Abstract
Objective
To study the potential role of subjective visual vertical (SVV) as a prognostic marker for canalith repositioning maneuver (CRM) in patients with posterior canal benign paroxysmal positional vertigo (PC-BPPV) for the Indian population.
Methods
SVV was examined in 30 patients with PC-BPPV before and after canalith repositioning maneuver and after complete resolution of PC-BPPV. Study parameters included the mean of 10 angular tilt readings and direction of deviation, which were compared before and after CRM and following complete resolution of PC-BPPV.
Results
The angle of SVV tilt was greater and deviated towards the affected ear before CRM in all patients, which decreased significantly shortly after CRM and continued to decrease after complete resolution of PC-BPPV (p < 0.0001).
Conclusions
SVV can be used to test utricular dysfunction in PC-BPPV. The angle of tilt improves in response to CRM, which may be used as a prognostic marker in patients with PC-BPPV receiving CRM.
Keywords: Benign paroxysmal positional vertigo (BPPV), Subjective visual vertical (SVV), Otoliths (canaliths), Canalith repositioning maneuver (CRM)
1. Introduction
Benign paroxysmal positional vertigo (BPPV) is a common vestibular disease that can originate from any of the semicircular canals (SCCs). The posterior canal (PC), being the most gravity-dependent part of the vestibular labyrinth, is most commonly associated with BPPV (80–90%). With head movements, free-floating canaliths/otoliths/otoconia (debris in the endolymph) can migrate from the utricular otolithic membrane into the posterior semicircular canal through its nonampullated end (You et al., 2018).
A Germany based study reported the lifetime prevalence of BPPV to be 3.2% in females, 1.6% in males and 2.4% overall (von Brevern et al., 2007). In the Indian rural population, the prevalence of otologic vertigo and BPPV is 0.08% and 0.05%, respectively (Abrol et al., 2001). In India, BPPV is more prevalent over the age of 45 years with a female preponderance (Swain et al., 2018).
The otoconia, calcium carbonate crystals, are embedded in the macula of the utricle and saccule. In BPPV, these are thought to accumulate in the SCC from the utricle, making them abnormally sensitive to gravity, which can lead to abnormal displacement of cupula and stimulation of corresponding vestibular afferents when head position changes with respect to gravity, resulting in nystagmus and vertigo (Schuknecht, 1969).
There are two theories explaining the possible pathophysiology. The "cupulolithiasis" theory suggests that the cupula, which becomes heavy due to attached otoliths, can be deflected by changes in head position thus causing nystagmus (Schuknecht, 1969). The "canalolithiasis" theory suggests that otoliths from the utricle migrate into the semicircular canal, evoking nystagmus and vertigo by moving freely inside the canal during changes of head position (Hall et al., 1979). Pathological studies have yielded evidence for both theories (Lee and Kim, 2010).
Dix Hallpike test is the gold standard test for PC-BPPV with characteristic nystagmus. The latency between the start of the test and the onset of nystagmus is about 2–5 s and due to the inertia of canaliths. Once canaliths migrate to the most dependent part, the nystagmus terminates. BPPV is generally managed nonsurgically with canalith repositioning maneuvers (CRMs). For PC-BPPV, certain maneuvers, e.g. Semont's and Epley's maneuvers, have been developed. Epley described the positional maneuver (Epley's manoeuvre) based on the canalolithiasis theory, which helps with the return of canaloliths from the posterior canal back to the utricle (You et al., 2018; Lee and Kim, 2010).
Videonystagmography (VNG) includes a test series to diagnose and report vestibular diseases causing vertigo. It is useful to support diagnosis and to document unilateral/bilateral loss of vestibular function, as well as to detect central lesions that may have been missed during physical examination (Mekki, 2014).
Currently, there are three methods to measure utricular functions: i.e. subjective visual vertical (SVV), ocular vestibular evoked myogenic potential (o-VEMP) and unilateral centrifugation test. SVV is a test to check a person's ability to adjust a line to be parallel to gravity in absence of any other visual cues. The utricle is the pivotal vestibular organ that gives sensory information for this test. Measurement of SVV can aid as a diagnostic indicator of utricular dysfunction. It can be done by various methods ranging from non-expensive conventional techniques to more sophisticated tests. The patient is seated in a dark room in front of a screen on which a fluorescent bar is projected. The patient uses a remote control to adjust the bar to his perceived vertical while keeping the head straight in a neutral position (0° position). Trials are allowed before performing the actual test for familiarization. Ten consecutive SVV readings are taken to generate a mean value. In the case of unilateral peripheral vestibular diseases, the bar tends to deviate to the affected side. This occurs due to ocular cyclotron where the patient has the impression that a bar placed in the vertical position is deviated to the healthy side and the patient ends up tilting it to the affected side, sometimes by many degrees (Faralli et al., 2011; Janky and Shepard, 2011) [Fig. 1]
Fig. 1.
SVV perception is tested in a dark room with the patient being seated in a chair in front of a display screen.
The SVV test is performed as either a static or dynamic protocol. The background is stationary in static assessment but rotating clockwise (CW) or counterclockwise (CCW) in the dynamic protocol. The normative values for static and dynamic SVV are 1.52° +/- 0.70° and 1.96° +/- 0.65° respectively for the Indian population, which suggest that deviation from the true vertical should be no more than ± 2.5° in a healthy individual (Ashish et al., 2016).
Since detached utricular otoliths enter the posterior canal, it is assumed that patients with PC-BPPV tend to have dysfunction of the utricle influencing their vertical perception. SVV test can be used to assesses utricular dysfunction and detect abnormalities in patients with PC-BPPV. Reduction in absolute SVV deviation values after CRM suggests a favorable effect of the procedure, consistent with the proposal that alteration of SVV after CRM would reflect the migration of canaliths back into the utricle (Faralli et al., 2011; El-Minawi et al., 2019).
We have found one article where SVV was recommended as a prognostic marker in PC-BPPV patients (El-Minawi et al., 2019). With this background, a prospective study was proposed to determine the role of SVV as a prognostic marker for CRM in patients with PC-BPPV in India.
2. Material and methods
The study was conducted at the Department of ENT & HNS, Army Hospital (Research & Referral), Delhi Cantonment with approval from the institutional ethics committee (IEC Registration Number- 68/2019) for a period of one and a half years (from October 2019 to March 2021) involving 30 patients with PC-BPPV.
Patients visiting the out-patient department (OPD) were registered and demographic data including name, age, sex, address, phone number, etc. were recorded along with appropriate history of giddiness and associated co-morbidities. General physical and otorhinolaryngology (ORL) examinations were completed. All patients were advised of impedance audiometry and pure tone audiometry to document hearing thresholds and to rule out pathologic causes of vertigo other than BPPV.
Among patients visiting the OPD, 30 patients diagnosed with PC-BPPV were conveniently recruited for the study. All the patients were explained about the study and an informed consent was taken. Patients with vertigo not related to PC-BPPV, with a history of ototoxic medication usage, loud noise exposure, consumption of drugs or alcohol within 72 h of the study including vestibular or labyrinthine inhibitors, and with any ophthalmologic, musculoskeletal or neurological diseases were excluded from the study.
Videonystagmography (VNG) test was completed with proper calibration using the Balance Eye goggles with infrared cameras and software modules designed by Cyclops Medtech Private Limited in the vestibular lab. VNG tests included spontaneous and gaze induced nystagmus and oculography tests (smooth pursuit, saccadic and optokinetic eye movements) to rule out central vestibular pathologies.
A positioning test was also performed using the VNG equipment, which included the Dix Hallpike maneuver for diagnosis of PC-BPPV. Patients with positive Dix Hallpike test were considered for further evaluation in the study. Dynamic subjective visual vertical test was done using the VNG equipment soon after positive Dix Hallpike test (i.e. in an acute episode of PC-BPPV), before performing any CRMs.
SVV test was conducted in a dark room to remove visual reference cues. The subject was made to sit upright in front of a display screen [Fig. 1]. A fluorescent line was shown on the dark screen. The patient was asked to use a remote control to adjust the line to his/her perceived vertical position.
Before the actual test, patients were allowed to do a trial test, which was not included in the statistical analysis. The patient perceived vertical line was compared with the true vertical and the angular deviation (measured in degrees) was marked as positive if deviated clockwise (right) or negative if deviated counterclockwise (left). Five SVV readings with clockwise (CW) and 5 with counterclockwise (CCW) background (dynamic SVV), for a total of 10 readings, were noted in each test. The mean of the 10 readings was calculated and the direction of summed deviation recorded.
The SVV testing was followed by Epley CRM as the therapy for PC-BPPV. A similar second SVV test was completed immediately after CRM. The patient was advised to complete bed rest for 24 h followed by Brandt Daroff exercises at home. No vestibular sedatives or other medications were prescribed. Patients were followed up 3 days later and the Dix Hallpike test repeated. In the case of a positive Dix Hallpike test, CRM was repeat without SVV testing. The third SVV test was done after complete resolution of PC-BPPV (i.e. Dix Hallpike test revealed no nystagmus/symptom).
The average of 10 SVV readings at each test was calculated for each patient before and after the CRM and following resolution of PC-BPPV. Values greater than 2°, towards either right or left, were considered abnormal. The direction of deviation at each test was represented by the sum of the 10 readings.
Data entry was done using Microsoft Excel and statistical analysis was done using the Statistical Package for Social Sciences software-21 (SPSS-21). A descriptive statistical analysis was performed to compare SVV readings before and after CRM and following resolution of PC-BPPV. Categorical variables were presented in the form of numbers and percentages (%), while quantitative data were presented as mean ± SD and as median with 25th and 75th percentiles (interquartile range). Paired t-test was used for comparison of SVV values. For statistical analysis, a p-value of less than 0.05 was considered significant.
3. Results
All 30 patients were between 28 and 66 years of age (mean = 48.60 years) [Table 1]. There were 14 males and 16 females (Male: Female = 1:1.28). All patients had symptoms within 12–72 h of the initial visit (mean = 24 h).
Table 1.
Age distribution among study subjects (years).
| Index | Value |
|---|---|
| Mean ± SD | 48.6 ± 10.5 |
| Median (25th-75th percentile) | 49.5 (40.25–54.75) |
| Range | 28–66 |
In all but two patients, history of BPPV was shorter than 2 years. All patients were hemodynamically stable and had normal otorhinolaryngology examinations. Tympanogram was type A bilaterally in all patients, indicating normal middle ear function. Pure tone audiometry thresholds were normal in 25 patients, showed bilateral high frequency sloping sensorineural hearing loss (SNHL) in 3 patients, bilateral mild SNHL at 2000, 4000 and 8000 Hz in 1 patient and bilateral moderate SNHL at 2000, 4000 and 8000 Hz in 1 patient who was also using hearing aids in both ears.
Dix Hallpike test was positive for the right side in 19 patients and positive for the left side in 11 patients. SVV deviated towards the affected ear in all of these patients. Table 2 shows dynamic SVV readings in the 30 patients before and after CRM and following resolution of PC-BPPV, without consideration of deviation direction. SVV deviation was at or greater than the normative value of 2.0° in 17 patients (56.66%) before CRM (average 2.0467+/-0.4160° for the group). After CRM, SVV readings in all patients were below 2.0° (average 1.2367+/-0.2029° for the group) [Table 2].
Table 2.
SVV deviation before and after CRM and following resolution of PC-BPPV (Mean ± SD).
| Case No. | Side of PC-BPPV | SVV deviation before CRM | SVV deviation after CRM | SVV deviation following PC-BPPV resolution |
|---|---|---|---|---|
| 1 | Right | 2.00 ± 1.29 | 1.35 ± 0.74 | 1.00 ± 0.74 |
| 2 | Left | 2.15 ± 1.33 | 1.50 ± 0.65 | 0.90 ± 0.93 |
| 3 | Right | 1.90 ± 1.28 | 1.00 ± 0.70 | 1.05 ± 0.79 |
| 4 | Right | 2.85 ± 1.33 | 1.40 ± 0.65 | 1.10 ± 0.43 |
| 5 | Left | 3.15 ± 0.62 | 1.50 ± 0.74 | 0.70 ± 0.48 |
| 6 | Right | 2.40 ± 0.87 | 1.05 ± 0.59 | 0.35 ± 0.41 |
| 7 | Right | 2.40 ± 1.41 | 1.60 ± 0.90 | 0.87 ± 0.65 |
| 8 | Right | 2.05 ± 1.27 | 1.30 ± 0.63 | 0.70 ± 0.48 |
| 9 | Left | 2.15 ± 1.33 | 0.90 ± 0.56 | 0.80 ± 0.53 |
| 10 | Right | 1.70 ± 0.75 | 1.10 ± 0.61 | 0.65 ± 0.47 |
| 11 | Left | 2.35 ± 1.37 | 1.20 ± 0.78 | 0.95 ± 0.59 |
| 12 | Right | 2.25 ± 1.42 | 1.20 ± 0.48 | 0.90 ± 0.73 |
| 13 | Right | 2.10 ± 1.61 | 1.35 ± 0.62 | 1.30 ± 0.75 |
| 14 | Left | 2.40 ± 1.55 | 1.55 ± 0.79 | 1.05 ± 0.76 |
| 15 | Right | 1.75 ± 1.75 | 1.05 ± 0.64 | 0.80 ± 0.53 |
| 16 | Left | 2.10 ± 1.39 | 1.40 ± 0.69 | 0.75 ± 0.58 |
| 17 | Right | 1.80 ± 1.35 | 1.45 ± 0.86 | 0.85 ± 0.70 |
| 18 | Right | 2.05 ± 1.46 | 1.40 ± 0.73 | 1.15 ± 0.75 |
| 19 | Left | 1.85 ± 1.37 | 1.05 ± 0.68 | 0.65 ± 0.62 |
| 20 | Right | 2.60 ± 1.39 | 1.50 ± 0.70 | 1.05 ± 0.68 |
| 21 | Right | 1.80 ± 1.20 | 1.20 ± 0.71 | 0.80 ± 0.75 |
| 22 | Left | 2.00 ± 1.49 | 1.15 ± 0.81 | 0.65 ± 0.74 |
| 23 | Right | 2.50 ± 1.84 | 1.35 ± 0.88 | 0.90 ± 0.69 |
| 24 | Left | 1.60 ± 1.12 | 0.95 ± 0.72 | 0.60 ± 0.56 |
| 25 | Right | 1.55 ± 1.14 | 1.00 ± 0.74 | 0.65 ± 0.71 |
| 26 | Right | 1.25 ± 0.97 | 1.15 ± 0.52 | 0.40 ± 0.45 |
| 27 | Right | 1.85 ± 1.47 | 1.35 ± 1.05 | 0.90 ± 0.84 |
| 28 | Right | 1.45 ± 1.06 | 1.05 ± 0.76 | 0.90 ± 0.65 |
| 29 | Left | 1.70 ± 1.11 | 1.00 ± 0.57 | 0.65 ± 0.55 |
| 30 | Left | 1.75 ± 1.31 | 1.05 ± 0.79 | 0.85 ± 0.70 |
| Group Average | 2.0467+/-0.4160 | 1.2367+/-0.2029 | 0.8290+/-0.2104 | |
At follow-up on day 3, 7 patients reported persistent symptoms of PC-BPPV and therefore received, repeated CRM with no immediate additional SVV tests. These patients had all demonstrated a reduction of SVV deviation immediately after the initial CRM [Table 2].
On the second follow-up visit after 2 weeks, all patients were free of symptoms (i.e. complete resolution of PC-BPPV), with additional reduction of SVV deviation (average 0.8290+/-0.2104° for the group) [Table 2]
Reduction of SVV deviation after CRM, as compared to before CRM, was statistically significant (P < 0.0001) [Table 3]. Reduction of SVV deviation after complete resolution of PC-BPPV, as compared to before CRM, was also statistically significant (P < 0.0001) [Table 3].
Table 3.
Group average SVV deviation readings before and after CRM and following complete resolution of PC-BPPV (degree), with comparison to pre-CRM readings.
| Time Point | Mean ± SD | Median (25th-75th percentile) | Range | P value |
|---|---|---|---|---|
| Before CRM | 2.05 ± 0.42 | 2.02(1.762–2.325) | 1.25–3.15 | |
| After CRM | 1.24 ± 0.2 | 1.2(1.05–1.4) | 0.9–1.6 | <.00001a |
| Following complete resolution of PC-BPPV | 0.83 ± 0.21 | 0.85(0.662–0.938) | 0.35–1.3 | <.00001a |
Paired t-test.
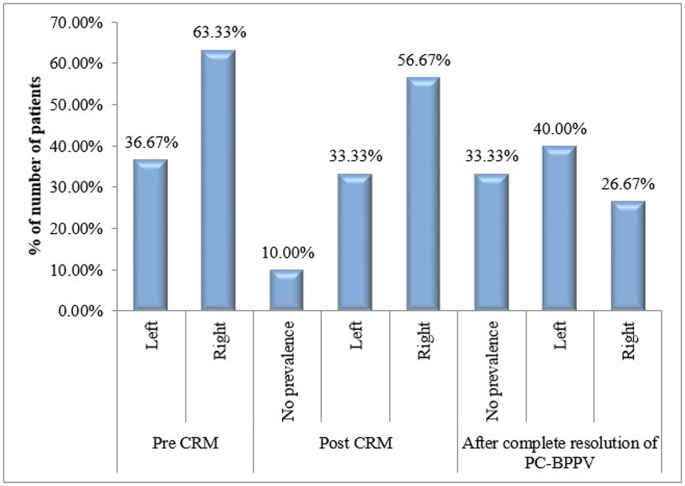
Immediately post-CRM, distribution pattern of direction of SVV deviation was not significantly different than before CRM, with only 3 patients showing no prevalence of deviation direction. After complete resolution of PC-BPPV, the number of patients showing no prevalence of deviation direction increased to 10 [Fig. 2].
Fig. 2.
Bar chart showing distribution of prevalence of SVV deviation direction.
4. Discussion
BPPV is a very common vestibular disease resulting from displacement of otoliths from the utricle into any of the SCCs. It is diagnosed by proper clinical history and Dix Hallpike test, and managed most commonly by Epley's CRM.
PC-BPPV can either be related to dysfunction of the utricle because of macular degeneration or to displacement of otoliths into posterior SCC or both, because several post mortem studies revealed signs of unilateral utricular damage on the side of BPPV (von Brevern et al., 2006). Utricular function can be assessed by SVV, which tests a person's ability to perceive gravitational vertical. Previous studies on SVV in patients with PC-BPPV diverge widely. While a few studies found no alteration, other studies showed altered SVV in a significant number of patients (El-Minawi et al., 2019). In the Indian population, the normative limits for SVV is ± 2.5° (Ashish et al., 2016). In our study, the normative SVV value using the Balance Eye VNG equipment was set at ± 2°, in accordance with the SVV normative data published by Akin et al. (El-Minawi et al., 2019; Akin et al., 2011; Böhmer and Rickenmann, 1995) A few studies have shown that there is a significant difference in SVV results between BPPV patients and healthy individuals (Faralli et al., 2011; El-Minawi et al., 2019; Sapountzi et al., 2017; Ferreira et al., 2017).
During an acute episode of BPPV, there can be direct stimulation of the posterior canal or possible damage to the utricular macula that can alter SVV test results. Vertigo can be explained by the presence of otoliths detached from the utricular macula in any of the canals (posterior SCC in this study). To conclude, otoliths that are responsible for vertigo likely have a direct or indirect role in SVV alteration (von Brevern et al., 2006).
In our study, mean SVV deviation was 2.05 ± 0.42° before CRM, which dropped to 1.24 ± 0.20° immediately after CRM (p < 0.0001), indicating a significant reduction in SVV deviation in a significant number of patients as compared to the acute phase of the disorder. Consistent to some of the existing studies (van Nechel et al., 2001; Boleas-Aguirre et al., 2005; Chetana and Jayesh, 2015), the direction of SVV deviation pointed to the affected side in all of our patients, although this does not agree with some other studies that reported a direction of SVV deviation to the contralateral side (Faralli et al., 2011; Böhmer and Rickenmann, 1995; van Nechel et al., 2001).
More than half of our patients (56.66%) had an angle of SVV deviation of more than the set normative value of 2° during acute episodes of BPPV. In those patients showing deviation less than 2° during the acute episode, their utricular dysfunction or disease might be less extensive. After CRM, SVV deviation in all of the patients, including those demonstrating greater than normal deviation earlier, decreased to a level below the set normative value, suggesting a favorable effect of the CRM.
Changes of SVV readings during follow-ups may be indicative of a dynamic relationship between canaliths and the utricle. The angle of SVV deviation in patients with PC-BPPV reflect dysfunction of a utricle that has lost otoliths from its macula or direct stimulation of the posterior semicircular canal by otoliths. We can probably assume that free otoliths, after being removed from the semicircular canal through CRM, may return to the macular structure and help restore its function with other secondary benefits (Faralli et al., 2011; El-Minawi et al., 2019; von Brevern et al., 2006; Sapountzi et al., 2017; Ferreira et al., 2017). In all of our cases, utricular dysfunction appeared to be brief, as normalization of SVV occurred within a couple of weeks. This may also support the efficacy of CRM in macular repair.
5. Limitations, conclusion and recommendations
It is assumed that, in PC-BPPV, otoliths are detached from the utricle and enter the posterior SCC causing the symptoms. A significant loss of otoconia in the utricular membrane leads to decreased stimulation of sensory receptors and causes SVV to tilt to the affected side, as shown in this study using VNG equipment during the acute phase of PC-BPPV. Although SVV deviation was not always greater than 2° in our patients, it consistently decreased after CRM and completely normalized in all of our patients after 2 weeks with complete resolution of PC-BPPV, suggesting that utricular dysfunction was brief, as well as possible efficacy of CRM in the repair of the otolithic membrane in the utricle. SVV test can therefore be used as a prognostic marker for CRM in patients with PC-BPPV. However, the possibility of SVV deviation due to direct posterior canal stimulation by otoliths should not be neglected.
One limitation of this study is its relatively small sample size of only 30 patients and lack of a healthy control group for comparison. Because of the small sample size, further studies with large sample size, and inclusion of a control group, are needed to confirm our findings. In place of the normal control, SVV readings after complete resolution of PC-BPPV might serve as normative values in this study, as the patients were symptom free with no residual giddiness at this time point.
Future studies may also assess SVV in patients with other types of BPPV and recurrent BPPV. o-VEMP may also be included in assessment of utricular dysfunction. Unilateral centrifugation for utricular function assessment may be combined with SVV test to confirm the results of the current study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interest
No conflicts of interest.
Footnotes
Peer review under responsibility of PLA General Hospital Department of Otolaryngology Head and Neck Surgery.
Contributor Information
Sanjeev Saxena, Email: saxena.iaf@gmail.com.
Bhaumik Patel, Email: patel_bhaumik93@hotmail.com.
Ravi Roy, Email: rroy76@yahoo.co.in.
Himanshu Swami, Email: hswami2003@yahoo.com.
Sanajit Kumar Singh, Email: sanajeetsingh@yahoo.in.
Sunil Goyal, Email: drsunilgoyal@yahoo.co.in.
Rajeev Chugh, Email: deeparajeevchugh@gmail.com.
Devendra Kumar Gupta, Email: docdk2000@yahoo.com.
Sween Banger, Email: shonabanger@gmail.com.
Mahesh Ravanikutty, Email: mahesh.rkty@gmail.com.
Sneha Yadav, Email: snehay22@gmail.com.
References
- Abrol R., Nehru V.I., Venkatramana Y. Prevalence and etiology of vertigo in adult rural population. Indian J. Otolaryngol. Head Neck Surg. 2001;53(1):32–36. doi: 10.1007/BF02910976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akin F.W., Murnane O.D., Pearson A., Byrd S., Kelly J.K. Normative data for the subjective visual vertical test during centrifugation. J. Am. Acad. Audiol. 2011;22:460–468. doi: 10.3766/jaaa.22.7.6. [DOI] [PubMed] [Google Scholar]
- Ashish G., Augustine A.M., Tyagi A.K., Lepcha A., Balraj A. Subjective visual vertical and horizontal: normative values using a software-based test in the Indian population. Indian J. Otol. 2016;22:208–212. [Google Scholar]
- Böhmer A., Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular diseases. J. Vestib. Res. 1995;5:35–45. [PubMed] [Google Scholar]
- Boleas-Aguirre F.M., Sánchez-Ferrándiz N., Perez N. The subjective visual vertical in benign paroxysmal positional vertigo. A preliminary study. Rev. Laryngol. Otol. Rhinol. 2005;126:253–255. [PubMed] [Google Scholar]
- Chetana N., Jayesh R. Subjective visual vertical in various vestibular disorders by using a simple bucket test. Indian J. Otolaryngol. Head Neck Surg. 2015;67:180–184. doi: 10.1007/s12070-014-0760-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed Sherif El-Minawi, Abeir Osman Dabbous, Mariam Magdy Medhat & Lamiaa Ahmed El-Dessokey Madkour (2019) Subjective visual vertical in posterior canal benign paroxysmal positional vertigo patients before and after Canalolith repositioning maneuvers, Hearing, Balance and Communication, 17:1, 69-82.
- Faralli M., Manzari L., Panichi R., Botti F., Ricci G., Longari F., Pettorossi V.E. Subjective visual vertical before and after treatment of a BPPV episode. Auris Nasus Larynx. 2011 Jun;38(3):307–311. doi: 10.1016/j.anl.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Ferreira M.M., Ganança M.M., Caovilla H.H. Subjective visual vertical after treatment of benign paroxysmalpositional vertigo. Braz J Otorhinolaryngol. 2017;83:659–664. doi: 10.1016/j.bjorl.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall S.F., Ruby R.R., McClure J.A. The mechanics of benign paroxysmal vertigo. J. Otolaryngol. 1979;8:151–158. [PubMed] [Google Scholar]
- Janky K.L., Shepard N.T. Unilateral centrifugation: utricular assessment and protocol comparison. Otol. Neurotol. 2011;32(1):116–121. doi: 10.1097/MAO.0b013e3181ff7549. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Kim J.S. Benign paroxysmal positional vertigo. J. Clin. Neurol. 2010 Jun;6(2):51–63. doi: 10.3988/jcn.2010.6.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekki S. The role of videonystagmography (VNG) in assessment of dizzy patient. Egypt J Otolaryngol. 2014;30:69–72. [Google Scholar]
- Sapountzi Z., Vital V., Psillas G. Subjective visual vertical in patients with benign positional paroxysmal vertigo. Hippokratia. 2017;21(3):159. [PMC free article] [PubMed] [Google Scholar]
- Schuknecht H.F. Cupulolithiasis. Arch Otolaryngol. 1969;90:765–778. doi: 10.1001/archotol.1969.00770030767020. [DOI] [PubMed] [Google Scholar]
- Swain S., Behera I., Sahu M. Prevalence of benign paroxysmal positional vertigo: our experiences at a tertiary care hospital of India. Egypt. J.Ear, Nose, Throat.Allied Sci. 2018;19(3):87–92. [Google Scholar]
- van Nechel C., Toupet M., Bodson I. The subjective visual vertical. Adv. Oto-Rhino-Laryngol. 2001;58:77–87. doi: 10.1159/000059113. [DOI] [PubMed] [Google Scholar]
- von Brevern M., Schmidt T., Schönfeld U., Lempert T., Clarke A.H. Utricular dysfunction in patients with benign paroxysmal positional vertigo. Otol. Neurotol. 2006;27(1):92–96. doi: 10.1097/01.mao.0000187238.56583.9b. [DOI] [PubMed] [Google Scholar]
- von Brevern M., Radtke A., Lezius F., Feldmann M., Ziese T., Lempert T., Neuhauser H. Epidemiology of benign paroxysmal positional vertigo: a population based study. J. Neurol. Neurosurg. Psychiatry. 2007 Jul;78(7):710–715. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P., Instrum R., Parnes L. Benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol. 2018;4(1):116–123. doi: 10.1002/lio2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]