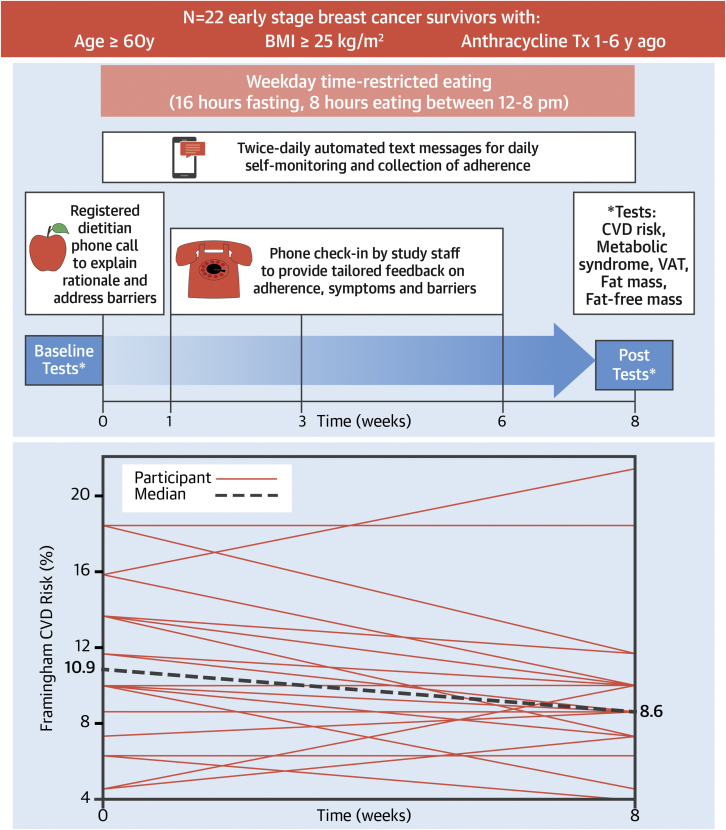
Central Illustration
Time-restricted eating (TRE) limits energy intake to within a given time window, commonly 8 hours, followed by fasting for 16 hours (16:8 TRE). It is a practical approach that could improve cardiometabolic health,1 but its effects in cancer populations or on cardiovascular disease (CVD) risk have not been studied. We performed a single-arm feasibility study to evaluate adherence, safety (symptoms, fat-free mass loss), and preliminary efficacy of 8 weeks of 16:8 TRE on CVD risk among breast cancer survivors (BCS).
Participants provided written informed consent. The Health Research Ethics Board of Alberta approved the study. We enrolled BCS with risk factors for CVD mortality2: older age (≥60 years), overweight or obesity (body mass index [BMI] ≥ 25 kg/m2), and completion of cardiotoxic treatment (anthracyclines within 1-6 years). Exclusion criteria included metastatic cancer, lipid- or glucose-lowering medications, magnetic resonance imaging (MRI) contraindications, self-reported diabetes, ≥15-lb weight loss in the previous 3 months, and currently working rotating or night shifts. Recruitment occurred during the COVID-19 pandemic, before vaccine availability (August 2020 to January 2021), by mailing an invitation letter to potentially eligible former patients of the local cancer hospital and by word of mouth.
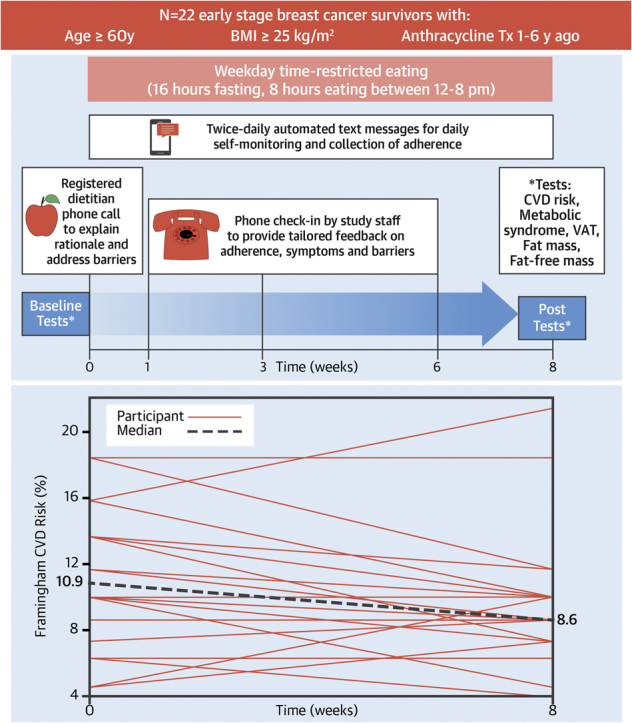
Participants were asked to eat ad libitum between 12 and 8 pm on weekdays and any time of day on weekends and to consume only water, black coffee, or black tea outside of those hours for 8 weeks. No other dietary or physical activity instructions were given. Behavioral support for the intervention included 1) a preintervention phone call from a registered dietitian; 2) check-in phone calls from study staff members at 1, 3, and 6 weeks; and 3) twice-daily (weekdays) automated text messages asking participants to respond with the time of day they started and stopped eating (Figure 1A). Phone calls involved discussion of adherence, symptoms, barriers, and facilitators. Adherence was determined by text message responses. Weekday caloric intake was evaluated by 3-day average of 24-hour diet records performed before and in the last week of the intervention.
Figure 1.
Study Design and Primary Result
(A) Study design and intervention behavioral support. (B) Individual and median Framingham 10-year cardiovascular risk at baseline and after 8 weeks of time-restricted eating. BMI = body mass index; CVD = cardiovascular disease; Tx = treatment; VAT = visceral adipose tissue.
Preliminary efficacy outcome measures were evaluated before (baseline) and after intervention and compared using paired Student’s t-tests or Wilcoxon signed rank tests, depending on data normality. Ten-year Framingham CVD risk was calculated using the Canadian Cardiovascular Society scoring system. Presence of metabolic syndrome was evaluated according to the National Cholesterol Education Program Adult Treatment Panel III definition. Age, smoking, and blood pressure treatment status were collected by questionnaire. Glucose, total cholesterol, and high-density lipoprotein were collected after overnight fasting (following a TRE day postintervention). Blood pressure and waist circumference were evaluated by averaging 2 measurements. Visceral adipose tissue (VAT) was acquired from chemical shift encoding–based water-fat MRI axial slices at the third lumbar vertebra (3.0-T Siemens Prisma, Siemens Healthineers) with image segmentation by semiautomated custom analysis. Whole-body fat-free and fat mass were estimated using 8-point bioelectric impedance (Seca).
Of 228 invited patients, 22 (10%) enrolled (mean age 66 ± 5 years, mean BMI 31 ± 5 kg/m2, n = 1 smoker, mean 3 ± 1 years after anthracyclines, none received trastuzumab, 50% received left-sided radiation, 91% currently on tamoxifen and/or aromatase inhibitors) and completed the study (100% retention). Participants responded to 99% of text messages and adhered to ≥16 hours of fasting for a median of 98% (range: 85%-100%) of prescribed days. Symptoms were minor (eg, headaches, irritability) and transient (lasting 5 minutes to 3 hours). Fat-free mass did not change (−0.1 ± 1.6 kg; P = 0.76). Calorie intake changed by a median of −450 kcal (IQR: −765 to −28 kcal), representing a −22% relative reduction (P < 0.001).
Median Framingham CVD risk decreased from 10.9% (IQR: 8.6% to 13.7%) to 8.6% (IQR: 7.6% to 10.0%), a −15% relative change (P = 0.037) (Figure 1B) at 8 weeks. The modifiable Framingham components (ie, total cholesterol, high-density lipoprotein, and systolic blood pressure) did not significantly change overall, indicating interindividual differences in each of these measures and the risk reduction etiology. Mean MRI-derived VAT (−5 ± 7%; P = 0.009), median bioelectric impedance–derived whole-body fat mass (−0.9 kg; IQR: −1.5 to 0.1 kg; P = 0.046), and median body mass (−1.0 kg; IQR: −2.3 to 0.2 kg; P = 0.025) decreased. Mean BMI did not change (−0.2 ± 0.7 kg/m2; P = 0.10).
At baseline, 15 of 22 participants (68%) were classified as cardiometabolically unhealthy, defined as meeting the criteria for metabolic syndrome or for pharmacologic preventive treatment of CVD risk (ie, statins) according to Canadian Cardiovascular Society guidelines. Following the 8-week intervention, 8 of 15 (53%) no longer met the criteria for pharmacologic treatment of CVD risk (ie, reclassification to “low” [<10%] Framingham risk) or metabolic syndrome.
We found that weekday 16:8 TRE was a highly feasible and low-symptom intervention that reduces calorie intake without fat-free mass loss or the need to count calories among BCS. Our preliminary efficacy findings include a 2% absolute or 15% relative CVD risk reduction within just 8 weeks among BCS at risk for CVD mortality because of overweight or obese status, older age, and receipt of anthracyclines. TRE also significantly decreased VAT, which our team has previously found to accumulate rapidly with cardiotoxic treatment and predict later cardiac events among BCS.3,4
BCS at low Framingham risk experience 38% fewer cardiac events than those at intermediate risk.5 Therefore, if reclassification to low Framingham risk (reversing the indication for medication) or the reversal of metabolic syndrome is sustained long term, TRE may reduce health care costs and improve outcomes.
Limitations of this study include participant selection bias, lack of a control group, and short duration. Randomized controlled trials are needed to confirm these findings and to evaluate the health benefits, including potential health care cost savings and safety of longer term TRE.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Moon S., Kang J., Kim S.H., et al. Beneficial effects of time-restricted eating on metabolic diseases: a systemic review and meta-analysis. Nutrients. 2020;12:1267. doi: 10.3390/nu12051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gernaat S.A.M., Ho P.J., Rijnberg N., et al. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164:537–555. doi: 10.1007/s10549-017-4282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkham A.A., Pituskin E., Thompson R.B., et al. Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: a secondary analysis of the MANTICORE trial. Eur Heart J Cardiovasc Pharmacother. 2022;8(2):130–139. doi: 10.1093/ehjcvp/pvab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cespedes-Feliciano E.M., Chen W.Y., Bradshaw P.T., et al. Adipose tissue distribution and cardiovascular disease risk among breast cancer survivors. J Clin Oncol. 2019;37:2528–2536. doi: 10.1200/JCO.19.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gernaat S.A.M., Boer J.M.A., van den Bongard D.H.J., et al. The risk of cardiovascular disease following breast cancer by Framingham risk score. Breast Cancer Res Treat. 2018;170:119–127. doi: 10.1007/s10549-018-4723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]