Abstract
Background
Estimated peak oxygen consumption (Vo2peak) is widely used in oncology; however, estimated Vo2peak equations were developed in noncancer settings.
Objectives
The aim of this study was to evaluate the validity of estimated Vo2peak in women with primary breast cancer and to develop oncology-specific estimated Vo2peak equations.
Methods
Vo2peak was directly measured (TrueOne 2400, Parvo Medics) during 380 cardiopulmonary exercise tests in women previously treated for breast cancer (mean age: 59 ± 10 years; 3.1 ± 1.2 years post-therapy). The American College of Sports Medicine (ACSM), the Fitness Registry and the Importance of Exercise National Database (FRIEND), and heart failure (HF)-FRIEND equations were used to estimate Vo2peak. New equations were developed using patient and peak (Oncpeak) or submaximal (Oncsub) exercise test characteristics.
Results
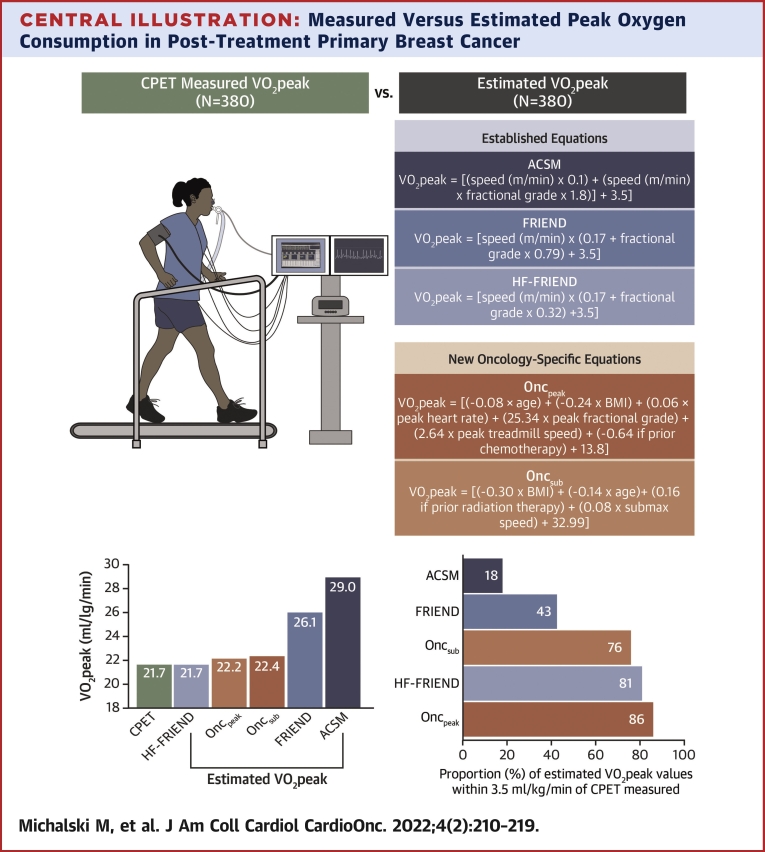
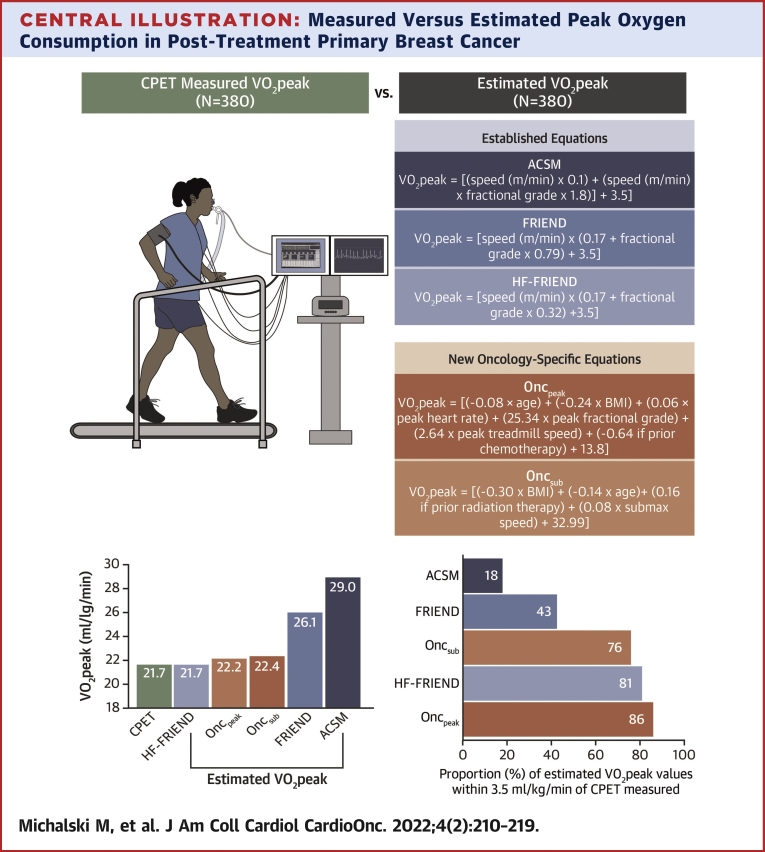
The median differences between measured and estimated Vo2peak were 7.0 mL O2·kg−1·min−1, 3.9 mL O2·kg−1·min−1, and −0.2 mL O2·kg−1·min−1 for ACSM, FRIEND, and HF-FRIEND, respectively. The number of estimated Vo2peak values within ±3.5 mL O2·kg−1·min−1 of the measured values was 70 (18%), 164 (43%), and 306 (81%) for ACSM, FRIEND, and HF-FRIEND, respectively. The Oncpeak and OncSub models included body mass index, age, a history of chemotherapy or radiation, the peak measured heart rate, and the treadmill grade and/or speed. The median differences between measured and estimated Vo2peak were 0.02 mL O2·kg−1·min−1 (Oncpeak) and −0.2 mL O2·kg−1·min−1 (Oncsub). Eighty-six percent (n = 325) and 76% (n = 283) estimated Vo2peak values were within ±3.5 mL O2·kg−1·min−1 of the measured Vo2peak values for Oncpeak and Oncsub, respectively.
Conclusions
HF-FRIEND or oncology-specific equations could be applied to estimate Vo2peak in patients previously treated for breast cancer in settings where cardiopulmonary exercise tests are not available. (Trial Comparing the Effects of Linear Versus Nonlinear Aerobic Training in Women With Operable Breast Cancer [EXCITE]; NCT01186367
Key Words: breast cancer, cancer survivorship, exercise capacity, peak oxygen consumption
Abbreviations and Acronyms: ACSM, American College of Sports Medicine; BMI, body mass index; CCC, Lin’s concordance correlation coefficient; CPET, cardiopulmonary exercise test; CRF, cardiorespiratory fitness; FRIEND, Fitness Registry and the Importance of Exercise National Database; HF, heart failure; Vo2peak, peak oxygen consumption
Central Illustration
Cardiorespiratory fitness (CRF) provides an integrative measure of the capacity of the pulmonary, cardiovascular, hematologic, and musculoskeletal systems to transport and use oxygen.1 For this reason, CRF is considered a “clinical vital sign,” and assessment is recommended for clinical decision making in many chronic diseases.2, 3, 4 In breast cancer, impaired CRF is a consequence of direct and indirect (ie, lifestyle perturbations) adverse effects of therapy on all organ components of the cardiopulmonary system.5 Poor CRF is associated with increased symptom burden6,7 and an increased risk of morbidity and mortality from cancer and noncancer conditions.8, 9, 10, 11 Therefore, accurate assessment of CRF in the large and rapidly growing population of patients with primary breast cancer is of high importance for risk stratification, toxicity monitoring, and evaluation of the efficacy of exercise interventions.12
A cardiopulmonary exercise test (CPET) coupled with automated gas exchange to directly measure peak oxygen consumption (Vo2peak) is the gold standard assessment of CRF.3 Nevertheless, the widespread applicability of the CPET is limited by requirements for specialized equipment and trained personnel.13,14 Accordingly, the American College of Sports Medicine (ACSM)15 and the Fitness Registry and the Importance of Exercise National Database (FRIEND)16 developed equations derived from patient and exercise test characteristics to estimate Vo2peak. Three commonly used estimated Vo2peak equations were developed based on exercise test characteristics from young adults (ie, 19-26 years old),15 older healthy adults (ie, no comorbidities),16 and patients with heart failure (ie, reduced or preserved ejection fraction).17 Estimated Vo2peak is widely used in oncology research and clinical practice settings18,19; therefore, there is a need to evaluate the validity of estimated Vo2peak equations in patients with a history of cancer.
We evaluated the validity of estimated Vo2peak equations in comparison with directly measured Vo2peak from CPETs in women with post-treatment primary breast cancer and developed oncology-specific equations derived from patient and peak (Oncpeak) or submaximal (Oncsub) exercise test characteristics. We hypothesized that oncology-specific equations would have improved accuracy relative to nononcology estimated Vo2peak equations.
Methods
Patients and eligibility
Full details regarding the study sample, recruitment, and procedures have been reported previously20 and are outlined in the Supplemental Methods. Eligible patients were ≥1 year to <5 years after the completion of primary adjuvant therapy and had Vo2peak below age- and sex-matched active levels.3,21 Patients enrolled in the 16-week randomized controlled exercise trial (NCT01186367) completed CPETs at baseline (prerandomization) and postintervention (week 17); a subset of patients completed a CPET at midpoint (week 8). All study procedures were reviewed and approved by Duke University Medical Center and Memorial Sloan Kettering Cancer Center Institutional Review Boards. All patients provided written informed consent.
CPET Vo2peak
Vo2peak (mL O2·kg−1·min−1) was assessed by an incremental walking CPET on an electronic motorized treadmill with 12-lead electrocardiographic monitoring (Mac 5000, GE Healthcare) according to standard procedures.3,22 Breath-by-breath (averaged every 30 seconds) expired gases were collected using a mouthpiece and analyzed continuously by a calibrated metabolic measurement system (TrueOne 2400, Parvo Medics). Before starting the test, a warm-up was completed to familiarize the patient with the treadmill and identify a comfortable walking speed between 1.5 and 4.0 mph. During warm-up, the heart rate response (∼20 beats/min above resting, varied with age), gait, and perceived level of exertion (rating of perceived exertion ∼8-10) were assessed to determine the CPET starting walking speed. After 3 minutes of rest, the test began using a personalized modified Balke protocol. Specifically, the test began at the individually identified warm-up speed and 0% grade for 2 minutes. During the first stage, metabolic metrics including minute ventilation, respiration rate, respiratory exchange ratio, fraction of expired oxygen content, and heart rate response compared with rest were assessed to select the increment of grade (2% or 3%) increase for subsequent stages. A 3% increase per stage was standard; if a patient demonstrated a large increase in minute ventilation and/or the respiratory exchange ratio stayed elevated, a 2% increase in grade was selected. The grade was subsequently increased every 2 minutes until a clear decrease in the fraction of expired carbon dioxide oxygen content from its highest value occurred; after this stage, the grade remained constant, and the speed was increased every minute until exhaustion. Speed increases were 0.2 or 0.3 depending on the patient’s gait and the perceived amount of effort left before self-terminating the test. Acceptable peak CPET criteria for this analysis included any 2 of the following3: 1) a plateau in VO2, concurrent with an increase in treadmill grade or speed; 2) a respiratory exchange ratio ≥1.10; 3) the attainment (±10 beats/min) of an age-predicted heart rate; and 4) volitional exhaustion as measured by a rating of perceived exertion ≥18 on the Borg scale. Upon CPET completion, a trained exercise physiologist identified the ventilatory threshold (ie, submaximal) as defined by the following criteria: 1) a drop in the fraction of expired carbon dioxide oxygen content after a peak or plateau; 2) a nonlinear increase in the minute ventilation; and 3) a respiratory exchange ratio between 0.98 and 1.02.
Estimated Vo2peak
Exercise test characteristics from the CPET were used to estimate Vo2peak using the following: ACSM15 (Vo2peak = [speed (m/min) × 0.1] + [speed (m/min) × fractional grade × 1.8] + 3.5), FRIEND16 (Vo2peak = [speed (m/min) × (0.17 + fractional grade × 0.79) + 3.5]), and HF-FRIEND17 (Vo2peak = [speed (m/min) × (0.17 + fractional grade × 0.32) + 3.5]) equations.
Statistical analysis
Data from all arms of the trial were combined for these analyses. Vo2peak as measured by CPETs and estimated models were summarized using descriptive statistics with the median (quartiles [Q1-Q3]) or mean ± SD for continuous variables and the number and percentage for categoric variables. Patient (eg, treatment history) and exercise test characteristics (eg, treadmill speed and grade) at Vo2peak and the ventilatory threshold were used to develop Oncpeak and Oncsub. Five-fold cross-validation was used to develop the oncology-specific estimated Vo2peak equations. The cross-validation was based on a linear model with an outcome of measured Vo2peak and a random intercept to account for repeated CPET measurements for the same patient at up to 3 time points. Variables were considered for inclusion on the basis of previous literature and the potential to impact Vo2peak16 (Supplemental Table 1). Variables retained by stepwise selection (P ≤ 0.20) in at least 50% of the models were included in the final models (Supplemental Table 2). As a sensitivity analysis, we also examined how results would differ if variables selected in at least 80% of the models were retained. Two variables introduced collinearity issues: the measured versus estimated heart rate and heart reserve. These variables were removed before fitting the stepwise selection model. The average root mean-squared error across the cross-validation models was evaluated to indicate model accuracy based on the difference between estimated and measured Vo2peak values.23 The average fixed effects from the random intercept model were used to generate estimated values for the oncology-specific equations.
Bland-Altman plots were used to display the difference between measured and all estimated Vo2peak measures along the y-axis and the average of measured and estimated observations along the x-axis, along with the average bias and 95% limits of agreement.24 The data were visually inspected and log transformed before computing the limits of agreement in order to meet the assumptions of the method; Vo2peak values were transformed back to the original scale for interpretation of the results. Estimates of the SD of the difference in Vo2peak between methods reflect the within- and between-participant variation to account for repeated Vo2peak measurements.24,25 The concordance between the estimated and measured Vo2peak values was evaluated by Lin’s concordance correlation coefficient (CCC)26 and SD, which accounts for the longitudinal experimental design.24 A CCC value of 1 indicates perfect agreement; values <0.6 were considered to be poor agreement.27 Sensitivity analyses of the CCC and Bland-Altman limits of agreement were performed among baseline CPET measurements only to assess whether the removal of repeated measurements changed the results. Based on previous work demonstrating that a 3.5 mL O2·kg−1·min−1 (ie, 1 metabolic equivalent) higher Vo2peak is associated with a ∼20% reduced risk of all-cause mortality,28 we used this value to serve as an acceptable difference threshold for estimated Vo2peak values (ie, a difference between the estimated and CPET Vo2peak of ≤3.5 mL O2·kg−1·min−1 considered an acceptable value). Analyses were performed in R version 4.0.0 (R Foundation for Statistical Computing).
Results
A total of 170 patients with primary breast cancer (mean post–primary adjuvant therapy: 3.1 ± 1.2 years; age: 59 ± 10 years; body mass index [BMI]: 29.8 ± 5.5 kg/m2) were included (Table 1).20
Table 1.
Characteristics of the Participants (N = 170)
| Time from surgery to enrollment, y | |
| Median (Q1-Q3) | 3.0 (2.1-3.9) |
| Mean ± SD | 3.1 ± 1.2 |
| Age, y | |
| Median (Q1-Q3) | 59 (51-65) |
| Mean ± SD | 59 ± 10 |
| BMI, kg/m2 | |
| Median (Q1-Q3) | 29.0 (25.5-33.5) |
| Mean ± SD | 29.8 ± 5.5 |
| Left ventricular ejection fraction, % | |
| Median (Q1-Q3) | 62.8 (59.4-65.1) |
| Mean ± SD | 62.1 ± 4.5 |
| Not available | 21 |
| Race | |
| Non-Hispanic White | 105 (62) |
| Other group | 65 (38) |
| Smoking | |
| Never | 106 (63) |
| Former | 55 (33) |
| Current | 7 (4.2) |
| Unknown | 2 (1.2) |
| Disease stage | |
| I | 96 (57) |
| II | 59 (35) |
| III | 14 (8) |
| Unknown | 1 (<1) |
| Clinical subtype | |
| ER+/PR+/HER2− | 101 (60) |
| HER2+ | 34 (20) |
| ER−/PR−/HER2− | 28 (17) |
| Other | 6 (3) |
| Unknown | 1 (<1) |
| Surgery | |
| Lumpectomy | 86 (51) |
| Mastectomy | 84 (49) |
| Previous chemotherapy | 99 (58) |
| Previous radiotherapy | 121 (71) |
| Current endocrine therapy | 123 (72) |
| Current medications | |
| Beta-blockers | 24 (14) |
| ACE inhibitors | 30 (18) |
| Angiotensin receptor blockers | 12 (7.1) |
| Diuretic | 33 (19) |
| Aspirin/antiplatelet | 34 (20) |
| Statins | 39 (23) |
| Calcium-channel blocker | 14 (8.2) |
| Pre-existing (controlled) cardiovascular conditions | |
| Coronary artery disease | 3 (1.8) |
| Osteoporosis | 13 (7.6) |
| Arthritis | 22 (13) |
| Type II diabetes | 18 (11) |
| Hyperlipidemia | 41 (24) |
| Hypertension | 68 (40) |
| Any | 93 (55) |
Values are n or n (%) unless otherwise indicated.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; BMI = body mass index; ER = estrogen receptor; HER2 = human epidermal growth factor; PR = progesterone receptor.
Measured Vo2peak and ventilatory threshold
At baseline, midpoint, and postintervention, 174, 77, and 156 CPETs were conducted, respectively. Of the 407 CPETs conducted, 27 did not meet criteria and were excluded, resulting in 380 included CPETs (Table 2). Across all time points, the median (Q1-Q3) peak treadmill grade and peak treadmill speed were 0.10 (Q1-Q3: 0.09-0.12) and 89 m/min (Q1-Q3: 80-99 m/min), respectively. The median treadmill grade and treadmill speed at the ventilatory threshold were 0.09 (Q1-Q3: 0.06-0.12) and 80 m/min (Q1-Q3: 70-89 m/min), respectively. The median difference between the measured and 80% of the age-predicted peak heart rate at the ventilatory threshold was −2 beats/min (Q1-Q3: −7 to −10 beats/min).
Table 2.
Cardiopulmonary Exercise Test Characteristics (N = 380)a
| Rest | 74 (68-83) |
| Heart rate, beats/min | 74 (68-83) |
| Ventilatory threshold | |
| Treadmill speed, mph | 3.00 (2.60-3.30) |
| Treadmill speed, m/min | 80 (70-89) |
| Treadmill grade, % | 9.0 (6.0-12.0) |
| Treadmill grade, decimal | 0.09 (0.06-0.12) |
| Treadmill speed x grade | 6.76 (5.19-8.37) |
| Measured heart rate, beats/min | 146 (136-156) |
| Heart rate reserve, beats/min | 70 (60-81) |
| Age-predicted heart rate at 80%, beats/min | 145 (139-150) |
| Difference between measured and age-predicted heart rate at 80%, beats/min | 2 (−7 to −10) |
| Peak | |
| Treadmill speed, mph | 3.30 (3.00-3.70) |
| Treadmill speed, m/min | 89 (80-99) |
| Treadmill grade, % | 10.0 (9.0-12.0) |
| Treadmill grade, decimal | 0.10 (0.09-0.12) |
| Treadmill speed × grade | 9.3 (7.5-11.3) |
| Measured heart rate, beats/min | 163 (151-176) |
| Heart rate reserve, beats/min | 89 (76-99) |
| Age-predicted peak heart, beats/min | 161 (155-168) |
| Difference between measured and age-predicted peak heart rate, beats/min | 2 (−7 to −9) |
Values are median (Q1-Q3).
Q = quartile.
7 patients were missing data on resting heart rate; 10 patients were missing data on ventilatory threshold treadmill speed (mph and m/min), grade, heart rate, heart rate reserve, and age-predicted heart rate at 80% beats/min.
Oncpeak and Oncsub estimated Vo2peak equations
Variables retained by stepwise selection (P ≤ 0.20) in at least 50% of the Oncpeak and Oncsub models included the peak measured heart rate (beats/min), BMI (kg/m2), age (years), a history of chemotherapy (yes/no) or radiation (yes/no), and treadmill grade (decimal) and/or speed (m/min). The resultant estimated Vo2peak equations were as follows: Oncpeak ([−0.08 × age (years)] + [−0.24 × BMI] + [0.06 × peak measured heart rate (beats/min)] + [25.34 × peak fractional grade] + [2.64 × peak treadmill speed (m/min)] + [−0.64 if previous chemotherapy] + 13.8) and Oncsub ([−0.30 × BMI] + [−0.14 × age (years)] + [0.16 if previous radiation therapy] + [0.08 × submaximal speed (m/min)] + 32.99). The average root mean-squared error across the cross-validation models was 2.53 (range: 2.43-2.65) for Oncpeak and 3.06 (range: 2.81-3.48) for Oncsub.
Validity of ACSM, FRIEND, HF-FRIEND, and Oncpeak and Oncsub estimated Vo2peak
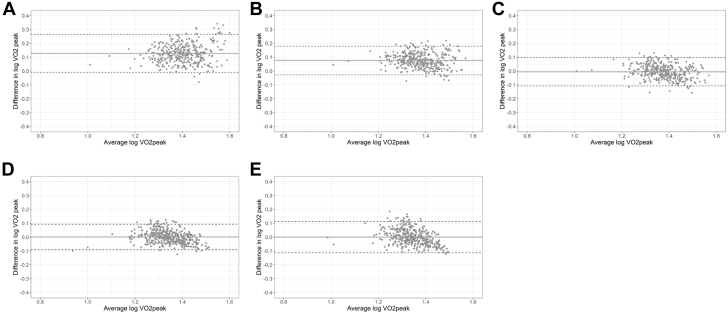
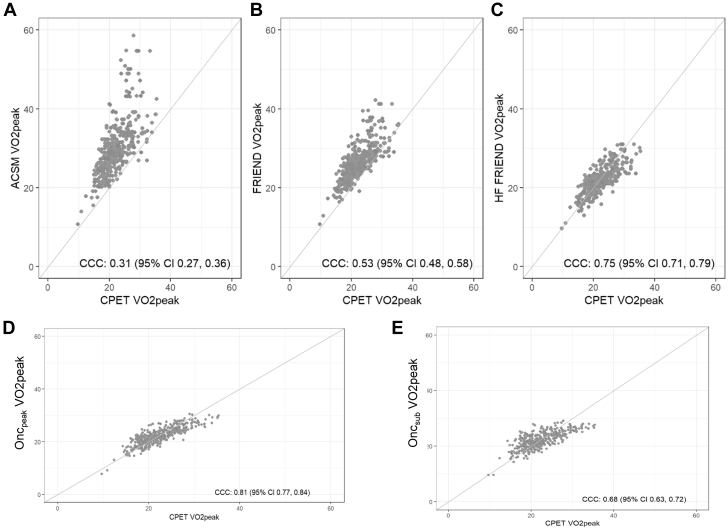
Measured and estimated Vo2peak values ar presented in the Central Illustration and Table 3. The median difference between measured and estimated Vo2peak was 7.0 mL O2·kg−1·min−1 and 3.9 mL O2·kg−1·min−1 in ACSM and FRIEND compared with −0.21 mL O2·kg−1·min−1, 0.02 mL O2·kg−1·min−1, and −0.23 mL O2·kg−1·min−1 in HF-FRIEND, Oncpeak, and Oncsub, respectively. The number of estimated Vo2peak values within ±3.5 mL O2·kg−1·min−1 of the measured values was below 50% for ACSM and FRIEND and above 75% for HF-FRIEND, Oncpeak, and Oncsub. ACSM and FRIEND overestimated Vo2peak with 95% limits of agreement ranging from −2% to 84% and −6% to 51%, respectively, whereas the limits of agreement were similar for HF-FRIEND (−22% to 25%), Oncpeak (−19% to 24%), and Oncsub (−23% to +30%) (Figures 1A-1E). There was a low CCC between measured and ACSM (CCC = 0.31; 95% CI: 0.27- 0.36) and FRIEND (CCC = 0.53; 95% CI: 0.48-0.58) estimated Vo2peak and a high CCC between measured and HF-FRIEND (CCC = 0.75; 95% CI: 0.71-0.79), Oncpeak (CCC = 0.81; 95% CI: 0.77-0.84), and Oncsub (CCC = 0.68; 95% CI: 0.63-0.72; Figures 2A-2E). In sensitivity analyses restricted to variables retained in at least 80% of the models, the number of estimated Vo2peak values within ±3.5 mL O2·kg−1·min−1 of the measured values was 80% and 71% for Oncpeak and Oncsub, respectively (Supplemental Table 3). Sensitivity analyses restricted to the baseline assessment did not vary from the primary results (Supplemental Table 4).
Central Illustration.
Measured Versus Estimated Peak Oxygen Consumption in Post-Treatment Primary Breast Cancer
(Top) Directly measured peak oxygen consumption (Vo2peak) using a CPET (n = 380) and estimated Vo2peak using established equations (American College of Sports Medicine [ACSM], Fitness Registry and the Importance of Exercise National Database [FRIEND], and heart failure [HF]-FRIEND) and oncology-specific equations developed from patient and exercise test characteristics were compared in women previously treated for breast cancer. (Bottom) ACSM and FRIEND equations overestimated Vo2peak and had poor accuracy compared with cardiopulmonary exercise test (CPET)-measured Vo2peak. HF-FRIEND and oncology-specific equations could be applied to estimate Vo2peak in settings where the CPET is not available. Oncpeak = oncology peak; Oncsub = oncology submaximal.
Table 3.
Measured and Estimated Vo2peak Using ACSM, FRIEND, HF-FRIEND, Oncpeak, and Oncsub
| CPET Measured | ACSM | FRIEND | HF-FRIEND | Oncpeak | Oncsub | |
|---|---|---|---|---|---|---|
| Vo2peak, mL O2·kg−1·min−1 | 21.7 (19.1-25.4) | 29.0 (25.3-33.4) | 26.1 (23.5-29.1) | 21.7 (19.9-23.6) | 22.2 (20.1-24.4) | 22.4 (20.3-24.3) |
| Difference between measured and estimated Vo2peak, mL O2·kg−1·min−1 | 7.0 (4.2-9.9) | 3.9 (2.3-5.9) | −0.2 (−2.1 to 1.5) | 0.02 (−1.7 to 1.5) | −0.2 (−2.3 to 1.9) | |
| Values within 3.5 mL O2·kg−1·min−1 | 70 (18) | 164 (43) | 306 (81) | 325 (86) | 283 (76) |
Values are median (Q1-Q3) or n (%).
ACSM = American College of Sports Medicine; CPET = cardiopulmonary exercise test; FRIEND = Fitness Registry and the Importance of Exercise National Database; HF = heart failure; Oncpeak = oncology peak; Oncsub = oncology submaximal; Q = quartile; Vo2peak = peak oxygen consumption.
Figure 1.
Bland-Altman Plots of Measured and Estimated Vo2peak
Estimated Vo2peak from (A) ACSM, (B) FRIEND, (C) HF-FRIEND, (D) Oncpeak, and (E) Oncsub. The difference between CPET measured Vo2peak and all estimated Vo2peak measures along the y-axis and the average of the measured and estimated observations along the x-axis, along with the average bias and 95% limits of agreement.24 ACSM and FRIEND overestimated Vo2peak with 95% limits of agreement ranging from −2% to 84% and −6% to 51%, respectively, whereas the limits of agreement were evenly distributed for HF-FRIEND (−20% to 25%), Oncpeak (−20% to 27%), and Oncsub (−23% to 30%). CPET = cardiopulmonary exercise test; Vo2peak = peak oxygen consumption; ACSM = American College of Sports Medicine; FRIEND = Fitness Registry and the Importance of Exercise National Database; HF = heart failure; Oncpeak = oncology peak; Oncsub = oncology submaximal.
Figure 2.
Concordance Plots of Measured and Estimated Vo2peak
Difference between CPET measured Vo2peak and estimated VO2peak from (A) ACSM, (B) FRIEND, (C) HF-FRIEND, (D) Oncpeak, and (E) Oncsub. The concordance estimated and measured Vo2peak was evaluated by Lin’s concordance correlation coefficient (CCC).26 A CCC value of 1 indicates perfect agreement; values <0.6 were considered to be poor agreement.27 There was a low CCC between measured and ACSM and FRIEND estimated Vo2peak and a high CCC between measured and HF-FRIEND, Oncpeak, and Oncsub. Abbreviations as in Figure 1.
Discussion
Poor Vo2peak is prevalent across the breast cancer continuum (ie, from diagnosis to survivorship)10,29,30 and correlates with heightened symptom burden.31 Vo2peak is also a significant predictor of all-cause10,32 and cause-specific mortality,33 even after adjustment for important clinical covariates. Despite the importance of accurate assessment of Vo2peak in the large and rapidly growing population of patients with cancer,34 prediction equations are commonly used in oncology clinical and research settings.13 For instance, in a systematic review evaluating exercise testing in cancer patients, Jones et al19 reported that among 90 studies, 49 (54%) used exercise tests other than the CPET. In a meta-analysis evaluating the effects of exercise therapy on Vo2peak in patients with adult-onset cancers,18 18 of 48 (38%) studies used prediction equations to estimate Vo2peak. Collectively, given that in noncancer clinical populations Vo2peak is considered a “clinical vital sign”2 and that an exercise-induced improvement in Vo2peak of 3.5 mL O2·kg−1·min−1 is associated with an adjusted 30%35 to 38%36 risk reduction in all-cause mortality, accurate estimation of Vo2peak is of high importance in research and clinical practice settings for risk stratification, toxicity monitoring, and evaluation of exercise intervention efficacy.
Our findings are consistent with results from prior studies demonstrating that estimated Vo2peak equations developed in individuals without comorbidities have low validity in noncancer clinical populations.37,38 In obese patients with metabolic syndrome, Debeaumont et al37 reported that estimated Vo2peak ranged from −5% to 31% of measured Vo2peak. Similarly, Moneghetti et al38 reported that FRIEND estimated Vo2peak was significantly different than CPET Vo2peak in 1,094 patients referred for CPET evaluation for HF symptoms. ACSM and FRIEND equations were developed in young (ie, 19-26 years old)15 and older healthy (ie, >40 years, free from cardiovascular disease)16 participants. The discrepancy between estimated and measured Vo2peak in clinical settings may be caused by the omission of potentially important clinical factors contributing to impaired Vo2peak. As such, our findings demonstrating that ACSM and FRIEND equations have poor validity in patients previously treated for primary breast cancer support the application of alternative equations to estimate Vo2peak.
Intriguingly, there was high validity between measured Vo2peak and estimated Vo2peak using the equation developed in patients with HF. The HF-FRIEND equation was developed from a cohort that included HF patients with both reduced and preserved ejection fraction.17 Although patients with primary breast cancer in the present trial had intact resting systolic function, whether the strong correlation between HF-FRIEND estimated and measured Vo2peak was caused by a preserved ejection fraction phenotype is not known. Patients with breast cancer reach Vo2peak for a particular age group approximately 20 to 30 years earlier than apparently healthy women without a history of breast cancer10; therefore, applying equations with treadmill grade and speed derived from patients with HF with similar Vo2peak is likely more accurate than those derived from nonclinical populations. Impaired Vo2peak in patients with breast cancer is also attributed, in part, to a blunted inotropic response.39,40 Thus, given that breast cancer patients have an intact chronotropic reserve,39,40 prediction equations derived from patients with similar exercise limitations such as HF with a reduced or preserved ejection fraction will likely improve the accuracy of Vo2peak estimations.41
Although there was high validity between measured and HF-FRIEND estimated Vo2peak, the inclusion of cancer treatment history in Vo2peak estimation formulas may be important given the potential of anticancer treatment regimens to impact all components of O2 transport and uptake.42 In addition to adverse cardiac effects, radiation and chemotherapy can result in pulmonary dysfunction, anemia, and vascular and skeletal muscle dysfunction.12 To this end, cancer treatment history was a key factor that distinguished oncology-specific from other estimated Vo2peak equations where Oncpeak includes previous chemotherapy and Oncsub includes previous radiation. The reason for the inclusion of disparate treatment modalities between Oncpeak and Oncsub is not known. However, chemotherapy and radiation were retained in 80% and 40% peak models, respectively, whereas in submaximal models, radiation was retained in 100% and chemotherapy in just 40% of the models. These findings suggest the differences are likely caused by specific treatment-related effects on maximal and submaximal exercise responses. For instance, the direct cardiac effects of chemotherapy are likely a primary factor contributing to impaired inotropic response at peak exercise,39,40 whereas the effects of radiation on skeletal muscle may contribute to altered anaerobic glycolysis at submaximal exercise.43 Additional research evaluating the effects of chemotherapy and/or radiation on the contributions of heart rate, stroke volume, and peripheral oxygen extraction at submaximal and peak exercise are needed. Confirmatory studies in larger cohorts are required; however, our findings support the recommendation of Oncpeak or HF-FRIEND in settings where treatment history is not available to estimate Vo2peak in patients with primary breast cancer. In settings where peak exercise tests cannot be performed, Oncsub is an acceptable alternative.
Study limitations
Our results are limited by a relatively small number of breast cancer patients; external validation of oncology-specific estimated Vo2peak equations in a larger cohort is warranted, as well as validation in other patient populations with cancer exposed to chemotherapy and/or radiation. External validation should be performed for the prediction models built using the 2 stepwise selection thresholds considered based on variables retained in 50% (primary results) and 80% (supplement) of models. Second, to develop oncology-specific equations, we used binary indicators of treatment exposure. Future studies should evaluate whether the inclusion of specific doses and agents improves estimated Vo2peak accuracy. Finally, most CPETs in the United States are performed on a treadmill; our findings will not extend to nonincremental and cycle ergometry CPETs.
Conclusions
In summary, equations developed in healthy noncancer populations to estimate Vo2peak are suboptimal in patients previously treated for breast cancer. HF-FRIEND or oncology-specific equations could be applied to estimate Vo2peak in settings where CPETs are not available.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Accurate assessment of CRF in the large and rapidly growing population of patients with primary breast cancer is of high importance for risk stratification, toxicity monitoring, and evaluation of the efficacy of exercise interventions. However, the widespread applicability of directly measured is limited by requirements for specialized equipment and trained personnel. We found that in post-treatment patients with primary breast cancer, equations developed in healthy noncancer populations to estimate CRF are suboptimal. Heart failure and oncology-specific estimated CRF equations had high validity and could be used to estimate CRF in breast cancer settings where CPETs are not available.
TRANSLATIONAL OUTLOOK: Further research evaluating the validity of heart failure and oncology-specific estimated CRF equations in other cancer settings is needed.
Funding Support and Author Disclosures
This study was supported by a research grant from the National Cancer Institute (R01-CA142566) awarded to Dr Jones and grants from AKTIV Against Cancer and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). Dr Jones has stock ownership in Pacylex, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Lakoski S.G., Eves N.D., Douglas P.S., et al. Exercise rehabilitation in patients with cancer. Nat Rev Clin Oncol. 2012;9:288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R., Blair S.N., Arena R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. doi: 10.1161/CIR.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 3.ATS/ACCP ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 4.Myers J., Forman D.E., Balady G.J., et al. Supervision of exercise testing by nonphysicians: a scientific statement from the American Heart Association. Circulation. 2014;130:1014–1027. doi: 10.1161/CIR.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones L.W., Haykowsky M.J., Swartz J.J., et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Cupit-Link M.C., Kirkland J.L., Ness K.K., et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2 doi: 10.1136/esmoopen-2017-000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herrero F., Balmer J., San Juan A.F., et al. Is cardiorespiratory fitness related to quality of life in survivors of breast cancer? J Strength Cond Res. 2006;20:535–540. doi: 10.1519/r-18215.1. [DOI] [PubMed] [Google Scholar]
- 8.Jones L.W., Haykowsky M., Pituskin E.N., et al. Cardiovascular reserve and risk profile of postmenopausal women after chemoendocrine therapy for hormone receptor--positive operable breast cancer. Oncologist. 2007;12:1156–1164. doi: 10.1634/theoncologist.12-10-1156. [DOI] [PubMed] [Google Scholar]
- 9.Jones L.W., Haykowsky M., Peddle C.J., et al. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev. 2007;16:1026–1031. doi: 10.1158/1055-9965.EPI-06-0870. [DOI] [PubMed] [Google Scholar]
- 10.Jones L.W., Courneya K.S., Mackey J.R., et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J Clin Oncol. 2012;30:2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groarke J.D., Payne D.L., Claggett B., et al. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6(4):315–322. doi: 10.1093/ehjqcco/qcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott J.M., Nilsen T.S., Gupta D., et al. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137:1176–1191. doi: 10.1161/CIRCULATIONAHA.117.024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott J.M., Stene G., Edvardsen E., et al. performance status in cancer: not broken, but time for an upgrade? J Clin Oncol. 2020;38(25):2824–2829. doi: 10.1200/JCO.20.00721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brawner C.A., Ehrman J.K., Keteyian S.J. Are international standards for exercise capacity ready for prime time? Mayo Clin Proc. 2020;95:218–220. doi: 10.1016/j.mayocp.2019.12.018. [DOI] [PubMed] [Google Scholar]
- 15.American College of Sports Medicine . 10th ed. Wolters Kluwer; 2018. ACSM’s Guidelines for Graded Exercise Testing and Prescription; pp. 226–267. [Google Scholar]
- 16.Kokkinos P., Kaminsky L.A., Arena R., et al. New generalized equation for predicting maximal oxygen uptake (from the Fitness Registry and the Importance of Exercise National Database) Am J Cardiol. 2017;120:688–692. doi: 10.1016/j.amjcard.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 17.Kokkinos P., Kaminsky L.A., Arena R., et al. New equations for predicting maximum oxygen uptake in patients with heart failure. Am J Cardiol. 2020;128:7–11. doi: 10.1016/j.amjcard.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 18.Scott J.M., Zabor E.C., Schwitzer E., et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones L.W., Eves N.D., Haykowsky M., et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008;9:757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 20.Scott J.M., Thomas S.M., Peppercorn J.M., et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: a randomized controlled trial. Circulation. 2020;141:560–570. doi: 10.1161/CIRCULATIONAHA.119.043483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald M.D., Tanaka H., Tran Z.V., et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: a meta-analysis. J Appl Physiol (1985) 1997;83:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 22.Balady G.J., Arena R., Sietsema K., et al. Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 23.Witten D., Tibshirani R., Hastie T., et al. Springer; 2013. An Introduction to Statistical Learning: With Applications in R. [Google Scholar]
- 24.Bland J.M., Altman D.G. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–582. doi: 10.1080/10543400701329422. [DOI] [PubMed] [Google Scholar]
- 25.Bland J.M., Altman D.G. Statistical methods for assessing agreement between 2 methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.Carrasco J.L., Jover L. Estimating the generalized concordance correlation coefficient through variance components. Biometrics. 2003;59:849–858. doi: 10.1111/j.0006-341x.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 28.Kodama S., Saito K., Tanaka S., et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 29.Peel A.B., Barlow C.E., Leonard D., et al. Cardiorespiratory fitness in survivors of cervical, endometrial, and ovarian cancers: The Cooper Center Longitudinal Study. Gynecol Oncol. 2015;138:394–397. doi: 10.1016/j.ygyno.2015.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Lakoski S.G., Barlow C.E., Koelwyn G.J., et al. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer 7 years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res Treat. 2013;138:909–916. doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood W.A., Deal A.M., Reeve B.B., et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013;48:1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 32.Jones L.W., Watson D., Herndon J.E., 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116:4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brunelli A., Pompili C., Salati M., et al. Preoperative maximum oxygen consumption is associated with prognosis after pulmonary resection in stage I non-small cell lung cancer. Ann Thorac Surg. 2014;98:238–242. doi: 10.1016/j.athoracsur.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 34.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laukkanen J.A., Zaccardi F., Khan H., et al. Long-term change in cardiorespiratory fitness and all-cause mortality: a population-based follow-up study. Mayo Clin Proc. 2016;91:1183–1188. doi: 10.1016/j.mayocp.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Imboden M.T., Harber M.P., Whaley M.H., et al. The influence of change in cardiorespiratory fitness with short-term exercise training on mortality risk from the Ball State Adult Fitness Longitudinal Lifestyle Study. Mayo Clin Proc. 2019;94:1406–1414. doi: 10.1016/j.mayocp.2019.01.049. [DOI] [PubMed] [Google Scholar]
- 37.Debeaumont D., Tardif C., Folope V., et al. A specific prediction equation is necessary to estimate peak oxygen uptake in obese patients with metabolic syndrome. J Endocrinol Invest. 2016;39:635–642. doi: 10.1007/s40618-015-0411-7. [DOI] [PubMed] [Google Scholar]
- 38.Moneghetti K.J., Hock J., Kaminsky L., et al. Applying current normative data to prognosis in heart failure: The Fitness Registry and the Importance of Exercise National Database (FRIEND) Int J Cardiol. 2018;263:75–79. doi: 10.1016/j.ijcard.2018.02.102. [DOI] [PubMed] [Google Scholar]
- 39.Koelwyn G.J., Lewis N.C., Ellard S.L., et al. Ventricular-arterial coupling in breast cancer patients after treatment with anthracycline-containing adjuvant chemotherapy. Oncologist. 2016;21:141–149. doi: 10.1634/theoncologist.2015-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khouri M.G., Hornsby W.E., Risum N., et al. Utility of 3-dimensional echocardiography, global longitudinal strain, and exercise stress echocardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res Treat. 2014;143:531–539. doi: 10.1007/s10549-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haykowsky M.J., Tomczak C.R., Scott J.M., et al. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol. 1985;119:739–744. doi: 10.1152/japplphysiol.00049.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koelwyn G.J., Jones L.W., Moslehi J. Unravelling the causes of reduced peak oxygen consumption in patients with cancer: complex, timely, and necessary. J Am Coll Cardiol. 2014;64:1320–1322. doi: 10.1016/j.jacc.2014.07.949. [DOI] [PubMed] [Google Scholar]
- 43.Adams G.R., Caiozzo V.J., Haddad F., et al. Cellular and molecular responses to increased skeletal muscle loading after irradiation. Am J Physiol Cell Physiol. 2002;283:C1182–C1195. doi: 10.1152/ajpcell.00173.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.