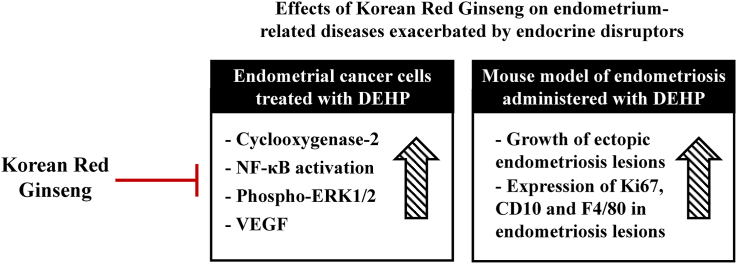
Abstract
Background
Di-(2-ethylhexyl) phthalate (DEHP) is the most common endocrine disrupting chemical used as a plasticizer. DEHP is associated with the development of endometrium-related diseases through the induction of inflammation. The major therapeutic approaches against endometrial cancer and endometriosis involve the suppression of inflammatory response. Korean Red Ginseng (KRG) is a natural product with anti-inflammatory and anti-carcinogenic properties. Thus, the purpose of this study is to investigate the effects of KRG on DEHP-induced inflammatory response in endometrial cancer Ishikawa cells and a mouse model of endometriosis.
Methods
RNA-sequencing was performed and analyzed on DEHP-treated Ishikawa cells in the presence and absence of KRG. The effects of KRG on DEHP-induced cyclooxygenase-2 (COX-2) mRNA levels in Ishikawa cells were determined by RT-qPCR. Furthermore, the effects of KRG on the extracellular signal-regulated kinases (ERKs) pathway, COX-2, and nuclear factor-kappa B (NF-κB) p65 after DEHP treatment of Ishikawa cells were evaluated by western blotting. In the mouse model, the severity of endometriosis induced by DEHP and changes in immunohistochemistry were used to assess the protective effect of KRG.
Results
According to the RNA-sequencing data, DEHP-induced inflammatory response-related gene expression was downregulated by KRG. Moreover, KRG significantly inhibited DEHP-induced ERK1/2/NF-κB/COX-2 levels in Ishikawa cells. In the mouse model, KRG administration significantly inhibited ectopic endometriosis growth after DEHP-induced endometriosis.
Conclusions
Overall, these results suggest that KRG may be a promising lead for the treatment of endometrial cancer and endometriosis via suppression of the inflammatory response.
Keywords: Cyclooxygenase-2, Di(2-ethylhexyl) phthalate, Endometrial cancer, Endometriosis, Korean Red Ginseng
Graphical abstract
1. Introduction
Endometrial cancer is the most common gynecological malignancy in developed countries and its incidence has recently been increasing [1]. Although the pathogenesis of endometrial cancer remains unclear, several risk factors, including late menopause onset, post-menopausal estrogen therapy, obesity, hypertension, and diabetes, appear to be related to the development and progression of endometrial cancer [2]. Endometrial cancer can be divided into type-I, estrogen-dependent endometrial cancer (80%), and type-II, non-estrogen-dependent endometrial cancer (20%) [3]. Endometrial cancer, and epithelial ovarian cancer (endometriosis-associated ovarian cancer), are also associated with endometriosis [4]. Endometriosis is a common and complex disease affecting about 6–10% of reproductive age women [5]. Endometriosis is an estrogen-dependent inflammatory disease, the etiology of which is not well known. However, the biological properties of endometriosis share similarities with cancer cell invasion and metastasis [6].
Di-(2-ethylhexyl)-phthalate (DEHP), an endocrine disrupting chemicals (EDCs), is a plasticizer that gives plastics flexibility and is used in a variety of industrial products [7]. Due to its extensive use, humans are exposed to DEHP through ingestion, inhalation, and dermal exposure, and maximum daily exposure to DEHP is about 2 mg/day in USA [8]. The levels of DEHP in plasma of Indian and Chinese women with endometriosis were significantly higher than those without endometriosis [9,10]. In a study of Korean women, the plasma concentration of DEHP in Korean women with advanced endometriosis was higher than that in women without endometriosis [11]. Moreover, the urinary concentration of metabolites of DEHP was higher in women with endometriosis [12]. Thus, the concentration of DEHP and its metabolites in plasma and urine is associated with endometriosis, suggesting that DEHP may have an aetiological association with endometriosis [13]. Although DEHP has no apparent estrogen activity, it may interfere with estrogen receptor (ER) α activity [14]. DEHP induces estrogen receptor α (ERα) protein expression in that ERα is a primary mediator of estrogenic action in endometrial stromal cells [15]. In silico docking experiments, DEHP binds with higher efficiency to human ERα and estrogen-related receptor γ (ERRγ) [16]. DEHP increases serum estradiol levels in adult Wistar rats [17] and promotes proliferation in ERα positive breast cancer cells [18]. Also, DEHP enhances proliferative activity and anti-apoptotic protein expression and cyclooxygenase-2 (COX-2) expression in human uterine leiomyoma cells [19]. Continuous exposure to DEHP has been reported to promote cell proliferation in hepatocytes via activated nuclear factor-kappa B (NF-κB) signaling pathway [20]. The endometriotic implant size was larger for with DEHP group compared with control group in the NOD/SCID mice [12]. DEHP exposure possibly cause an enhanced survival of endometrial cell in stressful conditions after retrograde menstruation, which eventually may lead to establishment of endometriosis by enhancing invasive and proliferative activities of endometrial cells [12,21]. Pathophysiological investigations of endometriosis suggest that the onset and progression of the disease include steroid-related mechanisms, involving hormone-related changes in the endometrium and peritoneal cavity, and excess estrogen production [22,23]. Therefore, it is believable that endocrine disruptors, such as DEHP, can affect the risk of endometriosis, and other diseases associated with the endometrium.
Endometriosis is a pelvic inflammatory disease caused by the evasion of the immune surveillance in the local peritoneal microenvironment [24]. Depending on the activation of macrophages and increased cytokine secretion in the peritoneal fluid, it triggers inflammatory reactions related to endometriosis [25]. The local inflammatory microenvironment enhances lesion proliferation, invasion and angiogenesis and subsequent progression to persistent endometrial lesions [26]. Inflammatory response to endometriosis may additionally cause pelvic pain and infertility [27]. In endometriosis, local pre-inflammatory mediators such as IL-1β and TNF-α will activate NF-κB and HIF-1α signaling pathways and enhance COX-2 transcription [24]. In addition, COX-2 promotes a proliferative local hormone environment by participating in a positive feed forward loop that enhances local estradiol (E2) production by increasing aromatase activity in endometriotic lesions [28]. Therefore, inhibition of COX-2 seems to be a promising strategy for treatment in endometriosis [29].
Korean Red Ginseng (KRG) has traditionally been used as an herbal medicine to treat various diseases in East Asia. Several studies have provided evidence that KRG possesses a variety of biological activities, including strengthening immunity, anti-inflammatory, and menopausal effects [30,31]. Ginsenoside Rg3, one of the components of KRG, has previously been demonstrated to suppress the growth of endometriosis lesions in rats, to reduce the volume of ectopic lesions, to inhibit expression of the vascular endothelial growth factor (VEGF), and to inhibit angiogenesis [32]. In addition, ginsenoside Rg3 has been shown to suppress endometriosis by regulating apoptosis and angiogenesis through NF-κB signaling in human ectopic endometrial stromal cells [33]. PPD can also attenuate the growth of ectopic lesions and eventually suppress the development of endometriosis [34]. We hypothesize that KRG may mediate its effects on DEHP-induced endometrial cancer and endometriosis through the TLR5/NF-κB pathway. Therefore, this study investigated the protective effects of KRG against endometrial cancer and endometriosis caused by DEHP.
2. Materials and methods
2.1. Reagents and antibodies
DEHP, dimethyl sulfoxide (DMSO), E2 and anti-β-actin were purchased from Sigma Aldrich (St. Louis, MO, USA). KRG was kindly supplied by the Korea Ginseng Corporation (KGC, Daejeon, Korea) [35]. The ginsenoside composition for KRG has been described previously [36]. Anti–NF–κB-p65, anti-Lamin B, anti-ERK1/2, anti-p-ERK1/2, anti-JNK1/2, anti-p-JNK1/2, anti-p38 and anti-p-p38 were purchased from Santa Cruz Biotechnology (CA, USA). Anti-COX-2 were purchased from Cayman Chemical Co. (Ann Arbor, MI, USA). Anti-VEGF-A were purchased from Abcam (Cambridge, UK).
2.2. Cell culture
The human endometrial adenocarcinoma Ishikawa cells were maintained in Dulbecco's Modified Eagle Medium (DMEM; WelGENE Inc., Daegu, Korea) containing 10% fetal bovine serum (FBS; GIBCO Invitrogen, NY, USA) and penicillin/streptomycin (GIBCO Invitrogen, NY, USA). The cells were incubated at 37 °C in the humidified 5% CO2 atmosphere.
2.3. Cell viability assay
Cell viabilities were measured using 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma Aldrich (St. Louis, MO, USA). Ishikawa cells were seeded at a density of 5000 cells/well in a 96-well plate. The following day, the cells were incubated with chemicals in the culture medium for 24 h. MTT assays were conducted as described in the previous study [35]. Cell viabilities were calculated as a percentage of control absorbance.
2.4. Quantitative real-time polymerase chain reaction
Total RNA extraction and cDNA synthesis were performed as described in the previous study [37]. qRT-PCR was performed with a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) using AccuPower® GreenStar™ qPCR PreMix (Bioneer Corporation, Daejeon, Korea) according to the manufacturer's instructions. The RT-qPCR primers were as follows: TLR-5, forward; 5′-ATTGCCAATATCCAGGATGC-3′ and reverse, 5′-CACCACCATGATGAGAGCAC-3′, myeloid differentiation factor 88 (MyD88), forward; 5′- TGGTTCTGGACTCGCCTTG-3′ and reverse, 5′- AGGAGGCAGGGCAGAAGTACAT-3’. The primers of COX-2 and β-actin have been previously described [38]. The β-actin was used as an endogenous control gene. The relative expression was calculated using the comparative cycle threshold (Ct).
2.5. Immunoblot analysis
Protein extraction and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) were performed as described in the previous study [39]. The blots were reacted with primary antibodies at 4 °C overnight. After washing steps, the blots were incubated Horseradish Peroxidase (HRP)-conjugated anti-IgG secondary antibody (Invitrogen, Grand Island, NY, USA). The band was visualized using Enhanced chemiluminescence (Amersham Pharmacia Biotech, Buckinghamshire, UK). Quantified protein levels were analyzed using Quantity One analysis software (Bio-Rad, Hercules, CA, USA).
2.6. Isolation of nuclear and cytoplasmic proteins
The proteins of cytoplasm and nuclear were isolated by the nuclear and cytosolic protein extraction kit (Abcam, Cambridge, UK) according to the manufacturer's instructions. Isolated proteins were analyzed by SDS-PAGE and immunoblot analysis.
2.7. Human VEGF enzyme-linked immunosorbent assay (ELISA)
The cell culture supernatants were collected in sterile test tubes and centrifuged at 3000 rpm. The supernatants were transferred to a new tube and stored at −20 °C until used. The secreted VEGF concentration was investigated to use VEGF ELISA kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2.8. RNA isolation and sequencing
Ishikawa cells were pre-treated with KRG for 1 h and treated with DEHP for 8 h. Each group's RNA was isolated using the Fast HQ RNA Extraction Kit (Intron technology). Serial test of RNA quality testing, quantification, library preparation, and sequencing was conducted as described in the previous study [36,40]. The ultimate RNA sequencing data were uploaded in the NCBI's GEO database (GSE154388).
2.9. Gene ontology analysis
Differentially expressed genes (DEGs) were analyzed using excel-based differentially expressed gene analysis (Ex-DEGA) software as shown in our previous study [36]. Genes clustered in the Venn diagram graph was determined considering fold change and normalized RC value (Fold change >2, Normalized RC (log2) = 1). As a result of DAVID functional annotation, component genes in inflammatory response were obtained from the Quick GO website (https://www.ebi.ac.uk/QuickGO/). Heat map graphs were expressed using the Multiexperimantal viewer software package.
2.10. Functional annotation
DAVID software version 6.8 was used for gene ontology analysis [41]. Genes were clustered in accordance with fold change and normalized RC value (Fold change >2, Normalized RC (log2) = 1). Clustered DEGs were submitted to categorize gene ontology, and the significant enrichment was estimated as per count >10 and EASE score of p-value < 0.05, which is a modified Fisher exact p-value. Significantly enriched gene ontologies were visualized with -log10 transformation of the p-value.
2.11. Mice
C57BL/6 mice (female, 6 weeks old, 20 g) obtained from ORIENT (Gapyeong, South Korea) were kept under the procedures ensured by the Konkuk University Institutional Animal Care and Use Committee (Ref. No.: KU19231-1). All the processes were conducted in compliance with ensured protocols and recommendations for the optimal use and care of animals at the specific pathogen-free housing facility at Konkuk University.
2.12. Mouse model for endometriosis
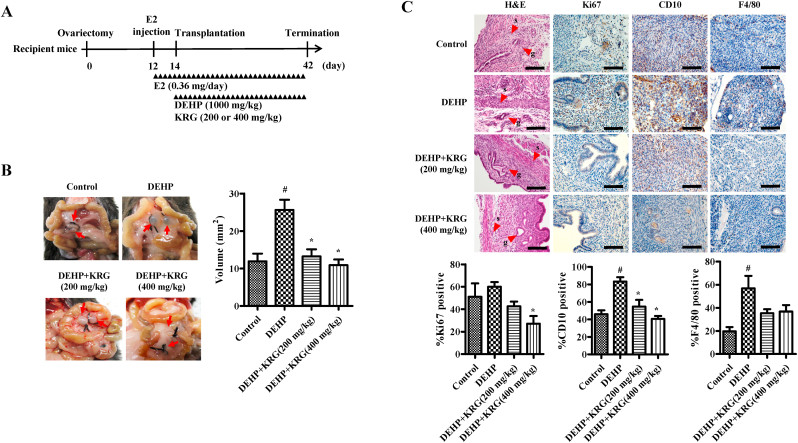
DEHP-induced endometriosis mouse model was developed according to schedule as shown in Fig. 5A. Ovary was resected from donor and recipient mice, and the mice were rested for 12 days. Sesame oil containing E2 (0.36 mg/mouse) was injected subcutaneously (s.c.) from 12 days to termination. To obtain uterine tissue, we first cut off uterine horn by 2 mm in size from donor mice, and the cut out uterine tissue was stored in PBS. For transplantation of uterine tissue, recipient mice were anesthetized and then abdominal cavity was opened. A sterile gauze was placed directly upper the incision site, and completely wetted by addition of sterile PBS. The small intestine was pulled up and placed on wet gauze to confirm intestinal artery. The uterine tissue from donor mouse was implanted by gently sutured into intestinal artery of recipient mice. After confirming that all organs returned to their position, the skin was closed using a wound clip [42,43]. To determine inhibition effect of endometriosis lesion, mice were randomly allocated to 4 groups as follows (n = 5/group): (1) control, (2) DEHP, (3) DEHP + KRG (200 mg/kg), (4) DEHP + KRG (400 mg/kg). After transplantation of uterine tissue to intestinal artery of mice, sesame oil containing E2 (0.36 mg/mouse) was continuously injected s.c. Daily. DEHP was injected intraperitoneally (i.p.) (1000 mg/kg). KRG was administrated by orally. The mice were sacrificed on day 42, and the lesions were carefully separated from intestinal mesentery. The volume of endometriosis lesion was recorded, and the lesion was fixed in 4% normal buffered formaldehyde for histological analysis.
Fig. 5.
Effects of KRG on DEHP-induced endometriosis. (A) Experimental design of endometriosis-bearing mouse model. (B) KRG was administered at 200 or 400 mg/kg/day by orally. The volume of endometriosis lesion was determined by callipers. (C) Histological analysis of morphology using formalin-fixed, paraffin-embedded lesion sections stained with H&E (g: gland, s: stroma). Immunohistochemical analyses of ectopic lesions using antibodies against Ki67, CD10, and F4/80 (scale bar: 100 μm). #p < 0.05, CON vs. DEHP; ∗p < 0.05, DEHP vs. DEHP + KRG.
2.13. Hematoxylin and eosin (H&E) staining and immunohistochemistry
H&E staining was conducted to confirm histological characterization of endometriosis tissue. Immunohistochemical (IHC) analysis of CD10 (Thermo Scientific, Waltham, MA, USA), cell proliferation (Ki67; Abcam, Cambridge, UK), and macrophage (F4/80; Thermo Scientific, Waltham, MA, USA) was performed on endometriosis lesion from the mice. 5 random fields in each lesion section slides were recorded. All staining was quantified by 2 blinded investigators.
2.14. Statistical analysis
The presented data were tested and validated by the means ± standard deviation as needed. We compared each group using one-way analysis of variance and Tukey's multiple-comparison posttest using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). Differences between groups at less than 0.05 of P-value regarded as significant.
3. Results
3.1. Expression levels of DEHP-induced inflammatory response genes were reduced by KRG
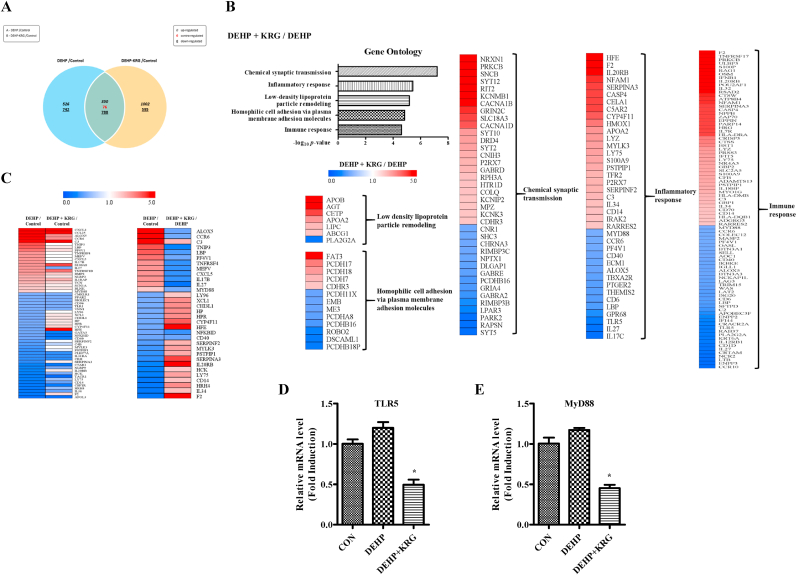
To determine the effects of KRG on DEHP-induced expression changes, we conducted a transcriptome analysis (assessing expression changes in tens of thousands of genes). The genes showing differential expression between the DEHP group and the control group and between the DEHP + KRG group and the control group are summarized in a Venn diagram (Fig. 1A). Functional annotation analysis was performed on the subsets of differentially expressed genes using the DAVID bioinformatics resource. Five gene ontology (GO) terms chemical synaptic transmission, inflammatory response, low-density lipoprotein particle remodeling, homophilic cell adhesion via plasma membrane adhesion molecules, and immune response were significantly enriched in the DEHP + KRG group compared to the DEHP group (Fig. 1B). In this study, we decided to focus on the inflammatory response-related gene sets. Among these gene sets, we identified a subset of genes in which expression level was more than two-fold different in the DEHP group compared to the control group. The expression levels of genes in this subset were then compared between the DEHP group and the DEHP + KRG group, and the results were expressed as a heat map (Fig. 1C). In most of the genes up-regulated or downregulated by DEHP, the expression level changes were attenuated by KRG treatment. The RNA seq results were subsequently verified using two inflammatory response-related genes (associated with the inflammatory response GO term) for which expression was reportedly decreased in the DEHP + KRG group compared to the DEHP group. Thus, RT-qPCR was performed on the TLR5 and MyD88 genes to confirm the RNA seq results (Fig. 1D and E). While TLR5 and MyD88 mRNA expression levels increased following DEHP treatment, the mRNA levels of both genes decreased following KRG treatment (500 μg/mL). The RT-qPCR results were similar to the RNA-seq data obtained. Taken together, transcriptome analysis with functional annotation analysis indicates that KRG attenuated changes in gene expression involved in DEHP-induced inflammatory response.
Fig. 1.
Differentially expressed genes and functional annotation from RNA-Seq in Ishikawa cells. The levels of mRNA transcripts in Ishikawa cells were determined by the library preparation and sequencing method. In every analysis, genes were clustered as per fold change and normalized RC value. (A) Venn diagrams show the set of differentially expressed genes in the DEHP and DEHP + KRG. (B) Gene ontology analysis of regulated DEGs in DEHP + KRG compared with those regulated by DEHP alone show the five most significant results, and heat map plots show the amount of expression of genes associated with each category. (C) Analysis was performed using the factors specified in the Quick GO database. Genes that expression levels were regulated more than 2 times by DEHP compared with negative control were clustered and presented as a heat map and which shows alleviated in DEHP + KRG group (former). Among these genes, genes that significantly regulated in the DEHP + KRG group against DEHP alone were selected and presented as a heat map (latter). (D and E) TLR5 and MyD88 mRNA levels were confirmed using qRT-PCR as the same RNA sample in which RNA-seq was performed. ∗p < 0.05, DEHP vs. DEHP + KRG.
3.2. KRG suppresses DEHP-induced COX-2 and nuclear NF-κB p65 expression in ishikawa cells
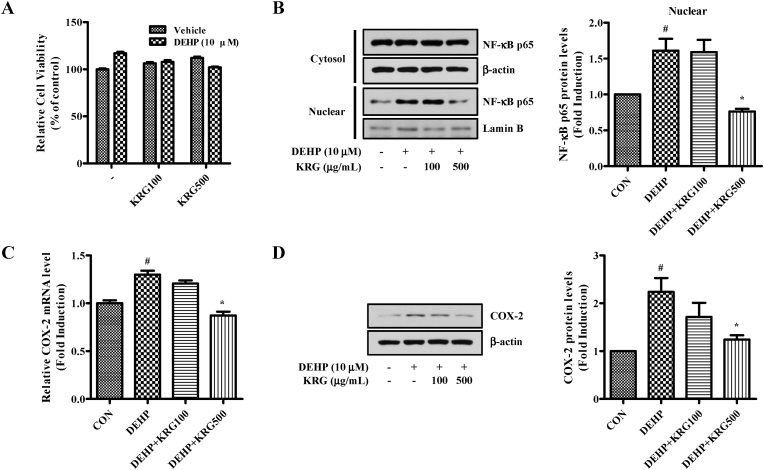
The effects of KRG on TLR5 and MyD88 were confirmed by RNA seq and RT-qPCR. Based on this result, the effect of KRG on the expression of NF-κB p65 downstream of TLR5 was analyzed. NF-κB p65 is an crucial regulatory transcription factor involved in the major inflammatory pathway [44]. First, we assessed the effect of DEHP and KRG on cell viability in Ishikawa cells using the MTT assay (Fig. 2A). At treatment concentrations, no cytotoxicity was observed for either DEHP or KRG. Although NF-κB is present in the cytoplasm in an inactive form, the NF-κB p65 subunit separates from IκBα and migrates to the nucleus upon various stimuli, inducing the transcription of multiple inflammatory genes [45]. When Ishikawa cells were treated with a range of DEHP concentrations, NF-kB p65 was observed to migrate into the nucleus at concentrations of DEHP of 10 μM (data not shown). We subsequently demonstrated that DEHP-induced NF-κB translocation to the nucleus was inhibited by KRG treatment (Fig. 2B). In addition, DEHP-induced NF-κB p65 expression was significantly inhibited by KRG (500 μg/mL). Moreover, DEHP-induced COX-2 (a subfactor of NF-κB) mRNA and protein expression levels were inhibited by KRG (500 μg/mL) (Fig. 2C and D). Thus, since KRG significantly attenuated COX-2 and NF-κB activation by DEHP, it may possess anti-inflammatory properties.
Fig. 2.
Effects of KRG on DEHP-induced NF-kB nuclear translocation and COX-2 expression in Ishikawa cells. (A) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and DEHP (10 μM) was treated 24 h. After incubation, cell viability was measured using MTT assay. (B) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 24 h. The proteins indicated above were evaluated by Western blot analysis. (C) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 8 h. COX-2 mRNA levels were confirmed by qRT-PCR. (D) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 24 h. COX-2 and β-actin were evaluated by Western blot analysis. ∗p < 0.05, DEHP vs. DEHP + KRG.
3.3. KRG mitigates DEHP-induced ERK1/2 signaling pathways in ishikawa cells
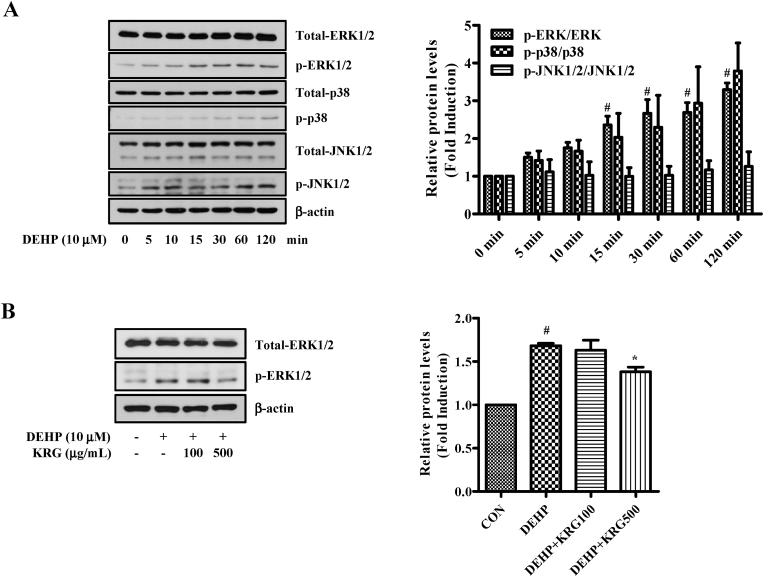
Activation of TLR can stimulate mitogen-activated protein kinases (MAPK) signaling pathways in addition to intracellular NF-κB, inducing the expression of a variety of inflammatory factors [46]. Before verifying that the KRG is involved in the MAPK pathway, we confirmed that MAPK is activated by DEHP (Fig. 3A). As a result of confirming phosphorylation of MAPK by treating DEHP (10 μM) at various times, it was confirmed that phosphorylated ERK1/2 was significantly increased. Next, the effect of KRG on DEHP-induced ERK1/2 signaling was analyzed by western blotting (Fig. 3B). Phosphorylated ERK1/2 by DEHP was significantly reduced by KRG (500 μg/mL). These data suggest that KRG significantly attenuates DEHP-induced ERK1/2 activation in Ishikawa endometrial cancer cells.
Fig. 3.
Effects of KRG on DEHP-induced phosphorylation of ERK1/2 in Ishikawa cells. (A) Ishikawa cells were treated with DEHP (10 μM) for indicated time. MAPK and phosphorylation of MAPK were evaluated by Western blot analysis. (B) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 60 min. ERK1/2, p-ERK1/2 and β-actin were evaluated by Western blot analysis. #p < 0.05, CON vs. DEHP; ∗p < 0.05, DEHP vs. DEHP + KRG.
3.4. KRG inhibits DEHP-induced VEGF expression in ishikawa cells
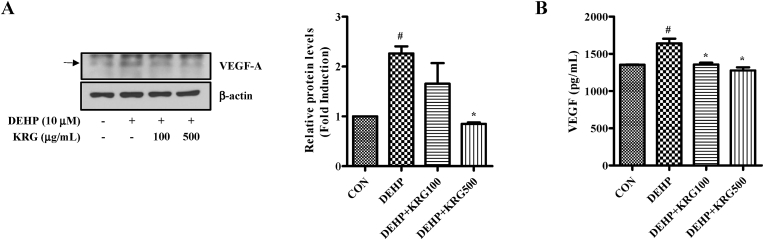
Activation of COX-2 regulate the expression of VEGF [47]. In addition, KRG was previously shown to inhibit DEHP-induced COX-2 in Ishikawa cells. Therefore, we investigated whether VEGF expression changed following treatment with DEHP and KRG. Although an increase in VEGF-A protein expression was induced by DEHP, the observed increase was reduced in cells treated with KRG (100, 500 μg/mL) (Fig. 4A). The levels of secreted VEGF protein in the supernatant following treatment with DEHP and KRG were also evaluated using VEGF ELISA (Fig. 4B). DEHP increased the levels of secreted VEGF protein compared to the control. DEHP-induced secretion of VEGF protein was significantly inhibited by KRG (100, 500 μg/mL).
Fig. 4.
Effects of KRG on DEHP-induced VEGF expression in Ishikawa cells. (A) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 24 h. VEGF-A and β-actin were evaluated by Western blot analysis. (B) Ishikawa cells were pre-treated with KRG (100, 500 μg/mL) for 1 h and treated with DEHP (10 μM) for 24 h. The concentration of secreted VEGF was determined in the cell supernatant using a VEGF ELISA. #p < 0.05, CON vs. DEHP; ∗p < 0.05, DEHP vs. DEHP + KRG.
3.5. KRG inhibits DEHP-induced endometriosis in a mouse model
In the endometriosis mouse model, growth of endometriosis lesions was significantly inhibited in the DEHP + KRG (200, 400 mg/kg) groups compared to the DEHP treatment only group (Fig. 5B). Endometriosis lesions containing endometrial glands (g) and stroma (s) were confirmed in mice through morphological validation using H&E staining (Fig. 5C). Cell proliferation (Ki67) of KRG (400 mg/kg) administrated groups was significantly decreased compared to DEHP alone. CD10 expression to confirm endometrial stroma cells was significantly reduced in KRG (200, 400 mg/kg) groups compared to DEHP alone. Likewise, inflammation-induced by macrophage (F4/80) was decreased in KRG groups compared to DEHP alone (Fig. 5C).
4. Discussion
In the present study, we investigated the protective effect of KRG against endometrial cancer and endometriosis exacerbated by DEHP. Previous experimental and epidemiological studies have shown that DEHP exposure may be associated with the development of endocrine-related diseases such as endometriosis [48]. A connection between endometriosis and endometrial cancer has long been assumed because of the many risk factors shared by both diseases [49]. Moreover, women previously diagnosed with endometriosis have an increased risk of developing endometrial cancer, consistent with a link between endometriosis and endometrial cancer [50]. DEHP increases the viability of endometrial cancer cells, and of endometrial stromal cells (ESCs), and the levels of inflammatory mediators such as TNF (tumor necrosis factor)-α in those cells [21,51]. The volume of ectopic endometrial lesions in the endometriosis animal model was significantly increased by DEHP (500, 1000 mg/kg) compared to control [12]. KRG possesses a range of biological activities, including anti-oxidant and anti -inflammatory effects [30,31]. Moreover, PPD, PPT, Ginsenoside Rg3, and Ginsenoside Rh2 could inhibit viability and growth in both ESCs and an endometriosis mouse model [34]. KRG was shown to decrease inflammation in endometrial cancer cells and to decrease ectopic endometriosis volume in a DEHP-induced model of endometriosis in mice. We also showed that KRG inhibited DEHP-induced expression of COX-2 and VEGF in endometrial cancer cells. Together, these results suggest that KRG may be a promising natural product for the treatment and prevention of endometriosis and endometrial cancer.
Our RNA seq data analysis confirmed that genes upregulated by DEHP were inhibited by KRG. Toll-like receptor5 (TLR5) and MyD88 were selected from the differentially regulated genes for validation and additional analysis. TLR5 plays a major role in the innate immune system and is expressed in lung and intestinal epithelial cells, human endometrium, and some cancer cells [52]. Downstream signaling of TLR5 involves activation of the MAPK and NF-κB pathways [53]. This signaling depends on the association of TLR5 with MyD88 through their homologous TLR domains [54]. DEHP-induced expression of TLR5 and MyD88 was inhibited by KRG, suggesting that KRG may inhibit activation of ERK1/2 and NF-κB. According to the present study, DEHP increased COX-2 levels in endometrial cancer cells following NF-κB activation. Increased expression of inflammatory proteins can trigger a variety of effects, including promoting tumor progression by increasing cell proliferation and apoptosis resistance [55]. COX-2 is overexpressed in endometrial cancers and COX-2 inhibitors are regarded as potential therapeutics [56]. Thus, these results suggest that COX-2 inhibition by KRG may inhibit cell proliferation in endometrial cancer cells.
We also investigated the effects of KRG on the DEHP-induced endometriosis mouse model. In the mouse endometriosis model, oral administration of KRG alleviated an increase in the size of the endometrial lesions, inflammation of the lesions, and increased cell proliferation by DEHP. In particular, CD10 were significantly reduced in the DEHP + KRG groups compared to the DEHP groups. CD10 is a sensitive immunohistochemical marker of endometrial stromal cells at ectopic sites and is used in confirming a diagnosis of endometriosis [57]. In addition, we found that the number of F4/80-positive macrophages was reduced following KRG treatment. Macrophages are involved in the development of endometriosis, and its depletion is known to significantly reduce the size of the lesion [58,59]. Therefore, a decrease of F4/80-positive macrophages may have contributed to the reduction of the size of the ectopic endometrium. These results suggest that KRG treatment can alleviate or prevent endometriosis caused by exposure to ubiquitous environmental endocrine disruptors. Our findings may prove useful for the development of a new therapeutic strategy for endometrial cancer and endometriosis.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported 2018 grant from the Korean Society of Ginseng to YJL and HDH.
Contributor Information
Hee Dong Han, Email: hanhd@kku.ac.kr.
YoungJoo Lee, Email: yjlee@sejong.ac.kr.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. 2017. [DOI] [PubMed] [Google Scholar]
- 2.Liu S.G., Wu X.X., Hua T., Xin X.Y., Feng D.L., Chi S.Q., Wang X.X., Wang H.B. NLRP3 inflammasome activation by estrogen promotes the progression of human endometrial cancer. OncoTargets Ther. 2019;12:6927–6936. doi: 10.2147/OTT.S218240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smuc T., Rizner T.L. Aberrant pre-receptor regulation of estrogen and progesterone action in endometrial cancer. Mol Cell Endocrinol. 2009;301(1–2):74–82. doi: 10.1016/j.mce.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Zaino R., Whitney C., Brady M.F., DeGeest K., Burger R.A., Buller R.E. Simultaneously detected endometrial and ovarian carcinomas--a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83(2):355–362. doi: 10.1006/gyno.2001.6400. [DOI] [PubMed] [Google Scholar]
- 5.Bulletti C., Coccia M.E., Battistoni S., Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–447. doi: 10.1007/s10815-010-9436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaco-Levy R., Sharabi S., Benharroch D., Piura B., Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139(2):226–232. doi: 10.1016/j.ejogrb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Halden R.U. Plastics and health risks. Annu Rev Publ Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- 8.Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361(1–2):20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Reddy B.S., Rozati R., Reddy B.V., Raman N.V. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006;113(5):515–520. doi: 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun J., Chen B., Zhang L.Q., Zhao D., Li S.G. Phthalate ester concentrations in blood serum, urine and endometrial tissues of Chinese endometriosis patients. Int J Clin Exp Med. 2016;9(2):3808–3819. [Google Scholar]
- 11.Kim S.H., Chun S., Jang J.Y., Chae H.D., Kim C.H., Kang B.M. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case-control study. Fertil Steril. 2011;95(1):357–359. doi: 10.1016/j.fertnstert.2010.07.1059. [DOI] [PubMed] [Google Scholar]
- 12.Kim S.H., Cho S., Ihm H.J., Oh Y.S., Heo S.H., Chun S., Im H., Chae H.D., Kim C.H., Kang B.M. Possible role of phthalate in the pathogenesis of endometriosis: in vitro, animal, and human data. J Clin Endocrinol Metab. 2015;100(12):E1502–E1511. doi: 10.1210/jc.2015-2478. [DOI] [PubMed] [Google Scholar]
- 13.Cobellis L., Latini G., De Felice C., Razzi S., Paris I., Ruggieri F., Mazzeo P., Petraglia F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18(7):1512–1515. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- 14.Park C., Lee J., Kong B., Park J., Song H., Choi K., Guon T., Lee Y. The effects of bisphenol A, benzyl butyl phthalate, and di(2-ethylhexyl) phthalate on estrogen receptor alpha in estrogen receptor-positive cells under hypoxia. Environ Pollut. 2019;248:774–781. doi: 10.1016/j.envpol.2019.02.069. [DOI] [PubMed] [Google Scholar]
- 15.Cho Y.J., Park S.B., Han M. Di-(2-ethylhexyl)-phthalate induces oxidative stress in human endometrial stromal cells in vitro. Mol Cell Endocrinol. 2015;407:9–17. doi: 10.1016/j.mce.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Josh M.K.S., Pradeep S., Adarsh V.K., Amma K.S.V., Devi R.S., Balachandran S., Sreejith M.N., Jaleel U.C.A., Benjamin S. In silico evidences for the binding of phthalates onto human estrogen receptor alpha, beta subtypes and human estrogen-related receptor gamma. Mol Simulat. 2014;40(5):408–417. [Google Scholar]
- 17.Somasundaram D.B., Manokaran K., Selvanesan B.C., Bhaskaran R.S. Impact of di-(2-ethylhexyl) phthalate on the uterus of adult Wistar rats. Hum Exp Toxicol. 2017;36(6):565–572. doi: 10.1177/0960327116657601. [DOI] [PubMed] [Google Scholar]
- 18.Chen F.P., Chien M.H. Lower concentrations of phthalates induce proliferation in human breast cancer cells. Climacteric. 2014;17(4):377–384. doi: 10.3109/13697137.2013.865720. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.H. Analysis of the in vitro effects of di-(2-ethylhexyl) phthalate exposure on human uterine leiomyoma cells. Exp Ther Med. 2018;15(6):4972–4978. doi: 10.3892/etm.2018.6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei N., Feng X., Xie Z., Zhang Y., Feng Y. Long-term di (2-ethylhexyl)-phthalate exposure promotes proliferation and survival of HepG2 cells via activation of NFkappaB. Toxicol Vitro. 2017;42:86–92. doi: 10.1016/j.tiv.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q., Zhang H., Chen Y.J., Chi Y.L., Dong S. The inflammation response to DEHP through PPARgamma in endometrial cells. Int J Environ Res Publ Health. 2016;13(3) doi: 10.3390/ijerph13030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulun S.E. Endometriosis. N Engl J Med. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 23.Ulukus M., Cakmak H., Arici A. The role of endometrium in endometriosis. J Soc Gynecol Invest. 2006;13(7):467–476. doi: 10.1016/j.jsgi.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Han S.J., Jung S.Y., Wu S.P., Hawkins S.M., Park M.J., Kyo S., Qin J., Lydon J.P., Tsai S.Y., Tsai M.J., et al. Estrogen receptor beta modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capobianco A., Rovere-Querini P. Endometriosis, a disease of the macrophage. Front Immunol. 2013;4:9. doi: 10.3389/fimmu.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klemmt P.A.B., Starzinski-Powitz A. Molecular and cellular pathogenesis of endometriosis. Curr Womens Health Rev. 2018;14(2):106–116. doi: 10.2174/1573404813666170306163448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloski T., Pierson R. Endometriosis and chronic pelvic pain: unraveling the mystery behind this complex condition. Nurs Womens Health. 2008;12(5):382–395. doi: 10.1111/j.1751-486X.2008.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bulun S.E., Zeitoun K.M., Takayama K., Sasano H. Estrogen biosynthesis in endometriosis: molecular basis and clinical relevance. J Mol Endocrinol. 2000;25(1):35–42. doi: 10.1677/jme.0.0250035. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y., Liu X., Guo S.W. Therapeutic potential of andrographolide for treating endometriosis. Hum Reprod. 2012;27(5):1300–1313. doi: 10.1093/humrep/des063. [DOI] [PubMed] [Google Scholar]
- 30.Seo S.K., Hong Y., Yun B.H., Chon S.J., Jung Y.S., Park J.H., Cho S., Choi Y.S., Lee B.S. Antioxidative effects of Korean Red Ginseng in postmenopausal women: a double-blind randomized controlled trial. J Ethnopharmacol. 2014;154(3):753–757. doi: 10.1016/j.jep.2014.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Hong M., Lee Y.H., Kim S., Suk K.T., Bang C.S., Yoon J.H., Baik G.H., Kim D.J., Kim M.J. Anti-inflammatory and antifatigue effect of Korean Red Ginseng in patients with nonalcoholic fatty liver disease. J Ginseng Res. 2016;40(3):203–210. doi: 10.1016/j.jgr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim M.K., Lee S.K., Park J.H., Lee J.H., Yun B.H., Park J.H., Seo S.K., Cho S., Choi Y.S. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Sci Rep. 2017;7(1):17670. doi: 10.1038/s41598-017-17956-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang R., Chen S., Zhao M., Li Z., Zhu L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. J Gynecol Obstet Hum Reprod. 2020;49(1):101642. doi: 10.1016/j.jogoh.2019.101642. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B., Zhou W.J., Gu C.J., Wu K., Yang H.L., Mei J., Yu J.J., Hou X.F., Sun J.S., Xu F.Y., et al. The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Dis. 2018;9(5):574. doi: 10.1038/s41419-018-0581-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song H., Lee Y.J. Inhibition of hypoxia-induced cyclooxygenase-2 by Korean Red Ginseng is dependent on peroxisome proliferator-activated receptor gamma. J Ginseng Res. 2017;41(3):240–246. doi: 10.1016/j.jgr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J., Park J., Lee Y.Y., Lee Y. Comparative transcriptome analysis of the protective effects of Korean Red Ginseng against the influence of bisphenol A in the liver and uterus of ovariectomized mice. J Ginseng Res. 2020;44(3):519–526. doi: 10.1016/j.jgr.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim H.J., Choi J.H., Hwang J.H., Kim K.S., Noh J.R., Choi D.H., Moon S.J., Kim H.Y., Kim S.W., Choi S., et al. 3,5-Di-C-beta-D-glucopyranosyl phloroacetophenone, a major component of Melicope ptelefolia, suppresses fibroblast activation and alleviates arthritis in a mouse model: potential therapeutics for rheumatoid arthritis. Int J Mol Med. 2018;42(5):2763–2775. doi: 10.3892/ijmm.2018.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim E.J., Kwon K.A., Lee Y.E., Kim J.H., Kim S.H., Kim J.H. Korean Red Ginseng extract reduces hypoxia-induced epithelial-mesenchymal transition by repressing NF-kappaB and ERK1/2 pathways in colon cancer. J Ginseng Res. 2018;42(3):288–297. doi: 10.1016/j.jgr.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Su G.Y., Zhao C., Qu F.Z., Wang P., Zhao Y.Q. Anticancer activity and potential mechanisms of 1C, a ginseng saponin derivative, on prostate cancer cells. J Ginseng Res. 2018;42(2):133–143. doi: 10.1016/j.jgr.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim B.B., Kim M., Park Y.H., Ko Y., Park J.B. Short-term application of dexamethasone on stem cells derived from human gingiva reduces the expression of RUNX2 and beta-catenin. J Int Med Res. 2017;45(3):993–1006. doi: 10.1177/0300060517701035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang D.W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 42.Greaves E., Cousins F.L., Murray A., Esnal-Zufiaurre A., Fassbender A., Horne A.W., Saunders P.T. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol. 2014;184(7):1930–1939. doi: 10.1016/j.ajpath.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilotas M., Meresman G., Stella I., Sueldo C., Baranao R.I. Effect of aromatase inhibitors on ectopic endometrial growth and peritoneal environment in a mouse model of endometriosis. Fertil Steril. 2010;93(8):2513–2518. doi: 10.1016/j.fertnstert.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 44.Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1(4):a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu Y.S., Kang K.A., Piao M.J., Ahn M.J., Yi J.M., Hyun Y.M., Kim S.H., Ko M.K., Park C.O., Hyun J.W. Particulate matter induces inflammatory cytokine production via activation of NFkappaB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019;21:101080. doi: 10.1016/j.redox.2018.101080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang H.S., Kim J.W., Oh S.H., Song J.H., Yang J.W., Zang Y., Kim Y.H., Lee S.E., Hwang Y.C., Koh J.T. TLR5 activation induces expression of the pro-inflammatory mediator Urokinase Plasminogen Activator via NF-kappaB and MAPK signalling pathways in human dental pulp cells. Int Endod J. 2019;52(10):1479–1488. doi: 10.1111/iej.13140. [DOI] [PubMed] [Google Scholar]
- 47.Xie C., Xu X., Wang X., Wei S., Shao L., Chen J., Cai J., Jia L. Cyclooxygenase-2 induces angiogenesis in pancreatic cancer mediated by prostaglandin E2. Oncol Lett. 2018;16(1):940–948. doi: 10.3892/ol.2018.8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Upson K., Sathyanarayana S., De Roos A.J., Thompson M.L., Scholes D., Dills R., Holt V.L. Phthalates and risk of endometriosis. Environ Res. 2013;126:91–97. doi: 10.1016/j.envres.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Painter J.N., O'Mara T.A., Morris A.P., Cheng T.H.T., Gorman M., Martin L., Hodson S., Jones A., Martin N.G., Gordon S., et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018;7(5):1978–1987. doi: 10.1002/cam4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mogensen J.B., Kjaer S.K., Mellemkjaer L., Jensen A. Endometriosis and risks for ovarian, endometrial and breast cancers: a nationwide cohort study. Gynecol Oncol. 2016;143(1):87–92. doi: 10.1016/j.ygyno.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y.H., Kim S.H., Lee H.W., Chae H.D., Kim C.H., Kang B.M. Increased viability of endometrial cells by in vitro treatment with di-(2-ethylhexyl) phthalate. Fertil Steril. 2010;94(6):2413–2416. doi: 10.1016/j.fertnstert.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 52.Caballero I., Boyd J., Alminana C., Sanchez-Lopez J.A., Basatvat S., Montazeri M., Maslehat Lay N., Elliott S., Spiller D.G., White M.R., et al. Understanding the dynamics of Toll-like Receptor 5 response to flagellin and its regulation by estradiol. Sci Rep. 2017;7:40981. doi: 10.1038/srep40981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tallant T., Deb A., Kar N., Lupica J., de Veer M.J., DiDonato J.A. Flagellin acting via TLR5 is the major activator of key signaling pathways leading to NF-kappa B and proinflammatory gene program activation in intestinal epithelial cells. BMC Microbiol. 2004;4:33. doi: 10.1186/1471-2180-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown J., Wang H., Hajishengallis G.N., Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90(4):417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K.H., Kao A.P., Chang C.C., Lee J.N., Chai C.Y., Hou M.F., Liu C.M., Tsai E.M. Modulation of tumorigenesis and oestrogen receptor-alpha expression by cell culture conditions in a stem cell-derived breast epithelial cell line. Biol Cell. 2010;102(3):159–172. doi: 10.1042/BC20090132. [DOI] [PubMed] [Google Scholar]
- 56.Hasegawa K., Ohashi Y., Ishikawa K., Yasue A., Kato R., Achiwa Y., Nishio E., Udagawa Y. Expression of cyclooxygenase-2 in uterine endometrial cancer and anti-tumor effects of a selective COX-2 inhibitor. Int J Oncol. 2005;26(5):1419–1428. [PubMed] [Google Scholar]
- 57.Onda T., Ban S., Shimizu M. CD10 is useful in demonstrating endometrial stroma at ectopic sites and in confirming a diagnosis of endometriosis. J Clin Pathol. 2003;56(1):79. doi: 10.1136/jcp.56.1.79-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bacci M., Capobianco A., Monno A., Cottone L., Di Puppo F., Camisa B., Mariani M., Brignole C., Ponzoni M., Ferrari S., et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175(2):547–556. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haber E., Danenberg H.D., Koroukhov N., Ron-El R., Golomb G., Schachter M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum Reprod. 2009;24(2):398–407. doi: 10.1093/humrep/den375. [DOI] [PubMed] [Google Scholar]