Abstract
Background
Cardiac rehabilitation (CR) is endorsed to improve cardiovascular outcomes in cancer survivors. The quality of CR-based research in oncology has not been assessed.
Objectives
The aim of this study was to evaluate the quality of reporting and evidence from CR-based intervention studies in oncology and to explore associations between intervention participation and outcomes.
Methods
Systematic searches of 5 databases were conducted (January 2020) and updated (September 2021). Randomized and nonrandomized studies evaluating CR-based interventions in adult cancer survivors during and after treatment were eligible. Independent reviewers extracted data using 2 reporting guidelines (Template for Intervention Description and Replication and Consolidated Standards for Reporting Trials Harms extension), risk of bias (ROB) assessment tools (Cochrane ROB 2.0 and Cochrane Risk of Bias in Non-Randomized Studies of Interventions), and a combined inventory (Tool for the Assessment of Study Quality and reporting in Exercise). A meta-analysis was used to explore pre-intervention/post-intervention differences for commonly assessed outcomes.
Results
Ten studies involving data from 685 survivors were included. The mean quality scores for intervention reporting (Template for Intervention Description and Replication) and harms (Consolidated Standards for Reporting Trials Harms extension) were 62% and 17%, respectively. There was moderate-to-high ROB across nonrandomized (Cochrane Risk of Bias in Non-Randomized Studies of Interventions score: 25%) and randomized (ROB 2.0 score: 50%) studies. The mean standardized cardiorespiratory fitness was higher (0.42; 95% CI: 0.27-0.57), fatigue was lower (−0.45; 95% CI: −0.55 to −0.34), and percent body fat (0.07; 95% CI: −0.23 to 0.38) was not different in survivors completing CR compared with those not completing CR.
Conclusions
CR-based studies in oncology have low-to-moderate reporting quality and moderate-to-high ROB limiting interpretation, reproducibility, and translation of this evidence into practice.
Key Words: bias, biomedical research standards, cardiology, data reporting, exercise therapy, oncology
Abbreviations and Acronyms: CONSORT, Consolidated Standards for Reporting Trials; CR, cardiac rehabilitation; CRF, cardiorespiratory fitness; CVD, cardiovascular disease; RCT, randomized controlled trial; ROB, risk of bias; ROBINS-I, Cochrane Risk of Bias in Non-Randomized Studies of Interventions; TESTEX, Tool for the Assessment of Study Quality and Reporting in Exercise; TIDieR, Template for Intervention Description and Replication; Vo2peak, peak oxygen consumption
Central Illustration
Cancer-specific mortality rates have decreased ∼30% in 3 decades due in part to advances in cancer diagnostics and therapeutics.1 However, common cancer therapies directly injure the cardiovascular, pulmonary, and skeletal muscle systems,2 leading to organ-specific and systemic cardiovascular toxicity.3 These direct treatment–related toxicities are exacerbated by pre-existing and indirect treatment–related factors4 like adverse lifestyle changes (eg, physical inactivity)4 and cardiovascular disease (CVD) risk factors (eg, hypertension),5,6 leading to increased CVD morbidity and mortality risk. CVD is a major source of chronic morbidity in vulnerable survivor groups (eg, pediatric, adolescent, and young adult cancers)7,8 and a leading cause of late-occurring noncancer mortality among survivors of select solid (eg, breast cancer4) and hematological (eg, Hodgkin and non-Hodgkin lymphoma9) malignancies. Despite these risks, highly effective approaches to preventing and treating cancer-related cardiovascular toxicity and CVD have not been established.
The American Heart Association and American Cancer Society recently endorsed a multimodal cardiac rehabilitation (CR)-based approach to improve cardiovascular outcomes in cancer survivors.10 Multimodal CR typically involves a comprehensive medical evaluation, exercise prescription, pharmaceutical and behavioral CVD risk factor management, and education/counseling support.11 This approach aims to improve cardiorespiratory fitness (CRF) and physical function, reduce CVD symptom burden and event risk, improve psychosocial well-being, and reduce CVD-related mortality.11 Thus, it appears CR may be ideally suited to address the multiple competing mechanisms of CVD risk in cancer survivors. The widespread adoption of CR-based programming to prevent and treat cancer-related CVD must be predicated on robust evidence from randomized controlled trials (RCTs) demonstrating the safety, tolerability, and efficacy of the intervention strategy—the empirical standard for all medical therapies. However, research supporting the specific benefits of CR-based interventions in oncology is scant, and the quality of the available evidence has not been evaluated. Two prior reviews have described the practical elements (eg, intervention components) of CR-based research in oncology.12,13 However, the reported associations12 and causal impact13 of the tested interventions on the evaluated outcomes were either minimally12 or not13 discussed in the context of the quality of the evidence (eg, high attrition rates and the majority were single-arm and nonrandomized studies), which has potentially led to biased interpretations of the safety, tolerability, and benefits of CR-based interventions for cancer survivors.14
Therefore, the objective of this study was to evaluate the quality of research reporting and evidence (ie, risk of bias [ROB]) from studies evaluating the effects of CR-based interventions in cancer survivors. Our secondary objective was to synthesize the evidence regarding whether participation in CR-based interventions was associated with changes in CRF, CVD risk factors, and patient-reported outcomes and interpret the findings within the context of the quality of evidence.
Methods
Data searches and sources
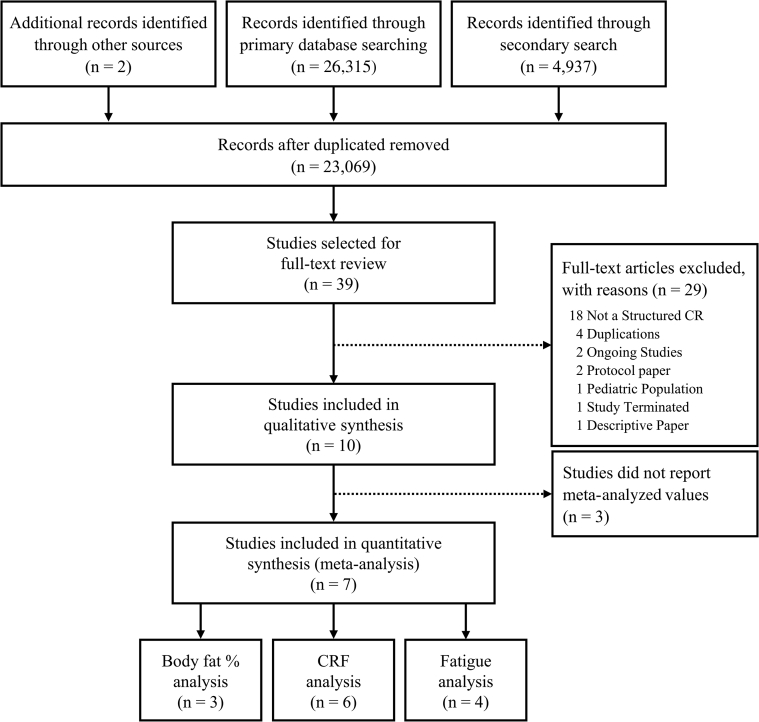
The study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines15 and the AMSTAR 2 inventory.16 Review methods were established and registered before initiating full-text extraction (PROSPERO ID#: CRD42020182679). Full details of study methods (including the quality checklists, search strategy, team training, data extraction methods and materials, and processing methods) are provided in the Supplemental Methods sections. Briefly, a research informationist (A.O.-C.) conducted a systematic search of Ovid MEDLINE, Ovid Embase, Cochrane Database of Systematic Reviews (Ovid), Cochrane Central Register of Controlled Trials (Ovid), and CINAHL (Ebsco) on January 16, 2020. Two component parts made up the search strategy: cardiac rehabilitation and cancer with relevant key words and controlled vocabulary. The search strategy for cancer was modified with permission from Howell et al.17 The search was repeated on September 15, 2021, to identify studies published after study inception. Because of database availability, the version of CINAHL searched in January 2020 was CINAHL with Full Text, and in September 2021, it was CINAHL Complete. Manual searches of reference lists from related publications were also performed (Figure 1).
Figure 1.
PRISMA Participant Flow Diagram
Flow diagram depicts the: 1) methods and results for 3 different search strategies; and 2) results of record screening and inclusion in the systematic review and meta-analysis. CR = cardiac rehabilitation; CRF = cardiorespiratory fitness; PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Study eligibility criteria
Given the limited evidence base, reports from RCTs, nonrandomized trials, single-arm trials, and both prospective and retrospective cohort studies that evaluated interventions explicitly described as involving or being modeled after CR within human adults (≥18 years) with previous diagnosis of cancer were eligible. Abstracts, reviews (eg, narrative, scoping, or systematic), meta-analyses, case studies, editorials, commentaries, and research letters were excluded.
Team training and study materials
Study reviewers (R.F. and S.S.P.) completed >20 hours of training in data extraction over a 6-week period. Similar to a previous study,14 the training consisted of 1) independent data extraction from sample articles using a custom data extraction reference guide and 2) regular investigator-led (S.C.A.) review sessions to evaluate the accuracy and completeness of data extractions and to improve the function of the data extraction tools (DistillerSR, Evidence Partners). The data extraction reference guide was updated regularly during the extraction training to improve the clarity and utility of its content.
Data extraction
Trained reviewers (R.F. and S.S.P.) independently screened titles and abstracts using Covidence (Covidence; ASTL). Full texts of potential articles were uploaded and independently reviewed using the DistillerSR web platform (Evidence Partners) to confirm eligibility. Data were extracted for all eligible studies from the primary article and all other related publicly available data sources (eg, study protocols and trial registries) using custom data extraction forms (via DistillerSR) and a data extraction reference guide. Discrepancies were resolved by consensus (R.F. and S.S.P.), and disagreements were adjudicated by a third party (S.C.A.).
Evaluation measures
Studies were evaluated using standardized inventories for assessing research reporting quality and ROB. Intervention reporting quality was assessed using an expanded (17-item) version of the Template for Intervention Description and Replication (TIDieR) checklist.18 Harms reporting quality was assessed via the 10-item Consolidated Standards of Reporting Trials Harms extension (CONSORT-Harms).19 Reporting quality items were rated (with equal weighting and a maximum score of 1 point per item) as “properly reported,” “incompletely reported,” “not reported,” “no information,” or “not applicable.” Properly reported items were assigned a 1, and all other reporting quality ratings were assigned a 0. Items rated as not applicable were excluded from subsequent quality score calculations.
ROB was assessed using the 5-domain Cochrane ROB 2.0 (ROB 2.0) inventory for randomized trials20,21 and the 7-domain Cochrane ROB in Non-Randomized Studies of Interventions (ROBINS-I) inventory for nonrandomized studies.22,23 Items within the ROB 2.0 and ROBINS-I domains were scored as “yes,” “probably yes,” “probably no,” “no,” “no information,” or “not applicable” per inventory guidelines. Domains were assigned an overall ROB rating. For ROB 2.0, the domains were rated as “low ROB,” “some concerns,” or “high ROB.” ROBINS-I domains were rated as “low ROB,” “moderate ROB,” “serious ROB,” “critical ROB,” or “no information.” Similar to previous research,14 ROB scores were then created (with equal weighting and a maximum score of 1 point per domain), with low ROB domains being assigned a 1 and all other possible ROB ratings assigned a 0.
Studies were also rated using the Tool for the Assessment of Study Quality and reporting in Exercise (TESTEX),24 a composite tool used to assess the overall quality of exercise trial reporting and ROB. TESTEX items were rated (and scored) as “yes” (properly reported = 1), “unclear” (insufficient information to make a determination = 0), “no” (not reported properly = 0), “no information” (not sufficient information = 0), or “not applicable.”
Percentage scores were calculated for all inventories by dividing the scores by the total number of applicable items, with higher percentage scores (ie, 100% being ideal) indicating better reporting quality and lower ROB.
Outcomes
The co-primary outcomes were studies 1) reporting quality scores measured via TIDieR18 and CONSORT-Harms19 inventories and (2) ROB scores measured via the ROB 2.020 or ROBINS-I22 inventories. Secondary outcomes included potential intervention impact (defined as the difference between pre- and post-intervention group means for the included outcomes), measures of intervention tolerability (including attendance, adherence, and intervention completion rates) and safety (number and severity of adverse events), and overall study quality measured via the TESTEX inventory.24
Data synthesis and statistical analysis
Study characteristics are presented using the mean and SD for continuous variables and counts with percentages for categoric variables. Reporting quality and ROB scores were calculated per inventory as the total achieved score relative to the total number of eligible items for each study. The maximum possible scores for each inventory were 17 for TIDieR, 10 for CONSORT-Harms, 5 for ROB 2.0, 7 for ROBINS-I, and 16 for TESTEX. All scores were calculated and presented numerically and as percentages. Items rated “not applicable” were subtracted from the denominators before calculating the percentage scores for each study. Associations between TESTEX scores and the scores of the other inventories were explored.
Intervention effects were pooled using Meta-Essentials,25 assuming a commonly applied correlation coefficient of 0.5 for repeated measures.26,27 We decided a priori to use random effects analyses given the anticipated heterogeneity in interventions and participant characteristics (eg, cancer diagnoses and treatments). We calculated standardized mean differences (SMDs), 95% CIs, and statistical significance for outcome measures that were reported by 3 or more studies. Statistical heterogeneity across studies was assessed via the Cochran Q test, Kendall tau correlation, and I2 statistic. The I2 values were interpreted as minimal (0% to <30%), moderate (30% to <50%), substantial (50%-90%), and considerable (>90%) heterogeneity.28 The z-test was used to assess the overall effect. A P value <0.05 was considered statistically significant. Publication bias was subjectively assessed via the visual inspection of funnel plots created for each of the assessed outcomes. Inter-rater reliability for each inventory was assessed by calculating the Cohen kappa statistic, with kappa values interpreted as poor (<0.20), fair (0.21-0.40), moderate (0.41-0.60), good (0.61-0.80), and very good (0.81-1.00).29
Results
Supplemental Tables 1 to 4 and Supplemental Figure 1 provide full details of studies, interventions, and results. A total of 26,315 and 4,937 records were identified via our primary (January 2020) and secondary (September 2021) searches, respectively. Subsequently, 8,183 duplicates were removed using EndNote citation management (Clarivate Analytics) and Covidence software, leaving 23,069 records for screening. Thirty-nine records underwent full-text review, and 10 studies were deemed eligible.30, 31, 32, 33, 34, 35, 36, 37, 38, 39 There was moderate to good inter-rater agreement across inventories (TIDieR [κ = 0.528; 95% CI: 0.476-0.580; P < 0.001], CONSORT-Harms [κ = 0.489; 95% CI: 0.416-0.562; P < 0.001], ROB [κ = 0.481; 95% CI: 0.448-0.514; P < 0.001], and TESTEX [κ = 0.675; 95% CI: 0.631-0.719; P < 0.001]).
Participant, study, and intervention characteristics
Participant characteristics are provided in Table 1. Studies included 741 total participants, with a mean sample size of 74.1 ± 77.9 participants (range 20-280). A total of 685 included participants (92%) had a previous cancer diagnosis. The majority of participants received CR-based interventions (n = 704, 95%) ranging from 6 to 26 weeks, whereas 37 (5%) were in a non-CR arm (Table 2). Participants were predominantly female (76%), had a history of breast cancer (61%), and had a mean age of 57.7 ± 5.5 years (range 24-86 years). Three of 10 studies reported the prevalence of CVD risk factors, which ranged from 13% to 40% in these studies (Table 1). Study designs included single-arm cohorts (n = 3 retrospective, n = 6 prospective) and 1 RCT. Only 2 prospective studies justified testing a CR-based model to specifically address CVD risk factors and biomarkers.37,39 Key intervention components (ie, frequency, intensity, time, type, location, and supervision) varied across studies (Table 2).
Table 1.
Participant Characteristics in Included Studies
| Studies Reporting Variable of Interest | Participants (N = 741) | |
|---|---|---|
| Age | 10 (100) | 57.7 (5.5) |
| Female | 10 (100) | 541 (75) |
| Smokers | 3 (30) | 38 (19) |
| CVD risk factors | ||
| Hypertension | 3 (30) | 56 (40) |
| Dyslipidemia | 1 (10) | 4 (13) |
| Type 2 diabetes mellitus | 1 (10) | 10 (19) |
| Medications | ||
| ACE inhibitor | 2 (20) | 47 (42) |
| Beta adrenergic antagonist | 2 (20) | 70 (63) |
| Statin | 2 (20) | 66 (59) |
| Cancer diagnosis | ||
| Breast cancer | 7 (70) | 449 (61) |
| Prostate cancer | 3 (30) | 45 (6) |
| Colorectal carcinoma | 3 (30) | 52 (7) |
| Hematologic | 3 (40) | 43 (6) |
| Othera | 2 (20) | 96 (13) |
| Cancer stage | ||
| Stage 0 | 1 (10) | 2 (1) |
| Stage 1 | 2 (20) | 30 (4) |
| Stage 2 | 2 (20) | 31 (4) |
| Stage 3 | 2 (20) | 15 (2) |
| Stage 4 | 1 (10) | 7 (1) |
| Treatment exposure | ||
| Surgery | 5 (50) | 483 (65) |
| Any chemotherapy | 7 (70) | 381 (51) |
| Anthracyclines | 1 (10) | 10 (1) |
| Trastuzumab | 2 (20) | 19 (3) |
| Any radiotherapy | 6 (60) | 379 (51) |
| Thoracic radiotherapy | 6 (60) | 7 (1) |
Values are n (%).
ACE = angiotensin-converting enzyme; CVD = cardiovascular disease.
Includes ovarian, endometrial, cervical, kidney, skin, lung, testicular, bladder, bone, and sarcoma.
Table 2.
Study and Intervention Characteristics
| Bonsignore et al, 201731 | Bonsignore et al, 201830 | De Jesus et al, 201732 | Dittus et al, 201533 | Dolan et al, 201834 | Hubbard et al, 201635 | Hubbard et al, 201836 | Rothe et al, 201837 | Young-McCaughan et al, 200338 | Zvinovski et al, 202139 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Study design | Retrospective cohort | Retrospective cohort | Prospective cohort | Prospective cohort | Retrospective cohort | RCT | Prospective cohort | Prospective cohort | Prospective cohort | Prospective cohort |
| Cancer diagnosis | Breast | Prostate | Breast | Mixeda | Breast | Colorectal | Breast | Hematological | Mixedb | Breast |
| Time since diagnosis | ∼90 months | NR | ∼10 monthsc | 30 months | ∼29 monthsc | NR | NR | NR | NR | NR |
| Participants/arm, n (%) | INT: 58 (100) | INT: 54 (100) | INT: 20 (100) | INT: 280 (100) | INT: 152 (100) | INT: 21 (51) CON: 20 (49) | INT: 3 (15) PA: 17 (85) | INT: 30 (100) | INT: 62 (100) | INT: 24 (100) |
| Age, y (mean) | 57 | 75 | 53 | 56 | 55 | 66 | 57 | 56 | 59 | 53 |
| Female, n (%) | 58 (100) | 0 (0) | 20 (100) | 240 (86) | 152 (100) | 14 (34) | 20 (100) | 6 (20) | 31 (50) | 24 (100) |
| Location(s) | Rehab center, public gym | Rehab center | Public gym | University | Rehab center | Medical center, public gym | Medical center, public gym | NR | Medical center, home | Medical center |
| Intervention supervision | Mixed | Mixed | Supervised | Mixed | Mixed | Supervised | Supervised | Supervised | Mixed | NR |
| Intervention modality | NR | NR | Treadmill, cycle and stepper | Walking | Walking | Cycle, walking, strength training | NR | NR | NR | NR |
| Training frequency | SUP: 1×/wk | SUP: 1×/wk | SUP: 3×/wk | SUP: 2×/wk | SUP: 1×/wk | SUP: 1-2×/wk | SUP: 1×/wk | SUP: 1×/wk | SUP: 2×/wk | 3×/wk |
| UNS: 4×/wk | UNS: 4×/wk | UNS: 2-3×/wk | UNS: 4×/wk | UNS: 3-5×/wk | ||||||
| Training intensity | 60%-80% Vo2peak | 60%-80% Vo2peak | 50%-70% Vo2peak | AET: 70%-85% HRR RET: 60%-70% 1RM | 60%-80% Vo2peak | 6-20 RPE | NR | NR | NR | 60%-85% Vo2peak |
| Training duration, min | 60 | 60 | 15-45 | 20-50 | NR | 60-90 | 60 | NR | NR | NR |
| Intervention duration, wk | 26 | 26 | 16 | 12 | 22 | 6-12 | 12 | 8 | 12 | 14 |
| Cointervention details | None | Behavioral counseling, nutrition | None | Behavior counseling, nutrition | Behavior counseling, nutrition | Behavior counseling, nutrition | Behavior counseling, nutrition | Behavior counseling, nutrition | Behavior counseling, nutrition | None |
| Attendance | NR | NR | NR | NR | 14/22 (64%) | S1: 100%; S2: 107%; S3: 92% | 23/36 (64%) | NR | 19/24 (79%) | NR |
| Adherence | NR | NR | NR | NR | NR | 13 (62%) | NR | NR | NR | NR |
| Attrition, n (%) | 20 (48) | 0 (0) | 11 (55) | 57 (26) | 122 (80) | 3 (7) | 7 (35) | 15 (33) | 16 (26) | 7 (29) |
| Total AEs | NR | NR | NR | INT: NR Non-INT: 6 | TEST: 12 (8%) INT: 0 | NR | NR | TEST: 2 (7%) INT: 0 | NR | INT: 1 Non-INT: 2 |
AET = aerobic exercise training; CON = control; INT = intervention related; Non-INT = nonintervention related; NR = not reported; PA = physical activity; RCT = randomized controlled trial; RET = resistance exercise training; RPE = rating of perceived exertion; S = site; SUP = supervised; TEST = testing related; UNS = unsupervised; Vo2peak = peak oxygen consumption.
Mixed cancer includes breast, colorectal, hematologic, lung, prostate, and others.
Mixed cancer includes breast, prostate, ovarian, colorectal, endometrial, cervical, kidney, non-Hodgkin lymphoma, skin, lung, testicular, bladder, bone, Hodgkin’s disease, leukemia, and sarcoma.
Time since cancer surgery.
Primary outcomes
Across studies, the mean intervention reporting quality score was 62% (9.5/15.3 mean eligible TIDieR items), and the mean harms reporting quality score was 17% (1.1/6.4 mean eligible CONSORT-Harms items). Within TIDieR, details regarding intervention progression and adherence, interventionist expertise and training, and supporting activities were missing or incompletely reported by >50% of studies. According to CONSORT-Harms, details of harms definitions, data collection, analysis plans, results, and harms-related discussions were incomplete or missing entirely for 60% to 100% of studies (Table 3).
Table 3.
Quality of Reporting Across All Studies
| Bonsignore et al, 201731 | Bonsignore et al, 201830 | De Jesus et al, 201732 | Dittus et al, 201533 | Dolan et al, 201834 | Hubbard et al, 201635 | Hubbard et al, 201836 | Rothe et al, 201837 | Young-McCaughton et al, 200338 | Zvinovski et al, 202139 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Reporting | ||||||||||
| CONSORT-Harms score | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 3 | 0 | 2 |
| Eligible itemsa | 7 | 6 | 6 | 10 | 7 | 4 | 7 | 5 | 3 | 9 |
| Percent | 0 | 0 | 0 | 0 | 29 | 100 | 0 | 60 | 0 | 22 |
| TIDieR score | 11 | 13 | 13 | 8 | 10 | 11 | 10 | 4 | 7 | 8 |
| Eligible itemsa | 14 | 16 | 15 | 15 | 15 | 16 | 16 | 15 | 16 | 15 |
| Percent | 79 | 81 | 87 | 53 | 67 | 69 | 63 | 27 | 38 | 53 |
| Risk of bias | ||||||||||
| Cochrane ROB-2 score | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | 3 | Not applicable | Not applicable | Not applicable | Not applicable |
| Eligible itemsa | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | 6 | Not applicable | Not applicable | Not applicable | Not applicable |
| Percent | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | 50 | Not applicable | Not applicable | Not applicable | Not applicable |
| Cochrane ROBINS-I score | 2 | 3 | 2 | 1 | 3 | Not applicable | 1 | 1 | 0 | 3 |
| Eligible itemsa | 7 | 7 | 7 | 7 | 7 | Not applicable | 7 | 7 | 7 | 7 |
| Percent | 29 | 43 | 29 | 14 | 43 | Not applicable | 14 | 14 | 0 | 43 |
| Composite scores | ||||||||||
| TESTEX | 8 | 7 | 6 | 2 | 5 | 9 | 3 | 4 | 4 | 4 |
| Eligible itemsa | 11 | 10 | 8 | 9 | 9 | 15 | 11 | 10 | 9 | 9 |
| Percent | 73 | 70 | 75 | 22 | 56 | 60 | 27 | 40 | 44 | 44 |
CONSORT = Consolidated Standards for Reporting Trials; ROB-2 = Risk of Bias 2; ROBINS-I = Cochrane Risk of Bias in Non-Randomized Studies of Interventions; TESTEX = Tool for the Assessment of Study Quality and Reporting in Exercise; TIDieR = Template for Intervention Description and Replication.
Eligible item totals reflect the total number of items rated as Not Applicable subtracted from the total number of inventory items.
The mean ROBINS-I percentage score across the 9 nonrandomized studies was 25%. Between 50% and 100% of the studies were rated as having moderate-to-critical ROB across 5 of the 7 domains within ROBINS-I (Table 4) (domains 1 [confounding], 2 [participant selection], 4 [intervention deviation], 5 [missing data], and 7 [selective reporting]), whereas the single RCT had a ROB-2 score of 50% and high ROB related to adherence and missingness of data.
Table 4.
Risk of Bias Ratings for Studies Using the Cochrane Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) and Risk of Bias (ROB) 2.0 Inventories
| First Author, Year, Ref. # | ROBINS-I Domains |
||||||
|---|---|---|---|---|---|---|---|
| Domain 1: Confounding | Domain 2: Selection of Participants | Domain 3: Classification of Intervention | Domain 4: Deviation From Intended Interventions | Domain 5: Missing Data | Domain 6: Measurement of Outcomes | Domain 7: Selection of the Reported Result | |
| Bonsignore et al, 201731 |  |
 |
 |
 |
 |
 |
 |
| Bonsignore et al, 201830 |  |
 |
 |
 |
 |
 |
 |
| De Jesus et al, 201732 |  |
 |
 |
 |
 |
 |
 |
| Dittus et al, 201533 |  |
 |
 |
 |
 |
 |
 |
| Dolan et al, 201834 |  |
 |
 |
 |
 |
 |
 |
| Hubbard et al, 201836 |  |
 |
 |
 |
 |
 |
 |
| Rothe et al, 201837 |  |
 |
 |
 |
 |
 |
 |
| Young-McCaughton et al, 200338 |  |
 |
 |
 |
 |
 |
 |
| Zvinovski et al, 202139 |  |
 |
 |
 |
 |
 |
 |
| First Author, Year, Ref. # | ROB 2.0 Domains |
|||||
|---|---|---|---|---|---|---|
| Domain 1: Randomization | Domain 2A: Deviations–Effect of Assignment | Domain 2B: Deviations–Effect of Adherence | Domain 3: Missing Data | Domain 4: Measurement of Outcome | Domain 5: Selection of Reported Result | |
| Hubbard et al, 201635 |  |
 |
 |
 |
 |
 |
ROBINS-I notes:  = low risk of bias;
= low risk of bias;  = moderate risk of bias;
= moderate risk of bias;  = serious risk of bias;
= serious risk of bias;  = critical risk of bias;
= critical risk of bias;  = no information.
= no information.
ROB 2.0 notes:  = low risk of bias;
= low risk of bias;  = some concerns;
= some concerns;  = high risk of bias.
= high risk of bias.
Secondary outcomes
Overall study quality
The mean TESTEX score was 51%. Items with consistently incomplete or missing information for >50% of eligible studies include assessor blinding, adherence, attendance, intention-to-treat analysis, relative exercise intensity, and exercise volume/energy expenditure. TESTEX scores were strongly correlated with TIDieR scores (r = 0.703, P = 0.02) but not correlated with ROB scores (r = 0.561, P = 0.09) or CONSORT-Harms scores (r = 0.036, P = 0.92).
Safety
Four studies reported adverse events. In 2 studies, all 14 adverse events occurred during preintervention testing and not during the intervention.34,37 A third study reported 6 cancer-related deaths during the observation period.25 The fourth study reported 2 serious arrhythmias that occurred during the study period although not during CR, and 1 headache that occurred during the intervention.39
Tolerability
Attendance was reported in 4 studies (range 64%-100%). One study reported intervention adherence (average 62%). All studies reported participant attrition (mean attrition rate of 37% ± 24.6%).
Intervention effect
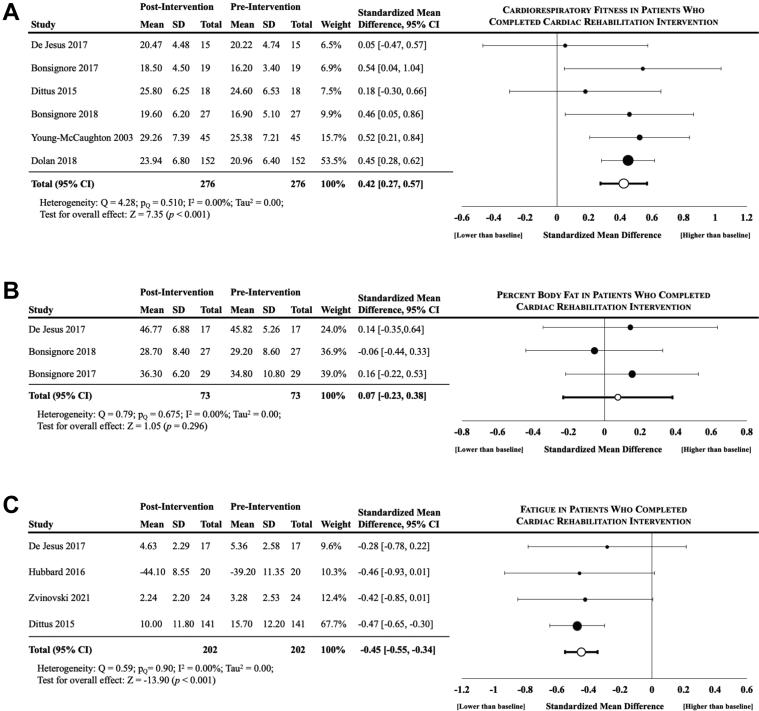
Three outcomes were reported at pre- and postintervention by ≥3 studies and were included in the meta-analysis (Figure 2).
Figure 2.
Differences in CRF, Body Fat Percentage, and Fatigue Following CR
Results of the meta-analysis evaluating the pre- and post-intervention values across studies that reported (A) CRF, (B) body fat percentage, and (C) fatigue. Significant differences were observed for cancer survivors who completed cardiac rehabilitation in CRF and fatigue but not percent body fat. Column header “Total” denotes the number of participants included in analyses. Abbreviations as in Figure 1.
Seven studies assessed CRF differences (n = 6 as peak oxygen consumption [Vo2peak],30, 31, 32, 33, 34,39 n = 1 as metabolic equivalents38); 1 study reported no difference in CRF but was not included in the analysis due to by missingness of data and despite contacting the authors to obtain the data.39 The standardized mean CRF was significantly higher postintervention (SMD = 0.42; 95% CI: 0.27-0.57; I2 = 0.0; P < 0.001) across the 6 analyzed studies. The mean relative Vo2peak was also higher at postintervention (+2.58 [±0.79] mL O2/kg/min) across the 5 studies reporting Vo2peak data.
Three studies reported percent body fat percentage differences.30, 31, 32 Postintervention percent body fat was not different from baseline (SMD = 0.07; 95% CI: −0.23 to 0.38; I2 = 0.0; P = 0.30).
Four studies reported fatigue differences.32,33,35,39 Cancer-related fatigue was significantly lower at postintervention (SMD = −0.45; 95% CI: −0.55 to −0.34; I2 = 0.0; P < 0.001).
Discussion
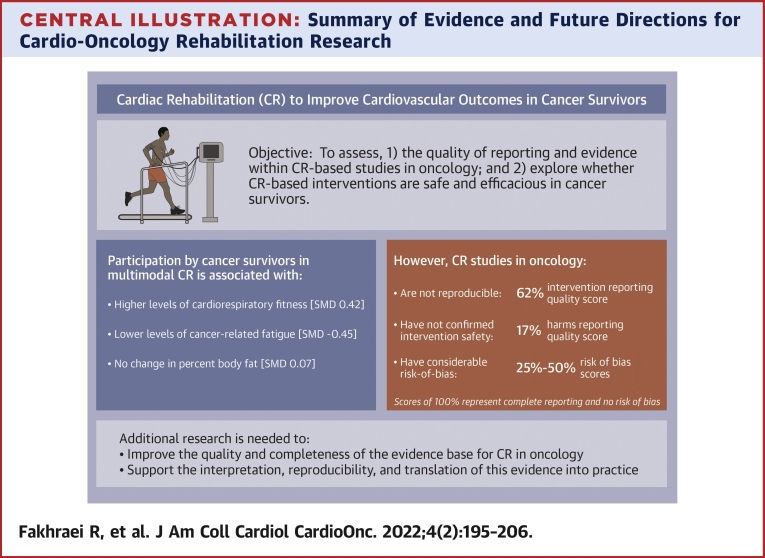
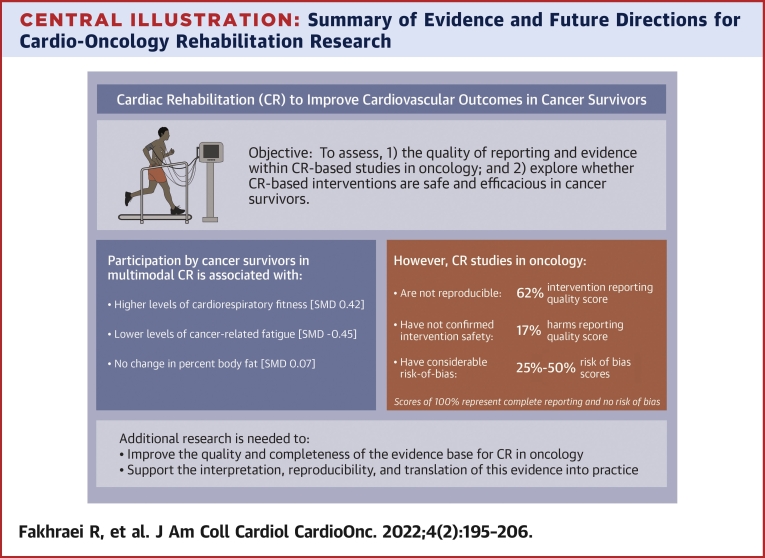
We evaluated reporting quality and ROB within studies exploring the feasibility and impact of CR-based interventions in cancer survivors. Overall, there was inadequate reporting of key intervention- and harms-related details and considerable ROB across studies (90% of which were single-arm cohorts). We also examined evidence of intervention safety, tolerability, and impact and found most articles did not include tolerability or safety data, whereas those that did reported variable testing and intervention-related adverse events, attendance levels, low adherence, and high attrition rates. Our meta-analysis results suggested higher CRF, lower fatigue, and no change in percent body fat after CR-based interventions in participants who completed them. However, non-randomized study designs and suboptimal reporting of critical study methods, intervention delivery parameters, and intervention safety data preclude a balanced interpretation of the available evidence (Central Illustration).
Central Illustration.
Summary of Evidence and Future Directions for Cardio-Oncology Rehabilitation Research
Leading cardiovascular and oncology organizations endorse the use of cardiac rehabilitation–based interventions to improve cardiovascular outcomes in cancer survivors. Despite the preliminary evidence of benefits, the completeness and rigor of the current evidence base are suboptimal. Future research is needed to establish the safety and efficacy of cardio-oncology rehabilitation, improve the interpretation and reproducibility of this evidence, and facilitate the translation of the evidence into practice. CR = cardiac rehabilitation; SMD = standardized mean difference.
Our findings are aligned with those of the 2 systematic reviews to date that evaluated the reporting quality of exercise oncology trials.14,40 A systematic review by Adams et al14 compared the quality of 48 exercise and 48 matched pharmaceutical RCTs in mixed medical fields (31% oncology and 43% cardiovascular medicine) and found exercise trial reports completely described only 57% of requisite intervention-related items assessed via TIDieR. An earlier review evaluated intervention reporting quality in 131 exercise oncology RCTs and found the completeness of TIDieR item reporting ranged from 42% to 96%.40 They further noted that 71% (range 42%-96%) of the items deemed necessary for replication (TIDieR items 3-9) were completely reported40 compared with 37% (range 18%-59%) reporting of these replication items by studies in the current review. Our findings highlight the specific need to improve intervention reporting completeness to facilitate study replication and translation of these interventions into clinical practice.
The reporting of harms- and tolerance-related information was also incomplete. To our knowledge, only 1 study to date evaluated the quality of harms reporting in exercise oncology RCTs; this study found that only 32% of the requisite harms-related information (assessed via CONSORT-Harms) was reported.14 The authors noted that key details related to harms monitoring and reporting were incompletely reported or completely missing from ≥75% of the included exercise RCTs and <50% of studies adequately considered and discussed the potential risks of the tested interventions.14 In our study, only 17% of harms-related information was completely reported, and 40% of the studies reported adverse events. These findings are consistent with previous work and highlight a trend that harms-related data are systematically under-reported across exercise RCTs in clinical populations, including cancer survivors. Incomplete harms reporting precludes the evaluation of risk-benefit ratios for interventions, a metric of considerable importance when evaluating cost-effectiveness and whether interventions should be adopted in practice for clinical populations. A balanced interpretation of the findings from the included studies in this review was further limited by the fact that 90% of the studies failed to provide details regarding intervention adherence and 80% of the studies had attrition rates ranging from 25% to 80%. Collectively, incomplete reporting of adherence data prevents the quantification of dose responses, whereas the incomplete reporting of harms data (eg, adverse event cause, frequency, and severity) and the reasons for participant attrition hinders efforts to confirm or refute the safety and tolerability of CR-based interventions in cancer survivors.
There was a notable discrepancy between inventories used to interpret the quality of the included studies. TESTEX24 was specifically developed to assess the overall quality of exercise trials and consists of items related to both reporting quality and potential ROB. We found TESTEX scores were highly correlated with TIDieR scores but not significantly correlated with harms or ROB scores. This may partially be explained by the fact that TESTEX and TIDieR were both developed to support the reporting and evaluation of behavioral interventions like exercise. However, our findings suggest that the TESTEX tool may provide a more balanced appraisal of exercise research by expanding the inventory to include additional items related to harms, intervention tolerability, and ROB.
Our meta-analysis found that participation in CR-based interventions was associated with more favorable levels of select outcomes in participants who completed the interventions. A scoping review of 9 CR-based studies that included data on 662 cancer survivors reported that participation in CR was associated with favorable effects on multiple health and psychosocial outcomes.12 Similarly, a recent meta-analysis of 33 studies assessing the impact of both cardiac and pulmonary rehabilitation programs in cancer survivors reported the interventions caused significant and clinically meaningful improvements in CRF, 6-minute walk distance, and quality of life.13 The magnitude of improvement in Vo2peak-defined CRF (2.58 mL O2/kg/min) shown here was similar to that reported by the aforementioned meta-analysis (2.9 mL O2/kg/min).13 This is encouraging; however, the findings of these reviews should be interpreted with caution. For instance, 1 study included in our review reported no intervention effect on CRF but could not be included in our meta-analysis because of missingness of data.39 More broadly, the moderate-to-high attrition rates (25%-80%) reported by 80% of our included studies bias the findings toward demonstrating an intervention effect—a factor that was unaddressed and likely biased the findings of the previously mentioned meta-analysis.13 In theory, this source of bias would have been less of an issue if the included studies were RCTs that followed an intention-to-treat analytic approach. However, there has only been a single unpowered pilot RCT published to date assessing the impact of CR-based interventions in cancer survivors.35 Ultimately, the combination of high and unexplained attrition rates together with the previously mentioned incomplete reporting of harms and adherence data make it impossible to confirm or deny whether CR-based interventions are safe, well tolerated, and beneficial for cancer survivors.
Notwithstanding these limitations, we agree with the American Heart Association and American Cancer Society statement that there is intriguing evidence suggesting a potential role for CR-based programs to improve cardiovascular outcomes in cancer survivors. Further research in this area is needed to address the notable gaps in evidence. Priorities for future research include the conduct of rigorous RCTs to confirm the safety, tolerability, efficacy, and effectiveness of CR-based interventions focused on improving CVD-related outcomes in more representative samples of cancer survivors (eg, studies involving a balance of women and men as well as cancer types other than breast). Subsequent meta-analyses, in turn, will then be needed to synthesize, interpret, and discuss the research in the context of the quality of the underlying evidence (like in the general field of exercise oncology41) to support the widespread implementation of CR-based interventions for cancer survivors.
Study limitations
Our study has several important strengths and limitations. To our knowledge, this is the first study to comprehensively assess the quality of research reporting and ROB within reports of CR-based interventions in cardio-oncology. We used rigorous and widely accepted inventories18, 19, 20,22 to comprehensively evaluate all currently available exercise studies in the field and contextualize the findings of our meta-analysis. However, items within the various inventories used to evaluate the studies were not always relevant to all studies. For example, the ROB-2 and ROBINS-I inventories were not necessarily designed to evaluate reports from exercise or retrospective trials. Consequently, select items (eg, participant blinding and harms reporting [for retrospective studies]) were not applicable and had to be excluded from our analyses, which may have introduced some measurement bias. Moreover, our moderate inter-rater agreement indicates that there was variability in primary data extraction by our team. However, we systematically addressed this discordance and achieved consensus via careful review of the extracted data within the context of our study reference guide, the supplemental data extraction notes taken by each team member, and oversight provided by the study lead (S.C.A.).
Conclusions
In summary, studies of CR-based interventions in cancer survivors have low-to-moderate overall quality of research reporting and moderate-to-high ROB, which limits the reproducibility, interpretation, and translation of this evidence into practice. Our meta-analysis confirms previous work that participation in CR-based interventions is associated with improvements in select outcomes. However, major limitations in the design, conduct, and reporting of studies preclude the interpretation of causation. There is a clear need for further research that is rigorously conducted and reported in order to better evaluate the safety, tolerability, and potential benefits of CR-based interventions in cancer survivors and, ultimately, facilitate the translation of this evidence into practice.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Prior studies suggest that cardiac rehabilitation can improve cardiorespiratory fitness and quality of outcomes for cancer survivors. However, most of these studies are of suboptimal rigor and confounded by a high risk of bias. Cardiac rehabilitation can be offered to cancer survivors meeting pre-existing criteria, but the recommendations are otherwise based on expert opinion rather than empirical data.
TRANSLATIONAL OUTLOOK: More rigorous research is needed to confirm the safety and efficacy of cardiac rehabilitation–based interventions in cancer survivors before it can be widely adopted as a standard of patient care.
Funding Support and Author Disclosures
Drs Abdel-Qadir, Thavendiranathan, Oh, Sabiston and Adams are supported by grants from the Canadian Cancer Society and the Canadian Institutes of Health Research (706710), the Heart and Stroke Foundation, Peter Munk Cardiac Centre Innovation Fund, and the MSH UHN AMO Innovation Fund. Drs Sabiston and Thavendiranathan are supported by the Canada Research Chair program. Dr Abdel-Qadir is supported by a National New Investigator Award (Heart and Stroke Foundation of Canada). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Jennifer A. Ligibel, MD, served as the Guest Editor for this paper. Anju Nohria, MD, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section and supplemental figures and tables, please see the online version of this paper.
Appendix
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Lyon A.R., Dent S., Stanway S., et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22:1945–1960. doi: 10.1002/ejhf.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zieff G.H., Wagoner C.W., Paterson C., et al. Cardiovascular consequences of skeletal muscle impairments in breast cancer. Sports (Basel) 2020;8(6):80. doi: 10.3390/sports8060080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones L.W., Haykowsky M.J., Swartz J.J., et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 5.Kaboré E.G., Guenancia C., Vaz-Luis I., et al. Association of body mass index and cardiotoxicity related to anthracyclines and trastuzumab in early breast cancer: French CANTO cohort study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Chow E.J., Oeffinger K.C., et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112(3):256–265. doi: 10.1093/jnci/djz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chao C., Xu L., Bhatia S., et al. Cardiovascular disease risk profiles in survivors of adolescent and young adult (AYA) cancer: the Kaiser Permanente AYA cancer survivors study. J Clin Oncol. 2016;34:1626–1633. doi: 10.1200/JCO.2015.65.5845. [DOI] [PubMed] [Google Scholar]
- 8.Lega I.C., Pole J.D., Austin P.C., et al. Diabetes risk in childhood cancer survivors: a population-based study. Can J Diabetes. 2018;42:533–539. doi: 10.1016/j.jcjd.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Boyne D.J., Mickle A.T., Brenner D.R., et al. Long-term risk of cardiovascular mortality in lymphoma survivors: a systematic review and meta-analysis. Cancer Med. 2018;7:4801–4813. doi: 10.1002/cam4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilchrist S.C., Barac A., Ades P.A., et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piepoli M.F., Corrà U., Benzer W., et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil. 2010;17:1–17. doi: 10.1097/HJR.0b013e3283313592. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbertson C.C., Pearce E.E., Valle C.G., et al. Cardiac rehabilitation programs for cancer survivors: a scoping review. Curr Epidemiol Rep. 2020;7(2):89–103. doi: 10.1007/s40471-020-00235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rickard J.N., Eswaran A., Small S.D., et al. Evaluation of the structure and health impacts of exercise-based cardiac and pulmonary rehabilitation and prehabilitation for individuals with cancer: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:739473. doi: 10.3389/fcvm.2021.739473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams S.C., McMillan J., Salline K., et al. Comparing the reporting and conduct quality of exercise and pharmacological randomised controlled trials: a systematic review. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-048218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shea B.J., Reeves B.C., Wells G., et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of health care interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howell D., Richardson A., May C., et al. Implementation of self-management support in cancer care and normalization into routine practice: a systematic scoping literature review protocol. Syst Rev. 2019;8(1):37. doi: 10.1186/s13643-019-0952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann T.C., Glasziou P.P., Boutron I., et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 19.Ioannidis J.P., Evans S.J., Gotzsche P.C., et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 20.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 21.Cochrane . The Cochrane Collaboration; 2017. Risk of Bias 2 (RoB 2) tool.https://methods.cochrane.org/risk-bias-2 [Google Scholar]
- 22.Sterne J.A., Hernán M.A., Reeves B.C., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane . The Cochrane Collaboration; 2017. ROBINS-I tool.https://methods.cochrane.org/methods-cochrane/robins-i-tool [Google Scholar]
- 24.Smart N.A., Waldron M., Ismail H., et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13:9–18. doi: 10.1097/XEB.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 25.Suurmond R., Van Rhee H., Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunnally J.C. Tata McGraw-Hill Education; 1994. Psychometric Theory 3E. [Google Scholar]
- 27.Pedhazur E.J., Schmelkin L.P. Psychology Press; 2013. Measurement, Design, and Analysis: An Integrated Approach. [Google Scholar]
- 28.Higgins J.P., Thomas J., Chandler J., et al. John Wiley & Sons; 2019. Cochrane Handbook for Systematic Reviews of Interventions. [Google Scholar]
- 29.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 30.Bonsignore A., Field D., Speare R., et al. The effect of cardiac rehabilitation in men with and without prostate cancer: a retrospective, comparative cohort study. J Phys Act Health. 2018;15:781–787. doi: 10.1123/jpah.2017-0362. [DOI] [PubMed] [Google Scholar]
- 31.Bonsignore A., Marzolini S., Oh P. Cardiac rehabilitation for women with breast cancer and treatment-related heart failure compared with coronary artery disease: a retrospective study. J Rehabil Med. 2017;49:277–281. doi: 10.2340/16501977-2203. [DOI] [PubMed] [Google Scholar]
- 32.De Jesus S., Fitzgeorge L., Unsworth K., et al. Feasibility of an exercise intervention for fatigued breast cancer patients at a community-based cardiac rehabilitation program. Cancer Manag Res. 2017;9:29–39. doi: 10.2147/CMAR.S117703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dittus K.L., Lakoski S.G., Savage P.D., et al. Exercise-based oncology rehabilitation: leveraging the cardiac rehabilitation model. J Cardiopulm Rehabil Prev. 2015;35:130–139. doi: 10.1097/HCR.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dolan L.B., Barry D., Petrella T., et al. The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev. 2018;38:246–252. doi: 10.1097/HCR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard G., Adams R., Campbell A., et al. Is referral of postsurgical colorectal cancer survivors to cardiac rehabilitation feasible and acceptable? A pragmatic pilot randomised controlled trial with embedded qualitative study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-009284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubbard G., Campbell A., Fisher A., et al. Physical activity referral to cardiac rehabilitation, leisure centre or telephone-delivered consultations in post-surgical people with breast cancer: a mixed methods process evaluation. Pilot Feasibility Stud. 2018;4:108. doi: 10.1186/s40814-018-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothe D., Cox-Kennett N., Buijs D.M., et al. Cardiac rehabilitation in patients with lymphoma undergoing autologous hematopoietic stem cell transplantation: a cardio-oncology pilot project. Can J Cardiol. 2018;34:S263–S269. doi: 10.1016/j.cjca.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Young-McCaughan S., Mays M.Z., Arzola S.M., et al. Research and commentary: change in exercise tolerance, activity and sleep patterns, and quality of life in patients with cancer participating in a structured exercise program. Oncol Nurs Forum. 2003;30:441–454. doi: 10.1188/03.ONF.441-454. discussion 441-454. [DOI] [PubMed] [Google Scholar]
- 39.Zvinovski F., Stephens J.A., Ramaswamy B., et al. A cardiac rehabilitation program for breast cancer survivors: a feasibility study. J Oncol. 2021;2021:9965583. doi: 10.1155/2021/9965583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meneses-Echavez J.F., Rodriguez-Prieto I., Elkins M., et al. Analysis of reporting completeness in exercise cancer trials: a systematic review. BMC Med Res Methodol. 2019;19(1):220. doi: 10.1186/s12874-019-0871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott J.M., Zabor E.C., Schwitzer E., et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36:2297–2305. doi: 10.1200/JCO.2017.77.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.