Abstract
One pathway by which environments of socioeconomic risk are thought to affect cognitive development is through stress physiology. The biological systems underpinning stress and attention undergo a sensitive period of development during infancy. Psychobiological theory emphasizes a dynamic pattern of context-dependent development, however, research has yet to examine how basal cortisol and attention dynamically covary across infancy in ecologically valid contexts. Thus, to address these gaps, we leveraged longitudinal, multilevel analytic methods to disentangle between- from within-person associations of hypothalamic–pituitary–adrenal (HPA) axis activity and executive attention behaviors across infancy. We use data from a large longitudinal sample (N = 1,292) of infants in predominantly low-income, non-urban communities at 7-, 15-, and 24-months of age. Using multilevel models, we investigated longitudinal associations of infant attention and basal cortisol levels and examined caregiving behaviors as moderators of this relationship. Results indicated a negative between- and within-person association between attention and cortisol across infancy and a within-person moderation by caregiver responsiveness. In other words, on the within-person level, higher levels of cortisol were concomitantly associated with lower infant attention across the first 2 years of life. However, variation in the caregiver’s level of responsiveness either buffered or sensitized the executive attention system to the negative effects of physiological stress.
Keywords: attention, cortisol, caregiving
Introduction
A wealth of literature has demonstrated that environments of socioeconomic disadvantage are associated with variation in cognitive abilities (Brooks-Gunn & Duncan, 1997), including the development of attention and executive functions (Blair & Raver, 2012b). One pathway by which the environment is thought to affect cognitive development is through the stress response, often measured physiologically through the hormone cortisol. Research has found cross-sectional and between-person associations between physiological stress and cognition in childhood (Blair et al., 2011; Davies, Sturge-Apple, Cicchetti, & Cummings, 2007; Finegood, Wyman, O’Connor, & Blair, 2017; Suor, Sturge-Apple, Davies, Cicchetti, & Manning, 2015). However, the neurobiological systems supporting attention, and the neurobiological systems supporting physiological response to stress undergo a sensitive period of development during infancy (Gunnar & Quevedo, 2007; Hensch, 2005). Psychobiological theories of development posit that attention in infancy is fundamentally a dynamic, adaptive, and context-dependent cognitive process that is organized through reciprocally interacting social and physiological demands (Feldman, 2007; Gottlieb, 1998; Vygotsky, 1979). As such, the dynamic and adaptive nature of attention development is best illustrated by modeling intraindividual associations of attention and physiological stress over time using within-person longitudinal methods (Hoffman & Stawski, 2009).
Attention
The development of attention in infancy is critical in supporting higher-order cognitive abilities in childhood (Casey & Richards, 1988; Ruff, 1986; Swingler, Perry, & Calkins, 2015). For instance, infants learn to selectively focus and sustain their attention to stimuli in their environment to support the volitional control of behavior (Rothbart & Rueda, 2005; Ruff & Capozzoli, 2003). Executive attention is a domain of attention delineated under Posner’s taxonomy of attention which refers to the specific cognitive process of volitional, goal-directed attention. Executive attention differs from alerting and orienting attention, which are considered to be primarily more bottom-up, reactive forms of attention (Petersen & Posner, 2012; Posner & Petersen, 1990). By engaging top-down processes of executive attention, infants are better able to resolve internal or external conflicts related to orienting and alerting responses to stimulation (Posner & Rothbart, 2007; Rosen, Hagen, et al., 2019; Rothbart, Sheese, Rueda, & Posner, 2011). These core executive attention abilities involving selective attention and conflict resolution are foundational for more complex self-regulation abilities such as executive function and emotion regulation (Brandes-Aitken, Braren, Swingler, Voegtline, & Blair, 2019).
Neurobiological research suggests a model of attention in infancy which is thought to be supported by the early emerging, yet protracted, development of the prefrontal cortex (PFC) (Posner & Rothbart, 2007). These biological underpinnings of attention are highly plastic in the first years of life, and as such are especially susceptible to environmental influence (Cerqueira, Mailliet, Almeida, Jay, & Sousa, 2007; Grossmann, 2013; Hodel, 2018). Previous studies have documented associations between environments of heightened risk and decreased attention abilities within the first year of life (Clearfield & Jedd, 2013; Lipina, Martelli, Vuelta, & Colombo, 2005). While this and similar research highlights attention as a particularly malleable and foundational cognitive process, further research is needed to identify the operating neurobiological mechanisms to better understand how environments shape attention beginning in infancy.
HPA physiology and cognition in childhood
Research has established that environmental risk, such as in conditions of poverty, can “get under the skin” via stress physiology to shape infant cognitive development (Blair & Raver, 2016; Hackman & Farah, 2009; Lipina & Posner, 2012). The hypothalamic–pituitary–adrenal (HPA) axis is a core physiological stress system which adaptively responds to environmental demands. Over the first years of life the HPA axis activity, and resulting cortisol levels, demonstrates rapid developmental flux (Gunnar & Donzella, 2002). The neonatal period is marked by relatively high levels of biologically active cortisol which generally decreases across the first year of life. By 12–24 months of age the HPA axis begins to follow a more mature pattern of activity. As such, experiences during infancy are critical for stress physiology development as research has shown that the HPA axis is strongly regulated by the social environment during this time (Gunnar & Donzella, 2002; Gunnar & Quevedo, 2008). Thus, the emerging infant HPA axis, and resulting basal cortisol levels, are thought to be the product of social programming from caregivers. In this way, the HPA axis is similar to the development of the PFC, which is thought to be especially susceptible to environmental influence early in life (Blair & Raver, 2016; Boyce & Ellis, 2005; Gunnar & Quevedo, 2007). For instance, individuals exposed to environments of stress during infancy tend to show longitudinal alterations of the HPA axis across development (VanTieghem & Tottenham, 2018). Early experiences that are unsupportive or highly chaotic are thought to result in repeated, chronic activation of the HPA axis to increase vigilance and responsiveness to a potentially threatening or uncontrollable environment. Evolutionary psychobiological theory posits that environmental stress-induced HPA axis sensitization likely confers short-term adaptive advantages by allowing the individual to respond to unpredictable and threatening environmental demands and challenges; however, long-term chronic HPA axis activity can result in wear in tear in the body and brain (McEwen, 1998; McEwen & Gianaros, 2011). It has been proposed that repeated exposure to environmental stress, particularly early in life, may lead to differences in resting cortisol levels, which, over time, may impact neurocognitive development (Blair et al., 2011; Perry et al., 2019; Suor et al., 2015).
The volitional control of attention, referred to here as “executive attention,” is a specific PFC-dependent cognitive process that is also modulated by environmental (Callaghan & Tottenham, 2016b; Gee et al., 2013) and physiological stress (Arnsten, 2009; Liston, McEwen, & Casey, 2009). Notably, executive attention is uniquely supported by the PFC. The modulatory influence of physiological stress on executive attention can be explained in part by the structural and functional neurobiological linkage between the HPA axis and the PFC (Perry, Finegood, Braren, & Blair, 2018). Cross-species research supports that the HPA axis is highly conserved across mammalian species and regulates extra hypothalamic structures, including the PFC (Herman & Tasker, 2016). Specifically, corticosteroid activity functionally connects the HPA axis to the PFC via glucocorticoid receptors (GR) which are particularly abundant in the medial PFC (Dedovic, Duchesne, Andrews, Engert, & Pruessner, 2009). Furthermore, the HPA axis coordinates with the PFC to regulate and respond to environmental demands (Arnsten, 2009; Pruessner et al., 2010). In particular, through the binding of corticosteroids to GR in the PFC, HPA axis activation in part modulates PFC-dependent top-down processes such as attention (Sullivan & Gratton, 2002).
Both the PFC and HPA axis demonstrate disproportional plasticity and environmental susceptibility during the first years of life (Hensch, 2005). As such, across infancy and childhood, PFC development and resulting executive attention processes are programmed by HPA axis activity. Over time, greater exposure to stress across development biases the HPA–PFC network to be more reactive to stimulation (Boyce & Ellis, 2005). As such, fluctuations in cortisol over time can regulate the development of cognitive control processes from infancy into childhood via HPA–PFC activity (McEwen, 2006). Existing research has demonstrated that chronically elevated basal cortisol reported from individuals living in low-income environments is associated with lower executive functioning and perceptual reasoning skills in children (Blair et al., 2011; Finegood et al., 2017; Suor et al., 2015). Despite the well-known plasticity of the infant HPA axis and attention systems, limited research has examined connections between basal cortisol and early emerging attention abilities in infancy. However, attention is one of the earliest developing cognitive abilities and is foundational for most, if not all, aspects of later behavior (Posner & Rothbart, 2007). Thus, examining how fluctuating cortisol levels across early life associates with emergent executive attention is key to understanding how the environment shapes infant attention development and later behavior.
Role of the caregiver in buffering stress
Caregivers are critical for regulating and guiding early infant cognitive abilities; thus, it is likely that aspects of Caregiver × Infant interactions contribute to the variability observed in correlations between stress and cognition (Bernier, Carlson, & Whipple, 2010; Wass et al., 2018). Behavioral research has found evidence suggesting that caregivers can promote cognitive development in children with a history of early-life stress (Afifi & Macmillan, 2011; DuMont, Widom, & Czaja, 2007; Troller-Renfree, McDermott, Nelson, Zeanah, & Fox, 2015), suggesting that caregivers can potentially offset the negative effects of early-life stress. Specifically, it has been shown that caregiving can buffer the effects of acute stress on cognitive processes in children (Roos et al., 2019). Caregiver responsiveness is a particular parenting behavior associated with reduced effects of physiological stress on cognitive processes (Callaghan & Tottenham, 2016a; Hostinar, Sullivan, & Gunnar, 2014; Perry, Blair, & Sullivan, 2017). Findings suggest that caregiver responsiveness buffers the effects of chronically elevated cortisol by downregulating the infant’s emotional arousal and reactivity, thus promoting top-down control in real time. The mechanism of this effect is related to the two types of corticosteroid receptors in the brain, GR and mineralocorticoid receptors (MR). GRs are less sensitive to (have a lower affinity for) corticosteroids than MRs and are largely unoccupied at low levels of stress. With increasing stress and moderate corticosteroid increase, however, GR occupation increases and synaptic long-term potentiation is facilitated. However, increases in corticosteroids beyond a moderate level, indicating increasingly high GR occupation, are associated with synaptic long-term depression rather than long-term potentiation (de Kloet, Oitzl, & Joëls, 1999; Erickson, Drevets, & Schulkin, 2003). In this way, the relation between the top-down control of attention and the stress response is best described by an inverted U-shaped curve (Arnsten, 2009; Blair, 2010). As such, responsive, sensitive caregiving can support the regulation of the stress response in ways that facilitate the top-down control of attention by avoiding chronic elevations of cortisol that will lead to increased occupation of GRs. Despite evidence that infant stress and attention systems are influenced by caregivers, to our knowledge, no study has investigated prospective longitudinal associations among the activity of the HPA axis, caregiving behaviors, and the development of executive attention across infancy to toddlerhood.
Between-person versus within-person models
Psychobiological theory posits that individual development unfolds across time through a system of bidirectional interacting levels, from the social to the biological (Gottlieb, 1997, 1998; Vygotsky, 1979). According to this theory of change, infant attention is modulated by reciprocally interacting processes at the physiological and caregiving level within the infant’s individual ecological system. In order to empirically test theories of the dynamic and systemic relations between physiological stress, caregiving, and attention development, longitudinal study designs can be leveraged to disentangle between- and within-person processes of change. Traditionally, much of the longitudinal research on child development has used analytic methods that confound within- and between-person effects (Berry & Willoughby, 2017; Hoffman & Stawski, 2009; Voelkle, Brose, Schmiedek, & Lindenberger, 2014). Specifically, the existing research linking stress to cognitive abilities is largely based on between-person findings, which suggest that, on average, children with chronically elevated cortisol tend to demonstrate lower cognitive abilities than children with lower cortisol (Blair et al., 2011; Finegood et al., 2017; Suor et al., 2015). In contrast, within-person relations can indicate how an individual’s cognitive abilities change in relation to their own changing levels of cortisol. These are two empirically distinct questions and far fewer studies have investigated stress and attention relations on the within-person level. Within-person analyses describe the dynamics of change within an individual and are thus essential to the study of development. Thus, the plastic, yet interrelated nature of both the stress physiology system and the executive attention system are well suited to be investigated on the intraindividual level.
Present study
The aim of the present study was to examine concurrent and longitudinal between-person and within-person relations between early-life fluctuations in cortisol and changes in observed executive attention behaviors in a sample composed of predominantly low-income families. Using a large longitudinal sample of families living in largely rural, low-income communities, we sought to disentangle the between- and within-person relations between basal cortisol levels and executive attention behaviors as they occur in the home environment. In doing so, we aimed to test the theory that attention processes are adaptively calibrated and dynamically modulated by physiological demands, specifically, basal cortisol levels across the first two years of life. In addition, based on existing literature documenting the biobehavioral effects of social buffering, we wanted to test the extent to which caregiver responsiveness moderated the longitudinal association between cortisol and attention.
Specifically, we investigated three primary research questions. (a) Between children, do those who experience higher levels of basal cortisol across infancy demonstrate lower concurrent executive attention than those who experience lower levels of basal cortisol across early development? We hypothesized a negative relation, such that children experiencing higher levels of cortisol between 7–24 months of age would have lower attention relative to their peers who experience lower levels of cortisol. (b) Are within-person changes in basal cortisol predictive of concurrent changes in infant’s executive attention? Similar to our between-person hypothesis, we hypothesized that intraindividual change in cortisol across infancy would be negatively associated with attention in infancy. (c) Does caregiver responsiveness moderate the association between basal cortisol and attention on the within-person level? We hypothesized that high within-person caregiver responsiveness would weaken the negative association between cortisol and attention. Moreover, we wanted to test for these associations while controlling for extraneous variables that could confound the effect of either infant cortisol or caregiving on observed attention behaviors. Previous research suggests that infant temperament, physical materials in the home, and broader family dynamics play potentially confounding roles in influencing observed infant attention, cortisol levels, and caregiving behaviors as measured in the home. As such, we included multiple covariate measures in our models to control all relevant confounding variables.
Method
Participants
The Family Life Project (FLP) is a prospective longitudinal study of families residing in six low-wealth counties in Eastern North Carolina and Central Pennsylvania (three counties per state) that were selected to be indicative of the Black South and Appalachia, respectively. The FLP adopted a developmental epidemiological design whereby complex sampling procedures were used to recruit a representative sample of 1,292 children whose families resided in one of the six counties at the time of the child’s birth. Detailed descriptions of the participating families and communities are available in Vernon-Feagans and Cox (2013).
Procedures
The data for this analysis were collected in participants’ homes at child ages 7, 15, and 24 months. During each home visit, primary caregivers provided information on demographics. The majority of primary caregivers enrolled in this study were the target child’s biological mother (97.9%). Biological fathers (0.4%), grandparents (1.1%), or other adults (foster parent, aunt/uncle, unrelated adult, adult sibling; combined 0.5%) comprised the rest of the primary caregivers in this sample. The average age of the caregivers during the first wave of data collection was 26.2 years (+/−6.35). Throughout the visit, trained research assistants conducted observations of caregiver responsiveness. In addition, at each time point, children and their primary caregivers participated in a book reading task from which observational indicators of infant attention were derived. Immediately following the home visits, research assistants (RAs) completed ratings of the children’s attention during the 2–3 hours of the data collection period. Further, at 7-, 15-, and 24-month visits, basal cortisol was assessed after data collectors had been in the home for at least one hour.
Measures
Cortisol
Infant resting cortisol levels were assayed from a saliva sample collected near the end of the 7-, 15-, and 24-month home visits for data collection (approximately 2 h duration). Samples were collected after data collectors had been in the home for a minimum of one hour, allowing the infants’ cortisol levels sufficient time to return to baseline following the arrival of the data collectors. Whole saliva was collected using cotton or hydrocellulose absorbent material and expressed into 2 mL cyrogenic vials using a needleless syringe (from cotton) or by centrifuge (from hydrocellulose). Prior studies have indicated no differences in cortisol concentrations associated with the two collection techniques (Granger et al., 2007; Harmon, Hibel, Rumyantseva, & Granger, 2007). Samples were then immediately placed on ice and stored frozen (−20°C). All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μl of saliva, had a range of sensitivity from 0.007 to 3.0 μg/dl, and average intra- and inter-assay coefficients of variation less than 10% and 15%, respectively. Samples were assayed in duplicate and the average of duplicates was used in all analyses.
Because families were seen at times that were convenient for them, time of day of saliva collection varied between families, although most families were seen in the afternoon. Time of day of saliva collection was regressed on cortisol to control for time of day and the residualized score was used. Natural log transformations were applied to the cortisol values to correct for positive skew. Cortisol values greater than ±3 SD after transformation were excluded from analyses. While the above methodological precautions are helpful, they do not completely protect our cortisol measurement from the diurnal effects on cortisol values. Thus, to explore morning/evening basal cortisol shifts, we conducted a sensitivity analysis with only subjects whose saliva was sampled between 10.00 a.m. and 4.00 p.m. at all three data collection points (see supplemental section). As the results did not differ substantively, we present these findings, as well as information about the range of saliva collection times, in the supplemental section.
Executive attention behaviors
Executive attention behaviors were assessed at 7-, 15-, and 24-months of age with the Infant Behavior Record (IBR; Bayley, 1969) as adapted for use by Stifter and Corey (2001) and completed independently by both RAs. The IBR was applied to infant behavior observed globally across the entire (2–3 h) home visit for RAs. The measure is intended to capture infant executive attention behaviors as they are observed in their natural home environment. The IBR consists of 11 items rated on a 9-point scale, with higher scores indicating greater attention skills. Three items were used in the current analysis to index child’s global attention: attention to objects, which assessed the degree to which the child demonstrated sustained interest in toys, test materials, or other objects (a score of 1 indicated the child did not look at or in any way indicate interest in objects, whereas a score of 9 indicated sustained interest in objects, to the point at which they were reluctantly relinquished); attention to activities, which assessed the child’s persistence in attending to activities with toys, objects or persons (a score of 1 indicated the child showed a fleeting attention span, whereas a score of 9 indicated long-continued absorption); and overall attention, which assessed the child’s attention across the demands of the home visit (a score of 1 indicated the child tired easily and quickly regresses to lower levels of functioning, whereas a score of 9 indicated that the child continued to respond well and with interest, even during pro-longed tasks at difficult levels). The mean of both home visitors’ ratings was used for each item; intra-class correlations ranged from 0.66 to 0.80. The final global executive attention score was calculated by summing the means across RAs for the three attention-related items. This attention behavior most accurately represents the executive domain of attention as it reflects the top-down and goal-directed aspects of attention (versus the more reactive alerting and orienting domains; Posner & Rothbart, 2007).
Caregiver responsiveness
To assess primary caregiver responsiveness, RAs scored the Home Observation for the Measurement of the Environment (HOME; Bradley, 1994) at 7-, 15-, and 24- months. The HOME consists of 28 items where higher scores represent higher quality and quantity of stimulation and support available in the home environment. The subscale of the HOME used in the current analysis is caregiver responsiveness (“Caregiver responds verbally to child’s vocalizations or verbalizations”). This subscale includes 11 items and each item is scored in binary fashion (yes/no). Information used to score the items is obtained during the course of the home visit by means of observation and semi-structured interview. Cronbach’s α for the total score of the caregiver responsiveness subscale was 0.75. The final caregiver responsiveness score was calculated by taking the average of all items on the subscale.
Covariates
Socioeconomic risk
To control for socioeconomic status (SES)-related risk factors on attention we include a composite measure of cumulative risk by creating an aggregate variable composed of multiple measures of poverty-related risk to encompass the many contributing environmental factors that are associated with living in poverty at 7-, 15-, and 24-months. Given the high likelihood of co-occurrence between risk factors associated with poverty and the difficulty in parsing them from each other, a cumulative risk model can better encompass the multidimensional nature of poverty-related risk. Thus, as with prior FLP data (see Vernon-Feagans & Cox, 2013), we created a cumulative risk index of seven measures collected at each time point: family income-to-needs ratio, maternal education, consistent partner, hours of employment, occupational prestige, household density, and neighborhood noise and safety (see Table 1 for descriptive statistics). These variables were chosen as indicators of social and economic resources that previous research has demonstrated are significantly related to the context of poverty, especially in rural communities (Burchinal, Roberts, Hooper, & Zeisel, 2000; Dill, 1999; Evans, 2004; Vernon-Feagans & Cox, 2013). Principal components analysis confirmed that these seven indicators each loaded significantly onto a single factor (Vernon-Feagans & Cox, 2013). The SES risk index was calculated by z-scoring each variable, reverse-scoring positively framed variables, and averaging the seven factors. For the current analysis, we used the cumulative risk scores to obtain a variable of early-life exposure to SES risk.
Table 1.
Descriptive statistics
| N | Mean | St. Dev. | Min | Median | Max | |
|---|---|---|---|---|---|---|
| Infant attention, 7 months | 1,193 | 17.68 | 2.47 | 4.50 | 18.00 | 24.00 |
| Infant attention, 15 months | 1,155 | 17.71 | 2.70 | 4.00 | 18.00 | 23.50 |
| Infant attention, 24 months | 1,116 | 17.63 | 3.15 | 4.50 | 18.25 | 24.25 |
| Infant age, 7 months | 1,201 | 7.73 | 1.48 | 5.03 | 7.56 | 15.38 |
| Infant age, 15 months | 1,169 | 15.75 | 1.35 | 13.50 | 15.38 | 22.34 |
| Infant age, 24 months | 1,144 | 24.89 | 1.95 | 22.24 | 24.31 | 35.38 |
| Infant cortisol, 7 months | 1,122 | −1.84 | 0.77 | −3.91 | −1.93 | 1.54 |
| Infant cortisol, 15 months | 1,007 | −1.95 | 0.84 | −4.96 | −2.09 | 1.59 |
| Infant cortisol, 24 months | 954 | −2.05 | 0.80 | −4.83 | −2.13 | 1.55 |
| Caregiver responsiveness, 7 months | 1,182 | 0.81 | 0.20 | 0.09 | 0.82 | 1.00 |
| Caregiver responsiveness, 15 months | 1,155 | 0.87 | 0.17 | 0.09 | 0.91 | 1.00 |
| Caregiver responsiveness, 24 months | 1,104 | 0.90 | 0.16 | 0.09 | 1.00 | 1.00 |
| Socioeconomic risk, 7 months | 1,201 | 0.01 | 0.66 | −2.24 | 0.01 | 1.98 |
| Socioeconomic risk, 15 months | 1,169 | 0.01 | 0.67 | −2.75 | 0.07 | 2.00 |
| Socioeconomic risk, 24 months | 1,144 | 0.01 | 0.65 | −2.57 | 0.05 | 2.17 |
| Family conflict, 7 months | 980 | 1.48 | 0.65 | 0.00 | 1.42 | 4.29 |
| Family conflict, 15 months | 932 | 1.38 | 0.61 | 0.00 | 1.28 | 3.88 |
| Family conflict, 24 months | 904 | 1.36 | 0.60 | 0.00 | 1.29 | 3.99 |
| Learning materials, 7 months | 1,179 | 0.84 | 0.21 | 0.00 | 0.89 | 1.00 |
| Learning materials, 15 months | 1,150 | 0.86 | 0.24 | 0.00 | 1.00 | 1.00 |
| Learning materials, 24 months | 1,072 | 0.89 | 0.18 | 0.00 | 1.00 | 1.00 |
| Infant reactivity, 7 months | 1,193 | 5.59 | 0.90 | 1.00 | 5.50 | 9.00 |
| Infant reactivity, 15 months | 1,155 | 6.03 | 0.96 | 2.00 | 6.00 | 9.00 |
| Infant reactivity, 24 months | 1,116 | 6.02 | 0.92 | 1.50 | 6.25 | 8.25 |
| Economic stress, 7 months | 1,188 | 2.29 | 0.70 | 1.00 | 2.17 | 4.33 |
| Economic stress, 15 months | 1,159 | 2.25 | 0.70 | 1.00 | 2.17 | 4.33 |
| Economic stress, 24 months | 1,125 | 2.19 | 0.70 | 1.00 | 2.17 | 4.33 |
| Saliva sample time of day (hh:mm), 7 months | 1,133 | 13:31 | 2:52 | 8:11 | 13.21 | 20:08 |
| Saliva sample time of day (hh:mm), 15 months | 1,070 | 13:57 | 2:54 | 8:45 | 13:43 | 20:24 |
| Saliva sample time of day (hh:mm), 24 months | 1,014 | 13:48 | 3:12 | 8:20 | 13:30 | 20:46 |
Perceived economic stress
To control for the effects of caregiver economic-related perceived stress, The Economic Strain Questionnaire (Conger & Elder, 1994) was completed by mothers at 7-, 15-, and 24-months. The questionnaire was modified from the Conger and Elder’s (1994) larger construct of economic pressure. This questionnaire is a six-item index assessing the degree to which families are able to make ends meet and the degree to which there is enough money in the household for bills, clothing, food, and medical care. Reliability for the measure was acceptable at 7, 15, and 24 months (Cronbach’s α ≥.81). Scores were rated on a Likert-type scale (range 1–5) and averaged across the six items. Higher scores indicated that families reported experiencing greater perceived economic stress.
Observed infant reactivity
To control for the potential confounding influence of an infant’s overall level of arousal and behavioral reactivity, we included a variable for observed global infant reactivity in our model. Infant reactivity was coded from the same IBR assessment that the infant attention measure was derived from at 7-,15-, and 24-months. One item from the assessment was used to index reactivity to assess the ease with which the infant is stimulated to react positively or negatively during the visit (a score of 1 indicated the child showed an unreactive pattern of behavior, whereas a score of 9 indicated a very reactive pattern of behavior).
Home learning materials
To control for the effect of home learning materials quality and quantity on infant’s attention behavior to objects in the environment, we included a learning materials variable taken from a subscale the HOME assessment. At 7-, 15-, and 24- months RAs assessed and coded for the quality and quantity of learning materials available in the child’s home (“Complex eye–hand coordination toys”). This subscale was made up of nine items and each item was scored in binary fashion (yes/no). The final home learning materials score was calculated by taking the average of all items on the subscale.
Family conflict and relations
To control for the potentially confounding effect of family conflict dynamics on infant cortisol and attention, the Conflict Tactics Scale (CTS; Straus, Hamby, Boney-McCoy, & Sugarman, 1996) was administered at 7-,15-, and 24-months to the primary caregiver. Caregivers reported their own use to their partner and also their partner’s use toward them of verbal aggression, physical aggression, and verbal reasoning in the past 12 months. A composite score of family conflict was created by averaging across subscales from the CTS.
Demographics
State of residence (PA = 0; NC = 1), sex (0 = male; 1 = female) and race (0 = not African American; 1 = African American) of the child were included as covariates to control for site and demographic differences in study variables.
Cortisol covariates
We assessed associations between infant cortisol and several factors known to influence basal cortisol levels. Namely we evaluated potential associations between cortisol, time since last feeding, time since last nap, caregiver tobacco usage, and mother or infant medication usage. We evaluated correlations for continuous variables and independent samples t tests for categorical variables. None of the variables demonstrated statistically significant associations and thus were not included in the final model to reduce model complexity (see supplemental section).
Missing data
The total sample size recruited at study entry was 1,292 with 1,204 children seen at age 7 months, 1,169 at 15 months, and 1,144 at 24 months. Participants were included in the analysis if the child had at least one attention rating at each agex (N = 1,073). This decline represents a 5% attrition rate between 6 and 24 months. We tested for selective attrition over time on the basis of key independent variables. We found no evidence of selective attrition over time on the basis of child cortisol; however, there was evidence of selective attrition based on caregiver responsiveness (t = −2.1; p = .04). As a robustness check, we ran a secondary model in which children missing attention data at 15 or 24 months were excluded (N = 108). The findings were substantively identical and we therefore report findings based on the less restricted sample. All multilevel models were fitted using maximum likelihood estimation.
Data analysis
To test our hypotheses, we constructed a two-level mixed model with random intercepts and a random slope for the cortisol. Our time-varying Level 1 predictors were group-mean (i.e., within-person) centered and our time-invariant Level 2 predictors were grand-mean (i.e., between-person) centered. At Level 1, we modeled infant executive attention (7, 15, and 24 months of age) as the dependent variable and all time-variant variables as within-person independent predictors. At Level 2, we entered all between-person time-invariant variables. Level 1 describes the within-person changes in attention and Level 2 describes the between-person differences in attention. To test for a between- and within-person moderation of the association between stress physiology and attention we included an interaction term of caregiver responsiveness and cortisol on Level 1 and Level 2. Our multilevel model is represented as the following:
| Level 1: |
| Level 2: |
On Level 1 Attentionti is the outcome at time t for person i that describes within-person variation in executive attention. Group-mean variables are entered as main effects on the Level 1 model. The Level 2 model describes between-person variation in average executive attention across the three time points where b00 refers to the grand mean of the sample, and α0i is a person-specific random intercept. Grand mean variables are entered as main effects in the Level 2 model.
To interpret the interaction, we used simple slopes analyses to evaluate effects at high and low levels of caregiver responsiveness. Specifically, within-person effects were tested at high (grand mean + 1) and low (grand mean - 1 SD) levels, to assess whether the within-person effect of cortisol differed in magnitude or directionality for those who experience high versus low levels of caregiver responsiveness. All models were fitted using continuous variables: the simple slopes are interpreted as conditional relations estimated from these models at high and low values in the (within-person average) cortisol distribution. All analyses were conducted in the R environment (R Core Team, 2013). The R code used for our model is included in the supplemental section.
Results
Descriptive statistics
Descriptive statistics for all key variables used in the analysis at each time point are displayed in Table 1 and rank-order correlations are shown in Table 2. Infant’s executive attention behavior demonstrated statistically significant correlations across time ranging from .16 to .29. Cortisol levels demonstrated small correlations across time that ranged from .03 to .10, suggesting little stability over time. In addition, caregiver responsiveness remained relatively stable over time, with correlations ranging from .21 to .36. Cortisol was negatively correlated with attention at each time point, although the correlations were relatively small (ranging from .04 to .11).
Table 2.
Computed Pearson correlations between variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | 13. | 14. | 15. | 16. | 17. | 18. | 19. | 20. | 21. | 22. | 23. | 24. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Infant attention, 7 months | ||||||||||||||||||||||||
| 2. Infant attention, 15 months | 0.19 | |||||||||||||||||||||||
| 3. Infant attention, 24 months | 0.15 | 0.35 | ||||||||||||||||||||||
| 4. Infant cortisol, 7 months | 0.05 | 0.03 | −0.02 | |||||||||||||||||||||
| 5. Infant cortisol, 15 months | −0.02 | −0.14 | −0.12 | 0.03 | ||||||||||||||||||||
| 6. Infant cortisol, 24 months | 0.03 | −0.08 | −0.15 | 0.03 | 0.10 | |||||||||||||||||||
| 7. Family conflict, 7 months | 0.08 | −0.12 | −0.07 | 0.01 | 0.02 | 0.01 | ||||||||||||||||||
| 8. Family conflict, 15 months | 0.01 | −0.15 | −0.05 | 0.00 | 0.08 | 0.02 | 0.66 | |||||||||||||||||
| 9. Family conflict, 24 months | −0.03 | −0.12 | −0.05 | −0.01 | 0.01 | 0.03 | 0.56 | 0.63 | ||||||||||||||||
| 10. Caregiver responsivity, 7 months | 0.11 | 0.10 | 0.04 | −0.02 | −0.05 | −0.02 | 0.01 | 0.01 | 0.02 | |||||||||||||||
| 11. Caregiver responsivity, 15 months | 0.06 | 0.19 | 0.07 | −0.09 | −0.03 | −0.10 | −0.05 | −0.10 | −0.11 | 0.36 | ||||||||||||||
| 12. Caregiver responsivity, 24 months | −0.00 | 0.08 | 0.20 | 0.01 | 0.01 | −0.08 | −0.13 | −0.12 | −0.09 | 0.21 | 0.22 | |||||||||||||
| 13. Learning materials, 7 months | 0.11 | 0.04 | 0.08 | −0.03 | −0.07 | −0.07 | −0.04 | −0.03 | −0.04 | 0.37 | 0.23 | 0.20 | ||||||||||||
| 14. Learning materials, 15 months | −0.03 | 0.10 | 0.06 | 0.02 | −0.05 | 0.01 | −0.06 | −0.07 | −0.08 | 0.22 | 0.34 | 0.09 | 0.31 | |||||||||||
| 15. Learning materials, 24 months | 0.00 | 0.04 | 0.09 | −0.05 | −0.01 | −0.12 | −0.11 | −0.12 | −0.10 | 0.10 | 0.12 | 0.26 | 0.17 | 0.19 | ||||||||||
| 16. Infant reactivity, 7 months | 0.54 | 0.20 | 0.11 | 0.05 | 0.02 | −0.02 | −0.03 | −0.07 | −0.12 | 0.18 | 0.14 | 0.05 | 0.05 | −0.01 | 0.01 | |||||||||
| 17. Infant reactivity, 15 months | 0.05 | 0.19 | 0.03 | 0.01 | −0.06 | −0.09 | −0.04 | −0.04 | −0.06 | 0.13 | 0.32 | −0.02 | 0.16 | 0.25 | 0.02 | 0.05 | ||||||||
| 18. Infant reactivity, 24 months | 0.17 | 0.15 | 0.46 | 0.07 | −0.07 | −0.03 | −0.07 | −0.08 | −0.02 | −0.04 | 0.03 | 0.24 | 0.01 | 0.01 | 0.11 | 0.18 | 0.04 | |||||||
| 19. SES, 7 months | −0.07 | −0.12 | −0.13 | 0.05 | 0.11 | 0.10 | 0.09 | 0.13 | 0.17 | −0.35 | −0.37 | −0.32 | −0.41 | −0.34 | −0.27 | −0.10 | −0.21 | −0.10 | ||||||
| 20. SES, 15 months | −0.05 | −0.07 | −0.13 | 0.04 | 0.13 | 0.13 | 0.11 | 0.16 | 0.15 | −0.34 | −0.34 | −0.30 | −0.39 | −0.31 | −0.24 | −0.07 | −0.20 | −0.14 | 0.92 | |||||
| 21. SES, 24 months | −0.06 | −0.11 | −0.15 | 0.04 | 0.10 | 0.17 | 0.15 | 0.19 | 0.16 | −0.34 | −0.33 | −0.33 | −0.36 | −0.30 | −0.24 | −0.09 | −0.19 | −0.13 | 0.87 | 0.92 | ||||
| 22. Economic stress, 7 months | −0.03 | −0.02 | −0.01 | 0.03 | −0.03 | −0.03 | 0.18 | 0.16 | 0.21 | −0.07 | −0.06 | −0.14 | −0.12 | −0.06 | −0.04 | 0.01 | −0.07 | −0.05 | 0.32 | 0.33 | 0.31 | |||
| 23. Economic stress, 15 months | −0.04 | 0.04 | −0.03 | −0.01 | −0.03 | 0.06 | 0.15 | 0.21 | 0.26 | −0.07 | −0.08 | −0.12 | −0.17 | −0.13 | −0.06 | −0.03 | −0.15 | −0.07 | 0.30 | 0.33 | 0.32 | 0.58 | ||
| 24. Economic stress, 24 months | −0.04 | −0.01 | −0.05 | −0.04 | −0.01 | −0.02 | 0.19 | 0.20 | 0.25 | −0.05 | −0.08 | −0.14 | −0.09 | −0.12 | −0.07 | 0.01 | −0.13 | −0.05 | 0.26 | 0.32 | 0.31 | 0.59 | 0.63 | |
SES = socioeconomic status.
Cortisol levels and executive attention across infancy
Between-person relations: Multilevel model results revealed a negative between-person relation between cortisol and attention (see Table 3). Specifically, children with higher mean levels of cortisol (relative to other children) across the first two years of life demonstrated lower attention on average (relative to other children). There was no association between average levels of caregiver responsiveness across all caregivers with levels of attention across all children. These between-person effects remained after controlling for all time-varying and time-invariant covariates. Specifically, on the between-person level, the model also demonstrated a significant positive association between infant reactivity and average infant attention, and a negative association between family conflict and average infant attention.
Within-person relations: Within-person relations in our multilevel model revealed a main effect of cortisol on attention, such that an infant’s cortisol level (relative to each infant’s own mean) was negatively associated with concurrent infant’s attention (see Table 3). In other words, when infants had higher levels of cortisol (relative to their own mean cortisol), they had simultaneously lower levels of attention (relative to their own mean attention). In addition, results showed a positive main effect of caregiver responsiveness on infant attention. In other words, increases in caregiver’s own level of responsiveness towards their infant was associated with contemporaneous increases in attention for that child. All within-person effects remained significant after controlling for all time-varying and time-invariant control covariates. In particular, the model demonstrated a significant within-person positive association with infant reactivity and attention.
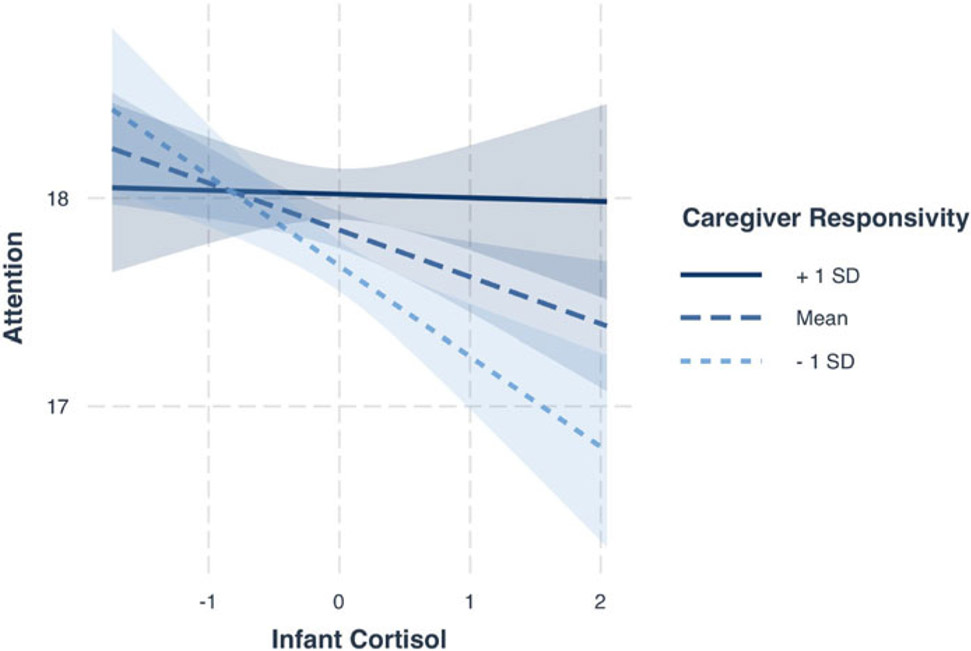
Level 1 interaction: moderation by responsive caregiving. As hypothesized, there was evidence of an interaction between within-person cortisol levels and within-person caregiver responsiveness on attention such that the within-person association between cortisol and attention differed depending on level of caregiver responsiveness. Simple slopes analysis of the cortisol and caregiver responsiveness interaction are displayed in Figure 1. Analysis of simple slopes indicated that when a child’s caregiver demonstrated low responsiveness (compared to their own average), there was evidence of a negative within-person relation between cortisol and attention (group mean − 1 SD; blow_responsiveness = −.43, p < .001). In other words, when caregivers demonstrated lower relative responsiveness, their infant’s cortisol was negatively associated with attention. However, when caregivers demonstrated higher than average responsiveness, within-infant cortisol levels were not significantly associated with concurrent levels of attention (group mean + 1 SD; bhigh_responsiveness = −.02, p = .86).
Table 3.
Results from multilevel model predicting infant executive attention
| Executive attention (7, 15, 24 months) |
|||
|---|---|---|---|
| Predictors | Estimates | Std. Beta |
Std. Error |
| Within-person (WP) effects (Level 1) | |||
| WP infant cortisol | −0.21* | −0.04 | .017 |
| WP caregiver responsiveness | 1.38** | 0.07 | .019 |
| WP SES | 0.09 | 0.01 | .018 |
| WP infant reactivity | 0.77*** | 0.21 | .019 |
| WP home enrichment materials | −0.02 | −0.00 | .018 |
| WP family conflict | 0.04 | 0.01 | .018 |
| WP economic stress | 0.11 | 0.02 | .018 |
| WP infant age | −0.01 | −0.04 | .019 |
| WP infant cortisol × WP responsiveness | 1.66* | 0.04 | .020 |
| Between-person (BP) effects (Level 2) | |||
| BP infant cortisol | −1.11** | −0.06 | .021 |
| BP caregiver responsiveness | 1.09 | 0.05 | .025 |
| BP SES | −0.14 | −0.03 | .028 |
| BP infant reactivity | 1.40*** | 0.31 | .022 |
| BP home enrichment materials | 0.09 | 0.00 | .027 |
| BP family conflict | −0.21* | −0.04 | .021 |
| BP economic stress | 0.05 | 0.01 | .023 |
| BP sex | −0.20 | −0.04 | .021 |
| BP race | 0.30 | 0.05 | .029 |
| BP state | 0.15 | 0.03 | .027 |
| BP infant cortisol × BP responsiveness | 0.95 | 0.02 | .017 |
| Random effects | |||
| σ2 | 4.90 | ||
| ICC | 0.16 | ||
| N S_ID | 1,073 | ||
| Observations | 2,397 | ||
| Marginal R2/Conditional R2 | 0.171/0.301 | ||
Note: σ2, Random effect variances; ICC, Intraclass correlation coefficient.
p < .05
p < .01
p < .001.
Figure 1.
Within-person infant cortisol and caregiver responsiveness interact to predict infant executive attention at 7, 15, and 24-months. Executive attention scores were lowest for infants with high cortisol and low caregiver responsiveness scores. Attention coefficients are unstandardized estimates. Shaded regions around the line represent 95% confidence intervals.
Discussion
In the present study, we assessed the extent to which between- and within-person infant basal cortisol levels were associated with observed executive attention behaviors in the context of early-life poverty-related risk. Further, we investigated moderation of these relations by the responsiveness of the primary caregiver. This research was motivated by existing psychobiological theory and empirical evidence suggesting that the executive attention system is dynamically shaped by early experiences, specifically, by interactions between stress response physiology and caregiving behaviors (Blair & Raver, 2012a; Boyce & Ellis, 2005; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). Using data from a large, longitudinal sample of infant–caregiver dyads, we demonstrated that intraindividual variation in infant cortisol was negatively associated with attention across the infant and toddler period but that this relation was attenuated by higher levels of responsive caregiving. To the best of our knowledge, this is the first study to investigate within-person relations among attention, cortisol, and caregiving within the first two years of life.
On the between-person level, results indicated that, on average, children who experienced higher average cortisol levels across infancy tended to have lower attention relative to infants with lower average cortisol levels. These results are largely consistent with previous research in children demonstrating cross-sectional and longitudinal relations between physiological and environmental stress with global cognitive and behavioral outcomes, with this (Blair et al., 2011; Finegood et al., 2017) and other data-sets (Davies et al., 2007; Suor et al., 2015). Moreover, previous research has shown that in laboratory settings, children exposed to acute environmental stressors demonstrated concomitant decreases in task-based sustained attention abilities (Roos et al., 2019). Here we expand on the existing research by providing evidence for a longitudinal association between cortisol at rest and observed executive attention behaviors in infancy within an ecologically valid context (i.e., in the infant’s home). These relations support the idea that resting cortisol levels may regulate attention (and vice versa) as it occurs within the home environment. Importantly, these findings show that elevated cortisol within the first two years of life is associated with differences in attention before infants reach childhood. This has important broader implications given the research demonstrating that early development of attention is a foundational cognitive ability and early alterations may have lasting effects on higher order self-regulation development (Amso & Scerif, 2015; Brandes-Aitken et al., 2019; Posner & Rothbart, 2007).
Our main question of interest in the current study involved the within-person associations between infant cortisol and attention as evidence for the dynamic flexibility that occurs between the stress regulation system and executive attention system. We found evidence that within-person deviations in cortisol levels negatively predicted concomitant change in attention across three time points in infancy. Specifically, increases in within-person cortisol levels were associated with concurrently decreased within-person attention. These findings speak to the dynamic, interdependent nature of the HPA axis and cognitive regulatory system that co-develop across infancy into childhood. The within-person component of the current analysis is particularly important given that we are studying families living in low-income contexts. Caregivers living in poverty face greater psychosocial and economic stressors and instability. Thus, caregiving behaviors and stress exposure are likely less predictable and more variable. In this way, it is more informative to investigate the developmental consequences of relative shifts in caregiving and stress where the baseline average is the individual instead of the population. Studying intra-dyad co-fluctuations may elucidate how interactions between change in a parent’s responsive caregiving and a child’s stress physiology shape the infant’s own pattern of self-regulation over time (Bernier et al., 2010). Moreover, our model did not reveal any associations between SES and executive attention on either the within- or between-person level. This finding suggests that focusing on specific variables within infant’s environment (i.e., physiology and caregiving) may be a better predictor of variations in early attention processes than SES alone. In general, within person analyses de-emphasize global assumptions and conclusions about populations and instead focus on relative shifts within individual families themselves.
Neurobiologically, PFC function and development do not follow a fixed trajectory for every child, but instead adapt and respond to internal and external environmental demands (Werchan & Amso, 2017). Our within-person, longitudinal analysis highlights the dynamic and adaptive nature of PFC-dependent attention processes. Specifically, psychobiological theory describes a process in which multiple aspects of stress response physiology, including the HPA axis, are calibrated early in development by aspects of the environment in which development occurs (Blair & Raver, 2012a; Del Giudice, Ellis, & Shirtcliff, 2011; Ellis & Del Giudice, 2019). Given the relation between corticosteroid levels and synaptic activity in the PFC, the relation between attention and cortisol demonstrated here is best interpreted within the framework of psychobiological theory. Relatedly, in the Adaptive Calibration Model (ACM; Del Giudice et al., 2011), a central tenet is the adaptive nature of behaviors engendered by early environmental stress. Under the ACM framework, it is assumed that each individual occupies their own unique but dynamic ecological niche. In contexts characterized by early-life stress, the HPA–PFC circuitry may adapt to these conditions to be more reactive, translating behaviorally to less top-down cognitive regulation or more dispersed patterns of attention (Frankenhuis & Del Giudice, 2012). For example, an infant living in a low-income home experiencing early-life stress may demonstrate chronic activation of the HPA axis and consequently decreased executive attention and increased alerting and orienting attention. Behaviorally this may manifest as a cognitive orientation biased towards increased vigilance or impulsivity to detect threat and respond quickly to opportunity (Frankenhuis, Panchanathan, & Nettle, 2016). These early-life programming patterns may potentially set the individual’s developmental trajectory towards a more reactive rather than reflective self-regulation style. As such, future research could aim to study the longitudinal implications of linkage between early-life physiological stress and attention. Collectively, highlighting within-person change when characterizing the effects of stress on child development shifts the emphasis away from deficit-based models of adversity towards models that highlight adaptation, likely providing a more complete understanding of the complex relation between stress and development (Ellis, Bianchi, Griskevicius, & Frankenhuis, 2017).
Results from the significant within-person interaction show that individual increases in resting cortisol are associated with concomitant decreases in attention but that this association is buffered by caregiver responsiveness, where the unit of analysis is the infant in the context of the caregiver–infant dyad. For example, for a given infant, higher than typical levels of cortisol would not affect their executive attention system if their caregivers were demonstrating higher than typical responsive caregiving behaviors. However, for the same infant, if their caregiver was practicing lower than typical responsive caregiving, the child’s executive attention would decrease to a greater extent. However, causal inferences can’t be assumed from this study given the correlational nature of this study. While the use of a within-person longitudinal design and the inclusion of covariates such as child reactivity, home enrichment materials, family conflict, and SES improve our ability to draw inferences, causal conclusions are not possible. Therefore, future experimental research is needed to evaluate causality regarding the links between stress physiology, caregiving, and infant attention.
The present findings provide further empirical support for interactions among biology, context, and behavior. Our results suggest that the relation between an infant’s cortisol levels and their attention abilities was moderated by their caregiver’s responsiveness levels. The negative association between cortisol and attention was attenuated when caregivers displayed higher responsiveness and amplified when caregivers displayed lower responsiveness. One interpretation of this finding is that although chronically stressful environments may lead to heightened resting HPA axis activity, responsive caregiving may regulate attention processes through cognitive scaffolding pathways (Rosen, Hagen, et al., 2019). In theory, responsive caregiving enables the downregulation of heightened cortisol levels in the infant, allowing for reduced occupation of GR receptors (de Kloet et al., 1999) and thereby supporting the top-down regulation of attention. That is, despite having heightened HPA axis activity, responsive caregivers are able to modulate their child’s self-regulation abilities through the structured scaffolding of attention, leading to increased attention across infancy (Gunnar & Donzella, 2002; Rosen, Amso, & McLaughlin, 2019; Swingler et al., 2015; Wass et al., 2018). Conversely, children with elevated resting HPA-activity and less responsive caregivers may be sensitized to the cognitive effects of elevated resting cortisol levels due to a lack of caregiver regulation. These infants may be receiving less external social stress buffering and thus be more likely to experience hyper-arousal, which is known to impede attention functions (Arnsten, 2009). In addition, a prior study using an empirically validated attention training procedure found that infants in the attention training treatment group had lower cortisol at post-test than did infants in the control group (Wass, Cook, & Clackson, 2017). Prior research has also established that maternal support for infant attention through joint attention interactions has direct benefits for sustained attention in infancy (Yu & Smith, 2016). This research clearly demonstrates the importance of social context for the development of attention regulation. Complementary research from Roos et al. (2019) demonstrated in a sample of young children that lack of caregiver stress regulation increased their children’s vulnerability to the effects of acute stressors on their sustained attention abilities. Collectively, these findings suggest that caregiving is a dynamic and interactive component of an infant’s developmental context which likely influences their infant’s stress and cognitive regulation.
On a theoretical level, the within-person component of this analysis highlights the dynamic nature of an infant’s social milieu and the functional linkage of the stress and attention systems in the infant and toddler periods. One of the main strengths of the present analysis is the use of a within-person analytic design to empirically test the combined effect of social context and the stress response on the development of executive attention across the first 2 years of life. We illustrate the interdependence of an infant’s ecological system’s levels by demonstrating a statistical interaction between intraindividual cortisol and caregiver responsiveness on attention. Further, the within-person component of this analysis helps elucidate the processes of relational change between caregiving, physiology, and cognition. An inherent component of the within-person systems perspective is the capacity for change. As such, implications from these results emphasize the plasticity of an infant’s developmental system, which has translational relevance for interventions aiming to promote change (Wass, Scerif, & Johnson, 2012). Specifically, although this work is correlational, we can consider the possible implications of intervening to promote the regulation of infant stress physiology through caregiver interactions to enhance infant attention abilities. Potentially, interventions aimed to increase economic capital or reduce psychological stress among lower-income families may result in enhanced caregiver responsiveness and, thus, improved early infant attention development. Moreover, results from this research have the potential to inform clinical applications as well. Both cognitive-related and emotion regulation-related psychopathologies have been tied back to early alterations in infant stress and attention (Isaksson, Nilsson, & Lindblad, 2013; Morales, Fu, & Pérez-Edgar, 2016; Sullivan et al., 2015). Early-life programming of attention in infancy due to stress exposure may affect the trajectories and likelihood for onset of attention and anxiety disorders. In such, this research also has the potential to inform clinical interventions aimed at reversing the effects of early-life stress, as this study highlights the malleability and receptivity of attention systems to positive social influences. However, because the measure of attention used in the present study is standardized based on age, the range of within-person longitudinal change in attention is relatively small. Thus, the clinical interpretability of our findings remains limited.
Disentangling between- from within-person associations fundamentally changes the interpretation of our results. In the current study, we can distinguish how relative shifts in cortisol and parenting relate to contemporaneous shifts in attention (within-person), versus how chronic cortisol levels and parenting behaviors relate to overall attention behaviors averaged from 7–24 months across a population (between-person). Moreover, while we did not find a significant main effect for caregiver responsiveness or a significant interaction for caregiver responsiveness and cortisol on the between-person level, we did on the within-person level. This suggests that the population may not operate homogeneously with respect to how caregiving predicts attention and interacts with the effect of cortisol. In other words, families with highly responsive caregivers do not necessarily have children with greater executive attention (between-person); however, infant executive attention is enhanced by relative increases in responsive caregiving, within a given dyad (within-person). Similarly, while relative increases in caregiver responsiveness may buffer the effects of increased relative cortisol levels for an individual infant, higher responsive caregiving on average does not reduce the negative cortisol effect on average attention across the population. Conversely, one of our control variables, intrafamily conflict, did not reveal an association with attention on the within-person level but did on the between-person level. This suggests that higher levels of average family conflict from 7–24 months was associated with lower infant executive attention across the population. However, for a given infant, instances of increased intrafamily conflict did not concomitantly decrease that infant’s executive attention behavior. The interpretations drawn from between- and within-person levels of analysis have vastly different implications, which highlights the importance of disentangling the relative contributions of both analytic levels.
Limitations and Conclusions
Although this study has a number of strengths, there are several limitations that need to be addressed. Namely, our operationalization of executive attention is a relatively global measure that might be capturing other aspects of infant self-regulation outside of attention. Similarly, aspects of the home environment, such as the number of distractors, could be influencing how the child is attending to their environment. This potential problem is minimized somewhat by the fact that we combined the ratings of two highly trained RAs who independently rated infant behavior over a 2- to 3-hour home visit across two child ages. Further, we included a measure of infant temperamental reactivity as a covariate in order to partition out variance explained by more global infant behavioral factors. Similarly, we included multiple variables for family-related conflict and perceived economic-related deprivation to control for potential environmental influences in the home. Future analyses would benefit from the inclusion of direct laboratory assessments of attention to understand how different domains of infant attention are modulated by physiological stress from a more mechanistically precise perspective.
Further, only one sample of child cortisol adjusted for time of day at each time point was used to measure resting stress HPA axis activity. However, salivary cortisol levels are known to fluctuate daily, therefore multiple samples are preferred to obtain a reliable estimate of resting activity. This limitation precludes our ability to conclude that cortisol levels were not influenced by an event prior to saliva collection. However, we have attempted to account for these limitations by including several important covariates known to influence cortisol and by the fact that saliva was collected after data collectors had been in the home for at least an hour. Although this methodology is not optimal, it is one of the tradeoffs when using large field-based studies such as this. We suggest that the benefits of assessing resting physiology and attention in a natural, ecologically valid context help offset these shortcomings. Future research should utilize an approach that would allow for the quantification of resting cortisol through the measurement of cumulative cortisol concentrations, waking or morning cortisol levels, or diurnal curves. In addition, the current study used a rural sample; however, it is known that aspects of urban environments such as density and noise likely play a role in the development of infant attention (Wass, Smith, Stubbs, Clackson, & Mirza, 2019). Thus, future studies should assess whether the findings currently presented would replicate in urban and suburban environments.
Despite these limitations, this study substantially advances the study of attention in infancy. Notably, these results provide novel evidence that changes in an infant’s resting cortisol levels dynamically predict variation in that infant’s executive attention behavior. Moreover, changes in caregiver responsiveness dynamically attenuate or sensitize their infant to the negative effects of elevated cortisol. These findings highlight the importance of assessing resting physiology and real-world attention behaviors within infants’ natural environments. Infant development occurs in a dynamic caregiving environment, which continuously interacts to influence the trajectory of infant cognitive development. Assessing gradual changes and interactions between the different levels (physiological, caregiving, home) of these systems across the first years of life provides a deeper description of complex relations involved in infant development. Using within-person analysis allows for a more comprehensive examination of how internal and external demands act on the infant across time to better understand how individuals adapt to their environment.
Psychobiological theory suggests that the attention system develops through the integration of interdependent relations between multiple levels of organization, spanning from the neurophysiological level to the social level (Bronfenbrenner & Morris, 2007; Gottlieb, 1991). Our results provide evidence that attention is dynamically related to resting HPA axis physiology and caregiver responsiveness across infancy. To the best of our knowledge this is the first study to investigate within-person longitudinal associations between resting cortisol levels and infant executive attention in naturalistic settings. This study expands the literature given that previous research has generally used cross-sectional data or laboratory-based measures of attention in childhood. Moreover, the investigation of within-person moderators of this relationship highlights the malleability of the association between physiological stress and attention as our results suggest the potential buffering role of the caregiving environment.
References
- Afifi TO, & Macmillan HL (2011). Resilience following child maltreatment: A review of protective factors. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie, 56, 266–272. doi: 10.1177/070674371105600505 [DOI] [PubMed] [Google Scholar]
- Amso D, & Scerif G (2015). The attentive brain: Insights from developmental cognitive neuroscience. Nature Reviews Neuroscience, 16, 606–619. doi: 10.1038/nrn4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews Neuroscience, 10, 410–422. doi: 10.1038/nrn2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N (1969). Bayley scales of infant development. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Bernier A, Carlson SM, & Whipple N (2010). From external regulation to self-regulation: Early parenting precursors of young children’s executive functioning. Child Development, 81, 326–339. doi: 10.1111/j.1467-8624.2009.01397.x [DOI] [PubMed] [Google Scholar]
- Berry D, & Willoughby MT (2017). On the practical interpretability of cross-lagged panel models: Rethinking a developmental workhorse. Child Development, 88, 1186–1206. doi: 10.1111/cdev.12660 [DOI] [PubMed] [Google Scholar]
- Blair C (2010). Stress and the development of self-regulation in context. Child Development Perspectives, 4, 181–188. doi: 10.1111/j.1750-8606.2010.00145.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT, … Fortunato CK & the FLP Investigators (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82, 1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2012a). Child development in the context of adversity: Experiential canalization of brain and behavior. The American Psychologist, 67, 309–318. doi: 10.1037/a0027493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2012b). Individual development and evolution: Experiential canalization of self-regulation. Developmental Psychology, 48, 647–657. doi: 10.1037/a0026472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Raver CC (2016). Poverty, stress, and brain development: New directions for prevention and intervention. Academic Pediatrics, 16, S30–S36. doi: 10.1016/j.acap.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17, 271–301. doi: 10.1017/S0954579405050145 [DOI] [PubMed] [Google Scholar]
- Bradley RH (1994). The home inventory: Review and reflections. Advances in Child Development and Behavior, 25, 241–288. doi: 10.1016/s0065-2407(08)60054-3 [DOI] [PubMed] [Google Scholar]
- Brandes-Aitken A, Braren S, Swingler M, Voegtline K, & Blair C (2019). Sustained attention in infancy: A foundation for the development of multiple aspects of self-regulation for children in poverty. Journal of Experimental Child Psychology, 184, 192–209. doi: 10.1016/J.JECP.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, & Morris PA (2007). The bioecological model of human development. In Handbook of child psychology: Theoretical models of human development; (Vol. 1, pp. 793). doi: 10.1002/9780470147658.chpsy0114 [DOI] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. The Future of Children/Center for the Future of Children, the David and Lucile Packard Foundation, 7, 55–71. [PubMed] [Google Scholar]
- Burchinal MR, Roberts JE, Hooper S, & Zeisel SA (2000). Cumulative risk and early cognitive development: A comparison of statistical risk models. Developmental Psychology, 36, 793. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016a). The neuro-environmental loop of plasticity: A cross-species analysis of parental effects on emotion circuitry development following typical and adverse caregiving. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41, 163–176. doi: 10.1038/npp.2015.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016b). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. doi: 10.1016/J.COBEHA.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, & Richards JE (1988). Sustained visual attention in young infants measured with an adapted version of the visual preference paradigm. Child Development, 59, 1514. doi: 10.2307/1130666 [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, & Sousa N (2007). The prefrontal cortex as a key target of the maladaptive response to stress. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clearfield MW, & Jedd KE (2013). The effects of socio-economic status on infant attention. Infant and Child Development, 22, 53–67. doi: 10.1002/icd.1770 [DOI] [Google Scholar]
- Conger RD, & Elder GH (1994). Families in troubled times: Adapting to change in rural America. Hawthorne, NY: Aldine de Gruyter. [Google Scholar]
- Davies PT, Sturge-Apple ML, Cicchetti D, & Cummings EM (2007). The role of child adrenocortical functioning in pathways between interparental conflict and child maladjustment. Developmental Psychology, 43, 918–930. doi: 10.1037/0012-1649.43.4.918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V, & Pruessner JC (2009). The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage, 47, 864–871. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, & Joëls M (1999). Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences, 22, 422–426. doi: 10.1016/s0166-2236(99)01438-1 [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The adaptive calibration model of stress responsivity. Neuroscience and Biobehavioral Reviews, 35, 1562–1592. doi: 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill BT (1999). Poverty in the rural U.S.: Implications for children, families, and communities. Annie E. Casey Foundation. Retrieved from http://www.aecf.org/KnowledgeCenter.aspx. [Google Scholar]
- DuMont KA, Widom CS, & Czaja SJ (2007). Predictors of resilience in abused and neglected children grown-up: The role of individual and neighborhood characteristics. Child Abuse & Neglect, 31, 255–274. doi: 10.1016/j.chiabu.2005.11.015 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Bianchi J, Griskevicius V, & Frankenhuis WE (2017). Beyond risk and protective factors: An adaptation-based approach to resilience. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 12, 561–587. doi: 10.1177/1745691617693054 [DOI] [PubMed] [Google Scholar]
- Ellis BJ, & Del Giudice M (2019). Developmental adaptation to stress: An evolutionary perspective. Annual Review of Psychology, 70, 111–139. doi: 10.1146/annurev-psych-122216-011732 [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets W, & Schulkin J (2003). Glucocorticoid regulation of diverse cognitive functions in normal and pathological emotional states. Neuroscience and Biobehavioral Reviews, 27, 233–246. doi: 10.1016/s0149-7634(03)00033-2 [DOI] [PubMed] [Google Scholar]
- Evans GW (2004). The environment of childhood poverty. American Psychologist, 59, 77. [DOI] [PubMed] [Google Scholar]
- Feldman R (2007). Parent-Infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, 48, 329–354. doi: 10.1111/j.1469-7610.2006.01701.x [DOI] [PubMed] [Google Scholar]
- Finegood ED, Wyman C, O’Connor TG, Blair CB, & The Family Life Project Investigators (2017). Salivary cortisol and cognitive development in infants from low-income communities. Stress, 20, 112–121. doi: 10.1080/10253890.2017.1286325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhuis WE, & Del Giudice M (2012). When do adaptive developmental mechanisms yield maladaptive outcomes? Developmental Psychology, 48, 628–642. doi: 10.1037/a0025629 [DOI] [PubMed] [Google Scholar]
- Frankenhuis WE, Panchanathan K, & Nettle D (2016). Cognition in harsh and unpredictable environments. Current Opinion in Psychology, 7, 76–80. doi: 10.1016/j.copsyc.2015.08.011 [DOI] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, & Telzer EH (2013). Early developmental emergence of human amygdala – prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America, 110, 15638–15643. doi: 10.1073/pnas.1307893110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb G (1991). Experiential canalization of behavioral development: Theory. Developmental Psychology, 27, 4–13. doi: 10.1037/0012-1649.27.1.4 [DOI] [Google Scholar]
- Gottlieb G (1997). Synthesizing Nature-nurture: Prenatal Roots of Instinctive Behavior. Psychology Press. [Google Scholar]
- Gottlieb G (1998). Normally occurring environmental and behavioral influences on gene activity: From central dogma to probabilistic epigenesis. Psychological Review, 105, 792–802. [DOI] [PubMed] [Google Scholar]
- Granger DA, Blair C, Willoughby M, Kivlighan KT, Hibel LC, Fortunato CK, & Wiegand LE (2007). Individual differences in salivary cortisol and alpha-amylase in mothers and their infants: Relation to tobacco smoke exposure. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 49, 692–701. [DOI] [PubMed] [Google Scholar]
- Grossmann T (2013). Mapping prefrontal cortex functions in human infancy. Infancy: The Official Journal of the International Society on Infant Studies, 18, 303–324. doi: 10.1111/infa.12016 [DOI] [Google Scholar]
- Gunnar MR, & Donzella B (2002). Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology, 27, 199–220. doi: 10.1016/s0306-4530(01)00045-2 [DOI] [PubMed] [Google Scholar]
- Gunnar M, & Quevedo K (2007). The neurobiology of stress and development. Annual Review of Psychology, 58, 145–173. doi: 10.1146/annurev.psych.58.110405.085605 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Quevedo KM (2008). Early care experiences and HPA axis regulation in children: A mechanism for later trauma vulnerability. Progress in Brain Research, 167, 137–149. doi: 10.1016/S0079-6123(07)67010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, & Farah MJ (2009). Socioeconomic status and the developing brain. Trends in Cognitive Sciences, 13, 65–73. doi: 10.1016/J.TICS.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon AG, Hibel LC, Rumyantseva O, & Granger DA (2007). Measuring salivary cortisol in studies of child development: Watch out—what goes in may not come out of saliva collection devices. Developmental Psychobiology: The Journal of the International Society for Developmental Psychobiology, 49, 495–500. [DOI] [PubMed] [Google Scholar]
- Hensch TK (2005). Critical period plasticity in local cortical circuits. Nature Reviews. Neuroscience, 6, 877–888. doi: 10.1038/nrn1787 [DOI] [PubMed] [Google Scholar]
- Herman JP, & Tasker JG (2016). Paraventricular hypothalamic mechanisms of chronic stress adaptation. Frontiers in Endocrinology, 7, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel AS (2018). Rapid infant prefrontal cortex development and sensitivity to early environmental experience. Developmental Review, 48, 113–144. doi: 10.1016/J.DR.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L, & Stawski RS (2009). Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development, 6, 97–120. doi: 10.1080/15427600902911189 [DOI] [Google Scholar]
- Hostinar CE, Sullivan RM, & Gunnar MR (2014). Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychological Bulletin, 140, 256–282. doi: 10.1037/a0032671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson J, Nilsson KW, & Lindblad F (2013). Early psychosocial adversity and cortisol levels in children with attention-deficit/hyperactivity disorder. European Child & Adolescent Psychiatry, 22, 425–432. doi: 10.1007/s00787-013-0383-0 [DOI] [PubMed] [Google Scholar]
- Lipina SJ, Martelli MI, Vuelta B, & Colombo JA (2005). Performance on the A-not-B task of Argentinean infants from unsatisfied and satisfied basic needs homes. Revista Interamericana de Psicología/Interamerican Journal of Psychology, 39(1), 49–60. [Google Scholar]
- Lipina SJ, & Posner MI (2012). The impact of poverty on the development of brain networks. Frontiers in Human Neuroscience, 6, 238. doi: 10.3389/fnhum.2012.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, & Casey BJ (2009). Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proceedings of the National Academy of Sciences of the United States of America, 106, 912–917. doi: 10.1073/pnas.0807041106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- McEwen BS (2006). Protective and damaging effects of stress mediators: Central role of the brain. Dialogues in Clinical Neuroscience, 8, 367–381. doi: 10.31887/DCNS.2006.8.4/bmcewen [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, & Gianaros PJ (2011). Stress- and allostasis-induced brain plasticity. Annual Review of Medicine, 62, 431–445. doi: 10.1146/annurev-med-052209-100430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales S, Fu X, & Pérez-Edgar KE (2016). A developmental neuroscience perspective on affect-biased attention. Developmental Cognitive Neuroscience, 21, 26–41. doi: 10.1016/J.DCN.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, & Buss K (1996). Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Development, 67, 508–522. [PubMed] [Google Scholar]
- Perry RE, Blair C, & Sullivan RM (2017). Neurobiology of infant attachment: Attachment despite adversity and parental programming of emotionality. Current Opinion in Psychology, 17, 1–6. doi: 10.1016/j.copsyc.2017.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Finegood ED, Braren SH, & Blair C (2018). The social neuroendocrinology and development of executive functions. In Schultheiss OC, & Mehta PH (Eds.), Routledge International Handbook of Social Neuroendocrinology (pp. 530–543). London: Routledge. doi: 10.4324/9781315200439-30 [DOI] [Google Scholar]
- Perry RE, Rincón-Cortés M, Braren SH, Brandes-Aitken AN, Opendak M, Pollonini G, … Sullivan RM (2019). Corticosterone administration targeting a hypo-reactive HPA axis rescues a socially-avoidant phenotype in scarcity-adversity reared rats. Developmental Cognitive Neuroscience, 40, 100716. doi: 10.1016/j.dcn.2019.100716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The attention system of the human brain: 20 years after. Annual Review of Neuroscience, 35, 73–89. doi: 10.1146/annurev-neuro-062111-150525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13, 25–42. doi: 10.1146/annurev.ne.13.030190.000325 [DOI] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (2007). Research on attention networks as a model for the integration of psychological science. Annual Review of Psychology, 58, 1–28. doi: 10.1146/annurev.psych.58.110405.085516 [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, … Lupien SJ (2010). Stress regulation in the central nervous system: Evidence from structural and functional neuroimaging studies in human populations-2008 Curt Richter award winner. Psychoneuroendocrinology, 35, 179–191. [DOI] [PubMed] [Google Scholar]
- R Core Team (2013). R: A language and environment for statistical computing.
- Roos LE, Giuliano RJ, Beauchamp KG, Berkman ET, Knight EL, & Fisher PA (2019). Acute stress impairs children’s sustained attention with increased vulnerability for children of mothers reporting higher parenting stress. Developmental Psychobiology, doi: 10.1002/dev.21915 [DOI] [PubMed] [Google Scholar]
- Rosen ML, Amso D, & McLaughlin KA (2019). The role of the visual association cortex in scaffolding prefrontal cortex development: A novel mechanism linking socioeconomic status and executive function. Developmental Cognitive Neuroscience, 39, 100699. doi: 10.1016/j.dcn.2019.100699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ML, Hagen MP, Lurie LA, Miles ZE, Sheridan MA, Meltzoff AN, & McLaughlin KA (2019). Cognitive stimulation as a mechanism linking socioeconomic status with executive function: A longitudinal investigation. Child Development, 91, e762–e779. doi: 10.1111/cdev.13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, & Rueda MR (2005). The Development of Effortful Control. In Mayr U, Awh E, & Keele SW (Eds.), Decade of behavior. Developing individuality in the human brain: A tribute to Michael I. Posner (pp. 167–188). American Psychological Association. doi: 10.1037/11108-009 [DOI] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, & Posner MI (2011). Developing mechanisms of self-regulation in early life. Emotion Review: Journal of the International Society for Research on Emotion, 3, 207–213. doi: 10.1177/1754073910387943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff HA (1986). Components of attention during infants ‘manipulative exploration. Child Development, 57, 105–114. [DOI] [PubMed] [Google Scholar]
- Ruff HA, & Capozzoli MC (2003). Development of attention and distractibility in the first 4 years of life. Developmental Psychology, 39, 877–890. doi: 10.1037/0012-1649.39.5.877 [DOI] [PubMed] [Google Scholar]
- Smith LB, & Thelen E (2003). Development as a dynamic system. Trends in Cognitive Sciences, 7, 343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Stifter CA, & Corey JM (2001). Vagal regulation and observed social behavior in infancy. Social Development, 10, 189–201. [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy SUE, & Sugarman DB (1996). The revised conflict tactics scales (CTS2): Development and preliminary psychometric data. Journal of Family Issues, 17, 283–316. doi: 10.1177/019251396017003001 [DOI] [Google Scholar]
- Sullivan RM, & Gratton A (2002). Prefrontal cortical regulation of hypothalamic–pituitary–adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology, 27, 99–114. [DOI] [PubMed] [Google Scholar]
- Sullivan EL, Holton KF, Nousen EK, Barling AN, Sullivan CA, Propper CB, & Nigg JT (2015). Early identification of ADHD risk via infant temperament and emotion regulation: A pilot study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56, 949–957. doi: 10.1111/jcpp.12426 [DOI] [PubMed] [Google Scholar]
- Suor JH, Sturge-Apple ML, Davies PT, Cicchetti D, & Manning LG (2015). Tracing differential pathways of risk: Associations Among family adversity, cortisol, and cognitive functioning in childhood. Child Development, 86, 1142–1158. doi: 10.1111/cdev.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler MM, Perry NB, & Calkins SD (2015). Neural plasticity and the development of attention: Intrinsic and extrinsic influences. Development and Psychopathology, 27, 443–457. doi: 10.1017/S0954579415000085 [DOI] [PubMed] [Google Scholar]
- Troller-Renfree S, McDermott JM, Nelson CA, Zeanah CH, & Fox NA (2015). The effects of early foster care intervention on attention biases in previously institutionalized children in Romania. Developmental Science, 18, 713–722. doi: 10.1111/desc.12261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanTieghem MR, & Tottenham N (2018). Neurobiological programming of early life stress: Functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Current Topics in Behavioral Neurosciences, 38, 117–136. doi: 10.1007/7854_2016_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernon-Feagans L, & Cox M (2013). I. Poverty, rurality, parenting, and risk: An introduction. Monographs of the Society for Research in Child Development, 78, 1–23. doi: 10.1111/mono.v78.5 [DOI] [PubMed] [Google Scholar]
- Voelkle MC, Brose A, Schmiedek F, & Lindenberger U (2014). Toward a unified framework for the study of between-person and within-person structures: Building a bridge between two research paradigms. Multivariate Behavioral Research, 49, 193–213. doi: 10.1080/00273171.2014.889593 [DOI] [PubMed] [Google Scholar]
- Vygotsky LS (1979). The development of higher forms of attention in childhood. Soviet Psychology, 18, 67–115. doi: 10.2753/RPO1061-0405180167 [DOI] [Google Scholar]
- Wass SV, Clackson K, Georgieva SD, Brightman L, Nutbrown R, Leong V, & Wass S (2018). Infants’ visual sustained attention is higher during joint play than solo play: Is this due to increased endogenous attention control or exogenous stimulus capture? Developmental Science, 21, e12667. doi: 10.1111/desc.12667 [DOI] [PubMed] [Google Scholar]
- Wass SV, Cook C, & Clackson K (2017). Changes in behavior and salivary cortisol after targeted cognitive training in typical 12-month-old infants. Developmental Psychology, 53, 815–825. doi: 10.1037/dev0000266 [DOI] [PubMed] [Google Scholar]
- Wass SV, Scerif G, & Johnson MH (2012). Training attentional control and working memory – Is younger, better?. Developmental Review, 32, 360–387. [Google Scholar]
- Wass S, Smith C, Stubbs L, Clackson K, & Mirza F (2019). Physiological stress, sustained attention and cognitive engagement in 12-month-old infants from urban environments. Unpublished Manuscript. School of Psychology, University of East London. Retrieved from doi: 10.31234/osf.io/7nv34 [DOI] [PubMed] [Google Scholar]
- Werchan DM, & Amso D (2017). A novel ecological account of prefrontal cortex functional development. Psychological Review, 124, 720. doi: 10.1037/rev0000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, & Smith LB (2016). The social origins of sustained attention in one-year-old human infants. Current Biology, 26, 1235–1240. doi: 10.1016/j.cub.2016.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]