Summary
Background
Cold exposure is one of the most important risk factors for atrial fibrillation (AF), and closely related to the poor prognosis of AF patients. However, the mechanisms underlying cold-related AF are poorly understood.
Methods
Various techniques including 16S rRNA gene sequencing, fecal microbiota transplantation, and electrophysiological examination were used to determine whether gut microbiota dysbiosis promotes cold-related AF. Metabonomics were performed to investigate changes in fecal trimethylamine (TMA) and plasma trimethylamine N-oxide (TMAO) during cold exposure. The detailed mechanism underlying cold-related AF were examined in vitro. Transgenic mice were constructed to explore the role of pyroptosis in cold-related AF. The human cohort was used to evaluate the correlation between A. muciniphila and cold-related AF.
Findings
We found that cold exposure caused elevated susceptibility to AF and reduced abundance of Akkermansia muciniphila (A. muciniphila) in rats. Intriguingly, oral supplementation of A. muciniphila ameliorated the pro-AF property induced by cold exposure. Mechanistically, cold exposure disrupted the A. muciniphila, by which elevated the level of trimethylamine N-oxide (TMAO) through modulation of the microbial enzymes involved in trimethylamine (TMA) synthesis. Correspondingly, progressively increased plasma TMAO levels were validated in human subjects during cold weather. Raised TMAO enhanced the infiltration of M1 macrophages in atria and increased the expression of Casp1-p20 and cleaved-GSDMD, ultimately causing atrial structural remodeling. Furthermore, the mice with conditional deletion of caspase1 exhibited resistance to cold-related AF. More importantly, a cross-sectional clinical study revealed that the reduction of A. muciniphila abundance was an independent risk factor for cold-related AF in human subjects.
Interpretation
Our findings revealed a novel causal role of aberrant gut microbiota and metabolites in pathogenesis of cold-related AF, which raises the possibility of selectively targeting microbiota and microbial metabolites as a potential therapeutic strategy for cold-related AF.
Funding
This work was supported by grants from the State Key Program of National Natural Science Foundation of China (No.81830012), and National Natural Science Foundation of China (No.82070336, No.81974024), Youth Program of the National Natural Science Foundation of China (No.81900374, No.81900302), and Excellent Young Medical Talents supporting project in the First Affiliated Hospital of Harbin Medical University (No. HYD2020YQ0001).
Keywords: Cold, Atrial fibrillation, Gut microbiota, Akkermansia muciniphila, TMAO
Abbreviations: AF, Atrial fibrillation; CF, Cardiac fibroblasts; CM, Cardiac myocytes; FMT, Fecal microbiota transplantation; NMDs, Non-metric multidimensional scaling; RT, Room temperature; SR, Sinus rhythm; TMAO, Trimethylamine N-oxide
Research in context.
Evidence before this study
Mounting studies have linked cold exposure to the higher risk of AF and poor prognosis of AF patients. Gut microbiota and its metabolites have been implicated in AF. It has also been reported that cold exposure led to the remarkable shift of the gut microbiota composition. However, the role of gut microbiota in cold-related AF and underlying mechanism remain unclear.
Added value of this study
We demonstrated that gut microbiota dysbiosis is responsible for cold-related AF, and oral administration of A. muciniphila prevents rats against cold-related AF. Mechanistically, we showed that cold exposure caused a significant decrease in A. muciniphila abundance, which elevated the levels of TMA and TMAO, thereby promoting the polarization and apoptosis of M1 macrophage, resulting in atrial structural remodeling and AF. More importantly, the decreased abundance of A.muciniphila in cold rat models are reproduced among human subjects, and been proved to be an independent risk factor for cold-related AF.
Implications of all the available evidence
Our findings provides new insights into the mechanisms underlying cold-related AF, highlighting a potentially essential role in the development of cold-related AF, which may pave an avenue for the treatment and prevention of cold-related AF.
Alt-text: Unlabelled box
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias worldwide, with a prevalence of 1-2% in the general population.1 Cold exposure is one of the most important independent risk factors for AF.2,3 As reported, when the ambient temperature decreases by 1℃, the incidence of AF increases by 3%.4 More importantly, low temperature is closely related to the poor prognosis of AF patients, including the significant increase in cardiovascular events and all-cause mortality.5 However, the mechanisms underlying cold-related AF remain largely unknown.
The gut microbiota consists of thousands of bacterial species and trillions of microbial cells, which may contribute to the cardiovascular health of the human host and, when aberrant, to the pathogenesis of various cardiovascular diseases including AF.6 Clinical data revealed that patients with AF showed imbalanced gut microbial function and correlated metabolic pattern changes, which raised the possibility that the differential gut microbiome signatures might be used to identify AF patients.7 Gut-derived metabolites, such as TMAO, have been implicated in AF. Local injection of TMAO has been proven to increase the instability of atrial electrophysiology in normal canines, and exacerbate the acute electrical remodeling in a rapid atrial pacing induced-AF model by aggravating autonomic remodeling.8 More importantly, TMAO has gained considerable attention as an independent predictive factor for thrombosis risk and ischemic stroke in patients with AF.9,10 Mounting studies demonstrated that cold exposure contributed to the remarkable shift of the gut microbiota composition.11,12 However, studies focusing on the relationship between gut microbiota and cold-related AF are not available.
Macrophages are the integral components of cardiac tissue, and resident cardiac macrophages account for approximately 6%-8% of the noncardiomyocyte population in the healthy heart of adult mice.13 It is well known that macrophages play paramount and distinct roles in modulation of the pathophysiological processes in the cardiovascular system. As reported, increased M1 macrophages accumulation occurred in the atria of AF patients.14 More importantly, pro-inflammatory M1 macrophages exacerbated atrial electrical remodeling in both canine and mouse AF models. It was demonstrated that TMAO promoted M1 polarization and induced inflammation, which enhanced the allogenic graft-versus-host reaction.15 However, the role of macrophages in cold-related AF and underlying mechanism have not yet been elucidated.
In the present study, we found that patients with AF during winter and rats with cold exposure showed a significant decrease in A. muciniphila abundance. Intriguingly, oral supplementation of A. muciniphila abolished the pro-AF effect elicited by cold exposure. Mechanistically, cold exposure increased TMAO by enhancement of intestinal-derived TMA generation. The elevated TMAO promoted M1 macrophages infiltration and induced pyroptosis in atria of rats, which exacerbated atrial structural remodeling, ultimately led to AF. In summary, our study provided a new insight into the mechanisms underlying cold-related AF, which may be helpful for the treatment and prevention of cold-related AF.
Methods
Human studies
There were two independent cohorts in our study. In the A. muciniphila test, fecal samples were collected from initial AF or SR patients during winter or summer in medical center of the First Affiliated Hospital of Harbin Medical University. Patients admitted with AF or SR from November to January are referred to as Winter AF or Winter SR, and patients admitted with AF or SR from June to August are referred to as Summer AF or Summer SR. The AF patients included in this study were diagnosed with initial AF. Individuals providing fecal samples in winter were required to continuously expose to outdoor cold environment for at least 2 hours a day for more than two weeks. In the A. muciniphila test, of the 862 participants, 521 were recruited into the study. Of those, 209 who had measurements of fecal abundance of A. muciniphila were included in the final analysis (see flowchart in Supplementary Figure S1A). In the TMAO test, blood samples were collected from patients hospitalized in cardiovascular ward of the First Affiliated Hospital of Harbin Medical University. 1873 individuals were consecutively recruited from January 2019 to December 2019, and 1305 subjects who had measurements of plasma TMAO were included for the final analysis (see flowchart in Supplementary Figure S1B). Exclusion criteria were predefined as follows: pregnant at the time of examination; antibiotics or probiotics used in the past three months; had a history of heart failure, cancer or inflammatory bowel diseases; had a gastrointestinal illness, vomiting, diarrhea, or atypical constipation in the past week; significantly altered diet compositions in the past week.
Animals
Male SD rats(200-250g)were purchased from Beijing Vital River Laboratory Animal Technology Co, Ltd (RRID: RDG_734476, Beijing, China) and raised at the Experimental Animal Center of Harbin Medical University (Harbin, China). The animals were kept in cages with light/dark cycles of 12 h and fed with food and water available ad libitum. After one week of adaptive feeding, rats were randomly divided into two groups using completely randomized design, the room temperature (RT) group were raised under 25 ± 1°C; and the Cold group were exposed to moderate cold (4 ± 1°C) in a temperature-controlled and ventilated chamber in SPF conditions using individually ventilated cages for two weeks. The sample sizes of rats were based on the previous studies which confirmed that equal to or more than 6 rats were enough to test the AF susceptibility in experimental studies related to AF.16,17 Our previous research also confirmed that 7 rats were enough to eliminate individuals differences and confounders when performing atrial electrophysiological testing and could effectively evaluate the susceptibility of atrial fibrillation.18 Therefore, we believed that 7 rats were enough to test AF susceptibility.
To testify the importance of TMAO in cold-related AF, Cold rats were administered with 1.0% DMB (cat#183105, Sigma AIdrich, St Louis, Missouri, USA)(a structural analogue of choline, inhibit TMA production)by drinking water for 2 weeks. In anti-pyroptosis experiments, NSA(cat#480073, Sigma AIdrich, St Louis, Missouri, USA)(a direct chemical inhibitor of gasdermin D)was administrated to Cold rats for 2 weeks via intraperitoneal injection (2g/kg/day). The control group rats were given the same amount of vehicle. In A. muciniphila supplementation experiment, the Cold rats were orally administered with A. muciniphila or pasteurised A. muciniphila at a dose of 1×109CFU/200 µL with anaerobic PBS daily for 2 weeks, respectively. The Cold rats in the control groups were administered with the PBS containing 2.5% glycerol.
Caspase1 floxed mice (Caspflox/flox) and Mef2c-Cre transgenic mice in C57BL/6 genetic background were purchased from the Cyagen Biosciences Inc. We generated the Mef2c-Cre‒driven atria and right ventricular-specific deletion of caspase1 by crossing Caspflox/flox mice with Mef2c-Cre transgenic mice, yielding Caspmef2c/mef2c and Caspflox/flox (control) offspring. Caspmef2c/mef2c offspring were born at the expected mendelian ratio and were viable.
At the end of the experiment, all animals were anaesthetized with pentobarbital sodium and sacrificed by cervical dyslocation, then their organ tissues were removed and collected for further biochemical analysis.
The 16S RNA sequencing and microbial analysis
Microbial DNA from fecal samples was extracted using the TIANamp Stool DNA Kit (cat#DP328-02, TIANGEN, Beijing, China) according to the manufacturer's instructions, then 16S rRNA was amplified at the V3 to V4 hypervariable region and sequenced with Illumina novaseq6000. For animal experiment, the DADA2 in QIIME2 were used to denoise the data after quality control, ASVs are filtered by default with a threshold of 0.005% of all sequences sequenced.19,20 Both the Shannon index and Chao1 represent alpha diversity. The principal coordinate analysis (PCoA) based on unweighted UniFrac distances reveal beta diversity. Permutational multivariate ANOVA (PERMANOVA) was used to test the differences between groups in PCoA plot. For human experiment, the raw sequences were preprocessed and quality controlled using QIIME2 with default parameters. The chao1, shannon and simpson represent alpha diversity. The non-metric multidimensional scaling(NMDs) based on unweighted UniFrac distances reveal beta diversity.
Fecal microbiota transplantation
Feces of 3 rats with significantly increased AF susceptibility after cold exposure were collected from different cages. The stool sample was diluted in sterile phosphate buffer (PBS, 50 mg stool/1 mL buffer) and homogenized for 5 minutes until a pasty consistency was reached. Then, the suspension was vortexed for 1 minute and centrifuged at 800 g for 3 minutes. Collect supernatant, aliquot, and freeze at -80℃. Rats were administered with omeprazole (50 mg/kg/d) for 3 days before FMT for decontamination. Fecal bacteria transplantation was performed by gavage in accordance with previous study.21 Fasting and 1 mL citrate (0.16 mg/mL sodium picosulfate, 51.2 mg/mL magnesium oxide) was administered to rats 24 hours before transplantation. Another 2 mL citrate was given to rats 12 hours prior to fecal bacteria transplantation. Then we performed a single oral gavage (2 mL) weekly for 4 weeks to re-colonization of transplanted gut microbiota. The rats gavaged with feces from RT rats were referred to the RT-FMT group and the rats gavaged with feces from Cold rats were referred to the Cold-FMT group.
Electrophysiology
Atrial fibrillation (AF) was induced with essentially the same protocol as described previously in detail.22 Briefly, rats underwent open-chest electrophysiological programmed stimulation under 1% sodium pentobarbital (30 mg/kg) anesthesia. The 1.9-F octapolar catheter (Transonic Systems Inc, New York, USA) was placed on the right atrium for programmed stimulation. To assess AF inducibility, 50-Hz burst pacing was applied for 3 seconds with 12 bursts separated by a 2 seconds interval. AF was defined as >1 second of irregular atrial electrograms (>800 beats/min) with irregular ventricular response. AF duration was defined as the mean duration of all AF episodes within 60 second s in each rat.
Culture of primary rat cardiomyocytes and fibroblasts
The primary atrial myocytes and fibroblasts were obtained from hearts of neonatal Sprague-Daw rats (1-3 days old) as described previously,23 and we have been validated the primary atrial myocytes and fibroblasts by immunofluorescence. In brief, The left atrium was separated from the exposed heart of neonatal rat under aseptic conditions, and cut to pieces approximately 1 mm3 with scissors. Then the heart tissues were digested in 0.25% tryspin with gently shaking. All digestive fluid was collected in DMEM(cat#D6429, Sigma AIdrich, St Louis, Missouri, USA) with 10% fetal bovine serum (cat#sv30087.02, Hyclone, Utah state, USA), centrifuged at 1200 rpm for 5 minutes. The cells were resuspended in DMEM containing 10% FBS, 1% penicillin (100 IU/mL) and streptomycin (100 mg/mL), which was inoculated in six-well plates for 90 minutes. The pre-seeding medium containing cardiomyocytes were seeded in new six-well plates, and the cardiac fibroblasts were left, which has been adhered to the culture plates. The cells stayed at 37°C in a incubator with 5% CO2. The cardiomyocytes were replaced with fresh culture medium after 48 hours. All cardiac fibroblasts in this study were treated within three passage cultures.
In vitro experiments, the cardiomyocytes and fibroblasts were cultured with TMAO (10 μmol/L) or DMSO for 48 hours at 37°C with 5% CO2, respectively.
Preparation of rat bone marrow-derived macrophages
To isolate rat bone marrow-derived macrophages (BMDMs), bone marrow was collected from the femurs of SD rats. First, hind limbs of rats were collected and muscle tissue was removed to expose femur and tibia. Bones were then flushed with RPMI 1640 using syringes to isolate bone marrow cells. Then cells were seeded in 6-well plates in RPMI 1640 supplemented with M-CSF(10μg/mL), 10% (v/v) FBS, and 1%(v/v)penicillin/streptomycin and incubated at 37°C 5% CO2. After 6 days, bone marrow cells were differentiated into macrophages. The subsequent experiments were performed after 48 hours incubation in the presence or absence of TMAO (10 μmol/L).
Cell co-culture model
Trans-well Chambers (Corning, New York, USA) were used to set the cell co-culture model with macrophages and cardiac fibroblasts/cardiomyocytes. Cardiac fibroblasts and cardiomyocytes were pretreated as abovementioned. BMDWs were seeded in 6-well plates and treated with M-CSF for 6 days to induce M0 macrophages. Then, the M0 macrophages were treated with TMAO, and fibroblasts/cardiomyocytes were simultaneously seeded into the upper chambers with 2mL culture medium. After 48 hours of co-culture, the cardiac fibroblasts/cardiomyocytes were lysed with RIPA reagent containing 1% protease inhibitor to extract total protein for western blot analysis.
MTT assay
The cell viability was determined by using the MTT Cell Proliferation Assay (Beyotime, Shanghai, China). Briefly, cells were seeded in 96-well plates and incubated for 48 hours in DMEM, in the presence or absence of TMAO. Then 10 μL tetrazolium salt (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT) was added to the cells in phenol red-free culturemedium and incubated for 4 hours at 37°C. After dimethyl sulfoxide was added, the absorbance was measured at wavelength of 570 nm using a microplate reader (Thermo, Massachusetts, USA) within 1 hour.
Immunofluorescent staining
The frozen sections of atrial tissue were fixed with 4% paraformaldehyde for 15 minutes, washed with 1×PBS for three times, and permeabilized in 1% TritonX-100 for 10 minutes. After washing, the sections were blocked with goat serum (Bioss, Beijing, China) for 1 hour and incubated with primary antibody, rabbit anti-CD163 (Bioss, cat#bs-2527R, RRID: AB_10856166) and mouse anti-CD86 (Santa Cruz Biotechnology, cat#sc-28347, RRID: AB_627200) at 4°C overnight. Following that, they were incubated with FITC Goat Anti-Rabbit IgG (H+L) antibody(ABclonal, cat#AS011, RRID: AB_2769476), and TRITC Goat Anti-Mouse IgG (H+L) antibody (ABclonal, cat#AS026, RRID: AB_2772721). Finally, 4′6-diamino-2-phenylindole (DAPI, Beyotime, China) was added to stain the nuclei. The sections were sealed with anti-fluorescence quencher. Imaging was performed by immunofluorescence microscope (Zeiss, Jena, Germany) and fluorescence intensity was analyzed with ImageJ.
Culture and pasteurisation of A. muciniphila
A. muciniphila was purchased from ATCC (BAA-835), which was cultured in brain heart infusion broth containing 10 mg/L resazurin under strictly anaerobic conditions. The CFU/mL was determined by plate counting using a mucin media containing 1% agarose under anaerobic conditions. The culture was diluted to a final concentration of 1×109CFU/200µL with anaerobic PBS which contains 2.5% glycerol. In addition, the pasteurisation of A. muciniphila was conducted at 70°C for 30 minutes to obtain the pasteurised A. muciniphila.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
The abundance of A. muciniphila in stool samples was quantified by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) in accordance with our previous study.24 Briefly, DNA from the fecal sample is extracted according to the instructions given by the TIANamp Stool DNA Kit,and DNA was stored at −80°C for further analysis. qRT-PCR assay (SYBR Green Assay, Roche, Switzerland) of A. muciniphila was performed on Applied Biosystem. The primers of A. muciniphila were as following, forward CAGCACGTGAAGGTGGGGAC, reverse CCTTGCGGTTGGCTTCAGAT. In addition, the mRNA expressions of microbial enzymes involved in TMA production were also detected by qRT-PCR assay and the primer sequences were listed in supplement table 2. The relative expression levels of DNA and mRNAs were calculated and quantified using the 2−ΔΔCT method after normalization with GAPDH.
LC-MS/MS analysis
Plasma samples and stool samples were collected in heparin tubes and sterile EP tubes respectively and stored at −80°C. 100 μL sample was transferred to an EP tube. After the addition of 400 μL of extract solution (acetonitrile: methanol = 1:1, containing isotopically-labelled internal standard mixture), the samples were vortexed for 30 seconds, sonicated for 10 minutes in ice-water bath, and incubated for 1 hour at -40℃ to precipitate proteins. After centrifugation, the supernatant was transferred to a fresh glass vial for analysis. LC-MS/MS analyses were performed using a UHPLC system (Vanquish, Thermo Fisher Scientific) with a UPLC BEH Amide column (2.1 mm × 100 mm, 1.7 μm) coupled to Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo). The QE HFX mass spectrometer was used for its ability to acquire MS/MS spectra on information-dependent acquisition (IDA) mode in the control of the acquisition software (Xcalibur, Thermo). In this mode, the acquisition software continuously evaluates the full scan MS spectrum.
Flow cytometry
The macrophages were collected with 0.25% pancreatic enzyme and single cell suspension was prepared. After washing by PBS, the macrophages were incubated with fluorescently labelled antibodies, including anti-CD11b/c-APC (Thermo Fisher Scientific, cat#MA5-17507, RRID: AB_2538897) (MФ), anti-CD86-PE (BD Biosciences, cat#551396, RRID: AB_394180) (M1 macrophags), anti-CD163-Percp (Thermo Fisher Scientific, cat#PA5-78961, RRID: AB_2746077) (M2 macrophages), anti-caspase1 p20(Bioss, cat#bs-10743R), dissolved in Flow Cytometry buffer (0.05% Sodium Azide with 1% BSA in 1X PBS) for 1 hour at room temperature in the dark. Goat anti-Rabbit IgG (H+L) Secondary Antibody, PE-Cyanine5.5 (Thermo Fisher Scientific, cat#L42018, RRID: AB_2536607) was also incubated for 1 hour in the dark. 10,000 cells were acquired from the macrophages population using BD FACS Calibur (BD Biosciences, USA) and detected by FlowJo. Dot Plots depict percentage of the cells that express indicated markers, while their expression levels are presented as Mean Fluorescence Intensity.
Histopathology
Fresh atrial samples were collected in 4% paraformaldehyde and then embedded with paraffin. The sections were continuously cut into 5 μm and stained with hematoxylin and eosin (HE)(cat#G1120, Solarbio, Beijing, China), Masson's trichrome staining(cat#G1340, Solarbio, Beijing, China), as described in our previous study.25 Histopathological changes were studied through light microscopy. The fibrosis was quantified by using software (Image-pro plus 6.0, Meida Cybernetics LP). Collagen volume fraction (CVF) was calculated as collagen area/total area × 100%.
Western blot
Western Blot was performed as described previously.26 Briefly, approximately 30∼50 μg of proteins were separated by electrophoresis on 8∼12% SDS–PAGE and then transferred moist to PVDF membranes (Millipore, Billerica, MA,USA).The primary antibodies were as following: anti-TGF-β1 (Bioss, cat#bs-0103R, RRID: AB_10855750), anti-α-SMA (Bioss, cat#bsm-52396R), anti-BAX (Proteintech, cat#60267-1-Ig, RRID: AB_2848213), anti-Bcl2 (abcom, cat#ab196495), anti-GAPDH (ZSGB-Bio, cat#TA-08, RRID: AB_2747414), anti-β-actin (ZSGB-Bio, cat#TA-09, RRID: AB_2636897), anti-ZO-1 (Proteintech, cat#21773-1-AP, RRID:AB_10733242), anti-Occludin(Abcam, cat#ab167161, RRID: AB_2756463), anti-Claudin-4 (Proteintech, cat#16195-1-AP, RRID: AB_2082969), anti-FMO3 (Proteintech, cat#17469-1-AP, RRID: AB_2878410), anti-Caspas1-p20 (Proteintech, cat#22915-1-AP, RRID: AB_2876874), anti-N-GSDMD (Santa Cruz Biotechnology, cat#sc-393581, RRID: AB_2819179). The secondary antibodies including anti-HRP Goat Anti-Mouse IgG(H+L) (ZSGB-Bio, cat#ZB-2305, RRID: AB_2747415), HRP Goat Anti-Rabbit IgG(H+L) (ZSGB-Bio, cat#ZB-2301, RRID: AB_2747412). The membranes were exposed to ECL buffer and chemiluminescent signals were developed with ECL kit and detected by ChemiDoc XRS gel documentation system (Bio-Rad, Hercules, CA, USA). Protein bands were analyzed by Bio-rad software and standardized with internal reference. All antibodies used in this study were purchased by the company, and all of which have been validated.
Statistical analysis
Statistical analysis is performed using GraphPad Prism 8.0 software (GraphPad Software, Inc, La Jolla, CA). Shapiro-Wilk was used for normality test. Continuous variables are expressed as the mean ± standard error of mean (SEM) or median and interquartile range. Categorical variables are represented as numbers and percentages, The comparison between two groups was assessed by Student's non-paired t-test or Wilcoxon (Mann-Whitney U) test. Variables with more than two groups were analyzed by one-way ANOVA followed by Tukey tests or Kruskal-Wallis followed by a post hoc test with Bonferroni adjustment. The chi-square test was used for comparison of categorical variables. Clinical correlation analysis was conducted by single factor and multi-factor Logistic regression. P<0.05 indicated statistical significance between two groups.
Ethics statement
Humans: The study protocol was approved by Research Ethics Committees of the First Affiliated Hospital of Harbin Medical University (Harbin, Heilongjiang, China) and performed in accordance with the Declaration of Helsinki (Ethical approval number: IRB-AF/SC-04/02.0). All subjects provided informed consent. Animals: The animal experiments in this study were performed according to the Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at the Harbin Medical University (Ethical approval number: 2020030).
Role of funding sources
Funders provide financial support for this study, and they did not involve in study design, data collection, data analyses, interpretation of data, or writing of manuscript.
Results
Cold exposure promotes AF by inducing gut microbiota dysbiosis
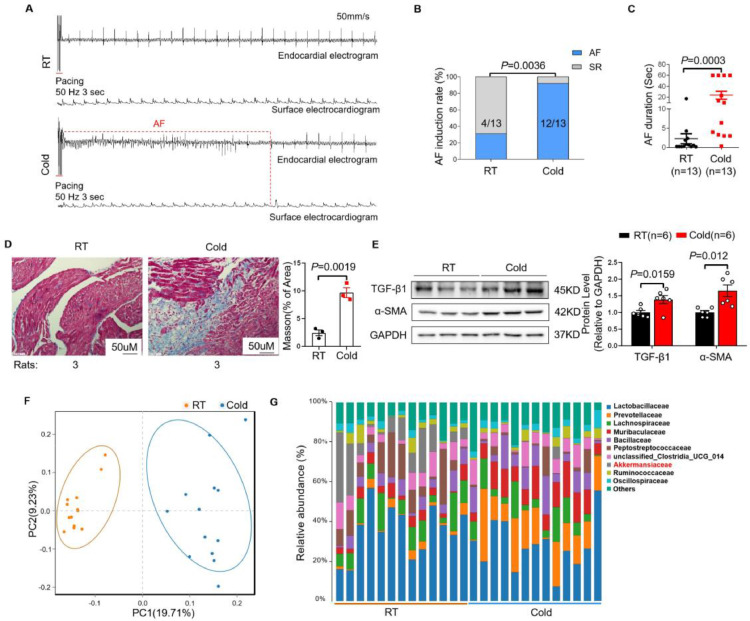
To study the effects of cold exposure on AF susceptibility in rats, we established a cold animal model where rats were exposed to cold temperature (4 ± 1℃) for 2 weeks, and control group rats were raised under room temperature (RT) (25 ± 1°C). As shown in Figure 1A, burst pacing rarely induced AF in RT rats, while it commonly induced AF in Cold rats. Compared with RT rats, the AF inducibility (Figure 1B) and AF duration (Figure 1C) in Cold rats were significantly increased, which is consistent with the clinical study.4 In addition, the greater atrial collagen deposition and collagen volume fraction (Figure 1D) were observed in Cold rats than that in RT rats. Correspondingly, cold exposure upregulated the expression of fibrosis-related proteins TGF-β1 and α-SMA in atria of rats (Figure 1E) . Mounting studies have highlighted the crucial role of gut microbiota in various cardiovascular diseases.27 Meanwhile, gut microbiota was reported to be influenced by ambient temperature.28 Therefore, we collected fecal samples on the 14th day of cold exposure for 16S rRNA analysis. Principal coordinates analysis(PCoA) based on unweighted UniFrac distance revealed distinct clustering between microbiota of RT and Cold rats (Figure 1F). We measured the α-diversity, the community richness showed no statistical difference between two groups (Supplementary Figure S2A), whereas the community diversity was higher in Cold rats than that in RT rats (Supplementary Figure S2B). Notably, the top 10 differential microbiotas in family level showed significant shifts in proportions between RT and Cold group. In particular, the abundance of A. muciniphila in Cold group was dramatically lower than that in RT group (Figure 1G and Supplementary Figure S2C). Furthermore, we found that Cold rats showed impaired intestinal permeability, evidenced by decreased expressions of zonulin-1,occludin and claudin in colon tissue (Supplementary Figure S2D).
Figure 1.
Cold exposure induces AF and gut microbiota dysbiosis in rats. (A) Endocardial and surface electrograms recordings in response to burst pacing in RT and Cold rats. (B) Number of rats in which AF could be reproducibly induced by right atrial burst pacing. (C) AF duration in RT and Cold groups (n=13 per group). (D) Representative images of Masson's trichrome staining and the collagen volume fraction in the atria of RT and Cold rats (n = 3 per group). Magnification × 200, scale bar = 50μm. (E) Representative bands and quantification of expressions of TGF-β1 and α-SMA in rats of RT and Cold rats groups (n = 6 per group). (F) Principal coordinate analysis (PCoA) of unweighted UniFrac revealed clustering of the gut microbiota after temperatures. Each dot represents a single sample of feces. (G) The top 10 of relative abundance of gut microbiota at family level in feces of RT and Cold rats (n = 13 per group). GAPDH was used for normalization. Data are expressed as mean ± SEM and compared by Student's t test (D and E) or Wilcoxon test (C). AF inducibility (B) was presented as numbers and compared by Fisher exact test. RT, room temperature. AF, atrial fibrillation. SR, sinus rhythm.
To investigate the importance of the microbiota changes in cold-related AF, we transplanted the microbiota from Cold or RT rats to wild type normal rats for 4 weeks, and the FMT efficiency was showed by PCoA analysis (Supplementary Figure S3A). Strikingly, rats transplanted with Cold microbiota showed a significant increase in the induction rate and duration of AF, while transplantation with RT microbiota did not lead to detectable changes in AF susceptibility (Supplementary Figure S3B, C). Those findings confirmed that cold-related AF was mediated by aberrant gut microbiota.
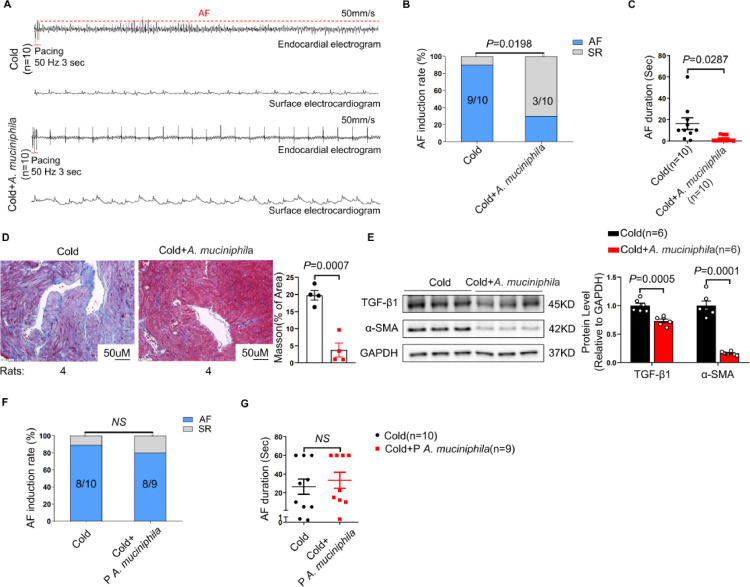
Oral administration of A. muciniphila protects against cold-related AF in rats
A. muciniphila has been considered as a promising probiotic ameliorating metabolic disorders and gut barrier function.29 We investigated whether this strain alone could prevent cold-related AF. As expected, the abundance of A. muciniphila in Cold rats administered with A. muciniphila was significantly higher than that in Cold group (Supplementary Figure S4). Strikingly, compared with Cold rats treated with vehicle, the AF inducibility and AF duration were significantly reduced in Cold rats administered with A. muciniphila (Figure 2A-C). Interestingly, A. muciniphila supplementation markedly ameliorated atrial fibrosis of Cold rats (Figure 2D, E). It has been reported that pasteurised A. muciniphila has the same function as A. muciniphila.30,31 However, oral supplementation of pasteurised A. muciniphila failed to reverse cold-related AF (Figure 2F, G).
Figure 2.
Oral supplementation of A. muciniphila protects Cold rats against AF. (A) Endocardial and surface electrograms recordings in response to burst pacing in Cold and Cold + A. muciniphila group. Supplementation with A. muciniphila reduced AF inducibility (B) and duration (C) in Cold rats (n = 10 per group). (D) Representative images of Masson's trichrome staining and the collagen volume fraction in the atria of Cold and Cold+A. muciniphila rats (n = 4 per group). Magnification × 200, scale bar = 50μm. (E) Representative bands and quantification of expressions of TGF-β1 and α-SMA in rats of Cold and Cold+A. muciniphila groups (n = 6 per group). AF inducibility (F) and AF duration (G) in Cold (n = 10) and Cold + pasteurised A. muciniphila group (n = 9). GAPDH was used for normalization. Data are expressed as mean ± SEM and compared by Student's t test (D and E) or Wilcoxon test (C and G). AF inducibility (B and F) was presented as numbers and compared by Fisher exact test.
A. muciniphila prevents cold-related AF by restraint of TMA/TMAO
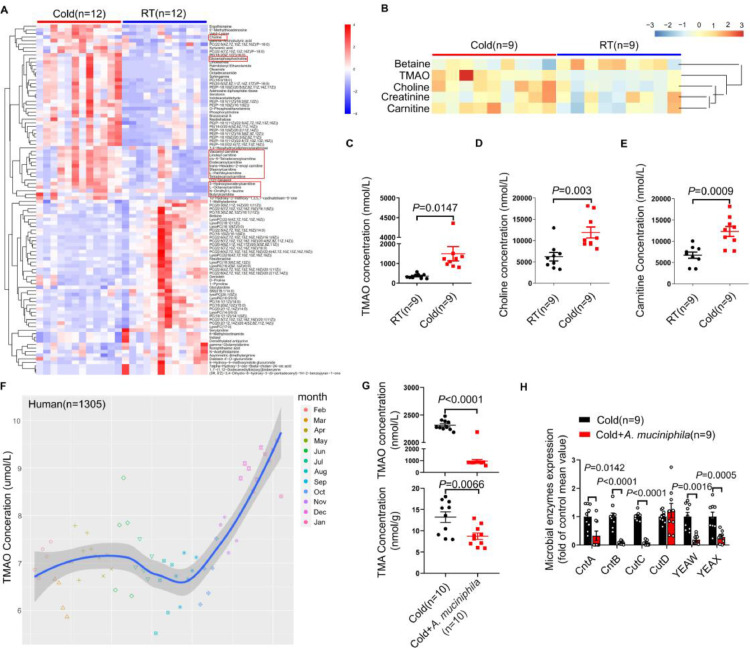
The gut microbiota produces bioactive metabolites by which impacts host physiology.32,33 The untargeted metabolomics showed that the levels of certain metabolites in plasma from RT and Cold rats were changed, of which the precursors of TMAO (choline and carnitine-related metabolites) were markedly increased (Figure 3A). As we know, TMAO has been identified as an prominent contributor facilitating the progression of AF.8,34 Targeted metabolomics showed that Cold rats had higher levels of TMAO, as well as its precursors(choline and carnitine), than RT rats (Figure 3B-E). To evaluate whether the level of TMAO in human can be influenced by ambient temperature, 1305 subjects were consecutively recruited from January 2019 to December 2019 for the TMAO test. The plasma TMAO levels progressively increased with cold weather and peaked at January (Figure 3F). In addition, the plasma TMAO levels were significantly higher in subjects during winter than that in subjects during summer(Supplementary Figure S5).
Figure 3.
A. muciniphila reverses the elevated TMAO induced by cold exposure through restraint of TMA synthesis. (A) Heat map of the nontargeted metabolomics in RT and Cold rats (n = 12 per group). (B) Heat map of relative abundances of TMAO-related metabolites in plasma samples from RT and Cold rats detected by targeted metabolomics (n = 9 per group). Comparison of TMAO (C), Choline (D), and Carnitine (E) levels in plasma between RT and Cold groups (n = 9 per group). (F) The results of plasma TMAO levels in human donors (n=1305) during 12 months. (G) Oral supplementation of A. muciniphila reduced the concentration of TMAO and TMA in Cold rats (n = 10 per group). (H) Statistical results for the expression levels of CntA, CntB, CutC, CutD, YEAX and YEAW in feces from rats of Cold and Cold+ A. muciniphila group (n = 9 per group). Throughout, data are expressed as mean ± SEM and compared by Student's t test. TMAO, trimethylamine N-oxide. TMA, trimethylamine.
To dissect the mechanism underline cold exposure increase TMAO, we detected FMO3, a key enzyme converted TMA into TMAO.35 However, there was no difference in the level of FMO3 in liver from RT and Cold rats(Supplementary Figure S6A). We further examined the fecal TMA by targeted metabolomics of feces, and found that the concentration of TMA was significantly higher in Cold rats than that in RT rats (Supplementary Figure S6B), and the correlation analysis of 16S rRNA sequencing and targeted metabolomics showed that A. muciniphila was negatively correlated with TMA(Supplementary Figure S7), suggesting that the augmented TMAO may be attributed to the high level of TMA. We treated Cold rats with 3,3-dimethyl-1-butanol (DMB) for 2 weeks to inhibit the production of TMA.36 Noticeably, DMB treatment almost completely reversed the cold-induced augment of TMAO and TMA in rats (Supplementary Figure S8A, B). Meanwhile, DMB treatment reduced the AF inducibility and AF duration in Cold rats (Supplementary Figure S8C, D). Interestingly, we found that oral supplementation of A. muciniphila reduced the levels of TMAO (964.71±138.8647 vs 3212.791±28.92857) and TMA (8.707±0.754358 vs 13.203±1.255851) in Cold rats (Figure 3G). The synthesis of TMA is modulated by several microbial enzymes, including choline TMA-lyase (CutC), choline TMA-lyase activating enzyme (CutD)37 and carnitine monooxygenase (CntA/CntB and YeaW/YeaX).38,39 Concurrently, oral supplementation of A. muciniphila dramatically restrained the expression of CntA,CntB,CutC,YeaX and YeaW in Cold rats (Figure 3H). Therefore, A. muciniphila prevent cold-related AF through inhibition of TMA synthesis.
TMAO enhances M1 macrophages infiltration and induces atrial pyroptosis
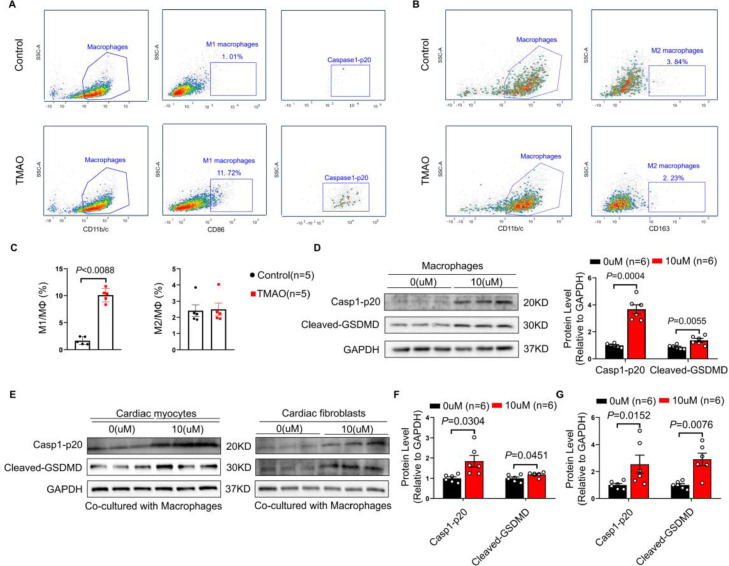
To elucidate the mechanisms through which TMAO enhances AF, we simulated the circulating TMAO concentration (10 μM) in winter subjects to treat cardiac myocytes (CM) and cardiac fibroblasts (CF) for 48 hours. However, CM and CF were tolerant to TMAO, demonstrating no significant difference in neither cell viability (Supplementary Figure S9A, B) nor structural change (Supplementary Figure S9C-E) after TMAO treatment. These results showed that TMAO at the concentration comparable to winter subjects is insufficient to directly cause CM and CF injury, indicating that other cell types may participate in TMAO-induced cardiac injury.
Macrophages are the crucial components of cardiac tissue,40 which has been proven to accumulate in the atria of AF patients and interact with atrial myocytes to promote AF.41 On observing the effects of TMAO on bone marrow derived macrophages (BMDMs), we found that the proportion of M1 macrophages was significantly increased in BMDMs after TMAO stimulation (Figure 4A and C), while that of M2 macrophages remained unchanged (Figure 4B and C). M1 macrophages have a pro-inflammatory effect to aggravate cardiac injury. Interestingly, we found that TMAO activated Casp1-p20 in M1 macrophages (Figure 4A), which means that pyroptosis was occurred.42 We further found that TMAO (10 μM) treatment upregulated the expressions of Casp1-p20 and cleaved-GSDMD in BMDMs (Figure 4D), while showed no influences on pyroptosis in CM and CF (Supplementary Figure S10A-C). More importantly, TMAO (10 μM) aggravated pyroptosis in CM and CF (Figure 4E-G), as well as apoptosis in CM and fibrosis in CF (Supplementary Figure S10D-G) when co-cultured with BMDMs. Nevertheless, those change was not observed when TMAO were at the concentration of 1, 2, 5 and 8μM (Supplementary Figure S10D-G). Taken together, TMAO promotes AF through enhancement of M1 macrophages infiltration and inducing pyroptosis.
Figure 4.
TMAO induces pyroptosis of cardiac myocytes and fibroblasts by enhancing M1 macrophages infiltration. BMDMs were cultured in the absence or presence of TMAO (10 μM) for 48 hours. (A) CD86+ M1 population were sorted from CD11b/c+ macrophages and caspase1-p20 were sorted from CD86+ M1 population. (B) CD163+ M2 population were sorted from CD11b/c+ macrophages. (C) The percentages of CD86+ M1 phenotype or CD163+ M2 phenotype out of CD11b/c+ macrophages were determined (n = 5 per group). (D) Representative bands and quantification of expressions of Casp1-p20 and Cleaved-GSDMD in macrophages in the absence or presence of TMAO (n = 6 per group). CM or CF were respectively co-cultured with macrophages in the absence or presence of TMAO (10 μM). (E) Representative bands of Casp1-p20 and Cleaved-GSDMD in CM and CF (n = 6 per group). Quantification of expressions of Casp1-p20 and Cleaved-GSDMD in CM (F) and CF (G). GAPDH was used for normalization. The data are given as mean ± SEM and compared by Student's t test. BMDMs, Bone marrow derived macrophages; CM, cardiac myocytes; CF, cardiac fibroblasts.
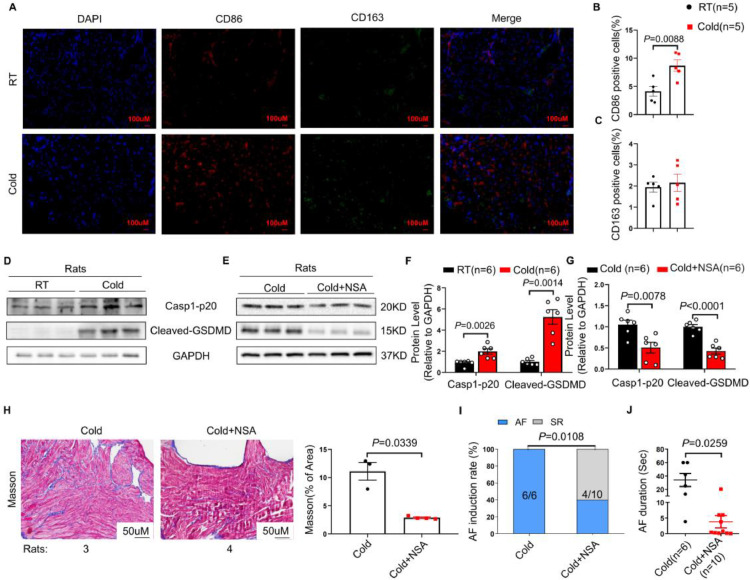
Consistently, the levels of proinflammatory M1 macrophages, rather than anti-inflammatory M2 macrophages were significantly increased in Cold rats, as revealed by increased fluorescence intensity of CD86 and unchanged CD163 (Figure 5A-C). Moreover, the enhanced expressions of Casp1-p20 and cleaved-GSDMD were observed in atria from Cold rats than that from RT rats (Figure 5D, F).
Figure 5.
NSA treatment protects against cold-related AF in rats by attenuation of atrial pyroptosis. Representative immunofluorescence staining of DAPI (blue), CD86 (red), CD163 (green) on the atrial tissue from Cold and RT rats (n = 5 per group). Scale bar = 100 μm. (B) and (C) Quantitative analysis of CD86 and CD163 positive cells. (D) Representative bands of Casp1-p20 and Cleaved-GSDMD expressions in atria of RT and Cold rats (n = 6 per group). (E) Representative bands of Casp1-p20 and Cleaved-GSDMD expressions in Cold and Cold + NSA rats (n = 6 per group). (F and G) Quantification of Casp1-p20 and Cleaved-GSDMD in rats of RT and Cold groups, or Cold and Cold + NSA groups. (H) Representative images of Masson staining, and quantification of collagen volume fractions in atria of Cold (n = 3) and Cold+NSA groups rats (n = 4). Magnification ×200, scale bar = 50 μm. AF induction rate (I) and AF duration (J) in Cold (n = 6) and Cold+NSA groups (n = 10). GAPDH was used for normalization. The data are given as mean ± SEM and compared by Student's t test (B, C, F, G and H) or Wilcoxon test (J). AF inducibility (I) compared by Fisher exact test. NSA, necrosulfonamide.
NSA treatment improves cold-related AF in rats by inhibition of pyroptosis
To further verify that pyroptosis is the key mechanism for cold-related AF, we conducted an experiment in which necrosulfonamide(NSA), a direct chemical inhibitor of gasdermin D, was administered to Cold rats for 2 weeks via intraperitoneal injection (2g/kg/day). NSA treatment suppressed the expressions of Casp1-p20 and cleaved-GSDMD in Cold rats (Figure 5E, G). Surprisingly, Cold rats exhibited enhanced atrial collagen deposition, contrary to Cold rats receiving NSA treatment where attenuated fibrosis were observed (Figure 5H). Furthermore, NSA treatment reduced AF susceptibility in Cold rats, as evidenced by the decrease of AF inducibility and AF duration (Figure 5I and J).
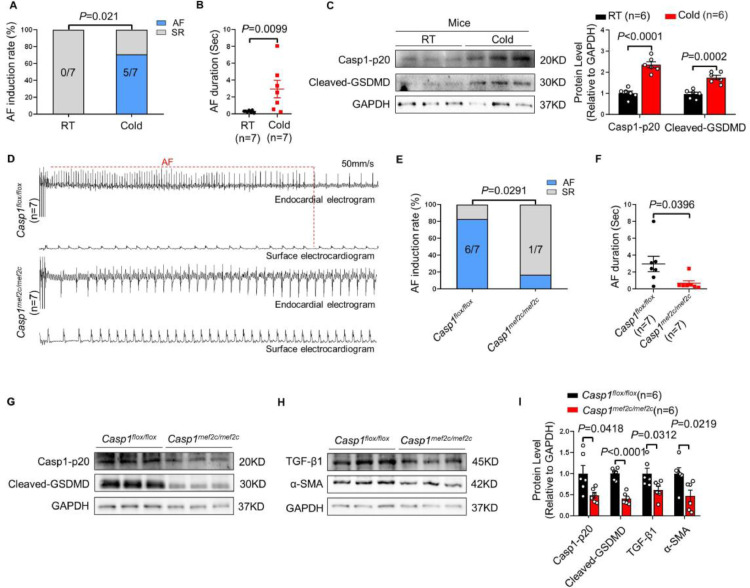
Caspase1 knockout prevents mice from cold-related AF
To further confirm the pivotal role of pyroptosis in cold-related AF, we used Casp1mef2c/mef2c mouse model, in which caspase1 is conditionally deleted in the atria and right ventricular, and western blot confirmed the successful knockout of caspase1 in atria (Supplementary Figure S11A, B). We found that a three-week cold exposure increased the AF susceptibility and exacerbated cardiac pyroptosis in wild type mice (Figure 6A-C). Then, Casp1flox/flox mice and Casp1mef2c/mef2c mice were exposed to cold for three weeks. The results showed that caspase1 knockout reduced the AF induction rate and AF duration of mice (Figure 6D-F). Concurrently, Casp1mef2c/mef2c mice showed a significant decrease in the expressions of atrial Casp1-p20 and cleaved-GSDMD in comparison with Casp1flox/flox mice (Figure 6G, I). More importantly, the alleviated atrial fibrosis were observed in Casp1mef2c/mef2c mice (Figure 6H, I). Furthermore, DMB treatment decrease the expression of Casp1-p20 and cleaved-GSDMD in Cold rats(Supplementary Figure S12A, B). Taken together, those findings confirmed the crucial role of pyroptosis in cold-related AF.
Figure 6.
Caspase1 knockout protects against cold-related AF in mice by restraint of atrial pyroptosis. AF induction rate (A) and AF duration (B) in RT and Cold mice (n=7 per group). (C) Representative bands and quantification of Casp1-p20 and Cleaved-GSDMD in atria of RT and Cold mice (n=6 per group). (D) Endocardial and surface electrograms recordings in response to burst pacing in Casp1flox/flox and Casp1mef2c/mef2c mice with a three-week cold exposure (n=7 per group). (E) AF induction rate. (F) AF duration. Representative bands of the protein expressions of Casp1-p20 and Cleaved-GSDMD (G), TGF-β1 and α-SMA (H) in atrial tissue from Casp1flox/flox and Casp1mef2c/mef2c mice (n=6 per group). (I) Quantification of Casp1-p20, Cleaved-GSDMD, TGF-β1 and α-SMA. GAPDH was used for normalization. The data are given as mean ± SEM and compared by Student's t test (C and I) or Wilcoxon test (B and F). AF inducibility (A and E) compared by Fisher exact test.
The decreased abundance of A. muciniphila was related to AF in patients during winter
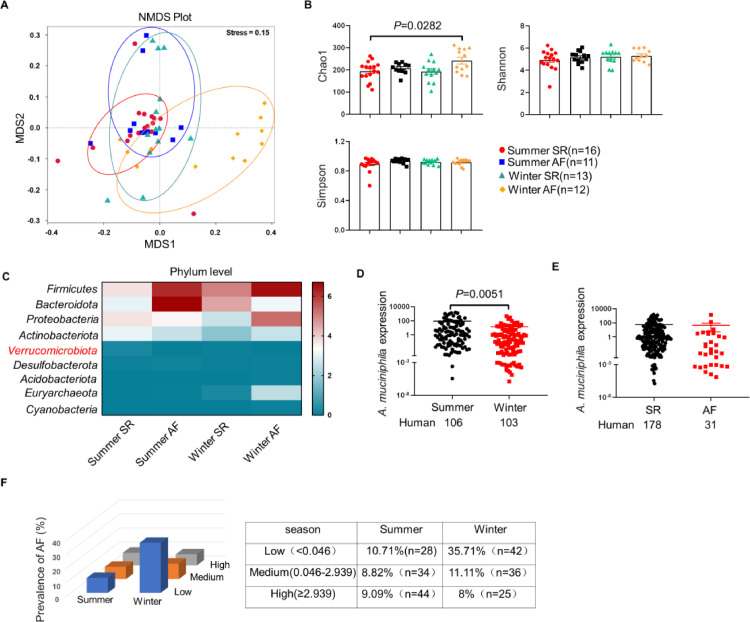
To further test whether gut microbiota dysbiosis is associated with cold-related AF, stool samples were collected from subjects with AF during winter (winter AF) or summer (summer AF), and subjects with sinus rhythm (SR) during winter (winter SR) or summer (summer SR). Notably, NMDS revealed significant difference in the microbial composition between winter AF group and other groups (Figure 7A). The richness (Chao1 indices) of gut microbiota was profoundly higher in winter AF group than other groups, whereas the diversity (Shannon and Simpson indices) showed no difference (Figure 7B). The sequencing data revealed the decreased abundance of A. muciniphila in winter AF group (Figure 7C). Subsequently, stool samples were collected from 103 winter subjects and 106 summer subjects to corroborate the change of A. muciniphila abundance, and the qRT-PCR data verified that winter subjects had lower abundance of A. muciniphila than summer subjects (Figure 7D). However, the abundance of A. muciniphila in SR group was comparable to that in AF group (Figure 7E). To further determine the predicted value of A. muciniphila in cold-related AF, we examined the prevalence of AF in clinical individuals according to A. muciniphila abundance stratified by tertiles of A. muciniphila. Interestingly, the prevalence of AF presented a trend of decreasing with A. muciniphila abundance within winter, but not within summer (Figure 7F), indicating that the decrease in A. muciniphila abundance is related to cold-related AF.
Figure 7.
The decreased A. muciniphila was associated with AF in human subjects during winter. (A) The NMDS of unweighted UniFrac showed that the gut taxonomic composition was significantly different between winter AF and other groups. The closer the spatial distance of the sample, the more similar the species composition of the sample. (B) Chao1, Shannon and Simpson indices were analysed in human. Chao1 indices reflect community richness, and the Shannon and Simpson indices represent community diversity. (C) Heatmap of fecal microbiota from summer SR(n=16), summer AF(n=11), winter SR(n=13) and winter AF(n=12) patients. (D) q-RT PCR validation of the relative abundance of A. muciniphila in feces collected from human subjects during summer (n = 106) or winter (n = 103). (E) The relative abundance of A. muciniphila in feces collected from subjects with SR (n = 178) or AF (n = 31). (F) Prevalence of AF according to A. muciniphila abundance stratified by season. A. muciniphila abundance was categorized into three levels according to the tertiles of A. muciniphila. Seasons were categorized into summer and winter. Throughout, data are expressed as mean ± SEM and compared by one-way ANOVA (B) and Student's t test (D and E). NMDS, non-metric multidimensional scaling; OTUs, operational taxonomic units; Summer SR, patients with sinus rhythm during summer; Summer AF, patients with atrial fibrillation during summer; Winter SR, patients with sinus rhythm during winter; Winter AF, patients with atrial fibrillation during winter; SR, sinus rhythm; AF, atrial fibrillation; A. muciniphila, Akkermansia muciniphila.
Discussion
In this study, we reveal the causative role of gut microbiota in cold-related AF and pave an avenue for the treatment and prevention of cold-related AF. Herein, we found that Cold rats exhibited the enhanced susceptibility to AF, accompanied by the diminished A. muciniphila abundance and augmented TMAO in comparison to RT rats. Mechanically, cold exposure led to reduced A. muciniphila, by which increased the synthesis of TMA and TMAO, the latter of which enhanced atrial M1 macrophages infiltration and promoted cardiac pyroptosis, eventually leading to atrial structural remodeling. Remarkably, such a pro-AF effect elicited by cold exposure is reversible, evidenced by the abrogation of pro-AF property following A. muciniphila supplementation.
In recent years, accumulating evidences from clinical studies have linked cold seasons or low ambient temperature to various cardiovascular diseases including AF.43, 44, 45, 46 When the ambient temperature decreases by 1℃, the incidence of AF increases by 3%.4 Cold exposure has been demonstrated to cause shift in the composition and metabolism of gut microbiota.11 We found that cold exposure led to AF and decreased A. muciniphila in rats. Patients in winter also presented lower abundance of A. muciniphila than that in summer, and the prevalence of AF presented a trend of decreasing with the A. muciniphila abundance within winter. Those findings implies that the abundance of A. muciniphila is closely associated with cold-related AF and might be further developed into non-invasive and inexpensive biomarkers for predicting cold-related AF.
A. muciniphila, beneficial bacteria residing in the mucus layer closed to host cells, is drawing increasing attention for its role as a promising probiotic in metabolic disorders and immune diseases.47 More importantly, oral supplementation of A. muciniphila prevents Cold rats from AF. Daily oral supplementation of A. muciniphila has been confirmed to be safe and well tolerated, which elicited favorable impacts on the metabolic parameter in overweight/obese insulin-resistant volunteers.48 In addition, it was reported that pasteurized A. muciniphila exerted stronger effects on the prevention of obesity and associated complications in HFD-fed mice through Amuc_1100, a specific protein isolated from its outer membrane.30 However, we found that pasteurized A. muciniphila failed to prevent cold-related AF, indicating that the protective effects of A. muciniphila are mediated by its biological activity rather than outer membrane protein.
In addition to gut microbiota composition, the bioactive microbial metabolites have been considered as a contributing factor in the progression of various cardiovascular diseases.49,50 Numerous pathways were described to be involved in the interaction between microbiota and the host, such as the TMA/TMAO pathway.51 Herein, our data demonstrated that the levels of plasma TMAO markedly elevated in Cold rats. More importantly, the plasma TMAO levels of human subjects progressively increased with cold weather, suggesting that cold-related AF may be mediated by TMAO. Our findings are consistent with previous studies which supported that TMAO was closely related to the initiation and maintenance of AF.34,52 Yu8 et al described that local injection of TMAO into dog ganglionated plexus aggravated atrial electrical remodeling, emphasizing the crucial role of TMAO in AF development.
TMAO is a gut microbe-dependent metabolite. Briefly, specific TMA-containing dietary nutrients, such as choline and carnitine, can be converted to TMA by gut microbiota, followed by TMA rapidly oxidized to TMAO by the flavin-containing monooxygenase (FMO) in the liver.53 Our study showed that the level of FMO3 in the liver of Cold rats remained unchanged, whereas TMA was increased in Cold rats. The generation of TMA is limited by several unique microbial lyases, including TMA lyase (such as CntA/B or YeaW/X) and choline TMA lyase (such as CutC/D or YeaW/X).54 Strikingly, oral administration of A. muciniphila significantly reduced circulating TMA, TMAO, as well as CntA, CntB, CutC, YeaW and YeaX in Cold rats, which supported the notion that A. muciniphila deminishes the level of plasma TMAO by restraint of the production of TMA. To our best knowledge, we demonstrate for the first time a crucial role of A. muciniphila in the regulation of TMA production.
Interestingly, we found that TMAO had no influences on the viability of CM and CF at the concentration of 10 μM, which was comparable with the physiological levels in subjects during winter. This is consistent with previous studies which confirmed that TMAO cannot directly cause or exacerbate CM damage, even when TMAO concentration was up to 10 mM.55 Moreover, Ge et al56 found that TMAO directly activated cardiac fibroblasts at 300 µM concentrations. Therefore, the discrepancy between our findings and Ge's study may be attributed to the different tested doses of TMAO. Taken together, TMAO at the concentration of that in subjects during winter can not directly cause CM and CF injury, which indicates that other cell types may be responsible for TMAO-induced cardiac injury.
Macrophages are the integral components of cardiac tissue,40,57 which can exert profound effects on the healthy and diseased heart.58 Macrophages were observed to accumulate in the atria of AF patients and interact with atrial myocytes to promote AF.41 Intriguingly, the atrial M1 macrophages rather than M2 macrophages dramatically increased in Cold rats as compared with RT rats. Consistently, similar results were obtained from the BMDMs treated with TMAO. More importantly, the aggravated injury in CM and CF were seen when they were co-cultured with BMDMs in the presence of TMAO (10 μM). However, the BMDMs failed to cause those changes in the absence of TMAO, or the concentration of TMAO lower than 10 μM. Those findings indicated that TMAO enhanced M1 macrophages infiltration which caused atrial structural remodeling, while the molecular mechanisms warrant further investigation.
M1 macrophages, characterized by proinflammatory properties, are accountable for the cardiac inflammation and adverse cardiac remodeling.59,60 We found that TMAO-induced M1 macrophages infiltration aggravated cardiac pyroptosis, the latter of which was closely associated with cardiovascular diseases, and some agents targeting the components related to pyroptosis have been clinically proven to bring cardiovascular benefits.61 In addition, inhibition of gasdermin D with NSA prevented cold-related AF and reversed atrial fibrosis. More importantly, conditional caspase1 knockout protected mice from cold-related AF. Therefore, we confirmed that TMAO-induced pyroptosis is largely responsible for cold-related AF and drugs targeting pyroptosis may become a potential novel target for therapeutics of cold-related AF.
There are several limitations in our study. The supplementation of A. muciniphila was not testified among human subjects, which yields therapeutic value of A. muciniphila based on animal studies. Besides, the clinical relevance between A. muciniphila abundance and cold-related AF was determined by a single centre study with a small number of individuals, which was not powered to justify a definite conclusion. Moreover, we failed to recruit the same subjects to provide feces both during winter and summer to analyze the clinical relevance. Therefore, multi-center and large sample clinical researches are urgently needed to further validate those findings.
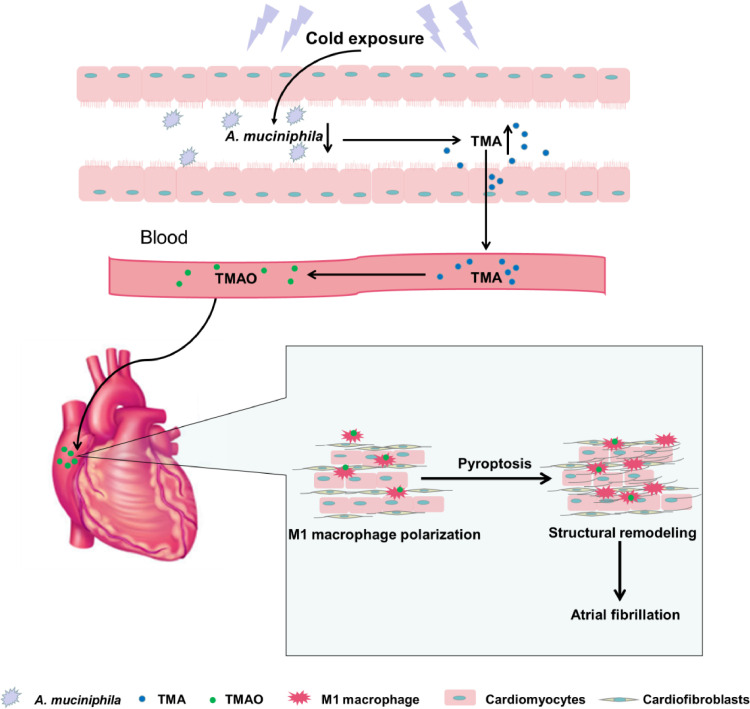
In the present study, we demonstrated that gut microbiota dysbiosis was associated with cold-related AF. The function of cold microbota and the pathogenesis of AF are extremely sophisticated. As is shown in Figure 8, cold exposure reduced the abundance of A. muciniphila, by which led to augmented TMAO through modulation of TMA generation. TMAO enhanced M1 macrophages infiltration and promoting atrial pyroptosis, which results in atrial structural remodeling. This study suggests that the gut microbiota may become a potential target for the prevention and treatment of cold-related AF.
Figure 8.
Summary scheme outlining AF-promoting mechanisms from cold microbiota dysbiosis. Cold exposure led to an decrease in A. muciniphila, by which promoted intestinal-derived TMA production and augmented the levels of TMAO in plasma, then TMAO enhanced M1 macrophages polarization and promoted atrial pyroptosis, ultimately leading to atrial structural remodeling and atrial fibrillation.
Contributors
Yingchun Luo, Yun Zhang and Xuejie Han performed the statistical analyses, interpreted the results and prepared the manuscript with inputs from all authors. Yue Li and Yongtai Gong designed the experiments and helped draft the manuscript. Yingchun Luo performed the cell experiments and Yun Zhang contributed to the animal experiments; Xuejie Han contributed to qRT-PCR experiments. Yunlong Gao and Hui Yu supervised and performed sample data collection. Jiawei Zhang and Song Zhang contributed to the animal experiments, Yue Yuan, Yun Zhou, Yiya Shi, Yu Duan, Xinbo Zhao, Sen Yan, Hongting Hao, Chenguang Dai, Shiqi Zhao, Jing Shi,Wenpeng Li, Wei Xu, Ning Fang performed ELISA and western blot experiments. Yingchun Luo, Yun Zhang, Xuejie Han, Yue Li and Yongtai Gong have accessed and verified the underlying data. All authors read and approved the final manuscript.
Data sharing statement
Datasets for this study have been deposited into the SRA database under accession numbers PRJNA822772 and PRJNA825827.
Declaration of interests
The authors declare no competing interests.
Acknowledgements
This work was supported by grants from the State Key Program of National Natural Science Foundation of China (No.81830012 to Yue Li), and National Natural Science Foundation of China (No.82070336 to Yue Li, No.81974024 to Yongtai Gong), Youth Program of the National Natural Science Foundation of China (No.81900374 to Jing Shi, No.81900302 to Yue Yuan), and Excellent Young Medical Talents supporting project in the First Affiliated Hospital of Harbin Medical University (No. HYD2020YQ0001 to Yue Yuan). We are grateful to Biotree (Shanghai) for the assistance with Metabonomic analysis.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104087.
Contributor Information
Yongtai Gong, Email: gongth@126.com.
Yue Li, Email: ly99ly@hrbmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Seccia TM, Caroccia B, Maiolino G, Cesari M, Rossi GP. Arterial Hypertension, Aldosterone, and Atrial Fibrillation. Curr Hypertens Rep. 2019;21(12):94. doi: 10.1007/s11906-019-1001-4. [DOI] [PubMed] [Google Scholar]
- 2.Fustinoni O, Saposnik G, Esnaola y Rojas MM, Lakkis SG, Sposato LA. Higher frequency of atrial fibrillation linked to colder seasons and air temperature on the day of ischemic stroke onset. J Stroke Cerebrovasc Dis. 2013;22(4):476–481. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Poletaev V, Antonelli D, Litskevich G, Turgeman Y. Monthly variation in emergency department admission for acute onset atrial fibrillation. Isr Med Assoc J. 2021;23(5):302–305. [PubMed] [Google Scholar]
- 4.Nguyen JL, Link MS, Luttmann-Gibson H, et al. Drier air, lower temperatures, and triggering of paroxysmal atrial fibrillation. Epidemiology. 2015;26(3):374–380. doi: 10.1097/EDE.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera-Caravaca JM, Roldán V, Vicente V, Lip G, Marín F. Particulate matter and temperature: increased risk of adverse clinical outcomes in patients with atrial fibrillation. Mayo Clin Proc. 2020;95(11):2360–2369. doi: 10.1016/j.mayocp.2020.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Fan Y, Pedersen O. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19(1):55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 7.Zuo K, Li J, Li K, et al. Disordered gut microbiota and alterations in metabolic patterns are associated with atrial fibrillation. Gigascience. 2019;8(6):giz058. doi: 10.1093/gigascience/giz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu L, Meng G, Huang B, et al. A potential relationship between gut microbes and atrial fibrillation: Trimethylamine N-oxide, a gut microbe-derived metabolite, facilitates the progression of atrial fibrillation. Int J Cardiol. 2018;255:92–98. doi: 10.1016/j.ijcard.2017.11.071. [DOI] [PubMed] [Google Scholar]
- 9.Liang Z, Dong Z, Guo M, et al. Trimethylamine N-oxide as a risk marker for ischemic stroke in patients with atrial fibrillation. J Biochem Mol Toxicol. 2019;33(2):e22246. doi: 10.1002/jbt.22246. [DOI] [PubMed] [Google Scholar]
- 10.Gong D, Zhang L, Zhang Y, Wang F, Zhao Z, Zhou X. Gut microbial metabolite trimethylamine n-oxide is related to thrombus formation in atrial fibrillation patients. Am J Med Sci. 2019;358(6):422–428. doi: 10.1016/j.amjms.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Chevalier C, Stojanović O, Colin DJ, et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell. 2015;163(6):1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Worthmann A, John C, Rühlemann MC, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23(7):839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 13.Aurora AB, Porrello ER, Tan W, et al. Macrophages are required for neonatal heart regeneration. J Clin Invest. 2014;124(3):1382–1392. doi: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He G, Tan W, Wang B, et al. Increased M1 Macrophages Infiltration Is Associated with Thrombogenesis in Rheumatic Mitral Stenosis Patients with Atrial Fibrillation. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0149910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu K, Yuan Y, Yu H, et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood. 2020;136(4):501–515. doi: 10.1182/blood.2019003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan YH, Chang GJ, Lai YJ, et al. Atrial fibrillation and its arrhythmogenesis associated with insulin resistance. Cardiovasc Diabetol. 2019;18(1):125. doi: 10.1186/s12933-019-0928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Ruan H, Rahmutula D, et al. Effect of acute and chronic ethanol on atrial fibrillation vulnerability in rats. Heart Rhythm. 2020;17(4):654–660. doi: 10.1016/j.hrthm.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang S, Li B, et al. Gut microbiota dysbiosis promotes age-related atrial fibrillation by lipopolysaccharide and glucose-induced activation of NLRP3-inflammasome. Cardiovasc Res. 2022;118(3):785–797. doi: 10.1093/cvr/cvab114. [DOI] [PubMed] [Google Scholar]
- 19.Bolyen E, Rideout JR, Dillon MR, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A, et al. Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology. 2018;67(4):1485–1498. doi: 10.1002/hep.29646. [DOI] [PubMed] [Google Scholar]
- 22.Hiram R, Naud P, Xiong F, Al-U'datt D, Algalarrondo V, Sirois MG. Right Atrial Mechanisms of Atrial Fibrillation in a Rat Model of Right Heart Disease. J Am Coll Cardiol. 2019;74(10):1332–1347. doi: 10.1016/j.jacc.2019.06.066. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M, Gong M, Zhang Z, et al. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/6569728. eCollection 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron HA, Waller PC, Ramsay LE. Ketanserin in essential hypertension: use as monotherapy and in combination with a diuretic or beta-adrenoceptor antagonist. Br J Clin Pharmacol. 1987;24(6):705–711. doi: 10.1111/j.1365-2125.1987.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhang S, Liu Z, et al. Resveratrol prevents atrial fibrillation by inhibiting atrial structural and metabolic remodeling in collagen-induced arthritis rats. Naunyn Schmiedebergs Arch Pharmacol. 2018;391(11):1179–1190. doi: 10.1007/s00210-018-1554-9. [DOI] [PubMed] [Google Scholar]
- 26.Yuan Y, Zhao J, Gong Y, et al. Autophagy exacerbates electrical remodeling in atrial fibrillation by ubiquitin-dependent degradation of L-type calcium channel. Cell Death Dis. 2018;9(9):873. doi: 10.1038/s41419-018-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Wang X, Feng W, et al. The gut microbiota and its interactions with cardiovascular disease. Microb Biotechnol. 2020;13(3):637–656. doi: 10.1111/1751-7915.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P. Gut microbiota structure differs between honeybees in winter and summer. ISME J. 2020;14(3):801–814. doi: 10.1038/s41396-019-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang T, Li Q, Cheng L, Buch H, Zhang F. Akkermansia muciniphila is a promising probiotic. Microb Biotechnol. 2019;12(6):1109–1125. doi: 10.1111/1751-7915.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23(1):107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Tang L, Feng Y, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut. 2020;69(11):1988–1997. doi: 10.1136/gutjnl-2019-320105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitai T, Tang W. Gut microbiota in cardiovascular disease and heart failure. Clin Sci (Lond) 2018;132(1):85–91. doi: 10.1042/CS20171090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8(1):36. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svingen G, Zuo H, Ueland PM, et al. Increased plasma trimethylamine-N-oxide is associated with incident atrial fibrillation. Int J Cardiol. 2018;267:100–106. doi: 10.1016/j.ijcard.2018.04.128. [DOI] [PubMed] [Google Scholar]
- 35.Janeiro MH, Ramírez MJ, Milagro FI, Martínez JA, Solas M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients. 2018;10(10) doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craciun S, Balskus EP. Microbial conversion of choline to trimethylamine requires a glycyl radical enzyme. Proc Natl Acad Sci U S A. 2012;109(52):21307–21312. doi: 10.1073/pnas.1215689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y, Jameson E, Crosatti M, et al. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc Natl Acad Sci U S A. 2014;111(11):4268–4273. doi: 10.1073/pnas.1316569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koeth RA, Levison BS, Culley MK, et al. γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 2014;20(5):799–812. doi: 10.1016/j.cmet.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavine KJ, Pinto AR, Epelman S, et al. The Macrophage in Cardiac Homeostasis and Disease: JACC Macrophage in CVD Series (Part 4) J Am Coll Cardiol. 2018;72(18):2213–2230. doi: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Z, Zhou D, Xie X, et al. Cross-talk between macrophages and atrial myocytes in atrial fibrillation. Basic Res Cardiol. 2016;111(6):63. doi: 10.1007/s00395-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci. 2017;42(4):245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Wang N, Farrell M, Hales S, et al. Prevalence and seasonal variation of precipitants of heart failure hospitalization and risk of readmission. Int J Cardiol. 2020;316:152–160. doi: 10.1016/j.ijcard.2020.04.084. [DOI] [PubMed] [Google Scholar]
- 44.Cheng J, Bambrick H, Tong S, Su H, Xu Z, Hu W. Winter temperature and myocardial infarction in Brisbane, Australia: Spatial and temporal analyses. Sci Total Environ. 2020;715 doi: 10.1016/j.ijcard.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Katayama Y, Tanaka A, Taruya A, et al. Increased plaque rupture forms peak incidence of acute myocardial infarction in winter. Int J Cardiol. 2020;320:18–22. doi: 10.1016/j.ijcard.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 46.Chung FP, Li HR, Chong E, et al. Seasonal variation in the frequency of sudden cardiac death and ventricular tachyarrhythmia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: the effect of meteorological factors. Heart Rhythm. 2013;10(12):1859–1866. doi: 10.1016/j.hrthm.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 47.Zhou JC, Zhang XW. Akkermansia muciniphila: a promising target for the therapy of metabolic syndrome and related diseases. Chin J Nat Med. 2019;17(11):835–841. doi: 10.1016/S1875-5364(19)30101-3. [DOI] [PubMed] [Google Scholar]
- 48.Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25(7):1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Velmurugan G, Dinakaran V, Rajendhran J, Swaminathan K. Blood Microbiota and Circulating Microbial Metabolites in Diabetes and Cardiovascular Disease. Trends Endocrinol Metab. 2020;31(11):835–847. doi: 10.1016/j.tem.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Heianza Y, Ma W, Manson JE, Rexrode KM, Qi L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J Am Heart Assoc. 2017;6(7) doi: 10.1161/JAHA.116.004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu W, Gregory JC, Org E, et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuo K, Liu X, Wang P, et al. Metagenomic data-mining reveals enrichment of trimethylamine-N-oxide synthesis in gut microbiome in atrial fibrillation patients. BMC Genomics. 2020;21(1):526. doi: 10.1186/s12864-020-06944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qian Q, Chowdhury BP, Sun Z, et al. Maternal diesel particle exposure promotes offspring asthma through NK cell-derived granzyme B. J Clin Invest. 2020;130(8):4133–4151. doi: 10.1172/JCI130324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang W, Li DY, Hazen SL. Dietary metabolism, the gut microbiome, and heart failure. Nat Rev Cardiol. 2019;16(3):137–154. doi: 10.1038/s41569-018-0108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Querio G, Antoniotti S, Levi R, Gallo MP. Trimethylamine n-oxide does not impact viability, ros production, and mitochondrial membrane potential of adult rat cardiomyocytes. Int J Mol Sci. 2019;20(12):3045. doi: 10.3390/ijms20123045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang W, Zhang S, Zhu J, et al. Gut microbe-derived metabolite trimethylamine N-Oxide accelerates fibroblast-myofibroblast differentiation and induces cardiac fibrosis. J Mol Cell Cardiol. 2019;134:119–130. doi: 10.1016/j.yjmcc.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Pinto AR, Ilinykh A, Ivey MJ, et al. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118(3):400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y, Mouton AJ, Lindsey ML. Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res. 2018;191:15–28. doi: 10.1016/j.trsl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mouton AJ, Li X, Hall ME, Hall JE. Obesity, Hypertension, and Cardiac Dysfunction: Novel Roles of Immunometabolism in Macrophage Activation and Inflammation. Circ Res. 2020;126(6):789–806. doi: 10.1161/CIRCRESAHA.119.312321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrett TJ. Macrophages in Atherosclerosis Regression. Arterioscler Thromb Vasc Biol. 2020;40(1):20–33. doi: 10.1161/ATVBAHA.119.312802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Q, Wu J, Zeng Y, et al. Pyroptosis: A pro-inflammatory type of cell death in cardiovascular disease. Clin Chim Acta. 2020;510:62–72. doi: 10.1016/j.cca.2020.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.