrated to the bottom of the gel at the highest denaturant concentration and thus formed the third group. While the difference in migration positions between the last two groups of PCR fragments was large, there was only a small difference between the first and second groups.
FIG. 4.
DGGE analysis of tdnQ fragments of different strains capable of degrading aniline and 3-CA.
To confirm the sequence differences of these tdnQ fragments, all five PCR products (without the GC clamp) were cloned and subsequently sequenced. Analysis of the DNA sequences is summarized in Table 2. The partial tdnQ genes of the five strains were related to the partial tdnQ gene of P. putida UCC22. While the sequence of the tdnQ-BN3.1 fragment was nearly identical to that of the tdnQ-UCC22 fragment, the sequences of the tdnQ-I2, tdnQ-LME1, tdnQ-B8c, and tdnQ-CA28 fragments were only 80 to 84% similar to that of the tdnQ-UCC22 fragment. When the partial sequences were translated to the amino acid sequence level, all the sequences were found to be highly similar to the amino acid sequence of the TdnQ protein of P. putida UCC22 (18) and less similar to a component of an aniline dioxygenase (glutamine synthetase-like protein) of Acinetobacter sp. strain YAA (17) (GenBank accession number D86080) (Table 2). Only the last 306 bp of the 384-bp tdnQ fragment was translated into the partial protein structure of 102 amino acids. A comparison of the DGGE and sequencing results showed that the DGGE approach can be useful for specifically amplifying and analyzing tdnQ-like genes from mixed cultures.
TABLE 2.
Levels of nucleotide and amino acid sequence identities for the 384-bp amplified portion of the tdnQ genes of aniline- and 3-CA-degrading bacteria and for database sequences
| Strain | % Nucleotide or amino acid identity witha:
|
||||||
|---|---|---|---|---|---|---|---|
| C. testosteroni I2 | D. acidovorans LME1 | D. acidovorans B8c | D. acidovorans CA28 | D. acidovorans BN3.1 | P. putida UCC22b | Acinetobacter sp. strain YAAb | |
| C. testosteroni I2 | 95/95 | 88/88 | 95/95 | 88/91 | 89/91 | 68/81 | |
| D. acidovorans LME1 | 98 | 91/92 | 98/98 | 92/95 | 91/95 | 69/82 | |
| D. acidovorans B8c | 94 | 95 | 93/93 | 86/89 | 86/89 | 67/81 | |
| D. acidovorans CA28 | 98 | 98 | 96 | 92/94 | 92/97 | 68/81 | |
| D. acidovorans BN3.1 | 83 | 84 | 80 | 84 | 99/100 | 70/84 | |
| P. putida UCC22b | 83 | 83 | 81 | 84 | 99 | 69/84 | |
| Acinetobacter sp. strain YAAb | NSS | NSS | NSS | NSS | NSS | NSS | |
Single entries indicate nucleotide identities. Double entries indicate amino acid identities/positives. NSS, no significant similarity was found.
Sequences were obtained from GenBank.
Differential expression of tdnQ
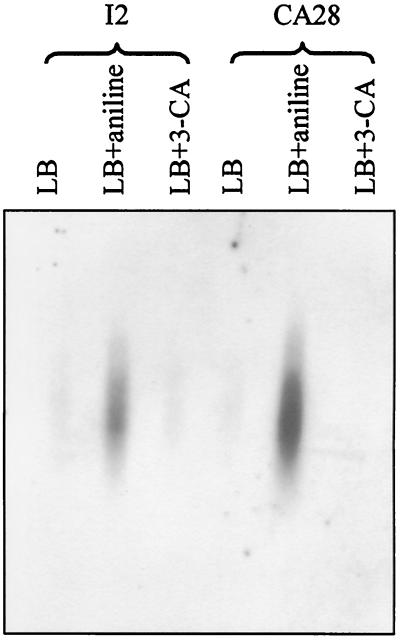
In order to investigate the role of tdnQ in the degradation of 3-CA, strains C. testosteroni I2 and D. acidovorans CA28 were grown in LB, LB-aniline, and LB–3-CA. No traces of aniline and 3-CA could be detected at the time of cell collection, prior to RNA extraction. This result indicates that at least one or several genes involved in the initial transformation steps had been transcribed. Total RNA was blotted and hybridized with the tdnQ probe (Fig. 5). With both strains, only the RNA that was extracted from the cells grown with aniline hybridized with the tdnQ probe. These results suggest that under the conditions used here, the tdnQ gene in these strains is induced by aniline or its metabolites (18) but not by 3-CA or its metabolites. This notion implies that oxidative deamination of 3-CA in these strains may involve genes different from those responsible for aniline degradation.
FIG. 5.
Hybridization with tdnQ of the total RNA of C. testosteroni I2 and D. acidovorans CA28 grown in LB, LB-aniline, and LB–3-CA.
DISCUSSION
Several bacterial species are known to degrade 3-CA (6, 32, 34, 53, 54, 64). Strains LME1 and B8c, isolated and described in this study, are two new strains of the species D. acidovorans that are able to metabolize aniline and 3-CA. C. testosteroni I2 (4) and D. acidovorans CA28 (34), BN3.1 (6), and LME1 show similar metabolic capacities. D. acidovorans B8c is not able to use 3-CA as a sole carbon source and thus is unable to degrade 3-CA completely. This situation leads to the accumulation of 4-chlorocatechol in the medium. During the dioxygenation of 3-CA, theoretically two different intermediates may be formed, e.g., 3-chlorocatechol and 4-chlorocatechol (32). The MS results obtained in this study, together with data from the literature (34, 50, 65), suggest that D. acidovorans B8c degrades 3-CA preferably through 4-chlorocatechol. D. acidovorans LME1 showed no accumulation of chlorinated catechols, probably because of the high level of activity of a chlorocatechol dioxygenase (34).
All the strains that were investigated harbor a large plasmid. Matings between D. acidovorans CA28 and the recipient JMP228gfp resulted in transconjugants with different phenotypes. Some could transform 3-CA and aniline; some could transform only aniline. However, no differences in the plasmid restriction patterns of the different transconjugant colonies could be shown. The cause of these different characteristics is currently under further investigation. When these transconjugants were grown in MMN-CAP, no formation of chlorocatechol was noted. However, complete mineralization of 3-CA apparently did not occur, since 3-CA could not be used as a carbon source. This result suggests that an aliphatic intermediate accumulated after ring cleavage. This aliphatic intermediate would likely have a six-carbon backbone because the release of any carbon should support some growth. It is clear that none of the plasmids codes for complete 3-CA mineralization. To our knowledge, plasmid pC1 of D. acidovorans CA28 is the first and only plasmid characterized so far that codes for partial 3-CA degradation. Plasmid pNB2, on the other hand, was the only plasmid that conferred complete degradation of aniline in R. eutropha JMP228gfp, allowing the strain to use the compound as a sole carbon source. This plasmid of C. testosteroni I2 is thus a new aniline catabolic plasmid, which also encodes mercury resistance. The observation that three plasmids could transfer the ability to use aniline but not 3-CA as a sole nitrogen source suggests that the genes carried on these plasmids are insufficient for the oxidative deamination of 3-CA. This suggestion leads to the hypothesis that other, as-yet-unknown chromosomally located genes are required for the deamination of 3-CA.
In order to confirm the involvement of the plasmids in aniline and/or 3-CA degradation, hybridization experiments were performed with a tdnQ probe. This particular gene was chosen as a representative of the tdn genes for several reasons. First, after primers were designed for tdnQ and tdnA, based on their sequences in the database, the only set that yielded amplification with the five strains was the set of tdnQ primers. An additional advantage was that the cloned tdnQ gene of P. putida UCC22 was provided to us by F. Fukumori, allowing us to make a tdnQ probe by PCR labeling. The other genes, such as tdnA2 and tdnT, were smaller and did not allow effective primer design. Out of the five plasmids, the four that were able to transfer the ability to use aniline as a nitrogen source also hybridized with the tdnQ gene. Only plasmid pB1 of D. acidovorans BN3.1, which could not transfer the ability to transform aniline and/or 3-CA, did not yield a hybridization signal. This result suggests either that plasmid pB1 does not carry the catabolic genes or that this plasmid carries catabolic genes involved in aniline or 3-CA but with lower sequence similarity to tdnQ and is not conjugative.
All plasmids in this study belong to the IncP-1 incompatibility group, and most of them could be assigned to the IncP-1β subclass by korA primers and probes. The incompatibility group and host range of several other catabolic plasmids are still not known. Interestingly, most plasmids involved in the degradation of chlorinated aromatics and for which the incompatibility group has been determined seem to belong to the IncP-1 group (often even IncP-1β), known to contain plasmids with a very broad host range (61). Examples are pJP4 (2,4-dichlorophenoxyacetic acid and 3-chlorobenzoic acid), pAC25 (3-chlorobenzoic acid), pBR60 (3-chlorobenzoic acid), and others (61). In our study, the only plasmid which yielded a korA PCR product that did not hybridize with the IncP-1α- or the IncP-1β-derived probe was plasmid pB1 from D. acidovorans BN3.1. This result could mean that plasmid pB1 belongs to an incompatibility group other than IncP-1, with a more restricted host range. Interestingly, pB1 is also the only one of the five plasmids which did not allow conjugative transfer of the 3-CA- or aniline-transforming phenotype and which did not hybridize with the tdnQ probe.
Results of recent studies have shown that a variety of catabolic genes and operons are flanked by insertion elements (10). IS1071 is an insertion sequence that has been found to bracket the class II transposable element Tn5271, first described for the 3- and 4-chlorobenzoate-degrading strain Alcaligenes sp. strain BR60 (40). Fulthorpe and Wyndham (19) observed that after the introduction of this host strain in lake water and sediment microcosms exposed to 4-chloroaniline, IS1071 was mobilized into different strains and was found in a plasmid unrelated to the donor, pBRC60. Also, in our study, insertion sequences strongly related to IS1071 were detected on the plasmids of the aniline- and 3-CA-degrading strains, probably on the same restriction fragment as the tdnQ gene. This finding suggests that in our strains, tdnQ is flanked by an insertion sequence fragment of the group IS1071. Other investigators (18, 43) also identified the tnpA transposase sequence, which is related to that of IS1071, near the tdnQ gene. Furthermore, Fujii et al. (17) found the transposase gene sequence of Tn1000 on the aniline catabolic plasmid pAS185 of Acinetobacter sp. strain YAA. These findings suggest that during bacterial evolution, the genes responsible for aniline degradation have been spread by horizontal transfer aided by transposons, such as Tn5271. The additional hybridization signal of a 2.7-kb fragment of plasmid pNB2 with the IS1071 probe could be related to the plasmid-encoded mercury resistance. This observation corroborates the findings of Pearson et al. (41), who observed that class II transposase genes are often associated with mercury resistance genes (mer genes).
A new approach to the study of the diversity of functional genes is the analysis of PCR products of these genes by DGGE (23, 47). To our knowledge, this is the first study that has used DGGE to examine the diversity of a gene involved in the degradation of an aromatic compound. The classification of the tdnQ-like gene fragments in three groups, based on their rates of migration in the DGGE gel (Fig. 4), did not correspond entirely with the degree of sequence similarity between the cloned fragments (Table 2). This situation is to be expected, since fragments with different DNA sequences may sometimes end up at the same location in the DGGE gel, while in many other cases, a 1-bp difference can be sufficient to separate two sequences (16). A comparison of DGGE and sequencing data demonstrates, however, that there was sufficient variation at the DNA sequence level to separate the different tdnQ-like genes in the DGGE gel. This DGGE approach, applied to total DNA from various environmental habitats, could be especially useful for further investigation of the diversity of tdnQ-like genes and other catabolic genes in microbial communities without prior cultivation of the degrading organisms.
Interestingly, different tdnQ sequences were found in strains of the same species (tdnQ-CA28 and tdnQ-BN3.1), while almost identical sequences were detected in two strains of different genera (tdnQ-I2 and tdnQ-LME1 or tdnQ-BN3.1 and tdnQ-UCC22). These results suggest again that horizontal gene transfer has played a role in the evolution of chloroaniline-degrading bacteria. The tdnQ gene products are quite conserved, and all belong to the group of glutamine synthetase-like proteins, involved in the oxidative deamination of aniline. None of the obtained tdnQ nucleotide sequences was related to the sequence of the aniline dioxygenase gene (glutamine synthetase-like protein) of Acinetobacter sp. strain YAA (17), while there was a good relationship at the level of the amino acid sequence. The tdnQ primers were probably too specific to detect possible genes responsible for the oxidative deamination of 3-CA in the strains. Work to identify the latter genes and their diversity within chloroaniline-degrading bacteria is currently under way.
In the present study and in previous reports (32), the relationship between the degradation of aniline and its chlorinated analogue, 3-CA, has been mentioned. The enzymes responsible for ortho-ring cleavage of catechol and chlorocatechols are different (26). However, it is not clear if the genes and enzymes responsible for the transformation of aniline and 3-CA into chlorocatechol (oxidative deamination) are also different. Some aniline-degrading bacteria were able to transform 3-CA into chlorocatechol, but these bacteria needed aniline or glucose as a cosubstrate and the cells had to be preincubated with aniline (45, 50). On the one hand, evidence in support of the hypothesis that the oxidative deamination of aniline and its chlorinated analogue is performed by the same enzyme was provided by the work of Latorre et al. (32). The authors obtained 2-chloroaniline-, 3-CA-, and 4-chloroaniline-degrading bacteria by natural gene exchange between an aniline- or a toluidine-degrading Pseudomonas strain and chlorocatechol-assimilating Pseudomonas sp. strain B13. Hybrid organisms were isolated through cocultivation of the parent strains in a chemostat as well as through conjugation on solid media in the presence of chloroanilines as selective substrates. On the other hand, some aniline-degrading bacteria have been reported to be unable to metabolize or cometabolize monochloroanilines (62), while all 3-CA-degrading bacteria described so far can use aniline as a sole carbon source (6, 32, 34, 64). This information suggests the existence of at least two different sets of enzymes, one that can transform only aniline and another that can transform both aniline and 3-CA. In our study, C. testosteroni I2 and D. acidovorans B8c and LME1 could transfer the genes encoding the oxidative deamination of aniline, while the genes encoding the oxidative deamination of 3-CA could not be transferred. These findings, together with the differential transcription of the tdnQ mRNA (Fig. 5), strongly suggest that two different sets of genes are involved in the oxidative deamination of aniline and 3-CA.
This work has shown that the catabolic plasmids and the tdnQ genes involved in the oxidative deamination of aniline in five strains of the family Comamonadaceae are quite diverse. We described a new plasmid encoding complete aniline degradation and two plasmids that code for the partial oxidative deamination of aniline. We also found evidence that the plasmid in D. acidovorans CA28 is the only one in the five strains that codes for partial 3-CA degradation. The importance of IncP-1 plasmids and insertion sequence elements in the spread of catabolic genes was confirmed. Increasing the understanding of new catabolic plasmids for future studies on the bioaugmentation of polluted environments is also relevant.
ACKNOWLEDGMENTS
This work was supported by project grant G.O.A. (1997–2002) of the Ministerie van de Vlaamse Gemeenschap, Bestuur Wetenschappelijk Onderzoek (Brussels, Belgium), by a research grant from the Flemish Fund for Scientific Research (F.W.O.-Vlaanderen), and by EU concerted action MECBAD. E. M. Top and P. De Vos are also indebted to the F.W.O-Vlaanderen for support.
We thank S. Maertens, B. Verbeke, and S. Tistaert for technical assistance; H. Van Limbergen for the construction of R. eutropha JMP228gfp; S. El Fantroussi for help in designing the tdnQ primers; D. Springael for the IS1071 probe; F. Fukumori for the tdnQ probe; H. Van Langenhove for the GC-MS analysis; and J. Robbens, W. Dejonghe, and J. Xu for helpful comments.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anson J G, Mackminnon G. Novel Pseudomonas plasmid involved in aniline degradation. Appl Environ Microbiol. 1984;48:868–869. doi: 10.1128/aem.48.4.868-869.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aoki K, Ohtsuka K, Shinke R, Nishina H. Rapid biodegradation of aniline by Frateuria species ANA-18 and its aniline metabolism. Agric Biol Chem. 1984;48:865–872. [Google Scholar]
- 4.Boon N, Goris J, De Vos P, Verstraete W, Top E M. Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading Comamonas testosteroni strain, I2gfp. Appl Environ Microbiol. 2000;66:2906–2913. doi: 10.1128/aem.66.7.2906-2913.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bron S, Venema G. Ultraviolet inactivation and excision-repair in Bacillus subtilis. I. Construction and characterization of a transformable eightfold auxotrophic strain and two ultraviolet-sensitive derivatives. Mutat Res. 1972;15:1–10. doi: 10.1016/0027-5107(72)90086-3. [DOI] [PubMed] [Google Scholar]
- 6.Brunsbach F R, Reineke W. Degradation of chloroanilines in soil slurry by specialized organisms. Appl Microbiol Biotechnol. 1993;40:2–3. doi: 10.1007/BF00902751. [DOI] [PubMed] [Google Scholar]
- 7.Chung C T, Niemela S L, Miller R H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci USA. 1989;86:2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Datta N, Hedges R W, Shaw E J, Sykes R B, Richmond M H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971;108:1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Vos P, Kersters K, Falsen E, Pot B, Gillis M, Segers P, De Ley J. Comamonas Davis and Park 1962 gen. nov., nom. rev. emend., and Comamonas terrigena Hugh 1962 sp. nov., nom. rev. Int J Syst Bacteriol. 1985;35:443–453. [Google Scholar]
- 10.Di Gioia D, Peel M, Fava F, Wyndham R C. Structures of homologous composite transposons carrying cbaABC genes from Europe and North America. Appl Environ Microbiol. 1998;64:1940–1946. doi: 10.1128/aem.64.5.1940-1946.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Don R H, Pemberton J M. Properties of six pesticide degradation plasmids isolated from Alcaligenes paradoxus and Alcaligenes eutrophus. J Bacteriol. 1981;145:681–696. doi: 10.1128/jb.145.2.681-686.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Fantroussi S, Verschuere L, Verstraete W, Top E M. Effect of phenylurea herbicides on soil microbial communities estimated by analysis of 16S rRNA gene fingerprints and community-level physiological profiles. Appl Environ Microbiol. 1999;65:982–988. doi: 10.1128/aem.65.3.982-988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Economic Community. 76/464/EEC Directive. Council directive of 4 May 1976 on pollution caused by certain dangerous substances discharged into the aquatic environment of the community. Official Journal L129, 18/05/1976. 1976. p. 0023. [Google Scholar]
- 14.Ezaki T, Hashimoto Y, Yabuuchi E. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int J Syst Bacteriol. 1989;39:224–229. [Google Scholar]
- 15.Federal Register. Priority Pollutant List (promulgated by the U.S. Environmental Protection Agency under authority of the Clean Water Act of 1977) Fed Regist. 1979;44:233. [Google Scholar]
- 16.Felske A, Wolterink A, van Lis R, de Vos W M, Akkermans A D L. Searching for predominant soil bacteria: 16S rDNA cloning versus strain cultivation. FEMS Microbiol Ecol. 1999;30:137–145. doi: 10.1111/j.1574-6941.1999.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujii T, Takeo M, Maeda Y. Plasmid-encoded genes specifying aniline oxidation from Acinetobacter sp. strain YAA. Microbiology. 1997;143:93–99. doi: 10.1099/00221287-143-1-93. [DOI] [PubMed] [Google Scholar]
- 18.Fukumori F, Saint C P. Nucleotide sequences and regulational analysis of genes involved in conversion of aniline to catechol in Pseudomonas putida UCC22(pTDN1) J Bacteriol. 1997;179:399–408. doi: 10.1128/jb.179.2.399-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulthorpe R R, Wyndham R C. Involvement of a chlorobenzoate-catabolic transposon, Tn5271, in community adaptation to chlorobiphenyl, chloraniline, and 2,4-dichlorophenoxyacetic acid in a freshwater ecosystem. Appl Environ Microbiol. 1992;58:314–325. doi: 10.1128/aem.58.1.314-325.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheewala S H, Annachhatre A P. Biodegradation of aniline. Water Sci Technol. 1997;36:53–63. [Google Scholar]
- 21.Götz A, Pukall R, Smit E, Tietze E, Prager R, Tschape H, van Elsas J D, Smalla K. Detection and characterization of broad-host-range plasmids in environmental bacteria by PCR. Appl Environ Microbiol. 1996;62:2621–2628. doi: 10.1128/aem.62.7.2621-2628.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg A E, Clesceri L S, Eaton A D, editors. Standard methods for the examination of water and wastewater. 18th ed. Washington, D.C.: American Public Health Association; 1992. [Google Scholar]
- 23.Henckel T, Friedrich M, Conrad R. Molecular analyses of the methane-oxidizing microbial community in rice field soil by targeting the genes of the 16S rRNA, particulate methane monooxygenase, and methanol dehydrogenase. Appl Environ Microbiol. 1999;65:1980–1990. doi: 10.1128/aem.65.5.1980-1990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey W J, Focht D D. Degradation of mono-, di-, and trihalogenated benzoic acids by Pseudomonas aeruginosa JB2. Appl Environ Microbiol. 1990;56:3842–3850. doi: 10.1128/aem.56.12.3842-3850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinteregger C, Loidl M, Streichsbier F. Characterization of isofunctional ring-cleaving enzymes in aniline and 3-chloroaniline degradation by Pseudomonas acidovorans CA28. FEMS Microbiol Lett. 1992;76:261–266. doi: 10.1016/0378-1097(92)90346-p. [DOI] [PubMed] [Google Scholar]
- 27.Horrowitz A, Suflita J M, Tiedje J M. Reductive dehalogenation of halobenzoates by anaerobic lake sediment microorganisms. Appl Environ Microbiol. 1983;45:1459–1465. doi: 10.1128/aem.45.5.1459-1465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kado C I, Liu S T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kearney P C, Kaufman D D. Degradation of herbicides. New York, N.Y: Marcel Dekker, Inc; 1969. [Google Scholar]
- 30.Konopka A, Knight D, Turco R F. Characterization of a Pseudomonas sp. capable of aniline degradation in the presence of secondary carbon sources. Appl Environ Microbiol. 1989;48:491–496. doi: 10.1128/aem.55.2.385-389.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lacorte S, Perrot M-C, Fraisse D, Barcelo D. Determination of chlorobenzidines in industrial effluent by solid-phase extraction and liquid chromatography with electrochemical and mass spectrometric detection. J Chromatogr A. 1999;833:181–194. [Google Scholar]
- 32.Latorre J, Reineke W, Knackmuss H J. Microbial metabolism of chloroanilines: enhanced evolution by natural genetic exchange. Arch Microbiol. 1984;140:159–165. [Google Scholar]
- 33.Logan N A, Lebbe L, Hoste B, Goris J, Forsyth G, Heyndrickx M, Murray B L, Syme N, Wynn-Williams D D, De Vos P. Aerobic endospore-forming bacteria from geothermal environments in northern Victoria Land, Antarctica, and Candlemas Island, South Sandwich archipelago, with the proposal of Bacillus fumarioli sp. nov. Int J Syst Evol Microbiol. 2000;50:1741–1753. doi: 10.1099/00207713-50-5-1741. [DOI] [PubMed] [Google Scholar]
- 34.Loidl M, Hinteregger C, Ditzelmueller G, Ferschl A, Streichsbier F. Degradation of aniline and monochlorinated anilines by soil-borne Pseudomonas acidovorans strains. Arch Microbiol. 1990;155:56–61. [Google Scholar]
- 35.Lyons C D, Katz S, Bartha R. Mechanisms and pathway of aniline elimination from aquatic environments. Appl Environ Microbiol. 1984;48:491–496. doi: 10.1128/aem.48.3.491-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus P, Talalay P. Induction and purification of alpha- and beta-hydroxysteroid dehydrogenases. J Biol Chem. 1956;218:661–674. [PubMed] [Google Scholar]
- 37.Meyer U. Biodegradation of synthetic organic colorants. London, England: Academic Press Ltd.; 1981. [Google Scholar]
- 38.Meyers N L. Molecular cloning and partial characterization of the pathway for aniline degradation in Pseudomonas sp. strain CIT1. Curr Microbiol. 1992;24:303–310. [Google Scholar]
- 39.Muyzer G, de Waal E C, Uitterlinden A. Profiling of complex microbial populations using denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol. 1993;59:695–700. doi: 10.1128/aem.59.3.695-700.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakatsu C, Ng J, Singh R, Straus N, Wyndham C. Chlorobenzoate catabolic transposon Tn5271 is a composite class-I element with flanking class-II insertion sequences. Proc Natl Acad Sci USA. 1991;88:8312–8316. doi: 10.1073/pnas.88.19.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson A J, Bruce K D, Osborn A M, Ritchie D A, Strike P. Distribution of class II transposase and resolvase genes in soil bacteria and their association with mer genes. Appl Environ Microbiol. 1996;62:2961–2965. doi: 10.1128/aem.62.8.2961-2965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peel C M, Wyndham R C. Selection of clc, cba, and fcb chlorobenzoate-catabolic genotypes from groundwater and surface waters adjacent to the Hyde Park, Niagara Falls, chemical landfill. Appl Environ Microbiol. 1999;65:1627–1635. doi: 10.1128/aem.65.4.1627-1635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poelarends G J, Kulakov L A, Larkin M J, van Hylckama Vlieg J E, Janssen D B. Roles of horizontal gene transfer and gene integration in evolution of 1,3-dichloropropene- and 1,2-dibromoethane-degradative pathways. J Bacteriol. 2000;182:2191–2199. doi: 10.1128/jb.182.8.2191-2199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pot B, Vandamme P, Kersters K. Analysis of electrophoretic whole organism protein fingerprints. In: Goodfellow M, O'Donnel A G, editors. Modern microbial methods. Chemical methods in prokaryotic systematics. Chichester, England: Wiley; 1994. pp. 493–521. [Google Scholar]
- 45.Reber H, Helm V, Karanth N G K. Comparative studies on the metabolism of aniline and chloroanilines by Pseudomonas multivorans strain An 1. Eur J Appl Microbiol. 1979;7:181–189. [Google Scholar]
- 46.Reddy K J, Webb R, Sherman L A. Bacterial RNA isolation with one hour centrifugation in a table-top ultracentrifuge. BioTechniques. 1990;8:250–251. [PubMed] [Google Scholar]
- 47.Rosado A S, Duarte G F, Seldin L, Van Elsas J D. Genetic diversity of nifH gene sequences in Paenibacillus azotofixans strains and soil samples analyzed by denaturating gradient gel electrophoresis of PCR-amplified gene fragments. Appl Environ Microbiol. 1998;64:2770–2779. doi: 10.1128/aem.64.8.2770-2779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saint C P, McClure N C, Venables W A. Physical map of the aromatic amine and m-toluate catabolic plasmid pTDN1 in Pseudomonas putida: location of a unique meta-cleavage pathway. J Gen Microbiol. 1990;136:615–625. doi: 10.1099/00221287-136-4-615. [DOI] [PubMed] [Google Scholar]
- 49.Sarasa J, Roche M P, Ormad M P, Gimeno E, Puig A, Ovelleiro J L. Treatment of a wastewater resulting from dye manufacturing with ozone and chemical coagulation. Water Res. 1998;32:2721–2727. [Google Scholar]
- 50.Schukat B, Janke D, Krebs D, Fritsche W, Springael D, Kreps S, Mergeay M. Cometabolic degradation of 2- and 3-chloroaniline because of glucose metabolism by Rhodococcus sp. An117. Curr Microbiol. 1983;9:81–86. [Google Scholar]
- 51.Segers P, Vancanneyt M, Pot B, Torck U, Hoste B, Dewettinck D, Falsen E, Kersters K, De Vos P. Classification of Pseudomonas diminuta Leifson and Hugh 1954 and Pseudomonas vesicularis Busing, Doll, and Freytag 1953 in Brevundimonas gen. nov. as Brevundimonas diminuta comb. nov. and Brevundimonas vesicularis comb. nov., respectively. Int J Syst Bacteriol. 1994;44:499–510. doi: 10.1099/00207713-44-3-499. [DOI] [PubMed] [Google Scholar]
- 52.Stanier R Y, Palleroni N J, Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 53.Surovtseva E G, Ivoilov V S, Vasileva G K, Belyaev S S. Degradation of chlorinated anilines by certain representatives of the genera Aquaspirillum and Paracoccus. Microbiology. 1996;65:553–559. [Google Scholar]
- 54.Surovtseva E G, Ivoilov V S, Karasevich Y N, Vasileva G K. Chlorinated anilines, a source of carbon, nitrogen and energy for Pseudomonas diminuta. Mikrobiologiya. 1985;54:948–952. [Google Scholar]
- 55.Süss A, Fuchsbichler G, Loidl C, Ferschl A, Streichsbier F. Degradation of aniline, 4-chloroaniline and 3,4-dichloroaniline in various soils. Z Planzenernäehr Bodenkd. 1978;141:421–428. [Google Scholar]
- 56.Tamaoka J, Ha D-M, Komagata K. Reclassification of Pseudomonas acidovorans den Dooren de Jong 1926 and Pseudomonas testosteroni Marcus and Talalay 1956 as Comamonas acidovorans comb. nov. and Comamonas testosteroni comb. nov., with an emended description of the genus Comamonas. Int J Syst Bacteriol. 1987;37:52–59. [Google Scholar]
- 57.Thomas P S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tombolini R, Unge A, Davey M E, De Bruijn F J, Jansson J K. Flow cytometric and microscopic analysis of GFP-tagged Pseudomonas fluorescens bacteria. FEMS Microbiol Ecol. 1997;22:17–28. [Google Scholar]
- 59.Top E, Mergeay M, Springael D, Verstraete W. Gene escape model transfer of heavy metal resistance genes from Escherichia coli to Alcaligenes eutrophus on agar plates and in soil samples. Appl Environ Microbiol. 1990;56:2471–2479. doi: 10.1128/aem.56.8.2471-2479.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Top E M, Holben W E, Forney L J. Characterization of diverse 2,4-dichlorophenoxyacetic acid-degradative plasmids isolated from soil by complementation. Appl Environ Microbiol. 1995;61:1691–1698. doi: 10.1128/aem.61.5.1691-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Top E M, Moënne-Loccoz Y, Pembroke T, Thomas C M. Phenotypic traits conferred by plasmids. Amsterdam, The Netherlands: Overseas Publisher Association; 2000. [Google Scholar]
- 62.Walker N, Harris D. Aniline utilization by a soil pseudomonad. J Appl Bacteriol. 1969;32:457–462. [Google Scholar]
- 63.Wen A, Fegan M, Hayward C, Chakraborty S, Sly L I. Phylogenetic relationships among members of the Comamonadaceae, and description of Delftia acidovorans (den Dooren de Jong 1926 and Tamaoka et al. 1987) gen. nov., comb. nov. Int J Syst Bacteriol. 1999;49:567–576. doi: 10.1099/00207713-49-2-567. [DOI] [PubMed] [Google Scholar]
- 64.Zeyer J, Kearny P C. Microbial degradation of para-chloroaniline as sole source of carbon and nitrogen. Pestic Biochem Physiol. 1982;17:215–233. [Google Scholar]
- 65.Zeyer J, Wasserfallen A, Timmis K N. Microbial mineralization of ring-substituted anilines through an ortho-cleavage pathway. Appl Environ Microbiol. 1985;50:447–453. doi: 10.1128/aem.50.2.447-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]