Abstract
Autism spectrum disorder (ASD) is a developmental disorder that is characterized by difficulties with social interaction and interpersonal communication. It has been argued that abnormal attentional function to exogenous stimuli precedes and contributes to the core ASD symptoms. Notably, the locus ceruleus (LC) and its noradrenergic projections throughout the brain modulate attentional function, but the extent to which this locus ceruleus–norepinephrine (LC–NE) system influences attention in individuals with ASD, who frequently exhibit dysregulated alerting and attention orienting, is unknown. We examined dynamic attention control in girls and boys with ASD at rest using the pupil dilation response (PDR) as a noninvasive measure of LC–NE activity. When gender- and age-matched neurotypical participants were passively exposed to an auditory stream, their PDR decreased for recurrent stimuli but remained sensitive to surprising deviant stimuli. In contrast, children with ASD showed less habituation to recurrent stimuli as well as a diminished phasic response to deviants, particularly those containing social information. Their tonic habituation impairment predicts their phasic orienting impairment, and both impairments correlated with the severity of ASD symptom. Because of the fact that these pupil-linked responses are observed when individuals passively listen without any task engagement, our findings imply that the intricate and dynamic attention allocation mechanism, mediated by the subcortical LC–NE system, is impaired in ASD.
SIGNIFICANCE STATEMENT Autistic individuals show attentional abnormalities to even simple sensory inputs, which emerge even before formal diagnosis. One possible mechanism behind these abnormalities is a malfunctioning pacemaker of their attention system, the locus ceruleus–norepinephrine pathway. Here we found, according to the pupillary response (a noradrenergic activity proxy), autistic children are hypersensitive to repeated sounds but hyposensitive to surprising deviant sounds when compared with age-matched controls. Importantly, hypersensitivity to repetitions predicts hyposensitivity to deviant sounds, and both abnormalities positively correlate to the severity of autistic symptoms. This provides strong evidence that autistic children have faulty noradrenergic regulation, which might underly the attentional atypicalities previously evidenced in various cortical responses in autistic individuals.
Keywords: attention, autism spectrum disorder, locus ceruleus, norepinephrine, pupil dilation response
Introduction
Alerting and attention orienting reflect our evolving awareness to environmental information. What naturally captures our attention and what we choose to focus on has an impact on our experiences and perceptions of the world around us as well as the direction in which our brain develops. Although autism spectrum disorder (ASD) is primarily defined and diagnosed by impaired social and communication skills and by repetitive and stereotypical behaviors (American Psychiatric Association, 2013), attentional abnormalities have been noticed since its initial description in 1943 (Kanner, 1943) and increasingly recognized as preceding and thus contributing to the core ASD symptoms (Bryson et al., 1990; Rogers and Ozonoff, 2005; Keehn et al., 2013). However, the role of the subcortical system in such unusual alerting response remains little understood (Orekhova and Stroganova, 2014; Bast et al., 2018).
Norepinephrine (NE), a neuromodulator released exclusively by the midbrain-located locus ceruleus (LC; Sara, 2009), is associated with arousal regulation and attention orienting (Sara and Bouret, 2012). Specifically, phasic noradrenergic activity in the LC has been implicated to signal a form of surprise and act as a resetting switch in the brain to interrupt existing internal models of the world (Ego-Stengel et al., 2002) and to effectively adapt to the environment (Bouret and Sara, 2005; Dayan and Yu, 2006; Hermans et al., 2011; Sara and Bouret, 2012; Marshall et al., 2016). The LC–NE system, as the pacemaker of the attention system, has hypothesized ties with autism since infancy (Cohen et al., 2013; Bast et al., 2018). However, it is still unknown whether and how the LC–NE system contributes to attention deficits in people with ASD, particularly in youngsters (de Vries et al., 2021).
Here, we sought to measure the pupil-linked phasic arousal response, pupil dilation response (PDR) to assess the functionality of LC–NE among children with ASD. Spiking activity in the LC and changes in pupil size are linked mechanistically, whether they occur spontaneously or as a result of external stimuli (Aston-Jones and Cohen, 2005; Joshi et al., 2016; Reimer et al., 2016). To examine the involvement of LC–NE in alerting, we used an auditory oddball paradigm, where the participants were presented with a stream of repetitive stimuli (standard) embedded with less-frequent, randomly interspersed oddball stimuli (deviant). Importantly, they listened to the stimuli passively, unconstrained by any volitional task or potential confounds of motivation, task performance, or cognitive differences, allowing for analysis of involuntary attention control as in its default resting state. This paradigm has been used extensively to research the attention processes among the ASD population. However, past research has mostly focused on cortical responses using EEG and fMRI (Keehn et al., 2013; Bast et al., 2018). Thus, it remains unknown whether ASD has a typically functioning LC–NE system underpinning automatic attention control. On the other hand, PDR has been examined among children with ASD as a window into the malfunctioning of their autonomic nervous system. However, most studies focused on responses to a simple light flash or to complex visual stimuli (de Vries et al., 2021).
We propose that neurotypical children are capable of allocating their attention resources in a normatively optimal fashion, that is, by decreasing their tonic reaction to repeated stimuli (i.e., standards) while maintaining their phasic response to surprising stimuli (i.e., deviants). By contrast, we hypothesize that children with ASD are less responsive to surprising stimuli based on previous findings on their cortical responses (e.g., Keehn et al., 2013) and that this diminished surprise reaction is accompanied by hypersensitivity to nonsurprising stimuli, manifesting as decreased habituation to repetitive standards. This is precisely what we saw here with pupil responses; although neurotypical children habituated to repetitive stimuli yet remain receptive to deviants, ASD children habituated less, predicting a decline in arousal response to deviants. Relevant to autism symptoms, this disruption in attention was more pronounced for stimuli that contained social information and predicted autistic severity on an individual level. Given that our participants listened passively without task engagements, our study offers support for a basic LC–NE system dysfunction underpinning the ASD-specific impairment in attention orienting (Dawson and Lewy, 1989; Keehn et al., 2013).
Materials and Methods
Ethics
The experiment was conducted according to the principles of the Declaration of Helsinki and was approved by the Ethical Committee of the School of Psychological and Cognitive Sciences at Peking University. We obtained written consent from the primary caregivers of the children and verbal consent from the children themselves. All children received toys and cartoon stickers as gifts for their participation.
Participants
Sample size
Because of the lack of knowledge in pupillary metrics in young ASD children responding to the stimuli used in the present study, we could not conduct a standard power analysis to estimate adequate sample size before data collection. Originally, we aimed for a sample size of ∼20 based on previous data from prior auditory pupillometry studies on healthy adults (Liao et al., 2016b; Zhao et al., 2019a) with similar auditory stimuli (brief sounds) and similar measurements (phasic PDR), where robust PDR effects evoked by brief sounds were observed using as few as 10 participants. However, somewhat unsurprisingly, there is a large amount of data loss in young children's pupil data, especially among individuals with ASD. To ensure that the observed effect is not caused by a difference in data quality, we used a strict data exclusion routine (details below). Eventually, we recruited as many participants as possible to maximize the effect size. This was not ideal but hard to prevent because of the nature of this study and its participants. We managed to recruit all young ASD children who attended the school on the day of data collection. We had intended to match the control sample size with the ASD, however data collection for the control participants was abruptly stopped because of the COVID-19 pandemic. This resulted in a nonideal sample size imbalance. In total, 52 children diagnosed with autism spectrum disorders and 35 chronological age-matched typically developed children took part in this study. Twelve ASD and four TD participants were excluded (see below, Data exclusion), providing a final sample size of 40 ASD participants (three females, mean age 5.8 years old, SD 0.5, range 4.6–7.0) and 31 TD participants (three females, mean age 5.8, SD 0.5, range 5.1–7.0).
To minimize the potential artifact caused by the unequal sample sizes (ASD 40 vs TD 31), we chose to perform the time series statistical analysis in a permutation-based manner and the correlation analysis on a collapsed dataset from both groups (see below, Data analysis).
All children had normal vision and hearing. Their general intelligence was measured using the Chinese version of the Wechsler Preschool and Primary Scale of Intelligence, fourth edition (Wechsler, 2012). All children with ASD had been previously diagnosed by two professional pediatric clinicians based on the Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, 2013). The control group consists of individuals with neurotypical development, defined as those who do not have any parent-reported psychiatric or neurodevelopmental diagnoses and do not exhibit characteristics of autism. We also measured the severity of autism symptoms using the Chinese version of the Autism Spectrum Quotient: Children's Version (AQ; Auyeung et al., 2008). All ASD participants scored higher than the cutoff score of 76 (Auyeung et al., 2008), and none of the TD children met the diagnosis criterion based on AQ scores. The Social Responsiveness Scale (SRS; Constantino et al., 2003) was also measured, and relevant results are reported in Extended Data Figures 1-1, 1-2, 1-3, 1-4. The ASD group scored significantly higher on the AQ [t(69) = 8.76, p < 0.0001, Bayesian factor (BF) > 107] and SRS (t(69) = 12.4, p < 0.0001, BF > 1012) than the TD group, but scored lower on full-scale IQ (t(69) = −2.8, p < 0.01, BF = 6.5). There were no significant differences between the ASD and TD groups in chronological age (t(69) = −0.2, p = 0.9, BF = 4.0) or gender (χ2(1,6) = 0.1, p = 0.7). Table 1 contains more details about the characteristics of the two groups.
Table 1.
Participant description
| Measure | ASD | TD | Statistics, ASD vs TD |
|---|---|---|---|
| N | 40 | 31 | NA |
| Gender, female (%) | 3 (7.5) | 3 (9.7) | χ2(1, N = 6) = 0.1, p = 0.7 |
| Age range | 4.6–7.0 | 5.1–7.0 | NA |
| Mean age (SD) | 5.8 (0.5) | 5.8 (0.5) | t(69) = −0.2, p = 0.9, BF = 4.0 |
| FSIQ, mean score (SD) | 111.9 (12.2) | 120.3 (12.9) | t(69) = −2.8, p < 0.01, BF = 6.5 |
| AQ, mean score (SD) | 87.8 (15.5) | 57.2 (13.3) | t(69) = 8.8, p < 0.0001, BF > 10^7 |
| SRS, mean score (SD) | 92.2 (21.4) | 39.0 (12.1) | t(69) = 12.4, p < 0.0001, BF > 10^12 |
Description of participants of the ASD group and the TD group; t and χ2 tests were used to assess between-group differences, with BF reported. Multiple comparisons were corrected. FSIQ and verbal IQ were measured by the Chinese version of Wechsler Preschool and Primary Scale of Intelligence, fourth edition. FSIQ, Full-scale intelligence quotient. NA, not applicable.
Standard_tone_500Hz Download Figure 1-1, WAV file (43.1KB, wav)
Deviant1_noise Download Figure 1-2, WAV file (43.1KB, wav)
Deviant2_laughter_female Download Figure 1-3, WAV file (43.1KB, wav)
Deviant2_laughter_male Download Figure 1-4, WAV file (43.1KB, wav)
Stimuli
We used a simple auditory oddball paradigm, with the standard stimulus as a 500 Hz pure tone (75%) against two deviant stimuli, a white noise (12.5%) and a real laughter sound (12.5%). The former deviant contains no social information, whereas the latter does. The reason to have these two distinct deviants is that a few previous studies have revealed that ASD-related abnormal cortical responses in the oddball paradigm differ between social (e.g., speech) and nonsocial stimuli (e.g., nonspeech tone) (Ceponiene et al., 2003; Whitehouse and Bishop, 2008). It remains unknown whether this distinction would persist for the LC–NE system-mediated attention response. All sounds were 500 ms long with 30 ms ramps on both sides and RMS equated (Extended Data Figures 1-1, 1-2, 1-3, 1-4, sound files). The sound duration was chosen because a previous study (Lima et al., 2019) showed that human laughter could be accurately recognized within 500 ms after the stimulus onset. To avoid the effect of specific gender voice on the result, we used two laughter stimuli, one female and one male, both Chinese speakers in their 20s. The recorded laughter was induced by watching funny videos to avoid possible confounds induced by fake laughter. The two laughter sounds were counterbalanced across participants, and no difference was observed between these two laughter sounds, thus in subsequent analysis, the data from these two laughter sounds were collapsed.
The intersound interval was randomized between 3 and 3.5 s. From a previous pupillometry study (Zhao et al., 2019b) using similar sound durations and experimental procedures (passive listening) in healthy young adults, this is a suitable silent interval to allow the sound-evoked pupil dilation response to evolve and return to baseline.
Participants completed two blocks of trials. Each block lasted ∼2.5 min, with 40 stimuli delivered in total. Thus, a block would include 30 standard trials, five white noise trials, and five laughter trials. The first five stimuli were the standard trials, and all other trials were presented in pseudorandom order to avoid the presentation of consecutive deviants; this constraint was set to avoid the effect of accumulated pupil dilation responses evoked by deviants.
Before the beginning of each block, we also measured the pupil diameter baseline for each individual over a 15 s resting state in silence.
Procedure
The experiment was conducted in a quiet classroom with controlled lighting. To ensure the luminosity of the room stayed unchanged, a digital light meter (TES-1332A, TES Electrical Electronic) was used to measure the luminosity of the room before each testing session to ensure the luminance level stayed at 35 lux.
Participants sat with their heads fixed on a chinrest ∼65 cm away from a monitor (23 inch LCD with a resolution of 1920 × 1080 pixels and a refresh rate of 60 Hz). The children with ASD were familiarized with the chinrest and headphones 1 week before the experiment with the help of their teachers and their caregivers. None of the children expressed verbal or physical aversion to the headphone or the chinrest during the experiment.
Eye movement and pupil diameter were recorded using a Tobii X120 binocular eye tracker (sampling rate, 120 Hz) during the experiment. All children completed a five-point calibration procedure before the start of testing. The calibration was thought to be successful when both eyes achieved good mapping on all five test positions (average error < 1 of visual angle).
Auditory stimuli were delivered diotically to the participants' ears with Sennheiser HD558 headphones via a Conexant SmartAudio HD sound card (Synaptics). The volume of the sound played through the headphones was adjusted by each participant to a comfortable level before the experiment. Stimuli were presented in a random order and controlled with the Psychtoolbox package (Psychophysics Toolbox Version 3; Brainard, 1997) on MATLAB (MathWorks, version R2018b).
The entire experimental session lasted ∼20 min. Before the experiment, children played in the classroom for 5–10 min to familiarize themselves with the environment. Then participants first completed a 15 s resting session in which they were instructed to fixate continuously on a black reciprocal number presented at the center of the screen against a gray background, flipping from 45 to 41. During the experiment, participants were required to passively listen to a series of sounds without any behavioral response and were instructed to remain quiet and static. Because of the difficulty of sustaining young children's attention for extended periods, it was challenging to keep participants fixating at the cross for >2.5 min (the length of one block) during passive listening. We thus presented a countdown number at the center of the screen to attract the participants' attention. During the resting state, the number changed every 3 s. During the experiment, the number changed 2 s after the sound onset, that is, in the middle of the intersound interval, and the change moment did not overlap with the sound presentation. Coupled with 40 sounds, the countdown number changed from 40 to 01. The size of the number was 200 × 200 pixels (4.7 of visual angle), and the brightness of all numbers was equalized and displayed in black. From our experience of the preliminary experiment, numbers attracted more attention than cartoon pictures for ASD children, and neither brightness change nor pupil diameter change was detected after flipping the number.
On average, our participants fixated around the center (i.e., viewing angle <2° in both horizontal and vertical axes) 86.9 ± 1.5% of the time while their eyes were open during the epochs. There was no group difference in the time on center (ASD = 85.9 ± 2.3%, TD = 88.2 ± 1.8%; t(69) = −0.7, p = 0.5, BF = 3.2).
Data analysis
Pupillometry data preprocessing
To measure the sound-evoked pupil dilation responses, the pupil diameter data of each trial was epoched from 0.5 s before to 2.5 s after sound onset. Intervals where the eye tracker detected full or partial eye closure (manifested as a loss of pupil signal), as well as a pupil diameter change velocity above 3 SDs, were automatically treated as missing data and recovered with shape-preserving piecewise cubic interpolation. Epochs with >50% missing data before sound onset or >50% missing data after sound onset were excluded from the analysis. On average, 13 trials per participant were flagged as bad trials. The first five trials (all standards) in each block were also excluded from further analysis.
The epoched data were then baseline corrected by subtracting the mean pupil diameter over the preonset interval of 0.5 s and then smoothed with an 83 ms Hamming window (sample rate = 120 Hz, we take every 10 points for the smoothed time window).
Data exclusion
All data exclusion was based on data loss because of excessive blinking, eye closure, gazing away from fixation, or unwanted head movements. If the baseline of a trial (0.5 s before the onset of sound) or the postsound epoch (0–2.5 s postsound onset) had >50% data loss, this trial was excluded. It was important to ensure that both baseline and the postsound epoch had sufficient data because (1) we are interested in both pre-event baseline and event-evoked PDR measures, and (2) baseline correction was applied for each trial to get a clean normalized PDR. If the participant had fewer than three trials for any of the two conditions, this participant would be excluded from further analysis. The number of trials for each participant in each experimental condition was statistically indifferent across groups [all p values > 0.2; number of trials, ASD standard = 37.8 (SD 12.0), TD standard = 41.1 (SD 8.7); ASD noise = 6.8 (SD 2.4), TD noise = 7.5 (SD 2.2); ASD laughter = 7.3 (SD 2.6), TD laughter = 7.4 (SD 1.9)]. There was no interact effect between group and deviant types (F(1,138) = 0.8, p = 0.4), no main effect of group (F(1,138) = 0.9, p = 0.3), or main effect of deviant types (F(1,138) = 0.2, p = 0.7).
Resting pupil diameter baseline
Before the beginning of each of the two blocks, 15 s resting state in silence was recorded for pupil diameter. To compute the preblock resting pupil diameter baseline, the first 5 s was excluded to ensure that the pupil has settled.
To examination the block effect on the resting pupil diameter baseline, we first computed the median over the 10 s resting state for each block. One ASD participant was excluded for the block-effect analysis because they closed their eyes over the resting period in the second block.
As shown later in Results (Fig. 1A), we did not observe any block effect in the resting baseline. Thus, the average over both two preblock resting periods (in total 20 s) was computed as the preblock resting pupil diameter baseline for each participant for further between-individual analysis (Fig. 1B).
Figure 1.
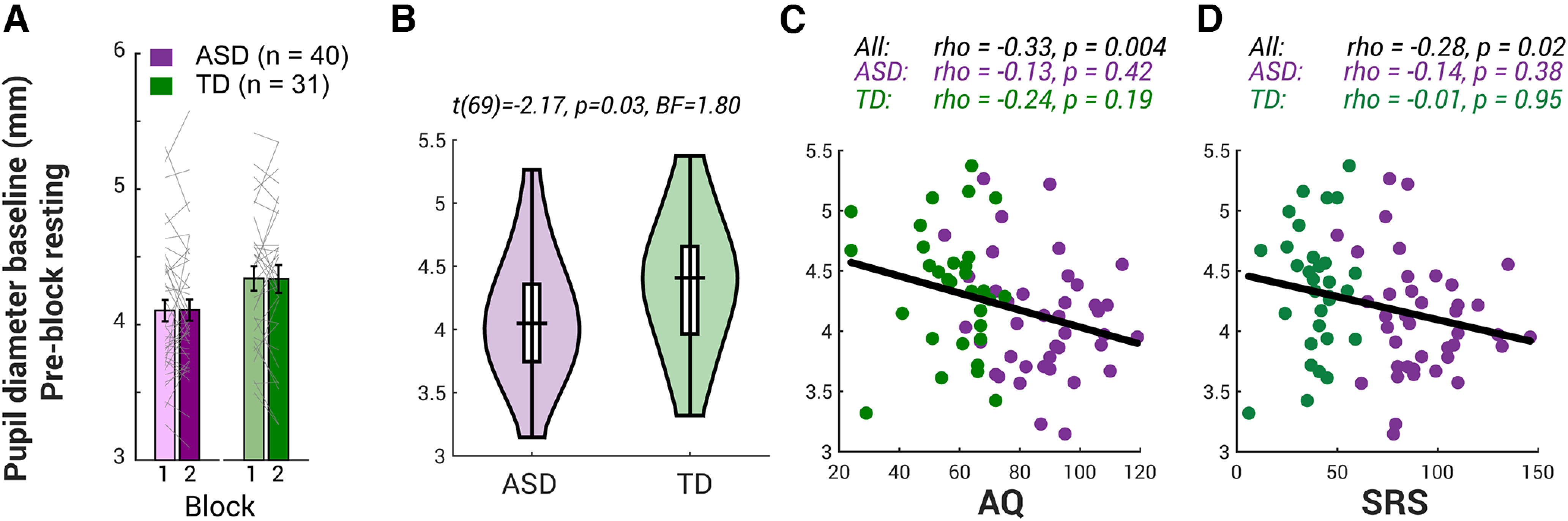
Pupil diameter baseline in ASD and TD children. A, The resting baseline was computed as the median of the 10 s preceding the first sound onset of each block. The gray lines indicate individual participant's data for both blocks. Error bar shows ±1 SEM. A two-way ANOVA on the baseline showed no significant interaction between group and block (F(1,137) = 0.009, p = 0.9, η2 < 0.001, observed power = 0.05) and no main effect of block (F(1,137) = 0.004, p = 1.0, η2 < 0.001, d = 0.05). B, The distribution of the baseline (averaged across two preblock resting periods) as a violin plot for ASD and TD children. Top, The t test results for group comparison. C, D, The baseline is strongly negatively correlated with the severity of the autistic traits AQ as well as SRS. Top, Spearman's correlation coefficients and their two-tailed p values.
To investigate the effect of the autistic severity, age, IQ, and group on the pupil baseline, a multiple linear regression was conducted using the MATLAB function fitlm.
Net PDR
A net effect measure (quantifying the size of the deviant-evoked PDR relative to its standard control) was computed for each participant by taking the difference between each deviant condition and the standard condition.
Time series statistical analysis
To identify time intervals in which a given pair of conditions exhibited PDR differences, we applied a nonparametric bootstrap-based statistical analysis (Efron and Tibshirani, 1994). The condition difference in time series was computed for each participant, and these differences were subjected to bootstrap resampling (1000 iterations with replacement). At each time point, the differences were deemed significant if the proportion of bootstrapped samples fell outside the 95% confidence interval centered on zero. For meaningful interpretation of our data, we were specifically interested in those clustered significant differences in PDR. Any significant differences in the preonset interval would be attributable to noise, and the largest number of consecutive significant samples in the preonset interval was used as the threshold for the statistical analysis for the entire epoch.
To compare the PDR between participant groups, we again used bootstrap-based resampling but based on independent samples. On each iteration, N samples (N denotes the number of participants in the ASD or TD group) were randomly selected (with replacement) from each group, and a group difference was computed based on this bootstrapped sampling. One thousand iterations were computed to derive the distribution of group differences for inferential statistics as illustrated above.
Group comparison
The t test statistics and BFs are reported. All p values reported are two tailed.
Correlation
To control for outlier effects, all reported bivariate and partial correlations were performed using the conservative Spearman's rank correlation method. Additionally, we conducted partial correlations after controlling for the effect of age, gender, baseline, and IQ when applicable. Our correlation analyses were based on collapsed data from both groups to examine the influence of relevant factors, that is, pupillometry response, severity of ASD core symptoms, and IQ, over the full, extended range in our samples. Differences in Spearman's correlation coefficients were tested using the procedures for testing statistical differences between correlations using the implementation in the R package cocor (Diedenhofen and Musch, 2015).
Data availability
The experimental datasets generated during the current study are available from the corresponding author on request.
Results
Autistic trait negatively correlates with pupil diameter baseline
We investigated the preblock resting-state baseline (median of the 10 s preceding the first sound onset of each block) for all participants. First, we examined the block effect on the resting pupil baseline (Fig. 1A), and a two-way ANOVA on the baseline showed no significant interaction between group and block (F(1,137) = 0.009, p = 0.9, η2 < 0.001, observed power(d) = 0.05) and no main effect of block (F(1,137) = 0.004, p = 1.0, η2 < 0.001, d = 0.05). However, there was a significant group effect on the baseline (F(1,137) = 7.7, p = 0.006, η2 = 0.05, d = 0.8). On average, ASD children's pupil size was 4.1 mm (SD 0.5 mm), slightly but significantly smaller than age-matched TD children (4.4 mm (SD 0.5 mm; Fig. 1B; t(69) = −2.2, p = 0.03, BF = 1.8); individuals with more severe autistic traits, quantified by AQ and SRS, have smaller tonic pupil diameter (Fig. 1C,D), whereas no such relationship was observed with age (Spearman's ρ = −0.1, p = 0.4) or IQ (ρ = 0.1, p = 0.3). However, the negative correlation between the autistic severity and the pupil diameter baseline must be taken with caution because this relationship was not significant within the diagnostic group. Moreover, we found that a multiple linear regression model with AQ, IQ, age, and the group could only explain 11.9% of the variance. The model was marginally significant (F(4,66) = 2.2, p = 0.07), and none of the individual predictors was significant (AQ, beta (standardized coefficient beta)=−0.2, p = 0.09; IQ, beta=−0.02, p = 0.7; age, beta=−0.09, p = 0.1; group, beta = 0.05, p = 0.7). This result suggests that the across-group correlation between baseline pupil diameter and AQ was largely drive by group difference.
ASD habituated less to repeating tones
To assess whether ASD exhibits a reduced repetition suppression to nonsocial stimuli, we examine the response evoked by the standards (pure tones). Figure 2A plots the group average of PDR (relative to preonset baseline) to the standards over time. In both ASD and TD groups, standards evoked a small transient PDR that started soon after the tone onset (t = 0), peaking at ∼0.6 s. After then, divergence emerged. TD children's pupils soon started to constrict, returning to baseline at ∼1.2 s on average with further continued miosis; in contrast, ASD pupils continued dilation, with across-group comparison showing a robust significant difference from 1 s onwards to the end of the epoch.
Figure 2.
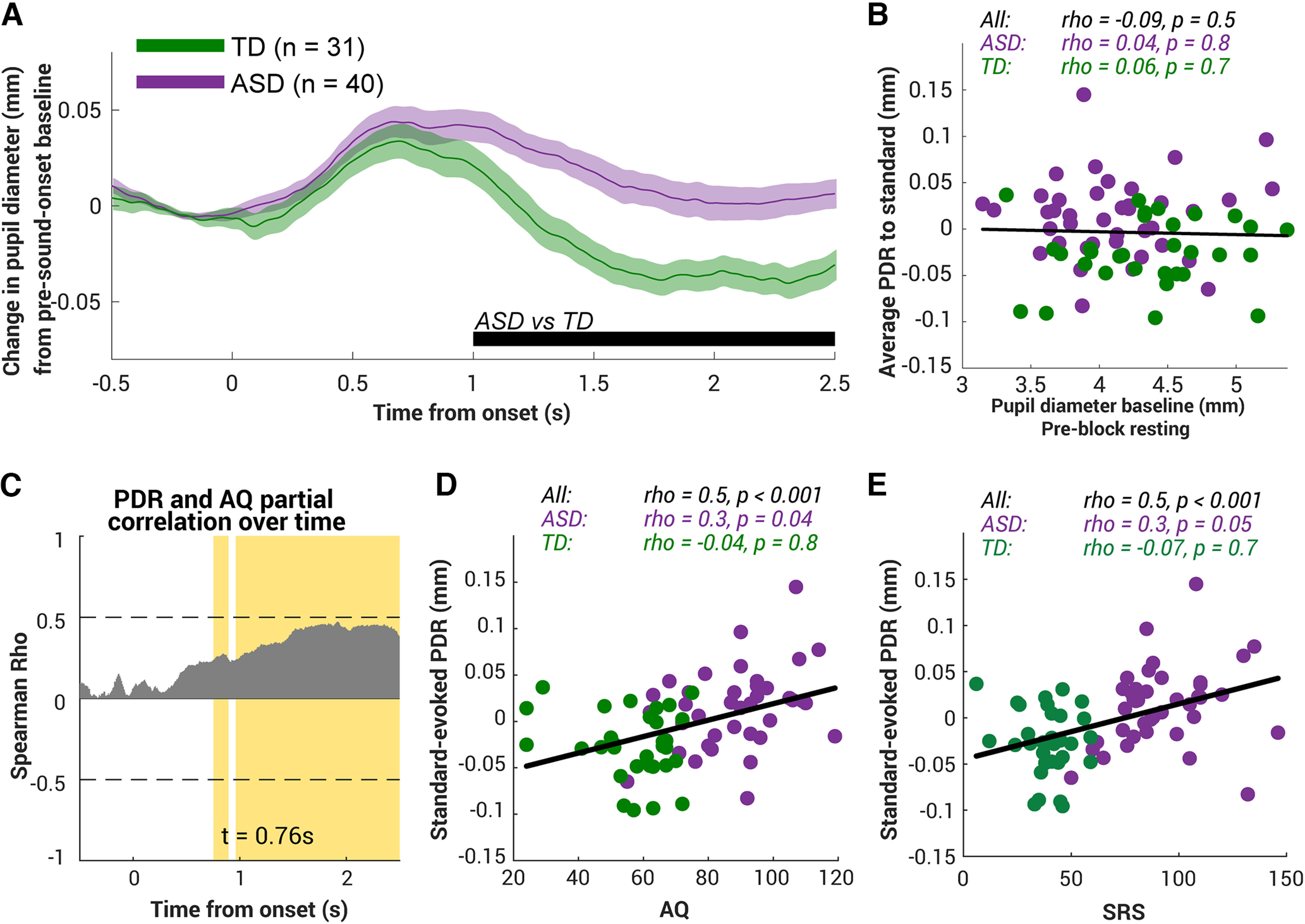
Standard-evoked pupil dilation responses in ASD and TD children. A, Averaged pupil dilation response to standard in the TD group (N = 31, green) and the ASD group (N = 40, purple). Bottom, The black horizontal line indicates the time interval where bootstrap resampling confirmed a significant difference between groups. The shaded area shows ±1 SEM. B, To investigate whether the difference observed in A is driven by the difference in tonic pupil diameter preblock, we computed the average PDR to standard from 1 s to 2.5 s postonset (the time interval chosen based on the significant interval shown in A). No correlation was found between this average PDR and the pupil diameter baseline. Each dot represents data from a single subject, green indicating the TD group and purple indicating the ASD group. Top, Spearman's correlation coefficients and their two-tailed p values. C, The standard-evoked PDR correlates with the autism-related measure AQ on an individual subject level, after controlling for the subject's age and IQ. Gray bars indicate Spearman's correlation coefficients at each time point. Yellow area indicates the time intervals where a significant correlation (p < 0.05; FWE uncorrected) was observed. This analysis was conducted over the entire trial duration with all significant time points indicated. The t value labels the start of the significant interval. D, E, Standard-evoked pupil dilation response positively correlated with the autistic traits AQ and SRS. Top, Spearman's correlation coefficients and their two-tailed p values.
The group difference in standard-evoked PDR could merely be driven by a difference in pupil baseline as smaller baselines provide more room to dilate, thus resulting in a larger PDR. Indeed, as mentioned above, individuals with more severe ASD tend to show a smaller pupil before the experiment started (Fig. 1C,D). However, the standard-evoked PDR (averaged over the postonset epoch period, 1 s to 2.5 s, chosen based on the significant interval shown in Fig. 2A) did not correlate with the baseline (ρ = −0.09, p = 0.5, Fig. 2B), suggesting that the ASD-specific larger PDR could not be simply explained by the baseline difference.
We further found that the standards evoked a larger PDR among individuals with more severe autistic traits. PDR was significantly and positively correlated with the severity of autistic traits, AQ (ρ = 0.5, p < 0.001; Fig. 2D) and SRS (ρ = 0.5, p < 0.001; Fig. 2E) at an individual level even within the ASD group (see statistics above; Fig. 2D,E). For a more time-sensitive analysis, we correlated the instantaneous standard-evoked PDR with AQ while regressing out the effects of preblock baseline and age for each participant (Fig. 2C). Partial correlation coefficients (Spearman's) are plotted as gray bars in Figure 2C. Significant time samples, familywise error (FWE) corrected, are highlighted in yellow. There are significant correlations ∼1 s postonset, in line with the group difference in Figure 2A, revealing that those individuals with more severe autistic traits were also those exhibiting a longer standard-evoked PDR.
One explanation for this larger standard-evoked PDR among ASD individuals is that their response had reduced habituation to standards. To examine this, we compared the first 30 standard trials with the last 30; in the case of habituation, the PDR in the last 30 should be smaller than the first 30. This is true among TD individuals (Fig. 3A, left), with the last 30 evoking a significantly smaller PDR. Divergence is significant from ∼ 0.5–2 s, suggesting strong habituation to repeating events. A 2 × 2 [time (first half, last half) × group (ASD, TD)] mixed ANOVA showed significant interaction between time and group on the standard-evoked PDR (F(1,69) = 7.6, p = 0.007, η2 = 0.1, d = 0.8; Fig. 3B).
Figure 3.
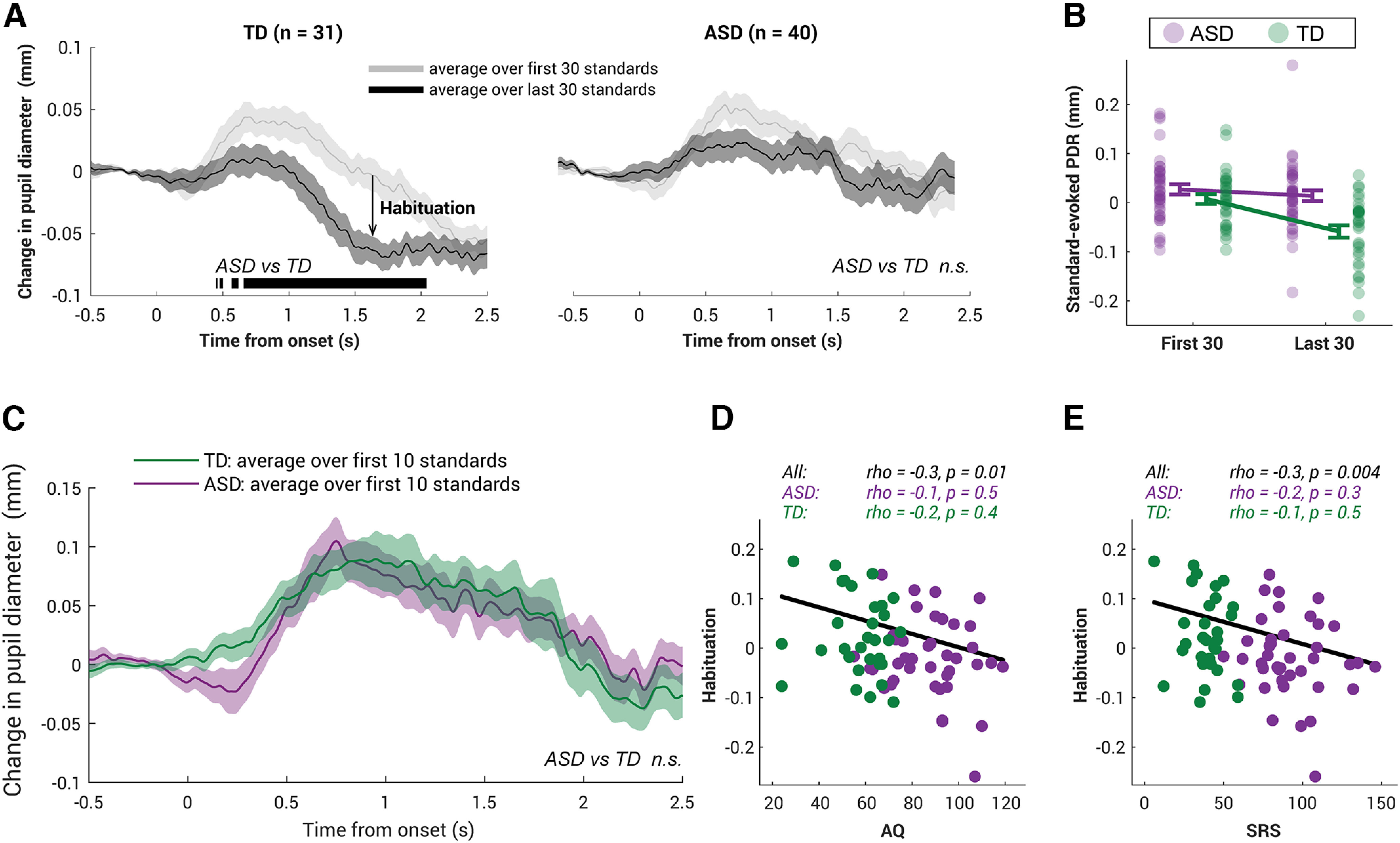
Habituation in standard-evoked pupil dilation in TD children but not in ASD children. A, Average PDR over the first 30 standard trials (dark gray) versus over the last 30 trials (light gray) for the TD group (left) and the ASD group (right). The solid lines represent the average pupil diameter related to the baseline (0.5 s preonset) as a function of time. Bottom, The black horizontal line indicates the time interval where bootstrap resampling confirmed a significant difference between the first half and the second half of the experiment. The shaded area shows ±1 SEM. n.s., not significant. B, Compared with the ASD group (green), the ASD group (purple) showed a significantly smaller reduction in standard-evoked PDR over time. The error bar is ±1 SEM. Each dot presents individual subject's data from each group. The size of habituation is quantified as the reduction in the standard-evoked PDR averaged over the epoch, namely subtracting the average amplitude of the last 30 trials from that of the first 30 trials for each subject. C, The first 10-trial standard-evoked pupil dilation response did not differ across ASD and TD groups. Permutation-based time-series analysis was done throughout the epoch, but no statistically significant intervals were found (p > 0.05). The shaded area shows ±1 SEM. D, E, The size of the habituation in standard-evoked PDR is negatively correlated with the autism-related measures—AQ in D and SRS in E— on an individual subject level. Each dot represents data from a single subject, green indicating the TD group and purple indicating the ASD group. Top, Spearman's correlation statistics are reported.
One concern is that this habituation effect is merely reflecting a potential difference in loudness perception between two groups. If the ASD group perceived the sounds louder than the TD group, it is unsurprising for the ASD group to be associated with a reduced habituation to the repeating sounds. Here, we used the fact that the size of sound-evoked phasic PDR is strongly associated with the loudness of the sound (e.g., Liao et al., 2016a); if the ASD group perceived the sound louder than the TD group, their standard-evoked PDR in the first few trials, before the habituation kicked in, should be larger than those from the TD group. However, this is not the case in the present sample. The average standard-evoked PDR for the first 10 trials (i.e., <1 min after the experiment onset) was statistically indifferent across the entire epoch between the two groups (Fig. 3C). This suggests that at least in the present sample, the habituation-related changes started with a common baseline for the two groups. Thus, it is unlikely that the individualized volume setting produces the group difference in the habituation effect.
For further analysis on an individual level, this habituation effect is quantified by subtracting the PDR (average over the epoch, 0.5–2.5 s) to the last 30 standards from the first 30 standards, with the effect of the preblock baseline regressed out. Among those in the TD group, 83.9% individuals showed habituation (i.e., a difference above zero), reaching significance at a group level (t(30) = 4.7, p < 0.0001, BF = 434.1, one-sample t test, two tailed). In contrast, only 60.0% of ASD individuals showed habituation and were not different from the floor (t(39) = 1.0, p = 0.3, BF = 3.6, one-sample t test, two tail). Overall, the ASD group exhibited a significantly smaller habituation than the TD group (t(69) = −2.8, p = 0.007, BF = 5.9). We further confirmed that this is not a result of age (ρ = −0.08, p = 0.5) but related to the severity of autistic traits across groups, both AQ (ρ = −0.3, p = 0.01; Fig. 3D) and SRS (ρ = −0.3, p = 0.004; Fig. 3E).
ASD exhibited a smaller pupillary response to surprise
Now we turn to the surprise response. In this study, the surprise response is measured by the PDR evoked by two types of possible deviants. One is a white noise that only differs from the standard in its acoustic feature, whereas the other, laughter, also carries social content.
To ensure that these two deviants had equal auditory salience, the sound files were root-mean-square equalized. They evoked same-size and same-latency PDR in individuals with TD (Fig. 4A, left). Both deviant conditions evoked a larger PDR compared with the standards; the pupil started to dilate ∼0.3 s postsound onset, forming an initial peak ∼0.6 s and rising again to a higher peak ∼1.3 s postonset. Thereafter, the pupil diameter gradually decreased, continuing through the silent intersound interval.
Figure 4.
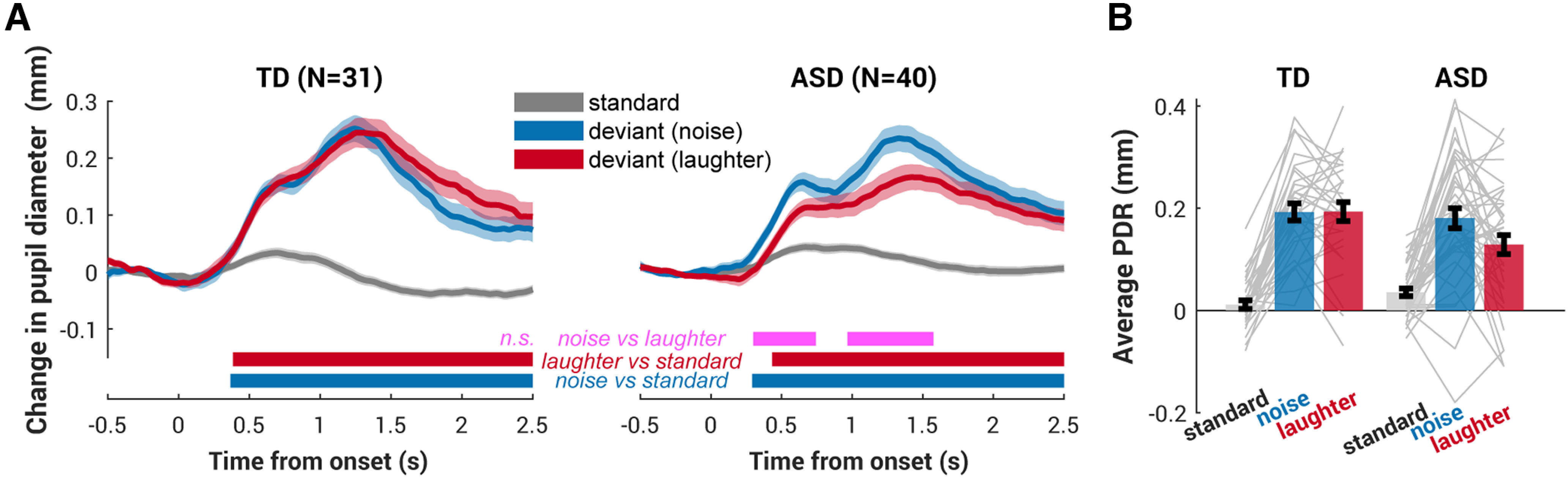
Auditory deviants evoked pupil dilation responses in ASD and TD children. A, Averaged pupil dilation results from the TD group (N = 31, left) and the ASD group (N = 40, right). The solid lines represent the average pupil diameter related to the baseline (0.5 s preonset) as a function of time. The shaded area shows ±1 SEM. Bottom, Colored horizontal lines indicate time intervals where bootstrap statistics confirmed a significant difference between the standard condition and each deviant condition. n.s., not significant. B, The average PDR to standard (gray) and two deviants (blue for noise and red for laughter) for TD and ASD groups are shown side by side. The average PDR was computed over the interval from 1 s to 2 s postonset. The gray lines indicate individual subjects. The error bar shows ±1 SEM.
Although there was no significant difference between the two deviant conditions in the TD group suggesting that these two sounds do not differ in their auditory salience to the TD individuals, laughter,– the deviant stimulus with social content, evoked a slightly but significantly smaller PDR in ASD (Fig. 4A, right, B, average PDR).
To compare the deviant-evoked pupil response across the two groups, a net effect measure quantifying the size of the PDR was computed for each participant by taking the difference between each deviant and the standard condition (Fig. 5A, noise, D, laughter). Although TD appeared to have a larger deviant-evoked PDR for both noise and laughter, the difference between groups is larger, longer, and earlier specifically to laughter, that is, the deviant containing social information; such a difference can be observed as early as 0.5 s postsound onset and lasting until 2 s (Fig. 5D). The difference in the net PDR (average over 1 s to 2 s postonset) between deviants is further confirmed by an on-threshold interaction in a 2 (group ASD, TD) × 2 (deviants noise, laughter) ANOVA (F(1, 69) = 3.6, p = 0.05, η2 = 0.05, d = 0.5) and a null main effect of deviant (F(1,69) = 0.6, p = 0.4, η2 = 0.009, d = 0.1). The main effect of group is also significant (F(1,69) = 6.9, p = 0.01, η2 = 0.09, d = 0.7).
Figure 5.
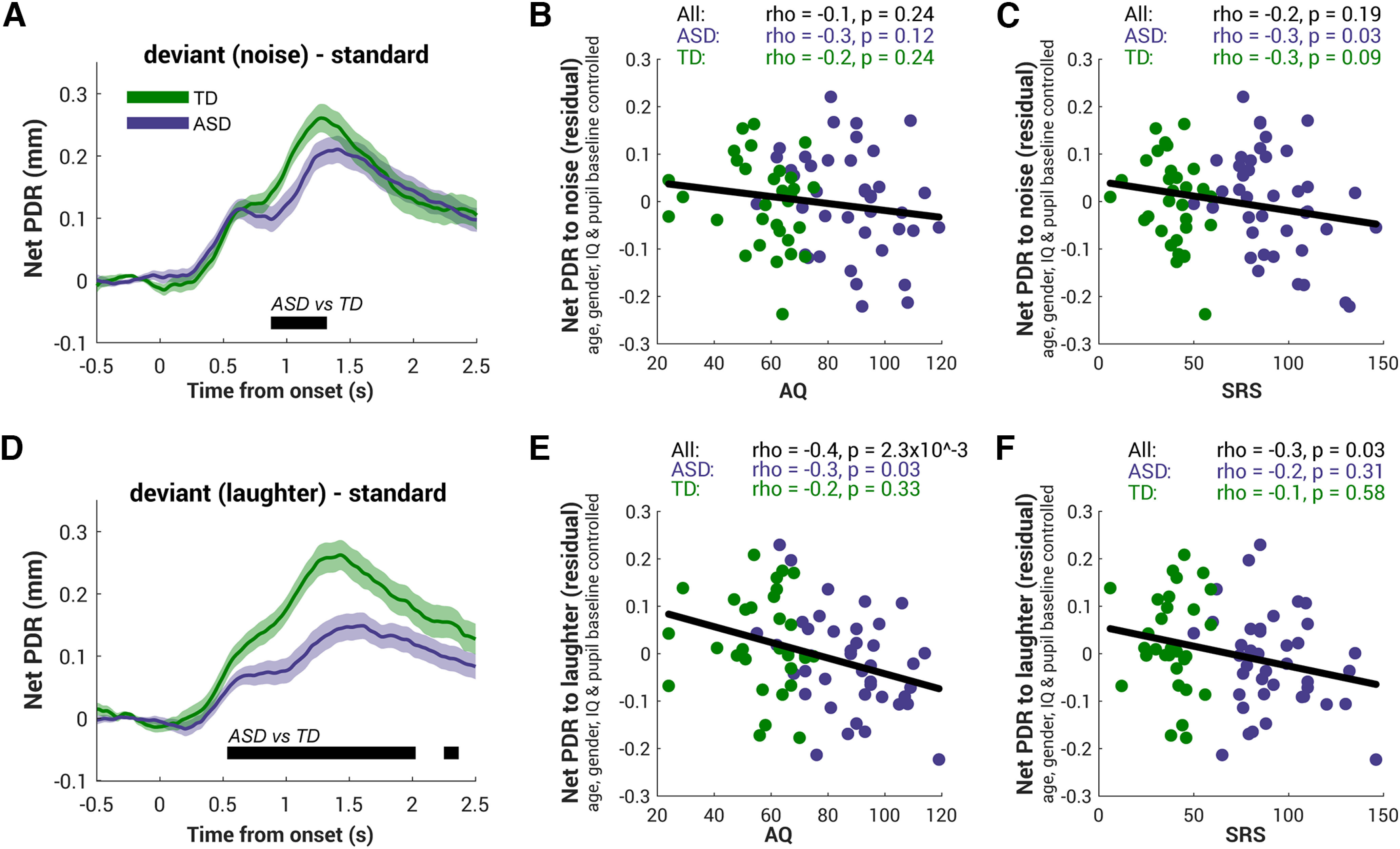
Deviant containing social information evoked pupil dilation response associated with autistic trait. A, D, Comparison of the net PDR to the noise deviant (A) and the laughter deviant (D) in the ASD group (purple) and the TD group (green). The net PDR is computed for each participant by taking the difference between each deviant and the standard condition and averaged across participants. Bottom, The black horizontal line indicates the time interval where bootstrap resampling confirmed significant differences between groups. The shaded area shows ±1 SEM. B, C, For every subject a net PDR to noise was computed by averaging over the interval from 1 s to 2 s postonset. To rule out the effect of baseline on phasic PDR, we regressed out the effect of baseline, age, gender, and IQ from the net PDR to noise. The residual of the net PDR to noise does not correlate with the autistic traits, neither AQ (B) nor SRS (C). Each dot presents the individual subject's data from each group, green for TD and purple for ASD. The black line indicates the correlation among all individuals. Top, Spearman's correlation statistics are reported. E, F, Same analysis was applied to the net PDR to laughter with AQ (E) and SRS (F). The autistic traits negatively correlated with the size of the surprise response to laughter but not to the response to noise.
Furthermore, we found that the net PDR to laughter and to noise were negatively correlated with the autistic traits, both AQ (noise, ρ = −0.3, p = 0.01; laughter, ρ = −0.5, p = 4.4 × 10−6) and SRS (noise, ρ = −0.3, p = 0.02; laughter, ρ = −0.4, p = 3.4 × 10−4). A multiple regression was then conducted to investigate whether AQ, IQ, age, and the group could significantly predict the net PDR to laughter. The results of the model showed that it explained 23.1% of the variance and that the model was a significant predictor of the net PDR to laughter (F(4,66) = 5.0, p = 0.001). Although AQ contributed significantly to the model (beta = −0.05, p = 0.007), the other three factors did not (IQ, beta = 0.02, p = 0.2; age: beta = −0.004, p = 0.8, group: beta = −0.008, p = 0.8). On the other hand, when predicting the net PDR to noise, the multiple regression model of these four factors (AQ, IQ, age, and group) could only explain 12.4% of variance, and the model was marginally significant (F(4,66)=2.3, p = 0.06). Nevertheless, AQ was still the only significant contributor to the model predicting the net PDR to noise (beta = −0.04, p = 0.02; IQ, beta = 0.005, p = 0.5; age, beta = 0.01, p = 0.3; group, beta = 0.02, p = 0.2). Although both net PDRs only marginally correlate with the preblock baseline (noise, ρ = 0.2, p = 0.1; laughter, ρ = 0.2, p = 0.05), we then regressed out the effect of baseline, age, gender, and IQ from the net PDR for each individual. The relationship remained highly significant for the net PDR to laughter (Fig. 5E; AQ, ρ = −0.4, p = 2.3 × 10−3; Fig. 5F; SRS, ρ = −0.3, p = 0.03) but not for the net PDR to noise (Fig. 5B, 5C). As tested using the R package cocor (Diedenhofen and Musch, 2015) with Hotelling's (1940) t and Hittner et al.'s (2003) method, the correlation between the net PDR to laughter and AQ was significantly greater than the AQ correlation with the net PDR to noise (laughter vs noise, Hotelling's t(68) = 1.9, p = 0.03; Hittner et al.'s z = 1.8, p = 0.03). This indicates that higher severity of core autistic symptoms reflected by AQ was associated with smaller laughter-evoked PDR, and this negative correlation was found significant within the ASD group (AQ, ρ = −0.3, p = 0.03; Fig. 5E; but not significant with SRS; Fig. 5F). Overall, the association between the severity of autistic symptoms and the phasic PDR response was significantly stronger for the laughter deviant.
Finally, to investigate whether the reduction in surprise response can be explained by the reduction in habituation, a linear mixed-effects model (LMM) using habituation and group as fixed effects and age, gender, and pupil baseline as random effects was applied to both noise-evoked and laughter-evoked net PDR. The LMM showed that the degree of reduction in habituation could significantly explain the noise-evoked net PDR (estimate = 0.5, F(1,68) = 0.005), but the group was not a significant predictor (group TD, estimate = 0.0003, F(1,68) = 0.0001, p = 1). In contrast, both habituation and group were predictive for the surprise response to laughter, with smaller habituation and being in the ASD group leading to smaller PDR responses (habituation, estimate = 0.4, F(1,68) = 4.7, p = 0.03; group TD, estimate = 0.08, F(1,68) = 7.1, p = 0.01). Together, these results suggest that habituation to repeated stimuli can predict the surprise response to noise independent of the group, and, importantly, reduction in habituation in the ASD group can predict the smaller surprise response to laughter.
Discussion
Our study used pupillometry to examine the attention function mediated by the LC–NE system in young children with ASD and the age-matched controls. When passively listening to the oddball stimuli, the neurotypical controls showed automatic and flexible allocation of attention, that is, a combination of a progressive reduction of PDR response to the standards and a persistently responsive PDR to the deviants. This reflects a default working mode of alerting and orienting that permits the nervous system to remain attuned to important events in the environment while reserving cognitive resources for relatively uninformative inputs. However, with ASD, this intricate and dynamic attention allocation is impaired. ASD participants lacked the same level of habituation to the standards (Fig. 3A), appearing persistently surprised by repetitive signals, and responded with significantly less attention resource to prominent social deviants (Fig. 5). Attenuated habituation predicted this poor phasic attention orienting, suggesting that aberrant processing of deviance in the environment is associated with dysregulation of alerting in children with ASD. Additionally, both habituation and phasic orienting atypicalities significantly correlate with the severity of autistic traits (Figs. 3D, 5E), demonstrating that the NE-related attention deficiencies may contribute to the development of core ASD symptoms. Critically, given that attention is assessed by pupillometry in the absence of a task goal or a motor response, our findings show that the subcortical network subserves the derailed attention in ASD at its default operating state. Furthermore, we also found that only the laughter evoked deviant response correlated with the severity of ASD symptoms, suggesting that the ASD-related abnormality in phasic PDR appears selective for socially relevant stimuli.
Increased tonic arousal and decreased phasic response to exogenous stimuli have been hypothesized to represent the primary issue of the LC–NE system in autism and potentially contribute to heterogenous behavioral symptoms among individuals with ASD (Bast et al., 2018). However, the direct support for the co-occurrence of these two abnormalities is scarce. On the one hand, people sought evidence for heightened tonic arousal by measures related to the autonomic nervous system, including baseline pupil size and skin conductance, but failed to find consistent results (see below). On the other hand, researchers examined the phasic response to exogenous stimuli with various attention tasks, including the Posner tasks or the auditory oddball paradigm (Keehn et al., 2013). The behavioral tasks, given their diversity, have not provided conclusive evidence (Keehn et al., 2013; de Vries et al., 2021), and many studies even reported normal phasic alerting (Odriozola et al., 2016; Kleberg et al., 2017). fMRI and ERP studies indeed found reduced neural activities in various cortical regions during phasic alerting or orienting (Courchesne et al., 1984; Gomot et al., 2006, 2008; Jeste and Nelson, 2009; Stroganova et al., 2013; Orekhova and Stroganova, 2014; Odriozola et al., 2016). For example, passive auditory oddball paradigms, similar to our experiment, have revealed that adults and children with ASD have reduced mismatch negativity (MMN) to unattended auditory deviants (Seri et al., 1999; Jansson-Verkasalo et al., 2003; Kujala et al., 2005, 2007; Lepistö et al., 2006; Dunn et al., 2008), suggesting that the ability to extract the corresponding information differing standard and deviant from the signal (Schröger, 1997; Näätänen and Winkler, 1999) is impaired in ASD. Nevertheless, despite the popularity of the passive oddball paradigm, traditionally, researchers have been focusing primarily on the deviant/novelty processing rather than its connection with the habituation to standards (but see Hudac et al., 2018 for habituation to deviant and Guiraud et al., 2011 for similar results in high-risk infants). Here, we show that despite its simplicity, the passive auditory oddball paradigm provides an ideal test bed for the investigation of tonic alerting and phasic orienting under one single task; it triggers automatic evaluation of incoming stimuli, which are either repetitive and thus subject to slow habituation or deviant and thus subject to reflexive attention orienting. In addition, as we pointed out earlier, these studies revealed abnormal cortical activities but did not target at the involvement of the LC–NE system. Our study, using a simple and unified paradigm, demonstrates that the co-occurrence of increased tonic alerting and reduced phasic orienting in ASD is related to the LC–NE system.
Furthermore, we found that the lower habituation predicts less attention orienting to deviant stimuli in ASD as measured by phasic PDR. Impaired habituation in ASD is typically found by presenting repeated stimuli to the participants (Lawson et al., 2015, 2018; Turi et al., 2015) and has been related to sensory overload prevalent in autism (Pellicano et al., 2007) and ASD participants' use of sensory avoidance strategies (Lawson et al., 2015). An oddball study also found that cortical responses to deviant sound habituated across trials in neurotypical controls but not in children with ASD (Hudac et al., 2018). Our previous study shows that the pupil-linked LC–NE system is essential for tracking unfolding sensory inputs without direct task engagement (Zhao et al., 2019a). In this aspect, diminished habituation can be viewed as failing to effectively update the internal representation of sensory statistics, which in turn leads to less surprise and consequently smaller PDR to the deviant. This is consistent with a pupillometry study (Lawson et al., 2017), which found adults with ASD underestimated the volatility of the sensory environment and had reduced surprise reaction to expectation violation. Our study made two advances from these studies. First, we established a relationship between habituation and the phasic response to deviants, implying that the habituation impairment is not simply a sensory adaptation problem but might reflect a deficit in dynamic attention allocation in ASD. Second, these attention deficits were observed in the absence of task engagement, suggesting that the dysfunctional sensory processing observed in the ASD population might be tied to the LC–NE-mediated attention at its default working state.
Previous studies may have overlooked the co-occurrence of reduced habituation and orienting to deviants because they focused on cortical responses and often required individuals with ASD to perform various tasks. Extensive research has demonstrated that neural signatures of alerting and attention orienting are influenced by a task or attention direction. Children with ASD, for example, have a smaller EEG response to novelty or deviant stimuli during passive listening, but this effect vanished when a behavioral response was required (Dunn et al., 2008; Whitehouse and Bishop, 2008). The BOLD signal yielded a similar result (Gomot et al., 2006, 2008). The active task not only alters neural signatures but also has a direct impact on pupil size variations, which are affected by cognitive effort that is demanded for various tasks (Anderson et al., 2006; Nuske et al., 2016; Bast et al., 2021). Thus, attributing any variation in pupillary response to a difference in attention processes would require reducing the confounds brought by active task engagement. For example, Granovetter et al. (2020) sought to investigate the role of LC–NE in attention regulation during a visual working memory task. Adults with ASD had substantially smaller task-evoked PDR than controls in the auditory distraction condition but not in the nondistraction condition. The authors ascribed the smaller PDR in the presence of distractions to dysregulation of LC activity, and they excluded the possible task confounds as both groups performed equally well. However, equivalent degrees of cognitive effort or task utility for the two populations cannot be guaranteed by an identical performance level (Lawson et al., 2017; Bast et al., 2021). As another example, in a cued reaction time task, children with ASD yielded a significantly larger stimulus-evoked PDR than controls (Boxhoorn et al., 2020). This abnormally larger PDR was observed exclusively when the visual cue was nonspecific, leading the authors to conclude that this impaired reflexive orienting is caused by a continuous hyperphasic state. However, because these effects were detected in a demanding task, this increase in PDR may merely reflect a greater effort for attention control during reflexive orienting. Our study circumvented these task-related confounds by using a passive, auditory oddball task to probe the involuntary attention regulation in the absence of performance demand or motoric response.
Along with the phasic response, the tonic level is an important component of the LC–NE activity. Prior support for enhanced alerting in ASD, however, has been sporadic (de Vries et al., 2021). Tonic arousal in children with ASD has been found to be excessively variable, ranging from abnormally high to unusually low, as measured by skin conductance levels (Schoen et al., 2008). Increased baseline pupil size, indicating increased tonic arousal, has been reported in individuals with ASD (Anderson and Colombo, 2009; Anderson et al., 2013; Blaser et al., 2014; Top et al., 2019) and has been linked to symptom severity (Anderson et al., 2013). Numerous other studies, on the other hand, either failed to find an increase in baseline pupil size (Nuske et al., 2016; Lynch et al., 2018) or found the opposite effect (Martineau et al., 2011). Compared with the control group, our ASD participants had a significantly smaller baseline pupil diameter, which was unrelated to the severity of autistic traits within the ASD group (Fig. 1C,D). Taking this by face value, our sample of children with ASD appeared to have a low level of tonic arousal. However, we discovered a higher, dynamic tonic pupillary activity as seen by a diminished habituation to recurring stimuli (Fig. 3A). This dynamic increase in tonic pupillary activity predicted a lower phasic orienting response to deviant stimuli, which is congruent with the concept of arousal dysregulation in the autistic population (Bast et al., 2018). Given that baseline pupil diameter, which probably depends on an individual's immediate state (de Vries et al., 2021), varies greatly among samples, our findings suggest that as compared with the baseline pupil diameter, the dynamic modulation of task-free attention may be a better indicator of tonic abnormalities in ASD.
The anomaly discovered in the LC–NE system in this study might have at least three important implications for the development of ASD. To begin, arousal regulation difficulties may hinder sensory processing, which is likely related to the hyposensititivy and hypersensitivity issues that are typical in people with ASD (Marco et al., 2011). Sluggish habituation, for example, is linked to sensory-seeking issues (Hudac et al., 2018), and early EEG response to deviances is linked to change intolerance in children with ASD (Guiraud et al., 2011). Second, because the alerting and executive networks interact more in ASD (Keehn et al., 2013), these attention deficits can impede performance in tasks requiring attention switches (Boxhoorn et al., 2020; Bast et al., 2021) while benefiting some tasks requiring focused attention and reduced distractibility (e.g., visual search; Blaser et al., 2014). Third, aberrant NE-mediated attentional function to external stimuli may precede and contribute to derailed social attention, which is crucial for social learning and development (Mehler and Purpura, 2009; Keehn et al., 2013; Bast et al., 2018). Lower phasic alertness has been associated with decreased gaze and facial processing (Nomi and Uddin, 2015), as well as diminished responsiveness to social cues (Dawson et al., 2004; Leekam and Ramsden, 2006; Keehn and Joseph, 2008). Given that children with ASD have a hyperarousal state because of attenuated habituation and a reduced phasic orienting to social signals, with both correlated with their core social symptoms, our findings implicate that the LC–NE system may contribute to the etiology of ASD.
Finally, although our findings point to a malfunctioning LC–NE system, other neuromodulator systems, such as nicotinic cholinergic arousal pathways, may also contribute to ASD-related atypical salient stimuli processing (Stroganova et al., 2013; Orekhova and Stroganova, 2014). Furthermore, our work is constrained by its reliance on the pupillary response, which is a proxy for noradrenergic activity in the brain. More research using direct measurement or pharmacological modification of the LC–NE system is needed to identify the extent to which an aberrant LC–NE system contributes to the attention-orienting difficulty associated with ASD.
Footnotes
This work was supported by the National Natural Science Foundation of China (Grants 62061136001, 32071047, 31871116, and C31622039 to K.W.). We thank the staff at Qingdao Elim School and Qingdao Shangwuting kindergarten, especially Ms. Wenyan Nie and Mr. Gong Wang, and the children and parents who participated in our study.
The authors declare no competing conflicts of interest.
References
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anderson CJ, Colombo J (2009) Larger tonic pupil size in young children with autism spectrum disorder. Dev Psychobiol 51:207–211. 10.1002/dev.20352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, Jill Shaddy D (2006) Visual scanning and pupillary responses in young children with autism spectrum disorder. J Clin Exp Neuropsychol 28:1238–1256. 10.1080/13803390500376790 [DOI] [PubMed] [Google Scholar]
- Anderson CJ, Colombo J, Unruh KE (2013) Pupil and salivary indicators of autonomic dysfunction in autism spectrum disorder. Dev Psychobiol 55:465–482. 10.1002/dev.21051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Auyeung B, Baron-Cohen S, Wheelwright S, Allison C (2008) The Autism Spectrum Quotient: Children's Version (AQ-Child). J Autism Dev Disord 38:1230–1240. 10.1007/s10803-007-0504-z [DOI] [PubMed] [Google Scholar]
- Bast N, Poustka L, Freitag CM (2018) The locus coeruleus–norepinephrine system as pacemaker of attention – a developmental mechanism of derailed attentional function in autism spectrum disorder. Eur J Neurosci 47:115–125. 10.1111/ejn.13795 [DOI] [PubMed] [Google Scholar]
- Bast N, Boxhoorn S, Supér H, Helfer B, Polzer L, Klein C, Cholemkery H, Freitag CM (2021) Atypical arousal regulation in children with autism but not with attention-deficit/hyperactivity disorder as indicated by pupillometric measures of locus coeruleus activity. Biol Psychiatry Cogn Neurosci Neuroimaging S2451-9022:00117–00118. [DOI] [PubMed] [Google Scholar]
- Blaser E, Eglington L, Carter AS, Kaldy Z (2014) Pupillometry reveals a mechanism for the autism spectrum disorder (ASD) advantage in visual tasks. Sci Rep 4:4301. 10.1038/srep04301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582. 10.1016/j.tins.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Boxhoorn S, Bast N, Supèr H, Polzer L, Cholemkery H, Freitag CM (2020) Pupil dilation during visuospatial orienting differentiates between autism spectrum disorder and attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry 61:614–624. 10.1111/jcpp.13179 [DOI] [PubMed] [Google Scholar]
- Brainard DH (1997) The Psychophysics Toolbox. Spat Vis 10:433–436. [PubMed] [Google Scholar]
- Bryson SEWainwright-Sharp JA, Smith IM (1990) Autism: a developmental spatial neglect syndrome? Adv Psychol 69: 405–427. [Google Scholar]
- Ceponiene R, Lepistö T, Shestakova A, Vanhala R, Alku P, Näätänen R, Yaguchi K (2003) Speech–sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc Natl Acad Sci U S A 100:5567–5572. 10.1073/pnas.0835631100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen IL, Gardner JM, Karmel BZ, Phan HTT, Kittler P, Gomez TR, Gonzalez MG, Lennon EM, Parab S, Barone A (2013) Neonatal brainstem function and 4-month arousal-modulated attention are jointly associated with autism. Autism Res 6:11–22. 10.1002/aur.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W (2003) Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord 33:427–433. 10.1023/a:1025014929212 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Kilman BA, Galambos R, Lincoln AJ (1984) Autism: processing of novel auditory information assessed by event-related brain potentials. Electroencephalogr Clin Neurophysiol 59:238–248. 10.1016/0168-5597(84)90063-7 [DOI] [PubMed] [Google Scholar]
- Dawson G, Lewy A (1989) Arousal, attention, and the socioemotional impairments of individuals with autism. In: Autism: Nature, diagnosis, and treatment (Dawson G, ed.), pp 49–74. New York: Guilford Press. [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J (2004) Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol 40:271–283. 10.1037/0012-1649.40.2.271 [DOI] [PubMed] [Google Scholar]
- Dayan P, Yu AJ (2006) Phasic norepinephrine: a neural interrupt signal for unexpected events. Network 17:335–350. 10.1080/09548980601004024 [DOI] [PubMed] [Google Scholar]
- de Vries L, Fouquaet I, Boets B, Naulaers G, Steyaert J (2021) Autism spectrum disorder and pupillometry: a systematic review and meta-analysis. Neurosci Biobehav Rev 120:479–508. 10.1016/j.neubiorev.2020.09.032 [DOI] [PubMed] [Google Scholar]
- Diedenhofen B, Musch J (2015) cocor: a comprehensive solution for the statistical comparison of correlations. PLoS One 10:e0121945. 10.1371/journal.pone.0121945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn MA, Gomes H, Gravel J (2008) Mismatch negativity in children with autism and typical development. J Autism Dev Disord 38:52–71. 10.1007/s10803-007-0359-3 [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ (1994) An introduction to the bootstrap. Boca Raton, FL: Chapman & Hall/CRC. [Google Scholar]
- Ego-Stengel V, Bringuier V, Shulz DE (2002) Noradrenergic modulation of functional selectivity in the cat visual cortex: an in vivo extracellular and intracellular study. Neuroscience 111:275–289. 10.1016/S0306-4522(02)00011-8 [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, Baron-Cohen S (2006) Change detection in children with autism: an auditory event-related fMRI study. Neuroimage 29:475–484. 10.1016/j.neuroimage.2005.07.027 [DOI] [PubMed] [Google Scholar]
- Gomot M, Belmonte MK, Bullmore ET, Bernard FA, Baron-Cohen S (2008) Brain hyper-reactivity to auditory novel targets in children with high-functioning autism. Brain 131:2479–2488. 10.1093/brain/awn172 [DOI] [PubMed] [Google Scholar]
- Granovetter MC, Burlingham CS, Blauch NM, Minshew NJ, Heeger DJ, Behrmann M (2020) Uncharacteristic task-evoked pupillary responses implicate atypical locus coeruleus activity in autism. J Neurosci 40:3815–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraud JA, Kushnerenko E, Tomalski P, Davies K, Ribeiro H, Johnson MH (2011) Differential habituation to repeated sounds in infants at high risk for autism. Neuroreport 22:845–849. 10.1097/WNR.0b013e32834c0bec [DOI] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJF, Ossewaarde L, Henckens MJAG, Qin S, van Kesteren MTR, Schoots VC, Cousijn H, Rijpkema M, Oostenveld R, Fernández G (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334:1151–1153. 10.1126/science.1209603 [DOI] [PubMed] [Google Scholar]
- Hittner JB, May K, Silver NC (2003) A Monte Carlo evaluation of tests for comparing dependent correlations. J Gen Psychol 130:149–168. 10.1080/00221300309601282 [DOI] [PubMed] [Google Scholar]
- Hotelling H (1940) The selection of variates for use in prediction with some comments on the general problem of nuisance parameters. Ann Math Statist 11:271–283. 10.1214/aoms/1177731867 [DOI] [Google Scholar]
- Hudac CM, DesChamps TD, Arnett AB, Cairney BE, Ma R, Webb SJ, Bernier RA (2018) Early enhanced processing and delayed habituation to deviance sounds in autism spectrum disorder. Brain Cogn 123:110–119. 10.1016/j.bandc.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson-Verkasalo E, Ceponiene R, Kielinen M, Suominen K, Jäntti V, Linna S-L, Moilanen I, Näätänen R (2003) Deficient auditory processing in children with Asperger syndrome, as indexed by event-related potentials. Neurosci Lett 338:197–200. 10.1016/s0304-3940(02)01405-2 [DOI] [PubMed] [Google Scholar]
- Jeste SS, Nelson CA (2009) Event related potentials in the understanding of autism spectrum disorders: an analytical review. J Autism Dev Disord 39:495–510. 10.1007/s10803-008-0652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, Gold JI (2016) Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron 89:221–234. 10.1016/j.neuron.2015.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L (1943) Autistic disturbances of affective contact. Nerv Child 2:217–250. [PubMed] [Google Scholar]
- Keehn B, Joseph RM (2008) Impaired prioritization of novel onset stimuli in autism spectrum disorder. J Child Psychol Psychiatry 49:1296–1303. 10.1111/j.1469-7610.2008.01937.x [DOI] [PubMed] [Google Scholar]
- Keehn B, Müller R-A, Townsend J (2013) Atypical attentional networks and the emergence of autism. Neurosci Biobehav Rev 37:164–183. 10.1016/j.neubiorev.2012.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleberg JL, Thorup E, Falck-Ytter T (2017) Reduced visual disengagement but intact phasic alerting in young children with autism. Autism Res 10:539–545. 10.1002/aur.1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala T, Lepistö T, Nieminen-von Wendt T, Näätänen P, Näätänen R (2005) Neurophysiological evidence for cortical discrimination impairment of prosody in Asperger syndrome. Neurosci Lett 383:260–265. 10.1016/j.neulet.2005.04.048 [DOI] [PubMed] [Google Scholar]
- Kujala T, Aho E, Lepistö T, Jansson-Verkasalo E, Nieminen-von Wendt T, von Wendt L, Näätänen R (2007) Atypical pattern of discriminating sound features in adults with Asperger syndrome as reflected by the mismatch negativity. Biol Psychol 75:109–114. 10.1016/j.biopsycho.2006.12.007 [DOI] [PubMed] [Google Scholar]
- Lawson RP, Aylward J, White S, Rees G (2015) A striking reduction of simple loudness adaptation in autism. Sci Rep 5:16157. 10.1038/srep16157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Mathys C, Rees G (2017) Adults with autism overestimate the volatility of the sensory environment. Nat Neurosci 20:1293–1299. 10.1038/nn.4615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson RP, Aylward J, Roiser JP, Rees G (2018) Adaptation of social and non-social cues to direction in adults with autism spectrum disorder and neurotypical adults with autistic traits. Dev Cogn Neurosci 29:108–116. 10.1016/j.dcn.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Ramsden CAH (2006) Dyadic orienting and joint attention in preschool children with autism. J Autism Dev Disord 36:185–197. 10.1007/s10803-005-0054-1 [DOI] [PubMed] [Google Scholar]
- Lepistö T, Silokallio S, Nieminen-von Wendt T, Alku P, Näätänen R, Kujala T (2006) Auditory perception and attention as reflected by the brain event-related potentials in children with Asperger syndrome. Clin Neurophysiol 117:2161–2171. 10.1016/j.clinph.2006.06.709 [DOI] [PubMed] [Google Scholar]
- Liao H-I, Yoneya M, Kidani S, Kashino M, Furukawa S (2016a) Human pupillary dilation response to deviant auditory stimuli: effects of stimulus properties and voluntary attention. Front Neurosci 10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H-I, Kidani S, Yoneya M, Kashino M, Furukawa S (2016b) Correspondences among pupillary dilation response, subjective salience of sounds, and loudness. Psychon Bull Rev 23:412–425. 10.3758/s13423-015-0898-0 [DOI] [PubMed] [Google Scholar]
- Lima CF, Anikin A, Monteiro AC, Scott SK, Castro SL (2019) Automaticity in the recognition of nonverbal emotional vocalizations. Emotion 19:219–233. 10.1037/emo0000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GTF, James SM, VanDam M (2018) Pupillary response and phenotype in ASD: latency to constriction discriminates ASD from typically developing adolescents. Autism Res 11:364–375. 10.1002/aur.1888 [DOI] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS (2011) Sensory processing in autism: a review of neurophysiologic findings. Pediatr Res 69:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Mathys C, Ruge D, Berker A. d, Dayan P, Stephan KE, Bestmann S (2016) Pharmacological fingerprints of contextual uncertainty. PLoS Biol 14:e1002575. 10.1371/journal.pbio.1002575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau J, Hernandez N, Hiebel L, Roché L, Metzger A, Bonnet-Brilhault F (2011) Can pupil size and pupil responses during visual scanning contribute to the diagnosis of autism spectrum disorder in children? J Psychiatr Res 45:1077–1082. 10.1016/j.jpsychires.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Mehler MF, Purpura DP (2009) Autism, fever, epigenetics and the locus coeruleus. Brain Res Rev 59:388–392. 10.1016/j.brainresrev.2008.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Winkler I (1999) The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull 125:826–859. 10.1037/0033-2909.125.6.826 [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ (2015) Face processing in autism spectrum disorders: from brain regions to brain networks. Neuropsychologia 71:201–216. 10.1016/j.neuropsychologia.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuske HJ, Vivanti G, Dissanayake C (2016) Others' emotions teach, but not in autism: an eye-tracking pupillometry study. Mol Autism 7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odriozola P, Uddin LQ, Lynch CJ, Kochalka J, Chen T, Menon V (2016) Insula response and connectivity during social and non-social attention in children with autism. Soc Cogn Affect Neurosci 11:433–444. 10.1093/scan/nsv126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova E, Stroganova T (2014) Arousal and attention re-orienting in autism spectrum disorders: evidence from auditory event-related potentials. Front Hum Neurosci 8:34. 10.3389/fnhum.2014.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E, Jeffery L, Burr D, Rhodes G (2007) Abnormal adaptive face-coding mechanisms in children with autism spectrum disorder. Curr Biol CB 17:1508–1512. 10.1016/j.cub.2007.07.065 [DOI] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 7:13289. 10.1038/ncomms13289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S (2005) Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry 46:1255–1268. 10.1111/j.1469-7610.2005.01431.x [DOI] [PubMed] [Google Scholar]
- Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223. 10.1038/nrn2573 [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S (2012) Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76:130–141. 10.1016/j.neuron.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, Brett-Green B, Hepburn SL (2008) Psychophysiology of children with autism spectrum disorder. Res Autism Spectr Disord 2:417–429. 10.1016/j.rasd.2007.09.002 [DOI] [Google Scholar]
- Schröger E (1997) On the detection of auditory deviations: a pre-attentive activation model. Psychophysiology 34:245–257. 10.1111/j.1469-8986.1997.tb02395.x [DOI] [PubMed] [Google Scholar]
- Seri S, Cerquiglini A, Pisani F, Curatolo P (1999) Autism in tuberous sclerosis: evoked potential evidence for a deficit in auditory sensory processing. Clin Neurophysiol 110:1825–1830. 10.1016/s1388-2457(99)00137-6 [DOI] [PubMed] [Google Scholar]
- Stroganova TA, Kozunov VV, Posikera IN, Galuta IA, Gratchev VV, Orekhova EV (2013) Abnormal pre-attentive arousal in young children with autism spectrum disorder contributes to their atypical auditory behavior: an ERP study. PLoS One 8:e69100. 10.1371/journal.pone.0069100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top DN Jr, Luke SG, Stephenson KG, South M (2019) Psychophysiological arousal and auditory sensitivity in a cross-clinical sample of autistic and non-autistic anxious adults. Front Psychiatry 9:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turi M, Burr DC, Igliozzi R, Aagten-Murphy D, Muratori F, Pellicano E (2015) Children with autism spectrum disorder show reduced adaptation to number. Proc Natl Acad Sci U S A 112:7868–7872. 10.1073/pnas.1504099112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (2012) Wechsler preschool and primary scale of intelligence, 4th ed. San Antonio: The Psychological Corporation. [Google Scholar]
- Whitehouse AJO, Bishop DVM (2008) Do children with autism “switch off” to speech sounds? An investigation using event-related potentials. Dev Sci 11:516–524. 10.1111/j.1467-7687.2008.00697.x [DOI] [PubMed] [Google Scholar]
- Zhao S, Chait M, Dick F, Dayan P, Furukawa S, Liao H-I (2019a) Pupil-linked phasic arousal evoked by violation but not emergence of regularity within rapid sound sequences. Nat Commun 10:16. 10.1038/s41467-019-12048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Yum NW, Benjamin L, Benhamou E, Yoneya M, Furukawa S, Dick F, Slaney M, Chait M (2019b) Rapid ocular responses are modulated by bottom-up-driven auditory salience. J Neurosci 39:7703–7714. 10.1523/JNEUROSCI.0776-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard_tone_500Hz Download Figure 1-1, WAV file (43.1KB, wav)
Deviant1_noise Download Figure 1-2, WAV file (43.1KB, wav)
Deviant2_laughter_female Download Figure 1-3, WAV file (43.1KB, wav)
Deviant2_laughter_male Download Figure 1-4, WAV file (43.1KB, wav)
Data Availability Statement
The experimental datasets generated during the current study are available from the corresponding author on request.


