This randomized clinical trial evaluates the use of transdermal cannabidiol vs placebo for adults with drug-resistant focal epilepsy.
Key Points
Question
What is the efficacy, safety, and tolerability of transdermally administered cannabidiol in adults with drug-resistant focal epilepsy?
Findings
In a randomized, double-blind, placebo-controlled, multicenter clinical trial of 188 patients, no difference was found in seizure frequency at week 12 of the double-blind period among the placebo, 195-mg cannabidiol, and 390-mg cannabidiol treatments. The open-label extension demonstrated the long-term safety, tolerability, and acceptability of transdermal cannabidiol delivery, with a seizure reduction of at least 50% in more than half of the patients by month 6 of the trial.
Meaning
Although cannabidiol did not perform significantly better than placebo in this trial, it was well tolerated and safe; future studies to assess the effect of higher doses may be warranted.
Abstract
Importance
Cannabidiol has shown efficacy in randomized clinical trials for drug-resistant epilepsy in specific syndromes that predominantly affect children. However, high-level evidence for the efficacy and safety of cannabidiol in the most common form of drug-resistant epilepsy in adults, focal epilepsy, is lacking.
Objective
To investigate the efficacy, safety, and tolerability of transdermally administered cannabidiol in adults with drug-resistant focal epilepsy.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled, multicenter clinical trial at 14 epilepsy trial centers in Australia and New Zealand. Participants were adults with drug-resistant focal epilepsy receiving a stable regimen of up to 3 antiseizure medications. Data were analyzed from July 2017 to November 2018.
Interventions
Eligible participants were randomized (1:1:1) to 195-mg or 390-mg transdermal cannabidiol or placebo twice daily for 12 weeks, after which they could enroll in an open-label extension study for up to 2 years.
Main Outcomes and Measures
Seizure frequency was self-reported using a daily diary. The primary efficacy end point was the least squares mean difference in the log-transformed total seizure frequency per 28-day period, adjusted to a common baseline log seizure rate, during the 12-week treatment period.
Results
A total of 188 patients (45% male [85 patients] and 54.8% female [103 patients]) with a mean (SD) age of 39.2 (12.78) years were randomized, treated, and analyzed (195-mg cannabidiol, 63 participants; 390-mg cannabidiol, 62 participants; placebo, 63 participants). At week 12 of the double-blind period, there was no difference in seizure frequency between placebo (mean [SD] 2.49 [1.31] seizures per 28 days) and 195-mg cannabidiol (mean [SD] 2.51 [1.15] seizures per 28 days; least squares mean difference, 0.014; 95% CI, −0.175 to 0.203; P = .89) or 390-mg cannabidiol (mean [SD] 2.59 [1.12] seizures per 28 days; least squares mean difference, 0.096; 95% CI, −0.093 to 0.285; P = .32). By month 6 of the open-label extension, 115 patients (60.8%) achieved a seizure reduction of at least 50%. Treatment-emergent adverse events occurred in 50.4% (63 of 125 participants) of the cannabidiol group vs 41.3% (26 of 63 participants) in the placebo group, with a treatment difference of 9.1% (95% CI, −6.0% to 23.6%), and occurred at similar rates in the cannabidiol groups. Few participants discontinued (7% [14 of 188 participants]), and most (98% [171 of 174 participants]) continued into the open-label extension.
Conclusions and Relevance
Both doses of transdermal cannabidiol were well tolerated and safe. No significant difference in efficacy was observed between cannabidiol and placebo during the double-blind treatment period. The open-label extension demonstrated the long-term safety, tolerability, and acceptability of transdermal cannabidiol delivery.
Trial Registration
ACTRN12616000510448 (double-blind); ACTRN12616001455459 (open-label).
Introduction
Focal seizures occur in more than 60% of patients with epilepsy and are the most common seizure type in adults.1 Despite the introduction of many new antiseizure medications (ASMs) over the past 3 decades, seizures remain uncontrolled in approximately one-third of patients.2,3 Cannabidiol is the primary noneuphoric cannabinoid found in the Cannabis sativa L plant. It has low affinity for cannabinoid type 1 and cannabinoid type 2 receptors and is thought to exert its effects via other mechanisms.4 Although the antiseizure effects of cannabidiol were noted more than 40 years ago,5,6 community and scientific interest in cannabidiol and other cannabinoids as potential treatments for drug-resistant epilepsy has intensified7 following demonstrations of efficacy in suppressing seizures in multiple in vivo models of epilepsy,8,9,10,11 as well as in open-label and randomized clinical trials in various patient populations.12,13,14,15 The 2018 US Food and Drug Administration approval of an oral solution (Epidiolex, Greenwich Biosciences) for the treatment of seizures associated with Lennox-Gastaut syndrome or Dravet syndrome in patients aged 2 years and older16 further supports the therapeutic potential of cannabidiol in epilepsy. However, there is currently no high-level evidence for the efficacy and safety of cannabidiol as a treatment for common types of seizures affecting adults.
Most cannabidiol-based therapies for the treatment of seizures are administered orally, but this can cause gastrointestinal adverse events (AEs; eg, decreased appetite and cause diarrhea),17,18 may reduce central nervous system availability as a result of first-pass metabolism,19 and may elevate liver aminotransferase concentrations in nearly 10% of patients.14,20 Nonoral formulations have potential to overcome all 3 of these limitations.
A pharmaceutically manufactured, clear, synthetic transdermal cannabidiol gel, ZYN002 (Zynerba Pharmaceuticals) has been developed to provide nonoral cannabidiol delivery with twice-daily dosing. In phase 1 controlled trials in adults and patients with epilepsy (single ascending dose, 7-day multiple ascending dose) and in healthy adult volunteers (14-day repeat application), transdermal cannabidiol doses ranging from 50 mg to 504 mg were safe and well tolerated, with no clinically meaningful drug-related changes observed.21 The objective of this trial was to evaluate the efficacy, safety, and tolerability of transdermal cannabidiol as adjunctive therapy for the treatment of adults with focal seizures.
Methods
Trial Design
This phase 2A, randomized, double-blind, placebo-controlled, multicenter clinical trial (STAR 1), with an open-label extension (OLE; STAR 2), assessed the safety, efficacy, and tolerability of cannabidiol administered as a transdermal gel to adults with focal seizures (see Supplement 1 for study protocol). This report follows the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
The trial was conducted at 10 clinical study sites in Australia and 4 sites in New Zealand, enrolling patients between June 1, 2016, and February 20, 2017, facilitated by the Australian Epilepsy Clinical Trials Network. The protocol, informed consent form, and participant information and recruitment materials were reviewed and approved by the Health and Disability Ethics Committees in New Zealand and the Human Research Ethics Committees in Australia. The trial was designed and monitored in accordance with the procedures of the designated contract research organization, Novotech Pty, Limited, which oversaw the study conduct. Participants provided written informed consent at the screening visit before any study-related procedures were undertaken.
Participants
Participants were aged 18 to 70 years at the time of screening and in generally good health, with a body mass index ranging from 18 to 35 (body mass index is calculated as weight in kilograms divided by height in meters squared). They had a diagnosis of drug-resistant focal seizures for at least 2 years, as documented by review of an electroencephalogram, magnetic resonance imaging scan, and narrative from the physician who managed the participant’s epilepsy, and averaged at least 3 observable focal seizures per month with not more than 20 consecutive seizure-free days. Participants had to be currently maintained with a stable regimen of up to 3 ASMs and could not be taking clobazam, ethosuximide, felbamate, or vigabatrin (benzodiazepines other than clobazam used as rescue medication were counted as ASMs if used more than 2 days per week). Complete inclusion and exclusion criteria are provided in the study protocol (Supplement 1).
Randomization
Participants were randomized via an interactive web response system according to a randomization scheme generated before study initiation. Once a participant qualified for study inclusion, the investigative site received the randomization number for the participant using the interactive web response system. A single randomization scheme was generated for use across all study sites.
Procedures
During the screening visit, a complete medical history and physical and neurological examinations were performed. Blood samples were collected for clinical laboratory assessments and to determine concomitant ASM plasma concentrations. All participants had their seizure history, seizure classification, and diagnosis reviewed and confirmed by The Epilepsy Study Consortium before randomization into the study. A paper seizure diary was used to record seizure type and daily frequency of each seizure type. Participants were instructed to complete the diary from the screening visit through the 8-week baseline period through day 84 of the treatment period. The seizures of interest included focal aware seizures with motor features, focal impaired awareness seizures, and focal to bilateral tonic-clonic seizures.
Following the 8-week baseline period, eligible participants were randomized in a 1:1:1 ratio to 195-mg transdermal cannabidiol (approximately 2.6 mg/kg), 390-mg transdermal cannabidiol (approximately 5.3 mg/kg), or placebo daily. These doses were chosen according to extrapolation from the literature of human plasma levels of cannabidiol following oral administration.22 Study treatments were supplied as a transdermal gel and contained in foil-lined sachets. Each sachet contained either 97.5 mg of cannabidiol (4.2% wt/wt) or placebo. During the double-blind portion of the study, participants applied 2 sachets of the study drug twice daily (ie, every 12 ± 2 hours) to the upper arms and shoulders for 12 weeks. No dose adjustments were made throughout the 12 weeks of treatment. A daily skin-check diary was used to record the degree of application-site erythema during the double-blind treatment period. Before each study dose, participants recorded the skin-check score in the diary, which was reviewed by investigators during study visits.
Before initial dosing, participants had a predose blood sample obtained for plasma concentrations of cannabidiol, tetrahydrocannabinol (THC), and ASMs. Blood samples for plasma concentrations of concomitant ASMs and trough cannabidiol/THC plasma levels were collected at 2, 4, 6, 8, and 12 weeks of the treatment period, as well as review of diaries, vital signs, targeted physical and neurological examinations (weeks 4 and 8), electrocardiograms (ECG; week 8), laboratory tests (week 8), pregnancy tests, concomitant medication review, study drug application, skin irritation assessments, and AE review. Skin erythema was rated on a 5-point scale where 0 denotes no erythema, 1 denotes minimal erythema, 2 denotes moderate erythema with sharply defined borders, 3 denotes intense erythema with or without edema, and 4 denotes intense erythema with edema and blistering or erosion. Participants were also administered the Columbia-Suicide Severity Rating Scale.23
Participants completing the 12-week, double-blind study were offered enrollment into a 2-year OLE study. Regardless of their allocation in the double-blind study, participants who continued into the OLE were started at a dose of 390-mg cannabidiol daily. Seizure diaries were required, with diary review occurring at the second week after entry; the first, second, and third month after entry; and every 3 months thereafter. During the OLE, blood samples for cannabidiol and THC trough analysis were collected at weeks 2, 4, 8, and 12 and then every 3 months for the remainder of the study. Five months into the OLE, the protocol was amended so investigators had the option of increasing the daily cannabidiol dose to 585 mg (approximately 7.9 mg/kg). After 1 month at 585 mg, the daily dose could be increased to 780 mg (approximately 10.5 mg/kg). The decision to increase the cannabidiol dose in a stepwise fashion was done to expand the assessment of tolerability and seizure control.
Participants who did not enroll in the OLE study underwent a 2-week blinded dose reduction beginning at the end of 12 weeks after randomization and ceased usage of the study drug by 15 weeks after randomization.
Study End Points
Efficacy
The primary efficacy end point was the log-transformed seizure frequency using the transformation natural log (ln)(SF28 + 1), where SF28 was the total seizure frequency per 28-day period during the 12-week treatment period, with 1 added to account for situations where SF28 = 0.24,25,26
Secondary efficacy end points were change from baseline in SF28, percentage change from baseline in SF28, response rate based on 50% or greater reduction from baseline in SF28 during the treatment period, and percentage of participants who were seizure-free during the treatment period.
Pharmacokinetics
Steady-state plasma trough concentrations were determined for cannabidiol and for participants’ current ASMs and THC. Analyses of cannabidiol and THC in plasma were done using a validated high-performance liquid chromatography with tandem mass spectrometry detection, with a lower limit of quantitation of 0.2 ng/mL.
Statistical Analysis
It was estimated that a sample size of 60 participants per treatment group would have a power of 88% to detect a difference between active treatment and placebo if the 50% responder rate was 40% for the active group and 15% for the placebo group. To account for an anticipated screening failure rate of 15%, 210 participants were enrolled to ensure a minimum of 180 participants.
Three populations were analyzed: intent-to-treat, pharmacokinetic, and safety. The intent-to-treat population was used to analyze efficacy; it included all participants who applied at least 1 dose of study medication and had at least 1 postbaseline seizure assessment. All analyses were based on 2-sided tests at the significance level of P < .05. The pharmacokinetic population was used to summarize plasma concentrations and included all participants who received at least 1 application of the study drug and had a plasma concentration obtained from at least 1 of the 3 postrandomization evaluations. The safety population, which was used to analyze safety, included all participants who received at least 1 dose of the study drug. All statistical analyses were performed using SAS statistical software version 9 or higher (SAS Institute).
The primary efficacy comparison was the adjusted least squares means difference from placebo for 195-mg cannabidiol and 390-mg cannabidiol. An analysis of covariance model was used on the log–transformed SF28 value (SF28 + 1), with treatment group and site as main effects and baseline ln(SF28 + 1) as a covariate. Pairwise comparisons of the active treatments with placebo were made using least squares means.
Secondary efficacy end points were analyzed as follows. For percentage change from baseline for the 12-week treatment period, a Mann-Whitney U rank sum nonparametric test was used to compare each active treatment group with the placebo treatment group. The maximal percentage change possible was 100%, corresponding to no seizures during treatment. Adjusted median difference with 95% CIs between each active treatment group and the placebo group were determined using the Hodges-Lehmann estimator technique. The analysis of 50% responder rates compared each active treatment with placebo using a logistic regression model with factors for treatment group and site. The number of patients who were completely seizure-free during treatment was summarized by treatment group using descriptive statistics.
Among those who chose to continue into the OLE, descriptive statistics were used to characterize changes in seizure frequency (quantified as percentage reduction from baseline). For simplicity, because of differences among original randomized groups in total drug exposure and duration of such exposure, participants were collapsed into a single OLE group among whom seizure frequency was tracked on a monthly basis. Data were analyzed for patients completing 18 months of OLE, as they represented the most current complete data at the time of manuscript preparation.
All AEs were coded using the Medical Dictionary for Regulatory Activities, version 19.127 and summarized using descriptive statistics by system organ class and preferred term. Data were analyzed from July 2017 to November 2018.
Results
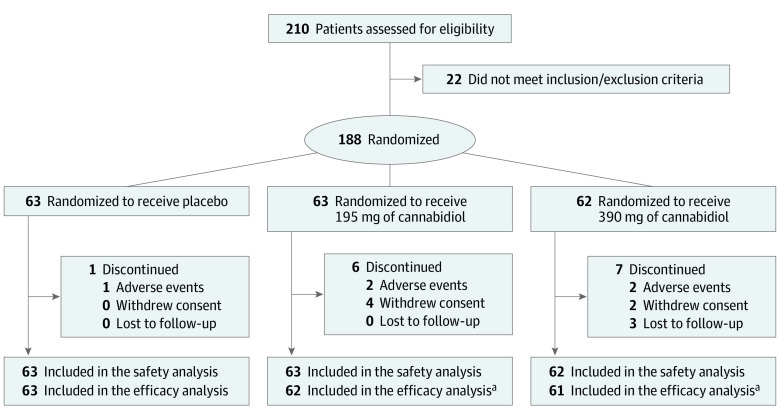
Of the 210 participants enrolled in the study, 54.8% (103 participants) were female, and the mean (SD) age was 39.2 (12.78). A total of 188 (89.5%) were randomized (195-mg cannabidiol, 63 participants; 390-mg cannabidiol, 62 participants; placebo, 63 participants) and received at least 1 application of the study drug, and 174 (92.6%) completed 12 weeks of double-blind treatment (Figure). Few participants discontinued (7% [14 of 188 participants]), and most (98% [171 of 174 participants]) continued into the open-label extension. Demographics and baseline characteristics are shown in Table 1.
Figure. Trial Patient Flow.
aTwo patients did not complete at least 1 postbaseline patient seizure diary and were excluded from the efficacy population. One patient was randomized to the 195-mg treatment group and 1 patient was randomized to the 390-mg treatment group.
Table 1. Demographics and Baseline Characteristics in the Safety Population.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Placebo (n = 63) | Cannabidiol | Total (N = 188) | ||
| 195 mg (n = 63) | 390 mg (n = 62) | |||
| Age, mean (SD), y | 40.3 (13.35) | 37.0 (12.55) | 40.4 (12.32) | 39.2 (12.78) |
| Sex | ||||
| Male | 27 (42.9) | 32 (50.8) | 26 (41.9) | 85 (45.2) |
| Female | 36 (57.1) | 31 (49.2) | 36 (58.1) | 103 (54.8) |
| Race | ||||
| Aboriginal or Torres Strait Islander | 0 | 0 | 1 (1.6) | 1 (0.5) |
| Asian | 3 (4.8) | 4 (6.3) | 5 (8.1) | 12 (6.4) |
| White | 56 (88.9) | 56 (88.9) | 54 (87.1) | 166 (88.3) |
| Othera | 4 (6.3) | 3 (4.8) | 2 (3.2) | 9 (4.8) |
| Weight, mean (SD), kg | 74.95 (15.40) | 76.50 (17.92) | 74.35 (18.88) | 75.27 (17.38) |
| Height, mean (SD), cm | 169.63 (10.46) | 170.69 (9.98) | 168.75 (12.12) | 169.69 (10.86) |
| Body mass index, mean (SD)b | 25.97 (4.54) | 26.03 (4.59) | 25.77 (4.57) | 25.93 (4.54) |
| Seizure frequency in past month, mean (SD) | ||||
| Focal impaired awareness | 16.7 (24.85) | 13.1 (22.83) | 16.7 (27.36) | 15.5 (25.00) |
| Focal aware with motor features | 12.4 (44.72) | 21.1 (82.97) | 8.1 (21.11) | 13.9 (55.68) |
| Focal to bilateral tonic-clonic | 0.8 (2.74) | 1.3 (7.76) | 4.8 (26.05) | 2.3 (15.66) |
Includes Dutch, Eurasian, Mixed Race Asian/White, Eurasian, Moroccan, Samoan, Eurasian, and Greek; there was 1 participant in all categories except Eurasian, for which there were 2.
Body mass index is calculated as weight in kilograms divided by height in meters squared.
On the primary efficacy end point (Table 2), the adjusted least squares mean difference from placebo was 0.014 for 195 mg of cannabidiol and 0.096 for 390 mg of cannabidiol. At week 12 of the double-blind period, there was no difference in seizure frequency between placebo (2.49 seizures per 28 days) and 195-mg cannabidiol (2.51 seizures per 28 days; least squares mean difference, 0.02; P = .89) or 390-mg cannabidiol (2.59 seizures per 28 days; least squares mean difference, 0.1; P = .32). These adjusted differences from placebo were not significant.
Table 2. Log-Transformed Seizure Rates During Treatment in the Intent-to-Treat Population.
| Time point | Seizure rate, (ln[seizure frequency per 28-d period + 1]) | ||
|---|---|---|---|
| Placebo (n = 63) | Cannabidiol | ||
| 195 mg (n = 62) | 390 mg (n = 61) | ||
| Baseline | |||
| Mean (SD) | 2.76 (1.17) | 2.87 (1.11) | 2.73 (1.05) |
| Median (IQR) | 2.44 (1.32-5.76) | 2.71 (0.88-6.03) | 2.41 (1.25-5.82) |
| Treatment | |||
| Mean (SD) | 2.49 (1.31) | 2.58 (1.15) | 2.56 (1.123) |
| Median (IQR) | 2.16 (0.00-5.70) | 2.42 (0.29-5.75) | 2.37 (0.70-6.05) |
| P value | NA | .89 | .32 |
| Adjusted LS mean ln seizure rate | 2.49 | 2.51 | 2.59 |
| LS mean difference from placebo (ln[seizure rates +1]) | NA | 0.014 | 0.096 |
Abbreviations: ln, natural log; LS, least squares; NA, not applicable.
As shown in Table 3, although the median percentage change in SF28 from baseline was greater for both the 195-mg and 390-mg cannabidiol groups than for the placebo group, the differences were not significant. Results on the remaining secondary efficacy end points are presented in eTable 1, eTable 2, eTable 3, and eTable 4 in Supplement 2.
Table 3. Change From Baseline in Seizure Rates in the Intent-to-Treat Population.
| Time point | Percentage change in seizure rate, (Seizure Frequency per 28-Day Period) | |||
|---|---|---|---|---|
| Placebo (n = 63) | Cannabidiol | |||
| 195 mg (n = 62) | 390 mg (n = 61) | Total (N = 123) | ||
| Baseline | ||||
| Mean (SD) | 34.84 (60.38) | 34.67 (61.85) | 30.88 (61.25) | 32.79 (61.33) |
| Median (IQR) | 10.50 (2.8 to 316.5) | 14.00 (1.4 to 416.0) | 10.14 (2.5 to 335.5) | 11.59 (1.4 to 416.0) |
| Treatment | ||||
| Mean (SD) | 27.36 (46.55) | 27.54 (51.25) | 27.71 (59.70) | 27.63 (55.37) |
| Median (IQR) | 7.67 (0.0 to 297.1) | 10.30 (0.3 to 314.3) | 9.67 (1.0 to 424.5) | 10.00 (0.3 to 424.5) |
| Percentage change | ||||
| Mean (SD) | 13.26 (48.83) | 19.92 (34.55) | 11.95 (34.96) | 15.97 (34.84) |
| Median (IQR) | 8.70 (−199.1 to 100.0) | 18.42 (−64.9 to 91.4) | 14.03 (−117.0 to 79.5) | 16.00 (−117.0 to 91.4) |
| P value | Not applicable | .43 | .85 | .73 |
Of the 174 participants who completed the 12-week, double-blind treatment period, 171 (98%) continued into the OLE. eFigure 1 in Supplement 2 shows that the median (range) percentage change in seizure frequency was −29% (−100% to 776%) at month 1 (169 participants), −57% (−100% to 87%) (98 participants) at month 8, and −67% (−100% to 180%) (49 participants) at month 17. Notably, by month 6 of the OLE, 60.8% (115 participants) of participants who remained in the study experienced a reduction in seizure frequency of approximately 50%.
Participants who completed 18 months of the OLE (63 participants) experienced a reduction in seizures by the third month after entry (49%) that remained stable throughout the 18-month treatment period (eFigure 2 in Supplement 2). By month 8, the seizure response in the cohort of patients who completed the OLE was similar to the overall population suggesting seizure response trajectory was not related to length of follow-up in the OLE study.
Among participants randomized to active treatment, predose trough plasma cannabidiol concentrations ranged from 0.72 ng/mL to 77.50 ng/mL. During the OLE, cannabidiol predose concentrations peaked at 235 ng/mL. The intent was to capture the cannabidiol trough concentrations. However, in some instances, patients took their morning dose before the study visit or samples were collected beyond the dosing interval. Plasma THC concentrations were below the limit of quantitation.
The overall incidence of treatment-emergent AEs (TEAEs) during the double-blind treatment period was 50.4% (63 of 125 participants) among participants receiving cannabidiol 195 mg or 390 mg and 41.3% (26 of 63 participants) in those who received placebo (Table 4). Treatment-related TEAEs were experienced by 25.6% (32 of 125 participants) of participants in the active treatment groups and 12.7% (8 of 63 participants) of participants in the placebo group.
Table 4. Treatment-Emergent Adverse Events During Double-Blind Treatment.
| Type of adverse event | Patients, No. (%) | |||
|---|---|---|---|---|
| Placebo (n = 63) | Cannabidiol | |||
| 195 mg (n = 63) | 390 mg (n = 62) | Total (N = 125) | ||
| Treatment-emergent adverse events | 26 (41.3) | 31 (49.2) | 32 (51.6) | 63 (50.4) |
| Mild | 15 (23.8) | 19 (30.2) | 19 (30.6) | 38 (30.4) |
| Moderate | 10 (15.9) | 12 (19.0) | 11 (17.7) | 23 (18.4) |
| Severe | 1 (1.6) | 0 | 2 (3.2) | 2 (1.6) |
| Adverse events related to treatment | 8 (12.7) | 11 (17.5) | 21 (33.9) | 32 (25.6) |
| Adverse events resulting in discontinuation | 1 (1.6) | 3 (4.7) | 2 (3.2) | 5 (4.0) |
| Serious adverse events | 3 (4.8) | 1 (1.6) | 4 (6.5) | 5 (4.0) |
In all 3 treatment groups, the majority of TEAEs (96.6% [86 of 89 participants]) were mild or moderate. The most common TEAEs during the double-blind treatment period occurring in more than 2% of cannabidiol-treated participants are listed in eTable 5 in Supplement 2.
The incidence of serious AEs was similar in the placebo and the combined cannabidiol groups (Table 4), all considered unrelated to the study drug. Serious TEAEs in all groups were recovered or resolved by end of study, except for 1 participant in the placebo group with abdominal distension adjudicated as a new medical condition of stage III high-grade ovarian cancer.
No clinically meaningful changes from baseline in clinical laboratory parameters (including free testosterone levels), vital signs, physical and neurological examination findings, or ECGs were observed in any of the treatment groups.
During the OLE period through 18 months, the TEAE profile of transdermal cannabidiol remained consistent with observations during the double-blind phase (eTable 6 in Supplement 2). Three serious AEs were considered possibly related to cannabidiol: tonic-clonic seizures requiring hospitalization (2 participants) and increased anxiety (1 participant). One death occurred following the visit at month 15 after entry; an autopsy revealed severe coronary atherosclerosis, which was not considered related to the study drug. There were no abnormal liver enzyme AEs and no alanine aminotransferase or aspartate aminotransferase levels greater than 3 times the upper limit of normal while receiving cannabidiol.
Discussion
To our knowledge, this is the first randomized, double-blind, placebo-controlled clinical trial of a cannabidiol treatment for adults with focal seizures and the first to investigate transdermal delivery of cannabidiol in this common group of patients. On the primary efficacy end point, there was no significant difference in seizure frequency between participants in the placebo group and those in the 195-mg or 390-mg cannabidiol treatment groups. The absence of a treatment effect with transdermal cannabidiol may be attributable to a number of factors. Participants in this trial could not use clobazam to avoid confounding the efficacy assessment, as cannabidiol is known to increase levels of norclobazam (an active metabolite of clobazam).28 The absence of clobazam may have reduced the response in the active treatment groups in this study compared with other cannabidiol trials.13,14 It is also possible cannabidiol doses were too low, time to onset of effect exceeded 12 weeks, or the response to cannabidiol in adults with focal epilepsy is not as robust as seen in patients with Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex.13,14,15 Efficacy has been observed with higher doses (500-1000 mg per day) of transdermal cannabidiol in patients with developmental epileptic encephalopathies, with a median reduction in focal impaired awareness seizures of 44.5%.29
Transdermal administration of cannabidiol successfully achieved plasma concentrations that overlap those reported with oral cannabidiol (5-10 mg/kg).15 The nominal rate of seizure reduction seen in the high-dose cannabidiol treatment group compared with the low-dose group suggests the lack of a dose-response relationship. The lack of separation from placebo by either active treatment group, the substantial variation in interindividual and intraindividual plasma concentrations (which has also been reported in trials of oral cannabidiol),15 and the wide variability in individualized dosing trajectories over the course of the OLE confound meaningful characterization of a dose-response relationship. Understanding the relative influence of cannabidiol dose on seizure outcomes remains an important next step in the overall assessment of transdermal cannabidiol.
Both doses of transdermal cannabidiol showed excellent tolerability and safety, substantially better than observed in clinical trials of orally administered cannabidiol.12,13,14,15,30 Although the incidence of TEAEs was slightly higher than placebo, few led to discontinuation (7% rate of discontinuation from the double-blind phase and the 98% rate of continuation into the OLE study, with more than one-third of participants continuing to take the study treatment for at least 17 months). There were no clinically meaningful changes from baseline in clinical laboratory assessments, vital signs, ECGs, physical examinations, or neurological findings. The lack of abnormal liver enzymes over 18 months of the OLE study may reflect an advantage of transdermal delivery vs oral administration; other advantages may include fewer gastrointestinal side effects (diarrhea, 4%, and vomiting, 5%, vs 31% and 15%, respectively, with cannabidiol oral solution)20 and reduced susceptibility to the first-pass metabolism.
Limitations
This study had several limitations. The documented persistence of placebo response makes it possible that the placebo effect was a factor in the seizure reductions observed in the OLE. This trial studied patients with focal epilepsy, which is more heterogeneous in cause than Dravet syndrome13,17 and tuberous sclerosis complex,15 which were studied in previous trials of oral cannabidiol. Focal epilepsies are the most common group of epilepsies in adults, as well as the most common studied in ASM trials.
Conclusions
Although the active treatment groups did not separate from placebo during blinded treatment, long-term seizure rates in cannabidiol-treated participants were reduced compared with what may be expected in a population of adults with focal seizures. As a result, additional randomized, well-controlled clinical trials using higher doses of transdermal cannabidiol in this patient population appear to be warranted.
Trial Protocol and Statistical Analysis Plan
eTable 1. Summary of 50% Responder Rate in Percent Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 2. Summary of Percent Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 3. Summary of Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 4. Summary of Patients Completely Seizure Free During Treatment (Efficacy Population)
eTable 5. Treatment-Emergent Adverse Events Occurring in More Than 2% of Participants Treated With ZYN002 During the Double-Blind Phase of the Study
eTable 6. Treatment-Emergent Adverse Events Occurring in More Than 2% of Participants Treated With ZYN002 During the Open-Label Extension
eFigure 1. Median Percent Reduction in Seizures Through Month 18 of the Open-Label Extension
eFigure 2. Median Percent Reduction in Seizures Among Those Completing 18 Months of the Open-Label Extension
STAR 1/STAR 2 Study Group
Data Sharing Statement
References
- 1.Hauser WA. Seizure disorders: the changes with age. Epilepsia. 1992;33(suppl 4):S6-S14. doi: 10.1111/j.1528-1157.1992.tb06222.x [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279-286. doi: 10.1001/jamaneurol.2017.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. doi: 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 4.Gaston TE, Szaflarski JP. Cannabis for the treatment of epilepsy: an update. Curr Neurol Neurosci Rep. 2018;18(11):73. doi: 10.1007/s11910-018-0882-y [DOI] [PubMed] [Google Scholar]
- 5.Cunha JM, Carlini EA, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175-185. doi: 10.1159/000137430 [DOI] [PubMed] [Google Scholar]
- 6.Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21(S1):417S-427S. doi: 10.1002/j.1552-4604.1981.tb02622.x [DOI] [PubMed] [Google Scholar]
- 7.Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373(11):1048-1058. doi: 10.1056/NEJMra1407304 [DOI] [PubMed] [Google Scholar]
- 8.Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7(2):61-76. doi: 10.14581/jer.17012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein BD, Jacobson CA, Metcalf CS, et al. Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42(7):1939-1948. doi: 10.1007/s11064-017-2287-8 [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017;70(pt B):319-327. 10.1016/j.yebeh.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A. 2017;114(42):11229-11234. doi: 10.1073/pnas.1711351114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270-278. doi: 10.1016/S1474-4422(15)00379-8 [DOI] [PubMed] [Google Scholar]
- 13.Devinsky O, Patel AD, Thiele EA, et al. ; GWPCARE1 Part A Study Group . Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204-e1211. doi: 10.1212/WNL.0000000000005254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devinsky O, Patel AD, Cross JH, et al. ; GWPCARE3 Study Group . Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888-1897. doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 15.Thiele EA, Bebin EM, Bhathal H, et al. ; GWPCARE6 Study Group . Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex: a placebo-controlled randomized clinical trial. JAMA Neurol. 2021;78(3):285-292. doi: 10.1001/jamaneurol.2020.4607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mechoulam R, Parker LA, Gallily R. Cannabidiol: an overview of some pharmacological aspects. J Clin Pharmacol. 2002;42(S1):11S-19S. doi: 10.1002/j.1552-4604.2002.tb05998.x [DOI] [PubMed] [Google Scholar]
- 17.Laux LC, Bebin EM, Checketts D, et al. ; CBD EAP study group . Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: expanded access program results. Epilepsy Res. 2019;154:13-20. doi: 10.1016/j.eplepsyres.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 18.Sekar K, Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Res. 2019;8:F1000. doi: 10.12688/f1000research.16515.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804. doi: 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011-2020. doi: 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 21.Sebree T, O’Neill C, Messenheimer J, Gutterman D. Safety and tolerability of ZYN002 (synthetic cannabidiol) transdermal permeation-enhanced gel in healthy subjects and patients with epilepsy: two phase 1, randomized, double-blind, placebo-controlled studies.. November 22, 2016. Accessed April 8, 2022. https://www.aesnet.org/abstractslisting/safety-and-tolerability-of-zyn002-(synthetic-cannabidiol)-transdermal-gel-in-healthy-subjects--two-phase-1--randomized--double-blind--placebo-controlled-studies
- 22.Consroe P, Laguna J, Allender J, et al. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol Biochem Behav. 1991;40(3):701-708. doi: 10.1016/0091-3057(91)90386-G [DOI] [PubMed] [Google Scholar]
- 23.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. doi: 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung S, Sperling MR, Biton V, et al. ; SP754 Study Group . Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia. 2010;51(6):958-967. doi: 10.1111/j.1528-1167.2009.02496.x [DOI] [PubMed] [Google Scholar]
- 25.Klein P, Schiemann J, Sperling MR, et al. A randomized, double-blind, placebo-controlled, multicenter, parallel-group study to evaluate the efficacy and safety of adjunctive brivaracetam in adult patients with uncontrolled partial-onset seizures. Epilepsia. 2015;56(12):1890-1898. doi: 10.1111/epi.13212 [DOI] [PubMed] [Google Scholar]
- 26.Krauss GL, Serratosa JM, Villanueva V, et al. Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology. 2012;78(18):1408-1415. doi: 10.1212/WNL.0b013e318254473a [DOI] [PubMed] [Google Scholar]
- 27.Brown EG, Wood L, Wood S, et al. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109-117. doi: 10.2165/00002018-199920020-00002 [DOI] [PubMed] [Google Scholar]
- 28.Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP; UAB CBD Program . Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586-1592. doi: 10.1111/epi.13852 [DOI] [PubMed] [Google Scholar]
- 29.Scheffer IE, Hulihan J, Messenheimer J, et al. Safety and tolerability of transdermal cannabidiol gel in children with developmental and epileptic encephalopathies: a nonrandomized controlled trial. JAMA Netw Open. 2021;4(9):e2123930. doi: 10.1001/jamanetworkopen.2021.23930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thiele E, Marsh E, Mazurkiewicz-Beldzinska M, et al. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia. 2019;60(3):419-428. doi: 10.1111/epi.14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable 1. Summary of 50% Responder Rate in Percent Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 2. Summary of Percent Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 3. Summary of Change From Baseline to Treatment in Seizure Rates (SF28) (Efficacy Population)
eTable 4. Summary of Patients Completely Seizure Free During Treatment (Efficacy Population)
eTable 5. Treatment-Emergent Adverse Events Occurring in More Than 2% of Participants Treated With ZYN002 During the Double-Blind Phase of the Study
eTable 6. Treatment-Emergent Adverse Events Occurring in More Than 2% of Participants Treated With ZYN002 During the Open-Label Extension
eFigure 1. Median Percent Reduction in Seizures Through Month 18 of the Open-Label Extension
eFigure 2. Median Percent Reduction in Seizures Among Those Completing 18 Months of the Open-Label Extension
STAR 1/STAR 2 Study Group
Data Sharing Statement