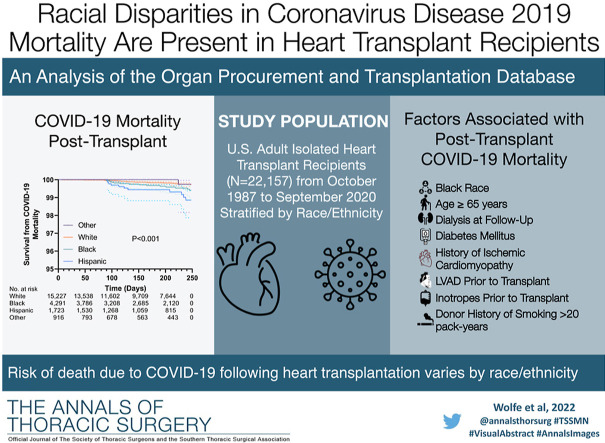
Abstract
Background
Studies have demonstrated the devastating effects of coronavirus disease 2019 (COVID-19) on vulnerable populations. Although they receive close follow-up, heart transplant recipients represent a particularly vulnerable population, given long-term immunosuppression and comorbid conditions. We sought to investigate the association between race/ethnicity and the probability of death due to COVID-19 in adult heart transplant recipients in the United States.
Methods
Adult isolated heart transplant recipients were identified using the Organ Procurement and Transplantation Network database. Recipients who were described as deceased or lost to follow-up before January 2020 were excluded. Recipients were stratified into 4 cohorts by race/ethnicity. The primary outcome of interest was death due to COVID-19.
Results
A total of 22 157 adult recipients were identified. During the course of follow-up, 153 recipients had COVID-19 reported as the primary cause of death. COVID-19 mortality was significantly different between race/ethnicity cohorts (Black, n = 34 [0.79%]; Hispanic, n = 23 [1.33%]; White, n = 92 [0.60%]; other, n = 4 [0.44%]; P = .007). COVID-19 was listed as a contributing cause of mortality in 0.12% of Black, 0.23% of Hispanic, 0.04% of White, and 0.33% of other recipients (P = .002). No significant difference in non-COVID mortality or all-cause mortality was observed. After multivariable adjustment, Black (hazard ratio, 2.78 [1.40-5.52]; P = .003) and Hispanic (hazard ratio, 3.92 [1.88-8.16]; P < .001) recipients were at higher risk of death due to COVID-19 compared with White recipients.
Conclusions
Compared with White recipients, Black and Hispanic recipients experienced higher rates of COVID-19 mortality after transplantation. These findings suggest that racial/ethnic disparities of COVID-19 mortality in the general population persist in adult heart transplant recipients.
Graphical abstract
Across the United States, vulnerable populations have experienced the devastating effects of coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Because of long-term immunosuppression and complex comorbidities, heart transplant recipients are at an increased risk of severe infection and adverse outcomes with COVID-19.1 Prior studies have demonstrated case-fatality rates in heart transplant recipients up to 25%.2, 3, 4
Racial/ethnic disparities in outcomes among heart transplant recipients have been established previously. An earlier study reported that Black and low socioeconomic status heart transplant recipients have higher rates of death and retransplantation.5 The same study found that long-term survival has improved during 21 years for White recipients but not for Black and Hispanic recipients. Additional studies have demonstrated that Black heart transplant recipients are at higher risk of posttransplantation death and that this disparity is greatest among recipients who are 18 to 30 years old.6 , 7 The COVID-19 pandemic and coinciding events have shed much-needed light on racial inequities, with recent studies exposing the disproportionate burden of COVID-19 infection, morbidity, and death among racial/ethnic minority groups in the nontransplant population.8, 9, 10, 11, 12
The aforementioned work has explored racial/ethnic disparities in posttransplantation outcomes and COVID-19 incidence and mortality, yet how race/ethnicity is associated with one’s risk of death due to COVID-19 after heart transplantation has not been described. Although heart transplant recipients are particularly vulnerable to COVID-19, they are also closely monitored by a multidisciplinary team after they receive the transplant.13 This close follow-up provides a unique opportunity to employ interventions to combat health and treatment inequities. In this study, we examined the association between race/ethnicity and COVID-19 mortality among adult heart transplant recipients in the United States. We hypothesized that racial/ethnic disparities in deaths due to COVID-19 observed in the general population would persist in heart transplant recipients despite their close follow-up.
Patients And Methods
Study Population
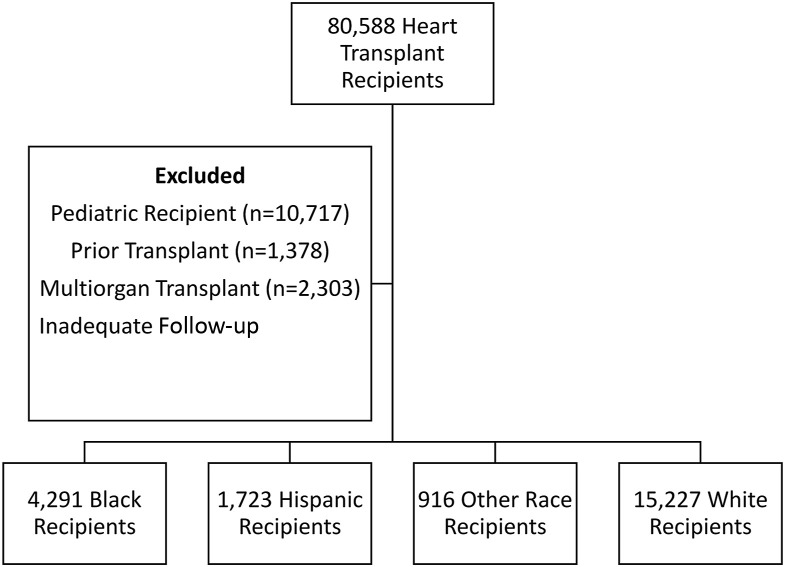
Adult heart transplant recipients (≥18 years old) in the United States were identified using the Organ Procurement and Transplantation Network (OPTN) database. Recipients who died or were lost to follow-up before January 2020 were excluded because they were not at risk of COVID-19 exposure or death. Multiorgan transplant recipients, recipients with a history of prior heart transplant, and patients with inadequate follow-up (<6 months) were also excluded (Figure 1 ). All heart transplant recipients with available follow-up were included, resulting in transplantation dates spanning October 1987 through September 2020.
Figure 1.
Flowchart of patient selection.
Study Design and Outcomes
Patients were stratified into cohorts by race/ethnicity according to the OPTN database variable ETHCAT that is collected during registration of the transplant candidate. Asian, American Indian/Native American, Native Hawaiian/Pacific Islander, and multiracial patients were combined into an “other” race category because of small sample size. The resulting cohorts were Black, Hispanic, other, and White. Baseline characteristics were extracted from the OPTN database. This study was approved by the Mass General Brigham Institutional Review Board, which provided a waiver of informed consent.
The primary outcome of interest was death due to COVID-19 as reported in the OPTN database under the primary cause of death (COD) variable. Deaths due to COVID-19 reported as free text (COD_OSTXT) were also extracted. Secondary outcomes included non–COVID-19 mortality, COVID-19 as a contributing cause of death, and all-cause mortality.
Statistical Analysis
Continuous data comparisons were performed by the Kruskal-Wallis test, and medians with interquartile ranges were reported. Binary variables were tested by the Fisher exact test, and categorical variable comparisons were performed by the χ 2 test. A 2-sided P value < .05 was used to define significance.
Survival analysis was conducted by Cox proportional hazards modeling. Because patients in the United States were not at risk of death due to COVID-19 before January 2020, the follow-up start date was set to January 1, 2020, for all patients receiving transplants on or before that date. Because of variations in follow-up reporting by centers to the OPTN database, follow-up was censored on September 5, 2020 (6 months before the most recently available data). Model building was carried out with a hybrid approach consisting of a univariate screen and inclusion of variables with a P value < .20 on univariate Cox regression modeling. In addition, variables with a P value > .20 were included in model building if they were identified as potential confounders or related to the outcome in prior literature including available social determinants of health (education level, citizenship status, and health care payer source). These variables were then entered into a backward selection model. In an attempt to account for differential COVID-19 risk by location, COVID-19 death rates by state were calculated by dividing total COVID-19 deaths as reported to the Centers for Disease Control and Prevention on September 30, 2020, by the state’s population as reported by the US Census Bureau.14 , 15 States were split into low, mild, moderate, and high risk of COVID-19 death by quartile, and this categorical variable was adjusted for in regression models. Proportionality of hazards was confirmed by Schoenfeld residuals test. The competing risk of non-COVID death was analyzed by Fine-Gray subhazards. Wald test was used to provide overall P values for categorical variables in regression models. All analyses were performed with Stata 16 statistical software, and GraphPad Prism was used for figure generation.16 , 17
Results
Baseline Characteristics
After exclusion criteria were applied, 22 157 heart transplant recipients were identified. Stratification by race/ethnicity resulted in cohorts consisting of 4291 Black, 1723 Hispanic, 916 other, and 15 227 White recipients. Baseline characteristics of the recipients are presented in Table 1 . The Black cohort had the lowest proportion of male recipients. Hispanic recipients reported lower rates of associate/bachelor’s degree, graduate degree, and US citizenship than the Black, White, and other cohorts. White recipients had the lowest rates of public insurance use and diabetes mellitus but were most likely to have ischemic cardiomyopathy listed as the indication for transplant. Black recipients had the highest rates of pretransplantation durable mechanical circulatory support. Donor characteristics were well balanced between cohorts (Table 2 ).
Table 1.
Demographic and Clinical Characteristics of Heart Transplant Recipients
| Variable | White (n = 15 227) | Black (n = 4291) | Hispanic (n = 1723) | Other (n = 916) | P Value |
|---|---|---|---|---|---|
| Age, y | 56 (47-63) | 53 (43-60) | 53 (42-60) | 54 (44-61) | <.001 |
| Male sex | 11 520 (75.7) | 2835 (66.1) | 1265 (73.4) | 705 (77.0) | <.001 |
| Education level | <.001 | ||||
| High school or less | 5261 (34.6) | 1913 (44.6) | 1015 (58.9) | 281 (30.7) | |
| Attended college/technical | 3777 (24.8) | 1187 (27.7) | 326 (18.9) | 214 (23.4) | |
| Associate/bachelor’s degree | 3077 (20.2) | 632 (14.7) | 166 (9.6) | 215 (23.5) | |
| Graduate degree | 1460 (9.6) | 246 (5.7) | 50 (2.9) | 131 (14.3) | |
| Missing | 1652 (10.8) | 313 (7.3) | 166 (9.6) | 75 (8.2) | |
| US citizen | 15 065 (98.9) | 4215 (98.2) | 1446 (83.9) | 815 (89.0) | <.001 |
| Public insurance | 6438 (42.3) | 2488 (58.0) | 1067 (61.9) | 431 (47.1) | <.001 |
| BMI, kg/m2 | 26.9 (23.7-30.4) | 27.5 (23.9-31.5) | 26.5 (23.4-30.0) | 24.3 (21.3-28.0) | <.001 |
| History of diabetes | 3004 (19.7) | 1077 (25.1) | 453 (26.3) | 256 (27.9) | <.001 |
| Ischemic cardiomyopathy | 5122 (33.6) | 655 (15.3) | 459 (26.6) | 279 (30.5) | <.001 |
| History of smoking | 6048 (39.7) | 1631 (38.0) | 616 (35.8) | 326 (35.6) | <.001 |
| Creatinine level at time of transplantation, mg/dL | 1.1 (0.9-1.4) | 1.2 (1.0-1.5) | 1.1 (0.8-1.3) | 1.1 (0.9-1.4) | <.001 |
| Bilirubin level at time of transplantation, mg/dL | 0.7 (0.5-1.1) | 0.7 (0.4-1.1) | 0.7 (0.5-1.2) | 0.7 (0.5-1.2) | <.001 |
| Days on waiting list | 87.0 (23.0-258.0) | 83.0 (22.0-258.0) | 67.0 (19.0-226.0) | 54.5 (15.0-189.5) | <.001 |
| On ventilator at transplantation | 238 (1.6) | 52 (1.2) | 19 (1.1) | 13 (1.4) | .22 |
| LVAD at transplantation | 5154 (33.8) | 1820 (42.4) | 591 (34.3) | 295 (32.2) | <.001 |
| Temporary MCS at transplantation | 1673 (11.0) | 591 (13.8) | 206 (12.0) | 121 (13.2) | <.001 |
| Receiving inotropes at transplantation | 5780 (38.0) | 1722 (40.1) | 754 (43.8) | 400 (43.7) | <.001 |
| Pretransplantation CO, L/min | 4.4 (3.6-5.4) | 4.3 (3.5-5.4) | 4.0 (3.2-5.0) | 3.9 (3.2-4.9) | <.001 |
| Pretransplantation PASP >40 mm Hg | 6986 (45.9) | 2171 (50.6) | 873 (50.7) | 430 (46.9) | <.001 |
| Pretransplantation PCWP >12 mm Hg | 10 985 (72.1) | 3088 (72.0) | 1288 (74.8) | 644 (70.3) | .06 |
| Pretransplantation TPG >12 mm Hg | 4540 (29.8) | 1498 (34.9) | 516 (29.9) | 279 (30.5) | <.001 |
| History of cardiac operation | 6586 (50.4) | 1747 (43.7) | 678 (42.9) | 382 (44.9) | <.001 |
| Waiting list status at transplantation | <.001 | ||||
| 1A | 6890 (45.2) | 1931 (45.0) | 793 (46.0) | 406 (44.3) | |
| 1B | 3916 (25.7) | 1090 (25.4) | 397 (23.0) | 243 (26.5) | |
| Old 2 | 1446 (9.5) | 169 (3.9) | 129 (7.5) | 74 (8.1) | |
| 1 | 236 (1.5) | 89 (2.1) | 25 (1.5) | 23 (2.5) | |
| 2 | 1328 (8.7) | 539 (12.6) | 187 (10.9) | 85 (9.3) | |
| 3 | 605 (4.0) | 224 (5.2) | 82 (4.8) | 54 (5.9) | |
| 4 | 628 (4.1) | 208 (4.8) | 91 (5.3) | 24 (2.6) | |
| 5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 6 | 147 (1.0) | 37 (0.9) | 18 (1.0) | 7 (0.8) | |
| Missing | 31 (0.2) | 4 (0.1) | 1 (0.1) | 0 (0.0) | |
| Blood group | <.001 | ||||
| A | 6987 (45.9) | 1227 (28.6) | 634 (36.8) | 289 (31.6) | |
| AB | 851 (5.6) | 262 (6.1) | 57 (3.3) | 66 (7.2) | |
| B | 1853 (12.2) | 974 (22.7) | 196 (11.4) | 232 (25.3) | |
| O | 5536 (36.4) | 1828 (42.6) | 836 (48.5) | 329 (35.9) | |
| Dialysis at follow-up | 613 (4.0) | 305 (7.1) | 59 (3.4) | 47 (5.1) | <.001 |
| Corticosteroid use at follow-up | 654 (4.3) | 278 (6.5) | 108 (6.3) | 53 (5.8) | <.001 |
| COVID death risk by state | <.001 | ||||
| Low | 1826 (12.0) | 156 (3.6) | 91 (5.3) | 67 (7.3) | |
| Mild | 3048 (20.0) | 872 (3.6) | 91 (5.3) | 67 (7.3) | |
| Moderate | 5747 (37.8) | 1673 (39.0) | 793 (46.0) | 460 (50.2) | |
| High | 4602 (30.2) | 1589 (37.0) | 694 (40.3) | 245 (26.8) |
Categorical variables are presented as number (percentage). Continuous variables are presented as median (interquartile range).
BMI, body mass index; CO, cardiac output; COVID, coronavirus disease 2019; LVAD, left ventricular assist device; MCS, mechanical circulatory support; PASP, pulmonary artery systolic pressure; PCWP, pulmonary capillary wedge pressure; TPG, transpulmonary gradient.
Table 2.
Clinical Characteristics of Donors
| Variable | White (n = 15 227) | Black (n = 4291) | Hispanic (n = 1723) | Other (n = 916) | P Value |
|---|---|---|---|---|---|
| Ischemia time, h | 3.2 (2.5-3.8) | 3.1 (2.4-3.8) | 3.2 (2.4-3.9) | 3.2 (2.4-3.9) | .19 |
| Donor age, y | 30 (22-40) | 30 (23-39) | 29 (22-39) | 30 (22-40) | .21 |
| History of hypertension | 2159 (14.5) | 648 (15.3) | 220 (13.0) | 111 (12.3) | .03 |
| History of myocardial infarction | 100 (0.7) | 33 (0.8) | 10 (0.6) | 10 (1.1) | .37 |
| Recipient-donor sex mismatch | 3604 (23.7) | 1067 (24.9) | 510 (29.6) | 287 (31.3) | <.001 |
| History of diabetes mellitus | 510 (3.4) | 126 (3.0) | 62 (3.7) | 27 (3.0) | .39 |
| History of cancer | 199 (1.3) | 49 (1.2) | 25 (1.5) | 15 (1.7) | .53 |
| Smoking history >20 pack-years | 2354 (16.0) | 538 (12.8) | 203 (12.1) | 112 (12.4) | <.001 |
| Cocaine use | 2848 (20.3) | 870 (21.2) | 341 (20.8) | 136 (15.5) | .001 |
Categorical variables are presented as number (percentage). Continuous variables are presented as median (interquartile range).
Unadjusted Outcomes
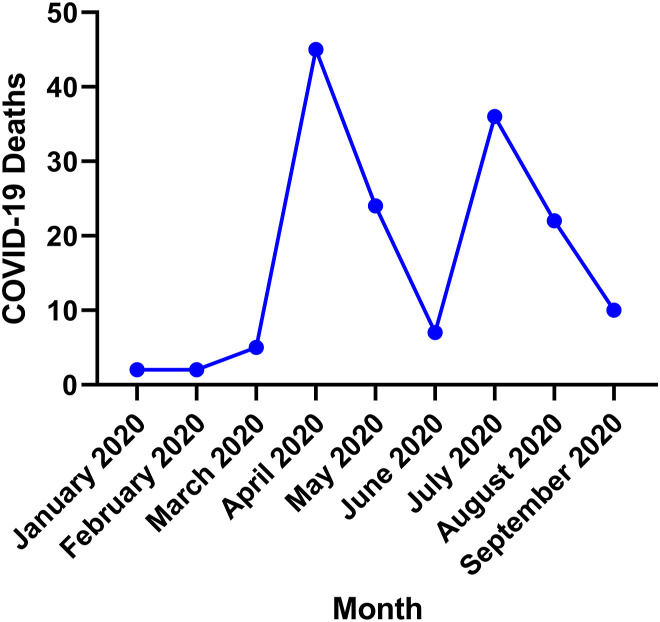
COVID-19 was reported as the primary cause of death in 153 recipients. Rates of death due to COVID-19 were significantly different between Black (n = 34 [0.79%]), Hispanic (n = 23 [1.3%]), White (n = 92 [0.60%]), and other (n = 4 [0.44%]; P = .007) cohorts (Table 3 ). Most COVID-19 deaths in this study occurred in April and July of 2020 (Figure 2 ). COVID-19 was reported as a contributing cause of death in 18 recipients (Black, n = 5 [0.12%]; Hispanic, n = 4 [0.23%]; White, n = 6 [0.04%]; and other, n = 3 [0.33%]; P = .002). No significant difference in non-COVID mortality was observed between Black (n = 251 [5.85%]), Hispanic (n = 85 [4.93%]), White (n = 892 [5.86%]), and other (n = 55 [6.00%]; P = .46) recipients. There was also no significant difference in all-cause (combined non-COVID and COVID) mortality between cohorts (P = .96). Recipients who died of COVID-19 were a median of 9.6 (interquartile range, 5.0-16.1) years after transplantation.
Table 3.
Unadjusted Outcomes
| White (n = 15 227) | Black (n = 4291) | Hispanic (n = 1723) | Other (n = 916) | P Value | |
|---|---|---|---|---|---|
| COVID mortality | 92 (0.60) | 34 (0.79) | 23 (1.33) | 4 (0.44) | .007 |
| COVID as contributing cause of death | 6 (0.04) | 5 (0.12) | 4 (0.23) | 3 (0.33) | .002 |
| Non-COVID mortality | 892 (5.86) | 251 (5.85) | 85 (4.93) | 55 (6.00) | 0.46 |
| All-cause mortality | 984 (6.46) | 285 (6.64) | 108 (6.27) | 59 (6.44) | .96 |
Values are reported as number (percentage).
COVID, coronavirus disease 2019.
Figure 2.
Coronavirus disease 2019 (COVID-19) deaths by month. April and July of 2020 had the highest rates of death due to COVID-19 in heart transplant recipients.
Survival Analysis
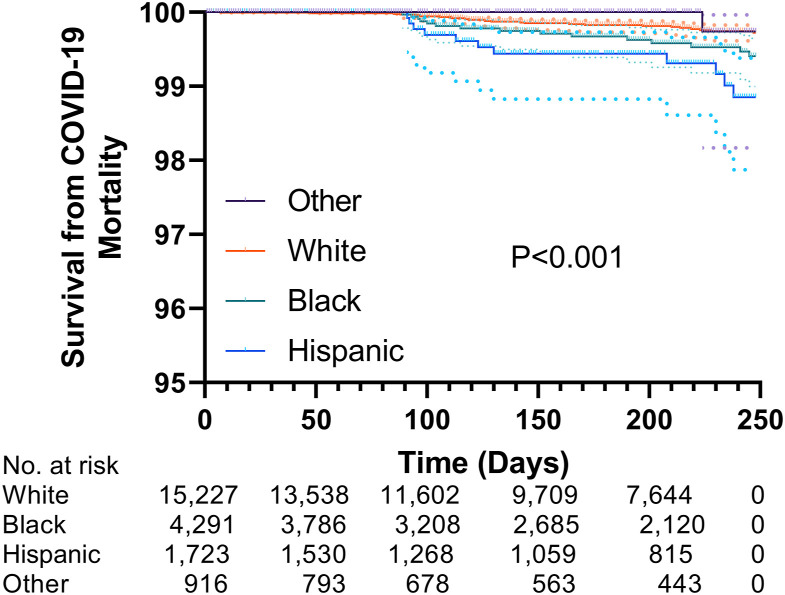
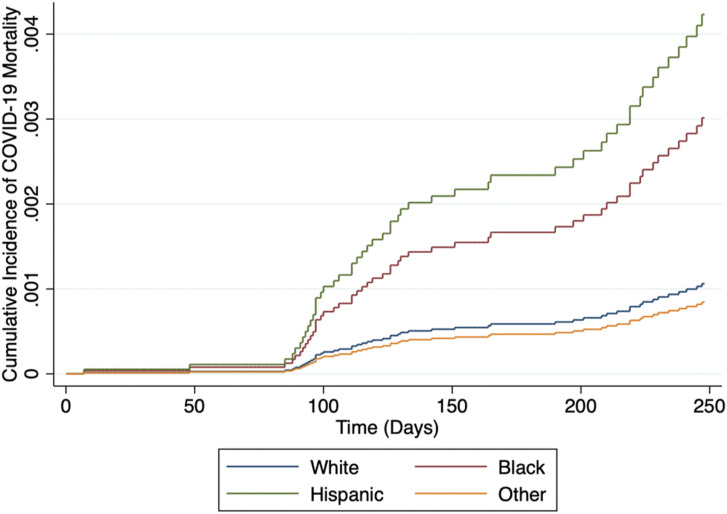
Kaplan-Meier survival estimates are illustrated in Figure 3 , in which the log-rank test indicated a significant difference in freedom from death due to COVID-19 between race/ethnicity cohorts (P < .001). The results of univariate and multivariable Cox proportional hazards models are presented in Table 4 . Univariable analysis revealed that Black (hazard ratio [HR], 2.07 [1.10-3.92]; P = .02) and Hispanic (HR, 3.91 [1.93-7.92]; P < .001) recipients were at a higher risk for posttransplantation death due to COVID-19 compared with White recipients. This finding persisted (Black: HR, 2.78 [1.40-5.52; P = .003]; Hispanic: HR, 3.92 [1.88-8.16; P < .001) after adjustment for characteristics before and after transplantation. Age ≥65 years, being on dialysis at most recent follow-up, diabetes mellitus, ischemic cardiomyopathy as an indication for heart transplantation, having a left ventricular assist device before transplantation, taking inotropes before transplantation, and receiving a heart from a donor with >20 pack-year smoking history were all associated with increased risk of death due to COVID-19 during the follow-up period in the multivariable model (all P < .05). Fine-Gray modeling was used to account for the competing risk of non-COVID death during the follow-up period. Figure 4 illustrates adjusted cumulative incidence of COVID-19 mortality being the highest in Black and Hispanic recipients, followed by White and other recipients. The increased risk of death due to COVID-19 in Black and Hispanic recipients compared with White recipients observed in the multivariable Cox model persisted after accounting for competing risks (Black: subhazard ratio, 2.84 [1.38-5.82; P = .004]; Hispanic: subhazard ratio, 3.98 [1.94-8.20; P < .001]; Table 5 ).
Figure 3.
Kaplan-Meier survival estimates for death due to coronavirus disease 2019 (COVID-19) stratified by race/ethnicity. During the study period, survival from death due to COVID-19 was highest in other race recipients, followed by White, Black, and Hispanic recipients, respectively.
Table 4.
Univariate and Multivariable Cox Proportional Hazards Models for COVID Mortality
|
Variable |
Univariate |
Multivariable |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Race/ethnicity | .001 | .001 | ||||
| White | 1 | 1 | . | |||
| Black | 2.07 | 1.10-3.92 | .02 | 2.78 | 1.40-5.52 | .003 |
| Hispanic | 3.91 | 1.93-7.92 | <.001 | 3.92 | 1.88-8.16 | <.001 |
| Other | 0.66 | 0.09-4.88 | .61 | 0.79 | 0.11-5.84 | .82 |
| Age ≥65 y | 2.99 | 1.67-5.39 | <.001 | 2.23 | 1.20-4.14 | .01 |
| BMI ≥30 kg/m2 | 1.34 | 0.76-2.37 | .31 | |||
| Dialysis at follow-up | 4.79 | 2.41-9.53 | <.001 | 3.65 | 1.80-7.37 | <.001 |
| Smoking history | 1.22 | 0.71-2.10 | .47 | |||
| COVID mortality risk by state | .004 | |||||
| Low | 1 | 1 | ||||
| Mild | 0.80 | 0.26-2.45 | .70 | 1.10 | 0.29-4.17 | .89 |
| Moderate | 0.51 | 0.17-1.48 | .21 | 0.59 | 0.16-2.19 | .43 |
| High | 1.79 | 0.69-4.61 | .23 | 1.79 | 0.53-5.99 | .35 |
| Education | .05 | |||||
| High-school diploma or less | 1 | |||||
| Attended college/technical school | 0.60 | 0.30-1.21 | .16 | |||
| Associate/bachelor’s degree | 0.30 | 0.10-0.85 | .02 | |||
| Graduate degree | 0.42 | 0.08-1.32 | .12 | |||
| US citizenship | 0.71 | 0.17-2.90 | .63 | |||
| Public health insurance | 2.07 | 1.18-3.63 | .01 | |||
| Diabetes mellitus | 4.10 | 2.39-7.02 | <.001 | 2.97 | 1.68-5.24 | <.001 |
| Ischemic cardiomyopathy | 3.95 | 2.27-6.89 | <.001 | 3.42 | 1.86-6.31 | <.001 |
| LVAD at transplantation | 1.40 | 0.81-2.41 | .22 | 2.01 | 1.02-3.96 | .04 |
| Inotropes at transplantation | 1.49 | 0.87-2.56 | .14 | 2.30 | 1.18-4.50 | .02 |
| Donor history of smokinga | 2.40 | 1.32-4.39 | .004 | 2.48 | 1.35-4.55 | .003 |
Variables with missing data in the multivariable model were removed during the backward selection process.
BMI, body mass index; COVID, coronavirus disease 2019; HR, hazard ratio; LVAD, left ventricular assist device.
More than 20 pack-years.
Figure 4.
Cumulative incidence function for death due to coronavirus disease 2019 (COVID-19) stratified by race/ethnicity. Competing risks regression was used to account for non-COVID death. The cumulative incidence of COVID-19 mortality was the highest tracked closely for Black and Hispanic recipients. Cumulative incidence of COVID-19 mortality was the lowest in the other race cohort.
Table 5.
Fine-Gray Competing Risks Model for COVID Death
| Variable | SHR | 95% CI | P value |
|---|---|---|---|
| Race/ethnicity | <.001 | ||
| White | 1 | ||
| Black | 2.84 | 1.38-5.82 | .004 |
| Hispanic | 3.98 | 1.94-8.20 | <.001 |
| Other | 0.80 | 0.11-5.86 | .82 |
| Age ≥65 y | 2.22 | 1.16-4.26 | .02 |
| Dialysis at follow-up | 3.40 | 1.66-6.96 | .001 |
| COVID mortality risk by state | .02 | ||
| Low | 1 | ||
| Mild | 1.09 | 0.28-4.17 | .91 |
| Moderate | 0.58 | 0.16-2.12 | .41 |
| High | 1.77 | 0.53-5.89 | .35 |
| Diabetes mellitus | 3.00 | 1.66-5.42 | <.001 |
| Ischemic cardiomyopathy | 3.41 | 1.78-6.55 | <.001 |
| LVAD at transplantation | 1.97 | 0.99-3.9 | .05 |
| Inotropes at transplantation | 2.28 | 1.15-4.51 | .02 |
| Donor history of smokinga | 2.45 | 1.33-4.54 | .004 |
COVID, coronavirus disease 2019; LVAD, left ventricular assist device; SHR, subhazard ratio.
More than 20 pack-years.
Comment
In this analysis of the OPTN database, we showcased that disparities in death due to SARS-CoV-2 infection between racial/ethnic groups observed in the general population are also present among heart transplant recipients. We had several notable findings. First, compared with White race, Black and Hispanic race was associated with higher rates of death due to COVID-19 despite adjusting for available social determinants of health and accounting for the competing risk of non-COVID death. Second, no significant difference in COVID-19 mortality was observed between White and the composite other cohort. Third, we also describe the association of race/ethnicity and COVID-19 mortality among heart transplant recipients across the United States using the OPTN database. Finally, factors associated with death due to COVID-19 (older age, diabetes, and history of ischemic cardiomyopathy) identified in the general population were significant predictors in the heart transplant population.18 , 19 These are in addition to transplant-specific factors (left ventricular assist device at transplantation, inotropes at transplantation, and donor history of smoking >20 pack-years) that reflect the recipient’s condition before heart transplantation.
These findings are consistent with the conclusions of previous studies that have analyzed racial disparities in COVID-19 mortality across the United States. Using national-level data, Karmakar and associates8 reported that counties with large populations of racial/ethnic minorities experienced higher rates of death due to COVID-19. This is supported by a second study demonstrating that although only 20% of US counties are disproportionally Black, they accounted for 58% of COVID-19 deaths in the United States.11 Black adults also experience higher rates of serious illness when infected with COVID-19 compared with White adults.9 A meta-analysis performed by Mackey and colleagues10 found that Black and Hispanic populations experience higher rates of COVID-19 infection and mortality than White populations do, although there was no difference in case-fatality rate.
As shown by recent studies, the sources of COVID-19 racial/ethnic disparities are multifactorial, involving medical risk factors, social determinants of health, and structural racism. Prior studies have highlighted the impact of social determinants of health on a number of health-related outcomes in the general population.20, 21, 22 In New York City, the highest rates of hospitalizations and deaths related to COVID-19 were reported in the Bronx, which has the highest proportion of minority residents and households living in poverty and the lowest levels of education attainment.23 Moreover, increased social vulnerability index has been linked to higher incidence of death due to COVID-19.8 , 24 The social determinants of health available in the OPTN are limited. As more studies demonstrate their importance on patients’ health, efforts should be made to better capture them in transplant patients. Before heart transplantation, patients undergo extensive evaluation and may be denied because of lack of social support mechanisms.25 This practice theoretically reduces variability in social determinants of health between heart transplant recipients compared with variability observed among the general population. We hypothesize that the observed disparities in COVID-19 mortality are not ascribed to biologic differences between races, as we recognize that race is a social construct, but rather to additional imbalances in social determinants of health that have not been accounted for. Regardless, it is imperative to detect racial disparities in health outcomes not only to identify vulnerable populations that necessitate close monitoring but also to motivate allocation of resources to confront sources of inequity.
This study has several limitations. First, the incidence of SARS-CoV-2 infection in this population is unknown. These data are not available in the OPTN database, and therefore racial disparities in COVID-19 mortality could be driven by disparities in COVID-19 incidence. However, we believe the disparity in mortality is concerning irrespective of potential differences in COVID-19 exposure or incidence. Second, this is a retrospective study and accordingly has inherent limitations. Third, as mentioned before, key social determinants of health including access to care, social support, and income are not available in the OPTN database, thereby resulting in residual confounding. Fourth, given the number of outcomes, we were unable to include geographic location in the model as this would have resulted in severe overfitting of the model. Fifth, whereas we used follow-up data when available, many covariates were recorded at the time of listing or transplantation and therefore may not represent the patient’s status at follow-up. Finally, this study captures only the first wave of COVID-19 in the United States; it does not include periods when vaccines were available or when variants had emerged. Thus, observed rates of COVID-19 mortality may not reflect current trends.
Unfortunately, our data demonstrate that disparities in COVID-19 mortality exist in heart transplant recipients despite their close follow-up. Black and Hispanic races were associated with higher rates of COVID-19 mortality than White patients after heart transplantation, even after risk adjustment. These findings highlight the need for clinicians and investigations to recognize that minority heart transplant recipients diagnosed with COVID-19 might be at a higher risk of death based on multifactorial risk factors on an individual and societal level. These data add to an accumulating body of evidence that COVID-19 does not affect all populations equally. Further resources should be allocated to identify and to combat imbalances in social determinants of health and other sources of racial health inequities.
Acknowledgments
Stanley B. Wolfe’s research fellowship is made possible by philanthropic support by the Martignetti family.
Funding Sources
This project was funded by the Massachusetts General Hospital Division of Cardiac Surgery.
Disclosures
The authors have no disclosures directly pertinent to this work. Dr D'Alessandro has received honoraria from Paragonix and Abiomed, which are not discussed in this manuscript.
References
- 1.Lima B., Gibson G.T., Vullaganti S., et al. COVID-19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transpl Infect Dis. 2020;22 doi: 10.1111/tid.13382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Latif F., Farr M.A., Clerkin K.J., et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol. 2020;5:1165–1169. doi: 10.1001/jamacardio.2020.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottio T., Bagozzi L., Fiocco A., et al. COVID-19 in heart transplant recipients. JACC Heart Fail. 2021;9:52–61. doi: 10.1016/j.jchf.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genuardi M.V., Moss N., Najjar S.S., et al. Coronavirus disease 2019 in heart transplant recipients: risk factors, immunosuppression, and outcomes. J Heart Lung Transplant. 2021;40:926–935. doi: 10.1016/j.healun.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh T.P., Almond C., Givertz M.M., Piercey G., Gauvreau K. Improved survival in heart transplant recipients in the United States: racial differences in era effect. Circ Heart Fail. 2011;4:153–160. doi: 10.1161/CIRCHEARTFAILURE.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu V., Bhattacharya J., Weill D., Hlatky M.A. Persistent racial disparities in survival following heart transplantation. Circulation. 2011;123:1642–1649. doi: 10.1161/CIRCULATIONAHA.110.976811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maredia H., Bowring M.G., Massie A.B., et al. Better understanding the disparity associated with Black race in heart transplant outcomes. Circ Heart Fail. 2021;14 doi: 10.1161/CIRCHEARTFAILURE.119.006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karmakar M., Lantz P.M., Tipirneni R. Association of social and demographic factors with COVID-19 incidence and death rates in the US. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaiser Family Foundation Disparities in health and health care: 5 key questions and answers. Published May 11, 2021. https://www.kff.org/racial-equity-and-health-policy/issue-brief/disparities-in-health-and-health-care-5-key-question-and-answers/
- 10.Mackey K., Ayers C.K., Kondo K.K., et al. Racial and ethnic disparities in COVID-19–related infections, hospitalizations, and deaths. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Millett G.A., Jones A.T., Benkeser D., et al. Assessing differential impacts of COVID-19 on black communities. Ann Epidemiol. 2020;47:37–44. doi: 10.1016/j.annepidem.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stokes A.C., Lundberg D.J., Elo I.T., Hempstead K., Bor J., Preston S.H. COVID-19 and excess mortality in the United States: a county-level analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Medicare & Medicaid Services (CMS) HHS. Medicare program; hospital conditions of participation: requirements for approval and re-approval of transplant centers to perform organ transplants. Final rule. Fed Regist. 2007;72:15197–15280. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention COVID data tracker. https://covid.cdc.gov/covid-data-tracker
- 15.US Census Bureau Explore Census data. https://data.census.gov/cedsci/
- 16.Stata Statistical Software: Release 17. StataCorp LLC; 2021.
- 17.Prism 9 for Windows. GraphPad Software, LLC; 2021.
- 18.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19–related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dessie Z.G., Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21:855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on Community-Based Solutions to Promote Health Equity in the United States; Baciu A, Negussie Y, Geller A, Weinstein JN, eds. The root causes of health inequity. In: Communities in Action: Pathways to Health Equity. National Academies Press (US); 2017. [PubMed]
- 21.Olshansky S.J., Antonucci T., Berkman L., et al. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood) 2012;31:1803–1813. doi: 10.1377/hlthaff.2011.0746. [DOI] [PubMed] [Google Scholar]
- 22.Braveman P. Racial disparities at birth: the puzzle persists. Issues in Science and Technology Winter 2008. https://issues.org/p_braveman/
- 23.Wadhera R.K., Wadhera P., Gaba P., et al. Variation in COVID-19 hospitalizations and deaths across New York City boroughs. JAMA. 2020;323:2192–2195. doi: 10.1001/jama.2020.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karaye I.M., Horney J.A. The impact of social vulnerability on COVID-19 in the U.S.: an analysis of spatially varying relationships. Am J Prev Med. 2020;59:317–325. doi: 10.1016/j.amepre.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ladin K., Emerson J., Butt Z., et al. How important is social support in determining patients’ suitability for transplantation? Results from a National Survey of Transplant Clinicians. J Med Ethics. 2018;44:666–674. doi: 10.1136/medethics-2017-104695. [DOI] [PMC free article] [PubMed] [Google Scholar]