Abstract
Objective:
Childhood exposure to acute, high-dose radiation has consistently been associated with risk of benign and malignant intracranial tumors of the brain and central nervous system, but data on risks following adulthood exposure to protracted, low-to-moderate dose radiation are limited. In a large cohort of radiologic technologists, we quantified the association between protracted, low-to-moderate dose radiation and malignant intracranial tumor mortality.
Methods:
The study population included 83,655 female and 26,642 male U.S. radiologic technologists who were certified for at least two years as of 1982. The cohort was followed from the completion date of the first or second survey (1983–1989 or 1994–1998) to the date of death, loss-to-follow-up, or December 31, 2012, whichever was earliest. Occupational brain doses through 1997 were based on work history, historical data, and, for most years after the mid-1970s, individual film badge measurements. Radiation-related excess relative risks (ERRs) and 95% confidence intervals (CIs) were estimated from Poisson regression models adjusted for attained age and sex.
Results:
Cumulative mean absorbed brain dose was 11.8 mGy (range 0–290.1 mGy). During follow-up (median 26.7 years), 193 technologists died of a malignant intracranial neoplasm. Based on models incorporating a five-year lagged cumulative brain dose, cumulative brain dose was not associated with malignant intracranial tumor mortality (overall ERR per 100 mGy=0.1, 95% CI <−0.3 to 1.5). No effect modification was observed by sex or birth cohort.
Conclusions:
In this nationwide cohort of radiologic technologists, cumulative occupational radiation exposure to the brain was not associated with malignant intracranial tumor mortality.
INTRODUCTION
From the early-1980s to the mid-2000s, average per-person exposure to ionizing radiation approximately doubled in the U.S., due mainly to the increasingly widespread use of medical radiation procedures [1]. This increase has raised concerns about the risks of cancer and other serious diseases associated with exposure within the low-to-moderate dose range (<100 milliSievert [mSv]), to which most of the general population is exposed. These risks have been directly evaluated in populations of patients undergoing repeated medical procedures [2] and occupationally-exposed groups, including nuclear workers [3–5] and medical workers [6].
Ionizing radiation is the only established modifiable risk factor for intracranial tumors, including malignant tumors (e.g., glioma), although a few non-modifiable (male sex, higher attained age, Caucasian race, taller height, some rare syndromes) and some other suggested risk factors (immune-related conditions, history of epilepsy) have been associated with increased risk [7]. Benign and malignant brain tumors are a particular health concern of physicians and other medical workers who perform or assist with fluoroscopically-guided interventional (FGI) procedures [8–10]. Unlike most of the rest of the body (which can be shielded using a lead apron and thyroid shield), the head and extremities are generally unprotected against ionizing radiation exposure during these procedures.
However, the evidence linking ionizing radiation exposure with risk of intracranial tumors is mostly based on studies of acute, mostly high-dose, exposure in childhood (e.g., children receiving radiation therapy for benign conditions and cancer), and these studies have generally found stronger dose-response associations for benign versus malignant tumors [11]. The Lifespan Study of atomic bomb survivors, including children and adults at the time of exposure, found that higher acute exposure within the low-to-moderate range was positively associated with incident meningioma, glioma, and other types of intracranial tumors, most notably schwannoma, a type of benign brain tumor [12]. However, only schwannoma was associated with radiation exposure in adulthood. Data on risks following adulthood exposure to protracted, low-to-moderate dose radiation, to which most of the population is exposed, are limited. Studies of occupationally-exposed groups (e.g., nuclear workers), which are generally exposed to radiation in the low-to-moderate dose range, have not shown convincing evidence of a dose-response association with malignant or benign intracranial neoplasms [3, 13–15].
The large, nationwide cohort of U.S. radiologic technologists (USRT study) has provided an opportunity to evaluate radiation-related risks of cancers, including intracranial neoplasms, in medical workers. In this cohort, a statistically significant 2.5-fold elevated risk of death from malignant intracranial tumors was observed for radiologic technologists who reported in the mid-1990s that they had ever performed or assisted with FGI procedures [16]. Given findings from other occupationally-exposed cohorts, it is unclear whether this observation was attributable to higher ionizing radiation exposure per se, or to confounding factors. General work history characteristics that have been associated with much higher doses in this cohort, including having worked in earlier decades (when doses and dose limits were much higher) and a greater total number of years worked were not found to be associated with risk of death from malignant intracranial tumors [6]. This cohort now includes an additional four years of mortality follow-up and estimated cumulative absorbed organ doses, including brain doses, which were reconstructed from film badge readings, where available, work history information, and a review of the literature [17]. Within this cohort of radiologic technologists, we quantified the association between protracted, low-to-moderate dose radiation and malignant intracranial tumor mortality.
METHODS
Study population
The U.S. Radiologic Technologists Study commenced in the mid-1980s when the U.S. National Cancer Institute, in collaboration with the University of Minnesota and the American Registry of Radiologic Technologists (ARRT) identified 146,022 radiologic technologists (73% female) who were certified by the ARRT for at least two years between 1926 and 1982. Details about the study have been previously described [18–19] and can be found online at http://www.radtechstudy.nci.nih.gov. Between 1983 and 2014, four mailed surveys were administered to the population of living and eligible radiologic technologists with a known postal address at the time of administration. The first survey, administered between 1983 and 1989, was completed by 90,305 technologists (68% response rate). The second survey was administered between 1994 and 1998, and completed by 90,972 technologists (72% response rate). The third survey was administered between 2003 and 2005 to those who had completed at least one of the first two surveys and was completed by 73,625 technologists (72% response rate). The fourth survey was administered between 2012 and 2014 to those who had completed at least one of the first two surveys and was completed by 58,587 technologists (62% response rate). The surveys elicited information about work history and practices, demographic characteristics, medical history, reproductive history and use of exogenous hormones, family medical history, tobacco and alcohol use, and height and weight.
The study population included in the present analysis was restricted to the 83,655 female and 26,642 male radiologic technologists who responded to the first and/or second cohort surveys and for whom annual and cumulative doses to the brain have been estimated.
Follow-up
Individual follow-up began at the date of first survey completed (baseline survey) and ended at the earliest of the date of death, last known vital status, or December 31, 2012, which was the last date for which vital status information was available. Vital status was ascertained from annual ARRT re-certification records and linkage with the Social Security Administration. For those known or presumed deceased, we matched their records with the National Death Index to confirm that they were deceased and to obtain their cause of death.
Cases were those for whom the underlying cause of death was listed as a malignant intracranial neoplasm of the brain and central nervous system (ICD-9 191, 192.0, 192.1; ICD-10 C70.0, C70.9, C71, C72.2-C72.9). Spinal tumors were excluded from the case definition. We identified 193 cases in total (ICD-9 191, n=67; ICD-10 C70.9, n=1; ICD-10 C71, n=125).
Dose estimation
Historical dose reconstruction was undertaken to estimate annual and cumulative radiation absorbed doses to specific organs, including the brain, from occupational exposure for each technologist [17]. The reconstruction was based on: (1) 921,134 annual badge dose measurements for 79,959 cohort members (72%) between 1960 and 1997 obtained from the nation’s largest commercial dosimetry provider, military radiation dose registries, civilian employers, and a major U.S. hospital; (2) detailed work history regarding procedures and protection practices obtained from the first three cohort surveys: and (3) for the years before 1960 when badge dose measurements were unavailable, literature-derived period-specific historical data on badge doses, x-ray imaging technology, radiation protection standards, protection practices, radiation energies and filtration, and other factors affecting exposure. The dosimetry system provides 1,000 realizations, or sets of dose records for the entire cohort. Each set of records contains annual doses (up to 1997) for each subject, and records are properly correlated to simulate similarities in exposure attributes.
Covariates
Information on demographic characteristics, lifestyle factors, medical history, and work history (including history of working with FGI procedures) was available from the mailed surveys. Of these, we considered the following suspected or possible risk factors as covariates in models of malignant brain tumor mortality: age, sex, year of birth (before 1930, 1930–39, 1940–49, 1950–66), race (White, non-White, unknown), marital status (never married, married, widowed, divorced/separated, unknown), height (sex-specific quartiles, unknown), body mass index (<18.5, 18.5–24.9, 25.0–29.9, 30.0–34.9, 35.0+, unknown), smoking (ever, never, unknown), alcohol consumption (none, <7 drinks/week, 7+ drinks per week, unknown), number of live births (none, 1, 2, 3, 4+, unknown), menopausal status (pre-, post-, unknown), menopausal hormone therapy use (no, yes, unknown), medical history of asthma (no, yes, unknown), and medical history of diabetes (no, yes, unknown). Sex, year of birth (before 1930, 1930–39, 1940–49, 1950–66), and year first worked as a radiologic technologist (before 1950, 1950–59, 1960–69, 1970+) were considered as potential effect modifiers due to differences in background mortality rates of malignant brain tumor mortality for men and women [20] and the strong inverse correlations between year of birth and year first worked with cumulative brain doses in this cohort (Spearman’s rho= −0.58 and −0.66, respectively).
Statistical analysis
Primary dose-response analyses used Poisson regression models to calculate excess relative risks (ERRs) and 95% confidence intervals (CIs) for malignant intracranial neoplasm deaths. Rates were described using ERR models of the form λ0 (a, b, zo)[1+ERR(d)] in which λ0 (a, b, zo) represents the background rates, which depend on age (a), sex (b), and other factors (zo). The ERR modeled five-year lagged cumulative brain dose (d) (in mGy). Maximum-likelihood estimates of the model parameters were computed using the AMFIT module of Epicure [21]. Two-sided hypothesis tests and confidence intervals were based on likelihood ratio tests.
Due to the small number of cases, parsimonious models were preferentially chosen to enable model convergence. The fit of the age- and sex-adjusted base model did not significantly improve with the inclusion of higher-order terms for age or year of birth, and thus these terms were not included in the final model. Of all of the covariates examined, only alcohol consumption and height were associated with risk. However, because additional adjustment for alcohol and height did not meaningfully change the ERR for brain dose, these additional covariates were not retained in the final age- and sex-adjusted model.
Effect modification by sex, year of birth, and year first worked as a radiologic technologist was evaluated by multiplying the linear dose term by a log-linear function of the potential effect modifier.
RESULTS
Most cohort participants were women (76%) and non-Hispanic White (94%). Most participants (78%) were born between 1940 and 1966, though a small proportion (12% of men and 7% of women) was born before 1930. The median follow-up between the baseline survey and loss-to-follow-up or death was 26.7 years (maximum 30.0 years). The median age at malignant brain tumor death was 60.2 years (range: 29.0 to 93.1 years). The age-adjusted mortality rate for malignant brain tumor death during the follow-up period were higher in men (20.5 per 100,000) than women (13.8 per 100,000), and increased linearly with increasing attained age.
Demographic and lifestyle characteristics of the cases and non-cases are shown in Table 1. Compared to non-cases, cases were more likely to be male and older at response to a baseline survey, to have first worked as a radiologic technologist in earlier time periods, and to have ever worked with FGI procedures.
Table 1.
Demographic and lifestyle characteristics of cases (participants who died of a malignant intracranial neoplasm) and non-cases during the follow-up period (1983 to 2012)
| Cases | Non-cases | ||||
|---|---|---|---|---|---|
| N | % | N | % | P-valuea | |
| Overall | 193 | 100 | 110,104 | 100 | --- |
| Sex | 0.003 | ||||
| Men | 64 | 33.2 | 26,587 | 24.1 | |
| Women | 129 | 66.8 | 83,588 | 75.9 | |
| Age at baseline (years) | <0.001 | ||||
| <30 | 15 | 7.8 | 16,413 | 14.9 | |
| 30–39 | 55 | 28.5 | 43,803 | 39.8 | |
| 40–49 | 62 | 32.1 | 28,555 | 25.9 | |
| 50+ | 61 | 31.6 | 21,404 | 19.4 | |
| Race/ethnicity | 0.38 | ||||
| White | 185 | 95.9 | 103,737 | 94.2 | |
| Black | 6 | 3.1 | 3,538 | 3.2 | |
| Other or unknown | 2 | 1.0 | 2,900 | 2.6 | |
| Smoking status at baseline | 0.04 | ||||
| Never | 90 | 46.6 | 52,175 | 47.4 | |
| Former | 52 | 26.9 | 32,067 | 29.1 | |
| Current | 45 | 23.3 | 24,776 | 22.5 | |
| Unknown | 6 | 3.1 | 1,157 | 1.1 | |
| Body mass index (kg/m 2 ) | 0.17 | ||||
| <25 | 110 | 57.0 | 70,862 | 64.3 | |
| 25–29 | 58 | 30.1 | 26,121 | 23.7 | |
| 30+ | 19 | 9.8 | 10,150 | 9.2 | |
| Unknown | 6 | 3.1 | 3,042 | 2.8 | |
| Year first worked as a radiologic technologist | <0.001 | ||||
| Pre-1950 | 19 | 9.8 | 6,306 | 5.7 | |
| 1950–59 | 45 | 23.3 | 16,199 | 14.7 | |
| 1960–69 | 59 | 30.6 | 32,687 | 29.7 | |
| 1970+ | 70 | 36.3 | 54,912 | 49.9 | |
| Ever worked with fluoroscopically-guided interventional (FGI) procedures b | 0.02 | ||||
| No | 65 | 52.0 | 58,580 | 64.5 | |
| Yes | 39 | 31.2 | 22,171 | 24.4 | |
| Unknown | 21 | 16.8 | 10,080 | 11.1 | |
| Number of times held patient for x-ray c | 0.09 | ||||
| <10 | 29 | 18.8 | 13,393 | 15.2 | |
| 10–49 | 38 | 24.7 | 28,747 | 32.5 | |
| 50+ | 87 | 56.5 | 46,232 | 52.3 | |
P-value from chi-squared test
Restricted to respondents to the second cohort survey (1994–98)
Restricted to respondents to the first cohort survey (1983–88)
The mean cumulative brain dose weighted by number of subjects was 11.8 mGy (range: 0–290.1 mGy). Mean cumulative brain doses were higher for radiologic technologists who began working in earlier versus more recent time periods. No clear differences in mean cumulative brain doses were observed between cases and non-cases within categories of year first worked (Figure 1).
Fig. 1—
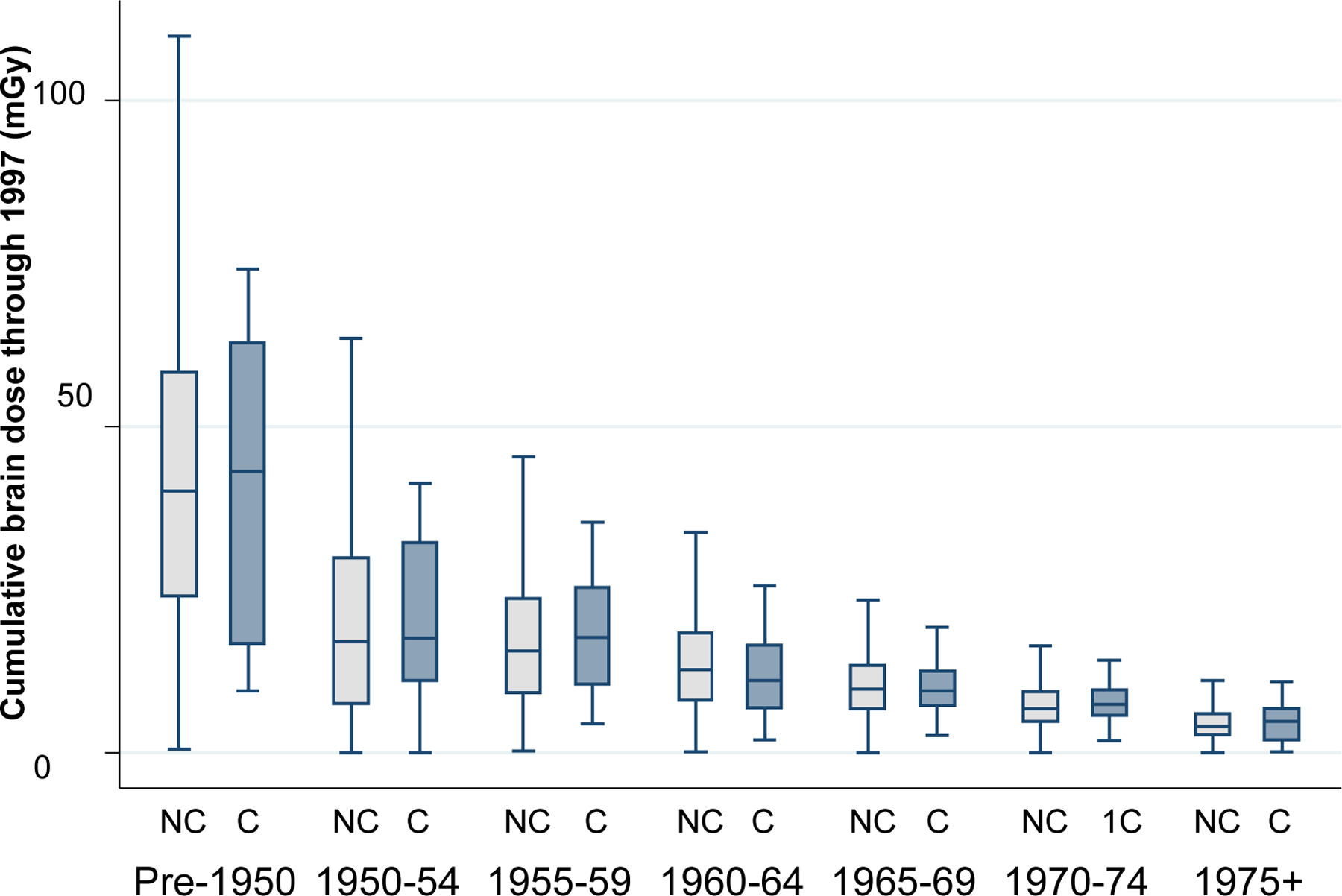
Cumulative mean doses to the brain through 1997 (mGy) according to year first worked as a radiologic technologist. Values for cohort participants who died of malignant intracranial neoplasms (cases, C) are shown in the darker-shaded bars and values for non-cases (NC) are shown in the lighter-shaded bars. The horizontal line in the box represents the median, the box represents the interquartile range (IQR), and the upper and lower lines perpendicular to the whiskers represent the upper (75th percentile plus 1.5*IQR) and lower (25th percentile minus 1.5*IQR) adjacent values, respectively.
In models incorporating the five-year lagged cumulative brain dose and adjusted for sex and attained age (continuous), we observed no evidence of a dose-response association with malignant intracranial neoplasm mortality (ERR per 100 mGy=0.1, 95% CI <−0.3 to 1.5) (Table 2). Results were essentially unchanged in models incorporating a 15-year lag or after excluding subjects with a cancer diagnosis (other than non-melanoma skin cancer) prior to the baseline survey (data not shown). We found no evidence of effect modification by sex (P=0.17), year of birth (P>0.50), or year first worked (P>0.50) (Table 2). Year first worked was highly correlated with year of birth (Spearman’s rho=0.88). We found no evidence of a dose-response among radiologic technologists who reported that they had ever performed or assisted with FGI procedures (ERR per 100 mGy=0.01, 95% CI <0 to 3.6), despite consistently higher estimated cumulative brain doses (Table 3) and higher relative risk (RR) of malignant intracranial neoplasm mortality (RR=1.7, 95% CI 1.1–2.3) in this group compared with those who reported never conducting FGI procedures. However, among participants who ever conducted FGI procedures, mean brain doses were similar among those who later died of a malignant brain tumor compared with those who did not (11 versus 12 mGy).
Table 2.
Dose-response estimates for malignant intracranial neoplasm mortality overall and by selected factors
| Events | ERR per 100 mGy (95% CI)a | |
|---|---|---|
| Overall | 193 | 0.1 (<−0.3 to 1.5) |
| Sex | ||
| Men | 64 | 0.9 (<−0.3 to 4.6) |
| Women | 129 | −0.3 (<0 to 1.0) |
| Year of birth | ||
| Before 1930 | 32 | 0.1 (<−0.3 to 2.4) |
| 1930–39 | 43 | −0.5 (<0 to 4.0) |
| 1940–49 | 69 | −0.2 (<0 to 7.8) |
| 1950–66 | 49 | −0.4 (<0 to 17.0) |
| Year first worked as a radiologic technologist | ||
| Before 1950 | 19 | 0.1 (<0 to 1.5) |
| 1930–39 | 45 | 0.5 (<0 to 3.0) |
| 1940–49 | 59 | −0.3 (<0 to 3.7) |
| 1950–66 | 70 | 2.8 (<0.3 to 9.0) |
| Work history characteristics | ||
| Ever worked with FGI proceduresb | 39 | 0.01 (<0 to 3.6) |
CI=confidence intervals; ERR=excess relative risks; FGI=fluoroscopically-guided interventional
Adjusted for attained age (continuous) and sex
As reported in the second cohort survey (1994–98)
Table 3.
Comparison of cumulative brain doses according to self-reported history of working with fluoroscopically-guided interventional (FGI) procedures, among 90,884 respondents to the second cohort survey (1994–98)
| Worked with FGI procedures | |||
|---|---|---|---|
| Never | Ever | Unknowna | |
| First worked before 1930 | |||
| No. of participants | 23 (48%) | 8 (17%) | 17 (35%) |
| Mean cumulative brain dose (mGy) | 137 | 159 | 158 |
| % dose from film badge | 0% | 0% | 0% |
| First worked between 1930 and 1934 | |||
| No. (%) of participants | 73 (55%) | 13 (10%) | 47 (35%) |
| Mean cumulative brain dose (mGy) | 97 | 134 | 104 |
| % dose from film badge | 0% | 0% | 0% |
| First worked between 1935 and 1939 | |||
| No. of participants | 269 (61%) | 30 (7%) | 145 (33%) |
| Mean cumulative brain dose (mGy) | 59 | 70 | 66 |
| % dose from film badge | 0% | 1% | 0% |
| First worked between 1940 and 1944 | |||
| No. of participants | 757 (63%) | 134 (11%) | 319 (26%) |
| Mean cumulative brain dose (mGy) | 39 | 51 | 43 |
| % dose from film badge | 1% | 2% | 1% |
| First worked between 1945 and 1949 | |||
| No. of participants | 1,713 (67%) | 309 (12%) | 542 (21%) |
| Mean cumulative brain dose (mGy) | 30 | 41 | 33 |
| % dose from film badge | 2% | 3% | 2% |
| First worked between 1950 and 1954 | |||
| No. of participants | 3,655 (66%) | 824 (15%) | 1,101 (20%) |
| Mean cumulative brain dose (mGy) | 18 | 26 | 18 |
| % dose from film badge | 3% | 6% | 3% |
| First worked between 1955 and 1959 | |||
| No. of participants | 5,135 (67%) | 1,319 (17%) | 1,236 (16%) |
| Mean cumulative brain dose (mGy) | 16 | 22 | 17 |
| % dose from film badge | 6% | 9% | 5% |
| First worked between 1960 and 1964 | |||
| No. of participants | 7,550 (66%) | 2,522 (22%) | 1,418 (12%) |
| Mean cumulative brain dose (mGy) | 13 | 17 | 13 |
| % dose from film badge | 9% | 13% | 7% |
| First worked between 1965 and 1969 | |||
| No. of participants | 10,491 (65%) | 3,992 (25%) | 1,614 (10%) |
| Mean cumulative brain dose (mGy) | 10 | 13 | 10 |
| % dose from film badge | 15% | 19% | 14% |
| First worked between 1970 and 1974 | |||
| No. of participants | 14,059 (63%) | 6,428 (29%) | 1,942 (9%) |
| Mean cumulative brain dose (mGy) | 7 | 9 | 7 |
| % dose from film badge | 25% | 31% | 22% |
| First worked between 1975 and 1979 | |||
| No. of participants | 14,108 (64%) | 6,428 (29%) | 1,942 (7%) |
| Mean cumulative brain dose (mGy) | 5 | 6 | 5 |
| % dose from film badge | 52% | 54% | 53% |
| First worked in 1980 or subsequently | |||
| No. of participants | 768 (62%) | 331 (27%) | 144 (12%) |
| Mean cumulative brain dose (mGy) | 2 | 3 | 2 |
| % dose from film badge | 59% | 57% | 61% |
| Total | |||
| No. of participants | 58,601 (64%) | 22,206 (24%) | 10,077 (11%) |
| Mean cumulative brain dose (mGy) | 11 | 12 | 15 |
| % dose from film badge | 24% | 31% | 18% |
Responded to second cohort survey but did not respond to work history question about FGI procedures
DISCUSSION
In this large, nationwide cohort of U.S. radiologic technologists, all of whom were first certified for at least two years as of 1982 and were prospectively followed for mortality outcomes for nearly 30 years, we found no evidence of a dose-response association between cumulative protracted occupational radiation and malignant intracranial tumor mortality. We also found no evidence of a dose-response association among radiologic technologists who reported having ever assisted with FGI procedures despite consistently higher doses compared with radiologic technologists who never assisted with FGI procedures.
Brain tumors have been a particular concern among medical workers, particularly those working with FGI procedures, because the brain is one of the few unshielded, radiosensitive organs. A few small case-series have suggested a higher incidence of brain tumors in these workers [8–10], but there has been limited evidence from well-designed observational studies on the association between estimated brain doses and brain tumor risks in medical workers or other exposed populations. A previous analysis of the U.S. radiologic technologist cohort, based on follow-up through 2008, revealed a statistically-significant approximately 2.5-fold increased risk of malignant intracranial neoplasm mortality for radiologic technologists who ever (versus never) performed or assisted with FGI procedures [16]. With an additional four years of follow-up, we found similar, albeit slightly weaker, relative risks from our current dataset. We found no evidence, however, of a radiation dose-response association within the subset of technologists who reported ever working with FGI procedures, including 39 individuals who later died of a malignant intracranial tumor. Considering the relatively small numbers of cases and the low range of estimated brain doses, statistical power may have been too limited to identify a positive dose-response association.
Alternatively, the elevated risk of malignant intracranial tumor deaths in technologists who ever conducted FGI procedures versus those who did not may be due to factors other than radiation. There are few known or suspected risk factors for malignant intracranial neoplasms [7], and none of the many potential risk factors that we examined appeared to be important confounders. Nonetheless, we cannot rule out the potential for confounding by differences in exposure to certain chemical or drugs used by radiologic technologists, including film-processing chemicals, which were not ascertained in the surveys. Film-processing chemicals include a wide range of substances, such as hydroquinone, aldehydes, acetic acid, glycol ethers, glycols, sulfur dioxide, and ammonia [22]. Some of these chemicals have been implicated in triggering dermal irritation, rhinitis, and asthma-like symptoms in medical workers processing x-ray films, particularly under working conditions characterized by heavy workloads, poorly-designed and ill-ventilated darkrooms, and lack of protective equipment or use of safe handling techniques [23]. Until the mid-1960s, x-ray films were processed in open tanks. Depending on the era and facility, this work was done either by radiologic technologists or darkroom technologists. As automatic film processors were introduced (starting in the mid-1950s) [24], the routine chemical exposure of non-FGI technologists diminished. FGI-technologists continued to work inside the darkroom through the 1980s performing film-subtraction angiography. Photographic subtraction angiography was then replaced by digital subtraction angiography. In addition, FGI-technologists were, and still are, exposed to a variety of drugs and iodinated agents at a much higher rate than non-FGI technologists. However, to date, extensive reviews of the published literature have not identified any specific occupational or industrial chemicals that are convincingly related to brain tumor development in humans [7,25]. Additional studies evaluating the carcinogenic potential of chemicals previously used (and still in use) by radiologic technologists are warranted.
Our finding of no association between low-to-moderate dose occupational radiation exposure and malignant intracranial neoplasms is consistent with the few other studies of populations exposed to acute or protracted radiation. These include studies of atomic bomb survivors [12] and occupationally-exposed nuclear workers [3, 13–15] (see Table 4). While a statistically-significant excess of malignant brain tumor incidence was observed in clean-up workers from the Baltic countries who arrived in the Chernobyl area early on (e.g., in 1986) and stayed longer (e.g., ≥90 days) (proportional incidence ratio=2.1, 95% CI 1.1–3.6), there was no clear dose-response association [26]. Several studies have found that childhood exposure more strongly influences risk of benign versus malignant intracranial neoplasms, though studies of benign intracranial neoplasms following adulthood exposure are scarce [12, 26–29]. The Lifespan Study of atomic bomb survivors found that adulthood exposure to ionization radiation was not associated with intracranial neoplasms apart from schwannoma, a type of benign tumor, while dose-response associations were more apparent for most types of intracranial neoplasms following childhood exposure [12]. Thus, evaluating the association between protracted radiation exposure and benign brain tumor incidence in our cohort will be an important next step.
Table 4.
Dose-response estimates for intracranial neoplasms (incidence and mortality) among populations with estimated doses from adulthood radiation exposure
| Events | ERR per Sv (95% CI)a | |
|---|---|---|
| Atomic bomb survivors [12] | 228 incident (benign/malignant) cases | All: 1.2 (0.6 to 2.1) Glioma: 0.6 (−0.2 to 2.0) Meningioma: 0.6 (−0.01 to 1.8) Schwannoma: 4.5 (1.9 to 9.2) Other nervous system: 0.5 (<−0.2 to 2.2) By age at exposure (years) All except schwannoma <20: 1.2 (0.3 to 2.9) 20–39: 0.3 (<−0.2 to 1.6) ≥40: 0.1 (<−0.2 to 1.2) Meningioma <20: 1.3 (0.01 to 4.5) 20–39: 0.5 (−0.05 to 2.8) ≥40: 0.3 (<−0.1 to 2.0) Schwannoma <20: 6.0 (2.1 to 14) 20–39: 2.6 (<−0.2 to 10) ≥40: 3.3 (0.3 to 11) |
| National Registry for Radiation Workers [14] | 337 incident cases; 278 deaths | Incidence: −0.9 (−1.6 to 0.7) Mortality: −1.4 (−1.9 to 0.6) |
| 15-Country Study [3] | 235 deaths | <0 (RR at 100 mSV=0.8, 90% CI 0.5–1.2) |
| Canadian nuclear power industry workers [15] | 25 deaths | −2.04 (<−2.1 to 9.0) |
| U.S. nuclear workers [13] | 23 deaths | −2.50 (<−2.5 to 27.1) |
CI=confidence interval; ERR=excess relative risk; RR=relative risk
Notable strengths of our study include the large sample of radiologic technologists, long length of follow-up, nearly complete ascertainment of mortality including causes of death, and individualized estimates of absorbed brain doses for all individuals in the cohort that have been corroborated by a dose-response evaluation of chromosomal translocation rate [30].
Our study has some limitations beyond those already described. Individuals were only included in the analysis if they survived or were otherwise able to receive and respond to a baseline cohort survey (administered during 1983–89 or 1994–98). Non-responders were not included in the analysis because work history information and/or consent to retrieve film badge doses were necessary for organ-specific dose reconstruction [17]. The extent to which non-response may have biased our results remains unknown, but we have no evidence that it was systematic by dose. Our findings are limited by uncertainties in the dose reconstruction, particularly for the earliest workers who lacked dose information from film badges and were also estimated to have much higher cumulative organ-specific doses compared to cohort participants who began working in more recent years [17]. The most recent dose reconstruction effort was limited to calendar years through 1997, reflecting the years for which we had access to film badge dose records; thus cohort members who survived to 2012 were missing the most recent 15 years of exposure information. While estimated annual doses in recent years for most cohort participants are expected to be negligible, it is possible that we have underestimated cumulative exposure for those who worked with FGI and other higher-dose procedures. Nonetheless, our results did not differ meaningfully when we used a 15-year versus a 5-year lagged dose. Although our case definition was restricted to mortality, the methods available for incidence ascertainment in this cohort are not as reliable for highly-fatal cancers like brain cancers (with a five-year relative survival of 34% [31] due to the long length of time between survey mailings. Systematic use of state cancer registries nationwide is currently impractical. However, in the U.S., brain cancer as a cause of death on death certificates has been shown to be highly concordant with the primary cancer site at diagnosis, as recorded in population-based cancer registries [32]; thus, it is unlikely that many of the deaths were metastases originating from other primary sites. Finally, the number of cases and the estimated brain doses may have been too low to detect a true association in this cohort. Pooled analyses of cohort studies with estimated doses received in adulthood would allow for a more powerful assessment of this association.
In summary, within this large cohort of radiologic technologists, we found no evidence of a positive association between estimated cumulative brain dose through 1997 and deaths from malignant intracranial neoplasms of the brain and central nervous system after 29 years of follow-up.
ACKNOWLEDGMENTS
The authors thank the radiologic technologists who participated in the study, Jerry Reid of the American Registry of Radiologic Technologists for continued support, and Diane Kampa and Allison Iwan of the University of Minnesota for data management and collection. This research was funded, in part, by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH.
Financial disclosures:
This research was funded, in part, by the Intramural Research Program of the National Cancer Institute, NIH.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.National Council on Radiation Protection and Measures (NCRP). Ionizing radiation exposure of the population of the United States. Bethesda, MD: NCRP, 2009. [Google Scholar]
- 2.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 2012; 380:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardis E, Vrijheid M, Blettner M, et al. The 15-Country Collaborative Study of Cancer Risk among Radiation Workers in the Nuclear Industry: estimates of radiation-related cancer risks. Radiat Res 2007;167:396–416. [DOI] [PubMed] [Google Scholar]
- 4.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vrijheid M, Cardis E, Ashmore P, et al. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol 2007;36:1126–35. [DOI] [PubMed] [Google Scholar]
- 6.Liu JJ, Freedman DM, Little MP, et al. Work history and mortality risks in 90,268 US radiological technologists. Occup Environ Med 2014;71:819–35. [DOI] [PubMed] [Google Scholar]
- 7.Bondy ML, Scheurer ME, Malmer B, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer 2008;113:1953–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smilowitz NR, Balter S, Weisz G. Occupational hazards of interventional cardiology. Cardiovasc Revasc Med 2013;14:223–8. [DOI] [PubMed] [Google Scholar]
- 9.Wenzl TB. Increased brain cancer risk in physicians with high radiation exposure. Radiology 2005;235:709–10; author reply 10–1. [DOI] [PubMed] [Google Scholar]
- 10.Roguin A, Goldstein J, Bar O, Goldstein JA. Brain and neck tumors among physicians performing interventional procedures. Am J Cardiol 2013;111:1368–1372. [DOI] [PubMed] [Google Scholar]
- 11.Braganza MZ, Kitahara CM, Berrington de Gonzalez A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol 2012;14:1316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preston DL, Ron E, Yonehara S, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst 2002;94:1555–63. [DOI] [PubMed] [Google Scholar]
- 13.Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res 2004;162:517–26. [DOI] [PubMed] [Google Scholar]
- 14.Muirhead CR, O’Hagan JA, Haylock RG, et al. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer 2009;100:206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zablotska LB, Ashmore JP, Howe GR. Analysis of mortality among Canadian nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res 2004;161:633–41. [DOI] [PubMed] [Google Scholar]
- 16.Rajaraman P, Doody MM, Yu CL, et al. JOURNAL CLUB: Cancer Risks in U.S. Radiologic Technologists Working With Fluoroscopically Guided Interventional Procedures, 1994. AJR Am J Roentgenol 2016:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon SL, Preston DL, Linet MS, et al. Radiation organ doses received in a nationwide cohort of U.S. radiologic technologists: methods and findings. Radiat Res 2014;182:507–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boice JD Jr., Mandel JS, Doody MM, Yoder RC, McGowan R. A health survey of radiologic technologists. Cancer 1992;69:586–98. [DOI] [PubMed] [Google Scholar]
- 19.Doody MM, Freedman DM, Alexander BH, et al. Breast cancer incidence in U.S. radiologic technologists. Cancer 2006;106:2707–15. [DOI] [PubMed] [Google Scholar]
- 20.Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 21.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure Version 2 User Guide. Ottawa, Ontario, Canada: RSI, 2015. [Google Scholar]
- 22.Scobbie E, Dabill DW, Groves JA. Chemical pollutants in X-ray film processing departments. Ann Occup Hyg 1996; 40:423–435. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt PJ. Occupational health problems in processing of X-ray photographic films. Ann Occup Hyg 1993; 37:287–295. [DOI] [PubMed] [Google Scholar]
- 24.Harris EL. The Shadowmakers: a History of Radiologic Technology. Albuquerque, NM: American Society of Radiologic Technologists, 1995. [Google Scholar]
- 25.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current condepts and review of the literatuer. Neuro Oncol 2002; 4:278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rahu M, Rahu K, Auvinen A, et al. Cancer risk among Chernobyl cleanup workers in Estonia and Latvia, 1986–1998. Int J Cancer 2006;119:162–8. [DOI] [PubMed] [Google Scholar]
- 27.Little MP, de Vathaire F, Shamsaldin A, et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer 1998;78:269–75. [DOI] [PubMed] [Google Scholar]
- 28.Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res 2005;163:424–32. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AJ, Little MP, Winter DL, et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol 2010;28:5287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little MP, Kwon D, Doi K, et al. Association of chromosome translocation rate with low dose occupational radiation exposures in U.S. radiologic technologists. Radiat Res 2014;182:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro Oncol 2015; 17 Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.German RR, Fink AK, Heron M, et al. Accuracy of Cancer Mortality Study Group. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol 2011; 35:126–31. [DOI] [PubMed] [Google Scholar]


