Abstract
Purpose
Registered trials and real-world evidence (RWE) studies provided evidence on the efficacy of once-weekly (OW) semaglutide on hyperglycaemia and cardiovascular risk factors as add-on or de-novo treatment in type 2 diabetes (T2D).
Methods
In a retrospective analysis of electronic data files from 258 T2D patients, this RWE study aimed to explore the impact of OW semaglutide on biochemical and anthropometric outcomes after 6 and 12 months in patients receiving at least one prescription of OW semaglutide between September 2019 and May 2021.
Results
During the study period, 154 and 56 consecutive patients completed the 6 and 12 months of OW semaglutide treatment. HbA1c levels decreased by -1.02±0.1% after 6 months and -1.1±0.1% after 12 months of OW semaglutide (p<0.0001 for both). At these time-points, HbA1c values were <7% in 61% and 57% of cases. HbA1c reduction was greater in patients with higher baseline HbA1c levels and it occurred irrespective of gender, age, insulin therapy and complications. The residual number of cases with HbA1c ≥9% by the study end was low (5.3% vs 18.9% at baseline). Weight loss occurred in 73.5% and 78.1% of cases and, compared to baseline, it was ≥5% in 21.2- 25.4% and ≥10% in 6.8-18.2% after 6 and 12 months, respectively. Significant predictors of HbA1c reduction after 6 months of OW semaglutide treatment were baseline HbA1c (p<0.0001), bodyweight reduction (p<0.0001) and disease duration (p<0.001), while baseline HbA1c was the only predictor of HbA1c response after 12 months (p<0.0001). Reported adverse events were consistent with the known safety profile of semaglutide.
Conclusions
Real-world evaluation of weekly subcutaneous treatment with semaglutide in a cohort of Italian diabetic patients.
Keywords: Type 2 diabetes mellitus, Obesity, Once-weekly semaglutide, GLP-1 receptor agonists
Introduction
Incretins are peptides secreted in response to meal ingestion which, at physiological concentrations, stimulate insulin release and reduce glucagon secretion. The incretin family comprises several peptide hormones, including secretin, VIP, pituitary adenylate cyclase-activating polypeptide (PACAP), glucagon as well as glucagon-like peptides, i.e. GLP-1 and GLP-2, glycentin, oxyntomodulin and gastric inhibitory peptide (GIP) [1]. Research has progressively focused on the development of drugs eliciting the so-called incretin effect through mono-, dual- and triple agonism of these peptides [2]. Glucagon-like peptide 1 receptor agonists (GLP-1RAs) are recognized glucose-lowering agents that act through pancreatic and extra-pancreatic mechanisms, these latter including decrease of gastric motility and central control of food intake [3]. In addition to achieving glycemic targets, chronic treatment with GLP-1RAs has proved the ability to promote cardiovascular (CV) benefits and reduce the risk of death from CV disease (CVD), nonfatal myocardial infarction, or nonfatal stroke in T2D patients [4–8]. Further, treatment with GLP-1RAs benefits kidney protection through reduction of albuminuria, as well as by controlling progression of diabetic kidney disease (DKD) to end-stage renal disease (ESRD) independent of albuminuria [9]. In turn, kidney effects advantage CV health independent of glycemic control [10]. Cumulatively, these effects appear internally consistent across the drug class [11] and highlight a potential dual advantage for patients with atherosclerotic CVD (ASCVD) and DKD [12]. Moreover, their intrinsic effects on insulin and glucagon are tailored in a glucose-dependent manner, thus posing a low risk of hypoglycemia and making them one of the most effective and safest options when a more intensive antidiabetic treatment is required. Finally, the once-weekly subcutaneous administration of some GLP-1RAs helps supporting patients’ compliance and adherence to treatment. Given this wide range of benefits, GLP-1RAs are currently recommended as a second-line therapy in T2D and can be prescribed as a first-line treatment when it is necessary to enhance therapy using an injectable agent [13]. In the presence of ASCVD or high/very high cardiovascular risk (CVR), GLP1-RA can be considered as first-line treatment [14].
Semaglutide is a long-acting GLP-1 analog with a once-weekly (OW) extended release, developed to treat T2D patients who are unable to achieve their hemoglobin A1c (HbA1c) goals with other anti-hyperglycemic medications. Marketing of OW semaglutide has been granted in Europe since February 2018 and has been available for therapeutic use in Italy since September 2019 [15, 16]. The efficacy of OW semaglutide as monotherapy or as add-on therapy has been extensively investigated in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) program, which comprises ten clinical trials that compared OW semaglutide to placebo or other antidiabetic treatments. Results showed a robust reduction in HbA1c up to 1.5–1.8%, a reduction in major adverse CV events (CVE) rates, kidney prevention and weight loss in patients treated with OW semaglutide as compared to placebo or other treatments [17–20]. Existing clinical trial data suggest a combined effect of OW semaglutide on the dual target of glucose and obesity control, implying a particular advantage for diabetic patients with obesity. There is a need for complementary real-world evidence to validate clinical trial results in a wider pool of patient types and for specific countries or regions [21]. So, to complement data generated by the SUSTAIN program, an analysis conducted on standard users of OW semaglutide with broad representativeness of the routine patient population referred to the clinical practice could support with real-world evidence the effectiveness of OW semaglutide and provide insights on response predictors. The purpose of this observational study was to expand the knowledge on OW semaglutide treatment in a real-world setting and to provide guidance on its efficacy on metabolic, anthropometric and hepato-renal outcomes in patients with T2D on other previous treatment schedules consecutively enrolled for up to 12 months. Change in HbA1c and HbA1c target attainment between pre- and post-index measurements were primary study aims. Secondary study aims included changes in bodyweight and kidney function tests, as well as analysis of interactions between HbA1c and predictors of responsiveness to OW semaglutide treatment.
Methods
Patients
This is a single-center, retrospective, observational cohort study conducted at the Endocrinology and Diabetology outpatient clinic, Ospedale Maggiore della Carità, Novara, Italy. The study included patients with an established diagnosis of T2D, who underwent OW semaglutide treatment between September 2019 and May 2021 and were followed over 12 months. The study was notified to the Ethics Committee and performed in accordance with the current legislation on Observational Studies and the Declaration of Helsinki. Patients provided their written consent to process personal data, and the publication of the study results. For this study, patients previously diagnosed with T2D aged ≥ 18 years who were on a standard-care treatment regimen with oral antidiabetic drug (OAD) or insulin therapy were considered eligible for OW semaglutide if not on target with standard OAD, i.e. HbA1c ≥ 7% (≥ 53 mmol/mol), or if any of the following inclusion criteria was accomplished: HbA1c < 7% but intolerance towards ≥ 1 OAD other than GLP-1RA; previous CVE or high/very high CVR according to ESC 2019 guidelines [13]; estimated glomerular filtration rate (eGFR) between ≥ 15 and ≤ 30 ml/min/1.73 m2; BMI ≥ 35 kg/m2. Patients were not excluded if on other GLP-1 RA or dipeptidyl peptidase 4 inhibitors (DPP4i), which were then replaced by OW semaglutide upon enrollment. According to regulatory restrictions at the time of the study, gliflozins and short-acting insulins were withdrawn at study entry. Exclusion criteria included gestational diabetes mellitus and T1DM, previous or current diagnosis/family history of medullary thyroid carcinoma or multiple endocrine neoplasia, incomplete anthropometric and/or biochemical data at baseline and during follow-up visits. Patients were excluded from the study if showing poor compliance to OW semaglutide (fear of daily subcutaneous injection, elderly people unable to self-administer the drug, people suffering from senile dementia and/or neurodegenerative diseases) or if they were suffering from ESRD requiring dialysis or kidney disease unrelated to diabetes or hypertension.
Study measures
Before enrollment, all patients were on stable OAD or insulin treatment for at least 3 months. At baseline, data collected for analysis included clinical history, antidiabetic drug history, anthropometric and biochemical parameters. Study measures included BMI, glucose, HbA1c, lipids, liver function tests, serum creatinine and microalbuminuria. Height was measured by the Harpenden stadiometer to the nearest mm with the subject head in Frankfurt plane and weight using electronic scale both taken in triplicate. Averaged BMI was calculated as body weight divided by squared height (kg/m2). The estimated glomerular filtrate rate (eGFR) was calculated with the MDRD [22] and CKD-EPI equation [23], yet kidney function was analyzed according to eGFR estimated by CKD-EPI, because MDRD equation has a low accuracy for an eGFR > 90 ml/min/1.73 m2 [22].
During treatment, sample data were evaluated as a whole and after dichotomization by categoric (gender, insulin therapy, previous CV events) and continuous variables (age, disease duration, baseline HbA1c, baseline BMI, baseline eGFR). Further, stratification by kidney function was performed according to eGFR CDK-EPI categories (< 60 ml/min, ≥ 60–89 ml/min, ≥ 90 ml/min).
Plasma glucose levels (mg/dl; 1 mg/dl: 0,05551 mmol/l) were measured by the gluco-oxidase colorimetric method (GLUCOFIX, by Menarini Diagnostici, Florence, Italy). Routine laboratory data included total cholesterol, high-density and low-density lipoprotein cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transpeptidase, measured by enzymatic methods (Roche Diagnostics, Mannheim, Germany). HbA1c levels were measured by high-performance liquid chromatography (HPLC), using a Variant machine (Biorad, Hercules, CA); intra- and inter-assay coefficients of variation are, respectively, lower than 0.6 and 1.6%. Linearity is excellent from 3.2 (11 mmol/mol) to 18.3% (177 mmol/mol). Serum creatinine levels were assessed with the enzymatic method of creatinine deamidase/GLDH (Advia Chemistry-Bayer).
Safety data were collected during the study period at each time visit.
Statistical analysis
Data obtained from computerized medical record system in use at Italian diabetes centers (Smart Digital Clinic, Meteda, Rome, Italy) were used for the analysis. Data are expressed as mean ± standard error of the mean (SEM), and as absolute or percent delta variations compared to baseline. Distributions of continuous variables were examined for skewness and were logarithmically transformed as appropriate. Differences between values obtained at baseline and during the follow-up were calculated by two-tailed unpaired t-test or repeated measures ANOVA. Multivariate analysis of variance (MANOVA) was used to test the statistical significance of the effect of one or more independent variables including HbA1c variation, sex, age and duration of disease within the eGFR CDK-EPI categories (dependent variable). Correlations analyses were calculated with Pearson’s coefficient. Analysis of repeated measures was used to determine differences in subjects before and after treatment with OW semaglutide. As covariates, we assessed the effect of gender, age, BMI, disease duration, presence of diabetic complication/s, use of insulin, previous CV events, number of drugs for hypertension, use of ACE-inhibitors, angiotensin receptor blockers (ARBs), or diuretics (yes/no). A stepwise regression was used to determine the association of delta variations between HbA1c and study covariates, so as to define independent predictors of HbA1c response to OW semaglutide. Statistical significance was assumed at p < 0.05. The statistical analysis was performed with SPSS for Windows V.21.0 (SPSS Inc., Chicago, IL, USA).
Results
Study population and baseline clinical characteristics
The original cohort of T2D patients receiving at least one prescription of OW semaglutide comprised 271 people, in 258 of whom a complete dataset allowed baseline analysis (Table 1 and Fig. 1). At study entry, 19 patients (7.3%) were naïve to any antidiabetic treatment, while OW semaglutide was added to 1 OAD in 136 (52.7%), to 2 OADs in 73 (28.2%), to 3 OADs in 11 cases (4.2%). Most but not all patients (84.5%) were GLP-1RA naïve. Biguanides (79.1%), followed by SGLT2i (20.1%), sulphonylureas (14.3%) and DPP4i (5.8%) were the non-insulin antidiabetic medications used in this cohort. Before OW semaglutide, metformin was used as single treatment in 74 cases (28.7%); long-acting insulin was overall used in 100 cases (38.7%) and as single treatment in 19 cases (7.3%).
Table 1.
Demographic, anthropometric and metabolic variables in the population as a whole and after stratification by gender
| Variables | Overall population | Males | Females | p-value |
|---|---|---|---|---|
| Sample (%) | 258 | 151 (58.5) | 107 (41.5) | 0.2 |
| Age (years) | 60.4 ± 0.5 | 61.0 ± 0.6 | 59.5 ± 0.9 | 0.2 |
| Disease duration (years) | 8.6 ± 0.4 | 8.9 ± 0.6 | 8.2 ± 0.7 | 0.4 |
| Weight (kg) | 92.5 ± 1.1 | 94.7 ± 1.3 | 89.4 ± 1.8 | 0.2 |
| BMI (kg/m2) | 32.7 ± 0.4 | 31.3 ± 0.4 | 34.7 ± 0.7 | < 0.0001 |
| Systolic blood pressure (mmHg) | 145 ± 1.2 | 145.8 ± 1.7 | 144.8 ± 1.8 | 0.7 |
| Diastolic blood pressure (mmHg) | 86.0 ± 0.6 | 86.7 ± 0.8 | 84.5 ± 0.9 | 0.1 |
| HbA1c (%) | 8.0 ± 0.6 | 8.0 ± 0.7 | 8.0 ± 0.7 | 0.2 |
| Glucose (mg/dL) | 163.7 ± 3.3 | 163.3 ± 3.8 | 164.2 ± 6.1 | 0.9 |
| Microalbuminuria (mg/L) | 30.4 ± 3.7 | 38.2 ± 5.6 | 19.3 ± 3.3 | 0.05 |
| Creatinine (mg/dL) | 0.9 ± 0.3 | 1.00 ± 0.02 | 0.72 ± 0.02 | 0.0001 |
| eGFR (mL/min) | 82.2 ± 1.9 | 68.3 ± 1.51 | 102 ± 3.3 | 0.0001 |
| Total CHO (mg/dL) | 173.0 ± 2.4 | 165.2 ± 3.1 | 183.7 ± 3.4 | 0.0001 |
| LDL CHO (mg/dL) | 91.0 ± 2.1 | 86.8 ± 2.7 | 96.8 ± 3.4 | 0.02 |
| HDL CHO (mg/dL) | 48.0 ± 1.2 | 44.7 ± 1.4 | 53.6 ± 1.8 | 0.0001 |
| TG (mg/dL) | 167.0 ± 5.4 | 170.5 ± 7.8 | 162.7 ± 6.9 | 0.9 |
| AST (UI/L) | 24.0 ± 0.6 | 24.6 ± 0.7 | 22.4 ± 0.9 | 0.3 |
| ALT (UI/L) | 30.5 ± 1.2 | 32.5 ± 1.2 | 27.7 ± 1.9 | 0.04 |
| GGT (UI/L) | 41.0 ± 2.4 | 44.1 ± 2.4 | 36.3 ± 3.4 | 0.1 |
| Urate (mg/dL) | 5.5 ± 0.12 | 5.8 ± 0.1 | 5.2 ± 0.2 | 0.02 |
| Antidiabetic therapy | ||||
| Metformin | 204 (79.1) | 121 (80.1) | 83 (77.6) | 0.6 |
| Sulfonylureas | 37 (14.3) | 17 (11.3) | 20 (18.7) | 0.09 |
| DPP4-i | 15 (5.8) | 11 (7.3) | 4 (3.7) | 0.2 |
| SGLT2-i | 52 (20.1) | 36 (23.8) | 16 (15.0) | 0.08 |
| Insulin | 101 (39.1) | 62 (41.1) | 39 (36.4) | 0.4 |
| Diabetes complications | ||||
| Nephropathy | 22 (8.5) | 18 (11.9) | 4 (3.7) | 0.02 |
| Retinopathy | 12 (4.7) | 6 (4.0) | 6 (5.6) | 0.5 |
| Stroke | 6 (2.3) | 3 (2.0) | 3 (2.8) | 0.7 |
| CHD | 30 (11.6) | 26 (17.2) | 4 (3.7) | 0.0009 |
| Peripheral arterial disease | 30 (11.6) | 21 (13.9) | 9 (8.4) | 0.2 |
| Neuropathy | 8 (3.1) | 6 (4.0) | 2 (1.9) | 0.3 |
| Concomitant therapies | ||||
| Anti-hypertensive drugs | 169 (65.5) | 101 (66.9) | 68 (63.6) | 0.6 |
| Lipid lowering drugs | 142 (55.0) | 86 (57.0) | 56 (52.3) | 0.5 |
| Cardiovascular events | 35 (13.6) | 27 (17.9) | 8 (7.5) | 0.02 |
Data are shown as mean ± SEM. P value is shown for gender-based analysis. Significant differences are shown in bold characters
BMI body mass index, CHO cholesterol, TG triglycerides, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyl transferase, DPP4-i dipeptidyl peptidase-4 inhibitor, SGLT2-i sodium-glucose cotransporter-2 inhibitors, CHD coronary heart disease
Fig. 1.
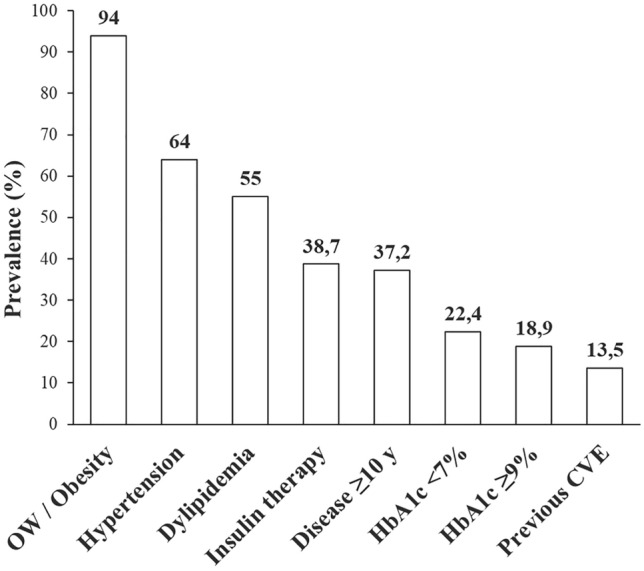
Baseline clinical features of TD2 patients included in the study. OW overweight, CVD cardiovascular disease
Kidney function, as assessed by eGFR CDK-EPI, was ≤ 30 ml/min in 1 case, > 30–59 ml/min in 62 (24.1%), ≥ 60–89 ml/min in 115 (44.4%) and ≥ 90 ml/min in 80 patients (30.9%).
At baseline, there were no differences in the tested variables between genders but for BMI, this being unexpectedly higher in females than males, as well as microalbuminuria, creatinine, lipids, ALT and urate levels (Table 1).
Outcomes of OW semaglutide treatment
Of the 258 consecutive patients, 154 completed the 6-month and 56 completed the 12-month follow-up. The samples scrutinized at the different timepoints hence reflected the patients consecutively enrolled until the study end with no dropouts. Following the standard dose-escalation protocol [16], OW semaglutide was given at the prefixed dose of 0.5 mg weekly in all cases for the first 6 months, and 1.0 mg weekly in 21.8% of the 12-month completers.
Improvements in glycemic control were significant in terms of HbA1c and glucose levels reduction (p < 0.0001 for both) both at 6 and 12 months (Table 2). HbA1c levels decreased by − 1.02 ± 0.1% after 6 months and − 1.1 ± 0.1% after 12 months of OW semaglutide. Target HbA1c values < 7% were achieved in 61% of 6-month completers and 57% of 12-month completers. The residual number of cases with HbA1c ≥ 9% by the study-end was low (5.3% vs 18.9% at baseline). After 6 months, HbA1c response to treatment was non-significantly different between GLP-1RA naïve patients compared to those previously exposed to any GLP-1RA (− 1.1 ± 0.1 vs − 0.64 ± 0.2%), while the study sample was too small for comparison at 12 months. Compared to study entry, the proportion of insulin users slightly decreased by 6 months (56 vs 53 cases: withdrawn in 10, newly added in 7, and continued in 46 cases) and 12 months (19 vs 16 cases: withdrawn in 6, newly added in 3, and continued in 13 cases).
Table 2.
Demographic, anthropometric and metabolic variables in the T2D population during OW semaglutide treatment
| Variables | Basal (N = 258) | 6 months (N = 154) | 12 months (N = 56) | Repeated measures ANOVA |
|---|---|---|---|---|
| HbA1c (%) | 8.0 ± 0.6 | 6.9 ± 0.1$ | 6.9 ± 0.1$ | < 0.0001 |
| Glucose (mg/dL) | 163.7 ± 3.3 | 130.2 ± 3.1$ | 128.8 ± 4.3$ | < 0.0001 |
| Weight (kg) | 92.5 ± 1.1 | 89.9 ± 1.5$ | 87.2 ± 2.5$ | 0.001 |
| BMI (kg/m2) | 32.7 ± 0.4 | 31.9 ± 0.5$ | 30.9 ± 0.8$ | < 0.0001 |
| Total CHO (mg/dL) | 173.0 ± 2.4 | 150.2 ± 3.4$ | 160.5 ± 6.6 | 0.09 |
| LDL CHO (mg/dL) | 91.0 ± 2.1 | 77.4 ± 2.9$ | 85.4 ± 55.2 | 0.2 |
| HDL CHO (mg/dL) | 48.0 ± 1.2 | 44.1 ± 1.2ϕ | 50.5 ± 2.7 | 0.4 |
| TG (mg/dL) | 167.0 ± 5.4 | 139.0 ± 5.9θ | 134.0 ± 7.5 | 0.4 |
| Microalbuminuria (mg/L) | 30.4 ± 3.7 | 22.2 ± 4.3 | 15.5 ± 1.88 | 0.08 |
| Creatinine (mg/dL) | 0.86 ± 0.3 | 0.85 ± 0.02 | 0.86 ± 0.03 | 0.6 |
| eGFR (mL/min) | 82.2 ± 1.9 | 80.7 ± 2.4 | 81.5 ± 4.2 | 0.2 |
| AST (UI/L) | 24.0 ± 1.3 | 21.9 ± 1.5 | 25.7 ± 2.7 | 0.6 |
| ALT (UI/L) | 30.5 ± 1.2 | 25.5 ± 1.3 | 25.7 ± 2.7 | 0.3 |
| GGT (UI/L) | 41.0 ± 2.4 | 28.2 ± 2.2$ | 27.9 ± 4.5ϕ | 0.06 |
| Urate (mg/dL) | 6.0 ± 0.12 | 5.4 ± 0.3 | 5.3 ± 0.3 | 0.4 |
| SBP (mmHg) | 145.0 ± 1.2 | 141.5 ± 1.7θ | 143.1 ± 2.3θ | 0.013 |
| DBP (mmHg) | 86.0 ± 0.6 | 83.8 ± 1.1 | 83.5 ± 1.1 | 0.1 |
Data are shown as mean ± SEM. The comparative analysis was performed at 6 and 12 months vs baseline using the corresponding index cases
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, CHO cholesterol, TG triglycerides, AST aspartate aminotransferase, ALT alanine aminotransferase, GGT gamma-glutamyl transferase
θp < 0.05; ϕp < 0.01; $p < 0.001 vs baseline. Significant differences are shown in bold characters
OW semaglutide treatment determined weight loss in 73.5% of patients after 6 months (ranging between − 0.5 and − 19 kg) and 78.1% after 12 months (ranging between − 0.2 and − 26 kg). The overall reduction in bodyweight was significant both in terms of body mass and BMI values (Table 2). Compared to baseline, weight loss was ≥ 5% in 21.2% and 25.4% of cases after 6 and 12 months, respectively, and ≥ 10% in 6.8% and 18.2% of cases after 6 and 12 months of OW semaglutide treatment, respectively.
The lipid profile improved during the first 6 months of treatment in terms of total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides when compared to baseline (Table 2). We did not record changes in kidney function, both in terms of eGFR and microalbuminuria. Likewise, no change was recorded in any liver function tests but for a reduction in GGT values (Table 2).
Subgroup analyses
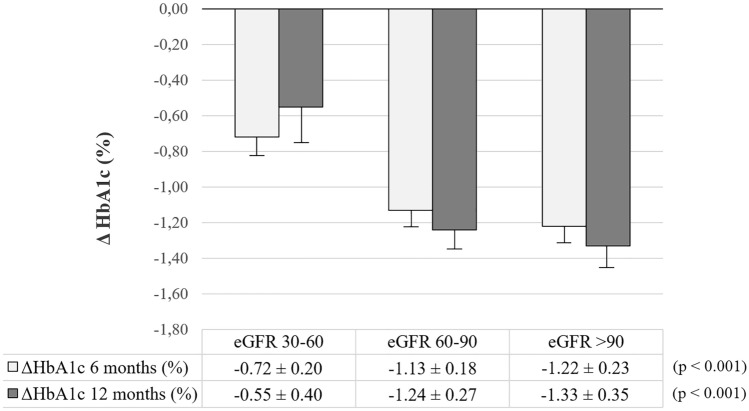
Subgroup analyses were conducted to test HbA1c responsiveness to OW semaglutide according to age, gender, baseline HbA1c and BMI, insulin, complications and previous CVE. We found no divergence in HbA1c response across subcategories except for patients with higher baseline HbA1c after 6 and 12 months, patients with higher disease duration after 6 months, and patients with previous CVE as compared to lower-end counterparts (Table 3). Stratification of patients according to the GFR CDK-EPI categories highlighted a significantly greater HbA1c response to OW semaglutide along with improving kidney filtration (Fig. 2). However, this difference in HbA1c response was lost after controlling for age, sex, BMI and duration of disease.
Table 3.
HbA1c response (%) to OW semaglutide in subgroups
| Variables | OW semaglutide | |||
|---|---|---|---|---|
| 6 months | 12 months | |||
| Bottom | Top | Bottom | Top | |
| Age (median, 61 y) | − 1.2 ± 0.2 | − 0.9 ± 0.1 | − 1.0 ± 0.3 | − 1.1 ± 0.2 |
| Gender (0, females; 1, males) | − 1.1 ± − 1.0 | − 1.0 ± 0.1 | − 1.3 ± 0.3 | − 1.0 ± 0.2 |
| Disease duration (median, 8.1 y) | − 1.3 ± 0.2 | − 0.8 ± 0.1θ | − 1.2 ± 0.3 | − 1.0 ± 0.2 |
| HbA1c (median, 7.9%) | − 0.4 ± 0.1 | − 1.8 ± 0.2$ | − 0.2 ± 0.2 | − 2.2 ± 0.2$ |
| BMI (median, 31.8 kg/m2) | − 0.8 ± 0.1 | − 1.2 ± 0.2 | − 1.1 ± 0.2 | − 1.1 ± 0.3 |
| Insulin treatment (0, no; 1, yes) | − 1.2 ± 0.1 | − 0.8 ± 0.2 | − 1.3 ± 0.2 | − 0.7 ± 0.3 |
| Complications (0, no; 1, yes) | − 1.2 ± 0.1 | − 0.9 ± 0.5 | − 1.2 ± 0.2 | − 0.5 ± 0.6 |
| Previous CVE (0, no; 1, yes) | − 1.1 ± 0.1 | − 0.4 ± 0.3θ | − 1.2 ± 0.2 | − 0.3 ± 0.3θ |
θp < 0.05; $p < 0.001 top vs bottom end. Significant differences are shown in bold characters
BMI body mass index, CVE cardiovascular event
Fig. 2.
Changes in HbA1c levels after 6 and 12 months of treatment with OW semaglutide in T2D patients stratified by glomerular filtration rate (eGFR CDK-EPI) uncontrolled for age, gender and disease duration. For a description of the results of the multivariate-controlled analysis see the text
Correlation and multivariate regression analyses
A wide number of associations were tested to assess correlates of HbA1c response to OW semaglutide. It should be noted that signs of association reflected deltas or absolute variables. Delta HbA1c response to OW semaglutide was well correlated with baseline HbA1c levels both after 6 and 12 months of the study (r = − 0.65 and r = − 0.72, respectively; p < 0.0001 for both) (Fig. 3A). Conversely, an association was observed between delta HbA1c and BMI variations only after 6 months (r = 0.38, p < 0.0001) (Fig. 3B). In turn, BMI deltas correlated with baseline BMIs after 6 months (r = − 0.22, p = 0.01) and after 12 months (r = − 0.31, p = 0.02) (Fig. 3C). At both time-points, associations related delta HbA1c to percent variations in blood glucose (r = 0.55 and r = 0.67; p < 0.0001 for both), triglycerides (r = 0.29, p = 0.002; and r = 0.45, p < 0.0001), and GGT levels (r = 0.33, p = 0.002; and r = 0.36, p < 0.05). Associations were only significant after 6 months between delta HbA1c and disease duration (r = 0.22, p < 0.05) as well as ALT variation (r = 0.43, p < 0.0001).
Fig. 3.
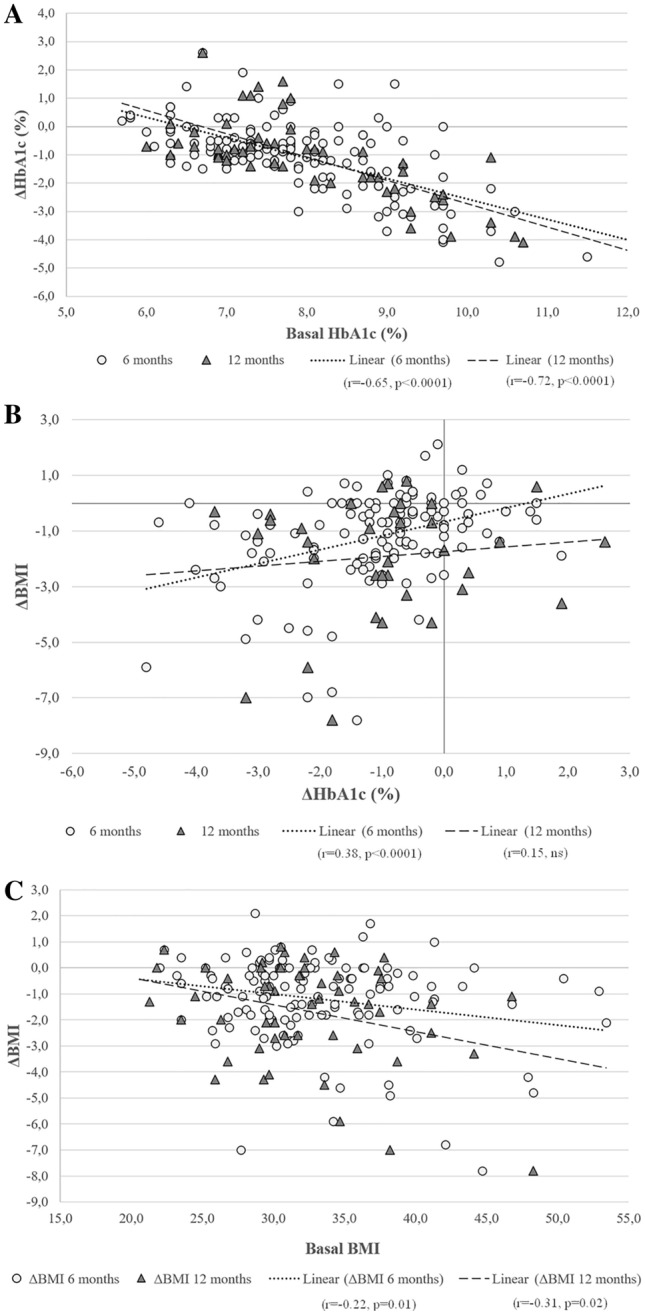
Correlation analyses between baseline HbA1c values and delta HbA1c reduction after 6 and 12 months of treatment with OW semaglutide (panel A); baseline BMI values and delta BMI reduction after 6 and 12 months of treatment with OW semaglutide (panel B); delta HbA1c and delta BMI variations after 6 and 12 months of treatment with OW semaglutide (panel C). Individual correlation coefficients and significance are reported in the figure
At stepwise multivariable regression analysis (Table 4), the most significant predictors of delta HbA1c variations after 6 months of OW semaglutide treatment included baseline HbA1c values (β = − 0.59, p < 0.0001), delta BMI variation (β = 0.24, p < 0.0001) and disease duration (β = 0.22, p < 0.001). The adjusted R2 for the model was 0.52.
Table 4.
Stepwise multivariable regression analysis showing independent predictors of delta HbA1c variations after 6 months of OW semaglutide treatment
| Dependent variables | Model | Independent variables | Unstandardized coefficients | Standardized coefficient | t | p-value | |
|---|---|---|---|---|---|---|---|
| B | SE | Beta | |||||
| ΔHbA1c after 6 months | 1 | Constant | 4.57 | 0.62 | – | 7.41 | < 0.0001 |
| Basal HbA1c | − 0.71 | 0.08 | − 0.64 | − 9.21 | < 0.0001 | ||
| 2 | Constant | 4.39 | 0.58 | − | 7.54 | < 0.0001 | |
| Basal HbA1c | − 0.65 | 0.07 | − 0.59 | − 8.89 | < 0.0001 | ||
| ΔBMI after 6 months | 0.21 | 0.05 | 0.27 | 4.09 | < 0.0001 | ||
| 3 | Constant | 3.97 | 0.57 | − | 6.94 | < 0.0001 | |
| Basal HbA1c | − 0.65 | 0.07 | − 0.59 | − 9.22 | < 0.0001 | ||
| ΔBMI after 6 months | 0.19 | 0.05 | 0.25 | 3.81 | < 0.0001 | ||
| Duration of disease | 0.04 | 0.11 | 0.22 | 3.41 | 0.001 | ||
| ΔHbA1c after 12 months | 1 | Constant | 5.55 | 0.89 | – | 6.22 | < 0.0001 |
| Basal HbA1c | − 0.83 | 0.11 | − 0.72 | − 7.53 | < 0.0001 | ||
Excluded variables: model 1: basal BMI, ΔBMI, age, duration of disease, sex, basal eGFR, complications, previous CVE; model 2: basal BMI, age, duration of disease, sex, basal eGFR, complications, previous CVE; model 3: basal BMI, age, sex, basal eGFR, complications, previous CVE
BMI body mass index, CVE cardiovascular event
At 12 months, baseline HbA1c levels were the only predictors of delta HbA1c variation (β = − 0.72, p < 0.0001) (Table 4), with an adjusted R2 for the model of 0.51.
Safety data
A total of 39 out of 258 patients (15.1%) receiving at least one dose of OW semaglutide withdrew from treatment. OW semaglutide was discontinued in 29 cases (11.2%) for gastrointestinal intolerance occurring within few weeks since therapy commencement (nausea and vomiting in 16, diarrhea or constipation in 7, abdominal cramps in 2 and malaise in 1 patient), while treatment was discontinued due to therapeutic inefficacy in 9 patients and pregnancy in 1 case. In 3 patients (0.1%), OW semaglutide was withdrawn for poor compliance. In 9 more patients, dose increment from 0.5 mg to 1.0 mg weekly was constrained due to gastrointestinal intolerance occurring during dose titration. However, gastrointestinal side effects did not appear to contribute to the reductions in HbA1c and/or body weight.
Discussion
RWE studies on GLP-1RAs have provided extensive evidence that OW semaglutide reduces HbA1c and body weight irrespective of previous GLP-1 RA use, with potentially higher persistence than other GLP-1 RAs [24–43]. Our results show that OW semaglutide induced significant reduction in mean HbA1c independent of age, gender, insulin therapy, prior history of GLP-1RA use and diabetic complications. Baseline HbA1c levels, disease duration and previous CVE appeared to influence HbA1c responsiveness to OW semaglutide. Our study also adds evidence that HbA1c response only initially reflects a legacy involving bodyweight changes, and hints at a potential regulatory role of kidney function on OW semaglutide effectiveness.
GLP‐1 analogs are recommended as add‐on therapy to metformin and other OADs and as first injectable therapy in preference to insulin [44, 45]. Among different GLP-1RA options, semaglutide occupies a prominent place in treating T2D patients at high CVD and DKD risk [46, 47]. Data from clinical trials highlighted the effectiveness and potency of OW semaglutide even in comparison to other same-class agonists [48–50]. Confirming the vast interest aroused by semaglutide in the treatment of diabetes and obesity, an oral formulation semaglutide has been recently introduced [51], which is particularly useful for patients who dislike injections, and major medicines regulatory agencies have recently granted approval for the use of OW semaglutide in the treatment of obesity [52].
The patient population herein scheduled to receive OW semaglutide was moderately aged, averagely complicated, and predominantly obese. Our results show that OW semaglutide was associated with clinically and statistically significant reduction in mean HbA1c, regardless of age, gender, insulin therapy, prior history of GLP-1RA use and diabetic complications. HbA1c decreased on average by 1.0–1.1%, and the magnitude of response was greater in patients with higher baseline HbA1c levels. Target HbA1c values < 7% were achieved in 61% and 57% of cases after 6 and 12 months, respectively. Our results seem comparable to those of other RWE studies. While the SUSTAIN studies reported a mean HbA1c reduction from baseline of 1.0–1.5% and HbA1c < 7% in 68.7–82.8% of cases after 6–12 months of treatment [53], recent retrospective observational studies documented a mean HbA1c reduction of 0.8–1.2% from baseline, with rates of patients achieving HbA1c < 7% ranging between 48.6–53% [24, 26]. Further, the number of patients with HbA1c persisting at ≥ 9% values by the end of treatment was 5.3%, a marginal figure that is comparable to rates recorded in registry-based studies [24, 26].
In addition to its anti-hyperglycemic effects, OW semaglutide outstands for its ability to reduce body weight thus aiding the global metabolic control. As a proof of this, we observed significant changes in body weight, with an average reduction of 3.1 kg after 6 months and 4.3 kg after 12 months as compared to baseline. Collectively, weight loss was ≥ 5% in 21 and 25.4% after 6 months and was ≥ 10% in 6.8 and 18.2% of cases after 12 months of treatment. Recent RWE have shown similar effects on weight [25, 32]. It is worth noting that a strong association related the variation between HbA1c and weight after 6 but not at the 12 months follow-up. On one hand, this implies a key role for OW semaglutide in controlling the whole metabolic spectrum while, on the other, it seems to disentangle the anti-hyperglycemic and weight-reducing effects of OW semaglutide over time.
Based on previous data from our group [54], a goal of our study was also the evaluation of renal function in terms of changes in eGFR and microalbuminuria. Current data showed no effect of OW semaglutide on these parameters, although a response trend emerged for microalbuminuria. Others reported similar results [25, 32] and post-hoc analyses suggested that OW semaglutide can initially decrease eGFR while promoting reductions in UACR [55]. While a conservative analysis of data stratified by kidney function hinted at a potential enhancing effect of kidney filtration rate on HbA1c response after 6 and 12 months, significance was lost when age, gender and disease duration were controlled for. We are therefore inclined to speculate on the significance of better kidney health as a function of younger age, gender difference, shorter duration of disease and overall more preserved β-cell function. As such, older patients with a long history of disease and previously exposed to insulin-secretagogues may show lower response to drugs other than insulin [56]. The open question remains if semaglutide can promote recovery of damaged β-cell function in case of long-term use of insulin-secretagogues, which may delay needs for insulin.
In insulin users, initiation of OW semaglutide led to a reduction in HbA1c and body as significant and comparable as in non-insulin users. Like another RWE analysis [57], we recorded a marginal insulin discontinuation rate. Yet, the insulin withdrawal rate was greater the newly add-on rate by the study end, suggesting the support provided by OW semaglutide in insulin de-intensification. It has been previously shown that patients on OADs are significantly more likely to reach target HbA1c and lose body weight when treatment is intensified with GLP-1 RA as compared to either OADs or insulin [53, 58–60]. An analysis capturing costs, mortality, and quality of life found similar results [61]. Together, these data confirm guidelines recommendations [45] to consider GLP1-RA therapy as an injective alternative to insulin therapy in patients not achieving treatment targets, allowing extended metabolic control associated with cardio-protection and weight loss, without the risks associated with hypoglycemia and possible weight gain deriving from the use of insulin.
Semaglutide was generally well-tolerated, though a lower proportion of persons experiencing side effects (18%) compared to the clinical studies were noted and this was predominated by gastrointestinal side effects, such as nausea, diarrhea or constipation, and abdominal cramps. No patient was admitted to hospital due to pancreatitis or for any other reason. The figure reported here is higher than that seen in the SUSTAIN-6 trial (13.1%) and some RWE studies [29, 32], while others reported rates similar to us [26]. Our study was not designed to assess hypoglycemic episodes, so we cannot draw conclusions on this issue.
This study presents limitations that are typical of retrospective observational studies, such as the lack of comparators and randomization, the access to utilization, and cost of health resources. Moreover, the low number of patients at the 12-month follow-up may hamper the conclusions of our analysis. It should also be acknowledged that the 1.0 mg dose was prescribed to only 22% of cases, implying that up-titration was incomplete in most cases possibly due to the choice of clinicians, concomitant therapies of the population sample, and underlying intentions to limit occurrence of side effects. Nevertheless, the effect of treatment on HbA1c and weight was similar to that reported in most RWE studies [24–31]. Points of potential strength of the study are constituted by the wide characterization of our cohort including 102 different variables and the broad representativeness of our patient population. Hence, this study sheds light on the impact of the different clinical and disease characteristics of T2D patients have on the efficacy and safety of OW semaglutide, which can help clinical decision‐making and individualization of treatment. Further real-world studies to evaluate semaglutide adherence and acceptability would be important and of clinical interest.
Acknowledgements
The authors wish to thank the nursing staff of the outpatient clinic for their assistance. Funding by Novo Nordisk Italy for the editing support of Polystudium SrL Milano is kindly acknowledged.
Funding
Open access funding provided by Università degli Studi del Piemonte Orientale Amedeo Avogrado within the CRUI-CARE Agreement. Unrestricted financial support provided by Novo Nordisk for manuscript draft preparation.
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the local Ethical Committee of University Hospital “Maggiore della Carità”, Novara (Italy). The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained from all participants.
Footnotes
The original online version of this article was revised to add missing OASIS funding note.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Brandt SJ, Götz A, Tschöp MH, Müller TD. Gut hormone polyagonists for the treatment of type 2 diabetes. Peptides. 2018;100:190–201. doi: 10.1016/j.peptides.2017.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolen-Doerr E, Stockman MC, Rizo I. Mechanism of glucagon-like peptide 1 improvements in type 2 diabetes mellitus and obesity. Curr Obes Rep. 2019;8:284–291. doi: 10.1007/s13679-019-00350-4. [DOI] [PubMed] [Google Scholar]
- 4.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB, LEADER Steering Committee; LEADER Trial Investigators Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T, SUSTAIN-6 Investigators Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa160714. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riesmeyer JS, Riddle MC, Rydén L, Xavier D, Atisso CM, Dyal L, Hall S, Rao-Melacini P, Wong G, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw JE, Sheu WH, Temelkova-Kurktschiev T, REWIND Investigators Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 7.Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, Jeppesen OK, Lingvay I, Mosenzon O, Pedersen SD, Tack CJ, Thomsen M, Vilsbøll T, Warren ML, Bain SC, PIONEER 6 Investigators Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 8.Iacobellis G, Baroni MG. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J Endocrinol Invest. 2022;45:489–495. doi: 10.1007/s40618-021-01687-1. [DOI] [PubMed] [Google Scholar]
- 9.Vitale M, Haxhi J, Cirrito T, Pugliese G. Renal protection with glucagon-like peptide-1 receptor agonists. Curr Opin Pharmacol. 2020;54:91–101. doi: 10.1016/j.coph.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 11.Kristensen SL, Rørth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Køber L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7:776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 12.Rangaswami J, Bhalla V, de Boer IH, Staruschenko A, Sharp JA, Singh RR, Lo KB, Tuttle K, Vaduganathan M, Ventura H, McCullough PA, American Heart Association Council on the Kidney in Cardiovascular Disease; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Lifestyle and Cardiometabolic Health Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: A scientific statement from the American Heart Association. Circulation. 2020;142:e265–e286. doi: 10.1161/CIR.0000000000000920. [DOI] [PubMed] [Google Scholar]
- 13.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa-Uva M, Valensi P, Wheeler DC, ESC Scientific Document Group 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323. doi: 10.1093/eurheartj/ehz486. [DOI] [PubMed] [Google Scholar]
- 15.Lau J, Bloch P, Schäffer L, Pettersson I, Spetzler J, Kofoed J, Madsen K, Knudsen LB, McGuire J, Steensgaard DB, Strauss HM, Gram DX, Knudsen SM, Nielsen FS, Thygesen P, Reedtz-Runge S, Kruse T. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem. 2015;58:7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 16.European Medicines Agency (EMA) (2018) Ozempic – Semaglutide. Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf. Accessed 24 June 2021
- 17.Tilinca MC, Tiuca RA, Niculas C, Varga A, Tilea I. Future perspectives in diabesity treatment: semaglutide, a glucagon-like peptide 1 receptor agonist (Review) Exp Ther Med. 2021;22:1167. doi: 10.3892/etm.2021.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aroda VR, Capehorn MS, Chaykin L, Frias JP, Lausvig NL, Macura S, Lüdemann J, Madsbad S, Rosenstock J, Tabak O, Tadayon S, Bain SC. Impact of baseline characteristics and beta-cell function on the efficacy and safety of subcutaneous once-weekly semaglutide: a patient-level, pooled analysis of the SUSTAIN 1–5 trials. Diabetes Obes Metab. 2020;22:303–314. doi: 10.1111/dom.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan X, Cao X, Zhou M, Zou P, Hu J. Efficacy and safety of once-weekly semaglutide for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2017;26:1083–1089. doi: 10.1080/13543784.2017.1360274. [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Holst AG, Vilsbøll T. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2017;376:891–892. doi: 10.1056/NEJMc1615712. [DOI] [PubMed] [Google Scholar]
- 21.de Lusignan S, Crawford L, Munro N. Creating and using real-world evidence to answer questions about clinical effectiveness. J Innov Health Inform. 2015;22:368–373. doi: 10.14236/jhi.v22i3.177. [DOI] [PubMed] [Google Scholar]
- 22.Florkowski CM, Chew-Harris JS. Methods of estimating GFR—different equations including CKD-EPI. Clin Biochem Rev. 2011;32:75–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visaria J, Uzoigwe C, Swift C, Dang-Tan T, Paprocki Y, Willey VJ. Real-world effectiveness of once-weekly semaglutide from a US commercially insured and Medicare advantage population. Clin Ther. 2021;43:808–821. doi: 10.1016/j.clinthera.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Brown RE, Bech PG, Aronson R. Semaglutide once weekly in people with type 2 diabetes: real-world analysis of the Canadian LMC diabetes registry (SPARE study) Diabetes Obes Metab. 2020;22:2013–2020. doi: 10.1111/dom.14117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansen KB, Svendstrup M, Lund A, Knop FK, Vilsbøll T, Vestergaard H. Once-weekly subcutaneous semaglutide treatment for persons with type 2 diabetes: real-world data from a diabetes out-patient clinic. Diabet Med. 2021;38:e14655. doi: 10.1111/dme.14655. [DOI] [PubMed] [Google Scholar]
- 27.Crabtree TSJ, Bickerton A, Sennik DK, Rohilla A, Sivappriyan S, Barnes D, Cull ML, Gallen IW, Adamson K, Ryder RE (2021) The Association of British Clinical Diabetologists (ABCD) United Kingdom Nationwide Semaglutide Audit. American Diabetes Association® (ADA) 81st Scientific Sessions, Poster 686
- 28.Lingvay I, Kirk AR, Lophaven S, Wolden ML, Shubrook JH. Outcomes in GLP-1 RA-experienced patients switching to once-weekly semaglutide in a real-world setting: The Retrospective, Observational EXPERT Study. Diabetes Ther. 2021;12:879–896. doi: 10.1007/s13300-021-01010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jain AB, Kanters S, Khurana R, Kissock J, Severin N, Stafford SG. Real-world effectiveness analysis of switching from liraglutide or dulaglutide to semaglutide in patients with type 2 diabetes mellitus: the retrospective REALISE-DM Study. Diabetes Ther. 2021;12:527–536. doi: 10.1007/s13300-020-00984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hepprich M, Zillig D, Florian-Reynoso MA, Donath MY, Rudofsky G. Switch-to-semaglutide study (STS-Study): a retrospective cohort study. Diabetes Ther. 2021;12:943–954. doi: 10.1007/s13300-021-01016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goncalves E, Bell DS. Efficacy of semaglutide versus liraglutide in clinical practice. Diabetes Metab. 2020;46:515–517. doi: 10.1016/j.diabet.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Williams DM, Ruslan AM, Khan R, Vijayasingam D, Iqbal F, Shaikh A, Lim J, Chudleigh R, Peter R, Udiawar M, Bain SC, Stephens JW, Min T. Real-world clinical experience of semaglutide in secondary care diabetes: a retrospective observational study. Diabetes Ther. 2021;12:801–811. doi: 10.1007/s13300-021-01015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tofé S, Argüelles I, Mena E, Serra G, Codina M, Urgelés JR, García H, Pereg V. An observational study evaluating effectiveness and therapeutic adherence in patients with Type 2 Diabetes initiating dulaglutide vs subcutaneous semaglutide in Spain. Endocrine and Metabolic Science. 2021;2:100082. doi: 10.1016/j.endmts.2021.100082. [DOI] [Google Scholar]
- 34.Holmes P, Bell HE, Bozkurt K, Catarig AM, Clark A, Machell A, Sathyapalan T. Real-world use of once-weekly semaglutide in type 2 diabetes: results from the SURE UK multicentre, prospective, observational study. Diabetes Ther. 2021;12:2891–2905. doi: 10.1007/s13300-021-01141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrer-García JC, Albalat Galera R, Arribas L, Tolosa Torrens M, Sánchez Lorente A, Portilla AJ, Artero A, Sánchez-Juan C (2020) Receptor agonist in type 2 diabetes: a study to evaluate real-world effectiveness. American Diabetes Association® (ADA) 80th Scientific Sessions, 69 (Suppl 1), Abstract 947-P
- 36.Tong J, Jain A, Stafford S. Comparative analysis of A1c and weight reduction potential of subcutaneous GLP-1 receptor agonists in a real-world setting. CJD. 2020;44:S23–S24. doi: 10.1016/j.jcjd.2020.08.061. [DOI] [Google Scholar]
- 37.Garcia de Lucas MD, Pérez-Belmonte LM, Aviles B, Jimenez AI, Fernandez JM, Rivas Ruiz F (2021) Semaglutide Achieves Better Metabolic and Weight Control than Other GLP-1 RA in Real Life after 12 Months of Follow-up. American Diabetes Association® (ADA) 81st Scientific Sessions, Poster 676
- 38.Balcazar CM, Zambrano J, Garcia AF, Casanova ME. Semaglutide once weekly in persons with type 2 diabetes: real-world analysis of the Colombian Diabetes Registry (COL-REAL 1 Study) Metab Clin Exp. 2021;116:154640. doi: 10.1016/j.metabol.2020.154640. [DOI] [Google Scholar]
- 39.Cárdenas-Salas JJ, Sierra R, Luca BL, Sanchez B, Modroño N, Casado C, Sanchez NM, Cruces E, Vazquez C (2021) Semaglutide in patients with type 2 diabetes: real-world data from Spain. American Diabetes Association® (ADA) 81st Scientific Sessions, Poster 690
- 40.Rudofsky G, Catarig AM, Favre L, Grau K, Häfliger S, Thomann R, Schultes B. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res Clin Pract. 2021;178:108931. doi: 10.1016/j.diabres.2021.108931. [DOI] [PubMed] [Google Scholar]
- 41.Yale JF, Catarig AM, Grau K, Harris S, Klimek-Abercrombie A, Rabasa-Lhoret R, Reardon L, Woo V, Liutkus J. Use of once-weekly semaglutide in patients with type 2 diabetes in routine clinical practice: Results from the SURE Canada multicentre, prospective, observational study. Diabetes Obes Metab. 2021;23:2269–2278. doi: 10.1111/dom.14468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uzoigwe C, Liang Y, Whitmire S, Paprocki Y. Semaglutide once-weekly persistence and adherence versus other GLP-1 RAs in patients with type 2 diabetes in a US real-world setting. Diabetes Ther. 2021;12:1475–1489. doi: 10.1007/s13300-021-01053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Hayek AA, Al Dawish MA. Evaluation of patient-reported satisfaction and clinical efficacy of once-weekly semaglutide in patients with type 2 diabetes: an ambispective study. Adv Ther. 2022 doi: 10.1007/s12325-022-02053-0. [DOI] [PubMed] [Google Scholar]
- 44.Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. Diabetes Care. 2022;45:S125–S143. doi: 10.2337/dc22-S009. [DOI] [PubMed] [Google Scholar]
- 46.Honigberg MC, Chang LS, McGuire DK, Plutzky J, Aroda VR, Vaduganathan M. Use of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease: a review. JAMA Cardiol. 2020;5:1182–1190. doi: 10.1001/jamacardio.2020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner H, Hamdy O. Oral GLP1 analog: where does the tide go? Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420984130. doi: 10.1177/1179551420984130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and safety of once-weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): a 56-week, open-label, randomized clinical trial. Diabetes Care. 2018;41:258–266. doi: 10.2337/dc17-0417. [DOI] [PubMed] [Google Scholar]
- 49.Pratley RE, Aroda VR, Lingvay I, Lüdemann J, Andreassen C, Navarria A, Viljoen A, SUSTAIN 7 Investigators Semaglutide versus dulaglutide once weekly in patients with type 2 diabetes (SUSTAIN 7): a randomised, open-label, phase 3b trial. Lancet Diabetes Endocrinol. 2018;6:275–286. doi: 10.1016/S2213-8587(18)30024-X. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Wen X, Duan W, Wang X, Chen Y, Dong J, Yang Z, Fang J, Zhou Z, Yao G, Fang Y, Huang Y. DR10601, a novel recombinant long-acting dual glucagon-like peptide-1 and glucagon receptor agonist for the treatment of obesity and type 2 diabetes mellitus. J Endocrinol Invest. 2020;43:653–662. doi: 10.1007/s40618-019-01153-z. [DOI] [PubMed] [Google Scholar]
- 51.Anderson SL, Beutel TR, Trujillo JM. Oral semaglutide in type 2 diabetes. J Diabetes Complications. 2020;34:107520. doi: 10.1016/j.jdiacomp.2019.107520. [DOI] [PubMed] [Google Scholar]
- 52.Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, Lingvay I, Thomsen M, Wadden TA, Wharton S, Wilding JPH, Rubino D. Semaglutide 2.4 mg for the treatment of obesity: key elements of the STEP Trials 1 to 5. Obesity (Silver Spring) 2020;28:1050–1061. doi: 10.1002/oby.22794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeSouza C, Cariou B, Garg S, Lausvig N, Navarria A, Fonseca V. Efficacy and safety of semaglutide for type 2 diabetes by race and ethnicity: a post hoc analysis of the SUSTAIN trials. J Clin Endocrinol Metab. 2020;105:72. doi: 10.1210/clinem/dgz072. [DOI] [PubMed] [Google Scholar]
- 54.Zavattaro M, Caputo M, Samà MT, Mele C, Chasseur L, Marzullo P, Pagano L, Mauri MG, Ponziani MC, Aimaretti G, Prodam F. One-year treatment with liraglutide improved renal function in patients with type 2 diabetes: a pilot prospective study. Endocrine. 2015;50:620–626. doi: 10.1007/s12020-014-0519-0. [DOI] [PubMed] [Google Scholar]
- 55.Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, Rossing P, Seufert J, Tadayon S, Vilsbøll T. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1–7 randomised controlled trials. Lancet Diabetes Endocrinol. 2020;28:880–893. doi: 10.1016/S2213-8587(20)30313-2. [DOI] [PubMed] [Google Scholar]
- 56.Cook MN, Girman CJ, Stein PP, Alexander CM, Holman RR. Glycemic control continues to deteriorate after sulfonylureas are added to metformin among patients with type 2 diabetes. Diabetes Care. 2005;28:995–1000. doi: 10.2337/diacare.28.5.995. [DOI] [PubMed] [Google Scholar]
- 57.Rajamand Ekberg N, Bodholdt U, Catarig AM, Catrina SB, Grau K, Holmberg CN, Klanger B, Knudsen ST. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: Results from the SURE Denmark/Sweden multicentre, prospective, observational study. Prim Care Diabetes. 2021;15:871–878. doi: 10.1016/j.pcd.2021.06.008. [DOI] [PubMed] [Google Scholar]
- 58.Pineda ED, Liao IC, Godley PJ, Michel JB, Rascati KL. Cardiovascular outcomes among patients with type 2 diabetes newly initiated on sodium-glucose cotransporter-2 inhibitors, glucagon-like peptide-1 receptor agonists, and other antidiabetic medications. J Manag Care Spec Pharm. 2020;26:610–618. doi: 10.18553/jmcp.2020.26.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aroda VR, Bain SC, Cariou B, Piletič M, Rose L, Axelsen M, Rowe E, DeVries JH. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol. 2017;5:355–366. doi: 10.1016/S2213-8587(17)30085-2. [DOI] [PubMed] [Google Scholar]
- 60.Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, Araki E, Chu PL, Wijayasinghe N, Norwood P. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018;103:2291–2301. doi: 10.1210/jc.2018-00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunt B, Malkin SJP, Moes RGJ, Huisman EL, Vandebrouck T, Wolffenbuttel BHR. Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ Open Diabetes Res Care. 2019;7:e000705. doi: 10.1136/bmjdrc-2019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.