Abstract
McConnell’s sign is a well-established, specific echocardiographic sign for acute pulmonary embolism. Multiple theories have been proposed regarding the mechanism of McConnell’s sign in the context of acute pulmonary embolism. Here, we present 2 patient cases in which McConnell’s sign was seen with right ventricular ischemia without pulmonary embolism. (Level of Difficulty: Beginner.)
Key Words: cardiology, echocardiogram, ischemia, right ventricular ischemia
Abbreviations and Acronyms: CT, computed tomography; PE, pulmonary embolism; RCA, right coronary artery; TTE, transthoracic echocardiogram
Central Illustration
In 1996, McConnell et al1 described an echocardiographic finding of right ventricular dysfunction, with akinesis of the mid–free wall and normal apical motion, that was highly specific for acute pulmonary embolism (PE). Multiple mechanisms have been proposed to explain this finding in the setting of acute PE. First, the preserved right apical motion can be explained by the right apex tethered to the hyperdynamic left ventricle.1 Another proposed mechanism is a spherical change in the right ventricle shape to help equalize regional wall stress caused by the increase in afterload.2,3 Localized ischemia of the right ventricular free wall caused by the increased wall stress may cause the wall motion abnormalities.1 Finally, by cardiac magnetic resonance, McConnell’s sign is proposed to be caused by substantial preload reduction and reflex hyperadrenergic up-regulation, with markedly augmented left ventricle septal intramyocardial deformations, such that tethered right ventricle insertion point fibers are acted upon via the hyperdynamic left ventricle apex with paradoxical right ventricle apical passive deformation.4
Learning Objectives
-
•
Recognize McConnell’s sign as a highly specific sign for acute PE.
-
•
Recognize McConnell’s sign as a potential indicator of right ventricular ischemia or infarction.
-
•
Discuss new insights into McConnell’s sign pathophysiology.
We present 2 different cases of McConnell’s sign in patients with proven right ventricular ischemia without acute PE.
Case 1
A 66-year-old man presented to a local emergency department with neck and chest pain. His medical comorbidities included impaired fasting glucose, hyperlipidemia, and asthma. The patient was afebrile, and his heart rate was 70/min, blood pressure was 146/72 mm Hg, and O2 saturation was 94% on room air. Physical examination revealed a well-nourished man in no acute distress, no focal neurologic deficits, regular heart rate and rhythm, lungs clear to auscultation bilaterally, abdomen that was soft and nondistended, and extremities without edema. Initial laboratory test results were significant for elevated troponin I to 2.4 ng/mL with continued increase to 3.9 ng/mL. He was treated with aspirin and clopidogrel for possible acute coronary syndrome and with inhaled ipratropium bromide/albuterol and prednisone for possible asthma exacerbation and transferred to our facility.
Upon presentation, he was hemodynamically stable and without chest pain, chest pressure, palpitations, or shortness of breath. Initial electrocardiogram did not show any significant ischemic changes. He was initiated on intravenous heparin infusion.
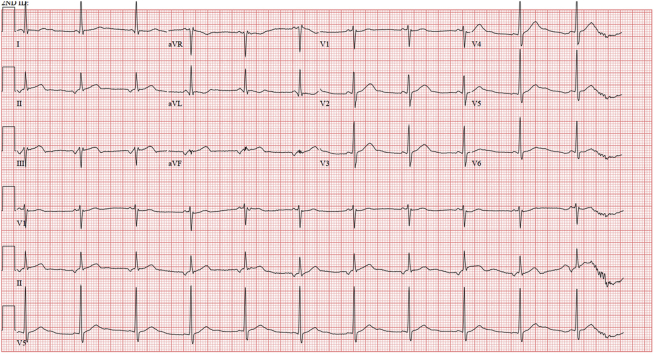
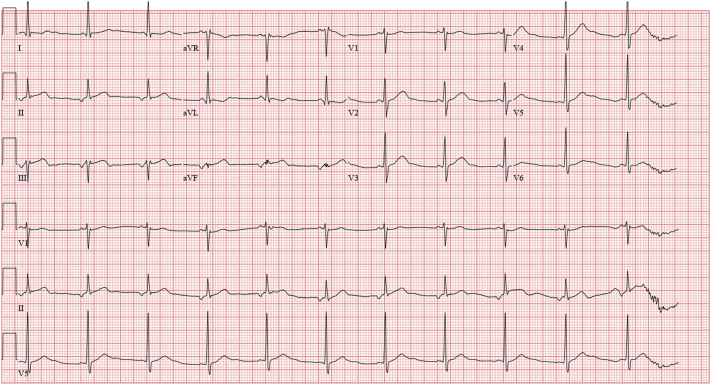
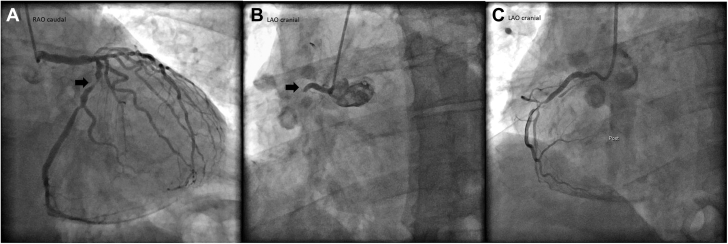
He was monitored with serial troponin T and electrocardiograms. A few hours after admission, he was noted to have slight ST-segment elevations in leads II, III, and aVF (Figure 1). He was taken to the cardiac catheterization laboratory for suspected inferior ST-segment elevation myocardial infarction. Coronary angiogram revealed a left dominant system with 100% occlusion of the proximal nondominant right coronary artery (RCA) (Figure 2, Videos 1, 2, and 3), 70% stenosis of the mid–left anterior descending artery, 90% stenosis of the proximal circumflex artery, 70% stenosis of the distal circumflex artery, and 70% stenosis of the ramus intermedius artery. The proximal RCA was stented (Figure 2, Video 3).
Figure 1.
Electrocardiogram
ST-segment elevation in inferior leads.
Figure 2.
Coronary Angiogram
(A) Dominant circumflex artery with critical proximal lesion (arrow) (Video 1). (B) Proximal right coronary artery with 100% occlusion (arrow) (Video 2). (C) Right coronary artery after proximal stenting (Video 3). LAO = left anterior oblique; RAO = right anterior oblique.
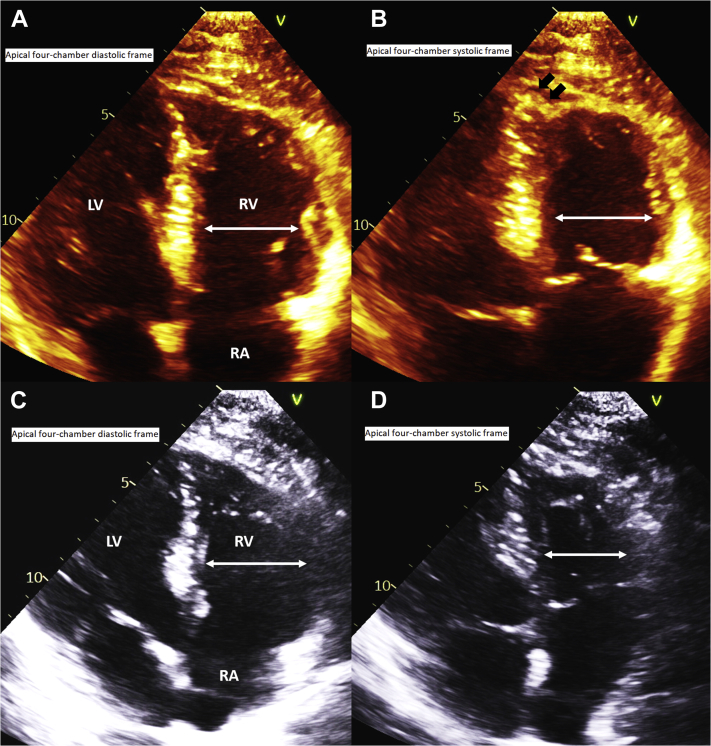
The following day, he underwent transthoracic echocardiogram (TTE), which was significant for enlarged right ventricular chamber size, moderately reduced systolic function, a prominent McConnell’s sign (Figure 3, Video 4), mild inferobasal and inferoseptal hypokinesis, and estimated ejection fraction of 63%. Because of the finding of McConnell’s sign, a computed tomography (CT) chest angiogram was obtained to assess for PE, and the result was negative. It was proposed that the McConnell’s sign was related to right ventricular ischemia (myocardial stunning or infarction) caused by the very proximal nature of the RCA occlusion supplying the right ventricular free wall and a wraparound left anterior descending artery supplying the apex.
Figure 3.
Transthoracic Echocardiogram
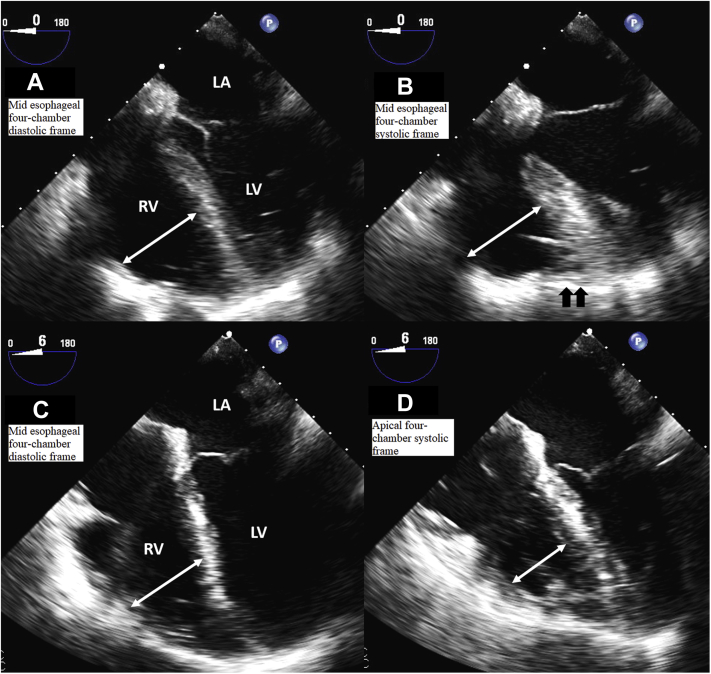
(A) Mid–right ventricular diameter (double-headed arrow) 1-day poststenting (Video 4). (B) Mid–right ventricular diameter (double-headed arrow) with prominent contraction of the apex (black arrows) 1-day poststenting (Video 4). (C) Mid–right ventricular diameter (double-headed arrow) 1-month poststenting (Video 5). (D) Decreased mid–right ventricular diameter (double-headed arrow) with absent McConnell’s sign 1-month poststenting (Video 5). LA = left atrium; LV = left ventricle; RV = right ventricle.
The patient was discharged with aspirin 81 mg daily, clopidogrel 75 mg daily, rosuvastatin 40 mg daily, and metoprolol tartrate 12.5 mg 2 times daily with a follow-up TTE scheduled in 1 month. Follow-up TTE was significant for complete resolution of McConnell’s sign and normal right ventricular systolic function (Figure 3, Video 5). He then underwent successful coronary artery bypass grafting for the residual left-sided lesions.
Case 2
A 50-year-old man with aortic root aneurysm presented to the cardiology clinic for follow-up. The patient was afebrile, with a heart rate of 64/min, blood pressure of 115/75 mm Hg, and O2 saturation of 99% on room air. The patient was alert and oriented, in no acute distress; heart rate was regular with regular rhythm; lungs were clear to auscultation bilaterally; and extremities were warm and without edema bilaterally. He had been followed up with serial CT angiograms to monitor the aneurysm size and rate of progression. At this follow-up, the aneurysm measured 5.1 cm. He was devoid of cardiac symptoms. Given that the size of the aneurysm was >5.0 cm, the patient met with the cardiovascular surgery team, and valve sparing root and ascending aorta/hemiarch replacement was recommended.
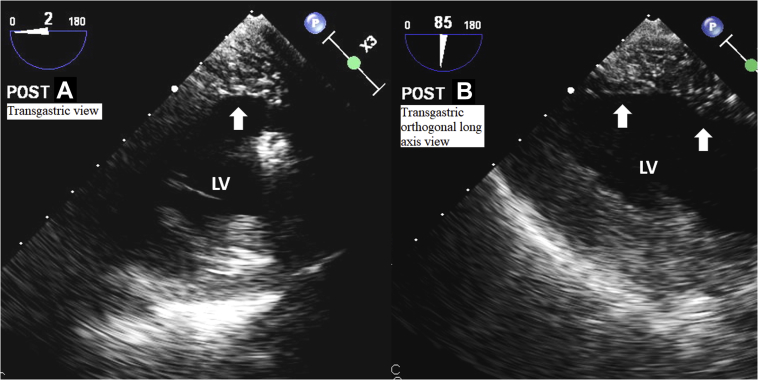
The patient proceeded with cardiovascular surgery without any interim complications or development of symptoms. Cardiovascular surgery revealed a dilated and thinned-out aortic root with a relatively normal-appearing tricuspid aortic valve. A Valsalva graft was secured in a remodeling fashion with a basal ring annuloplasty band to stabilize the annulus, thereby preserving the native aortic valve. The remainder of the ascending aorta was excised into the arch during a brief period of circulatory arrest and reconstructed in a standard ascending-hemiarch fashion. During de-airing maneuvers before coming off cardiopulmonary bypass, the intraoperative transesophageal echocardiogram revealed hypokinetic inferior/posterior walls and a prominent McConnell’s sign (Figure 4, Video 6). There was evidence of air in the proximal RCA (Video 7) with confirmed air embolism in the inferior wall of the left ventricle (Figure 5, Videos 8 and 9). After de-airing maneuvers and post–cardiopulmonary bypass, there was restoration of normal right ventricular systolic function (Figure 4, Video 10). The patient tolerated the procedure well. We re-reviewed the predischarge chest CT angiogram, and there was no evidence of PE. Predischarge TTE revealed normal right ventricular systolic function.
Figure 4.
Intraoperative Transesophageal Echocardiogram
(A) Mid–right ventricular diameter (double-headed arrow) post–right coronary air embolism (Video 6). (B) Mid–right ventricular diameter (double-headed arrow) with prominent contraction of the apex (black arrows) post–right coronary air embolism (Video 6). (C) Mid–right ventricular diameter (double-headed arrow) post–air embolism recovery (Video 10). (D) Decreased mid–right ventricular diameter (double-headed arrow) with absent McConnell’s sign after air embolism recovery (Video 10). LA = left atrium; LV = left ventricle; RV = right ventricle.
Figure 5.
Intraoperative Transgastric Views
(A) Typical speckled appearance of air within the inferior/posterior walls (arrow) (Video 8). (B) Typical speckled appearance of air within the inferior/posterior walls (arrow) (Video 9). LV = left ventricle.
Discussion
Although occasionally encountered in clinical practice, published cases of McConnell’s sign related to right ventricular ischemia are limited.5, 6, 7, 8 McConnell’s sign has long been established as an echocardiographic sign with high specificity for acute PE.1 A recent meta-analysis in patients with suspected PE confirmed that McConnell’s sign had a sensitivity of 22% and a specificity of 97% for the detection of acute PE.9 However, other conditions, such as right ventricle ischemia (as shown by our cases) or infarction, can give rise to McConnell’s sign. Indeed, a comparison between patients with PE vs patients with right ventricular ischemia/infarction demonstrated the presence of McConnell’s sign in approximately 70% of both groups.10 The patient cases described herein provide clear evidence of McConnell’s sign without evidence of PE by CT angiogram. In both cases, the cause of right ventricular ischemia is clearly demonstrated (ie, in case 1, acute coronary thrombosis and in case 2, RCA air embolism), and its temporal relation to the McConnell’s sign detection is suggestive of causality (ie, 1 day in case 1 and immediate in case 2). Further suggesting causality, the right ventricular ischemia was transient and terminated by a specific intervention (ie, a stent in case 1 and de-airing in case 2), resulting in complete resolution of the McConnell’s sign on reimaging.
Our cases support right ventricular free wall ischemia (related to increased wall stress) as a possible contributing mechanism to the McConnell’s sign observed in PE. Most importantly, our cases demonstrate that McConnell’s sign can be elicited in response to right ventricular ischemia: “the right ventricular ischemia McConnell’s sign.” Therefore, in clinical practice, the differential diagnosis of McConnell’s sign should include right ventricular ischemia once PE has been ruled out. Evidently, the clinical context in which McConnell’s sign is observed (suspected PE vs suspected right ventricular ischemia) becomes critical.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Right Anterior Oblique Caudal View. Dominant circumflex artery with critical proximal lesion.
Left Anterior Oblique Cranial View Before Stenting. Proximal RCA, 100% occlusion.
Left Anterior Oblique Cranial View After Stenting. RCA after stenting.
TTE. Moderately reduced systolic function and prominent McConnell’s sign.
Follow-Up TTE. Resolution of McConnell’s sign and normal right ventricular systolic function.
Intraoperative Transesophageal Echocardiogram. Prominent McConnell’s sign.
Right Coronary Artery. Air in the proximal RCA.
Transgastric Left Ventricle. Air within the inferior/posterior walls of the left ventricle.
Transgastric Orthogonal Long-Axis Left Ventricle. Confirmed air embolism in the inferior wall of the left ventricle.
Postcardiopulmonary Bypass and De-airing Maneuvers. Restoration of normal right ventricular systolic function.
References
- 1.McConnell M.V., Solomon S.D., Rayan M.E., Come P.C., Goldhaber S.Z., Lee R.T. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469–473. doi: 10.1016/s0002-9149(96)00339-6. [DOI] [PubMed] [Google Scholar]
- 2.Calvin J.E., Jr. Pressure segment length analysis of right ventricular function: influence of loading conditions. Am J Physiol Heart Circ Physiol. 1991;260:H1087–H1097. doi: 10.1152/ajpheart.1991.260.4.H1087. [DOI] [PubMed] [Google Scholar]
- 3.Janz R.F., Kubert B.R., Pate E.F., Moriarty T.F. Effect of shape on pressure-volume relationships of ellipsoidal shells. Am J Physiol Heart Circ Physiol. 1980;238:H917–H926. doi: 10.1152/ajpheart.1980.238.6.H917. [DOI] [PubMed] [Google Scholar]
- 4.Ivanova V., Doyle M., Yamrozik J., et al. McConnell’s sign unveiled. J Cardiovasc Magnet Res. 2012;14:P88. [Google Scholar]
- 5.Kuznetsova N.S., Rabinovich R.M., Mazur V.V., Mazur E.S. Diagnostic difficulties of isolated right ventricular myocardial infarction. Kardiologiia. 2021;61:66–70. doi: 10.18087/cardio.2021.9.n1601. [DOI] [PubMed] [Google Scholar]
- 6.Longo S.A., Echegaray A., Acosta C.M., Rinaldi L.I., Cabrera Schulmeyer M.C., Olavide Goya I. McConnell’s sign in intra-operative acute right ventricle ischaemia: an under-recognized aetiology. Rev Esp Anestesiol Reanim. 2016;63:528–532. doi: 10.1016/j.redar.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Zareh M., Dhillon A., Girn H., Chukumerije M., Narayanan M., Mehra A. McConnel’s sign: an under-recognized finding of right ventricular infarction. J Am Coll Cardiol. 2018;71 A2238-A2238. [Google Scholar]
- 8.Shah P., Schleifer J.W., Mookadam F., Chandrasekaran K. Right ventricular myocardial infarction: an underrecognized aetiology of McConnell’s sign. Eur Heart J Cardiovasc Imag. 2015;16:225. doi: 10.1093/ehjci/jeu186. [DOI] [PubMed] [Google Scholar]
- 9.Fields J.M., Davis J., Girson L., et al. Transthoracic echocardiography for diagnosing pulmonary embolism: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2017;30:714–723.e4. doi: 10.1016/j.echo.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Casazza F., Bongarzoni A., Capozi A., Agostoni O. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echocardiogr. 2005;6:11–14. doi: 10.1016/j.euje.2004.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Right Anterior Oblique Caudal View. Dominant circumflex artery with critical proximal lesion.
Left Anterior Oblique Cranial View Before Stenting. Proximal RCA, 100% occlusion.
Left Anterior Oblique Cranial View After Stenting. RCA after stenting.
TTE. Moderately reduced systolic function and prominent McConnell’s sign.
Follow-Up TTE. Resolution of McConnell’s sign and normal right ventricular systolic function.
Intraoperative Transesophageal Echocardiogram. Prominent McConnell’s sign.
Right Coronary Artery. Air in the proximal RCA.
Transgastric Left Ventricle. Air within the inferior/posterior walls of the left ventricle.
Transgastric Orthogonal Long-Axis Left Ventricle. Confirmed air embolism in the inferior wall of the left ventricle.
Postcardiopulmonary Bypass and De-airing Maneuvers. Restoration of normal right ventricular systolic function.