Abstract
Background and aims
Coronavirus disease 2019 (COVID-19) imposes a substantial and ongoing burden on the US healthcare system and society. Molnupiravir is a new oral antiviral for treating COVID-19 in outpatient settings. This study evaluated the cost-effectiveness profile of molnupiravir versus best supportive care in the treatment of adult patients with mild-to-moderate COVID-19 at risk of progression to severe disease, from a US payer’s perspective.
Methods
The model was developed using a decision tree for the short-term acute phase of COVID-19 and a Markov state transition model for the long-term post-acute phase. This model compared molnupiravir with best supportive care as consistent with the MOVe-OUT trial. Costs were reported in 2021 US dollars. Transition probabilities were derived from the phase III MOVe-OUT trial and the TriNetX real-world electronic health records database. Costs were derived from the TriNetX database and utility values from a de novo, vignette-based utility study. Deterministic and probabilistic sensitivity analyses (DSA/PSA) were conducted. Primary outcomes included proportion hospitalized, proportion who died overall and by highest healthcare setting at the end of the acute phase, quality-adjusted life-years (QALYs), and incremental costs per QALY gained over a lifetime (100 years) horizon, discounted at 3% annually and assessed at a willingness-to-pay (WTP) threshold of $100,000 per QALY.
Results
In this model, the use of molnupiravir led to an increase in QALYs (0.210) and decrease in direct total medical costs (−$895) per patient across a lifetime horizon, compared with best supportive care in COVID-19 outpatients. Molnupiravir was the dominant intervention when compared with best supportive care. Patients treated with molnupiravir were less likely to be hospitalized (6.38% vs. 9.20%) and more likely to remain alive (99.88% vs. 98.71%) during the acute phase. Through DSA, molnupiravir treatment effect of hospitalization reduction was identified to be the most influential parameter, and through PSA, molnupiravir remained dominant in 84% of the total simulations and, overall, 100% cost effective.
Conclusion
This analysis suggests that molnupiravir is cost effective compared with best supportive care for the treatment of adult outpatients with COVID-19. However, our study was limited by the unavailability of the most recent information on the rapidly evolving pandemic, including new viral variants, patient populations affected, and changes in standards of care. Further research should explore the impact of vaccination on the cost effectiveness of molnupiravir and other therapies, based on real-world data, to account for these changes, including the impact of vaccination and immunity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40273-022-01168-0.
Key Points for Decision Makers
| Given the high economic burden in this setting in the US, this study intends to assess whether molnupiravir is cost effective versus best supportive care in the MOVe-OUT trial population from a US payer perspective. |
| Molnupiravir compared with best supportive care resulted in a dominating (lower costs and higher quality-adjusted life-years [QALYs]) incremental cost-effectiveness ratio. |
| Molnupiravir was estimated to result in longer life expectancy and to be cost effective at a willingness-to-pay threshold of $100,000 per QALY gained in the overall MOVe-OUT population. |
Introduction
As of March 2022, the United States (US) has reported over 78,000,000 coronavirus disease 2019 (COVID-19) cases and over 900,000 COVID-19-related deaths, more than any other country [1]. The direct cost to US healthcare systems and the indirect cost to society are substantial and ongoing [2–4]. Severe COVID-19 disease in particular is estimated to comprise approximately 7% of total cases but approximately 49% of total treatment costs (approximately $2.6 billion per 1000 treated cases) [3].
Mass vaccination with RNA and adenovirus vector vaccines in the US has helped to reduce morbidity and mortality related to COVID-19 [5, 6]. However, the risk of progression to severe or critical COVID-19 remains substantial in the unvaccinated and immunocompromised [7–9], and the emergence of new viral variants may further increase this risk for vaccinated individuals. Therefore, safe and effective pharmacological treatments are needed for prompt management of infection to prevent severe COVID-19 disease on a health system level.
Furthermore, the COVID-19 pandemic has also impacted the global economy, leading to an estimated shrinkage of 5.2% during 2020 [10]. In the US specifically, it has been forecast that COVID-19 will result in almost $8 trillion in economic losses over the next decade [11]. Over 60 million initial unemployment claims were filed in 2020, and the US federal government has spent $2.4 trillion in the form of COVID-19 relief bills [12, 13]. National healthcare spending increased by 9.7% in 2020 to $4.1 trillion, however private health insurance spending decreased by 1.2% to $1.15 trillion (28% of total national health spending) [14]. These changes were due to decreased enrollment and decreased use of healthcare services during the initial stages of the COVID-19 pandemic. Both Medicare and Medicaid spending increased (3.5% and 9.2%, respectively), with Medicare private health plan spending increasing by 17.1%. The increase in Medicaid was due to a rapid increase in spending for hospital care in the pandemic, however this was offset by a decline in private health insurance and out-of-pocket spending; therefore, hospital spending remained steady and realized a spending of $1.3 trillion in 2020.
Molnupiravir is an oral antiviral treatment that has been shown to improve clinical outcomes in outpatients with COVID-19, through inhibition of viral replication [15, 16]. In the phase III MOVe-OUT trial, a double-blind, parallel-group, randomized, placebo-controlled trial, molnupiravir demonstrated significant efficacy versus placebo in reducing hospitalization or death among unvaccinated adults with mild-to-moderate COVID-19 and at least one risk factor for progression. In this trial, supportive care treatment with antipyretic agents, anti-inflammatory agents, glucocorticoids, or a combination was permitted in both arms [15]. This treatment has now been approved in several countries, such as the UK and Japan, and is approved for emergency use in the US [17–19].
Despite large-scale vaccination programs, the incidence of COVID-19 could remain significant in both the US and globally, driven by limited uptake of vaccination in some areas [20] and potential waning immunity that must be supplemented with booster doses 6–12 months after initial vaccination [21]. In addition, the emergence of new viral variants is also likely to reduce the global effectiveness of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [22]. Individuals willfully remaining unvaccinated due to multiple reasons may lead to moral outrage among healthcare workers. This could be due to violation of social contract, high influx of unvaccinated patients in hospitals, and disproportionate utilization of healthcare resources [23]. Therefore, availability of effective pharmacological treatment options for prompt management of mild-to-moderate disease is likely to be a key contributing factor to successful control of COVID-19 on a heath-system level. However, assessment of the cost effectiveness of these therapies is also required to demonstrate the value that may be provided.
This analysis therefore aimed to evaluate the cost effectiveness, from a US payer perspective, for molnupiravir versus best supportive care in the treatment of adult patients with mild-to-moderate COVID-19 at risk of progression to severe disease.
Methods
A cost-effectiveness model was developed to assess the cost effectiveness of molnupiravir versus best supportive care in the treatment of adult patients with mild-to-moderate COVID-19 at high risk of progression. A decision tree was employed to model patients with a starting age of 45 years, as per the average age of the MOVe-OUT trial, in the short-term acute phase (30 days) of COVID-19, and a Markov state transition model was developed to model patients in the long-term post-acute phase. The decision tree was modeled to reflect the results of the MOVe-OUT trial to fully account for the observed mortality benefit of molnupiravir, including the reduced number of patients requiring higher levels of care following hospitalization, if previously treated with molnupiravir (post hoc analysis of hospitalized patients in the MOVe-OUT trial is presented in electronic supplementary material [ESM] Table S1). Modeling was implemented in Microsoft® Excel® 365 (Microsoft Corporation, Redmond, WA, USA).
Although other antiviral therapies are approved for use in the outpatient setting, molnupiravir was compared with best supportive care because there is no widely recognized consensus on the use of these other therapies due to a rapidly evolving treatment landscape. Furthermore, other trials for outpatient therapies were standalone trials and were designed to test their respective hypotheses versus placebo, with disparate characteristics in trial designs and populations. This analysis only compared molnupiravir with best supportive care, corresponding to the design of the MOVe-OUT study. The analysis was conducted from the perspective of healthcare payers in the US at a willingness-to-pay (WTP) threshold of $100,000 per QALY, and costs were expressed in US dollars (US$) in 2021.
Model Structure
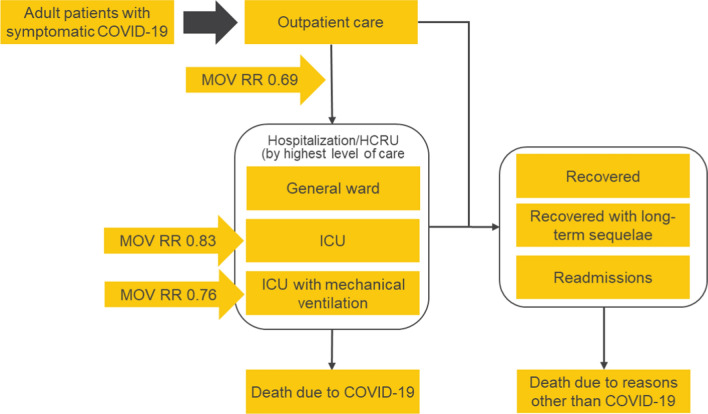
This cost-effectiveness analysis comprised a decision tree model of the acute phase of COVID-19 (30 days), followed by a Markov model with a lifetime horizon limited to 100 years of age or death, whichever occurred first (see Fig. 1).
Fig. 1.
Structure of analysis. COVID-19 coronavirus disease 2019, ICU intensive care unit, HCRU health care resource use, RR risk ratio
At simulation initiation, all patients experienced mild-to-moderate COVID-19 and were managed in the outpatient setting. Patients could then progress to hospitalization due to COVID-19, which included three possible highest levels of care (general ward, intensive care, or intensive care with mechanical ventilation)—each with an associated mortality rate. Long-term sequelae (defined as cardiovascular, pulmonary, and neurologic conditions or other general symptoms related to COVID-19 that remained up to 6 months after the initial onset of symptoms) and all-cause readmissions after the initial COVID-19 hospitalization were also modeled for patients who had survived the acute phase.
Adverse events related to treatment were not modeled as no safety concerns were raised following completion of the phase III MOVe-OUT trial of molnupiravir [15].
Model Inputs
For the base-case analysis, patient characteristics, hospitalization rate, and in-hospital mortality rates were estimated using data from the phase III MOVe-OUT trial of molnupiravir, alongside the TriNetx database study for parameters that were not captured in the trial (see Sect. 2.2.1, Clinical Parameters). Utilities were derived using the Euro-QoL-5D-5L study (see Sect. 2.2.2, Utilities). These sources provided parameter estimates for the base-case analysis, while other sources were explored for scenario analyses (see Sect. 3.2, Sensitivity, Scenario and Threshold Analyses).
Published literature, further real-world database findings, and a primary research study were used to estimate disease progression probabilities, treatment costs, and data on quality of life (see utilities and cost parameters).
Clinical Parameters
Transition probabilities for the base-case analysis were informed by the MOVe-OUT study [15], and the TriNetX real-world electronic health records database in the US informed the parameters (such as outpatient and emergency department (ED) visit rates, proportion experiencing long-term sequelae, and readmission rate) that were not fully captured in the MOVe-OUT study (see Table 1) [24].
Table 1.
Demographic characteristics and clinical parameters
| Parameters | MOVe-OUT trial (post hoc analysis) [N = 699] (for the base-case analysis) | US real-world data (TriNetX) [N = 177,049] (for the base-case analysis where data are not available from the MOVe-OUT trial) |
|---|---|---|
| Age, years (SE) | 45.3 (0.57) | 48.3 |
| Age at death, years (SE) | 61 (5.86) | 72 |
| Female (SE) | 49% (0.02) | 56% |
| Proportion with pre-existing diabetes and serious heart conditions | 3% | 6% |
| Hospitalization rate (SE) | 9.2% (0.01) | 9.3% |
| Proportion, by highest hospital setting [% (SE)] | ||
| General ward | 70% (0.06) | 70% |
| Intensive care unit | 17% (0.05) | 14% |
| Mechanical ventilation | 13% (0.04) | 16% |
| Length of stay, by highest hospital setting, days (SE) | ||
| General ward | 10 (0.82) | 6 (0.09) |
| Intensive care unit | 14 (1.13) | 21 (0.53) |
| Mechanical ventilation | 14 (1.33) | 22 (0.51) |
| Other parameters (real-world data only) | ||
| Outpatient visits [n (SE)] | 4 (0.01) | |
| Emergency department visit [n (SE)] | 2 (0.01) | |
| Patients with emergency department visit (%) | 28% | |
| Length of stay in readmitted (days) | 7a | |
| Long-term sequelae in alive patients in their respective health states after the acute phase (%) [real-world data only] | ||
| Outpatient | 44% | |
| General ward | 77% | |
| Intensive care unit | 97% | |
| Mechanical ventilation | 96% | |
| Proportion of alive patients in their respective health states experiencing readmissions after the acute phase (%) [real-world data only] | ||
| General ward | 14% | |
| Intensive care unit | 36% | |
| Mechanical ventilation | 36% | |
When SE was not available, this was assumed to be 5% of the mean value
SE standard error
aBased on previous observational evidence [23]
The MOVe-OUT trial was a double-blind, parallel group, randomized, placebo-controlled trial evaluating the safety and efficacy of molnupiravir in non-hospitalized adults with COVID-19 [15].
The TriNetX database includes anonymized records for adult patients treated for COVID-19 at academic medical centers across the US (a total of 37 organizations, at the time of analysis) [24]. Both outpatient and inpatient records are included and are longitudinally linked [24]. The study population included adults who had at least one positive COVID-19 polymerase chain reaction (PCR) laboratory test or COVID-19 diagnosis code on or after 20 January 2020 to 25 January 2021. The study excluded patients with vaccination status, patients with no record of any healthcare encounter, despite the diagnosis, prior to COVID-19 admission, or if they were enrolled in a clinical trial anytime during their COVID-19 treatment.
Demographic characteristics derived from these sources included age, sex, and age at death. Clinical parameters estimated using these sources included the key transition probabilities of hospitalization rate and highest level of care, as well as length of stay (by highest level of care). Further parameters that were only available from TriNetX included rate of outpatient visits and ED visits, rates of long-term sequelae (namely cardiovascular, pulmonary, and neurologic conditions as well as other general symptoms), and readmission rates [25]. The length of stay on readmission was defined as 7 days, as suggested by observational evidence [26].
In-hospital mortality rates due to COVID-19 (by highest level of care) were estimated using the data of patients from the MOVe-OUT trial who initiated therapy in the outpatient setting but were hospitalized for COVID-19 (see Table 2), while background mortality rates for patients in the post-acute phase were estimated from 2017 US life tables [27]. In addition, a standardized mortality ratio of 1.5 was applied to the background mortality rate for those individuals with serious severe cardiovascular conditions or diabetes, as recent studies have demonstrated that comorbidities increase mortality risk in hospitalized patients surviving the acute phase of COVID-19 [28–32]. The reduced mortality for those who were hospitalized following molnupiravir treatment in the outpatient setting (versus best supportive care) reported in the MOVe-OUT trial was also modeled. This benefit resulted from reduced disease severity among patients treated with molnupiravir, even after hospitalization. In particular, the WHO-11 ordinal scale analysis showed treatment with molnupiravir was associated with less intensive hospital care before death (see ESM Table S1).
Table 2.
Mortality rate parameters
| Parameters | Mortality ratea |
|---|---|
| General ward (SE) | 2.2% (0.02) |
| Intensive care unit (SE) | 27.3% (0.13) |
| Mechanical ventilation (SE) | 62.5% (0.17) |
SE standard error
aDerived from MOVe-OUT trial (post hoc analysis). Modelled effect on mortality is dependent on the highest score reached on WHO Clinical Progression Scale (11-point); WHO scores 4–5 assumed to represent general ward; WHO score 6 assumed to represent ICU; WHO scores 7–9 assumed to represent mechanical ventilation
The direct impact of molnupiravir on hospitalization (and further progression), and therefore its indirect impact on mortality, were estimated from the MOVe-OUT study (see Table 3 and ESM Table 1) [15]. A post hoc analysis was conducted for the subpopulation hospitalized for reasons related to COVID-19 through day 29. This analysis population included 45 participants assigned to receive molnupiravir 800 mg and 64 participants assigned to receive placebo. The small sample sizes are reflected in broad confidence intervals for these estimates (see Table 3).
Table 3.
Efficacy of molnupiravir based on the MOVe-OUT trial
| Treatment effect | Risk ratio [confidence interval] |
|---|---|
| Progression to COVID-19 related hospitalization | 0.69 [0.48–1.00] |
| Progression to score 6 (ICU) | 0.83 [0.33–2.08] |
| Progression to scores 7–9 (mechanical ventilation) | 0.76 [0.24–2.37] |
Note: Estimates are derived from post hoc analysis of MOVe-OUT trial data through day 29 (see Supplementary information Table 11).
ICU intensive care unit, WHO World Health Organization
Utilities
To estimate quality-adjusted life-years (QALYs) accrued by patients, the time spent in each health state was multiplied by the utility for each health state. A utility value of 0.0 typically corresponds to death and a utility value of 1.0 corresponds to the (hypothetical) state of perfect health. As health state utility values for COVID-19 patients were not available from MOVe-OUT or from the published literature, a de novo vignette-based utility study was conducted to derive appropriate values for use in this analysis (see Table 4). In this study, a series of health state descriptions, or vignettes, were developed to depict a range of health states pertinent to the natural history of COVID-19, and reflective of the health states in the cost-effectiveness model (see ESM Tables S2 and S3). Members of the general public in the UK (n = 500) then completed the EQ-5D-5L questionnaire, a generic, preference-based health-related quality of life (HRQoL) tool, for these health states, acting as proxies for patients. The existing US EQ-5D-5L value set was then applied to generate utility values [33].
Table 4.
Health state utility parameters
| Health states | Utility valuesa |
|---|---|
| Mild/moderate symptoms (SE) | 0.51 (0.007) |
| General ward (SE) | 0.16 (0.009) |
| Intensive care unit (SE) | 0.23 (0.009) |
| Mechanical ventilation (SE) | 0.00 (0.005) |
| Long-term sequelae (SE) | 0.46 (0.008) |
| Recovered without long-term sequelae (SE) | 0.89 (0.005) |
| Readmission | Assumed same as general ward health state |
SE standard error
aDerived from de novo primary research study as specified above
Cost Parameters
Cost per course of treatment for molnupiravir ($707) was derived from the US Advance Purchase Agreement. Based on this agreement, the US government purchased 3.1 million courses of molnupiravir for approximately $2.2 billion by early 2022 [34].
The direct medical cost of COVID-19 management was derived from encounter data within the TriNetX database and imputed using proxy costs from Centers for Medicare & Medicaid Services (CMS)-generated Medicare fee-for-service claims data (see Table 5) [25]. Specifically, costs were those listed as total allowed amounts paid from insurers to healthcare facilities. For patients under the age of 65 years, adjustment factors to proxy costs from the CMS specific to the physician, hospital outpatient department, and hospitalization level were applied for estimating costs for commercially insured patients. Costs were calculated as total costs for each encounter recorded in the electronic medical records (EMRs) and summed to estimate the total cost of medical care. Total direct medical costs were stratified by inpatient costs (general ward, intensive care unit [ICU], and ICU with mechanical ventilation), outpatient costs, and ED costs. Costs were adjusted from 2018 to 2021 US$ using the medical care component of the Consumer Price Index.
Table 5.
Direct medical cost of managing COVID-19
| Parameters | Costa |
|---|---|
| Outpatient visit (per visit) | $351 (3.66) |
| Emergency department visit (per visit) | $2468 (14.18) |
| Cost of COVID-19 hospitalization, by highest hospital setting (SE) | |
| General ward | $32,543 (104.94) |
| Intensive care unit | $54,867 (611.61) |
| Mechanical ventilation | $101,401 (1104.23) |
| Cost of readmission for patients surviving from highest hospital setting (SE) | |
| General ward | $54,691 (954.36) |
| Intensive care unit | $71,324 (1856.51) |
| Mechanical ventilation | $119,342 (2381.77) |
| Long-term sequelae management cost, patients surviving highest level of care (SE) | |
| Outpatient | $829 (17.31) |
| General ward | $1130 (27.48) |
| Intensive care unit | $1951 (105.15) |
| Mechanical ventilation | $2199 (127.54) |
The cost of COVID-19 hospitalization and readmission includes the cost of management of a patient by highest hospital setting during the entire stay and was obtained from claims data. Management includes costs of type of oxygen support received and medication. The cost of long-term sequelae management is the average cost per patient per month for patients surviving the highest level of care. The costs include outpatient visit cost and pharmacy costs. Pharmacy costs were based on electronic medical records of prescribed medications normally used to treat each condition of the long-term sequelae
COVID-19 coronavirus disease 2019, SE standard error
aDerived from TriNetX (encounter data imputed with proxy costs)
A conservative approach was undertaken where long-term sequelae imposed only outpatient medical costs for 5 months following hospital discharge (and not inpatient medical costs, for which there is inadequate evidence).
Outcomes
Outputs included the proportion of patients remaining in the outpatient setting, undergoing hospitalization, remaining alive, dying, experiencing long-term sequelae, and experiencing all-cause readmission following the acute phase.
Any mortality benefit of molnupiravir was modeled indirectly as a consequence of its reduction on the rate of hospitalization (and inpatient mortality) among treated outpatients.
Total costs per patient were calculated by healthcare resource type and health state, and total QALYs per patient were calculated by health state. The incremental cost-effectiveness ratio (ICER) of molnupiravir versus best supportive care was calculated and a cost-effectiveness acceptability curve was developed. A WTP threshold of $100,000 per QALY gained was used to assess a strategy’s cost effectiveness, an incremental cost per QALY threshold below which interventions are typically considered to be favorably cost effective [35]. Costs and QALYs were discounted at 3% per year.
Sensitivity Analysis
Both a deterministic sensitivity analysis (DSA) and a probabilistic sensitivity analysis (PSA) were conducted, including a base-case analysis for different time horizons (1, 5, 10, and 35 years) [36]. The DSA varied parameters within their 95% confidence intervals. Where the variance information was unavailable (literature-based parameters), the standard error was assumed to be 5% of the mean (see ESM Table S4). This analysis also conducted value-of-information analysis. The overall expected value of perfect information (EVPI) was calculated by subtracting the net benefit of the optimal strategy given perfect information from the net benefit of the non-optimal strategy that would be adopted given the current information, which was averaged over all model iterations.
The predefined distributions for the PSA reflect recommendations by Briggs et al. (see ESM Table S4) [36]. A beta distribution was used for probabilities (Dirichlet for polytomous parameters) and health state utility values, and a gamma distribution was used for costs related to COVID-19 management. For other variables (continuous variables) such as length of stay, a normal distribution was utilized. For treatment effect parameters (relative risk), a log-normal distribution was utilized. The PSA sampled from the distribution of each model parameter in 10,000 iterations. The results for each probabilistic model run were also represented using a cost-effectiveness acceptability curve (see ESM Fig. SF3).
Scenario Analyses
Further scenario analyses were also conducted to explore the impact of structural model changes on the ICER for molnupiravir (see Table 6 for summary of analyses, and ESM Tables S5, S6, and S7 for inputs).
Table 6.
Scenario analyses conducted to assess the cost effectiveness of molnupiravir
| Scenario 1: Analysis applying MOVe-OUT subgroup characteristics and outcomes to all patients (tested for 10 subgroups: > 60 years of age; ≤ 60 years of age; BMI ≥ 30; BMI < 30; with diabetes; without diabetes; symptom onset to randomization ≤ 3 days; symptom onset to randomization >3 days (≤ 5 days); mild COVID-19 severity at baseline; moderate COVID-19 severity at baseline) |
| Scenario 2: Analysis using health state utility values from the published literature, rather than from de novo primary research |
| Scenario 3: Analysis not including long-term sequelae and readmission events |
| Scenario 4: Analysis using baseline risk data from US real-world data (TriNetX) rather than MOVe-OUT |
| Scenario 5: Analysis using baseline risk data from US real-world data rather than MOVe-OUT, and cost data from the Premier Health Database or published literature rather than TriNetX |
| Scenario 6: Societal perspective: analysis incorporating the societal impact of productivity losses among inpatients or symptomatic outpatients |
| Scenario 7: Variations of the above where vaccine effectiveness against hospitalization and mortality were varied |
BMI body mass index, COVID-19 coronavirus disease 2019
Scenario 1: Subgroups
Ten prespecified subgroups from the MOVe-OUT trial were tested in this scenario analysis, including those above or below 60 years of age, those with a body mass index (BMI) ≥ 30, those with or without diabetes, those with symptomatic onset < 3 days or between 3 and 5 days, those with mild COVID-19 at baseline, and those with moderate COVID-19 at baseline. The analysis did not include the subgroup of patients who had positive antibody at baseline because of low event rate (only one patient was hospitalized).
Scenario 2: Alternative Health State Utility Values
The analysis explored quality-of-life parameters from published literature other than de novo vignette-based utility studies (see ESM Table S7) [37–40]. We ran base-case-like analysis using the alternate health utility values (assuming 25% standard error of the mean) and assessed its impact on the results.
Scenario 3: Exclusion of Long-Term Sequelae and Readmissions
Evidence on long-term sequelae and readmissions in COVID-19 patients is well established [41, 42]; however these outcomes were not captured in the MOVe-OUT trial. In this scenario, the analysis excluded these outcomes and explored its impact on the results.
Scenario 4: Alternative Patient Characteristics and Baseline Risks
In this scenario analysis, patient characteristics and baseline risks were taken from the US real-world data (TriNetX), rather than from the MOVe-OUT trial (see Table 2 and ESM Table S5).
Scenario 5: Alternative Patient Characteristics, Baseline Risks, and Cost
This scenario analysis tested two alternative sources of cost parameters (see ESM Table S6), together with the parameters varied in the previous section (baseline risks from US real-world data [TriNetX]).
The first source of cost parameters was an unpublished analysis of confirmed COVID-19 cases registered within the Premier Health Database between 1 February 2020 and 12 December 2020. The Premier Health Database is a US hospital-based, service-level, all-payer database containing inpatient and outpatient encounter data primarily from non-profit, non-governmental, community and teaching hospitals, and health systems from rural and urban areas [43, 44]. Costs were reported for the COVID-19-associated health care resource use, including mean associated cost (calculated from the hospital billing data within the electric medical records). Several assumptions were made for parameters such as that the cost of ED was assumed to be twice the outpatient cost, the cost of management for long-term sequelae was assumed to be the same as that of the COVID-19 outpatient cost, and the cost of readmission was assumed to be the same as that of the general ward.
The second source of cost parameters was based on published literature (see ESM Table S6) [45, 46]. Similarly, in this analysis, several assumptions had to be made: (1) cost of COVID-19 hospitalization with no oxygen support and low-flow oxygen support was assumed to be the same as that of the general ward; (2) cost of COVID-19 hospitalization with high-flow oxygen support or non-invasive ventilation was assumed to be the same as that of the ICU; and (3) cost of managing long-term sequelae was assumed to be that of an outpatient visit of complexity reported in the CMS Physician Fee Schedule [47].
Scenario 6: Inclusion of Productivity Loss among Inpatients or Symptomatic Outpatients
This scenario analysis is a societal perspective incorporating short-term productivity loss, assuming that COVID-19 patients were not working for the duration of their symptoms. The productivity loss was estimated by multiplying the average daily wage (considering level of employment) by the number of symptomatic days (in outpatients) or length of stay (in hospitalized patients). The income data were obtained from the Bureau of Labor Statistics (BLS) [48].
Threshold Analysis
As the outcome in COVID-19 patients is evolving due to the continued vaccination program and variants that are associated with milder disease, a threshold analysis was performed to estimate the baseline hospitalization and mortality rate threshold at which molnupiravir became cost effective (at a WTP threshold of $100,000 per QALY gained) or dominant, when compared with best supportive care [49–51]. In this analysis, different rates of hospitalization (1–7%) and mortality (95% reduction in baseline mortality rate to 70% reduction) were used. The range of hospitalization rate and reduction in mortality rate was based on assumption as it was challenging to obtain accurate numbers given the evolving pandemic along with waning vaccine effectiveness. Additionally, the hospitalization rate in the at-risk population was also difficult to locate. In this approach, the model was re-run each time to get an ICER value when the hospitalization rate and mortality rate (applied to baseline mortality in the general ward, ICU and mechanical ventilation) was changed. Additionally, costs associated with COVID-19 management were assumed to be the lowest estimates among the three sources used in base-case and scenario analyses for each level of healthcare (see Table 5 and ESM Table S6).
Results
Base-Case Analysis
The effects of molnupiravir on reduction of hospitalization rate, and reduction of disease severity after hospitalization, were modeled. Overall, molnupiravir increased per patient QALYs (0.210) and reduced per patient total healthcare costs (−$895) over the lifetime horizon compared with best supportive care; therefore, treatment with molnupiravir dominated treatment with best supportive care (improved health outcomes and reduced cost) [see Table 7]. Patients treated with molnupiravir were less likely to be hospitalized compared with patients receiving best supportive care (6.38% vs. 9.20%), and were therefore more likely to remain alive (99.88% vs. 98.71%) during the acute phase (see Table 8).
Table 7.
Overall QALY, cost, and ICER estimates for molnupiravir versus best supportive care, in the base-case analysis
| Treatment | Total discounted QALYs | Total discounted costs | Incremental QALYs (molnupiravir vs. BSC) | Incremental costs (molnupiravir vs. BSC) | ICER (molnupiravir vs. BSC) |
|---|---|---|---|---|---|
| Molnupiravir | 17.721 | $8795 | 0.210 | −$895 | Dominating |
| BSC | 17.512 | $9690 |
BSC best supportive care, ICER incremental cost-effectiveness ratio, QALY quality-adjusted life-years
Table 8.
Disease outcomes
| Proportion of patients (%) | Molnupiravir (%) | Best supportive care (%) |
|---|---|---|
| Total alive, acute phase | 99.88 | 98.71 |
| Total dead, acute phase | 0.12 | 1.29 |
| Proportion hospitalized | 6.38 | 9.20 |
| Proportions in those who were alive at the end of the acute phase, by highest level of care | ||
| Outpatient | 93.62 | 90.80 |
| General ward | 4.84 | 6.32 |
| Intensive care unit | 0.87 | 1.15 |
| Mechanical ventilation | 0.55 | 0.43 |
| Proportions in those who died at the end of the acute phase, by highest level of care | ||
| General ward | 0.02 | 0.14 |
| Intensive care unit | 0.04 | 0.43 |
| Mechanical ventilation | 0.06 | 0.72 |
| Proportion readmitted | 1.20 | 1.47 |
| Proportions of those who survived the acute phase and experienced long-term sequelae, by highest level of care | ||
| Outpatient | 41.12 | 39.88 |
| General ward | 3.71 | 4.84 |
| Intensive care unit | 0.85 | 1.12 |
| Mechanical ventilation | 0.52 | 0.41 |
Treatment with molnupiravir reduced costs due to lower hospitalization rates ($2696 vs. $4139) and because it reduced subsequent readmissions and long-term sequelae ($838 + $2177 vs. $975 + $2239) [see Table 9]. Over the lifetime horizon, treatment with molnupiravir increased QALYs (17.721 vs. 17.512) [see Table 10].
Table 9.
Direct medical cost outcomes
| Cost outcomes | Molnupiravir ($) | Best supportive care ($) |
|---|---|---|
| Total costs | 8795 | 9690 |
| Acute phase | ||
| Drug cost | 707 | 0 |
| Outpatient costa | 2378 | 2338 |
| Total hospitalization cost | 2696 | 4139 |
| General ward | 1582 | 2105 |
| Intensive care unit | 499 | 868 |
| Mechanical ventilation | 614 | 1166 |
| Post-acute phase | ||
| Readmission cost | 838 | 975 |
| Total long-term sequelae cost | 2177 | 2239 |
aIncludes outpatient visit cost and Emergency Department visit cost
Table 10.
QALY outcomes
| Health state | Molnupiravir | Best supportive care |
|---|---|---|
| Total QALYs | 17.72168 | 17.51153 |
| QALYs in the acute phase | ||
| Outpatient | 0.01438 | 0.01395 |
| Hospitalization, overall | 0.00077 | 0.00098 |
| Hospitalization, general ward | 0.00059 | 0.00076 |
| Hospitalization, intensive care unit | 0.00013 | 0.00019 |
| Hospitalization, mechanical ventilation | 0.00004 | 0.00003 |
| QALYs in the post-acute phase | ||
| Readmission | 0.00425 | 0.00529 |
| Long-term sequelae | 0.28678 | 0.28742 |
QALYs quality-adjusted life-years
Sensitivity, Scenario, and Threshold Analyses
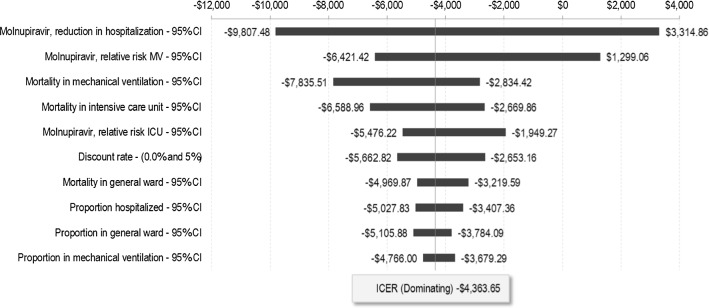
DSA, which varied each parameter individually (as described in the Methods section), found that under all alternative assumptions tests, molnupiravir continued to dominate best supportive care (see Fig. 2 and ESM Fig. SF4). However, the DSA results suggested that the ICER value was most sensitive to the treatment effect of hospitalization reduction by molnupiravir, followed by the treatment effect of molnupiravir relative risk of mechanical ventilation, mortality rate in the highest hospital setting (among patients not treated with molnupiravir), and molnupiravir relative risk of ICU.
Fig. 2.
Deterministic sensitivity analysis of the cost effectiveness of molnupiravir versus best supportive care. A negative ICER indicates that molnupiravir is dominating best supportive care (that is, leading to increased QALYs alongside reduced cost). The list of parameters presented in the DSA figure are based on the most impactful figures on the ICER value (top to bottom). ICU intensive care unit, MV mechanical ventilation, ICER incremental cost-effectiveness ratio, QALYs quality-adjusted life-years, DSA deterministic sensitivity analysis, CI confidence interval
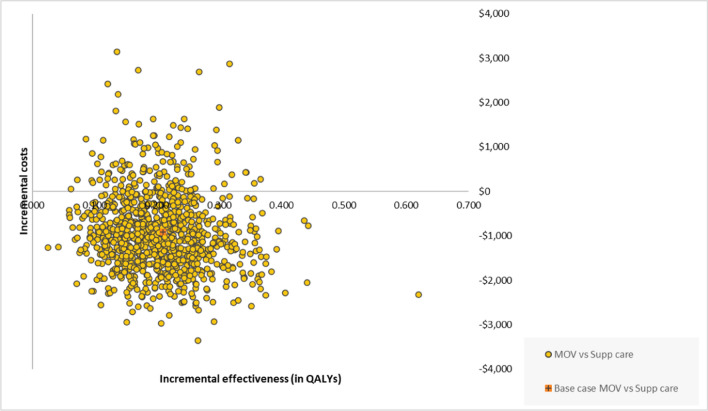
Figure 3 presents total discounted costs and QALYs for all 10,000 iterations of the model run in the PSA. The ICERs for molnupiravir versus best supportive care remained largely in the southeast quadrant, which indicates that molnupiravir is more effective and less costly than best supportive care. The cost-effectiveness acceptability curve (not presented in this article) demonstrated that molnupiravir consistently remained the strategy with the greatest (100%) probability of being the most cost effective at WTP thresholds ranging up to $100,000 per QALY gained. Of 10,000 iterations of the model run in the PSA, 84% of iterations showed molnupiravir dominated best supportive care.
Fig. 3.
Probabilistic sensitivity analysis of the cost effectiveness of molnupiravir versus best supportive care. MOV molnupiravir treatment, Supp supportive (care), QALYs quality-adjusted life-years
The value of information analysis depicts the amount a decision maker would be willing to pay knowing all the information influencing the decision of selecting a prefered treatment from this analysis. In other words, the EVPI is showing us how much a decision maker will lose if the optimal strategy is wrong (see ESM Figs. SF7 and SF8). Thus, the expected monetary loss per patient by a decision maker who chooses best supportive care as an optimal strategy is $21,464 (at $100,000 per QALY); however, there is no loss to a decision maker for molnupiravir.
Results for the cost effectiveness of molnupiravir versus best supportive care in a number of scenario analyses also showed that molnupiravir remains dominant and/or cost effective in every scenario (see ESM Table S8 and ESM Fig SF2). At 1-, 5-, 10-, and 35-year time horizons, molnupiravir dominated best supportive care (see ESM Table S8).
The threshold analysis showed that molnupiravir remained cost effective in all scenarios, with a baseline hospitalization rate ≥ 3% and a reduction in mortality rate of 95%, and remained dominant in all scenarios, with a baseline hospitalization rate ≥ 6% and a reduction in mortality rate of 95% (see ESM Fig. SF2). The baseline hospitalization threshold reduced when the reduction in mortality rate decreased.
Validation
Both internal and external validation were conducted. For external validation, validation with real-world data was carried out on the outcomes of patients with COVID-19; however, validation of long-term outcomes of COVID-19 could not yet be carried out due to paucity of such data in the MOVe-OUT patient population.
For internal validation, a comparison of the predicted model results and the observed trial results per the MOVe-OUT study showed an alignment in the results (see ESM Table S10), especially for the incremental outcomes between the molnupiravir and best supportive care arm at the acute phase [15]. Lastly, a quality check of the model was conducted using the TECHnical VERification (TECH-VER) checklist (see ESM Table S11) [52].
Discussion
Following the US FDA emergency use authorization based on the results of the MOVe-OUT trial [18], molnupiravir represents an innovative treatment that fulfils an unmet need for treating outpatients with COVID-19. This analysis demonstrated that treatment with molnupiravir reduced COVID-19 hospitalization, disease severity, and in-hospital mortality, and that surviving patients who were hospitalized were less likely to experience long-term sequelae or readmission. It concluded that when compared with best supportive care, treatment with molnupiravir was associated with cost savings and QALY gains, and can be considered a cost-effective treatment option in the management of outpatients with COVID-19 at risk of progression to severe disease in the US. Recent evidence suggests that hospitalized patients who were treated with molnupiravir experienced reduced hospital length of stay (median 3 days reduction), demonstrating its additional clinical benefits [53]. However, this analysis did not apply treatment effect, leading to a reduction in hospital length of stay.
In the current analysis, the price of molnupiravir was based on the US Advance Purchase Agreement price ($707), as its commercial price is not yet available. A threshold analysis was conducted as part of this research, suggesting that molnupiravir remains cost effective up to a price of approximately $22,500 per treatment course, at the commonly accepted US WTP threshold of $100,000 per QALY gained.
In February 2022, the Institute for Clinical and Economic Review published a draft report on the cost-effectiveness profile of several COVID-19 therapies (including molnupiravir), individually against usual care in the US [54]. While the Institute for Clinical and Economic Review’s model structure was similar to the structure of the current study’s model, in that draft report the ICER of molnupiravir versus usual care was estimated at $55,000 per QALY gained. There are several key differences in the assumptions between the Institute for Clinical and Economic Review model and the current analysis. First, in the Institute for Clinical and Economic Review model, the baseline risk in the usual care arm was based on a pooled estimate across different trials of COVID-19 therapies, weighted by the number of US participants in each individual trial. This approach was considered as the Institute for Clinical and Economic Review believed it would represent the patient population in the US that would receive these outpatient treatments for COVID-19. However, this approach may not be considered appropriate as trials and their participants had disparate characteristics which should have been accounted for. Second, the Institute for Clinical and Economic Review model did not replicate the MOVe-OUT trial results in terms of COVID-19-associated mortality. This approach averted mortality through preventing hospitalization [55]. Third, the Institute for Clinical and Economic Review’s model included a proportion of patients who were vaccinated, and adjusted the baseline hospitalization in the base-case analysis, despite vaccinated individuals being excluded from the trials from which analysis parameters were drawn. This inclusion of vaccinated individuals in the Institute for Clinical and Economic Review approach was on the basis that these outpatient therapies for COVID-19 are eligible for all at-risk populations regardless of vaccine status. Finally, the Institute for Clinical and Economic Review model included unrelated health care costs for patients surviving the acute phase, thereby applying a penalty to interventions that save lives.
Since the beginning of 2020, the COVID-19 landscape has been evolving rapidly, especially after the advent of the mass vaccination program from the end of 2021. Nevertheless, in the MOVe-OUT trial, vaccinated patients were not included. This was because of the small size of the vaccinated population when the trial began and the unknown impact of vaccination under Emergency Use Authorization on the primary endpoint at the time of the study. This also reflected those most likely to need antiviral treatment, in order to facilitate more rapid evaluation of the therapeutic efficacy of molnupiravir. Additionally, in the MOVe-OUT trial, approximately 56% of sequenced infections were of the Delta variant [15], which was associated with more severe disease than the currently circulating Omicron variant. Recently, a study conducted in Houston, TX, USA, reported that the Omicron variant was associated with a 54% lower hospitalization rate when compared with the Delta variant (19.8% vs. 43.1%) in symptomatic patients, and the mortality in the same population was 83% lower (0.9% vs. 5.3%) [50]. In this study, 40.6% of those who were infected with the Omicron variant were not vaccinated (72.6% for the Delta variant). Similarly, a study from southern California, USA, estimated the adjusted hazard ratio of symptomatic hospitalization between the Omicron and Delta variant at 0.56 (7.2% vs. 31.6%) in those who had a non-zero Charlson Comorbidity Index [49]. Among those who were infected with the Omicron variant, 29.5% were not vaccinated (48.1% for the Delta variant).
In the current analysis, the effect of vaccination at the population level and the effect of variants that are associated with a milder disease were approximated by reducing the baseline risks of hospitalization and mortality. As shown earlier in the analysis, molnupiravir remained cost effective when the baseline hospitalization rate was ≥ 3% (a reduction of 67% from the current baseline rate of 9.2%) and a reduction in mortality rate of 95%, and dominant when the rate was ≥ 6% (a reduction of 35% from the current baseline rate of 9.2%) and a reduction in mortality rate of 95%, when compared with best supportive care. Therefore, even when the impact of vaccination and milder variants are considered, molnupiravir may still be considered as a cost-effective treatment option for those in need. Given the waning vaccine effectiveness, potential future variants that may impact vaccine effectiveness, and the presence of a large unvaccinated population, especially among the immunocompromised population in the US [56–58], molnupiravir should reside in the armamentarium of COVID-19 containment strategy in the country.
Besides the direct impact on population health, the COVID-19 pandemic has also imposed an immense societal burden. The current analysis (in scenario 6, specifically) only incorporated productivity loss due to COVID-19 hospitalization (molnupiravir $86 vs. best supportive care $107), which is very likely a conservative approach.
Research has documented the prolonged impact of ‘long COVID’ on both economic and quality-of-life outcomes for individuals [59]. Some patients with COVID-19 may experience strokes or become diabetic, and individuals experiencing an episode of critical COVID-19 requiring ICU treatment may suffer permanent damage to the heart, lungs, or brain [60]. In addition, patients with COVID-19 experience a higher risk of mental health problems—it has been reported that almost half of patients with COVID-19 experienced depression [61]. Furthermore, patients with infected family members were more likely to be impacted by depression, potentially due to feeling guilty about transmitting SARS-CoV-2 to their family members and the community [61, 62].
Outbreaks of COVID-19 have overwhelmed health care resources and ICU capacity in several regions. Thus, health care systems have faced difficult choices in terms of prioritization, and the ability to continue providing high-quality care for other conditions has been more limited [63]. Interestingly, it has been suggested that outpatient COVID-19 therapies that reduce hospitalization risk could play an important role in reducing this strain on ICU capacity and reducing overall health system burden [64, 65]. In addition to its effect on private individuals, COVID-19 is also associated with an impact on health care practitioners; those exposed to patients with COVID-19 have been estimated to have a duration of absenteeism of between 7.5 and 25.8 days [66], to which anxiety and depression may be influencing factors [67]. Absenteeism among health care practitioners will further impact the quality of care experienced by patients with COVID-19 or other conditions [10–13]. Restrictions such as lockdowns and social distancing have also been imposed on the public in order to reduce transmission rates and therefore the number of patients requiring hospitalization; however, these restrictions are associated with huge economic and health consequences for society [68, 69]. These elements were not intentionally included in the current study as it was difficult to either quantify them or quantify them systematically. Being an outpatient treatment, molnupiravir could be of significant assistance in such consequences. The extra value of molnupiravir, as part of a COVID-19 containment strategy, is therefore expected to be greater than what this study shows.
This study has several limitations. Given the dynamic nature of the ongoing SARS-CoV-2 pandemic, the data sources used to define the model parameters may be superseded by the time of publication. This may be especially true for estimates of vaccine effectiveness and associated hospitalization rates in at-risk COVID-19 patients, as these have been observed to vary according to vaccine type, time since vaccination, and viral variant. Other pharmacological therapies for outpatient COVID-19 could not reasonably be included for comparison due to differences in trial designs and populations. Appropriate health state utility data for COVID-19 patients, especially for patients hospitalized due to COVID-19, for this analysis were unavailable within the existing literature and required de novo research to elicit. The de novo was carried out using survey responses from the UK general population acting as proxies for COVID-19 patients, and these utility values were cross-walked to derive US utility values using US value sets. Based on the results of the de novo, the utility value of the general ward was observed less than the ICU, which cannot be easily explained. This could be due to the perception of the health state vignettes developed. The description of the health state vignettes of the general ward and the ICU shared similarity except how the supplemental oxygen was delivered. It was mentioned that the supplemental oxygen in the general ward setting was delivered to the lungs through a tube through the nose, whereas in the ICU, supplemental oxygen was delivered through a face mask. Therefore, it seems oxygen delivery through the nose into the lungs was perceived to be invasive, which possibly explained the lower utility values for the general ward than the ICU. The utility value of the mechanical ventilation health state was equivalent to the death state. We utilized these utility values in the base-case analysis as the appropriateness of the influenza or pneumonia cannot be simply applied to COVID-19, despite sharing similar symptoms. We considered this approach for deriving utility values because COVID-19 had never been experienced before. We should account for the perspective of the patients who have experienced COVID-19 when there was no treatment available. However, the de novo surveyed the UK general population and captured these responses, including the fear of COVID-19 in individuals who are not infected and a relief of being recovered without experiencing long-term sequelae. Although, to check the robustness of the model, in the scenario analysis where alternate sources for utility values (25% standard error of the mean) were used, replacing de novo utility values, the results of the analysis did not deviate qualitatively. Re-infections and transmissions were not captured in this analysis due to limited information. Lastly, as mentioned above, this model may not have captured all economic and health benefits associated with the outpatient treatment of COVID-19 and may therefore have underestimated of the true value of molnupiravir.
Conclusion
This economic evaluation suggested that treatment with molnupiravir is cost effective when compared with best supportive care in the management of adult patients with mild-to-moderate COVID-19 at risk of progression to severe disease in the US.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the patients, along with their families and caregivers, who participated in the MOVe-OUT trial, from which the clinical inputs of this study were obtained. They thank Angeliki Zarotiadou, Anjela Tzontcheva, Lidia Mukina, Mikkel Oestergaard, and Svetlana Bizjajeva, employees of MSD Innovation & Development GmbH, as well as Paul Picq, employee of Aixial, and Geoffrey Johnson and Weilin Meng, employees of Merck & Co., Inc., for statistical consultation and data inputs. The authors also thank Georgie Weston, Ashley Enstone, Ben Rousseau, and Robin Wyn, employees of Adelphi Values, Bollington, Cheshire, UK, for editorial assistance with the manuscript.
Declarations
Funding
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Conflict of interest
Hardik Goswami, Adnan Alsumali and Amy Puenpatom are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, who may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Yiling Jiang is an employee of MSD (UK) Ltd, who may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Matthias Schindler is an employee of MSD (Switzerland) Ltd., who may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. Elizabeth Duke was the site principal investigator for the clinical trial from which these data were drawn and for the ongoing MK4482-013 clinical trial studying molnupiravir for prevention of COVID-19 in household contacts. Her institution, Fred Hutchinson Cancer Research Center, received funding from Merck & Co., Inc. to conduct these studies but the funding was not tied to study outcomes. Joshua Cohen received compensation from Merck & Co., Inc. as a consultant for this work. Andrew Briggs received compensation from Merck & Co., Inc. as a consultant for this work. He has also been contracted by Bayer, Eisai, Janssen, Novartis, Sword Health, Amgen, and Daichii Sankyo and received compensation outside of the submitted work.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Ethics approval
This was a cost-effectiveness study and no ethics approval was required.
Consent to participate
Consent to participate was not required for this study.
Consent to publish
Consent to publish was not required for this study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.World Health Organization. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/table. Accessed 4 Feb 2022.
- 2.CNBC. Uninsured Americans could be facing nearly $75,000 in medical bills if hospitalized for coronavirus. 2020. https://www.cnbc.com/2020/04/01/covid-19-hospital-bills-could-cost-uninsured-americans-up-to-75000.html. Accessed 2 Feb 2022.
- 3.McKinsey & Company. Understanding the hidden costs of COVID-19’s potential impact on US healthcare. 2020. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/understanding-the-hidden-costs-of-covid-19s-potential-impact-on-us-healthcare. Accessed 2 Feb 2022.
- 4.CNN Health. An average Covid-19 hospitalization costs Medicare about 150 times more than it does to vaccinate one beneficiary. 2021. https://edition.cnn.com/2021/09/09/health/covid-19-hospitalization-cost-vaccination/index.html.
- 5.Centers for Disease Control and Prevention. Different COVID-19 Vaccines. 1 September 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines.html.
- 6.Ledford H. Six months of COVID vaccines: what 1.7 billion doses have taught scientists. Nature. 2021;594(7862):264–167. doi: 10.1038/d41586-021-01505-x. [DOI] [PubMed] [Google Scholar]
- 7.Anjan S, et al. Breakthrough COVID-19 infections after mRNA vaccination in solid organ transplant recipients in Miami, Florida. Transplantation. 2021;105(10):e139. doi: 10.1097/TP.0000000000003902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juthani PV, et al. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect Dis. 2021;21(11):1485–1486. doi: 10.1016/S1473-3099(21)00558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tenforde MW, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The World Bank. COVID-19 to Plunge Global Economy into Worst Recession since World War II. https://www.worldbank.org/en/news/press-release/2020/06/08/covid-19-to-plunge-global-economy-into-worst-recession-since-world-war-ii#:~:text=According%20to%20World%20Bank%20forecasts,shrink%20by%205.2%25%20this%20year.&text=That%20would%20represent%20the%20deepest,June%202020%20Global%20Economic%20Prospects. Accessed 8 June 2020.
- 11.Congressional Budget Office. Comparison of CBO’s May 2020 Interim Projections of Gross Domestic Product and Its January 2020 Baseline Projections. https://www.cbo.gov/publication/56376. Accessed 1 June 2020.
- 12.Congressional Budget Office. Budgetary Effects of the 2020 Coronavirus Pandemic. 2020. https://www.cbo.gov/publication/56388. Accessed 2 Feb 2022.
- 13.United States Department of Labor. Unemployment insurance weekly claims. 2020; https://oui.doleta.gov/unemploy/claims_arch.asp.
- 14.Office of the Actuary, Centers for Medicare & Medicaid Services. National Health Spending in 2020 Increases due to Impact of COVID-19 Pandemic. https://www.cms.gov/newsroom/press-releases/national-health-spending-2020-increases-due-impact-covid-19-pandemic.
- 15.Jayk Bernal A, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med. 2021;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitley R. Molnupiravir—a step toward orally bioavailable therapies for Covid-19. N Engl J Med. 2022;386(6):592–593. doi: 10.1056/NEJMe2117814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GOV.UK. First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA. 2021. https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra. Accessed 2 Feb 2022.
- 18.US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Additional Oral Antiviral for Treatment of COVID-19 in Certain Adults. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-oral-antiviral-treatment-covid-19-certain.
- 19.厚生労働省. 新型コロナウイルス治療薬の特例承認について. https://www.mhlw.go.jp/stf/newpage_23047.html. Accessed 3 Dec 2021.
- 20.The New York Times. See How Vaccinations Are Going in Your County and State. https://www.nytimes.com/interactive/2020/us/covid-19-vaccine-doses.html. Accessed 7 Feb 2022.
- 21.Centers for Disease Control and Prevention. COVID-19 Vaccine Booster Shots. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html. Accessed 2 Feb 2022.
- 22.Collie S, et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudzinski DM. Moral outrage toward willfully unvaccinated COVID-19 patients. J Gen Intern Med. 2022;37(8):2070–2071. doi: 10.1007/s11606-022-07496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.TriNetX. Coronavirus. 2022. https://trinetx.com/real-world-resources/coronavirus/.
- 25.Puenpatom A et al. Healthcare utilization, mortality, and care pathway among patients with COVID-19 in the US [Manuscript in preparation.]
- 26.Atalla E, et al. Readmissions among patients with COVID-19. Int J Clin Pract. 2021;75(3):e13700. doi: 10.1111/ijcp.13700. [DOI] [PubMed] [Google Scholar]
- 27.Arias E. United States Life Tables, 2017. Natl Vital Stat Rep. 2019;68(7):1–66. [PubMed] [Google Scholar]
- 28.Mason KE, et al. Age-adjusted associations between comorbidity and outcomes of COVID-19: a review of the evidence from the early stages of the pandemic. Front Public Health. 2021;9:584182. doi: 10.3389/fpubh.2021.584182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai Y, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, et al. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020;286:198043. doi: 10.1016/j.virusres.2020.198043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen N, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.EuroQol. EQ-5D-5L | Valuation | Crosswalk Index Value Calculator. 2019. https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/valuation-standard-value-sets/crosswalk-index-value-calculator/. Accessed 2 Feb 2022.
- 34.Merck & Co. Merck and Ridgeback Announce U.S. Government to Purchase 1.4 Million Additional Courses of Molnupiravir, an Investigational Oral Antiviral Medicine, for the Treatment of Mild-to-Moderate COVID-19 in At Risk Adults. https://www.merck.com/news/merck-and-ridgeback-announce-u-s-government-to-purchase-1-4-million-additional-courses-of-molnupiravir-an-investigational-oral-antiviral-medicine-for-the-treatment-of-mild-to-moderate-covid-19-in-a/. Accessed 6 Nov 2021.
- 35.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 36.Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- 37.Falk Hvidberg M, et al. The health-related quality of life for patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) PLoS One. 2015;10(7):e0132421. doi: 10.1371/journal.pone.0132421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Institute for Clinical and Economic Review. Alternative Pricing Models for Remdesivir and Other Potential Treatments for COVID-19. https://icer.org/wp-content/uploads/2020/11/ICER-COVID_Updated_Report_11102020.pdf. Accessed 10 Nov 2020.
- 39.Poteet S, Craig BM. QALYs for COVID-19: a comparison of US EQ-5D-5L value sets. Patient Patient Centered Outcomes Res. 2021;14(3):339–345. doi: 10.1007/s40271-021-00509-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Mak. 2006;26(4):410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshijima H, et al. Incidence of long-term post-acute sequelae of SARS-CoV-2 infection related to pain and other symptoms: a living systematic review and meta-analysis. MedRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 42.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(7862):259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 43.Premier. Premier Healthcare Database being used by National Institutes of Health to evaluate impact of COVID-19 on patients across the U.S. 2020. https://www.premierinc.com/newsroom/press-releases/premier-healthcare-database-being-used-by-national-institutes-of-health-to-evaluate-impact-of-covid-19-on-patients-across-the-u-s. Accessed 2 Feb 2022.
- 44.Premier. Data that Informs and Performs. 2020. https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper. Accessed 2 Feb 2022.
- 45.Ohsfeldt RL, et al. Inpatient Hospital Costs for COVID-19 patients in the United States. Adv Ther. 2021;38(11):5557–5595. doi: 10.1007/s12325-021-01887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore BJ, Liang L. Costs of Emergency Department Visits in the United States, 2017, in Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2020. [PubMed]
- 47.Centers for Medicare & Medicaid Services. CMS Physician Fee Schedule (HCPCS code: 99205 for office visit of high complexity). https://www.cms.gov/files/document/physician-fee-schedule-pfs-payment-officeoutpatient-evaluation-and-management-em-visits-fact-sheet.pdf. Accessed 11 Jan 2022.
- 48.US Bureau of Labor Statistics. Data Retrieval: Labor Force Statistics (CPS). 2015. https://www.bls.gov/webapps/legacy/cpswktab3.htm. Accessed cited 2020.
- 49.Lewnard JA, et al. Clinical outcomes among patients infected with Omicron (B.1.1.529) SARS-CoV-2 variant in southern California. medRxiv. 2022:2022.01.11.22269045.
- 50.Christensen PA, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. doi: 10.1016/j.ajpath.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paredes MI, et al. Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington State: a retrospective cohort study. medRxiv. 2022:2021.09.29.21264272. [DOI] [PMC free article] [PubMed]
- 52.Büyükkaramikli NC, et al. TECH-VER: a verification checklist to reduce errors in models and improve their credibility. Pharmacoeconomics. 2019;37(11):1391–1408. doi: 10.1007/s40273-019-00844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson MG, et al. Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19. Ann Internal Med. 2022 doi: 10.7326/M22-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Institute for Clinical and Economic Review. Special Assessment of Outpatient Treatments for COVID-19 Response to Public Comments on Draft Evidence Report. Institute for Clinical and Economic Review; 2022.
- 55.Yeung K, Whittington MD, Beinfeld M, Mohammed R, Wright A, Nhan E, et al. Special Assessment of Outpatient Treatments for COVID-19. Institute for Clinical and Economic Review; 2022.
- 56.Tartof SY, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang SY, et al. Severe breakthrough COVID-19 cases in the SARS-CoV-2 delta (B.1.617.2) variant era. Lancet Microbe. 2022;3(1):e4–e5. doi: 10.1016/S2666-5247(21)00306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenberg ES, et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386(2):116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Office of National Statistics. Coronavirus and the social impacts of ‘long COVID’ on people’s lives in Great Britain: 7 April to 13 June 2021. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronavirusandthesocialimpactsoflongcovidonpeopleslivesingreatbritain/7aprilto13june2021#well-being-loneliness-and-long-covid.
- 60.Higgins V, et al. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58(5):297–310. doi: 10.1080/10408363.2020.1860895. [DOI] [PubMed] [Google Scholar]
- 61.Ma Y-F, et al. Prevalence of depression and its association with quality of life in clinically stable patients with COVID-19. J Affect Disord. 2020;275:145–148. doi: 10.1016/j.jad.2020.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang Y-T, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.IHME COVID-19 Health Service Utilization Forecasting Team, Murray CJ. Forecasting COVID-19 impact on hospital bed-days, ICU-days, ventilator-days and deaths by US state in the next 4 months. MedRxiv. 2020.
- 64.Moon RC, Brown H, Rosenthal N. Healthcare resource utilization of patients with COVID-19 visiting US Hospitals. Value Health. 2022;25(5):751–760. doi: 10.1016/j.jval.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.French G, et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic—United States, July 2020–July 2021. Morb Mortal Wkly Rep. 2021;70(46):1613. doi: 10.15585/mmwr.mm7046a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maltezou HC, et al. Costs associated with COVID-19 in healthcare personnel in Greece: a cost-of-illness analysis. J Hosp Infect. 2021;114:126–133. doi: 10.1016/j.jhin.2021.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salari N, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health. 2020;18(1):1–14. doi: 10.1186/s12960-020-00544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonaccorsi G, et al. Economic and social consequences of human mobility restrictions under COVID-19. Proc Natl Acad Sci. 2020;117(27):15530–15535. doi: 10.1073/pnas.2007658117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giuntella O, et al. Lifestyle and mental health disruptions during COVID-19. Proc Natl Acad Sci. 2021;118(9):e2016632118. doi: 10.1073/pnas.2016632118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.