Abstract
Background
Triglyceride-glucose (TyG) index as a reliable surrogate of insulin resistance (IR) has been shown to be related to adverse clinical outcomes in patients with acute coronary syndrome, heart failure, ischemic stroke and so on. However, the relationship between TyG index and all-cause mortality in intensive care unit (ICU) patients remains unknown. The purpose of this study was to investigate the correlation between TyG index and all-cause mortality to evaluate the impact of IR on the prognosis of this population.
Methods
This was a retrospective observational study that included 3026 patients who had an initial triglyceride and glucose data on the first day of ICU admission, and all data were extracted from the Medical Information Mart for Intensive Care III (MIMIC-III) database. These patients were grouped into quartiles (Q1–Q4) according to TyG index. The Kaplan–Meier analysis was used to compare all-cause mortality among the above four groups. Cox proportional hazards analyses were performed to examine the association between TyG index and all-cause mortality.
Results
During 10.46 years of follow-up, 1148 (37.9%) patients died, of which 350 (11.6%) occurred during the hospital stay and 258 (8.5%) occurred during the ICU stay. Kaplan–Meier analysis showed that the risk of all-cause mortality was significantly higher in patients with higher TyG index (log-rank P = 0.021). Multivariable Cox proportional hazards analyses showed that the TyG index was an independent risk predictor of ICU death (HR: 1.72, 95% CI 1.18–2.52, P = 0.005) and hospital death (HR: 2.19, 95% CI 1.59–3.03, P < 0.001), and each 1-unit increased in the TyG index, a 1.19-fold increase in the risk of death during the hospital stay.
Conclusions
TyG index is strongly related to the all-cause mortality increasing in critically ill patients. This finding indicates that the TyG index might be useful in identifying people at high risk of ICU death and hospital death.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01563-z.
Keywords: Triglyceride-glucose index, Insulin resistance, Intensive care unit, All-cause mortality
Introduction
Patients in intensive care unit (ICU) usually have a longer duration of mean stay and extremely high mortality rates, placing a heavy burden to the family and society [1]. Risk prediction in advance is important to guide medical treatment. Severity of illness scores have gained considerable interest for their use in predicting outcomes such as mortality and length of stay [2–4]. However, these scores are far from perfect to predict the outcomes of critical illness [5]. Furthermore, these scores encompass various clinical information including patients’ symptoms, signs, laboratory tests, microbiology findings. In the absence of any of this information, these scores cannot be used [4]. Therefore, we urgently need to explore novel biomarkers, mechanisms, and targeted measures in ICU patients. Compared with these scores, the triglyceride-glucose (TyG) index is an easily available, inexpensive and reliable test, which may facilitate its use in the clinical practice [6].
Insulin resistance (IR), defined as the decreased insulin sensitivity of peripheral tissues, plays a critical role in many metabolic abnormalities associated with critical illness [7, 8], and is linked to increased morbidity and mortality [9]. Previous studies have found that critically ill patients had a 50% to 70% of reduction in insulin sensitivity compared to healthy controls [10–12]. Zauner et al. [12] demonstrated that the critically ill patients occurred severe IR on the first day after admission to ICU, and IR was related to the severity of their condition rather than the different admission diagnoses of ICU patients. IR, with time, contributes to micro- and macroangiopathy, neuropathy, and organ failure [13]. Accordingly, it is critical to identify early IR in critically ill patients.
TyG index based on fasting blood glucose (FBG) and triglycerides has been used in clinical practice as a simple and reliable surrogate marker of IR, and former studies have proved that it has high correlation with hyperinsulinaemic–euglycaemic clamp (the gold standard technique for assessing IR) [14, 15]. Previous studies have noted that the TyG index was related to adverse clinical outcomes in patients with acute coronary syndrome (ACS), heart failure (HF), ischemic stroke and so on [16–18]. However, current data about associations between TyG index and critically ill patients are limited, whether TyG index was an independent factor of prognosis in ICU patients has not been determined yet. The purpose of this study was to investigate the relationship between TyG index and all-cause mortality to evaluate the impact of IR on the prognosis of ICU patients.
Methods
Study population
This study is a retrospective observational study. Data were extracted from an online international database, the Medical Information Mart for Intensive Care III (MIMIC-III) [19]. The MIMIC-III is a longitudinal, single-center database which is maintained by the Laboratory of Computational Physiology at Massachusetts Institute of Technology (MIT). This database comprises information related to patients admitted to critical care units at a large tertiary care hospital located in Boston between June 1, 2001 and October 10, 2012. Access to this database is granted by passing an examination and obtaining certification. One author (YL) obtained permission to access the dataset (Record ID 36132841) and was responsible for data extraction. The project was approved by the institutional review boards of the MIT and Beth Israel Deaconess Medical Center (BIDMC) and was granted a waiver of informed consent.
We included 38,511 patients (aged ≥ 18 years) admitted to the ICU in MIMIC-III, and patients with missing triglyceride and glucose data on the first day of admission were excluded. Additionally, we analyzed only the first ICU stay for patients who were admitted to the ICU more than once. A total of 3026 patients were included in the final study cohort and divided into four groups based on the quartiles of the first day of ICU stay TyG index. Patient screening flow chart is displayed in Fig. 1.
Fig. 1.
Flow chart of patients selection for analytic
Variable extraction
Data on baseline characteristics within first 24 h of ICU admission were extracted from the MIMIC-III database, including sex, age, ethnicity, height, weight, body mass index, first care unit, severity at admission measured by Sequential Organ Failure Assessment (SOFA) score, Logistic Organ Dysfunction System (LODS) score, Systemic inflammatory response syndrome (SIRS) score, Oxford Acute Severity of Illness Score (OASIS), Acute physiology score III (APSIII), Simplified acute physiological score II (SAPSII). Initial TyG index [20] and vital signs were also extracted. The TyG index was calculated as the ln [Fasting triglyceride (mg/dL) × fasting glucose (mg/dL)]/2. If a variable was recorded more than once in the first 24 h, we used its average value. Comorbidities identified based on documented ICD-9 codes included coronary heart disease (CHD), HF, hypertension, atrial fibrillation (AF), dyslipidemia, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), renal failure (RF), liver disease (LD), chronic kidney disease (CKD), sepsis, cancer. Acute kidney injury (AKI) was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) guidelines as an increase in serum creatinine (Scr) by ≥ 0.3 mg/dL from baseline within 48 h [21]. Routine blood, complete set of biochemistry and laboratory indicators related to glucose and lipid metabolism were collected for analysis.
Primary outcome and secondary outcomes
The primary outcome of this study was in hospital all-cause mortality. Of these, hospital death included ICU death and general ward inpatient death. The secondary outcomes included long-term follow-up death and length of stay in ICU and hospital. Patient mortality information for discharged patients was accessed from the US Social Security Death Index.
Statistical analysis
The study population was divided into four groups based on the quartiles of the first day of ICU stay TyG index. Data were presented as the mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables and quantity and frequency (%) for categorical variables. Continuous variables were compared by Student’s t test and categorical variables were compared using the Pearson chi-square test or Fisher’s exact test. The propensity score matching (PSM) was used to adjust for covariates to ensure the comparability across groups in the analysis of baseline characteristics. Baseline characteristics of the original and matched cohorts were presented separately.
After testing the normality of the TyG index, multifactorial linear regression was used to analyze the relationship between TyG index with length of ICU stay and length of hospital stay. To evaluate the association between the primary outcomes and TyG index (1 unit and quartile), we used Kaplan Meier survival analysis to evaluate the incidence rate of primary outcome events among groups according to different levels of the TyG index, and discrepancies among groups were evaluated by log-rank test. We used Cox proportional hazards models to estimate the hazard ratio (HR) and 95% confidence interval (CI) between the risk of TyG index and primary outcomes. Moreover, the restricted cubic spline (RCS) regression model with assumed three knots was used to outline the relations between TyG index and HR. Further stratified analyses according to sex, age (≤ 65 and > 65 years), body mass index (BMI) (< 30 and ≥ 30 kg/m2), DM and hypertension were conducted to identify the consistency of the prognostic impact of TyG index for primary endpoint. The interaction between TyG index and stratified variables was further tested. The baseline variables were used as candidate predictors for the multivariate regression model. Considering the possibility of overfitting, we quantified the multicollinearity between variables using variance inflation factors (VIF). Variables with VIF ≥ 5 were excluded. Finally, the model was adjusted for age, sex, ethnicity, first care unit, SOFA score, LODS score, laboratory tests [white blood cell (WBC), red blood cell (RBC), hemoglobin, serum sodium, serum potassium, total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), albumin, Scr], and co-morbidities [CHD, HF, hypertension, dyslipidemia, DM, COPD, RF, LD, CKD, AKI, sepsis, cancer].
To assess whether the accuracy of predicting adverse outcome events would be improved by adding TyG index to the existing severity of illness scores (SOFA score, LODS score, OASIS score, SIRS score, APSIII, SAPSII), the area under the curve (AUC) was calculated. The results of AUC were generated from the matched cohort.
All data analyses were performed using R software (version 4.0.4; R Foundation for Statistical Computing, Vienna, Austria) and SPSS statistical software (IBM SPSS Statistics, Version 24.0; Armonk, NY, USA). A two-sided P-value < 0.05 was considered statistically significant for all analyses.
Results
After reviewing the data of 38,511 patients who were admitted into the ICU from the MIMIC-III database, a total of 3026 were included in our study. The mean age of the enrolled patients was 65.44 ± 16.07 years, including 1,240 (41%) female patients. All enrolled patients’ average TyG index was 9.16 ± 0.74. During 10.46 years of follow-up, 1148 (37.9%) experienced all-cause mortality, of which 350 (11.6%) occurred during the hospital stay and 258 (8.5%) occurred during the ICU stay.
Baseline characteristics
Baseline characteristics grouped according to quartiles of the TyG index are shown in Table 1. Patients with higher TyG index were generally younger, higher severity of illness scores on admission, higher prevalence of DM, COPD, RF, LD, AKI, CKD, sepsis, higher levels of WBC, Scr, TC, LDL, hemoglobin A1c and blood urea nitrogen, lower levels of albumin and HDL compared to the lower group. With increasing TyG index, ICU length of stay (3.68 days vs. 3.78 days vs. 4.16 days vs. 5.49 days, P < 0.001), hospital length of stay (7.68 days vs. 7.83 days vs. 8.82 days vs. 10.50 days, P < 0.001), ICU mortality (5.6% vs. 7.8% vs. 10.2% vs. 10.6%, P = 0.001), hospital mortality (7.5% vs. 11.3% vs. 13.5% vs. 14.0%, P < 0.001), and long-term follow-up mortality (35.1% vs. 35.8% vs. 40.4% vs. 40.6%, P = 0.040) increased gradually. Similar results were observed for the baseline characteristics grouped by the TyG index and baseline characteristics of the matched cohorts (Additional file 1: Table S1).
Table 1.
Baseline characteristics of ICU patients grouped according to TyG index quartiles
| Categories | Overall (N = 3026) | Q1 (N = 756) | Q2 (N = 755) | Q3 (N = 758) | Q4 (N = 757) | P-value |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, years, mean (SD) | 65.44 (16.07) | 68.32 (16.44) | 66.83 (15.90) | 65.62 (15.58) | 60.99 (15.45) | < 0.001 |
| Male, n (%) | 1786 (59.0) | 451 (59.7) | 437 (57.9) | 455 (60.0) | 443 (58.5) | 0.819 |
| Ethnicity, n (%) | 0.003 | |||||
| Asian | 617 (20.4) | 135 (17.9) | 139 (18.4) | 170 (22.4) | 173 (22.9) | |
| Black | 58 (1.9) | 21 (2.8) | 10 (1.3) | 19 (2.5) | 8 (1.1) | |
| White | 202 (6.7) | 62 (8.2) | 44 (5.8) | 36 (4.7) | 60 (7.9) | |
| Hispanic/Latino | 83 (2.7) | 15 (2.0) | 25 (3.3) | 21 (2.8) | 22 (2.9) | |
| Other | 2066 (68.3) | 523 (69.2) | 537 (71.1) | 512 (67.5) | 494 (65.3) | |
| Weight, kg, mean (SD) | 82.25 (22.71) | 77.00 (19.72) | 80.49 (22.39) | 82.28 (21.75) | 88.98 (24.93) | < 0.001 |
| Height, cm, mean (SD) | 169.92 (14.47) | 171.03 (20.16) | 169.30 (13.36) | 169.27 (12.04) | 170.14 (11.20) | 0.319 |
| BMI, kg/m2, mean (SD) | 31.35 (76.35) | 36.20 (156.02) | 29.21 (12.59) | 29.18 (13.20) | 31.10 (12.03) | 0.581 |
| ICU admission | ||||||
| SOFA score, mean (SD) | 3.32 (2.91) | 2.86 (2.45) | 3.00 (2.59) | 3.42 (2.88) | 4.01 (3.48) | < 0.001 |
| LODS score, mean (SD) | 3.49 (2.56) | 3.08 (2.25) | 3.20 (2.26) | 3.64 (2.61) | 4.05 (2.94) | < 0.001 |
| OASIS score, mean (SD) | 30.94 (8.96) | 29.81 (8.58) | 30.45 (8.38) | 31.29 (9.04) | 32.22 (9.61) | < 0.001 |
| SIRS score, mean (SD) | 2.51 (1.06) | 2.32 (1.04) | 2.40 (1.06) | 2.57 (1.03) | 2.73 (1.05) | < 0.001 |
| APSIII, mean (SD) | 39.29 (18.74) | 36.61 (16.12) | 37.10 (17.36) | 39.37 (19.18) | 44.05 (21.01) | < 0.001 |
| SAPSII, mean (SD) | 32.83 (13.23) | 31.50 (12.09) | 32.08 (12.33) | 33.39 (13.59) | 34.37 (14.58) | < 0.001 |
| First Care Unit, n (%) | < 0.001 | |||||
| CCU | 1259 (41.6) | 332 (43.9) | 304 (40.3) | 333 (43.9) | 290 (38.3) | |
| CSRU | 152 (5.0) | 27 (3.6) | 36 (4.8) | 45 (5.9) | 44 (5.8) | |
| MICU | 708 (23.4) | 152 (20.1) | 162 (21.5) | 159 (21.0) | 235 (31.0) | |
| SICU | 643 (21.2) | 180 (23.8) | 176 (23.3) | 154 (20.3) | 133 (17.6) | |
| TSICU | 264 (8.7) | 65 (8.6) | 77 (10.2) | 67 (8.8) | 55 (7.3) | |
| Vital signs | ||||||
| HR, bmp, mean (SD) | 81.13 (16.20) | 78.03 (15.21) | 79.34 (15.65) | 82.02 (15.79) | 85.15 (17.18) | < 0.001 |
| SBP, mmHg, mean (SD) | 123.12 (18.63) | 122.37 (18.77) | 123.36 (18.51) | 123.58 (19.21) | 123.17 (18.03) | 0.612 |
| DBP, mmHg, mean (SD) | 63.82 (11.43) | 63.21 (10.93) | 63.78 (11.51) | 64.17 (11.50) | 64.14 (11.76) | 0.321 |
| SpO2, %, mean (SD) | 97.27 (2.02) | 97.35 (1.65) | 97.28 (1.83) | 97.28 (2.26) | 97.18 (2.26) | 0.435 |
| Comorbidities | ||||||
| CHD, n (%) | 1618 (53.5) | 403 (53.3) | 384 (50.9) | 434 (57.3) | 397 (52.4) | 0.080 |
| HF, n (%) | 879 (29.0) | 216 (28.6) | 209 (27.7) | 218 (28.8) | 236 (31.2) | 0.483 |
| Hypertension, n (%) | 1611 (53.2) | 386 (51.1) | 424 (56.2) | 408 (53.8) | 393 (51.9) | 0.198 |
| AF, n (%) | 724 (23.9) | 207 (27.4) | 186 (24.6) | 182 (24.0) | 149 (19.7) | 0.005 |
| Dyslipidemia, n (%) | 689 (22.8) | 153 (20.2) | 176 (23.3) | 175 (23.1) | 185 (24.4) | 0.248 |
| DM, n (%) | 828 (27.4) | 68 (9.0) | 135 (17.9) | 235 (31.0) | 390 (51.5) | < 0.001 |
| COPD, n (%) | 45 (1.5) | 8 (1.1) | 5 (0.7) | 16 (2.1) | 16 (2.1) | 0.037 |
| RF, n (%) | 481 (15.9) | 73 (9.7) | 96 (12.7) | 135 (17.8) | 177 (23.4) | < 0.001 |
| LD, n (%) | 143 (4.7) | 21 (2.8) | 36 (4.8) | 39 (5.1) | 47 (6.2) | 0.016 |
| AKIa, n (%) | 1547 (51.1) | 345 (45.6) | 345 (45.7) | 412 (54.4) | 445 (58.8) | < 0.001 |
| CKD, n (%) | 310 (10.2) | 71 (9.4) | 58 (7.7) | 75 (9.9) | 106 (14.0) | 0.001 |
| Sepsis, n (%) | 162 (5.4) | 24 (3.2) | 32 (4.2) | 40 (5.3) | 66 (8.7) | < 0.001 |
| Cancer, n (%) | 360 (11.9) | 98 (13.0) | 103 (13.6) | 88 (11.6) | 71 (9.4) | 0.054 |
| Laboratory tests | ||||||
| WBC, K/µL, mean (SD) | 11.75 (5.88) | 10.65 (4.75) | 11.50 (6.86) | 12.01 (5.29) | 12.85 (6.18) | < 0.001 |
| RBC, m/µL, mean (SD) | 4.19 (0.73) | 4.17 (0.68) | 4.21 (0.71) | 4.17 (0.75) | 4.20 (0.78) | 0.568 |
| Platelet, K/µL, mean (SD) | 252.91 (110.48) | 245.85 (110.86) | 254.08 (109.33) | 255.96 (109.13) | 255.75 (112.49) | 0.236 |
| Hemoglobin, g/dL, mean (SD) | 12.69 (2.15) | 12.69 (2.00) | 12.77 (2.15) | 12.64 (2.22) | 12.67 (2.24) | 0.645 |
| Serum potassium, mEq/L, mean (SD) | 4.20 (0.81) | 4.19 (0.82) | 4.18 (0.78) | 4.16 (0.72) | 4.29 (0.89) | 0.007 |
| Serum sodium, mEq/L, mean (SD) | 138.43 (4.58) | 138.30 (4.74) | 138.69 (4.29) | 138.57 (4.13) | 138.15 (5.10) | 0.084 |
| TC, mg/dL, mean (SD) | 163.17 (54.46) | 151.66 (42.60) | 160.83 (46.99) | 165.97 (47.13) | 175.50 (74.60) | < 0.001 |
| TG, mg/dL, mean (SD) | 143.99 (168.28) | 66.55 (21.27) | 104.21 (74.80) | 138.64 (48.33) | 266.35 (286.95) | < 0.001 |
| LDL, mg/dL, mean (SD) | 91.57 (40.10) | 86.06 (35.61) | 94.04 (40.53) | 95.14 (40.98) | 91.17 (42.83) | < 0.001 |
| HDL, mg/dL, mean (SD) | 45.78 (16.71) | 52.62 (18.01) | 46.43 (14.84) | 43.82 (14.89) | 39.42 (16.10) | < 0.001 |
| HbA1c, %, mean (SD) | 6.43 (1.59) | 5.86 (0.82) | 5.97 (0.79) | 6.31 (1.27) | 7.63 (2.32) | < 0.001 |
| Glucose, mg/dL, mean (SD) | 162.37 (105.57) | 128.03 (45.85) | 138.73 (47.71) | 159.99 (77.66) | 222.79 (169.83) | < 0.001 |
| Albumin, g/dL, mean (SD) | 3.43 (0.64) | 3.49 (0.59) | 3.47 (0.65) | 3.40 (0.63) | 3.38 (0.67) | 0.013 |
| Ucr, mg/dL, mean (SD) | 97.90 (71.45) | 93.62 (67.53) | 100.90 (66.69) | 100.79 (76.90) | 96.48 (72.77) | 0.745 |
| Scr, mg/dL, mean (SD) | 1.30 (1.24) | 1.19 (1.06) | 1.19 (1.09) | 1.29 (1.31) | 1.51 (1.45) | < 0.001 |
| BUN, mg/dL, mean (SD) | 24.31 (17.61) | 21.94 (12.78) | 22.66 (17.55) | 24.74 (16.94) | 27.91 (21.49) | < 0.001 |
| Uric acid, mg/dL, mean (SD) | 5.66 (2.86) | 5.74 (2.89) | 4.93 (1.90) | 5.58 (3.05) | 6.01 (3.10) | 0.377 |
| TyG index, mean (SD) | 9.16 (0.74) | 8.30 (0.33) | 8.87 (0.12) | 9.32 (0.15) | 10.13 (0.49) | < 0.001 |
| Events | ||||||
| LOS ICU, days, mean (SD) | 4.28 (6.08) | 3.68 (6.38) | 3.78 (5.35) | 4.16 (4.95) | 5.49 (7.21) | < 0.001 |
| LOS Hospital, days, mean (SD) | 8.71 (10.12) | 7.68 (8.71) | 7.83 (8.58) | 8.82 (9.71) | 10.50 (12.68) | < 0.001 |
| ICU death, n (%) | 258 (8.5) | 42 (5.6) | 59 (7.8) | 77 (10.2) | 80 (10.6) | 0.001 |
| Hospital death, n (%) | 350 (11.6) | 57 (7.5) | 85 (11.3) | 102 (13.5) | 106 (14.0) | < 0.001 |
| Follow-up death, n (%) | 1148 (37.9) | 265 (35.1) | 270 (35.8) | 306 (40.4) | 307 (40.6) | 0.040 |
TyG index: Q1 (6.23–8.65), Q2 (8.65–9.08), Q3 (9.08–9.59), Q4 (9.59–12.43)
TyG index triglyceride glucose index, BMI body mass index, ICU intensive care unit, SOFA sequential organ failure assessment, LODS logistic organ dysfunction system, OASIS Oxford acute severity of illness, SIRS systemic inflammatory response syndrome, APSIII acute physiology score III, SAPSII simplified acute physiological score II, CCU coronary care unit, CSRU cardiac surgery recovery unit, MICU medical intensive care unit, SICU surgical intensive care unit, TSICU trauma/surgical intensive care unit, HR heart rate, bmp beats per minute, SBP systolic blood pressure, DBP diastolic blood pressure, SpO2 pulse blood oxygen saturation, CHD coronary heart disease, HF heart failure, AF atrial fibrillation, DM diabetes mellitus, COPD chronic obstructive pulmonary disease, RF respiratory failure, LD liver disease, AKI acute kidney injury, CKD chronic kidney disease, WBC white blood cell, RBC red blood cell, TC total cholesterol, TG triglyceride, LDL low-density lipoprotein, HDL high-density lipoprotein, HbA1c hemoglobin A1c, Ucr urine creatinine, Scr serum creatinine, BUN blood urea nitrogen, LOS length of stay
aAKI was defined according to KDIGO guidelines as an increase in serum creatinine (Scr) by ≥ 0.3 mg/dL (≥ 26.5 μmol/L) from baseline within 48 h
Incidence rate of all-cause mortality among different groups
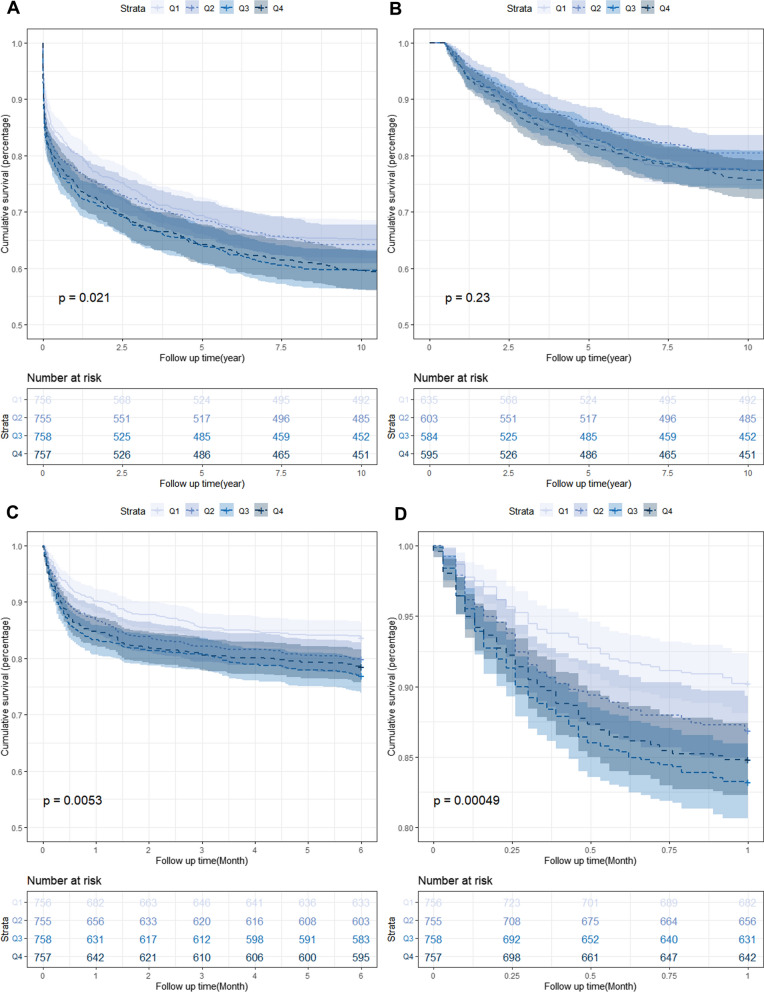
The Kaplan–Meier survival analysis curves for assessing the incidence of all-cause mortality among groups based on the quartile groupings of the TyG index are shown in Fig. 2. There was a statistically significant difference in mortality rate in the groups (Q1: 35.1% vs. Q2: 35.8% vs. Q3: 40.4% vs. Q4: 40.6%, log-rank P = 0.021, Fig. 2A). Higher mortality driven by high TyG index disappeared after 6 months, and no difference was found among groups (log-rank P = 0.230, Fig. 2B). Applying the landmark method, during the 6 months of follow-up, the all-cause mortality of patients with higher TyG index was obvious higher than lower TyG index (Q1: 16.3% vs. Q2: 19.8% vs. Q3: 23.1% vs. Q4: 21.4%, log-rank P = 0.005, Fig. 2C). Notably, more significantly result was observed during the short-term follow-up of 1 month (Q1: 9.8% vs. Q2: 13.1% vs. Q3: 16.8% vs. Q4: 15.2%, Log-rank P < 0.001, Fig. 2D).
Fig. 2.
Kaplan–Meier survival analysis curves for all-cause mortality. TyG index: Q1 (6.23–8.65), Q2 (8.65–9.08), Q3 (9.08–9.59), Q4 (9.59–12.43). Kaplan–Meier curves showing cumulative probability of all-cause mortality according to groups at 10 years (A), landmark analysis from 6 months to 10 years (B), landmark analysis at 6 months (C), and Kaplan–Meier survival analysis curves for all-cause mortality according to groups at 1 month (D)
Association between the all-cause mortality and TyG index
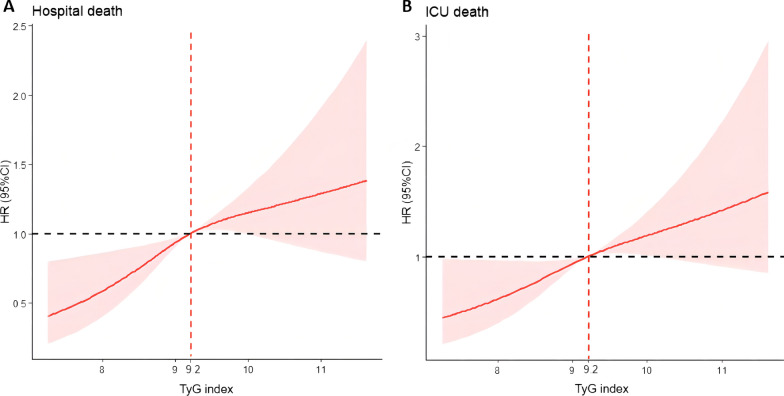
Cox proportional risk analysis showed the significant association between TyG index and hospital death both in unadjusted model (HR, 1.29 [95% CI 1.12–1.48] P < 0.001) and fully adjusted model (HR, 2.19 [95% CI 1.59–3.03] P < 0.001). Furthermore, TyG index was also associated ICU death in unadjusted model (HR, 1.31 [95% CI 1.12–1.53] P < 0.001) and fully adjusted model (HR, 1.72 [95% CI 1.18–2.52] P = 0.005). The risk of hospital death of TyG index Q2, Q3 and Q4 was higher than TyG index Q1, and showed a tendency of increasing with the TyG index (Q1 vs. Q2: HR, 1.70 [95% CI 1.06–2.70]; Q3: HR, 2.08 [95% CI 1.29–3.33]; Q4: HR, 2.80 [95% CI 1.53–5.13]; P for trend < 0.001). Similar results were obtained in Cox proportional risk analysis of the TyG index and ICU death (Q1 vs. Q2: HR, 1.52 [95% CI 0.88–2.64]; Q3: HR, 1.88 [95% CI 1.09–3.24]; Q4: HR, 1.95 [95% CI 0.96–3.99]; P for trend = 0.036) (Table 2). The RCS regression model revealed that higher levels of TyG index (> 9.2) was associated with an increased risk of hospital death and ICU death (Fig. 3).
Table 2.
Cox proportional hazard ratios (HR) for all-cause mortality
| Categories | Events (%) | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | P for trend | HR (95% CI) | P-value | P for trend | HR (95% CI) | P-value | P for trend | ||
| Hospital death | ||||||||||
| Continuous variable per 1 unit | 1.29 (1.12–1.48) | < 0.001 | 1.26 (1.09–1.46) | 0.002 | 2.19 (1.59–3.03) | < 0.001 | ||||
| Quartilea | 350 (11.57) | < 0.001 | 0.004 | < 0.001 | ||||||
| Q1 (N = 756) | 57 (7.54) | Ref. | Ref. | Ref. | ||||||
| Q2 (N = 755) | 85 (11.26) | 1.57 (1.12–2.20) | 0.008 | 1.57 (1.12–2.21) | 0.009 | 1.70 (1.06–2.70) | 0.027 | |||
| Q3 (N = 758) | 102 (13.46) | 1.73 (1.25–2.39) | < 0.001 | 1.69 (1.22–2.35) | 0.002 | 2.08 (1.29–3.33) | 0.002 | |||
| Q4 (N = 757) | 106 (14.00) | 1.75 (1.27–2.42) | < 0.001 | 1.69 (1.20–2.37) | 0.003 | 2.80 (1.53–5.13) | < 0.001 | |||
| ICU death | ||||||||||
| Continuous variable per 1 unit | 1.31 (1.12–1.53) | < 0.001 | 1.21 (1.02–1.43) | 0.029 | 1.72 (1.18–2.52) | 0.005 | ||||
| Quartile | 258 (8.53) | 0.002 | 0.030 | 0.036 | ||||||
| Q1 (N = 756) | 42 (5.56) | Ref. | Ref. | Ref. | ||||||
| Q2 (N = 755) | 59 (7.81) | 1.46 (0.98–2.17) | 0.060 | 1.44 (0.96–2.15) | 0.074 | 1.52 (0.88–2.64) | 0.136 | |||
| Q3 (N = 758) | 77 (10.16) | 1.75 (1.20–2.54) | 0.004 | 1.65 (1.13–2.42) | 0.010 | 1.88 (1.09–3.24) | 0.023 | |||
| Q4 (N = 757) | 80 (10.57) | 1.78 (1.22–2.58) | 0.003 | 1.55 (1.04–2.29) | 0.030 | 1.95 (0.96–3.99) | 0.066 | |||
Model 1: unadjusted
Model 2: adjusted for age, sex, ethnicity, first care unit
Model 3: adjusted for age, sex, ethnicity, first care unit, SOFA score, LODS score, white blood cell, red blood cell, hemoglobin, serum sodium, serum potassium, total cholesterol, low-density lipoprotein, high-density lipoprotein, albumin, serum creatinine, coronary heart disease, heart failure, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, respiratory failure, liver disease, chronic kidney disease, acute kidney injury, sepsis, cancer
aTyG index: Q1 (6.23–8.65), Q2 (8.65–9.08), Q3 (9.08–9.59), Q4 (9.59–12.43)
Fig. 3.
Restricted cubic spline regression analysis of TyG index with in hospital all-cause mortality. Heavy central lines represent the estimated adjusted hazard ratios, with shaded ribbons denoting 95% confidence intervals. TyG index 9.2 was selected as the reference level represented by the vertical dotted lines. The horizontal dotted lines represent the hazard ratio of 1.0. A Restricted cubic spline for hospital death. B Restricted cubic spline for ICU death. HR hazard ratio, CI confidence interval, TyG triglyceride-glucose
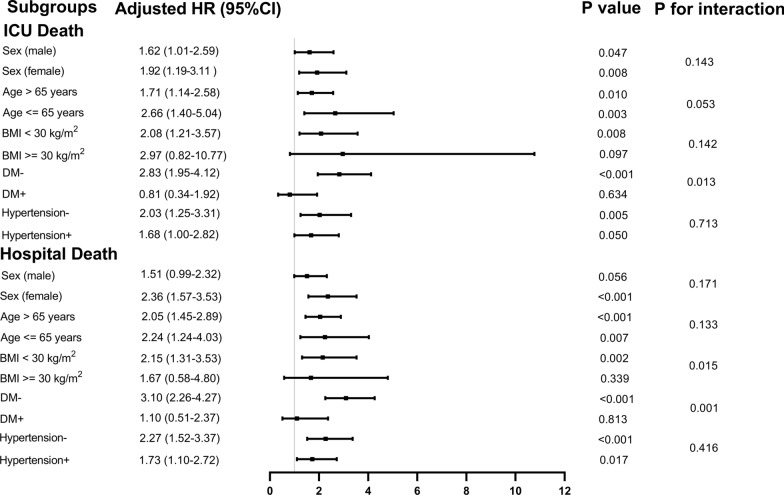
In addition, we conducted a stratified analyses of the relationship between the TyG index and all-cause mortality according to the potential modifiers, including sex, age, BMI, DM and hypertension (Fig. 4). And the TyG index was significantly associated with an increased risk of ICU death in a subgroup of female [HR (95% CI) 1.92 (1.19–3.11)], male [HR (95% CI) 1.62 (1.01–2.59)], those aged ≤ 65 years [HR (95% CI) 2.66 (1.40–5.04)] or > 65 years [HR (95% CI) 1.71 (1.14–2.58)], those with BMI < 30 kg/m2 [HR (95% CI) 2.08 (1.21–3.57)], those without DM [HR (95% CI) 2.83 (1.95–4.12)], and those without hypertension [HR (95% CI) 2.03 (1.25–3.31)] (all P < 0.05). Interestingly, the predictive value of TyG index seemed to be more prominent in patients without DM [HR (95% CI) without DM 2.83 (1.95–4.12) vs. with DM 0.81 (0.34–1.92), P for interaction = 0.013]. Similar results were obtained in stratified analyses of the TyG index and hospital death (Fig. 4).
Fig. 4.
Forest plots of hazard ratios for the primary outcome in different subgroups. HR hazard ratio, CI confidence interval, TyG triglyceride-glucose, ICU intensive care unit, BMI body mass index, DM diabetes mellitus
Incremental effect of TyG index on predictive value for primary outcomes
The TyG index had an incremental effect on the AUC of existing severity of illness scores to predict all-cause mortality, including SOFA score (hospital: 0.765 vs. 0.772; ICU: 0.811 vs. 0.815), LODS score (hospital: 0.791 vs. 0.796; ICU: 0.834 vs. 0.837), OASIS score (hospital: 0.798 vs. 0.801; ICU: 0.810 vs. 0.812), SIRS score (hospital: 0.689 vs. 0.706; ICU: 0.708 vs. 0.722), APSIII (hospital: 0.794 vs. 0.798; ICU: 0.829 vs. 0.831) and SAPSII (hospital: 0.834 vs. 0.838; ICU: 0.854 vs. 0.856). All P-values < 0.001 (Table 3).
Table 3.
Discrimination of each predictive model for outcomes using AUC
| Models | AUC (95% CI) | P-value | Models | AUC (95% CI) | P-value |
|---|---|---|---|---|---|
| Hospital death | |||||
| SOFA Score | 0.765 (0.734–0.797) | < 0.001 | + TyG index | 0.772 (0.742–0.802) | < 0.001 |
| LODS Score | 0.791 (0.763–0.820) | < 0.001 | + TyG index | 0.796 (0.768–0.824) | < 0.001 |
| OASIS Score | 0.798 (0.771–0.824) | < 0.001 | + TyG index | 0.801 (0.774–0.828) | < 0.001 |
| SIRS Score | 0.689 (0.659–0.720) | < 0.001 | + TyG index | 0.706 (0.676–0.736) | < 0.001 |
| APSIII | 0.794 (0.766–0.822) | < 0.001 | + TyG index | 0.798 (0.771–0.825) | < 0.001 |
| SAPSII | 0.834 (0.811–0.858) | < 0.001 | + TyG index | 0.838 (0.815–0.861) | < 0.001 |
| ICU death | |||||
| SOFA Score | 0.811 (0.780–0.842) | < 0.001 | + TyG index | 0.815 (0.785–0.846) | < 0.001 |
| LODS Score | 0.834 (0.806–0.862) | < 0.001 | + TyG index | 0.837 (0.810–0.865) | < 0.001 |
| OASIS Score | 0.810 (0.780–0.840) | < 0.001 | + TyG index | 0.812 (0.782–0.842) | < 0.001 |
| SIRS Score | 0.708 (0.675–0.742) | < 0.001 | + TyG index | 0.722 (0.690–0.755) | < 0.001 |
| APSIII | 0.829 (0.800–0.857) | < 0.001 | + TyG index | 0.831 (0.803–0.858) | < 0.001 |
| SAPSII | 0.854 (0.828–0.879) | < 0.001 | + TyG index | 0.856 (0.831–0.881) | < 0.001 |
AUC area under curve, SOFA sequential organ failure assessment, LODS logistic organ dysfunction system, OASIS Oxford acute severity of illness score, SIRS systemic inflammatory response syndrome, APSIII acute physiology score III, SAPSII, simplified acute physiological score II, TyG index triglyceride-glucose index, ICU intensive care unit
Relationship between TyG index and length of stay
Multiple linear regression analysis showed that the TyG index was positively associated with length of stay even in the fully adjusted model among patients who survived the hospital stay (β = 1.36, P = 0.008) and those who survived the ICU stay (β = 0.87, P = 0.004, Table 4).
Table 4.
Relationship between TyG index and length of stay (LOS)
| Coef. | S. E | t-value | P-value | |
|---|---|---|---|---|
| LOS hospitala | ||||
| Model 1 | 1.66 | 0.25 | 6.65 | < 0.001 |
| Model 2 | 1.55 | 0.25 | 6.25 | < 0.001 |
| Model 3 | 1.36 | 0.51 | 2.68 | 0.008 |
| LOS ICUb | ||||
| Model 1 | 0.96 | 0.14 | 6.91 | < 0.001 |
| Model 2 | 0.91 | 0.14 | 6.46 | < 0.001 |
| Model 3 | 0.87 | 0.30 | 2.87 | 0.004 |
Model 1: unadjusted
Model 2: adjusted for age, sex ethnicity, first care unit
Model 3: adjusted for age, sex, ethnicity, first care unit, SOFA score, LODS score, white blood cell, red blood cell, hemoglobin, serum sodium, serum potassium, total cholesterol, low-density lipoprotein, high-density lipoprotein, albumin, serum creatinine, coronary heart disease, heart failure, hypertension, dyslipidemia, diabetes, chronic obstructive pulmonary disease, respiratory failure, liver disease, chronic kidney disease, acute kidney injury, sepsis, cancer
aThe relationship between TyG index and length of hospital stay was analyzed in a cohort that survived the hospital stay (n = 2676)
bThe relationship between TyG index and length of ICU stay was analyzed in a cohort that survived the ICU stay (n = 2768)
Discussion
To the best of our knowledge, this study was the first to explore the relationship between TyG index and all-cause mortality in ICU patients. In the present study, the information of unselected ICU adult patients were extracted from the MIMIC III database, the primary finding is that increased TyG index was a strong independent predictor of greater mortality in ICU patients. This association remained after adjustment for a wide variety of clinical and laboratory variables. Most importantly, this study provides a novel, simple and efficient biomarker for the early diagnosis of IR in critically ill patients.
The severity of IR in vivo can be determined using the hyperinsulinaemic–euglycaemic clamp technique which is the gold standard for evaluating IR [22]. However, the performance of the hyperinsulinaemic–euglycaemic clamp technique is time-consuming, costly and complex [14]. Alternatives for estimating IR include the Quantitative Insulin Sensitivity Check Index (QUICKI) and the Homeostasis model assessment of IR (HOMA-IR) [23, 24]. However, constrained to the complex mathematical calculation or the requirement of insulin concentration examination, the clinical popularity of QUICKI and HOMA-IR remain challenging. Recently, the TyG index, based on the FBG and triglycerides, is a novel index that has been well-recognized as a simple and reliable surrogate of IR [14]. Former studies have proved that TyG index has high correlation with hyperinsulinaemic–euglycaemic clamp, either in individuals with or without DM [16, 25]. In addition, compared with the HOMA-IR, TyG index showed better evaluation efficiency [26, 27]. Among them, serum triglyceride and glucose are low-cost routine biochemical detection items [6], therefore, the immense advantage of using such a simple method of IR identification is obviously that it is easily accessible in any clinical settings and has a good application prospect [28].
Recent studies have widely used the TyG index as a marker of IR [29]. Previous studies conducted in Asia and Europe validated the strong association between TyG index and incidence of DM, suggesting that TyG index might be an important predictor of early identification of individuals at high risk for diabetes and prediabetes, even better than other risk factors such as fasting glucose and triglycerides [30–32]. Zhao et al. [33] and Chiu et al. [34] showed that TyG index was associated with macrovascular and microvascular damage in both elderly community-dwelling Chinese population and diabetic population. Hu et al. [35] found that, regardless of diabetes status, patients with high TyG index had a significantly higher risk of cardiovascular events. For patients with ACS undergoing percutaneous coronary intervention, TyG index might be a better predictor of cardiovascular risk than FBG or glycated hemoglobin. A recent study demonstrated that the TyG index was directly correlated with poor prognosis in patients with acute decompensated heart failure regardless of DM status [17]. Furthermore, Liu et al. [36] found that elevated level of TyG index reflected a more severe IR and was non-linear associated with all-cause and cardiovascular mortality in the general population. However, current data about associations between TyG index and critically ill patients are limited. Zhang et al. [20] demonstrated that TyG index was a potential predictor of hospital and ICU mortality in a study involving only patients with critical stroke. Additionally, in our study of unselected ICU adult patients, we found that TyG index was an independent predictor of hospitalization and ICU mortality in critically ill patients, which makes the study be great agreement and complement to previous literature [12], which considered that IR was related to the severity of their condition rather than the different admission diagnoses of ICU patients.
IR is defined as unresponsiveness of anabolic processes to the normal effects of insulin, and it has been postulated that many metabolic abnormalities associated with critical illness are related to a loss of tissue sensitivity to insulin [8, 12], and which is not already reflected in severity scores [37]. Severity of illness scores and their use in predicting outcomes have gained considerable favor worldwide and have been proven effective for predicting mortality in ICU patients [4, 38, 39]. However, whether the addition of TyG index has an incremental effect on the prediction of all-cause mortality in ICU patients at the basis of severity scores is uncertain. This study revealed a significant prognostic impact of the TyG index and its incremental effect on risk stratification based on severity scores in critically ill patients. In addition, some of these scores encompass various clinical information including patients’ symptoms, signs, laboratory tests, microbiology findings. In the absence of any of this information, these scores cannot be used [4]. Compared with above scores, the TyG index is an easily available, inexpensive and reliable test [6], and could be used as an independent predictor of hospitalization and ICU mortality in critically ill patients. Therefore, routine assessment of TyG index may improve risk stratification and facilitate decision making in ICU patients.
Intriguingly, some previous studies have found that insulin treatment and lipid-lowering drugs were not associated with TyG index in non-critically ill patients. They believe that the above unexpected results may be related to the history of insulin treatment, and lipid-lowering drugs can not directly reflect the observed level of TyG index [40, 41]. However, our current study found that the predictive value of IR evaluated by TyG index seemed to be more prominent in patients without DM [HR (95% CI) without DM 2.83 (1.95–4.12) vs. with DM 0.81 (0.34–1.92), P for interaction = 0.013], suggesting that antidiabetic treatment may have an important effect on the predictive performance of TyG index for adverse clinical outcomes. This inconsistency may be due to the fact that critically ill diabetic patients are more likely to receive intensive insulin therapy, Van den Berghe et al. [42] showed that intensive insulin therapy reduced mortality during intensive care from 8.0 percent with conventional treatment to 4.6 percent. Another important finding of our study was that patients with higher TyG index were younger, and the relationship between TyG index and all-cause mortality seemed to be more pronounced in younger patients, which was consistent with the previous study [14]. Contrary to conventional wisdom, clinicians may pay more attention to older patients because they may have more comorbidities, whereas our study calls for the same attention to be given to younger patients because they may have a higher mortality rate.
IR is a clinical condition characterized by impaired glucose processing in the presence of normal or elevated serum insulin concentrations [7, 8]. The development of IR was no disease-specific reaction but a prevailing response to critical illness [12]. Although the mechanism underlying the close connection between the TyG index and all-cause mortality in ICU patients has not been elucidated, it might be attributed to the relationship between the IR status represented by the TyG index and the severity of the disease. First, IR has been widely demonstrated to be well correlated with endothelial dysfunction, oxidative stress, cardiovascular remodeling, coagulation imbalance and inflammation response [43–45], and all of which were important reasons for the aggravation of ICU patients. Second, the relationship between severity of IR and illness could be explained by an increased productions of serum cytokines. These productions have been shown to increase with disease severity, and as the key components of systemic inflammation and stress response induced IR [8, 46]. Third, hyperglycemia associated with IR was common in critically ill patients, even in those without diabetes previously [42]. Hyperglycemia promoted tissue acidosis, production of reactive oxygen species and nitrogen, and inflammatory cell infiltration, leading to more severe tissue structural dysfunction. Fourth, IR was associated with macrovascular disease, neuropathy, and organ failure [13], which led the continued deterioration in critically ill patients ultimately. Studies have shown that FBG levels mainly reflected IR from the liver, whereas fasting triglycerides levels mainly reflected IR from adipose cells. Therefore, the TyG index might reflect IR from two aspects and thus be closely related to IR [47, 48]. Furthermore, a recent review showed that it would be interesting to explore whether a postprandial TyG index might have clinical significance, because increased postprandial levels of triglyceride and glucose are metabolically abnormal responses to IR [49].
Our study confirmed that TyG index could be used as an effective predictor in clinical practice, and was independent risk predictor of ICU death and hospital death. However, we must acknowledge some limitations. Firstly, this was a retrospective analysis derived from an observational study, which could not definitively establish causality, but we have carried out careful, multifaceted and rigorous statistical methods to produce valid and reliable and results. Further studies need to be performed to determine whether interventions for TyG index have a positive impact on improving clinical prognosis. Secondly, due to the limitation of the database, there is no way to confirm that all glucose and lipids are the results of fasting. Thirdly, the data were from the United States, and thus the results might not be completely applicable to ICUs in other countries, but the enrolled patients came from different races, therefore, it had a certain representative.
Conclusions
Increased IR extent presented by TyG index is a prominent risk predictor of all-cause mortality in ICU patients. Our findings indicate that this simple index facilitates early identification of IR in critically ill patients, which can improve risk stratification and guide subsequent interventions. All of these findings strongly support the importance of including TyG index in physicians’ daily work. Further prospective studies are required to confirm our findings.
Supplementary Information
Additional file 1: Table S1. Comparisons of baseline characteristics between the original cohort and matched cohort.
Acknowledgements
None.
Abbreviations
- ICU
Intensive care unit
- TyG
Triglyceride-glucose
- IR
Insulin resistance
- FBG
Fasting blood glucose
- ACS
Acute coronary syndrome
- HF
Heart failure
- MIMIC-III
Medical Information Mart for Intensive Care III
- MIT
Massachusetts Institute of Technology
- BIDMC
Beth Israel Deaconess Medical Center
- SOFA
Sequential organ failure assessment
- LODS
Logistic organ dysfunction system
- SIRS
Systemic inflammatory response syndrome
- OASIS
Overall anxiety severity and impairment scale
- APSIII
Acute physiology score III
- SAPSII
Simplified acute physiological score II
- CHD
Coronary heart disease
- AF
Atrial fibrillation
- DM
Diabetes mellitus
- COPD
Chronic obstructive pulmonary disease
- RF
Respiratory failure
- LD
Liver disease
- CKD
Chronic kidney disease
- AKI
Acute kidney injury
- KDIGO
Kidney Disease: Improving Global Outcomes
- Scr
Serum creatinine
- SD
Standard deviation
- IQR
Interquartile range
- PSM
Propensity score matching
- HR
Hazard ratio
- CI
Confidence interval
- RCS
Restricted cubic spline
- BMI
Body mass index
- VIF
Variance inflation factor
- WBC
White blood cell
- RBC
Red blood cell
- TC
Total cholesterol
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- AUC
Area under the curve
- QUICKI
Quantitative Insulin Sensitivity Check Index
- HOMA-IR
Homeostasis model assessment of IR
Author contributions
RTZ, SSS, YKZ, LHL, XQL, QG and YNW were responsible for the study concept and YF, KHC, SHL, YL, RTZ, and SSS for study design. Data extraction was undertaken by YL. RTZ, SSS, YKZ and YBH were responsible for data analysis. Drafting of the manuscript: RTZ, SSS and YKZ. Critical revision of the manuscript for important intellectual content: RTZ, SSS, YKZ, YBH, KHC, SHL, YF, YL, WGL and LLC. All authors read and approved the final manuscript.
Funding
This research was funded and sponsored by Longyan City Science and Technology Plan Project (Grant Numbers: 2021LYF17025, 2021LYF17039). The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the principals of the Declaration of Helsinki. The use of the MIMIC-III database was approved by the review committee of Massachusetts Institute of Technology and Beth Israel Deaconess Medical Center. The data is publicly available (in the MIMIC-III database) hence ethical approval statement and the informed consent is not required for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Liao, Rongting Zhang and Shanshan Shi contributed equally to this work and share first authorship
Contributor Information
Shihai Li, Email: 13959002329@139.com.
Kaihong Chen, Email: chenkaihong1964@163.com.
Yong Fang, Email: fjly7008@163.com.
References
- 1.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. Lancet Respir Med. 2014;2(5):380–386. doi: 10.1016/s2213-2600(14)70061-x. [DOI] [PubMed] [Google Scholar]
- 2.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, et al. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU Scoring Group. JAMA. 1996;276(10):802–810. doi: 10.1001/jama.276.10.802. [DOI] [PubMed] [Google Scholar]
- 4.Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med. 2013;41(7):1711–1718. doi: 10.1097/CCM.0b013e31828a24fe. [DOI] [PubMed] [Google Scholar]
- 5.Han YQ, Yan L, Zhang L, Ouyang PH, Li P, Goyal H, et al. Red blood cell distribution width provides additional prognostic value beyond severity scores in adult critical illness. Clin Chim Acta Int J Clin Chem. 2019;498:62–67. doi: 10.1016/j.cca.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, et al. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320. doi: 10.1038/s41598-019-43776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter SJ, Garvey WT. Insulin action and insulin resistance: diseases involving defects in insulin receptors, signal transduction, and the glucose transport effector system. Am J Med. 1998;105(4):331–345. doi: 10.1016/s0002-9343(98)00300-3. [DOI] [PubMed] [Google Scholar]
- 8.Carlson GL. Insulin resistance and glucose-induced thermogenesis in critical illness. Proc Nutr Soc. 2001;60(3):381–388. doi: 10.1079/pns200193. [DOI] [PubMed] [Google Scholar]
- 9.Yahia A, Szlávecz Á, Knopp JL, Norfiza Abdul Razak N, Abu Samah A, Shaw G, et al. Estimating enhanced endogenous glucose production in intensive care unit patients with severe insulin resistance. J Diabetes Sci Technol. 2021 doi: 10.1177/19322968211018260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black PR, Brooks DC, Bessey PQ, Wolfe RR, Wilmore DW. Mechanisms of insulin resistance following injury. Ann Surg. 1982;196(4):420–435. doi: 10.1097/00000658-198210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed M, Carlson GL, Little RA, Irving MH. Selective impairment of glucose storage in human sepsis. Br J Surg. 1999;86(6):813–821. doi: 10.1046/j.1365-2168.1999.01140.x. [DOI] [PubMed] [Google Scholar]
- 12.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Schiefermeier M, Ratheiser K, et al. Severity of insulin resistance in critically ill medical patients. Metab Clin Exp. 2007;56(1):1–5. doi: 10.1016/j.metabol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Investig. 2000;106(4):453–458. doi: 10.1172/jci10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146. doi: 10.1186/s12933-014-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic–hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19(1):108. doi: 10.1186/s12933-020-01086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang R, Wang Z, Chen J, Bao X, Xu N, Guo S, et al. Prognostic value of triglyceride glucose (TyG) index in patients with acute decompensated heart failure. Cardiovasc Diabetol. 2022;21(1):88. doi: 10.1186/s12933-022-01507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi W, Xing L, Jing L, Tian Y, Yan H, Sun Q, et al. Value of triglyceride-glucose index for the estimation of ischemic stroke risk: insights from a general population. Nutr Metab Cardiovasc Dis. 2020;30(2):245–253. doi: 10.1016/j.numecd.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi: 10.1038/sdata.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Liu L, Ruan H, Zhu Q, Yu D, Yang Y, et al. Triglyceride-glucose index linked to hospital mortality in critically ill stroke: an observational multicentre study on eICU database. Front Med. 2020;7:591036. doi: 10.3389/fmed.2020.591036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17(1):204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30(5):387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 23.Brun JF, Ghanassia E, Fédou C, Bordenave S, Raynaud de Mauverger E, Mercier J. Assessment of insulin sensitivity (S I) and glucose effectiveness (S G) from a standardized hyperglucidic breakfast test in type 2 diabetics exhibiting various levels of insulin resistance. Acta Diabetol. 2013;50(2):143–153. doi: 10.1007/s00592-010-0232-2. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 25.Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic–euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–465. doi: 10.1111/pedi.12303. [DOI] [PubMed] [Google Scholar]
- 26.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Jiao Y, Su Y, Shen J, Hou X, Li Y, Wang J, et al. Evaluation of the long-term prognostic ability of triglyceride-glucose index for elderly acute coronary syndrome patients: a cohort study. Cardiovasc Diabetol. 2022;21(1):3. doi: 10.1186/s12933-021-01443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, et al. Triglyceride-glucose index in the development of peripheral artery disease: findings from the atherosclerosis risk in communities (ARIC) study. Cardiovasc Diabetol. 2021;20(1):126. doi: 10.1186/s12933-021-01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang M, Wang B, Liu Y, Sun X, Luo X, Wang C, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol. 2017;16(1):30. doi: 10.1186/s12933-017-0514-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramírez-Vélez R, Pérez-Sousa M, González-Ruíz K, Cano-Gutierrez CA, Schmidt-RioValle J, Correa-Rodríguez M, et al. Obesity- and lipid-related parameters in the identification of older adults with a high risk of prediabetes according to the American diabetes association: an analysis of the 2015 health, well-being, and aging study. Nutrients. 2019 doi: 10.3390/nu11112654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. 2016;86:99–105. doi: 10.1016/j.ypmed.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, et al. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the northern Shanghai study. Cardiovasc Diabetol. 2019;18(1):95. doi: 10.1186/s12933-019-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, et al. Associations between triglyceride-glucose index and micro- and macro-angiopathies in type 2 diabetes mellitus. Nutrients. 2020 doi: 10.3390/nu12020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, et al. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. 2020;19(1):116. doi: 10.1186/s12933-020-01091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7:628109. doi: 10.3389/fcvm.2020.628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knopp JL, Chase JG, Shaw GM. Increased insulin resistance in intensive care: longitudinal retrospective analysis of glycaemic control patients in a New Zealand ICU. Ther Adv Endocrinol Metab. 2021;12:20420188211012144. doi: 10.1177/20420188211012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Zhang Z, Hu T. Effectiveness of LODS, OASIS, and SAPS II to predict in-hospital mortality for intensive care patients with ST elevation myocardial infarction. Sci Rep. 2021;11(1):23887. doi: 10.1038/s41598-021-03397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu T, Lv H, Jiang Y. The association between four scoring systems and 30-day mortality among intensive care patients with sepsis: a cohort study. Sci Rep. 2021;11(1):11214. doi: 10.1038/s41598-021-90806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, et al. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20(1):82. doi: 10.1186/s12933-021-01274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira ÂC, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. 2019;18(1):89. doi: 10.1186/s12933-019-0893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 43.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuñiga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 45.Markus MRP, Rospleszcz S, Ittermann T, Baumeister SE, Schipf S, Siewert-Markus U, et al. Glucose and insulin levels are associated with arterial stiffness and concentric remodeling of the heart. Cardiovasc Diabetol. 2019;18(1):145. doi: 10.1186/s12933-019-0948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Presterl E, Staudinger T, Pettermann M, Lassnigg A, Burgmann H, Winkler S, et al. Cytokine profile and correlation to the APACHE III and MPM II scores in patients with sepsis. Am J Respir Crit Care Med. 1997;156(3 Pt 1):825–832. doi: 10.1164/ajrccm.156.3.9607131. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Q, Cheng YJ, Xu YK, Zhao ZW, Liu C, Sun TN, et al. Comparison of various insulin resistance surrogates on prognostic prediction and stratification following percutaneous coronary intervention in patients with and without type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):190. doi: 10.1186/s12933-021-01383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J, Lin Y, Li H, Fan R, Lin L, Wang X, et al. A retrospective study of the relationship between the triglyceride glucose index and myocardial revascularization for new-onset acute coronary syndromes. Front Cardiovasc Med. 2022;9:862252. doi: 10.3389/fcvm.2022.862252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Comparisons of baseline characteristics between the original cohort and matched cohort.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.