Abstract
The temperature-driven adaptation of the bacterial community in peat was studied, by altering temperature to simulate self-heating and a subsequent return to mesophilic conditions. The technique used consisted of extracting the bacterial community from peat using homogenization-centrifugation and measuring the rates of thymidine (TdR) or leucine (Leu) incorporation by the extracted bacterial community at different temperatures. Increasing the peat incubation temperature from 25°C to 35, 45, or 55°C resulted in a selection of bacterial communities whose optimum temperatures for activity correlated to the peat incubation temperatures. Although TdR and Leu incorporations were significantly correlated, the Leu/TdR incorporation ratios were affected by temperature. Higher Leu/TdR incorporation ratios were found at higher temperatures of incubation of the extracted bacterial community. Higher Leu/TdR incorporation ratios were also found for bacteria in peat samples incubated at higher temperatures. The reappearance of the mesophilic community and disappearance of the thermophilic community when the incubation temperature of the peat was shifted down were monitored by measuring TdR incorporation at 55°C (thermophilic activity) and 25°C (mesophilic activity). Shifting the peat incubation temperature from 55 to 25°C resulted in a recovery of the mesophilic activity, with a subsequent disappearance of the thermophilic activity. The availability of substrate for bacterial growth varied over time and among different peat samples. To avoid confounding effects of substrate availability, a temperature adaptation index was calculated. This index consisted of the log10 ratio of TdR incorporation at 55 and 25°C. The temperature index decreased linearly with time, indicating that no thermophilic activity would be detected by the TdR technique 1 month after the temperature downshift. There were no differences between the slopes of the temperature adaptation indices over time for peat samples incubated at 55°C 3 or 11 days before incubation at 25°C. Thus, different levels of bacterial activity did not affect the temperature-driven adaptation of the bacterial community.
Peat is a common substrate used for plant cultivation. After peat is harvested, it is usually collected into stockpiles, which sometimes during the storage period can undergo self-heating. This results not only in substantial losses of material (losses of up to 50% of the substance have been reported [20]) but also in a peat product that may inhibit seed germination and plant growth (23, 38).
From a microbiological point of view, self-heating and the subsequent cooling period can be considered two phases with extremely different selection pressures, the first selecting for a more thermophilic community and the second selecting for a mesophilic community when the temperature drops. The usual approach to studying such transition phenomena has been to use agar plates incubated at different temperatures, e.g., at around room temperature to indicate the mesophilic community and at a higher temperature to indicate the thermophilic one (for examples, see references 22 and 37). Although this method may have given some insights into changes in the microbial community due to temperature changes, there are several problems associated with plate counts. Besides the well-known problem regarding how representative plate counts are of the total community, it is also a quite laborious method, and one has to wait several weeks in order to maximize counts (21). Even more problematic is the fact that plate counts do not indicate only active organisms. One cannot be certain whether a colony emanates from an active bacterial cell or from a dormant cell or from a spore. This is especially problematic after a heating period, since thermophilic organisms, at least those found in compost, often belong to the genus Bacillus (9, 34, 35). Spores of Bacillus can survive for a long time, long after the conditions permissive for growth have ended.
One way of avoiding the problem with plate counts was tested by McKinley and Vestal (25), who used short-term [14C]-acetate incorporation into microbial lipids of compost samples incubated at different temperatures to assess the extent of thermophilicity of the microbial community. This ensured that only active organisms, capable of synthesizing lipids, were included in the measurements. However, the technique was rather laborious, since lipids had to be extracted before measurements, and furthermore, it was not possible to separate the fungal and bacterial parts of the community. The latter problem also holds for the 14C-labeled glutamate mineralization technique used by Derikx et al. (11) to study the temperature dependence of a phase I compost.
Díaz-Raviña et al. (16) studied the relationship between temperature and bacterial activity in soil, using thymidine (TdR) and leucine (Leu) incorporation. Bacteria were extracted from the soil by a combination of homogenization and low-speed centrifugation. Incorporation rates of TdR and leucine were then assessed at several temperatures during a short period of time. This was a fast and simple way to study bacterial community response to changes in temperature. In water habitats, this method has been used several times to study temperature control of bacterial growth (for examples, see references 24, 32, 33, and 36). Modifications of this technique have also been used to indicate bacterial community response to changes in pH (5, 6, 7, 29) or metal concentration (3, 8, 10, 13, 15, 28).
The objectives of this study were to determine if the TdR incorporation technique could be used to monitor temperature-driven adaptations of bacterial communities in peat and to compare the TdR and leucine incorporation techniques in this respect. Furthermore, we wanted to examine the rates of changes in the development of bacterial communities at different temperatures in order to compare the effects due not only to increased or decreased temperature but also to different time periods of incubation at high temperatures before temperature decreases. Self-heating was therefore simulated by incubating peat samples at 55°C, and the reestablishment of the mesophilic community after self-heating was studied by incubation of the heated peat samples at 25°C. Growths of thermophilic and mesophilic communities were then assessed using the TdR incorporation technique.
MATERIALS AND METHODS
Peat samples and chemical measurements.
The peat samples came from different Norwegian mires which were being exploited for horticultural use. All peat samples were from stockpiles on the mires and were taken 4 to 12 months after harvesting. The peat samples were either naturally self-heated (above 35°C) or not exposed to self-heating. Peat samples incubated in the laboratory studies had not previously been exposed to self-heating.
pH was measured in a peat-water suspension (1:1.5, vol/vol). Dissolved organic carbon (DOC) was measured in the water extract from the pH measurement. The C/N ratio was determined after dry combustion. Organic matter (OM) content was calculated after combustion at 550°C for 24 h. NO3-N and NH4-N levels were measured after extraction in a 2 M KCl solution. Background data for the peat samples incubated in the laboratory are presented in Table 1. Data for the other peat samples (naturally self-heated or not) are not shown.
TABLE 1.
Description of peat samples
| Mire location | Production method | pHa | C/N | DOCab | % OM | NO3-Nab | NH4-Nab |
|---|---|---|---|---|---|---|---|
| Bliksrud | Harrowed and vacuumed | NDc | ND | ND | 98 | ND | ND |
| Gjφdalsmosan | Block cut and milled | 4.2 (3.8) | 45 | 4,170 (13,200) | 99 | 17 (19) | 180 (445) |
| Grenimosan | Harrowed and vacuumed | 4.5 (3.9) | 51 | 3,300 (9,180) | 95 | 13 (16) | 211 (388) |
| Killingmo | Harrowed and vacuumed | 4.4 (4.0) | 53 | 3,160 (4,710) | 97 | 25 (21) | 100 (200) |
| Komnes | Harrowed and vacuumed | 4.9 (4.9) | 61 | 2,020 (6,450) | 99 | 4 (26) | 243 (312) |
Values in parentheses are for samples after incubation at 55°C.
Values are in milligrams per kilograms of dry matter.
ND, not determined.
Extraction of bacteria from peat.
Bacteria were extracted from peat using the method of Fægri et al. (18) with the modification by Bååth (2) which we adapted for peat samples. Briefly, 1 g of peat was homogenized with 20 ml of Ultra Pure Milli Q Water for 1 min at 80% of maximum speed in a Sorvall Omnimixer. The peat suspension was centrifuged at 1,000 × g for 10 min. The supernatant was poured through a small amount of glass wool to get rid of floating organic particles, and the resulting bacterial suspension was divided into 2-ml portions.
TdR and leucine incorporation.
The incorporation levels of labeled TdR and Leu were determined simultaneously on subsamples of the same bacterial suspension using the method of Bååth (2, 4). The extent of isotope dilution and the distribution of radioactivity among different macromolecules were not measured. The bacterial suspensions obtained from the extractions were incubated in water baths at their respective incubation temperatures for about 10 min before the labeled substrates were added. [methyl-3H]TdR (925 GBq mmol−1) and l-[U-14C]leucine (11.9 GBq mmol−1) were added to 2 ml of the bacterial suspensions to final concentrations of 100 and 395 nM, respectively. The bacterial suspensions were then incubated at 3, 15, 25, 31, 45, 55, and 64°C for 26, 9, 3, 2, 1, 1, and 1 h, respectively. In some instances, temperatures of 5 instead of 3°C and 35 instead of 31°C were used. Incubation was stopped by adding 1 ml of 5% formalin. To determine the level of nonspecific labeling, controls in which formalin was added together with the labeled substrate were included in each temperature assay. Filtration, washing of filters, and scintillation counting were done as described by Díaz-Raviña et al. (16).
Experiments.
In an initial experiment, three peat samples (from Bliksrud, Killingmo, and Komnes, Norway) were incubated at 25, 35, 45, and 55°C. Ultra Pure Milli Q Water was added to increase the water content of the peat to 80% before the incubation. Bacteria were extracted after 72 h, and TdR and Leu incorporation levels were measured at different temperatures as described above.
We also tested the effect of an initial incubation of peat at 55°C for 3 days followed by a shift to 25, 15, or 5°C for 7 days. Substrate incorporation of the bacterial suspensions was then measured as described above. Two peat samples (from Killingmo and Grenimosan) were used.
In the main experiment, three peat samples (from Gjødalsmosan, Killingmo, and Komnes, adjusted to 80% water content) were incubated at 55°C for about 1 month to study the development of thermophilic bacteria. After 3 and 11 days at 55°C, samples were shifted to 25°C to simulate the cooling phase, thus introducing a selection pressure favoring mesophilic bacteria. This was done in order to compare the rates of reestablishment of a mesophilic community after a short period and a long period at elevated temperatures. The incubation temperature of the peat was reached within 1 h. At each sampling time point, bacteria were extracted and levels of incorporation of TdR at 25 and 55°C were measured. During the experiment, the peat samples were kept in autoclave bags and weighted to maintain a constant weight and avoid changes in moisture content.
In an additional test, 32 different peat samples, about half of which had been naturally self-heated to between 35 and 55°C 6 to 12 months before, were tested for bacterial activity (TdR and Leu incorporation) at 25 and 55°C. These peat samples were also characterized by pH and DOC content as described above (data not shown).
Statistics.
The basic replication unit, used to calculate standard errors, was one peat sample from one stockpile from different mires. Usually two or three different peat samples were used for each experiment. However, in the main experiment only one peat sample was measured at the last time point (34 days) for samples incubated at 25°C. These data were therefore not used in further calculations.
RESULTS
Initial experiments.
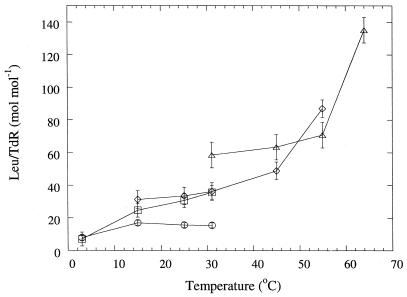
The TdR and Leu incorporation rates at different bacterial suspension incubation temperatures from peat incubated for 72 h at 25, 35, 45, or 55°C are shown in Fig. 1. Leu and TdR incorporation rates were correlated to each other (r2 = 0.78, n = 76 for all samples from the three peats). Except for peat incubated at 25°C, the highest incorporation rates were always achieved when the bacterial suspension incubation temperature corresponded with the peat incubation temperature.
FIG. 1.
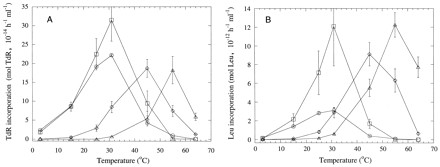
Mean TdR and Leu incorporation rates of bacterial communities in peat samples from Bliksrud, Killingmo, and Komnes incubated for 72 h at different temperatures. The x axis shows the incubation temperature of the extracted bacterial suspensions during incorporation of labeled substrate. Different symbols denote the incubation temperatures of peat before measurements as follows: ○, 25°C; □, 35°C; ◊, 45°C; and ▵, 55°C. Bars indicate standard errors (n = 3).
Despite the good correlation between TdR and Leu incorporation rates, there were differences in the ratios of Leu incorporation to TdR incorporation at different temperatures (Fig. 2). Only data points for which the activity was significantly higher than that of controls were included. Increasing the temperature increased the ratio of the Leu/TdR incorporation. This was found both when comparing the ratios at different peat incubation temperatures, where the lowest ratio was found for bacteria extracted from peat incubated at 25°C and the highest was found for bacteria from peat incubated at 55°C, and when comparing the ratios at different bacterial suspension incubation temperatures. Bacteria from peat incubated at 25 and 35°C had a nearly constant ratio of Leu/TdR incorporation at a bacterial suspension incubation temperature of 15 to 31°C, but the ratio was significantly (P < 0.01) lower at 3°C. At the two highest peat incubation temperatures, a significant (P < 0.05) increase in the ratio was observed at the highest bacterial suspension incubation temperature.
FIG. 2.
Leu/TdR incorporation ratios of bacterial communities in peat samples incubated for 72 h at different temperatures. The ratios are mean values of samples from Bliksrud, Killingmo, and Komnes. The x axis shows the incubation temperature of the extracted bacterial suspensions during incorporation of labeled substrate. Different symbols denote the incubation temperatures of peat before measurements as follows: ○, 25°C; □, 35°C; ◊, 45°C; and ▵, 55°C. Bars indicate standard errors (n = 3) from separate analyses of variance for each peat incubation temperature.
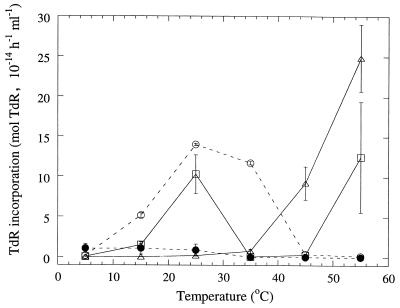
The incubation temperature of the peat samples after a period at 55°C had a large impact on the temperature dependency of the resulting bacterial communities (Fig. 3). As a comparison, the temperature dependency curves for the initial community in peat incubated at 25 and at 55°C for 5 days are given. The last two treatments had results very similar to those obtained before (Fig. 1). Incubating peat at 25°C for 7 days after heating at 55°C resulted in two different communities: a mesophilic community with optimum bacterial suspension incorporation rates at 25°C and a thermophilic community with highest activity at 55°C. Incubating the peat samples at 15°C destroyed all thermophilic activity and made the recovery of mesophilic activity much slower than with the 25°C peat incubation. The optimum temperature for the bacterial suspension incorporation rate was also lower with the 15°C treatment than with the 25°C treatment. Shifting the peat sample incubation temperature from 55 to 5°C destroyed all thermophilic activity and slowed the recovery of mesophilic activity to the point that no activity at all could be detected at any bacterial suspension incubation temperature after 7 days. Only TdR incorporation rates are given, since effects on Leu incorporation rates were similar.
FIG. 3.
Mean TdR incorporation rates of bacterial communities in peat samples from Killingmo and Grenimosan. The x axis shows the incubation temperature of the extracted bacterial suspensions during incorporation of labeled substrate. ▵, peat incubated at 55°C for 5 days; ○, peat incubated at 25°C; □ and ●, peat incubated at 55°C for 3 days and then at 25 and 15°C, respectively, for 7 days. Bars indicate standard errors (n = 2).
Main experiment.
The initial experiments (Fig. 1 and 3) showed that peat incubated at 25°C had virtually no activity at a bacterial suspension incubation temperature of 55°C, indicating that the initial mesophilic bacterial community was inactive at 55°C. On the other hand, peat incubated at 55°C for 72 h had very little activity at 25°C. Thus, in the main experiment we used a 25°C incubation temperature for the extracted bacterial suspensions as an indicator of mesophilic activity and a 55°C incubation temperature as an indicator of thermophilic activity.
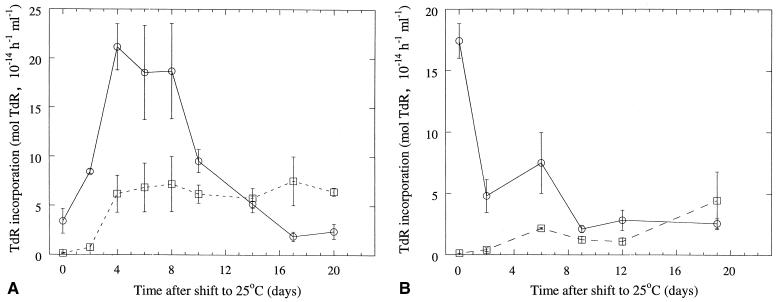
Shifting the peat incubation temperature from 25 to 55°C at day zero resulted in all bacterial activity disappearing, both mesophilic and thermophilic. Then the thermophilic activity increased to maximum values after 6 to 10 days and then slowly decreased. The bacterial community reached maximum activity values comparable to the initial activity values in unheated peat samples and stabilized at values about half of these initial activity values after about 15 days at 55°C. The mesophilic activity remained close to zero during the entire peat incubation period at 55°C.
The mesophilic activity recovered slowly after the peat incubation temperature was shifted to 25°C (after 3 days at 55°C, as shown in Fig. 4A). After 2 days the activity was still low, but then the mesophilic activity increased to maximum values about 7 days after the shift in peat incubation temperature. This maximum activity was lower than both the initial activity in unheated peat and the maximum activity in peat incubated at 55°C throughout the experiment. The thermophilic activity was measured at the same time as the mesophilic activity (Fig. 4A). The thermophilic activity increased for about 4 days, reaching levels similar to those of the corresponding thermophilic activity of the peat samples incubated at 55°C throughout. Then the thermophilic activity decreased, and about 14 days after the shift from 55 to 25°C, the mesophilic activity became higher than the thermophilic one.
FIG. 4.
Mean TdR incorporation rates of bacterial communities in peat incubated for 3 days (A) and 11 days (B) at 55°C and then at 25°C. Time zero is the time of transfer of the peat samples to 25°C. □, activity at 25°C (mesophilic activity at the corresponding bacterial suspension temperature); ○, activity at 55°C (thermophilic activity at the corresponding bacterial suspension temperature). Bars indicate standard errors (n = 3).
When the peat incubation temperature was shifted to 25°C after 11 days at 55°C, the mesophilic activity also recovered slowly (Fig. 4B) but never reached the activity levels found after only 3 days at 55°C (Fig. 4A). The thermophilic activity also decreased with this treatment, and after about 14 days the mesophilic activity became higher than the thermophilic one.
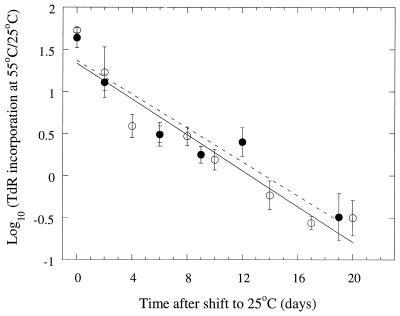
Because of the fresh substrate released by heat killing of the microorganisms in the peat due to the incubation at 55°C, the bacterial activity varied much over time. Thus, to be able to distinguish the selective effect of temperature from that of varying substrate availability, a temperature adaptation index was calculated (Fig. 5). This index consisted of the log10 ratio of TdR incorporation levels of the bacterial suspensions incubated at 55 and 25°C. A high value thus indicates a mainly thermophilic community, and a low value indicate a mainly mesophilic one. After the peat incubation temperature was shifted from 55 to 25°C, the temperature adaptation index decreased linearly with time for all three peat samples, with no differences in the slopes. There were no significant differences in the slopes of the indices for the peat samples incubated for 3 and 11 days at 55°C before the peat incubation temperature was altered to 25°C (Fig. 5). This indicates that the length of exposure time at 55°C did not affect the rates of reappearance of the mesophilic community and disappearance of the thermophilic one.
FIG. 5.
Temperature adaptation index (the log10 ratio of TdR incorporation measured at 55 and 25°C) of bacterial communities in peat incubated for 3 days (open circles and solid line) and 11 days (filled circles and dashed-line) at 55°C and then at 25°C. Time zero is the time of transfer of the peat samples to 25°C. Bars indicate standard errors (n = 3).
Self-heated peat.
Leu and TdR incorporation rates measured at 25°C for the additional 32 peat samples were correlated to each other (r2 = 0.66, P < 0.001). The variations in the actual incorporation values were mainly due to pH, since peat samples with a pH below 3.2 had very low bacterial activities. Excluding these samples, there was a weak (P < 0.05) correlation between bacterial activity and DOC concentration. No bacterial activity was found at a bacterial suspension incubation temperature of 55°C, whether the peat samples had been self-heated previously or not.
DISCUSSION
Temperature relationships of single isolates, as well as of bacterial communities, do not usually show patterns of symmetry around the optimum temperature for growth. This asymmetry bears two characteristics. First, increasing the temperature above optimum decreases activity much faster than does decreasing the temperature below optimum. Second, and more important in the present study, increasing the temperature above the temperature permissive for growth will kill the organisms, while decreasing the temperature to just below the permissive temperature will make the organisms only temporarily inactive. They may immediately become active if the temperature is raised again. When the peat incubation temperature was increased above optimum for the initial mesophilic community (from 25 to 55°C [Fig. 1 and 3]), mesophilic activity was destroyed and did not recover for the whole period during which the peat samples were incubated at 55°C. On the other hand, decreasing the incubation temperature of the peat samples from 55 to 25°C did not immediately decrease the thermophilic activity to zero (Fig. 4). Thus, temperature differences of 30°C might be believed to exert similar levels of selection pressure whether they are increases or decreases. This was not the case, however, and instead the direction of temperature alteration was found to be of utmost importance.
Another characteristic of the temperature asymmetry is seen in Fig. 3, which shows that an increased temperature of incubation of the peat samples resulted in temperature relation curves with only one optimum, while a decrease in the peat incubation temperature resulted in curves with two optima. Temperature relationship curves with two optima were found to be frequent in a seasonal study of bacteria in a temperate lake, where the lower optimum was closer to the actual temperature of the lake (33). This was interpreted as resulting from the presence of two different communities with different temperature relationships, the one with a higher temperature optimum being considered made up of survivors from earlier higher temperature conditions. Our study also indicated that one reason for temperature relation curves with two optima is that the community with the higher optimum survives under nongrowth conditions.
Although bringing the temperature slightly below the minimum required for growth did not necessarily kill the organisms, lowering it further away from the temperature range permissive for growth appeared to have lethal effects. A shift from a peat incubation temperature of 55 to 15 or 5°C rather than to 25°C destroyed the thermophilic activity completely (Fig. 3). Also, communities with different temperature relationships appeared to be selected for, with optimum activity close to the peat incubation temperature (15 or 25°C).
The thermophilic community showed maximum activity levels after only 3 to 7 days at a peat incubation temperature of 55°C. Thermophilic bacteria are known to appear quickly, at least when grown on agar plates (1, 22, 26). However, the actual peak activity levels were similar to the initial activity levels in the unheated peat. Regarding the mesophilic activity taking place after the peat incubation temperature was shifted from 55 to 25°C, it never became as high as in the unheated peat. This was despite the fact that the heat treatment would kill most of the microorganisms, thus releasing energy and nutrient resources for the survivors. In the present study, DOC increased by a factor of 1.5 to 3.2 with the 55°C treatment (Table 1). Autoclaving and fumigation, which kill soil organisms, are known to result, after reinoculation, in peak activities that are much higher than in nontreated soils (30, 31). It has also been found that in an agricultural soil, autoclaving and recolonization resulted in TdR and Leu incorporation rates 5 to 20 times higher than preautoclaving values after 1 week (12). One explanation for the relatively low peak TdR incorporation rates in heated peat is that toxic substances were produced during the heating of the peat. It is well known that heating or burning of soil produces compounds toxic to the microbial community (17, 19, 39). Although the temperature used here was lower than in these studies, heat together with the high OM content of the peat may have resulted in enough toxic compounds being released to affect TdR incorporation by the bacteria. Peat, which undergoes self-heating, is known to produce compounds toxic to plants, and the toxicity increases with increasing temperatures (38).
Although TdR and Leu incorporation rates were well correlated to each other at the different peat and bacterial suspension incubation temperatures (compare Fig. 1A and B), lowering the bacterial suspension incubation temperature affected Leu incorporation more than TdR incorporation (Fig. 2). This was reflected in a lower Leu/TdR incorporation ratio at low bacterial suspension incubation temperatures. This phenomenon was reported first by Díaz-Raviña et al. (16) for soil bacterial communities and later by Tibbles (36) for different aquatic bacterial communities and pure cultures of bacteria. In the present study, these observations were extended to cover not only mesophilic but also thermophilic communities, indicating that the variation in the Leu/TdR incorporation ratio as a function of temperature is a common trait. The reason for this temperature-dependent variation is unclear, but altered community composition, unbalanced growth, different nonspecific labeling of macromolecules, and substrate limitation were ruled out as explanations by previous studies (16, 36).
The Leu/TdR incorporation ratio was affected not only by the bacterial suspension incubation temperature but also by the incubation temperature used for the peat from which the bacterial community was extracted (Fig. 2). The higher the peat incubation temperature, the higher was the Leu/TdR incorporation ratio of the bacterial community. In this case, different abilities of different communities to incorporate Leu or TdR cannot be ruled out as the cause of this, since different communities did exist in peat incubated at 25°C and, for example, 55°C. Large shifts in the Leu/TdR incorporation ratio were also found in soils contaminated with large amounts of heavy metals (14) and appeared to be related to changes in the microbial community composition.
One unexpected result was the increase in the thermophilic activity (measured at a bacterial suspension incubation temperature of 55°C) during the 4 days following the transition of the peat samples to 25°C after 3 days at 55°C (Fig. 4A). We have no explanation for this. It might be that some activity of the thermophilic community was still possible at 25°C and that the excess of carbon and nutrients present in the beginning of the experiment (note the increase in DOC after heating [Table 1]) allowed increased thermophilic activity.
No differences between the rates of adaptation to mesophilic conditions, irrespective of the amount of time the peat samples were incubated at 55°C (3 or 11 days), were observed when the temperature adaptation indices were compared (Fig. 5). This was despite the fact that the peat that was shifted to 25°C after 3 days at 55°C had a higher activity during the next 20 days at the new temperature than did the peat shifted after 11 days. Thus, the total activity, and presumably the turnover rate of the bacterial community, did not affect the rate of adaptation to the new temperature regimen in our study. This is in contrast to the rate of heavy metal adaptation of a bacterial community, which was slower when the activity was decreased by decreasing the temperature (13). This difference could of course be due to the types of selection pressures, i.e., temperature and heavy metals, affecting the bacterial community differently in this respect.
The temperature adaptation index (Fig. 5) was a fast and sensitive way of estimating rates of changes in the bacterial community, without confounding factors like different TdR incorporation rates during the experiment due to changing energy and nutrient availability. Nedwell and Floodgate (27) used a similar temperature adaptation index with CFU counted on agar plates incubated at 10 and 20°C to show seasonal temperature adaptation of sediment bacterial communities. However, the TdR technique is much faster than plate count techniques, since the assay can easily be performed within 1 day. The variations between samples are also lower with the TdR method, since TdR incorporation is measured for more than 107 bacteria, while usually not more than 100 CFU can be counted on one agar plate.
The linear decrease in the temperature adaptation index (Fig. 5) indicates that it would be difficult to detect any thermophilic activity in self-heated peat after a fairly short period of time after the temperature has returned to more mesophilic conditions. A value of around −1.7 is the lowest possible value for the temperature adaptation index (using the lowest values measurable above zero for the thermophilic activity). Extrapolating from the graph (Fig. 5), this value is reached after about 1 month, indicating that at that time no thermophilic activity will remain. This is consistent with the fact that no thermophilic activity was detected in the peat samples, which had undergone natural self-heating 6 to 12 months earlier. A temperature lower than 25°C after the self-heating phase could also be a reason for a fast disappearance of the thermophilic activity in these samples (Fig. 3). Thus, the use of the TdR incorporation technique to monitor the development of mesophilic and thermophilic communities is a method that detects present or fairly recent activity without including dormant or dead organisms reflecting past events.
ACKNOWLEDGMENTS
This study was supported by grants to E.B. from the Swedish Natural Science Research Council. S.B.R. was financially supported by the Jiffy Pro. Ltd., NGF, and Norwegian Research Council project no. 103.195.
We thank Katarina H. Söderberg for assistance at the laboratory.
REFERENCES
- 1.Allen S D, Brock T D. The adaptation of heterotrophic microcosms to different temperatures. Ecology. 1968;49:343–346. [Google Scholar]
- 2.Bååth E. Thymidine incorporation into macromolecules of bacteria extracted from soil by homogenization-centrifugation. Soil Biol Biochem. 1992;24:1157–1165. [Google Scholar]
- 3.Bååth E. Measurement of heavy metal tolerance of soil bacteria using thymidine incorporation into bacteria extracted after homogenization-centrifugation. Soil Biol Biochem. 1992;24:1167–1172. [Google Scholar]
- 4.Bååth E. Measurement of protein synthesis of soil bacterial assemblages using the leucine incorporation technique. Biol Fertil Soils. 1994;17:147–153. [Google Scholar]
- 5.Bååth E. Adaptation of soil bacterial communities to prevailing pH in different soil. FEMS Microbiol Ecol. 1996;19:227–237. [Google Scholar]
- 6.Bååth E, Frostegård Å, Fritze H. Soil bacterial biomass, activity, phospholipid fatty acid pattern, and pH tolerance in an area polluted with alkaline dust deposition. Appl Environ Microbiol. 1992;58:4026–4031. doi: 10.1128/aem.58.12.4026-4031.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bååth E, Arnebrant K. Growth rate and response of bacterial communities to pH in limed and ash treated forest soils. Soil Biol Biochem. 1994;26:995–1001. [Google Scholar]
- 8.Bååth E, Díaz-Raviña M, Frostegård Å, Campbell C D. Effect of metal-rich sludge amendments on the soil microbial community. Appl Environ Microbiol. 1998;64:238–245. doi: 10.1128/aem.64.1.238-245.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beffa T, Blanc M, Aragno M. Obligately and facultatively autotrophic, sulfur- and hydrogen-oxidizing thermophilic bacteria isolated from hot composts. Arch Microbiol. 1996;165:34–40. [Google Scholar]
- 10.Dahlin S, Witter E, Mårtensson A, Turner A, Bååth E. Where's the limit? Changes in the microbiological properties of agricultural soils at low levels of metal contamination. Soil Biol Biochem. 1997;29:1405–1415. [Google Scholar]
- 11.Derikx P J L, Op den Camp H J M, van der Drift C, van Griensven L J L D, Vogels G D. Biomass and biological activity during the production of compost used as a substrate in mushroom cultivation. Appl Environ Microbiol. 1990;56:3029–3034. doi: 10.1128/aem.56.10.3029-3034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Raviña M, Bååth E. Response of soil bacterial communities pre-exposed to different metals and re-inoculated in an unpolluted soil. Soil Biol Biochem. 2001;33:241–248. [Google Scholar]
- 13.Díaz-Raviña M, Bååth E. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl Environ Microbiol. 1996;62:2970–2977. doi: 10.1128/aem.62.8.2970-2977.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Raviña M, Bååth E. Thymidine and leucine incorporation into bacteria from soils experimentally contaminated with heavy metals. Appl Soil Ecol. 1996;3:225–234. [Google Scholar]
- 15.Díaz-Raviña M, Bååth E, Frostegård Å. Multiple heavy metal tolerance of soil bacterial communities and its measurement by a thymidine incorporation technique. Appl Environ Microbiol. 1994;60:2238–2247. doi: 10.1128/aem.60.7.2238-2247.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Díaz-Raviña M, Frostegård Å, Bååth E. Thymidine, leucine and acetate incorporation into soil bacterial assemblages at different temperatures. FEMS Microbiol Ecol. 1994;14:221–232. [Google Scholar]
- 17.Díaz-Raviña M, Prieto A, Bååth E. Bacterial activity in a forest soil after soil heating and organic amendments measured by the thymidine and leucine incorporation techniques. Soil Biol Biochem. 1996;28:419–426. [Google Scholar]
- 18.Fægri A, Lid Torsvik A, Goksøyr J. Bacterial and fungal activities in soil: separation of bacteria and fungi by a rapid fractionated centrifugation technique. Soil Biol Biochem. 1977;9:105–112. [Google Scholar]
- 19.Fritze H, Pennanen T, Kitunen V. Characterization of dissolved organic carbon from burned humus and its effects on microbial activity and community structure. Soil Biol Biochem. 1998;30:687–693. [Google Scholar]
- 20.Gärdenäs S, Thörnqvist T. Spontaneous combustion and dry matter losses in peat storage. Report no. 156. Uppsala, Sweden: Department of Forest Products, The Swedish University of Agricultural Sciences; 1984. [Google Scholar]
- 21.Hattori T. Mathematical equations describing the behavior of soil bacteria. Soil Biol Biochem. 1982;14:523–527. [Google Scholar]
- 22.Horwath W R, Elliott L F. Microbial C and N dynamics during mesophilic and thermophilic incubations of ryegrass. Biol Fertil Soils. 1996;22:1–9. [Google Scholar]
- 23.Kurzmann P. Sonderdruck aus Jahresbericht Weihenstephan. Freising, Germany: Institut für Bodenkunde and Pflanzenernährung.; 1981. Auswirkung selbsterhitzer Torfe auf gärtnerische Kulturen; pp. 33–34. [Google Scholar]
- 24.Li W K W, Dickie P M. Temperature characteristics of photosynthetic and heterotrophic activities: seasonal variations in temperate microbial plankton. Appl Environ Microbiol. 1987;53:2282–2295. doi: 10.1128/aem.53.10.2282-2295.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKinley V L, Vestal J R. Biokinetic analyses of adaptation and succession: microbial activity in composting municipal sewage sludge. Appl Environ Microbiol. 1984;47:933–941. doi: 10.1128/aem.47.5.933-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakasaki K, Sasaki M, Shoda M, Kubota H. Change in microbial numbers during thermophilic composting of sewage sludge with reference to CO2 evolution rate. Appl Environ Microbiol. 1985;49:37–41. doi: 10.1128/aem.49.1.37-41.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedwell D B, Floodgate G D. The seasonal selection of heterotrophic bacteria in an intertidal sediment. Mar Biol. 1971;11:306–310. [Google Scholar]
- 28.Pennanen T, Frostegård Å, Fritze H, Bååth E. Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl Environ Microbiol. 1996;62:420–428. doi: 10.1128/aem.62.2.420-428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pennanen T, Fritze H, Vanhala P, Kiikkilä O, Neuvonen S, Bååth E. Structure of a microbial community in soil after prolonged addition of low levels of simulated acid rain. Appl Environ Microbiol. 1998;64:2173–2180. doi: 10.1128/aem.64.6.2173-2180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powlson D S, Jenkinson D S. The effects of biocidal treatments on metabolism in soil. II. Gamma radiation, autoclaving, air-drying and fumigation. Soil Biol Biochem. 1976;8:179–188. [Google Scholar]
- 31.Salonius P O, Robinson J B, Chase F E. A comparison of autoclaved and gamma-irradiation soils media for colonizing experiments. Plant Soil. 1967;27:239–248. [Google Scholar]
- 32.Shiah F-K, Ducklow H W. Bacterioplankton growth responses to temperature and chlorophyll variations in estuaries measured by thymidine:leucine incorporation ratio. Aquat Microb Ecol. 1997;13:151–159. [Google Scholar]
- 33.Simon M, Wünsch C. Temperature control of bacterioplankton growth in a temperature large lake. Aquat Microb Ecol. 1998;16:119–130. [Google Scholar]
- 34.Strom P F. Effect of temperature on bacterial species diversity in thermophilic solid-waste composting. Appl Environ Microbiol. 1985;50:899–905. doi: 10.1128/aem.50.4.899-905.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strom P F. Identification of thermophilic bacteria in solid-waste composting. Appl Environ Microbiol. 1985;50:906–913. doi: 10.1128/aem.50.4.906-913.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibbles B J. Effects of temperature on the incorporation of leucine and thymidine by bacterioplankton and bacterial isolates. Aquat Microb Ecol. 1996;11:239–250. [Google Scholar]
- 37.Vuorinen A H, Saharinen M H. Evolution of microbiological and chemical parameters during manure and straw co-composting in a drum composting system. Agric Ecosyst Environ. 1997;66:19–29. [Google Scholar]
- 38.Wever G, Hertogh-Pon M H. Effects of self-heating on biological, chemical and physical characteristics of peat. Acta Hortic. 1993;342:15–24. [Google Scholar]
- 39.Widden P, Parkinson D. The effects of a forest fire on soil microfungi. Soil Biol Biochem. 1975;7:125–138. [Google Scholar]