Abstract
Background
Patients with intermediate- and advanced-stage hepatocellular carcinoma (HCC) represent a highly heterogeneous patient collective with substantial differences in overall survival.
Purpose
To evaluate enhancing tumor volume (ETV) and enhancing tumor burden (ETB) as new criteria within the Barcelona Clinic Liver Cancer (BCLC) staging system for optimized allocation of patients with intermediate- and advanced-stage HCC to undergo transarterial chemoembolization (TACE).
Materials and Methods
In this retrospective study, 682 patients with HCC who underwent conventional TACE or TACE with drug-eluting beads from January 2000 to December 2014 were evaluated. Quantitative three-dimensional analysis of contrast-enhanced MRI was performed to determine thresholds of ETV and ETB (ratio of ETV to normal liver volume). Patients with ETV below 65 cm3 or ETB below 4% were reassigned to BCLC Bn, whereas patients with ETV or ETB above the determined cutoffs were restratified or remained in BCLC Cn by means of stepwise verification of the median overall survival (mOS).
Results
This study included 494 patients (median age, 62 years [IQR, 56–71 years]; 401 men). Originally, 123 patients were classified as BCLC B with mOS of 24.3 months (95% CI: 21.4, 32.9) and 371 patients as BCLC C with mOS of 11.9 months (95% CI: 10.5, 14.8). The mOS of all included patients (including the BCLC B and C groups) was 15 months (95% CI: 12.3, 17.2). A total of 152 patients with BCLC C tumors were restratified into a new BCLC Bn class, in which the mOS was then 25.1 months (95% CI: 21.8, 29.7; P < .001). The mOS of the remaining patients (ie, BCLC Cn group) (n = 222; ETV ≥65 cm3 or ETB ≥4%) was 8.4 months (95% CI: 6.1, 11.2).
Conclusion
Substratification of patients with intermediate- and advanced-stage hepatocellular carcinoma according to three-dimensional quantitative tumor burden identified patients with a survival benefit from transarterial chemoembolization before therapy.
© RSNA, 2022
Summary
Restratification of patients with advanced-stage hepatocellular carcinomas according to enhancement-based, three-dimensional, quantitative tumor burden measurements identified patients who would benefit from transarterial chemoembolization.
Key Results
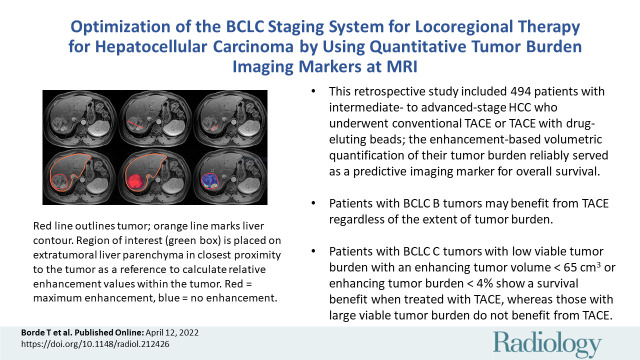
■ This retrospective study included 494 patients with intermediate- to advanced-stage hepatocellular carcinoma who underwent conventional transarterial chemoembolization (TACE) or TACE with drug-eluting beads; the enhancement-based volumetric quantification of their tumor burden reliably served as a predictive imaging biomarker for overall survival.
■ Patients with Barcelona Clinic Liver Cancer (BCLC) B tumors may benefit from TACE regardless of the extent of tumor burden.
■ Patients with BCLC C tumors with low viable tumor burden with an enhancing tumor volume of less than 65 cm3 or enhancing tumor burden of less than 4% show a survival benefit when treated with TACE, whereas patients with a large viable tumor burden do not benefit from TACE.
Introduction
Hepatocellular carcinoma (HCC) is the fourth most common cause of cancer-related death worldwide. More than 70% of patients with HCC are diagnosed at intermediate- or advanced-stage disease and are ineligible for curative treatment (1,2). The Barcelona Clinic Liver Cancer (BCLC) staging system was introduced as the standard staging system for HCC (3,4). Accordingly, the recommended treatment option for patients with BCLC B cancer is transarterial chemoembolization (TACE). For patients with BCLC C cancer, systemic therapy and immune checkpoint inhibitors are the current standard of care (3). However, the BRIDGE (Bridge to Better Outcomes in HCC) and GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of Its Treatment with Sorafenib) studies identified that TACE is frequently applied beyond BCLC stage B (5,6), with 46% of patients with BCLC C tumors undergoing TACE (6). Survival benefits were observed in patients treated with TACE in whom the BCLC C categorization was assigned solely based on segmental macrovascular portal vein invasion (7–9). This finding demonstrates that both BCLC stages B and C consist of highly heterogeneous cancers with vastly different extents of tumor burden. Such heterogeneity of disease results in differences in expected overall survival among treated patients (10–12). Therefore, optimized subcategorization of this heterogeneous population is necessary to achieve meaningful refinement of therapeutic decisions for each individual patient (13).
The BCLC stratification algorithm currently relies on one-dimensional tumor size measurements and number of lesions to help quantify tumor burden and recommend the most appropriate treatment in early and intermediate stages (4). However, tumor burden and lesion size are no longer taken into consideration for substratification between intermediate and advanced stages (BCLC B vs C) and are formally irrelevant for clinical decision-making, which has been recognized as a major limitation of the system in a recently proposed amendment (4). These one-dimensional tumor measurements have limitations in their ability to reflect true tumor size, viability, and growth potential (14,15). The known discrepancy between the single-axis diameter of a target lesion and the actual viable tumor volume prompted the introduction of modified Response Evaluation Criteria in Solid Tumors and, more recently, the development of three-dimensional quantitative tumor assessment methods (16).
Volumetric quantification of the enhancing tumor tissue at multiparametric MRI has been shown to predict survival more reliably after TACE (15,17,18). Thus, in this study, we evaluated the role of three-dimensional quantitative tumor burden analysis for subcategorization of the BCLC system with the goal of establishing potentially improved treatment allocation of patients with intermediate- to advanced-stage HCC treated with TACE.
Materials and Methods
Study Design
This retrospective single-institution study was approved by the institutional review board. Data collection and analysis were conducted in compliance with the Health Insurance Portability and Accountability Act. The study was designed in agreement with the Standards for Reporting of Diagnostic Accuracy guidelines. Because of the retrospective nature of this study, the requirement for written informed consent was waived by the institutional review board committee. The patient sample in our study has partial overlap with the patients in the study by Tacher et al (15), where the primary focus was tumor response according to three-dimensional quantitative criteria without any restratification according to BCLC.
Study Sample
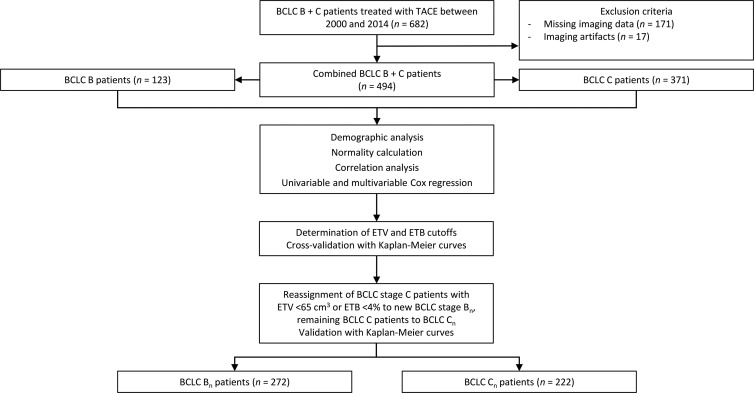
This study initially considered 682 patients with BCLC B or C tumors and diagnosed with HCC who underwent either conventional TACE or drug-eluting bead TACE as first-line therapy between January 2000 and December 2014. Patients were treatment-naive regarding prior locoregional and systemic therapy. Overall, 188 patients (27.6%) were excluded due to missing, technically insufficient, or artifactually distorted MRI data. BCLC stages were determined by rereviewing the imaging, laboratory, and hepatic functional parameters. The final analyzed study sample (n = 494) was stratified according to BCLC B (123 [24.1%]) and BCLC C (371 [75.9%]) (3) (Fig 1). Data on race and ethnicity were collected from the electronic health record. For further information, including disease staging and intra-arterial therapy, see Appendix E1 (online).
Figure 1:
Study flowchart. Following the exclusion criteria, 494 patients were included in the analyses and stratified according to Barcelona Clinic Liver Cancer (BCLC) stages B and C. Statistical analysis was performed on clinical data from each group. Enhancing tumor volume (ETV) and enhancing tumor burden (ETB) cutoffs were determined, and patients were reassigned to new BCLC Bn and Cn classes according to their volumetrically quantified tumor burden. TACE = transarterial chemoembolization.
MRI Acquisition
Details on image acquisition parameters and scanners are found in Appendix E1 (online).
Image Analysis
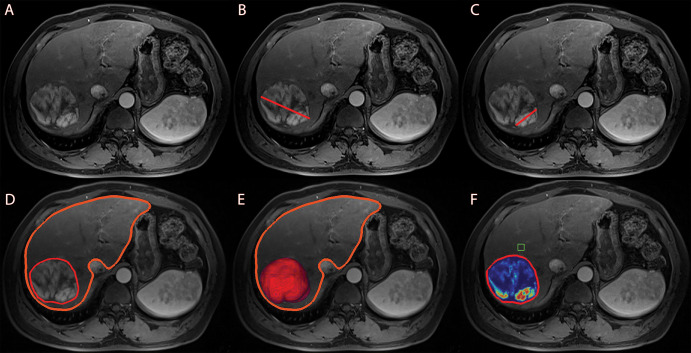
Tumor assessment, including one- and three-dimensional measurements, was performed independently by two radiologic readers (N.N. and R.D., with 5 and 6 years of experience in abdominal MRI and volumetric image analysis), blinded to all clinical data and survival outcomes. The interobserver reliability was calculated by using Cohen κ (κ, 0.8). Tumor diameters were determined on Digital Imaging and Communications in Medicine files, as previously described (19) (Fig 2B, 2C).
Figure 2:
Anatomic and enhancement-based assessment methods on axial-view images. (A) Baseline arterial enhanced T1-weighted MRI scan, preprocedural for anatomic orientation. (B) One-dimensional measurement of the largest overall tumor diameter illustrated by a red line. (C) One-dimensional measurement of the largest enhancing tumor diameter illustrated by a red line. Enhancement was defined as hyperintense areas at the arterial phase that were not visible at the precontrast phase. (D) Semiautomated three-dimensional tumor and whole-liver segmentation to create a segmentation mask, which involves the entire lesion and liver. The fine red line outlines the tumor, and the thick orange line marks the liver contour. (E) Three-dimensional segmentation mask represents the total tumor volume in red at maximum intensity projection. (F) Automatically generated three-dimensional quantification of enhancing tumor volume based on image subtraction. A region of interest (green box) was placed on extratumoral liver parenchyma in the closest proximity to the tumor as a reference to calculate the relative enhancement values within the tumor. Color coding varied from red, representing maximum enhancement, to blue, representing no enhancement.
Total liver and tumor volume, enhancing tumor volume (ETV, in cubic centimeters), and enhancing tumor burden (ETB, in percentages) were obtained at baseline MRI with use of a semiautomatic tumor segmentation software and quantitative European Association for the Study of the Liver software (IntelliSpace Portal, version 8; Philips Healthcare) (16,20). In short, the software used a volumetric segmentation mask obtained on portal-venous phase images to quantify the liver and tumor volume, respectively, and the ETV at arterial-phase T1-weighted imaging (Fig 2D, 2E) (17). Tumor burden was volumetrically quantified by measuring all enhancing (viable) tumor lesions within the liver to account for complete tumor burden. Infiltrative disease with poorly defined margins was quantified by including the entire liver into volumetric segmentation (21). Portal vein tumor thrombus was not separately segmented. However, for large tumor masses with infiltration of the portal vein, the tumor thrombus portion was included into the overall tumor segmentation mask. A region of interest (1 cm3) was placed into extratumoral liver parenchyma as a reference to identify the volume of hyperenhancing voxels within the segmented tumor. Enhancing regions were expressed as a percentage of the previously calculated tumor volume and visualized using a color map overlay (Fig 2F). The ETB was defined as the ratio of ETV to the total liver volume, calculated using the following formula:
 |
Statistical Analysis
Descriptive statistics were summarized as absolute numbers and percentages. Counts with frequencies were used for categorical variables, and medians with IQRs for continuous variables. The median overall survival (mOS) was measured until date of death, last available follow-up, or end-of-observation date. Gaussian distribution was assessed by using density plots and the Shapiro-Wilk test. The predictive value of mOS of each variable was assessed with use of Cox proportional hazard ratios at univariable and multivariable analyses (22). Statistically significant variables at univariable analysis were included in the multivariable Cox regression under further stepwise forward selection of significant predictors of mOS.
ETV and ETB cutoffs were calculated using Q statistics of residuals visualized with use of locally estimated scatterplot smoothing, or LOESS, fit. Subsequently, a plateau of several significant ETV values was demarcated in the range of 57–123 cm3, all of which represented a significant cutoff to stratify the patient sample based on mOS. In reference to the most frequently described and tested diameter (d) threshold of 5 cm, initially introduced in the Milan criteria and consecutively adopted in the Hong Kong Liver Cancer classification, 65-cm3 volume (V) was extrapolated using the spherical formula V = 1/6πd3 and previously validated in a large patient sample (23–26). Therefore, 65 cm3 was determined as the most clinically relevant volumetric threshold for patient stratification.
Regarding ETB, Q statistics of residuals revealed a cutoff of 4% to significantly stratify the patient sample, which was consequently determined to be the final cutoff value. Cross-validation was performed using Kaplan-Meier survival curves based on mOS (27) and compared using the log-rank test. Two-tailed P < .05 was considered indicative of statistically significant difference. Patients whose cancer was classified as BCLC B or C were stratified according to the previously determined ETV and ETB cutoffs and reassigned to new BCLC Bn or Cn groups by means of stepwise verification of mOS and validation using Kaplan-Meier plots and the log-rank test. Statistical analysis was performed using R software, version 1.3.959/2020 (R Project for Statistical Computing) and SPSS, version 22 (IBM).
Results
Patient and Tumor Characteristics
Table 1 summarizes demographic and tumor characteristics of all patients (n = 494) and the BCLC B (n = 123) and C (n = 371) groups. For all patients (ie, both the BCLC B and C groups), the median age was 62 years (IQR, 56–71 years), with 401 men (81.2%) and 93 women (18.8%). Race and ethnicity categories included African American, Asian, Caucasian, Hispanic, and other (patients who self-identified as Arab, Indian, non-Hispanic, or South American individuals or as more than one race). There was no evidence of any differences in demographic characteristics between the patients with BCLC B and C tumors. Volumetric quantification showed a median ETV of 57 cm3 (IQR, 25–165 cm3) and a median calculated ETB of 4% (IQR, 1%–9%) for patients with BCLC B tumors and a median ETV of 130 cm3 (IQR, 27–429 cm3) and a median calculated ETB of 7% (IQR, 2%–20%) for patients with BCLC C tumors.
Table 1:
Baseline Patient and Tumor Characteristics
Survival Analysis
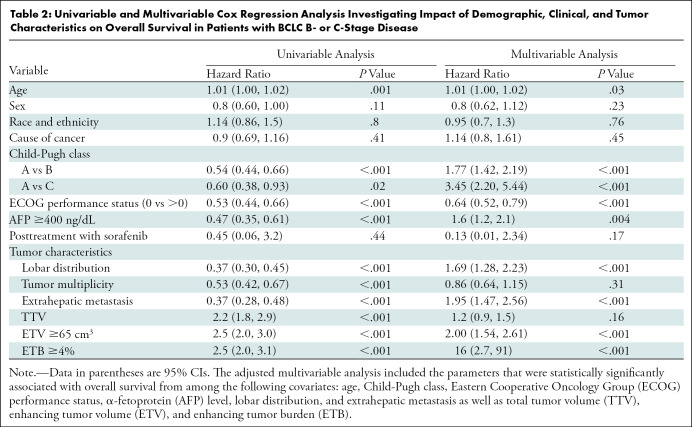
Univariable analyses of clinical parameters identified a significant association between mOS and the following parameters: age, Child-Pugh class, Eastern Cooperative Oncology Group performance status, α-fetoprotein levels, presence of bilobar disease (affecting both liver lobes), multifocal disease (multiple lesions independent of their localization), extrahepatic metastasis, and one- and three-dimensional tumor quantifications (Table 2). Sex, race and ethnicity, cause, and posttreatment with sorafenib were not associated with mOS. After stepwise selection of significant predictors in the multivariable Cox regression model, the Child-Pugh class, Eastern Cooperative Oncology Group performance status, α-fetoprotein levels, lobar distribution, extrahepatic metastasis, and three-dimensional tumor quantifications were identified as predictive variables of mOS (Table 2). Volumetric tumor enhancement quantifications showed a statistically highly significant predictive association with mOS (ETV hazard ratio, 2.00; 95% CI: 1.54, 2.61; P < .001). Interestingly, the highest hazard ratio calculated was for ETB (hazard ratio, 16; 95% CI: 2.7, 91; P < .001), confirming a strong association between ETB and increased risk of death.
Table 2:
Univariable and Multivariable Cox Regression Analysis Investigating Impact of Demographic, Clinical, and Tumor Characteristics on Overall Survival in Patients with BCLC B- or C-Stage Disease
ETV and ETB Cutoff Values
After the calculation of the thresholds for ETV (65 cm3) and ETB (4%), all patients (n = 494) were stratified into groups of high (≥65 cm3; n = 281 [57%]) and low (<65 cm3; n = 213 [43%]) ETV, resulting in a separation of the survival curves (mOS [ETV <65 cm3], 28.4 months vs mOS [ETV ≥65 cm3], 10.5 months; P < .001) (Fig 3A). Regarding ETB, the cohort was stratified into two groups of patients with low (<4%; n = 217 [43.9%]) and high (≥4%; n = 277 [56.1%]) ETB, which also significantly separated the Kaplan-Meier curves (mOS [ETB <4%], 26.7 months vs mOS [ETB ≥4%], 10.6 months; P < .001) (Fig 3B). In the BCLC B and BCLC C groups separately, the log-rank test showed a significant separation of all survival curves (Fig 3C–3F).
Figure 3:
Kaplan-Meier survival curves show the comparison of patients with high (enhancing tumor volume [ETV] ≥65 cm3 and enhancing tumor burden [ETB] ≥4%) and low tumor burden (ETV <65 cm3 and ETB <4%) in (A, B) all patients (the Barcelona Clinic Liver Cancer [BCLC] B + C group), (C, D) only patients with BCLC B tumors, and (E, F) only patients with BCLC C tumors.
The mOS Based on Original BCLC Stages
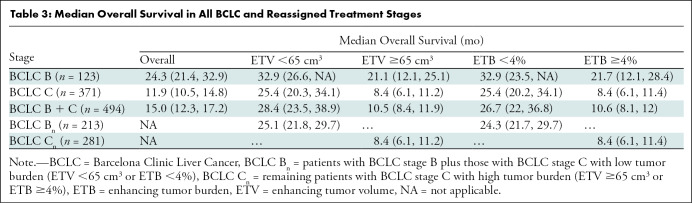
Table 3 presents a structured summary of the mOS in patients according to original and reassigned BCLC stages. The mOS of all patients was 15.0 months (95% CI: 12.3, 17.2). The original mOS for patients with BCLC B cancers (n = 123) was 24.3 months (95% CI: 21.4, 32.9) and for patients with BCLC C cancers (n = 371) was 11.9 months (95% CI: 10.5, 14.8) (Fig 4). Patients with BCLC B cancers with an ETV of less than 65 cm3 (n = 64) had a mOS of 32.9 months (95% CI: 26.6, not applicable), and patients with an ETV of 65 cm3 or greater (n = 59) had a mOS of 21.1 months (95% CI: 12.1, 25.1). Patients with BCLC C cancers with an ETV of less than 65 cm3 (n = 149) had a mOS of 25.4 months (95% CI: 20.3, 34.1), and patients with an ETV of 65 cm3 or greater (n = 222) had a mOS of 8.4 months (95% CI: 6.13, 11.2). Regarding the ETB threshold, there was no difference in mOS for patients with BCLC B and C cancers.
Table 3:
Median Overall Survival in All BCLC and Reassigned Treatment Stages
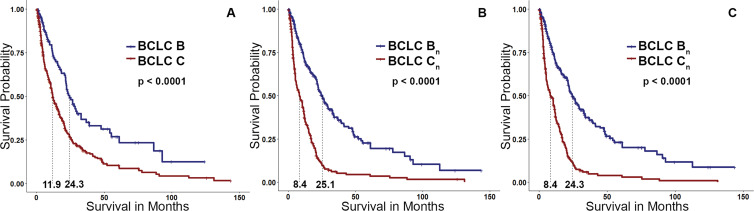
Figure 4:
Kaplan-Meier curves. (A) Group of patients with Barcelona Clinic Liver Cancer (BCLC) B or C cancers. (B) Survival curve after reassignment of patients with BCLC C cancers with an enhancing tumor volume (ETV) of less than 65 cm3 to BCLC Bn compared with the remaining high–tumor burden BCLC Cn group. (C) Survival curve after reassignment of patients with BCLC C cancers with enhancing tumor burden of less than 4% to BCLC Bn. Patient reallocation based on either ETV of less than 65 cm3 or enhancing tumor burden of less than 4% resulted in a greater separation of the survival curves than the original separation achieved based on conventional BCLC classification.
The mOS Based on Reassigned BCLC Stages
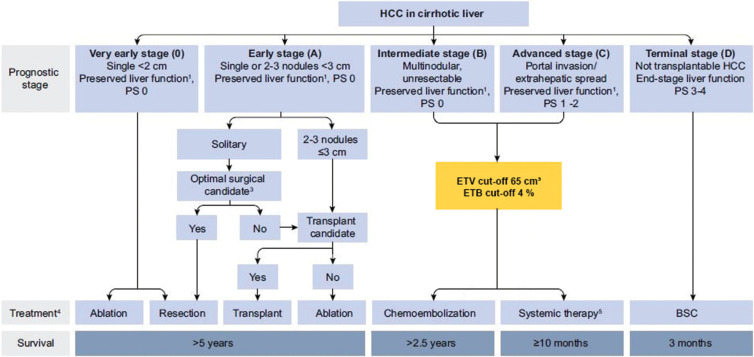
A total of 149 patients (40.2%) initially classified with BCLC C cancer who had an ETV of less than 65 cm3 were reassigned to BCLC stage Bn. The new mOS for patients in the BCLC Bn group (including newly assigned patients whose cancer was initially categorized as BCLC C) was 25.1 (95% CI: 21.8, 29.7) (Table 3). Likewise, 152 patients (40.9%) with an ETB of less than 4% were reassigned from BCLC C to Bn, with a mOS of 24.3 months (95% CI: 21.7, 29.7) for patients with BCLC Bn cancers (Fig 4). Because of the longer survival of patients with high tumor burden (ETV ≥65 cm3 or ETB ≥4%) in BCLC B treated with TACE, these patients remained in their original BCLC stage and were not reassigned to BCLC Cn. Figure 5 illustrates the updated version of the BCLC staging classification in accordance with the results of our study.
Figure 5:
Flowchart shows proposed update to the Barcelona Clinic Liver Cancer (BCLC) classification. This figure is based on the original BCLC classification and has been modified by adding the enhancing tumor volume (ETV) threshold of 65 cm3 and enhancing tumor burden (ETB) threshold of 4% (yellow box), which precisely stratifies patients with BCLC B and C tumors and potentially improves patient survival and outcome after transarterial chemoembolization. BSC = best supportive care, HCC = hepatocellular carcinoma, PS = performance status. 1 Liver function defined by Child-Pugh score and class; 3 Candidacy for resection determined by clinical parameters and co-morbidities; 4 Therapy determined by multi-disciplinary tumor board; 5 Multiple first- and second-line systemic therapy options as outlined by the BCLC 2022 update.
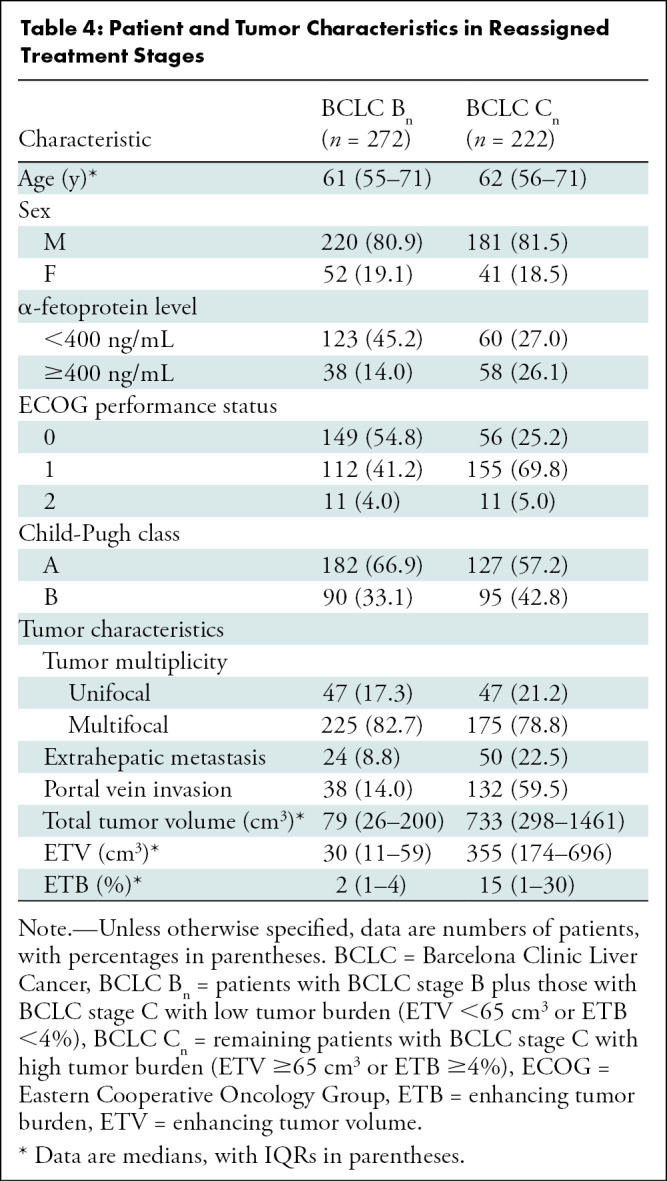
Patient and Tumor Characteristics for the Reassigned BCLC Stages
There was no difference in tumor multiplicity (unifocal vs multifocal; multifocal disease in 82.7% [225 of 272 patients] of the BCLC Bn group and 78.8% [175 of 222 patients] of the BCLC Cn group; P = .27) between the newly assigned BCLC Bn and BCLC Cn stages. In terms of tumor extent, approximately three-quarters of the patients who were restaged as having BCLC Cn cancers presented with multifocal disease with portal vein invasion (P < .001) (Table 4). Interestingly, patients with BCLC Bn cancers also differed from patients with BCLC Cn cancers in terms of α-fetoprotein values, with 45.2% of the patients (123 of 272) having an α-fetoprotein value below 400 ng/mL, compared with only 27.0% (60 of 222) in patients with BCLC Cn cancers (P < .001).
Table 4:
Patient and Tumor Characteristics in Reassigned Treatment Stages
Discussion
The purpose of this study was to evaluate the potential of subcategorizing tumors classified as Barcelona Clinic Liver Cancer (BCLC) stages B and C. The principal finding of this study was that enhancement-based volumetric quantification of tumor burden could serve as a predictive imaging biomarker for overall survival and could be used to improve selection of patients with hepatocellular carcinoma for transarterial chemoembolization (TACE). Our results support that patients with BCLC B cancers, as determined by the original staging system, may benefit from TACE regardless of the extent of their tumor burden. In BCLC C, patients with low viable tumor burden (enhancing tumor volume [ETV] <65 cm3 and enhancing tumor burden [ETB] <4%) show a substantial survival benefit when treated with TACE, whereas patients with BCLC C cancers with large viable tumor burden (ETV ≥65 cm3 and ETB ≥4%) did not benefit from locoregional therapy compared with reported data for systemic therapies.
Volumetric tumor assessment is a prognostic instrument for patients with HCC that is more accurate than one- and two-dimensional caliper-based techniques for assessing tumor response (14,15,24). However, all previously published versions of the BCLC staging system use unidirectional measurements of the greatest tumor diameter to estimate tumor burden as a qualifying element of disease stage and progression (28). Measurements of two-dimensional tumor size do not account for the heterogeneous, nonspherical, central tumor tissue necrosis that is inherently observed in HCC (29,30). Our study enables an estimation of the total tumor burden in relation to the liver volume while additionally reducing the known interreader variability of manual measurements (31–33). Tumors with more enhancing (viable) tissue have a greater proliferative potential, which negatively impacts patient survival. Therefore, patient selection for TACE according to ETV and ETB may be more reliable than using total tumor volume or simple diameter measurements alone. Facilitated by computational advances in image analysis and automated segmentation, enhancement-based tumor volumetry has become an increasingly feasible and efficient workflow in clinical practice (16,17,29,32), and therefore helps redirect patients to a more favorable treatment option.
Our study included a large sample of patients with HCC treated with TACE. Other published attempts to further stratify patient allocation to TACE (eg, Bolondi subclassifications [12]) focus on patients with BCLC B cancers, following the current BCLC recommendations for TACE. However, the BRIDGE and GIDEON studies reported that TACE was regularly used in patients with BCLC C (5,6) in consensus with the Hong Kong Liver Cancer staging system. Moreover, previous studies have shown favorable therapeutic outcomes for TACE in symptomatic patients (mostly BCLC C) (7–9). This circumstance prompted the decision to include patients with portal vein thrombosis to fully represent the spectrum of patients with BCLC C cancers. As a result, notwithstanding almost half the patients with advanced stage BCLC C cancers being reallocated to BCLC Bn because of low tumor burden (ETV <65 cm3 or ETB <4%), no negative impact on the overall survival outcome of the new BCLC Bn stage was observed. Despite a significant survival curve separation, patients with BCLC B cancers with a high tumor burden (ETV ≥65 cm3 or ETB ≥4%) continued to show a survival benefit from TACE and therefore remained categorized as BCLC Bn. Our results demonstrate that patients with BCLC C cancers with low tumor burden may benefit from TACE despite being considered as having advanced-stage disease for various reasons other than extent of the tumor burden (eg, Child-Pugh class and Eastern Cooperative Oncology Group performance status).
Interestingly, reallocated patients with BCLC C cancers demonstrated less frequent vascular tumor invasion and infiltrative-appearing disease, as well as lower α-fetoprotein values at diagnosis, compared with the remaining patients in the BCLC Cn group. Thus, patients with advanced-stage HCC with low viable tumor burden may still show a substantial survival advantage from TACE despite their suboptimal clinical parameters that initially categorized them as ineligible for TACE. At the same time, the imaging biomarkers clearly demonstrated that patients in the BCLC Cn group had a poor mOS after TACE compared with reported systemic treatment outcomes or best supportive care known in the published literature (34), suggesting that this particular patient group does not benefit from TACE.
Our study has several limitations. First, it is a single-institution retrospective analysis of patients with HCC treated with chemoembolization only. Patients with heterogeneous BCLC C cancers with main portal vein thrombosis and extrahepatic metastases were included in the treatment regimen in this study. However, because TACE is the most commonly used treatment of unresectable HCC, our study represents clinical reality and may provide a more standardized approach. In addition, in recent years, new systemic therapies, including several oral tyrosine kinase inhibitors and immune checkpoint inhibitors, have been used as first-line therapy in patients with advanced-stage HCC (35). Further studies to investigate treatment outcomes of new systemic and locoregional therapies, particularly in patients with low–tumor burden BCLC C cancers, would help to further refine treatment allocation for HCC.
In conclusion, our findings demonstrate that restratification of patients initially classified as having Barcelona Clinic Liver Cancer B- or C-stage hepatocellular carcinoma (HCC), according to enhancement-based, three-dimensional, quantitative tumor burden measurements, reliably differentiated between those who stand to benefit from transarterial chemoembolization and those with poor postprocedural outcome, particularly in advanced-stage disease. These findings demonstrate the potential application of this imaging biomarker as a triage tool for improved patient selection for transarterial locoregional therapy for HCC.
Acknowledgments
Acknowledgments
We thank Geliang Gan, PhD, and Yanhong Deng, MPH, for their exceptional advice, support, and supervision of the statistical analyses.
L.J.S., M.L., and J.C. received grants from the National Institutes of Health (National Cancer Institute, R01 CA206180). L.J.S. and J.C. also received grants from the Society of Interventional Oncology and Guerbet Healthcare. J.C. additionally received grants from the German-Israeli Foundation for Scientific Research and Development, Boston Scientific, and Philips Healthcare.
Data sharing: Data generated or analyzed during the study are available from the corresponding author by request.
Disclosures of conflicts of interest: T.B. Grant from the National Institutes of Health. N.N. No relevant relationships. F.L.G. No relevant relationships. L.J.S. Grants from the Leopoldina Foundation, Society of Interventional Oncology, Rolf W. Guenther Stiftung, Berlin Institute of Health (Clinician Scientist Program), and Berliner Krebsgesellschaft. T.T. No relevant relationships. A.J. No relevant relationships. M.S. Travel support from the European Society of Organ Transplantation as an invited speaker; member of the advisory board for Engitix (no payment received). M.L. Board member of Tau Beta Pi (engineering honors society; no payment received); stockholder in Visage Imaging. R.D. Grants from the Society of Interventional Oncology, Boston Scientific, and Guerbet; consulting fees from Boston Scientific and Guerbet; payment for lectures from Boston Scientific and Guerbet. C.G. Consulting fees from Boston Scientific and Guerbet; patent planned for novel embolization gel; chair of the Publications Committee of the Society of Interventional Oncology. K.H. Grant support from Boston Scientific and Merit Medical; participation on a data safety monitoring board or advisory board for Boston Scientific, Varian Medical, and AstraZeneca. C.J. Grants from Guerbet, Boston Scientific, Philips Healthcare, Society of Interventional Oncology, Radiological Society of North America, and National Institutes of Health; consulting fees from Guerbet, Bayer, and AstraZeneca; payment for lectures from Guerbet and Bayer; patent or patent pending with Philips; equipment, materials, gifts, or services from Boston Scientific, Guerbet, and Philips.
Abbreviations:
- BCLC
- Barcelona Clinic Liver Cancer
- ETB
- enhancing tumor burden
- ETV
- enhancing tumor volume
- HCC
- hepatocellular carcinoma
- mOS
- median overall survival
- TACE
- transarterial chemoembolization
References
- 1. Bray F , Ferlay J , Soerjomataram I , Siegel RL , Torre LA , Jemal A . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries . CA Cancer J Clin 2018. ; 68 ( 6 ): 394 – 424 . [Published correction appears in Cancer J Clin 2020;70(4):313.] [DOI] [PubMed] [Google Scholar]
- 2. Bruix J , Llovet JM . Prognostic prediction and treatment strategy in hepatocellular carcinoma . Hepatology 2002. ; 35 ( 3 ): 519 – 524 . [DOI] [PubMed] [Google Scholar]
- 3. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma . J Hepatol 2018. ; 69 ( 1 ): 182 – 236 . [Published correction appears in J Hepatol 2019;70(4):817.] [DOI] [PubMed] [Google Scholar]
- 4. Reig M , Forner A , Rimola J , et al . BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update . J Hepatol 2022. ; 76 ( 3 ): P681 – 693 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JW , Chen M , Colombo M , et al . Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study . Liver Int 2015. ; 35 ( 9 ): 2155 – 2166 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lencioni R , Kudo M , Ye SL , et al . GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis . Int J Clin Pract 2014. ; 68 ( 5 ): 609 – 617 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao Y , Duran R , Chapiro J , et al . Transarterial chemoembolization for the treatment of advanced-stage hepatocellular carcinoma . J Gastrointest Surg 2016. ; 20 ( 12 ): 2002 – 2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinter M , Hucke F , Graziadei I , et al . Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib . Radiology 2012. ; 263 ( 2 ): 590 – 599 . [DOI] [PubMed] [Google Scholar]
- 9. Chern MC , Chuang VP , Liang CT , Lin ZH , Kuo TM . Transcatheter arterial chemoembolization for advanced hepatocellular carcinoma with portal vein invasion: safety, efficacy, and prognostic factors . J Vasc Interv Radiol 2014. ; 25 ( 1 ): 32 – 40 . [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver ; European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma . J Hepatol 2012. ; 56 ( 4 ): 908 – 943 . [DOI] [PubMed] [Google Scholar]
- 11. Takayasu K , Arii S , Kudo M , et al . Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines . J Hepatol 2012. ; 56 ( 4 ): 886 – 892 . [DOI] [PubMed] [Google Scholar]
- 12. Bolondi L , Burroughs A , Dufour JF , et al . Heterogeneity of patients with intermediate (BCLC B) hepatocellular carcinoma: proposal for a subclassification to facilitate treatment decisions . Semin Liver Dis 2012. ; 32 ( 4 ): 348 – 359 . [DOI] [PubMed] [Google Scholar]
- 13. Huitzil-Melendez FD , Capanu M , O’Reilly EM , et al . Advanced hepatocellular carcinoma: which staging systems best predict prognosis? J Clin Oncol 2010. ; 28 ( 17 ): 2889 – 2895 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chockalingam A , Duran R , Sohn JH , et al . Radiologic-pathologic analysis of quantitative 3D tumour enhancement on contrast-enhanced MR imaging: a study of ROI placement . Eur Radiol 2016. ; 26 ( 1 ): 103 – 113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tacher V , Lin M , Duran R , et al . Comparison of existing response criteria in patients with hepatocellular carcinoma treated with transarterial chemoembolization using a 3D quantitative approach . Radiology 2016. ; 278 ( 1 ): 275 – 284 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chapiro J , Lin M , Duran R , Schernthaner RE , Geschwind JF . Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI . Expert Rev Anticancer Ther 2015. ; 15 ( 2 ): 199 – 205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sahu S , Schernthaner R , Ardon R , et al . Imaging biomarkers of tumor response in neuroendocrine liver metastases treated with transarterial chemoembolization: can enhancing tumor burden of the whole liver help predict patient survival? Radiology 2017. ; 283 ( 3 ): 883 – 894 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleckenstein FN , Schernthaner RE , Duran R , et al . 3D quantitative tumour burden analysis in patients with hepatocellular carcinoma before TACE: comparing single-lesion vs. multi-lesion imaging biomarkers as predictors of patient survival . Eur Radiol 2016. ; 26 ( 9 ): 3243 – 3252 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lencioni R , Llovet JM . Modified RECIST (mRECIST) assessment for hepatocellular carcinoma . Semin Liver Dis 2010. ; 30 ( 1 ): 52 – 60 . [DOI] [PubMed] [Google Scholar]
- 20. Tacher V , Lin M , Chao M , et al . Semiautomatic volumetric tumor segmentation for hepatocellular carcinoma: comparison between C-arm cone beam computed tomography and MRI . Acad Radiol 2013. ; 20 ( 4 ): 446 – 452 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu C , Smolka S , Papademetris X , et al . Predicting infiltrative hepatocellular carcinoma patient outcome post-TACE: MR bias field correction effect on 3D-quantitative image analysis . J Clin Transl Hepatol 2020. ; 8 ( 3 ): 292 – 298 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox DR . Regression models and life-tables . J R Stat Soc Series B Stat Methodol 1972. ; 34 ( 2 ): 187 . https://www.jstor.org/stable/2985181 . [Google Scholar]
- 23. Kashkoush S , El Moghazy W , Kawahara T , Gala-Lopez B , Toso C , Kneteman NM . Three-dimensional tumor volume and serum alpha-fetoprotein are predictors of hepatocellular carcinoma recurrence after liver transplantation: refined selection criteria . Clin Transplant 2014. ; 28 ( 6 ): 728 – 736 . [DOI] [PubMed] [Google Scholar]
- 24. Chapiro J , Duran R , Lin M , et al . Identifying staging markers for hepatocellular carcinoma before transarterial chemoembolization: comparison of three-dimensional quantitative versus non-three-dimensional imaging markers . Radiology 2015. ; 275 ( 2 ): 438 – 447 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazzaferro V , Regalia E , Doci R , et al . Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis . N Engl J Med 1996. ; 334 ( 11 ): 693 – 699 . [DOI] [PubMed] [Google Scholar]
- 26. Prajapati HJ , Kim HS . Treatment algorithm based on the multivariate survival analyses in patients with advanced hepatocellular carcinoma treated with trans-arterial chemoembolization . PLoS One 2017. ; 12 ( 2 ): e0170750 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaplan EL , Meier P . Nonparametric estimation from incomplete observations . J Am Stat Assoc 1958. ; 53 ( 282 ): 457 – 481 . [Google Scholar]
- 28. Bogaerts J , Ford R , Sargent D , et al . Individual patient data analysis to assess modifications to the RECIST criteria . Eur J Cancer 2009. ; 45 ( 2 ): 248 – 260 . [DOI] [PubMed] [Google Scholar]
- 29. Chapiro J , Duran R , Lin M , et al . Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver . Eur Radiol 2015. ; 25 ( 7 ): 1993 – 2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Forner A , Ayuso C , Varela M , et al . Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009. ; 115 ( 3 ): 616 – 623 . [DOI] [PubMed] [Google Scholar]
- 31. Kielar A , Fowler KJ , Lewis S , et al . Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm . Abdom Radiol (NY) 2018. ; 43 ( 1 ): 218 – 230 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luedemann WM , Geisel D , Gebauer B , et al . Comparing HCC arterial tumour vascularisation on baseline imaging and after lipiodol cTACE: how do estimations of enhancing tumour volumes differ on contrast-enhanced MR and CT? Eur Radiol 2020. ; 30 ( 3 ): 1601 – 1608 . [DOI] [PubMed] [Google Scholar]
- 33. van Breugel JMM , Geschwind JF , Mirpour S , et al . Theranostic application of lipiodol for transarterial chemoembolization in a VX2 rabbit liver tumor model . Theranostics 2019. ; 9 ( 13 ): 3674 – 3686 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rimassa L , Santoro A . Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial . Expert Rev Anticancer Ther 2009. ; 9 ( 6 ): 739 – 745 . [DOI] [PubMed] [Google Scholar]
- 35. Li D , Sedano S , Allen R , Gong J , Cho M , Sharma S . Current treatment landscape for advanced hepatocellular carcinoma: patient outcomes and the impact on quality of life . Cancers (Basel) 2019. ; 11 ( 6 ): E841 . [DOI] [PMC free article] [PubMed] [Google Scholar]



![Kaplan-Meier survival curves show the comparison of patients with high (enhancing tumor volume [ETV] ≥65 cm3 and enhancing tumor burden [ETB] ≥4%) and low tumor burden (ETV <65 cm3 and ETB <4%) in (A, B) all patients (the Barcelona Clinic Liver Cancer [BCLC] B + C group), (C, D) only patients with BCLC B tumors, and (E, F) only patients with BCLC C tumors.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/9a2a/9270683/55d467271626/radiol.212426.fig3.jpg)