Graphical abstract
Keywords: Circumflex extrinsic compression, Mycotic aneurysm of aorta, Endocarditis, TTE, TEE
Highlights
-
•
Mycotic aneurysm of the aortic sinus is a rare complication of aortic valve endocarditis.
-
•
Mycotic aneurysm of left sinus causing LM compression and circumflex occlusion is shown.
-
•
We present TTE and TEE approaches to making the diagnosis of this complex lesion.
-
•
Sketches of complications of aortic valve endocarditis are presented.
Introduction
The majority of myocardial ischemia and infarction occurs due to coronary atherothrombosis or myocardial oxygen supply and demand imbalance.1 Myocardial ischemia and infarction due to extrinsic compression of a left coronary artery are unusual and have been the subject of rare case reports. They have been described as being due to severe pulmonary artery dilation in patients with pulmonary hypertension,2 anomalous origin of left coronary artery from right aortic sinus with interarterial course,3 congenital aortic sinus of Valsalva aneurysms (SVAs),4 pseudoaneurysm following ascending aortic replacement surgery,5 and submitral left ventricular aneurysms.6 Aortic valve endocarditis can be complicated by a variety of subaortic (Figure 1)7, 8, 9 and periaortic (Figure 2)10, 11, 12, 13 structural complications. In this report, we describe a case of aortic valve endocarditis that presented with acute lateral wall myocardial infarction due to extrinsic compression of left main (LM) and occlusion of the circumflex coronary artery by a mycotic aneurysm of the left aortic sinus of Valsalva (Figure 2I). Additionally, an aorta to left ventricular fistula was noted due to rupture of the aneurysm through the zone of mitral-aortic intervalvular fibrosa (MAIVF; Figure 2J). The complex diagnosis was made by transthoracic (TTE) and transesophageal echocardiography (TEE), and findings on selective coronary angiography were complementary.
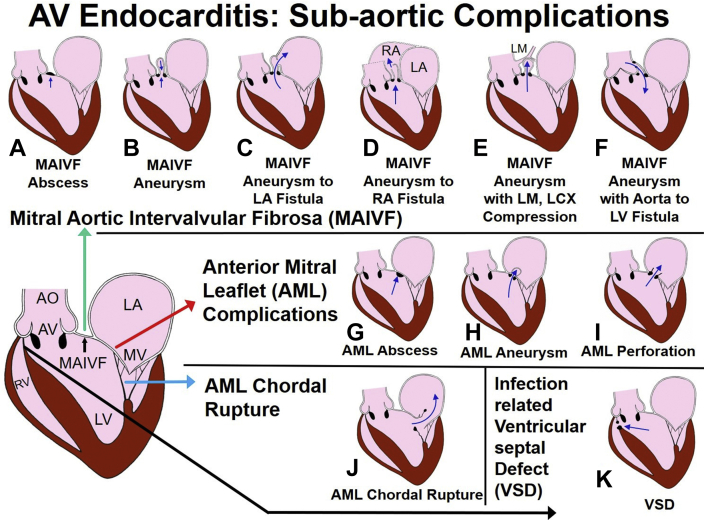
Figure 1.
Spectrum of subaortic complications of AV endocarditis. The infected jet of aortic regurgitation can strike the subaortic structures and lead to secondary infection of MAIVF, AML, chordae tendineae of AML, and ventricular septum. MAIVF infection will produce abscess (A), a mycotic aneurysm from expansion of the infected structure, and this aneurysm shows systolic filling (upward arrow) and diastolic emptying (downward arrow) (B); fistula by rupture into the LA (C); fistula by rupture into the RA (D); compression of LM and branches with ischemia (E); and aorta to LV fistula (F). An AML infection can produce abscess (G), AML aneurysm (H), and perforation (I). Infection of chordae tendineae may cause chordal rupture and flail AML (J). Infection can lead to septal involvement and VSD (K). AML, Anterior mitral leaflet; AO, aorta; AV, aortic valve; LA, left atrium; LCX, left circumflex; LV, left ventricle; MV, mitral valve; RA, right atrium; RV, right ventricle.
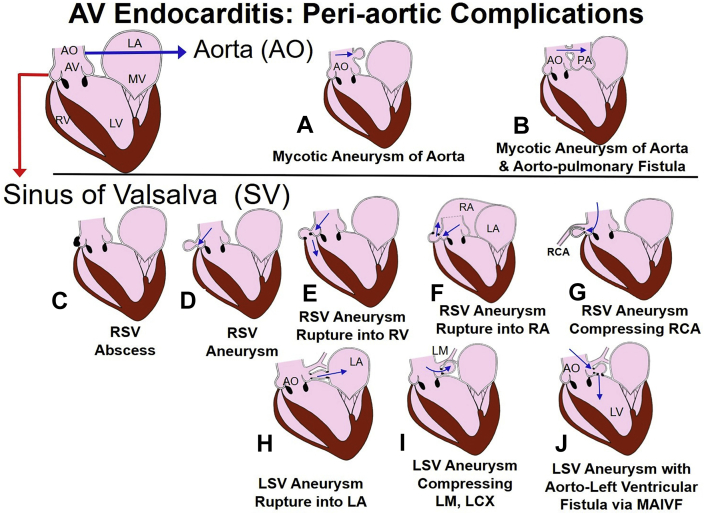
Figure 2.
Spectrum of periaortic complications of aortic valve endocarditis. Mycotic aneurysm of the AO (A) and rupture of the aneurysm into the PA with shunt (B). Infection can involve aortic ring and any SV and cause abscess (involvement of RSV is shown in panel C), RSV aneurysm (D), and RSV aneurysm rupture into the RV (E) and RA (F). Involvement of RSV can cause compression of the RCA and ischemia (G). An LSV aneurysm can rupture into the left atrium (H), cause compression of the LM and LCX (I), and erode into MAIVF with formation of aorta to left ventricular fistula (J). AO, Aorta; LCX, left circumflex; LSV, left sinus of Valsalva; PA, pulmonary artery; RA, right atrium; RCA, right coronary artery; RSV, right sinus of Valsalva; RV, right ventricle; SV, sinus of Valsalva.
Case Presentation
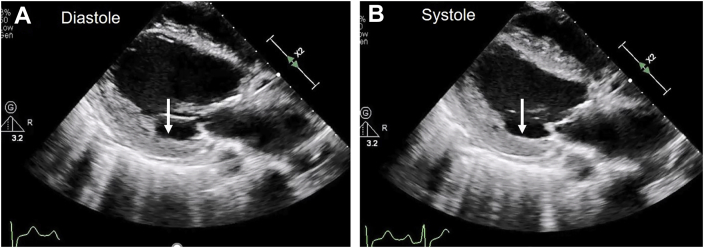
A 42-year-old man with history of end-stage renal disease on hemodialysis for many years via left arm fistula was initially admitted with a 2-day history of pleuritic chest pain, dyspnea, and chills (admission 1, Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Videos 1–5). On examination his heart rate was 117 beats per minute, blood pressure 120/60 mm Hg, and temperature 101.4°F. He had a diastolic murmur in the aortic area. His hemoglobin was 10 g/dL, white blood cell count was 10.17 × 109 cells/L, and multiple blood cultures were positive for methicillin-sensitive Staphylococcus aureus. A TTE showed a small pericardial effusion, normal wall motion, left ventricular estimated ejection fraction of 65%, and severe aortic regurgitation14 (Figure 3, Videos 1 and 2). TTE and TEE demonstrated a large, thickened, and redundant left cusp with severe prolapse (Figure 3, Figure 4, Figure 5, Video 3) and diastolic vibrations suggestive of flail motion (Figure 3). Severe aortic regurgitation was related to a prolapsing left cusp (Figure 3, Figure 4, Figure 5, Videos 3–5). The TEE also showed a large abscess and a moderate-size mycotic aneurysm in the left aortic sinus close to the LM coronary artery (LMCA; Figure 5, Video 4). A TEE Doppler study showed low-velocity LMCA flow indicating no obstruction of flow by the moderate-size mycotic aneurysm. A cardiac computed tomographic (CT) scan showed no significant obstructive coronary artery disease and confirmed the left aortic sinus mycotic aneurysm and its proximity to LMCA (Figure 6). He underwent surgical drainage and debridement of the mycotic aneurysm. The ostium of the aneurysm was closed with a patch of autologous pericardium, and the aortic valve was replaced with a no. 21 On-X bileaflet disk mechanical prosthesis (On X disc valve, CryoLife, Kennesaw, GA). Surgical inspection confirmed echocardiographic findings. A large, thickened, and infected left cusp sagged down from its basal annular attachment and showed severe prolapse (Figure 7). He also had anterior pericardiectomy due to severe fibrinous pericarditis. His postoperative course was uncomplicated, and he was discharged 5 days after surgery. He was treated with a 6-week course of intravenous cefazolin. A TTE performed at 7 weeks after the first surgery showed intact patch repair of the mycotic aneurysm and normal function of the prosthetic aortic valve.
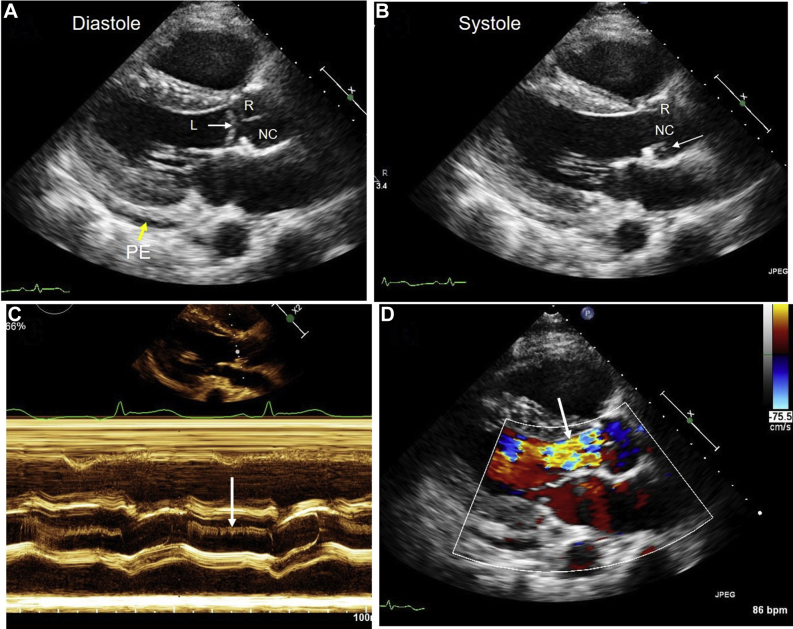
Figure 3.
Admission 1. Two-dimensional, color Doppler, and M-mode images from parasternal approach. (A) Parasternal long-axis diastolic frame shows trivial PE and normal coaptation of R and NC cusps. The L cusp is below the other cusps and shows prolapse. (B) Long-axis systolic frame shows opening of R and NC cusps. A portion of L cusp is noted (arrow) behind the NC cusp. (C) M-mode shows diastolic fluttering of the L cusp. (D) Severe aortic regurgitation is noted (arrow). The aortic regurgitation vena contracta width is >0.6 cm, and the jet width/left ventricular outflow tract ratio is ≥0.65. L, Left; NC, noncoronary; PE, pericardial effusion; R, right.
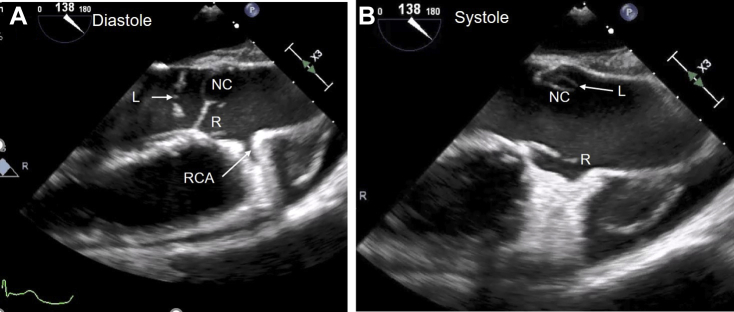
Figure 4.
Admission 1. Midesophageal TEE long-axis image of aorta at 138°. (A) Diastolic image of the aorta and aortic valve shows normal coaptation of R and NC cusps. Severe prolapse and flail motion of the L cusp (arrow) is noted. The RCA ostium is seen. (B) Systolic frame shows normal opening of R and NC cusps. A portion of L cusp is seen behind the NC cusp (arrow). L, Left; NC, noncoronary; R, right; RCA, right coronary artery.
Figure 5.
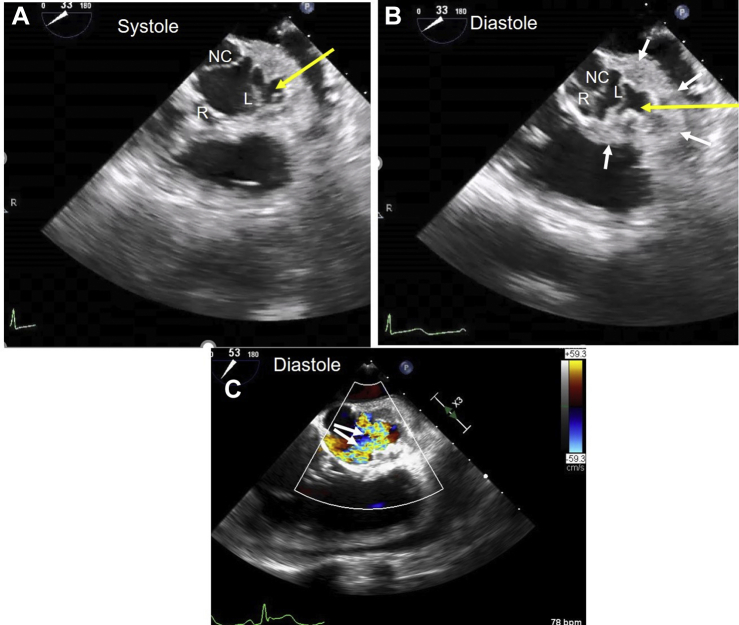
Admission 1. Midesophageal TEE short-axis two-dimensional images with color Doppler. (A) Short-axis image of open aortic valve cusps. (B) Diastolic frame shows good coaptation of R and NC cusps. The L cusp margin is pushed inferiorly due to prolapse and incompletely closed. White arrows outline the extent of mycotic aneurysm in left aortic sinus. Yellow arrows in panels A and B show the ostium of left sinus mycotic aneurysm (SVA). (C) Two large jets of aortic regurgitation originate from the L cusp (arrows). L, Left; NC, noncoronary; R, right.
Figure 6.
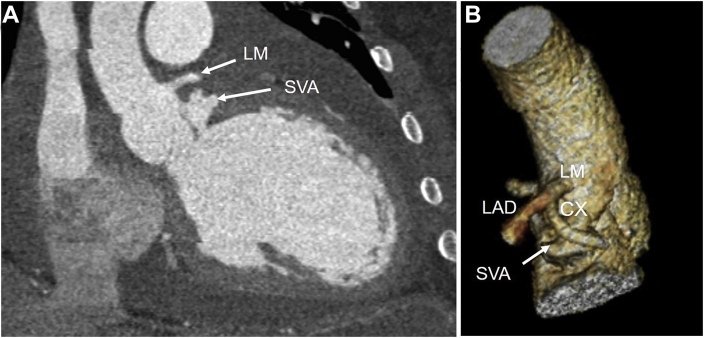
Admission 1. Cardiac CT scan. (A) Coronal image shows separation between LM ostium and SVA. (B) Three-dimensional volume-rendered cardiac CT image shows the proximity of LM, LAD, CX, and coronary artery to SVA but no compression. CX, Circumflex; LAD, left anterior descending artery.
Figure 7.
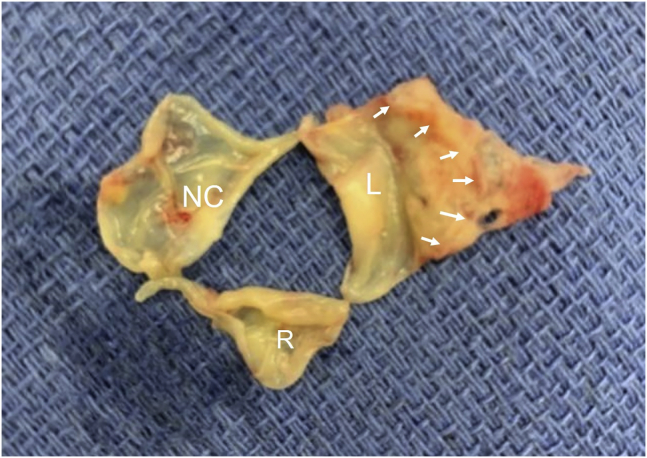
Admission 1. The aortic valve explanted at surgery; leaflets are shown to correspond to the TEE short-axis view. The right cusp is the smallest. The left cusp is very large. No vegetation is noted. Basal attachment of the left cusp to the left sinus (outlined by arrowheads) is shown. The cusp appeared thick, redundant, and inflamed and showed severe prolapse at surgical inspection. L, Left; R, right.
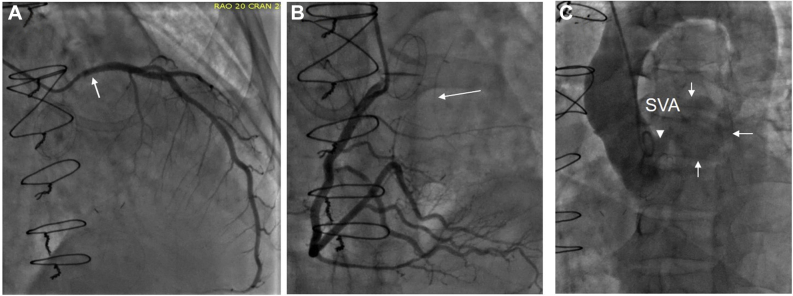
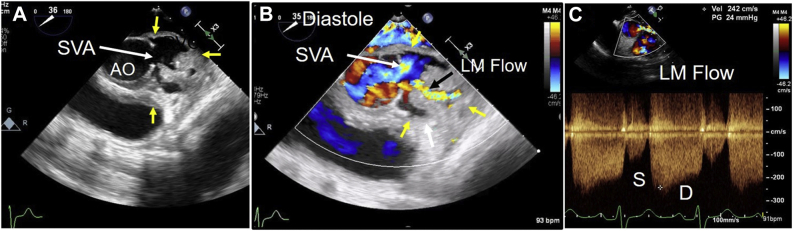
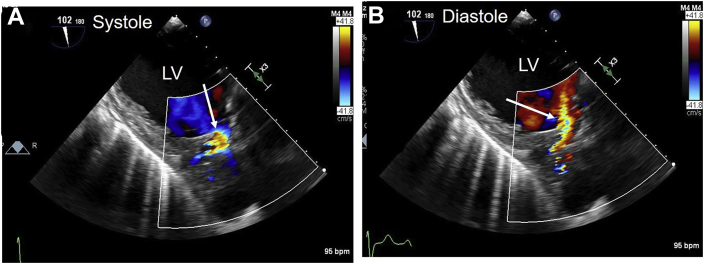
He was readmitted 3 months later due to acute shortness of breath (admission 2, Figure 8, Figure 9, Figure 10, Figure 11, Videos 6–8). Electrocardiogram showed ST segment elevation in leads aVR and V5-6, and troponin-T was elevated to 4.8 ng/mL. He was intubated, and his blood pressure was stabilized on vasopressors. Emergent coronary angiography revealed compression of proximal LM, ostial occlusion of the circumflex artery, and collateral filling from the right system (Figure 8). The findings on aortography were abnormal, but a complete understanding of abnormal anatomy and blood flow was realized only after completion of echocardiography. A TTE showed severe hypokinesis of the basal and midinferolateral walls due to myocardial infarction, an estimated ejection fraction of 30% (Figure 9, Video 6), normal function of the disk aortic prosthesis, and severe mitral regurgitation.14 A TEE showed patch dehiscence of previously repaired mycotic aneurysm, and a large communication was seen between the aorta and a large mycotic aneurysm 3 cm in size (Figure 10, Video 7). The turbulent and high-velocity LM flow was suggestive of compression from the aneurysm (Figure 10, Video 7). The mycotic aneurysm also had ruptured via the MAIVF into the left ventricle resulting in the formation of an aorta to left ventricular fistula (Figure 2, Figure 11, Video 8). Aortographic findings were better understood after echocardiography (Figure 8, Figure 10 and 11). His blood cultures from this admission were positive for Enterococcus, suggesting a new episode of infective endocarditis. He was taken to the operating room for the second time and placed on bypass via femoral artery and venous cannulation. He was noted to have a large left sinus mycotic aneurysm of 3 × 4 cm. It was resected, and the root was replaced with a no. 28 Vascutek graft and LM was reimplanted as per the Cabrol technique using an 8 mm Dacron aortic graft (Terumo Aortic, Sunrise, FL). He also had mitral valve replacement with a no. 27 Epic St. Jude porcine bioprosthesis (Abbott, Plymouth, MN). While rewarming, his lateral left ventricular wall dehisced from the graft anastomosis and he expired in the operating room.
Figure 8.
Admission 2. Images of coronary angiography during second admission 3 months after first surgery when the patient presented with lateral infarction. (A) Compression of the proximal LM (arrow) and absence and complete occlusion of left circumflex coronary artery. (B) Right coronary artery angiogram shows filling of circumflex via collaterals (arrow). (C) Aortogram shows opacification left SVA outlined by thin arrows (SVA) via a connection (arrowhead) in the left aortic sinus. The bottom of the SVA showed a small connection with the left ventricle through MAIVF in real-time angiography that was not appreciated in the still image.
Figure 9.
Admission 2. Two-dimensional TTE parasternal long-axis images acquired during second admission when the patient presented with acute lateral wall myocardial infarction. (A) Diastole. (B) Systole. There is preserved myocardial thickness with regional akinesis of the basal and midinferolateral wall segments (arrows) consistent with an acute myocardial infarction.
Figure 10.
Admission 2. Midesophageal TEE two-dimensional, color Doppler, and continuous-wave Doppler images. (A) Short-axis image of the aortic root shows a large left SVA due to patch dehiscence. The ostium of the SVA is shown by a large arrow, and its extent by yellow arrows. (B) Color flow imaging shows emptying of SVA in blue in diastole (arrow). The extent of the SVA is shown by yellow arrows. Turbulent, mosaic color, high-velocity flow (black arrow) suggests LM obstruction due to extrinsic compression. (C) Color-guided continuous-wave Doppler examination of the LM flow shows mildly increased systolic (S) and very high diastolic (D) velocity of > 2 m/sec. The LM is very long, and no left circumflex is noted. Findings are consistent with LM compression and likely left circumflex occlusion. AO, aorta.
Figure 11.
Admission 2. TEE transgastric long-axis two-dimensional and color Doppler images. (A) Blood flows toward the LV outflow tract and aorta in blue (arrow) in systole. (B) Blood returns toward the LV outflow tract in red (arrow) in diastole. This finding combined with a normal functioning prosthetic aortic valve suggested that the left SVA had eroded into the MAIVF and created an aorta to LV fistula (which was confirmed at surgery). LV, Left ventricular, ventricle.
Discussion
Extrinsic compression of the LMCA as a cause of acute coronary syndrome is rare and unusual. It may be caused by pressure from a dilated main pulmonary artery in patients with pulmonary hypertension.2 This is described as LMCA compression syndrome, and, in some patients, stenting is an effective option.2 Compression of the LMCA between the aorta and pulmonary artery has been demonstrated by multimodality imaging in anomalous origin of left coronary artery from right sinus and interarterial course.3 The LMCA can also be compressed by aneurysmal structures in the vicinity of the aorta such as congenital and pseudoaneurysms of the aortic sinus of Valsalva,4,5 submitral left ventricular aneurysm,6 and MAIVF aneurysm in aortic valve endocarditis.7, 8, 9 The most commonly reported lesion is the MAIVF aneurysm (Figure 1E). Sudhakar et al,9 in their review of 88 cases of MAIVF aneurysm, reported compression of one or more coronary arteries in 10 cases (11%): circumflex in all, LM in seven, and left anterior descending artery in three.
Aortic SVA is rare cardiac anomaly that may be congenital or acquired. Congenital is more common in Asians and involves right sinus in 82%, posterior in 17%, and left sinus in <1%.4 Acquired aneurysms are much less common than congenital and may be spontaneous due to cystic medial necrosis, postsurgical status, trauma, Takayasu's arteritis, or endocarditis.5,10, 11, 12, 13 Unruptured SVA may be discovered as an incidental finding10 or cause symptoms related to right ventricular outflow obstruction, left ventricular outflow obstruction, complete heart block, or ventricular tachycardia. Ruptured aneurysms may present with heart failure due to left-to-right shunt.11 There is one case report of LM compression by mycotic aneurysm of the left sinus, where the diagnosis was made by cardiac CT.13 In this report, we describe the echocardiographic findings of LM compression by a mycotic aneurysm of left sinus and aorta to left ventricular fistula. Combined use of TTE, TEE, and all echocardiographic modalities allowed a complete diagnosis of complex structural complications in this patient with aortic valve endocarditis.
Our case was previously presented as a poster abstract for American College of Cardiology scientific session in 2020.15 In this publication, we provide a detailed illustration of subaortic (Figure 1) and periaortic complications (Figure 2) of infective endocarditis. We also provide detailed discussions of echocardiographic diagnosis of two previously undescribed complications (Figure 2I and J).
Conclusion
Extrinsic compression of the coronary artery by mycotic aneurysm of the sinus of Valsalva is rare and unusual. This report describes a case of a 42-year-old man who presented with acute lateral wall myocardial infarction. Coronary angiography showed compression of LM and occlusion of circumflex coronary artery. He was on hemodialysis, and blood cultures were positive for Enterococcus. Echocardiography established the diagnosis of coronary compression by a large mycotic aneurysm of the left aortic sinus and revealed the abnormal flow related to aorta to left ventricular fistula. Urgent surgery was performed, and findings were confirmed. Familiarity with complex structural complications associated with aortic valve endocarditis (Figures 1 and 2) and use of all the echocardiographic tools should allow an experienced echocardiographer to make the complex diagnosis required for surgical planning.
Footnotes
Conflicts of Interest: None.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2021.11.009.
Supplementary Data
Admission 1. Parasternal long-axis view shows trivial pericardial effusion, normal wall motion, and normal systolic LV function. There is normal coaptation of right and noncoronary cusps. Left cusp is seen in left ventricular outflow tract below the other cusps and shows severe prolapse.
Admission 1. Parasternal long-axis view with color flow imaging shows severe aortic regurgitation.
Admission 1. TEE midesophageal long-axis view shows normal coaptation of right and noncoronary cusps. Severe prolapse and flail motion of the left cusp are noted.
Admission 1. TEE midesophageal short-axis view shows good coaptation of the right and noncoronary cusps. The left cusp margin is pushed inferiorly due to prolapse and closes incompletely during diastole. A large mycotic aneurysm and abscess are noted in the left aortic sinus.
Admission 1. Midesophageal TEE short-axis image of aortic root with color flow imaging shows eccentric jet of severe aortic regurgitation originating from the left cusp.
Admission 2. TTE long-axis view 3 months after initial surgery shows preserved thickness and akinesis of the basal and midinferolateral walls of the left ventricle.
Admission 2. TEE short-axis view of aorta with color flow imaging shows systolic filling and diastolic emptying of SVA. Turbulent, mosaic, high-velocity flow is consistent with LM compression.
Admission 2. TEE transgastric long-axis view with color flow imaging shows blood flow toward aorta in blue in systole and return toward left ventricular outflow tract in red in diastole. These findings are suggestive of left SVA eroding into MAIVF and creating an aorta to left ventricular fistula (Figure 2J). These findings were confirmed at re-do surgery.
References
- 1.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 2.Labin J.E., Saggar R., Yang E.H., Lluri G., Channick R., Ardhali A., et al. Left main coronary artery compression in pulmonary hypertension. Catheter Cardiovasc Interv. 2021;97:E956–E966. doi: 10.1002/ccd.29401. [DOI] [PubMed] [Google Scholar]
- 3.Chang H.R., Hsieh J.C., Chao S.F., Wang J.H., Huang S.K.S. Sudden cardiac death associated with anomalous origin of the left main coronary artery from the right sinus, with an intramural course. Tex Heart Inst J. 2015;42:554–557. doi: 10.14503/THIJ-14-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H.W., Xiong H., Xu J.P., Wang X.Q., Hu S.S. A new and simple classification for sinus of Valsalva aneurysms and the corresponding surgical procedure. Eur J Cardiothorac Surg. 2013;43:1188–1193. doi: 10.1093/ejcts/ezs673. [DOI] [PubMed] [Google Scholar]
- 5.Gannon M.P., Boutis L.S., Brinster D.R., Esposito R.A., Saba S.G., Makaryus J.N. Aortic pseudoaneurysm causing compression of the left main coronary artery. J Invasive Cardiol. 2018;30:E103–E104. [PubMed] [Google Scholar]
- 6.Kumar P., Jana S., Kenchappa K., Manik G. Large submitral aneurysm compressing left main coronary artery: rare presentation of a rare disease. J Assoc Phys India. 2018;66:90–91. [PubMed] [Google Scholar]
- 7.Bansal R.C., Moloney P.M., Marsa R.J., Jacobson J.G. Echocardiographic features of a mycotic aneurysm of the left ventricular outflow tract caused by perforation of mitral-aortic intervalvular fibrosa. Circulation. 1983;67:930–934. doi: 10.1161/01.cir.67.4.930. [DOI] [PubMed] [Google Scholar]
- 8.Karalis D.G., Bansal R.C., Hauck A.J., Ross J.J., Applegate P.M., Jutzy K.R., et al. Transesophageal echocardiographic recognition of subaortic complications in aortic valve endocarditis. Circulation. 1992;86:353–362. doi: 10.1161/01.cir.86.2.353. [DOI] [PubMed] [Google Scholar]
- 9.Sudhakar S., Sewani A., Agrawal M., Uretsky B.F. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): a comprehensive review. J Am Soc Echocardiogr. 2010;23:1009–1018. doi: 10.1016/j.echo.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Batiste C., Bansal R.C., Razzouk A.J. Echocardiographic features of an unruptured mycotic aneurysm of the right aortic sinus of Valsalva. J Am Soc Echocardiogr. 2004;17:474–477. doi: 10.1016/j.echo.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Bansal R.C., Wangsness K.M., Bailey L. Right aortic sinus of Valsalva–to–right ventricle fistula complicating bacterial endocarditis of membranous ventricular septal defect: evaluation by two-dimensional, color flow, and Doppler echocardiography. J Am Soc Echocardiogr. 1993;6:308–311. doi: 10.1016/s0894-7317(14)80068-0. [DOI] [PubMed] [Google Scholar]
- 12.Lacalzada-Almeida J., Rosa-Hernandez A., Izquierdo-Gomez M.M., Garcia-Niebla J., Hernandez-Betancor I., Bonilla-Arjona J.A., et al. Compression of the right coronary artery by an aortic pseudoaneurysm after infective endocarditis: an unusual case of myocardial ischemia. Clin Interv Aging. 2018;13:9–11. doi: 10.2147/CIA.S144840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faillace B.L.R., Galon M.Z., Oliveira M.D.P., Prado G.F.A., Jr., Truffa A.A.M., Ribeiro E.E., et al. Left main compression by a giant aneurysm of the left sinus of Valsalva: an extremely rare reason for myocardial infarction and cardiogenic shock. Case Rep Cardiol. 2015;2015:703646. doi: 10.1155/2015/703646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoghbi W.A., Adams D., Bonow R.O., Enriquez-Sarano M., Foster E., Grayburn P.A., et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Ali S., Bansal R.C., Ghatnekar N., Prasad V., Floridia R., Razzouk A. Extrinsic compression of circumflex coronary artery by mycotic aortic sinus aneurysm. J Am Coll Cardiol. 2020;75(11 Suppl 1):2427. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Admission 1. Parasternal long-axis view shows trivial pericardial effusion, normal wall motion, and normal systolic LV function. There is normal coaptation of right and noncoronary cusps. Left cusp is seen in left ventricular outflow tract below the other cusps and shows severe prolapse.
Admission 1. Parasternal long-axis view with color flow imaging shows severe aortic regurgitation.
Admission 1. TEE midesophageal long-axis view shows normal coaptation of right and noncoronary cusps. Severe prolapse and flail motion of the left cusp are noted.
Admission 1. TEE midesophageal short-axis view shows good coaptation of the right and noncoronary cusps. The left cusp margin is pushed inferiorly due to prolapse and closes incompletely during diastole. A large mycotic aneurysm and abscess are noted in the left aortic sinus.
Admission 1. Midesophageal TEE short-axis image of aortic root with color flow imaging shows eccentric jet of severe aortic regurgitation originating from the left cusp.
Admission 2. TTE long-axis view 3 months after initial surgery shows preserved thickness and akinesis of the basal and midinferolateral walls of the left ventricle.
Admission 2. TEE short-axis view of aorta with color flow imaging shows systolic filling and diastolic emptying of SVA. Turbulent, mosaic, high-velocity flow is consistent with LM compression.
Admission 2. TEE transgastric long-axis view with color flow imaging shows blood flow toward aorta in blue in systole and return toward left ventricular outflow tract in red in diastole. These findings are suggestive of left SVA eroding into MAIVF and creating an aorta to left ventricular fistula (Figure 2J). These findings were confirmed at re-do surgery.