Abstract
Hepatocellular carcinoma (HCC) and metabolic syndrome (MetS) have a rising prevalence worldwide. The relationship between these two entities has long been studied and understanding it has become a public health and clinical priority. This association follows, in most patients, the path through non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cirrhosis and finally HCC. Nonetheless, increasing evidence has been found, that shows MetS as an independent risk factor for the development of HCC. This review brings together the clinical evidence of the relationship between these highly prevalent diseases, with a particular interest in the impact of each component of MetS on HCC; It aims to summarize the complex physiopathological pathways that explain this relationship, and to shed light on the different clinical scenarios of this association, the impact of treating the different components of MetS on the risk of HCC and what is known about screening for HCC in patients with MetS. By doing so, it hopes to improve awareness on this topic.
Keywords: metabolic syndrome, hepatocellular carcinoma, excess body weight, diabetes mellitus, non-alcoholic fatty liver disease
Introduction
In 2020, liver cancer was the sixth most incident type of cancer globally and had the third highest mortality rate, with 905,677 new cases and 830,180 deaths.1 HCC is the most common type of liver cancer, accounting for 75% of cases.2 Trends in the incidence of HCC in Western populations are upward, and that remains true for both developed and developing countries in this region. In Asia, the incidence has decreased in the last few years owing to the improvement in hepatitis B virus (HBV) vaccination rates.3 Furthermore, the prognosis of liver cancer is dismal, with an 18% and 0.8% relative 5 and 10-year survival rates, respectively, in the US.2,4
The etiology and risk factors for HCC are well established. They include hepatitis C virus (HCV), HBV, NAFLD, MetS, genetic factors, lifestyle factors (alcohol consumption, smoking, dietary factors), and other less common causes. Historically, the most common etiology is viral, followed by alcohol and around 5–30% of cases are of unknown etiology.5 Virological liver diseases are preventable and treatable, thus their incidence has reduce over time. On the other hand, NAFLD and MetS are gaining importance as etiological factors of HCC because of their worldwide increase in incidence, a better understanding of these diseases, and an increased awareness among healthcare workers.6
MetS is a cluster of metabolic abnormalities that includes hypertension, central obesity, insulin resistance, and atherogenic dyslipidemia. It is associated with an increased risk of cardiovascular disease, diabetes mellitus, cancer, and NAFLD, among others.7,8 The prevalence of MetS has increased dramatically over the last three decades. It varies according to definition, but in 2018, it was estimated that over 1 billion people worldwide were affected by MetS.9 Its prevalence is estimated to be between 10% and 30% in both developed and developing countries.10
In the context of an alarming and ubiquitous increase in the prevalence of MetS, NAFLD, and HCC, understanding the relationship between these entities has become a clinical and public health priority.
Association Between Metabolic Syndrome and Hepatocellular Carcinoma
The relationship between MetS and HCC has been previously described in the context of, not only MetS leading to NAFLD, non-alcoholic steatohepatitis, and eventually bridging fibrosis or cirrhosis, but also in patients with MetS as their only risk factor for chronic hepatic disease. In this case, histopathological studies have shown that 65% of HCCs are present without an advanced fibrosis background.11 This evidence raises questions about the role of MetS as an independent risk factor in HCC.
In 2011, a large population-based study in the US using multiple logistic regression showed that having MetS increased the risk of HCC 2.58-fold (95% confidence interval [CI]: 2.40–2.76. P < 0.0001).12 Similarly, a 2014 meta-analysis of three cohort studies and one case-control study that included 829,651 participants aged between 30–84 years concluded that there is an increased risk of HCC in patients with MetS with a relative risk (RR) of 1.81 (95% CI: 1.37–2.41). Although that study included many patients, the evidence level was low and it did not account for multiple confounding variables, such as hepatitis virological status and alcohol ingestion.5 Another meta-analysis and systematic review that included 11 cohort studies revealed an increased HCC risk in patients with, compared with those without MetS (RR: 1.76, 95% CI: 1.33–2.33). Nevertheless, this result was obtained with high heterogeneity (I2 = 87.6%).13 Another 2017 meta-analysis that included six cohorts and 127,198 participants with 1293 HCCs also showed a significantly higher risk of HCC in patients with MetS. This was found in patients that fulfilled either the revised National Cholesterol Education Program’s Adults Treatment Panel III (NCEP-ATP III) criteria (RR: 1.43, 95% CI: 1.19–1.72, P < 0.001; I2 = 29%) or the International Diabetes Federation (IDF) criteria definition of MetS (RR: 1.59, 95% CI: 1.13–2.23, P = 0.008; I2 = 0%). When a sub-analysis was conducted for gender, the latter conclusion held for males (RR: 1.75, 95% CI: 1.28–2.38, P < 0.001), but not for females (RR: 1.18, 95% CI: 0.76–1.84, P = 0.46).10 Gender differences were also reported in a 2012 systematic review and meta-analysis, with a moderate quality of evidence. Male participants had an RR of 1.43 (95% CI: 1.23–1.65, P = 0.000; I2 = 0%), whereas female participants had an RR of 1.42 (95% CI: 0.80–2.52, P = 0.224; I2 = 71%).8
As previously shown, MetS, as a cluster including all its metabolic disarrays, is a risk factor for incident HCC. The follow up question that arises, is whether all its components act as risk factors for HCC? And if some of them are more significant than others?
Metabolic Syndrome and Its Components as Independent Risk Factors of Hepatocellular Carcinoma
Excess Body Weight
The prevalence of excess body weight, including overweight and obesity, has increased since the 1970s. In 2016, approximately 40% of adults (2 billion) and 18% of children aged 5–19 years (340 million) suffered from either one of these conditions. In 2015, an estimated 4 million deaths were attributable to excess body weight, and approximately 2 trillion USD were spent globally on associated healthcare problems.14
The relationship between excess body weight and multiple types of cancer has been described. A model developed using epidemiological data from 30 European countries showed that in 2002, 2.5% of cancer cases in men and 4.1% in women were attributable to excess body weight and accounted for 70,000 new cancer cases in that year.15 Similarly, a study published in 2018 found that 3.9% of all cancer cases in 2012 were attributable to excess body weight alone. Of cancer cases reported in 2012, 31.9% were due to increased body weight prevalence between 1980 and 2002.16 It is expected that as excess body weight increases in global populations, the number of cancer cases attributable to this condition will also rise.
After this association was proven, the burden of excess body weight specifically on liver cancer was published. In a 2010 systematic review of 13 studies on the effect of obesity on HCC risk, Saunders found that out of 10 cohorts, eight reported a statistically significant increase in HCC risk, and two found no association. For the obese population (body mass index [BMI] ≥ 30 kg/m2), the effect size ranged from a hazard ratio (HR) of 1.4 to an RR of 4.1, depending on sex and other risk factors for HCC. In general, the effect size was more significant in men than in women.17 A 2012 prospective study of 578,700 adults showed a positive dose-response pattern for risk of cancer as BMI increased, with a P trend of 0.001 and an RR of 1.92 (95% CI: 1.23–2.96) in the group with a mean BMI of 31.3 kg/m2.18 An odds ratio (OR) of 1.69 (95% CI: 1.36–2.09) per 5 kg/m2 of BMI increase was found in a 2016 study. An RR of 1.3 (95% CI: 1.16–1.46) reported in a 2019 meta-analysis added evidence to the positive dose effect.14,19 In a 2015 risk assessment study, it was estimated that one in every four liver cancer cases is attributable to diabetes and a high BMI, and 13.5% to a high BMI alone (10% in men and 14% in women). That study makes a 2025 projection of cancer cases; if diabetes and excess body weight continue as expected, liver cancer will have the largest increase in prevalence, alongside gallbladder and endometrial cancers.16 A 2021 study, based on a British cohort (UK Biobank) of 502, 656 adults, found that obesity had an HR of 2.14 (95% CI:1.32–3.39) for HCC when defined by the IDF 2005 criteria and 1.58 (95% CI:1.07–2.34) when defined by the NCEP-ATP III criteria.20 This evidence strongly suggests that excess body weight is a significant risk factor for developing HCC. It is, nonetheless, a preventable and reversible condition that allows patients, governments, and medical communities the opportunity to work together toward reducing its incidence and thereby its toll on the prevalence of HCC.
Diabetes Mellitus
The prevalence of diabetes mellitus has also increased substantially in the past few decades. According to World Health Organization statistics, in 2014, 8.5% of adults aged 18 years and older had diabetes. In 2019, 1.5 million deaths were caused directly by diabetes, and 48% were in people younger than 70 years of age.21 As shown by these data, diabetes mellitus is also a growing epidemic. As part of MetS, its prevalence has grown, therefore, its relationship with hepatic diseases becomes more critical, and understanding its role in the development of HCC becomes more urgent.
In 2016, the Liver Cancer Pooling Project published a case-control analysis nested in a cohort of 1.57 million adults from the US. This showed a twofold increase in the risk of HCC in patients with diabetes mellitus, with an adjusted HR (aHR) of 2.61 (95% CI: 2.34–2.91) that was significant in both men (aHR: 2.66, 95% CI: 2.34–3.02) and women (aHR: 2.39, 95% CI: 1.91–3.00). That study also showed the combined effect of diabetes and excess body weight on HCC risk. For patients with diabetes with a normal BMI (18.5 to < 25 kg/m2), the HR was 2.93 (95% CI: 1.28–3.22). For overweight patients (BMI: 25 to < 30 kg/m2) with diabetes, the aHR was 3.31 (95% CI: 2.77–3.95). Furthermore, in obese patients (BMI: ≥ 30 kg/m2), the aHR was 4.28 (95% CI: 3.59–5.1).19 The British Biobank cohort reported a multivariable-adjusted analysis where abnormal glucose metabolism (glycated hemoglobin ≥ 5.7% regardless of diabetes status) had an HR of 2.19 (95% CI: 1.48–3.25) for HCC.20 The Me-Can project described a dose-dependent increase in the risk of HCC as glucose increases, only reaching statistical significance in the group with mean glucose of 6.7 mmol/L (HR: 3.88, 95% CI: 1.11–13.5); however, when adjusted for BMI quintiles, it lost statistical significance (HR: 2.78, 95% CI: 0.78–9.96). It is important to consider that the higher glucose quantile in this study did not reach the American Diabetes Association criteria for a diagnosis of diabetes. Regardless, the dose-dependent response is shown in both adjusted and unadjusted models.18
The apparent association between diabetes mellitus and HCC has been proven in multiple studies, as with excess body weight. The actions taken by the medical community and patients toward reducing the incidence of diabetes mellitus are of the utmost importance to the health of individual patient and healthcare systems in general.
Hypertension
The association between hypertension, another component of MetS, and HCC is unclear, and scarce evidence has been published. SEER-Medicare analysis, which included patients from 1993 to 2005, found an adjusted OR of hypertension for HCC of 2.22 (2.04–2.42, P < 0.0001).22 Nevertheless, other publications have reported conflicting data. The Me-Can project, which includes 578,700 adults from Norway, Austria, and Sweden shows that, when adjusted for BMI, mean blood pressure (average between systolic and diastolic blood pressure) in its highest quantile of 124.5 mmHg had an RR for HCC of 2.08 (95% CI: 0.95–4.73), with no statistical significance.18 Similarly, also showing no significance, the UK Biobank cohort found an HR of hypertension for HCC of 1.13 (95% CI: 0.76–1.69).20
Prospective studies that analyze this association are needed to better the quality of the evidence and to understand the impact that hypertension has on HCC and the possible benefits of controlling blood pressure on the incidence of HCC.
Dyslipidemia
High-density lipoprotein and triglyceride levels are part of the definition of MetS and have also become a major public health concern. In the US, the Centers for Disease Control and Prevention reported that between 2015 and 2018, 12% of adults over the age of 20 years had total cholesterol above 240 mg/dL and 17% had high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL.23 The prevalence of hypertriglyceridemia (≥ 150 mg/dL) in the US is even higher. According to the National Health and Nutrition Examination Survey, from 2007 to 2014, 25% of adults had triglycerides above 150 mg/dL.24 Cholesterol and its metabolism have been implicated in carcinogenic pathways based on both pathophysiological and clinical evidence.25 The Me-Can study described a protective effect of total cholesterol on the risk of HCC, with a RR of 0.53 (95% CI: 0.40–0.69) per unit Standard deviation; this did not hold for triglycerides, where the findings did not show statistical significance, with an RR of 0.85 (95% CI: 0.60–1.19).18 In a 2021 cohort study including 8,528,790 adults, an inverse correlation was also found between total cholesterol, low-density lipoprotein cholesterol (LDL-C), and the risk of HCC. Unlike the previous study, this cohort study did find the same correlation with triglycerides. On the other hand, all levels of HDL-C tested (0 to ≥ 67 mg/dL) were protective against HCC. For all these HRs, an increasing dose-dependent risk reduction was found. The aHRs shown were 0.36 (95% CI: 0.34–0.37) for total cholesterol ≥ 200 mg/dL, 0.59 (95% CI: 0.54–0.65) for triglycerides ≥ 158 mg/dL, 0.35 (95% CI: 0.34–0.36) for LDL-C ≥ 132 mg/dL, and between 0.83–0.87 with 95% CIs that showed statistical significance, for HDL-C from 49 to ≥ 67 mg/dL.25 In the Biobank cohort, the HR of low HDL-C for HCC was 1.17 (95% CI: 0.78–1.76) and of high triglycerides was 0.84 (95% CI: 0.58–1.23), showing no signs of dyslipidemia as a risk factor for HCC.20
The evidence suggests that higher total cholesterol, HDL-C and LDL-C levels are protective factors against HCC, this is not clear for triglycerides levels. A hypothesis that may explain this is that low cholesterol levels are a preclinical phase of cancer, and that they are, therefore, not an actual protective factor for HCC. This has been proven for other types of cancer, but not for gastrointestinal tumors.26 This hypothesis is based on the fact that tumor cells in the liver use lipids as part of their metabolism.27 More research is needed to elucidate this relationship.
Pathophysiology of the Association Between Metabolic Syndrome and Hepatocellular Carcinoma
Multiple mechanisms have been proposed to provide a tumor-promoting environment in MetS. Recent evidence suggests that excess body weight plays an essential role in the increased risk of premalignant conditions, such as NASH. Excess body weight seems to be not only an independent risk factor for HCC development, but also an independent risk factor for its recurrence.28,29 Excess body weight can initiate hepatic carcinogenesis via NASH without cirrhosis.30
Excess adiposity, particularly visceral fat, and liver carcinogenesis are related via complex processes that include insulin and insulin-like growth factor signaling, chronic inflammation, adipokine signaling, and alterations of the intestinal microbiome.29
Insulin resistance links all the components of MetS and leads to fat accumulation in hepatocytes by lipolysis and hyperinsulinemia, predisposing them to the development of NAFLD. Adipose tissue growth promotes an oncogenic cascade where pro-inflammatory cytokines, such as interleukin-6, which regulates inflammation and activates pro-oncogenic pathways, and STAT3, which favors immune evasion and tumor growth; induce vascular endothelial growth factor, thus stimulating angiogenesis, which favors metastasis.31 Also, activated STAT3 inhibits sp53-mediated growth control32 and increases the expression of Mcl-1, which is associated with resistance to apoptosis, allowing tumor progression.33 Due to this pro-inflammatory environment, the inhibition of anti-inflammatory cytokines (eg, adiponectin) may not be sufficient to suppress inflammatory signaling. The expression of adipokines is related to the development of HCC and is inversely proportional to the size of the tumor. Adiponectin exerts chemoprotective and hepatoprotective effects through sulfatase 2 and inhibits the oncogenic effects of leptin on cell proliferation, migration, and invasion in HCC.34,35 An imbalance in the homeostasis of pro- and anti-inflammatory substances due to excess adipose tissue lipotoxicity ensues, which promotes systemic and hepatic insulin resistance, hyperinsulinemia, and the activation of prooncogenic pathways, including c-Jun N-terminal kinase (JNK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and the mammalian target of rapamycin (mTOR), resulting in a tumorigenic-prone environment. Hyperinsulinemia also leads to the upregulation of the insulin growth factor-1 pathway resulting in proliferative, antiapoptotic, and angiogenic effects, which promote cancer cell proliferation. High circulating leptin levels secondary to hyperinsulinemia in NAFLD exert pro-inflammatory and profibrogenic effects.28 Also, regulatory T-cells (Tregs), effector CD4(+), and CD8(+) T-cells stimulate the expression of the leptin receptor after HCC induction;36 thus, adiponectin and leptin deregulation promotes fibrosis and HCC progression.
Fetuin-A and Fetuin-B (α2-HS-Glycoproteins),37 Retinol Binding Protein 4,38 and Fibroblast Growth Factor 1939 are associated with hepatic lipid accumulation, insulin resistance, and inflammatory signaling pathways. Furthermore, ANGPTL8 interacts with SREBP-1, promoting lipogenesis and tumor cell proliferation, which plays an essential role in HCC progression.38 Liver and adipose tissue are the main organs associated with lipid metabolism. Therefore, it is crucial to investigate hepatokines and adipokines, which could be both diagnostic and therapeutic targets, along with the signaling pathways targeted by current treatments.
One of the critical components of Western diets is high cholesterol, which is harmful when it accumulates excessively in cells because of signal transduction alterations through altered cell membrane fluidity and mitochondrial dysfunction. Cholesterol overload in the liver is associated with HCC development. However, its onset and progression require further study.40 Four genes, Slc41a3, Fabp5, Mthfd1l, and Igdcc4, are elevated in HCC and indicate a metabolic adaptation, compromising mitochondrial adaptation and macrophage participation, particularly M2-like polarization in a process induced and driven by cholesterol overload. These genes turn out to be clinically relevant in human HCC as they are associated with poor survival. Metabolic reprogramming is suggested as a critical option in the fight against cancer.41
There is also evidence of the role of the gut–liver axis and dysbiosis in the development of HCC. Animal models have proven that microbiota regulate bile acid synthesis and metabolism, affecting hepatic homeostasis and contributing to the pathogenesis of liver cancer.42 Endotoxins such as lipopolysaccharides are associated with gram-negative bacteria, which are pro-inflammatory substances that induce epithelial–mesenchymal transition by activating JNK and MAPK through TLR4 in HCC cells.43
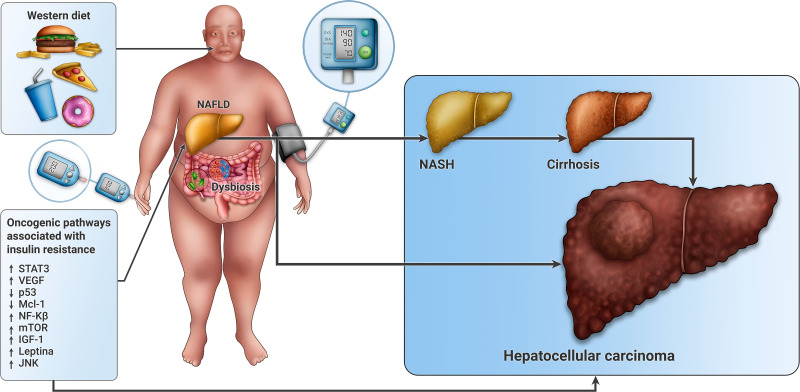
The relationship between MetS and HCC is complex (Figure 1), which makes its understanding more difficult, but its treatment far more promising. Further studies are needed to elucidate all the metabolic and oncogenic pathways that unite both clinical entities.
Figure 1.
Pathophysiology of the association between metabolic syndrome and hepatocellular carcinoma. Excess body weight, hypertension, diabetes mellitus and visceral adiposity are all components of MetS. At the center of this phenomenon lays insulin resistance and fat accumulation in hepatocytes leading to NAFLD. These factors activate prooncogenic pathways, including JNK, NF-kB, mTOR and STAT3, resulting in a tumorigenic prone environment. There is also an upregulation of IGF-1 and leptin, that results in proliferative, antiapoptotic (p53) and angiogenic effects (VEGF) that lead to cancer cell proliferation. Western diet is associated to signal transduction alterations through changes in cell membrane fluidity and to mitochondrial dysfunction that has also been associated with carcinogenesis. Microbiota is involved in the regulation of bile acids synthesis and metabolism, thus affecting hepatic homeostasis. These processes can lead to HCC through cirrhosis or independently.
Clinical Scenarios
The spectrum of backgrounds in which HCC can develop is very diverse, and from a previously seemingly healthy patient with no history of hepatic disease, an individual can become a decompensated cirrhotic with liver cancer as part of their terminal chronic hepatopathy.
The most common form of HCC arises in the context of a cirrhotic patient. Even so, about 20% of HCCs develop in a non-cirrhotic liver.44 The clinical characteristics in patients with HCC and MetS as the sole risk factor for hepatic disease represent a population that tends to be older and have more cardiovascular comorbidities compared with those with other chronic liver diseases (CLDs).
Some studies have found an elevated prevalence of HCC associated with non-cirrhotic NAFLD in males (OR: 7.774, 95% CI: 2.176–27.775), light drinkers (OR: 4.893, 95% CI: 1.923–12.449), high FIB4 index (OR: 2.634, 95% CI: 1.787–3.884),45 age > 65 years (adjusted OR: 3.37, 95% CI: 2.47–4.59), elevated alanine aminotransferase (2.69; 2.14–3.37), current smoker status (1.75; 1.23–2.49), and diabetes (1.56; 1.15–2.11) (all P < 0.05).30,46 These studies have also described a gender disparity in liver fibrosis status. While 80.5% of women with NAFLD-HCC present with advanced fibrosis, only 58.8% of males do (P = 0.03). Thus, male patients appear to develop HCC regardless of advanced fibrosis, while HCCs seem to be dependent or at least accompanied by advanced fibrosis in women.47
These data show the significant impact that HCCs in non-cirrhotic patients have on the prevalence of the disease. Regarding the clinical presentation, 50% of patients initially report impaired performance status, and 47% present with symptoms due to the tumor. These symptoms are nonspecific, requiring high clinical suspicion because there are no clear screening guidelines for this set of patients. HCCs tend to be diagnosed at later stages compared with their cirrhotic counterparts, and consequently, these patients have a worse prognosis.48
Among patients with NASH, a cross-sectional multicenter study examined 87 patients with histologically proven diagnoses who developed HCC. The relevant clinical data collected at the time of HCC diagnosis indicated a median age of 72 years, 62% male, and the presence of obesity, diabetes, dyslipidemia, and hypertension in 62%, 59%, 28%, and 55% of patients, respectively. In nontumorous liver tissue, the degree of fibrosis was stage 1 in 11%, stage 2 in 17%, stage 3 in 21%, and stage 4 in 51%.46,49 This evidence shows, once again, that components of MetS are highly prevalent in patients with CLDs that progress to HCC and that bridging fibrosis or cirrhosis is not required for the development of HCC.
Treatment of Metabolic Syndrome and Its Effects on Hepatocellular Carcinoma Risk
Each component of MetS may increase cancer risk, and when components interact with each other, a synergistic effect can occur. Even though this can be discouraging, it is essential to keep in mind that these risk factors are modifiable, and if lifestyle modifications are made and maintained, the risk of HCC can be minimized.
Among the components, excess body weight and insulin resistance have the highest level of evidence. In a recent systematic review, the potential role of diet in the development of HCC was suggested. Exposure to aflatoxin, heavy alcohol drinking, and possibly the intake of dairy products (except yogurt) increase the risk of liver cancer. By contrast, the intake of coffee, fish, tea, light-to-moderate alcohol drinking, and several healthy dietary patterns may decrease liver cancer risk. Among the dietary patterns, the Mediterranean diet (OR: 0.51, 95% CI: 0.34–0.75), Chinese healthy eating index (OR: 0.43, 95% CI: 0.38–0.50), urban prudent dietary pattern (OR: 0.25, 95% CI: 0.18–0.35), and traditional Cantonese diet (OR: 0.61, 95% CI: 0.46–0.82;), which include food groups such as vegetables, whole grains, fish, coffee, poultry, monounsaturated fatty acids, vitamin E, folate, β-carotene, manganese, and potassium, are associated with a reduced risk of HCC.50,51
Previous evidence of dietary patterns in MetS, specifically hypertension and diabetes, escape the objectives of this review, but are also crucial in modifying risk factors that may contribute to the development of HCC. Weight loss with lifestyle interventions, diet, and exercise is crucial for improving liver histology, biochemical tests, and cancer risk, specifically in NAFLD and NASH. The extent of weight loss is proportional to NASH resolution. In patients with weight loss of > 7–10% of body weight, 82–100% had a decreased NAFLD Activity score, 58–90% showed resolution of NASH, and 45% showed regression of fibrosis, thereby reducing the risk of progression to fibrosis and liver cancer. Unfortunately, only 30% of patients maintained these long-term lifestyle modifications.52
Bariatric surgery is associated with significant reductions in the risk of any cancer. There is evidence that compared with nonsurgical management, bariatric surgery is associated with a significantly lower risk of incident major adverse liver outcomes (progression to clinical or histological cirrhosis, development of HCC, liver transplantation, or liver-related mortality) and major adverse cardiovascular events (MACE; a composite of coronary artery events, cerebrovascular events, heart failure, or cardiovascular death) at 10 years. A previous study showed a 2.3% (95% CI: 0–4.6%) probability of having either of these outcomes in patients with bariatric surgery compared with 9.6% (95% CI: 6.1–12.9%) for nonsurgical treatment (adjusted absolute risk difference: 12.4% [95% CI: 5.7–19.7%]; aHR: 0.12 [95% CI: 0.02–0.63]; P = 0.01).53 In a recent retrospective cohort study, the adjusted risk of any cancer and obesity-related cancer was reduced by 18% (HR: 0.82; 95% CI: 0.76–0.89) and 25% (HR: 0.65; 95% CI: 0.56–0.75), respectively, in patients with compared to those without bariatric surgery. The adjusted risks of any cancer and obesity-related cancer were significantly lower in cirrhotic versus non-cirrhotic patients who underwent surgery.54
In patients with type 2 diabetes mellitus and MetS, studies show that regular aerobic exercise and weight loss reduce insulin resistance, improve inflammatory activity, inhibit mTOR, and activate adenosine monophosphate-activated protein kinase, which are all involved in cell growth and proliferation. Metformin has been reported to possess anti-cancer properties and glucose-lowering activity, and numerous systematic reviews and meta-analyses have studied the association between its use and cancer incidence or survival outcomes independent of the diabetic load in the population.55 Metformin appears to be associated with a lower risk of HCC in patients with type 2 diabetes (OR: 0.30, 95% CI: 0.17, 0.52; P < 0.001). The proposed antitumor mechanisms are weight loss, reduction of endogenous reactive oxygen species, and reduction of hyperinsulinemia. The evidence for metformin use for HCC risk reduction is scarce and heterogeneous, and its use is not recommended for all patients. More targeted studies are needed to elucidate the true impact of metformin in this context.56
Several studies have suggested a potential neoplastic effect for statins, independent of their lipid-lowering effect. This is probably because of the anti-inflammatory properties of statins mediated through the inhibition of JAK kinase and its pro-apoptotic effect via protein kinases, rapidly accelerated fibrosarcoma/mitogen-activated protein kinase 1/extracellular signal-regulated kinase.57 In a meta-analysis that included 18 studies, the overall result showed a significantly reduced risk of HCC (RR: 0.54, 95% CI: 0.42–0.66) in statin users compared with non-statin users. A dose-dependent effect of statin use to reduce the risk of HCC is suggested, but the dose of statins and their pharmacokinetics can only partially explain the heterogeneity in these studies.58 Another recent study of adult patients with NAFLD showed a 53% risk reduction in HCC (HR: 0.47, 95% CI: 0.36–0.60). A dose-dependent response was also seen, with a 70% risk reduction in patients in the group with the highest accumulated dose. This was shown to be true for both hydrophilic and lipophilic statins, and the effect was higher in patients with a high risk of advanced fibrosis according to the FIB4 score (HR: 0.45, 95% CI: 0.23–0.88).59
Given the pathogenesis of HCC related to MetS and NAFLD, all preventive measures should focus on following a healthy lifestyle to prevent decompensation of metabolic status and progression to NAFLD, non-alcoholic steatohepatitis, fibrosis, and cirrhosis.
Clinical Outcomes in Patients with Hepatocellular Carcinoma and Metabolic Syndrome
As described above, MetS, particularly obesity, increases the risk of atherosclerotic disease, MACE, and adverse liver outcomes. Patients with NAFLD-HCC have a worse prognosis than CLD-HCC with other etiologies, and this is related to comorbidities that limit the use of curative treatments.60
Current HCC surveillance programs are inadequate because they only screen for HCC in patients with cirrhosis. By contrast, in MetS and NAFLD, a significant proportion of HCCs develop in the absence of cirrhosis, and these patients are not considered for screening. For this reason, patients with MetS and NAFLD often present with a more advanced stage of HCC and a poorer prognosis compared with other etiologies. NAFLD-related HCC has characteristics that worsen the prognosis, such as an infiltrative pattern, larger tumor size, and more macrovascular invasion. Therefore, patients with non-cirrhotic HCC are not commonly eligible for liver transplantation, as they do not meet the Milan criteria; moreover, they are less frequently diagnosed during tumor surveillance, further decreasing the likelihood of liver transplantation.49,61
Patients with NAFLD-HCC who undergo liver resection or orthotopic liver transplantation have worse perioperative and short-term cardiovascular, respiratory, and infectious complications linked to obesity and diabetes. However, their overall long-term survival is comparable with HCC with other etiologies.49,61
Careful consideration is also essential for nonsurgical treatments. Although evidence supporting treatment selection is frequently lacking, as these patients tend to be poorly represented in clinical trials, locoregional therapies such as percutaneous ablation and transarterial chemoembolization may be less well-tolerated and less effective in patients with NAFLD with obesity or diabetes. The tyrosine kinase inhibitor, sorafenib, may also be less effective.60,62
The recurrence rate was significantly lower in the non-cirrhotic HCC than in the cirrhotic HCC group, with risk factors being des-γ-carboxy prothrombin and the number of HCCs. The non-cirrhotic HCC group showed significantly better survival because of the absence of noncancerous liver failure.63
Screening for Hepatocellular Carcinoma in Patients with Metabolic Syndrome
With the advent of curative treatments for chronic hepatitis C infection, and large-scale vaccination efforts for HBV, MetS is anticipated to become the most common cause of HCC in developed countries.64 The prognosis is dismal except in patients diagnosed early and receiving curative treatment. Therefore, HCC surveillance is recommended in high-risk patients as defined by chronic HBV infection, advanced fibrosis, or cirrhosis. The American Association for the Study of Liver Diseases and the European Association for the Study of the Liver guidelines recommend screening by performing liver ultrasonography and measuring serum α-fetoprotein every 6 months in at-risk populations.65,66 HCC surveillance is associated with increased tumor detection (OR: 2.08; 95% CI: 1.80–2.37), increased receipt of curative treatment (OR: 2.24; 95% CI: 1.99–2.52), and improved 3-year survival (OR: 1.90; 95% CI: 1.67–2.17).67
Some concerns remain about the surveillance of patients with NAFLD-related cirrhosis. A higher prevalence of obesity may further impair ultrasound performance and an increased risk of liver and non-liver-related mortality in these patients. Finally, several cohort studies and decision analyses suggest that screening patients with NAFLD with bridging fibrosis or cirrhosis could be reasonable and cost-effective, but not in patients with F0–F2.68 Studies of screening in non-cirrhotic patients with NAFLD and patients with MetS are not sufficient. Nevertheless, patients with NAFLD and MetS need to be followed closely and signs of chronic hepatic disease should be monitored proactively, thereby increasing the possibility of a timely diagnosis of any liver complications secondary to these diseases.
Conclusion
HCC is the sixth most common type of cancer, and its prognosis is very poor when its diagnosis is delayed. Its relationship with another growing pandemic: MetS is clear, especially with excess body weight and diabetes mellitus. The role that hypertension and dyslipidemia play are less understood. More research is needed to improve the quality of evidence on this pressing matter. Lifestyle modifications, such as physical activity and certain diets, are associated with risk improvement; and pharmacologic and chirurgical treatments have shown promising results. Screening in patients with MetS without bridging fibrosis or cirrhosis is not recommended so far, but its benefits has been scarcely researched.
The evidence of the intertwined relationship between MetS and HCC has increased in the past decade. With the increasing prevalence MetS, healthcare professionals need to be aware of the complexities of this relationship and should keep patients with MetS under close surveillance with tight control of their metabolic comorbidities, thereby reducing their cardiovascular, cirrhotic, and diabetic risks, but perhaps more importantly, their cancer risk, including that of HCC.
Disclosure
The authors declare that they have not received specific funding or aid from the public or private sector to carry out this review. The authors declare that they have no conflict of interest.
References
- 1.World Health Organization. Globocan; 2020. Available from: https://gco.iarc.fr/today/online-analysis-pie?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=total&sex=0&cancer=39&type=0&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17items=7&group. Accessed September 5, 2022.
- 2.Petrick JL, McGlynn KA. The changing epidemiology of primary liver cancer. Curr Epidemiol Rep. 2019;6(2):104–111. doi: 10.1007/s40471-019-00188-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;10:1–17. doi: 10.3389/fonc.2020.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61(1):191–199. doi: 10.1002/hep.27388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jinjuvadia R, Patel S, Liangpunsakul S. The association between metabolic syndrome and hepatocellular carcinoma: systemic review and meta-analysis. J Clin Gastroenterol. 2014;48(2):172–177. doi: 10.1097/MCG.0b013e3182a030c4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Wspolczesna Onkol. 2018;22(3):141–150. doi: 10.5114/wo.2018.78941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soiza RL, Donaldson AIC, Myint PK. Vaccine against arteriosclerosis: an update. Ther Adv Vaccines. 2018;9(6):259–261. doi: 10.1177/https [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012;35(11):2402–2411. doi: 10.2337/dc12-0336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elabbassi WN, Haddad HA. The epidemic of the metabolic syndrome. Saudi Med J. 2005;26(3):373–375. [PubMed] [Google Scholar]
- 10.Chen Y, Li X, Wu S, Ye W, Lou L. Metabolic syndrome and the incidence of hepatocellular carcinoma: a meta-analysis of cohort studies. Onco Targets Ther. 2018;11:6277–6285. doi: 10.2147/OTT.S154848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paradis V, Zalisnski S, Chelbi E, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49(3):851–859. doi: 10.1002/hep.22734 [DOI] [PubMed] [Google Scholar]
- 12.Welzel T, Graubard B, McGlynn K. Metabolic syndrome increases the risk of primary liver cancer (HCC, CCC) in the United States: a population-based case-control study. Z Gastroenterol. 2009;47(06):463–471. doi: 10.1055/s-0029-1225717 [DOI] [Google Scholar]
- 13.Ren H, Wang J, Gao Y, Yang F, Huang W. Metabolic syndrome and liver-related events: a systematic review and meta-analysis. BMC Endocr Disord. 2019;19(1):1–13. doi: 10.1186/s12902-019-0366-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung H, Siegel RL, Torre LA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2018;69:88–112. doi: 10.3322/caac.21499 [DOI] [PubMed] [Google Scholar]
- 15.Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer. 2010;126(3):692–702. doi: 10.1002/ijc.24803 [DOI] [PubMed] [Google Scholar]
- 16.Pearson-Stuttard J, Zhou B, Kontis V, Bentham J, Gunter MJ, Ezzati M. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol. 2018;6(6):e6–e15. doi: 10.1016/S2213-8587(18)30150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma - Epidemiological evidence. Aliment Pharmacol Ther. 2010;31(10):1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x [DOI] [PubMed] [Google Scholar]
- 18.Borena W, Strohmaier S, Lukanova A, et al. Metabolic risk factors and primary liver cancer in a prospective study of 578,700 adults. Int J Cancer. 2012;131(1):193–200. doi: 10.1002/ijc.26338 [DOI] [PubMed] [Google Scholar]
- 19.Campbell PT, Newton CC, Freedman ND, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for U.S. adults. Cancer Res. 2016;76(20):6076–6083. doi: 10.1158/0008-5472.CAN-16-0787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothwell JA, Jenab M, Karimi M, et al. Metabolic syndrome and risk of gastrointestinal cancers: an investigation using large-scale molecular data. Clin Gastroenterol Hepatol. 2021;20(6):e1338–e1352. doi: 10.1016/j.cgh.2021.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Diabetes. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed May 9, 2022.
- 22.Welzel TM, Graubard BI, Zeuzem S, El-Serag HB, Davila JA, Mcglynn KA. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-medicare database. Hepatology. 2011;54(2):463–471. doi: 10.1002/hep.24397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. High cholesterol facts | cdc.gov. Available from: https://www.cdc.gov/cholesterol/facts.htm. Accessed May 9, 2022.
- 24.Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Prevalence of US adults with triglycerides ≥ 150 mg/dl: NHANES 2007–2014. Cardiol Ther. 2020;9(1):207–213. doi: 10.1007/s40119-020-00170-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho Y, Cho EJ, Yoo JJ, et al. Association between lipid profiles and the incidence of hepatocellular carcinoma: a nationwide population-based study. Cancers. 2021;13(7):1599. doi: 10.3390/cancers13071599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasak AM, Pfeiffer RM, Brant LJ, et al. Time-dependent association of total serum cholesterol and cancer incidence in a cohort of 172 210 men and women: a prospective 19-year follow-up study. Ann Oncol. 2009;20(6):1113–1120. doi: 10.1093/annonc/mdn736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang JT, Xu N, Zhang XY, Wu CP. Lipids changes in liver cancer. J Zhejiang Univ Sci B. 2007;8(6):398–409. doi: 10.1631/jzus.2007.B0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rahman R. Primary hepatocellular carcinoma and metabolic syndrome: an update. World J Gastrointest Oncol. 2013;5(9):186. doi: 10.4251/wjgo.v5.i9.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kant P, Hull MA. Excess body weight and obesity-The link with gastrointestinal and hepatobiliary cancer. Nat Rev Gastroenterol Hepatol. 2011;8(4):224–238. doi: 10.1038/nrgastro.2011.23 [DOI] [PubMed] [Google Scholar]
- 30.Pinyopornpanish K, Khoudari G, Saleh MA, et al. Hepatocellular carcinoma in nonalcoholic fatty liver disease with or without cirrhosis: a population-based study. BMC Gastroenterol. 2021;21(1):1–7. doi: 10.1186/s12876-021-01978-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, He J, Tao X, et al. miR-20b negatively regulates VEGF expression by targeting STAT3 in H22 hepatocellular carcinoma cells. Oncol Rep. 2018;40(5):2806–2813. doi: 10.3892/or.2018.6651 [DOI] [PubMed] [Google Scholar]
- 32.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 33.Domínguez-Pérez M, Simoni-Nieves A, Rosales P, et al. Cholesterol burden in the liver induces mitochondrial dynamic changes and resistance to apoptosis. J Cell Physiol. 2019;234(5):7213–7223. doi: 10.1002/jcp.27474 [DOI] [PubMed] [Google Scholar]
- 34.Sharma D, Wang J, Fu PP, et al. NIH public access. Hepatology. 2011;52(5):1713–1722. DOI: 10.1002/hep.23892 [DOI] [Google Scholar]
- 35.Al-Gayyar MMH, Abbas A, Hamdan AM. Chemopreventive and hepatoprotective roles of adiponectin (SULF2 inhibitor) in hepatocellular carcinoma. Biol Chem. 2016;397(3):257–267. doi: 10.1515/hsz-2015-0265 [DOI] [PubMed] [Google Scholar]
- 36.Wei R, Hu Y, Dong F, Xu X, Hu A, Gao G. Hepatoma cell-derived leptin downregulates the immunosuppressive function of regulatory T-cells to enhance the anti-tumor activity of CD8 + T-cells. Immunol Cell Biol. 2016;94:388–399. doi: 10.1038/icb.2015.110 [DOI] [PubMed] [Google Scholar]
- 37.Peter A, Kovarova M, Staiger H, et al. The Hepatokines fetuin-A and fetuin-B are upregulated in the state of hepatic steatosis and may differently impact on glucose homeostasis in humans. Am J Physiol Endocrinol Metabol. 2018;314:E266–E273. doi: 10.1152/ajpendo.00262.2017 [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Chen X, Zhang H, et al. Circulating retinol-binding protein 4 is associated with the development and regression of non-alcoholic fatty liver disease. Diabetes Metab. 2020;46(2):119–128. doi: 10.1016/j.diabet.2019.04.009 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhang W, Doughtie A, et al. Up-regulation of fibroblast growth factor 19 and its receptor associates with progression from fatty liver to hepatocellular carcinoma. Oncotarget. 2016;7(32):52329–52339. doi: 10.18632/oncotarget.10750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Ruiz C, Conde de la Rosa L, Ribas V, Fernandez-Checa JC. Mitochondrial Cholesterol and Cancer. Semin Cancer Biol. 2021;73:76–85. doi: 10.1016/j.semcancer.2020.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simoni-Nieves A, Salas-Silva S, Chávez-Rodríguez L, et al. The consumption of cholesterol-enriched diets conditions the development of a subtype of hcc with high aggressiveness and poor prognosis. Cancers. 2021;13(7):1721. doi: 10.3390/cancers13071721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu L, Feng J, Li J, et al. The gut microbiome-bile acid axis in hepatocarcinogenesis. Biomed Pharmacother. 2021;133:111036. doi: 10.1016/j.biopha.2020.111036 [DOI] [PubMed] [Google Scholar]
- 43.Li H, Li Y, Liu D, Liu J. LPS promotes epithelial–mesenchymal transition and activation of TLR4/JNK signaling. Tumor Biol. 2014;35(10):10429–10435. doi: 10.1007/s13277-014-2347-5 [DOI] [PubMed] [Google Scholar]
- 44.Desai A, Sandhu S, Lai JP, Sandhu DS. Hepatocellular carcinoma in non-cirrhotic liver: a comprehensive review. World J Hepatol. 2019;11(1):1–18. doi: 10.4254/wjh.v11.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tobari M, Hashimoto E, Taniai M, et al. The characteristics and risk factors of hepatocellular carcinoma in nonalcoholic fatty liver disease without cirrhosis. J Gastroenterol Hepatol. 2020;35(5):862–869. doi: 10.1111/jgh.14867 [DOI] [PubMed] [Google Scholar]
- 46.Yasui K, Hashimoto E, Komorizono Y, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9(5):428–433. doi: 10.1016/j.cgh.2011.01.023 [DOI] [PubMed] [Google Scholar]
- 47.Zampaglione L, Ferrari J, Pedica F, Goossens N. Hcc in metabolic syndrome: current concepts and future directions. Hepatoma Res. 2021;7. doi: 10.20517/2394-5079.2021.22 [DOI] [Google Scholar]
- 48.Schütte K, Schulz C, Poranzke J, et al. Characterization and prognosis of patients with hepatocellular carcinoma (HCC) in the non-cirrhotic liver. BMC Gastroenterol. 2014;14(1):1–10. doi: 10.1186/1471-230X-14-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn SY, Kim SB, Song IH. Clinical patterns and outcome of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Can J Gastroenterol Hepatol. 2020;2020:1–9. doi: 10.1155/2020/4873875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang WS, Zeng XF, Liu ZN, et al. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124(3):330–340. doi: 10.1017/S0007114520001208 [DOI] [PubMed] [Google Scholar]
- 51.George ES, Sood S, Broughton A, et al. The association between diet and hepatocellular carcinoma: a systematic review. Nutrients. 2021;13(1):1–23. doi: 10.3390/nu13010172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Promrat K, Kleiner DE, Niemeier HM, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aminian A, Al-Kurd A, Wilson R, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA. 2021;326(20):2031–2042. doi: 10.1001/jama.2021.19569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rustgi VK, Li Y, Gupta K, et al. Bariatric surgery reduces cancer risk in adults with nonalcoholic fatty liver disease and severe obesity. Gastroenterology. 2021;161(1):171–184.e10. doi: 10.1053/j.gastro.2021.03.021 [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Zhong X, Gao P, et al. The potential effect of metformin on cancer: an umbrella review. Front Endocrinol. 2019;10. doi: 10.3389/fendo.2019.00617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, type 2 diabetes, and cancer risk. Front Oncol. 2021;10. doi: 10.3389/fonc.2020.615375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mak L, Cruz-ramón V, Chinchilla-lópez P, et al. Global epidemiology, prevention, and management of global epidemiology of HBV. Am Soc Clin Oncol. 2019;262–279. doi: 10.1200/EDBK_200939 [DOI] [PubMed] [Google Scholar]
- 58.Chang Y, Liu Q, Zhou Z, et al. Can statin treatment reduce the risk of hepatocellular carcinoma? A systematic review and meta-analysis. Technol Cancer Res Treat. 2020;19(403):1–11. doi: 10.1177/1533033820934881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou B, Odden MC, Nguyen MH. Statin use and reduced hepatocellular carcinoma risk in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2022. doi: 10.1016/j.cgh.2022.01.057 [DOI] [PubMed] [Google Scholar]
- 60.Geh D, Manas DM, Reeves HL. Hepatocellular carcinoma in non-alcoholic fatty liver disease—a review of an emerging challenge facing clinicians. Hepatobiliary Surg Nutr. 2021;10(1):59–75. doi: 10.21037/hbsn.2019.08.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labenz C, Prenosil V, Koch S, et al. Impact of individual components of the metabolic syndrome on the outcome of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Dis. 2017;36(1):78–88. doi: 10.1159/000477578 [DOI] [PubMed] [Google Scholar]
- 62.Li X, Wang Y, Ye X, Liang P. Locoregional combined with systemic therapies for advanced hepatocellular carcinoma: an inevitable trend of rapid development. Front Mol Biosci. 2021;8:1–11. doi: 10.3389/fmolb.2021.635243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bengtsson B, Stål P, Wahlin S, Björkström NK, Hagström H. Characteristics and outcome of hepatocellular carcinoma in patients with NAFLD without cirrhosis. Liver Int. 2019;39(6):1098–1108. doi: 10.1111/liv.14087 [DOI] [PubMed] [Google Scholar]
- 64.Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: a multicenter matched analysis with HCV-related HCC. J Hepatol. 2015;63(1):93–101. doi: 10.1016/j.jhep.2015.01.024 [DOI] [PubMed] [Google Scholar]
- 65.Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 66.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 67.Singal AG, Pillai A, Tiro J, Klenerman P. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624. doi: 10.1371/journal.pmed.1001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Singal AG, El-Serag HB. Rational HCC screening approaches for patients with NAFLD. J Hepatol. 2022;76(1):195–201. doi: 10.1016/j.jhep.2021.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]