Abstract
Background
In physical activity or labor, the human body is in a state of high intensity stress, and all parts or physiological functions of the body respond positively to maintain or balance the need for movement. The human body has many physiological changes in the process of movement, and fatigue is the external manifestation of various complex changes inside the human body. Fatigue is also a physiological mechanism of self-protection after the body reaches a certain level of activity, which can prevent the occurrence of life-threatening excessive functional failure. The generation of fatigue is a very complex process, and its mechanism has not been concluded yet. Therefore, it is an important work to search and screen the effective components of natural plants that have anti-fatigue effect and to explore their mechanism.
Methods
This was a 8-week, randomized, double-blind, placebo-controlled clinical trial. A total of 110 subjects who passed physical examination were included according to the scheme design, and randomly divided into a test group which was given KRG and a placebo control group. The calculation is carried out according to the standard of sub-high-intensity exercise test.
Results
There was no adverse effect on safety index of subjects after taking red ginseng capsule. After KRG treatment, subjective strength grade is significant lower than placebo treatment. Blood lactic acid content is significantly get lower after trial in KRG group, and significant lower than placebo group. Creatine phosphokinase(CK) content is significantly get lower after trial in KRG group, and significant lower than placebo group.
Conclusion
According to the criterion in the test scheme, the result shows that KRG is helpful on relieving physical fatigue.
Keywords: Physical fatigue, Korean Red Ginseng, Blood lactic acid, Creatine phosphokinase
Graphical abstract
1. Introduction
In physical activity or labor, the human body is in a state of high intensity stress, and all parts of the body or physiology respond positively to maintain or balance the needs of movement. The physiological changes of human body during sports are mainly manifested in energy consumption, water loss, electrolyte loss, lactic acid accumulation, free radical damage and internal environment disorder [[1], [2], [3]]. Fatigue is the external manifestation of various complex internal changes in the body, which is related to load intensity, exercise amount, psychology, age, environment, climate, constitution, diet and living habits and other factors. Fatigue is also a physiological mechanism of self-protection after the body reaches a certain level of activity, which can prevent the occurrence of excessive function failure that threatens life. The generation of fatigue is a very complex process, and the mechanism has not been definitively concluded [[4], [5], [6], [7], [8], [9], [10], [11]]. Therefore, it is a significant work to search and screen the effective components with anti-fatigue effect in natural plants and to explore their mechanism of action [12].
The main active ingredients of Korean ginseng include ginsenosides (more than 30 kinds), sugars, amino acids, notoginseng element, volatile oil, etc., which have various physiological effects such as anti-fatigue, improving immunity, protecting injured muscles, improving atherosclerosis [[1], [2], [3], [4], [5], [6], [7]]. Animal experiments have found that the anti-fatigue mechanisms of the active ingredient ginsenoside in Korean Red Ginseng are mainly reflected in several aspects: Ginsenoside Rg1 can slow down the blood glucose drop in rats and increase the reserve of muscle glycogen and liver glycogen; Ginsenoside can reduce the level of lactic acid in rats and reduce the accumulation of lactic acid; Ginsenoside can significantly increase the content of DA and Ach in the rat hypothalamus, and reduce the content of 5-hydroxytryptamine (5-HT) and γ-aminobutyric acid (GABA), thereby resisting central neurotransmitter disorders and maintaining exercise capacity [13]. Human clinical trials also confirmed the effect of Korean ginseng on relieving physical fatigue [[13], [14], [15], [16], [17]]. According to Bei Yan and other experiments, Korean ginseng can reduce human serum creatine kinase, blood urea nitrogen and regulation of serum metabolic profile to relieve physical fatigue [1]. Zhang Li et al. designed a randomized double-blind clinical trial in which Korean Red Ginseng and a placebo were compared. After the test, the symptoms and fatigue of the experimental group were significantly improved [16]. In this experimental study, gradient load exercise is carried out according to the standard of sub-high-intensity exercise test. The subjective physical strength sensory rating scale was combined with blood biochemical indicators such as blood lactate, creatine kinase, cortisol, etc., to jointly evaluate the effect of Zheng guanzhuang Korean Red Ginseng capsules in relieving physical fatigue. It was a great significance for the study of the mechanism of Korean Red Ginseng products in human body anti-fatigue, and was a great significance to the in-depth development and product promotion of Korean Red Ginseng related products.
2. Materials and methods
2.1. Ethical statement
This randomized, double-blind and placebo-controlled clinical trial study was performed in the Tianjin Third Central Hospital, Tianjin, China. The study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects have always been observed.
2.2. Materials
Zheng guanzhuang Korean Red Ginseng capsule, which is provided by the Korea Ginseng Corporation. It has obtained the State Food and Drug Administration health food registration approval, and its approval number is “Wei Shi Jian Jin Zi (1999) No. 0035”. Sample appearance: hard capsule, contents are yellowish-brown, product specification: 0.465 g/granule × 90 granule/bottle, total saponin content 3.5–4.8 g/100 g. Batch number: 20191223. Storage method:Keep away from light and keep in a cool, dry place. Shelf life: 36 months. The placebo capsule, also provided by the same organization, was identical to the test product in dosage form, taste, appearance, and packaging, except for its efficacy. The ingredients of placebo capsule contain lactose, microcrystalline cellulose, silica, magnesium stearate, tartrazine, allura red, brilliant blue, food flavoring essence. The sample provider is solely responsible for any safety problems caused by the test product.
2.3. Participants
2.3.1. Criteria of included subjects
2.3.1.1. Aged 18–65 years old, in good physical health, no obvious brain, heart, liver, lung, kidney, or blood disease, volunteers for the test, and guarantee for cooperation
.
2.3.1.2. The safety indicator test was qualified. Safety indicators include mental, sleep, diet, urine and bowel, heart rate, blood pressure, blood routine, urine routine, and ECG tests
.
2.3.1.3. Sign informed consent
.
2.3.2. Criteria of excluded subjects
2.3.2.1. Pregnant or breastfeeding women, and subjects who are allergic to the test sample
.
2.3.2.2. Patients with serious systemic diseases such as heart, liver, kidney and hematopoietic system
.
2.3.2.3. Subjects who took something related to the tested function in a short period of time, which will affect the judgment result
.
2.3.2.4. Subjects who do not meet the inclusion criteria, fail to consume the tested samples as required, fail to determine the efficacy or have insufficient data that affect the efficacy or determine the safety
.
2.3.2.5. Subjects who have heart disease, high blood pressure, hyperglycemia, epilepsy and other diseases easily attacked after strenuous exercise
.
2.4. Test design and grouping requirements
Subjects were randomly divided into two control groups: a study group and a control group. Intragroup comparisons before and after the test were also used. The main factors affecting the results, such as gender, age, body mass index (BMI) and body fat percentage, were considered as far as possible to conduct equilibrium test to ensure the comparability between groups. There were no less than 50 subjects in each group. Professional statisticians (non-final statisticians) use SPSS 21.0 software to generate random numbers, which can be used for random grouping of subjects. Put all random grouping numbers into two sets of emergency letters, one set was then handed over to the sample manager to issue the corresponding numbered test samples or placebo to the subjects, and the other set was handed over to the data manager for safekeeping, in case of unblind use.
2.5. Dose and duration
Two control designs were used: a study group and a control group. Intragroup comparisons before and after the test were also used. The test group took Zheng guanzhuang Korean Red Ginseng capsule, 2 times a day, 3 tablets each time, with warm water for 8 weeks. The control group received placebo, 3 tablets twice a day, in warm water for 8 weeks.
2.6. Method to induce exercise fatigue
Induce exercise fatigue is realized by gradient load exercise. The calculation is carried out according to the standard of sub-high-intensity exercise test. Using power bicycle (Ergoline 100) as the auxiliary device, the exercise heart rate (195 - age) was calculated according to the age of the subjects. During operation, the warm-up time of the power bicycle was set as 60 s and the initial power was 25 w. The blood pressure and heart rate at the beginning were read and recorded. After the warm-up time, power bicycle automatic increasing with every 50 w as a ladder, each step to keep the 120 s, read the record in the process of the participants' blood pressure and heart rate, and arrive in time automatically stop when the heart rate (maximum heart rate ∗ 0.8), bicycle automatic recovery to 25 w, 60 s and let the participants to keep motion state, read the record of blood pressure and heart rate. Monitor subjects’ blood pressure and heart rate during exercise to ensure their safety.
3. Observation indicators
3.1. Safety indicators
3.1.1. General conditions (including mental, sleep, diet, urine and urine, blood pressure, etc.)
Good mental state is manifested in full mentation, brisk thinking and focused attention. Ordinary mental state is manifested in acceptable mentation and general attention. Poor mental state is manifested in decreased understanding, memory loss and unfocused attention. Good sleep condition shows high spirits the next day, no difficulty in falling asleep and returning, and no more dreams. Ordinary sleep condition shows a lack of energy the next day, no difficulty in falling asleep and returning, and no more dreams. Poor sleep condition shows difficulty in falling asleep and returning, and more dreams. Good diet status means good appetite. Ordinary diet status means general appetite. Poor diet status means poor appetite accompanied by postprandial distension and fullness. Good urine and urine condition is manifested as unobstructed defecation 1–2 times a day. Ordinary urine and urine condition is manifested as unsmooth stool once every 2–3 days. Poor urine and urine condition is manifested as constipation and diarrhea.
3.1.2. Blood and urine routines
.
3.1.3. Liver and kidney function tests
.
3.1.4. Heart rate and blood pressure (measured during gradient load exercise)
According to the standard of sub-high-intensity exercise test, the safety standard of heart rate was set as the upper limit of no more than 195 - age during the gradient load exercise. The safety standard of blood pressure is that the participants have no discomfort after gradient load exercise.
3.1.5. ECG, B-ultrasound, chest X-ray (only checked once before the start of the test)
.
3.2. Efficacy indicators
3.2.1. Cortisol (measured before gradient load exercise)
.
3.2.2. Creatine kinase, blood lactate (measured after gradient load exercise)
.
3.2.3. Subjective physical strength rating scale (measured after gradient load exercise)
The specific content of subjective physical strength rating scale is mainly divided into 7 types (Table 1).
Table 1.
Subjective physical strength rating scale.
| Sense of self | Main symptoms | Points |
|---|---|---|
| Very relaxed | No difference between before and after exercise | 6 |
| Self control the speed and heart rate of completing the action accurately | 7 | |
| Sight shortness of breath | 8 | |
| Maintain coordination and stability of movement | 9 | |
| Relaxed | Slightly sore muscles | 10 |
| Just sweating and breathing faster | 11 | |
| A little relaxed | Increased perspiration and heavy walking | 12 |
| Sweating heavily and moving normally | 13 | |
| A little tired | Slow in action and late response | 14 |
| Decreased attention and uncoordinated movements | 15 | |
| Tired | Sticky tongue and sore muscles | 16 |
| Extremely sore muscles and difficulty in lifting legs | 17 | |
| Very tired | Dyspnea and dizziness | 18 |
| Respiratory disturbance, nausea and vomiting | 19 | |
| Exhausted | Blurred vision and inability to move | 20 |
4. Clinical trial results
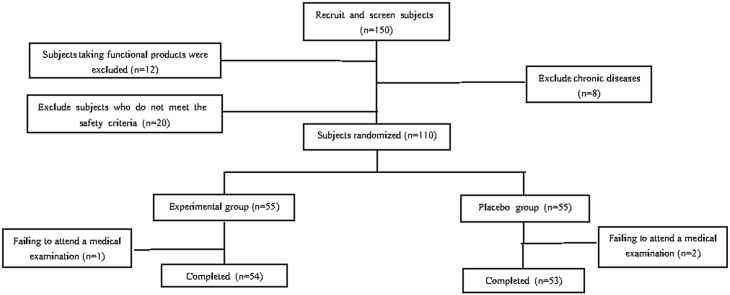
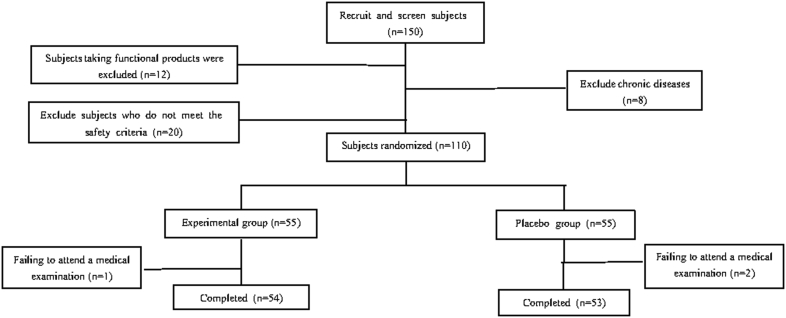
4.1. Recruiting and screening of the subjects (Fig. 1)
Fig. 1.
Screening process of subjects.
After preliminary screening, 150 subjects were sent to the hospital for the re-screening. According to the inclusion and exclusion criteria in the human feeding trial plan, 40 subjects did not meet the requirements, among which 20 did not meet the requirements for safety indicators, 12 were taking function-related products, and 8 were patients with chronic diseases. Finally, 110 subjects signed the informed consent. 110 subjects were randomly divided into a test group and a control group.1 case in the test group and 2 cases in the control group did not participate in the physical examination at the specified time, which met the exclusion criteria of the subjects. There were 54 valid subjects in the test group and 53 cases in the control group. The food group and the control group had deletion rates of 1.8% and 3.6% respectively.
4.2. General condition
Before the test, the subjects' general condition, blood routine test, urine routine test, liver and kidney functions, chest X-ray, ECG, abdominal B-ultrasound and other examinations were basically within the normal range. The grouping of the subjects is shown in Table 2. The age and gender of the two groups of patients were not significantly different and comparable before the test (Table 2).
Table 2.
Comparison of equilibrium between the two groups before the test(¯x±SD).
| Test group (n = 54) | Control group (n = 53) | |
|---|---|---|
| Cases | 54 | 53 |
| Male/Female | 18/36 | 23/30 |
| Age (years old) | 51.80 ± 12.03 | 53.02 ± 9.54 |
| BMI | 25.41 ± 3.49 | 25.36 ± 3.81 |
| Body fat rate | 34.93 ± 7.52 | 33.71 ± 7.47 |
Comparison between groups, P > 0.05.
Observation of the general condition of the two groups before and after the test revealed no significant changes in the mental status, sleep, diet, defecation, urine routine and defecation routine of the subjects before and after the test (Table 3).
Table 3.
Comparison of general conditions in the two groups before and after the test.
| Test group (n = 54) |
Control group (n = 53) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before test |
After test |
Before test |
After test |
|||||||||
| Poor | Ordinary | Good | Poor | Ordinary | Good | Poor | Ordinary | Good | Poor | Ordinary | Good | |
| Mental condition | 0 | 2 | 52 | 0 | 2 | 52 | 2 | 4 | 47 | 2 | 4 | 47 |
| Sleep condition | 1 | 1 | 52 | 0 | 1 | 53 | 0 | 2 | 51 | 0 | 1 | 52 |
| Diet condition | 0 | 1 | 53 | 0 | 1 | 53 | 0 | 0 | 53 | 0 | 0 | 53 |
| Defecation | 0 | 0 | 54 | 0 | 0 | 54 | 0 | 0 | 53 | 0 | 0 | 53 |
| Urine routine | Normal | Normal | Normal | Normal | ||||||||
| Stool routine | Normal | Normal | Normal | Normal | ||||||||
4.3. Observation results of efficacy indicators (Table 4)
Table 4.
Changes of efficacy indicators before and after the test (¯x±SD).
| Test group (n = 54) |
Control group (n = 53) |
|||
|---|---|---|---|---|
| Before test | After test | Before test | After test | |
| Cortisol | 116.71 ± 31.47 | 125.42 ± 32.56 | 121.16 ± 36.04 | 119.64 ± 29.26 |
| Creatine phosphokinase | 175.20 ± 38.42 | 163.30 ± 26.84∗# | 165.51 ± 41.53 | 174.53 ± 25.76 |
| Blood lactate | 214.52 ± 30.22 | 199.17 ± 34.80∗∗# | 214.27 ± 34.04 | 211.32 ± 21.75 |
| Subjective physical perception | 10.96 ± 0.43 | 10.46 ± 0.61 ∗∗## | 11.04 ± 0.39 | 10.92 ± 0.47 |
Comparison within groups ∗∗P<0.01 ∗ P<0.05,comparison between groups #P<0.05 ##P<0.01.
It could be seen from Table 4 that there was no significant difference on scores of subjective physical perception scale in the two groups before the test (P > 0.05), and the score of subjective physical scale in the test group after the test was lower than that before the test (P < 0.01). There was no significant difference on scores of subjective physical perception scale in the control group before and after the test (P > 0.05), and the score of subjective physical scale in the test group was lower than that in the control group after the test, indicating significant difference (P < 0.01).
It could be also seen from Table 4 that there was no significant difference on cortisol, CK, and blood lactate in the two groups before the test. In self-comparison, the blood lactate content in the test group after the test was significantly lower than that before the test (P < 0.01), and the content of CK in the test group after the test was significantly lower than that before the test (P < 0.05). Compared between the groups after the test, the blood lactate content in the test group was significantly lower than that in the control group (P < 0.05), and the content of CK in the test group was significantly lower than that in the control group (P < 0.05). There was no significant change in the cortisol content between or within the test group and the control group before and after the test.
4.4. Observation results of safety indicators
4.4.1. The comparison of blood pressure and heart rate during the gradient load exercise in subjects before and after the test (Table 5)
Table 5.
Comparison of blood pressure and heart rate before, after and during the test (¯x±SD).
| Test group (n = 54) |
Control group (n = 53) |
||||
|---|---|---|---|---|---|
| Before test | After test | Before test | After test | ||
| Before exercise | Systolic pressure | 129.57 ± 15.49 | 129.41 ± 15.48 | 130.58 ± 14.38 | 130.30 ± 14.27 |
| Diastolic pressure | 76.94 ± 10.30 | 76.91 ± 9.85 | 77.23 ± 11.58 | 77.28 ± 11.29 | |
| Heart rate | 73.98 ± 8.25 | 73.87 ± 8.01 | 73.57 ± 9.96 | 73.60 ± 9.55 | |
| During exercise | Systolic pressure | 162.35 ± 27.94 | 162.46 ± 28.71 | 162.57 ± 28.27 | 165.85 ± 26.03 |
| Diastolic pressure | 90.56 ± 19.21 | 89.41 ± 19.32 | 89.28 ± 22.26 | 95.23 ± 19.97 | |
| Heart rate | 119.41 ± 13.32 | 123.20 ± 13.81 | 115.15 ± 21.89 | 115.77 ± 17.35# | |
| After Exercise | Systolic pressure | 141.00 ± 24.77 | 140.17 ± 21.72 | 145.26 ± 27.66 | 144.81 ± 27.97 |
| Diastolic pressure | 83.19 ± 10.24 | 84.89 ± 11.03 | 84.64 ± 10.03 | 89.70 ± 12.35∗# | |
| Heart rate | 100.28 ± 18.00 | 102.06 ± 14.33 | 102.60 ± 15.00 | 101.09 ± 11.28 | |
Compared with pre-test ∗P<0.05 Comparison between groups # represents P<0.05.
According to the standard of sub-high-intensity exercise test, the exercise heart rate (195 - age) was calculated according to the age of the subjects. There are significant differences in intra-group or inter-group comparison on blood pressure and heart rate (P < 0.05). However, blood pressure and heart rate are only considered as safety indicators and have no practical significance for the test results.
4.4.2. The comparison of blood routine, urine routine and blood biochemical indexes of the subjects before and after the test (Table 6)
Table 6.
Changes of blood routine, urine routine and blood biochemical indexes before and after the food test (¯x±SD).
| Test group (n = 54) |
Control group (n = 53) |
|||
|---|---|---|---|---|
| Before test | After test | Before test | After test | |
| Leukocyte count( × 109/L) | 6.36 ± 1.54 | 4.48 ± 1.79 | 7.21 ± 1.73 | 6.93 ± 1.43 |
| Erythrocyte count ( × 1012/L) | 4.64 ± 0.38 | 4.64 ± 0.41 | 4.77 ± 0.46 | 4.74 ± 0.44 |
| Hemoglobin (g/L) | 139.57 ± 14.07 | 138.30 ± 14.70 | 141.38 ± 16.28 | 140.00 ± 15.76 |
| Platelet count ( × 109/L) | 261.07 ± 79.67 | 257.72 ± 83.77 | 263.70 ± 67.78 | 261.15 ± 61.98 |
| Urea (mmol/L) | 5.05 ± 1.28 | 4.78 ± 1.19 | 5.11 ± 1.65 | 4.98 ± 1.87 |
| Creatinine (μmol/L) | 69.83 ± 16.38 | 70.35 ± 16.97 | 70.81 ± 21.42 | 72.72 ± 30.86 |
| Uric acid (μmol/L) | 344.96 ± 87.00 | 343.24 ± 96.53 | 318.21 ± 91.01 | 327.11 ± 95.48 |
| Blood glucose (mmol/L) | 5.59 ± 1.21 | 5.39 ± 0.72 | 5.75 ± 1.04 | 5.57 ± 1.17 |
| Total protein (g/L) | 73.22 ± 3.28 | 74.36 ± 4.39 | 71.92 ± 5.09 | 73.11 ± 4.60 |
| Albumin (g/L) | 47.70 ± 1.97 | 47.18 ± 2.27 | 46.89 ± 2.96 | 46.46 ± 2.55 |
| ALT (U/L) | 23.24 ± 13.39 | 23.00 ± 11.40 | 20.85 ± 15.00 | 22.23 ± 16.63 |
| AST (U/L) | 20.46 ± 6.60 | 19.63 ± 5.79 | 19.38 ± 7.13 | 20.23 ± 8.93 |
| Triglycerides (mmol/L) | 1.93 ± 1.31 | 1.95 ± 0.91 | 1.68 ± 1.00 | 1.79 ± 1.06 |
| Total cholesterol (mmol/L) | 5.00 ± 1.13 | 4.85 ± 0.97 | 4.98 ± 1.03 | 4.83 ± 0.89 |
| High-density lipoprotein (mmol/L) | 1.19 ± 0.24 | 1.18 ± 0.19 | 1.22 ± 0.24 | 1.22 ± 0.24 |
| Low-density lipoprotein (mmol/L) | 3.01 ± 0.89 | 2.96 ± 0.81 | 2.96 ± 0.79 | 2.85 ± 0.70 |
It could be seen from Table 6 that the indicators of the test group and the control group were in the normal range before and after the test, and there was no significant change before and after the test (P > 0.05).
4.4.3. The abdominal B-ultrasound, ECG and chest X-ray in the test group and the control group were all within the normal range
.
4.4.4. Observation results of adverse reactions: there were no allergies and other adverse reactions in the test group and the control group during the test
The test group took Zheng guanzhuang Korean Red Ginseng capsules, 2 times a day, 3 capsules each time, with warm water for 8 weeks. The control group took placebo, 2 times a day, 3 capsules each time, with warm water for 8 weeks. Through follow-up interviews, the test group (n = 54) and the control group (n = 53) had no adverse reactions and allergic reactions such as nausea, flatulence, diarrhea, and abdominal pain occurred during the test period.
5. Conclusion
No allergy or other adverse reactions were observed in the subjects after eating Zheng guanzhuang Korean Red Ginseng capsule. Before and after the test, blood routine, urine routine, stool routine and blood biochemical indexes were all in the normal range, indicating that this product had no adverse effects on the health of the subjects. 110 eligible subjects were included and randomly divided into a trial group and a control group, with 107 effective cases, including 54 in the trial group and 53 in the control group. The experimental group was required to take Zheng guanzhuang Korean Red Ginseng capsule, and the control group was given placebo. The scores of subjective physical strength rating scale in the experimental group were lower than those before the trial (P < 0.01), and there was no significant difference in the scores of the control group before and after the trial (P > 0.05). After the trial, the scores of subjective physical strength rating scale in the experimental group were lower than those of the control group (P < 0.01). Cortisol in the trial group increased after the trial than before, but the difference was not significant [14]. The serum lactic acid content of the test group was lower than that before the feeding (P < 0.01), and the serum lactic acid content of the test group was lower than that of the control group (P < 0.05), indicating that Zheng guanzhuang Korean Red Ginseng capsule could reduce the blood lactic acid production and delay the physical fatigue of the body. The content of CK in the trial group was lower than that before (P < 0.05), and the content of CK in the trial group was lower than that in the control group (P < 0.05), indicating that Zheng guanzhuang Korean Red Ginseng capsule can reduce the production of CK, so as to delay the physical fatigue of the body. Comprehensive evaluation, according to the criteria in the test scheme, the results show that Zheng guanzhuang Korean Red Ginseng capsule has the effect of anti-fatigue.
6. Discussion
Exercise-induced fatigue is a temporary decrease in the body's working ability caused by exercise itself, and a physiological phenomenon that can be restored after proper time of rest and adjustment. It is an extremely complex comprehensive reaction process of body changes. Sports fatigue is a combination of physical and mental fatigue. Therefore, the evaluation of sports fatigue often includes the evaluation of subjective feelings and the determination of physiological and biochemical objective indicators that can reflect fatigue. In this study, a sub-high-intensity exercise test was used to make the subjects suffer from exercise fatigue, combined with the subject's physical perception scale, heart rate, and blood pressure, to judge the fatigue of the subjects. To study and evaluate the effect of Zheng guanzhuang Korean Red Ginseng capsules in relieving physical fatigue.
Studies have found that fatigue can cause excessive secretion of cortisol in the body, leading to disorders of fat and protein metabolism in the body, which can lead to physical discomfort and even diseases [14,15]. Cortisol can accelerate the catabolism and accelerate the decomposition during intense stress to meet the needs of exercise, but it shall be reduced to the original basic range as soon as possible during the post-exercise recovery period to avoid excessive consumption in the body [18]. In this study, the content of cortisol in the test group increased after the test, while the content of cortisol in the control group decreased slightly after the test, and there was no significant change. It is speculated that there are two reasons. One reason may be that taking Zheng guanzhuang Korean Red Ginseng capsules or placebo did not affect the changes in cortisol content of the subjects. The difference in cortisol content between the test group and the control group is the change in the subjects' normal cortisol content. The other reason may be that cortisol is a kind of glucocorticoid. Through literature review, we found that red ginseng may have two-way regulatory effect on glucocorticoid. Shi Zhe et al. Summarized that ginsenoside can regulate glucocorticoid receptor and enhance the effect of glucocorticoid [19]. Kim JH et al. have shown that red ginseng has a therapeutic effect on glucocorticoid-induced osteoporosis [20]. Therefore, the specific influencing factors and mechanism of Zheng guanzhuang Korean Red Ginseng capsules on cortisol index need to be further explored.
CK is an energy-converting enzyme, which is one of the more sensitive indicators for changes in exercise training. Studies have found that the muscular soreness of the body during high-intensity exercise is closely related to the activity of serum CK after exercise [21]. With increase of fatigue during exercise, the activity of serum CK may continue to rise. Change in CK activity within a certain range can well reflect the fatigue level during exercise. In this study, it indicated that Zheng guanzhuang Korean Red Ginseng capsule can reduce the production of CK, so as to delay the physical fatigue of the body.
Many studies have confirmed that the accumulation of lactate is one of the main factors leading to peripheral fatigue [22]. The production of blood lactate is due to that the body's oxygen supply cannot meet the energy requirements and accelerates the energy supply process of glycolysis [23]. The large accumulation of lactate increases the concentration of H+ ions in the muscles, resulting in ache fatigue of muscles. Therefore, the recovery from fatigue is promoted by assisting the body to remove lactate. In this study, it indicated that Zheng guanzhuang Korean Red Ginseng capsules could reduce the blood lactic acid production and delay the physical fatigue of the body.
When strenuous exercise causes the accumulation of lactic acid, it will cause a significant increase of free radicals. Excess free radicals may cause cell damage or tissue damage and it will cause the process of lipid peroxidation [24]. As the final product of lipid peroxidation, malondialdehyde can be used to evaluate the damage to cells caused by exercise fatigue and excessive free radicals [25]. Therefore, it can be explored in follow-up studies that whether Zheng guanzhuang Korean Red Ginseng capsules can reduce the production of malondialdehyde in the process of relieving physical fatigue.
The effect of Korean Red Ginseng on health maintenance has been concerned by researchers. At present, some studies from different directions have confirmed that Korean Red Ginseng has the potential of anti fatigue effect. Sung WS et al. provided the objective evidence for the treatment of Korean Red Ginseng on middle-aged individuals with chronic fatigue [26]. Hong MG et al. evaluated the antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease, their results show that Korean Red Ginseng can effectively improve adiponectin levels, reduce proinflammatory cytokines and fatigue [27]. Other studies have found that Korean Red Ginseng has some effects on metabolic syndromeon and related diseases, such as obesity, cardiovascular disease, insulin resistance, diabetes, dyslipi demia, non-alcoholic fatty liver disease [28]. However, more clinical trials are needed to confirm the clinical safety and effectiveness of Korean Red Ginseng.
Further analysis found that a possible limitation of this experimental study that the age of the test population is mostly concentrated in the middle-aged and elderly people. The next step of the study can be to expand the age range of the population, combined with metabolic profile analysis, and in-depth study the mechanism of Korean Red Ginseng capsules to relieve physical fatigue and the target population.
Declaration of competing interest
All authors have no conflicts of interest to declare.
Acknowledgements
The study was supported by a grant from the Korea Ginseng Corporation.
References
- 1.Yan B., Liu Y., Shi A., Wang Z., Aa J., Huang X., Liu Y. Investigation of the antifatigue effects of Korean ginseng on professional athletes by gas chromatography-time-of-flight-mass spectrometry-based metabolomics. J AOAC Int. 2018;101:701–707. doi: 10.5740/jaoacint.17-0220. [DOI] [PubMed] [Google Scholar]
- 2.Oyagi A., Ogawa K., Kakino M., Hara H. Protective effects of a gastrointestinal agent containing Korean red ginseng on gastric ulcer models in mice. BMC Compl Alternative Med. 2010;10 doi: 10.1186/1472-6882-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabral De Oliveira A.C., Perez A.C., Prieto J.G., Duarte I.D.G., Alvarez A.I. Protection of Panax ginseng in injured muscles after eccentric exercise. J Ethnopharmacol. 2005;97 doi: 10.1016/j.jep.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Voces J., Cabral de Oliveira A.C., Prieto J.G., Vila L., Perez A.C., Duarte I.D., Alvarez A.I. Ginseng administration protects skeletal msucle from oxidative stress induced by acute exercise in rats. Braz J Med Biol Res. 2004;37 doi: 10.1590/S0100-879X2004001200012. [DOI] [PubMed] [Google Scholar]
- 5.Kim S.H., Park K.S., Chang M.J., Sung J.H. Effects of Panax ginseng extract on exercise-induced oxidative stress. J Sports Med Phys Fit. 2005;45 [PubMed] [Google Scholar]
- 6.Jovanovski E., Jenkins A., Dias A.G., Peeva V., Sievenpiper J., Arnason J.T., Rahelic D., Josse R.G., Vuksan V. Effects of Korean red ginseng (Panax ginseng C.A. Mayer) and its isolated ginsenosides and polysaccharides on arterial stiffness in healthy individuals. Am J Hypertens. 2010;23 doi: 10.1038/ajh.2010.5. [DOI] [PubMed] [Google Scholar]
- 7.Qi B., Liu L., Zhang H., Zhou G.X., Wang S., Duan X.Z., Bai X.Y., Wang S.M., Zhao D.Q. Anti-fatigue effects of proteins isolated from Panax quinquefolium. J Ethnopharmacol. 2014;153 doi: 10.1016/j.jep.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 8.Evans R.K., Knight K.L., Draper D.O., Parcell A.C. Effects of warm-up before eccentric exercise on indirect markers of muscle damage. Med Sci Sports Exerc. 2002;34 doi: 10.1097/00005768-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Dolezal B.A., Potteiger J.A., Jacobsen D.J., Benedict S.H. Muscle damage and resting metabolic rate after acute resistance exercise with an eccentric overload. Med Sci Sports Exerc. 2000;32 doi: 10.1097/00005768-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hartz A.J., Bentler S., Noyes R., Hoehns J., Logemann C., Sinift S., Butani Y., Wang W., Brake K., Ernst M., et al. Randomized controlled trial of Siberian ginseng for chronic fatigue. Psychol Med. 2004;34 doi: 10.1017/S0033291703008791. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan R.K., Evans W.J., Kirwan J.P. Impaired substrate oxidation in healthy elderly men after eccentric exercise. J Appl Physiol. 2003;94 doi: 10.1152/japplphysiol.00746.2002. [DOI] [PubMed] [Google Scholar]
- 12.Choi K.T. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. 2008;29 doi: 10.1111/j.1745-7254.2008.00869.x. [DOI] [PubMed] [Google Scholar]
- 13.Genlan Y., Yaping Z., Liufeng O., Kexuan Z., Yunan Z. Research progress on anti-fatigue effect of ginseng. J Chem Inf Model. 2015;10:1174–1177. doi: 10.13935/j.cnki.sjzx.150844. [DOI] [Google Scholar]
- 14.Qian Y., Kaiyuan S., Yongfen L., Xuguang L., Shuguang Y., Hongfa X., Yong H. A study on the effects of tonifying prescription and clearing prescription of Chinese herbs on anti-physical fatigue. Chinese J Sport Med. 1999;18:300–304. [Google Scholar]
- 15.Anhui X., Hongjun W., Fengwen G., Yu Y., Liqiang W., Rui Z., Chunyan L. Experimental study on the promoting motion fatigue recover of the cordyceps militaris extract on the chemical indicators in blood of human. Lishizhen Med Mater Medica Reseach. 2010;21:2481–2483. doi: 10.3969/j.issn.1008-0805.2010.10.027. [DOI] [Google Scholar]
- 16.Surong X. Lanzhou University; 2016. The human feeding study on relieving physical fatigue of multi-vitamins amino acid functional beverage. [Google Scholar]
- 17.Zhang L., Chen X., Cheng Y., Chen Q., Tan H., Son D., Chang D., Bian Z., Fang H., Xu H. Safety and antifatigue effect of Korean Red Ginseng: a randomized, double-blind, and placebo-controlled clinical trial. J Ginseng Res. 2019;43:676–683. doi: 10.1016/j.jgr.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilkens L., Bock J.D., Kretzers J., Alwine F.M.K., Esther G.F.-V., Petra A.M.J.S., Astrid M.H.H., Luc J.C.V.L., Jan-Willem V.D. Whey protein supplementation does not accelerate recovery from a single bout of eccentric exercise. J Sports Sci. 2020 doi: 10.1080/02640414.2020.1820184. [DOI] [PubMed] [Google Scholar]
- 19.Shi Z., Li Y. Chinese Archives of Traditional Chinese Medicine; 2021. A review of ginsenosides regulating glucocorticoid receptor pathway. [Google Scholar]
- 20.Kim J., Lee H., Kang K.S., Chun K.H., Hwang G.S. Protective effect of Korean Red Ginseng against glucocorticoid-induced osteoporosis in vitro and in vivo. Journal of Ginseng Research. 2015;39:46–53. doi: 10.1016/j.jgr.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahtiainen J.P., Lehti M., Hulmi J.J., Kraemer W.J., Alen M., Nyman K., Selänne H., Pakarinen A., Komulainen J., Kovanen V., et al. Recovery after heavy resistance exercise and skeletal muscle androgen receptor and insulin-like growth factor-I isoform expression in strength trained men. J Strength Condit Res. 2011;25:767–777. doi: 10.1519/JSC.0b013e318202e449. [DOI] [PubMed] [Google Scholar]
- 22.Pengfei L., Baoxin F., Wenyuan S., Weiying Z., Pifang Z., Guoqiang S. Comparative study of three different incremental exercises for evaluating aerobic capacity of cyclist on a cycle ergometer. China Sport Sci Technol. 2010;46:123–125. [Google Scholar]
- 23.Caslin H.L., Abebayehu D., Qayum A.A., Haque T.T., Taruselli M.T., Paez P.A., Pondicherry N., Barnstein B.O., Hoeferlin L.A., Chalfant C.E., et al. Lactic acid inhibits lipopolysaccharide-induced mast cell function by limiting glycolysis and ATP availability. J Immunol. 2019;203:453–464. doi: 10.4049/jimmunol.1801005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junwei W. Jilin Uniersity; 2016. The campare study of 4 weeks fixed load and incremental load on college students' aerobic capacity effect. [Google Scholar]
- 25.Liu R., Wu L., Du Q., Ren J.W., Chen Q.H., Li D., Mao R.X., Liu X.R., Li Y. Small molecule oligopeptides isolated from walnut (juglans regia L.) and their anti-fatigue effects in mice. Molecules. 2018;24:45. doi: 10.3390/molecules24010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung W.S., Kang H.R., Jung C.Y., Park S.S., Lee S.H., Kim E.J. Efficacy of Korean red ginseng (Panax ginseng) for middle-aged and moderate level of chronic fatigue patients: a randomized, double-blind, placebo-controlled trial. Compl Ther Med. 2020;48:102246. doi: 10.1016/j.ctim.2019.102246. [DOI] [PubMed] [Google Scholar]
- 27.Hong M., Lee Y.H., Kim S., Suk K.T., Bang C.S., Yoon J.H., Baik G.H., Kim D.J., Kim M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J Ginseng Res. 2016;40:203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoon S.J., Kim S.K., Lee N.Y., Choi Y.R., Kim H.S., Gupta H., Youn G.S., Sung H., Shin M.J., Suk K.T. Effect of Korean red ginseng on metabolic syndrome. J Ginseng Res. 2021;45:380–389. doi: 10.1016/j.jgr.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]