Abstract
Purpose
The clinical utility of plasma methylated septin 9 (mSEPT9) DNA in screening and recurrence monitoring for colorectal cancer (CRC) is highly promising. The present study was performed to determine the diagnostic value of mSEPT9 in CRC detection and recurrence monitoring in Chinese patients.
Methods
Overall, 616 patients newly diagnosed with CRC and 122 individuals with no evidence of disease were recruited from October 1, 2019, to May 31, 2021, at Fujian Medical University Union Hospital. Plasma and serum samples were collected for analyzing mSEPT9, carcinoembryonic antigen (CEA), and carbohydrate antigen-19-9 (CA19-9). Data on clinicopathological characteristics were collected and analyzed. Sensitivity and specificity were calculated to evaluate the diagnostic potential of each marker; the receiver operating characteristic (ROC) curve was applied for the assessment of diagnostic value, and comparisons among mSEPT9, CEA, CA19-9, and their combination were assessed via ROC curves.
Results
mSEPT9 achieved an overall sensitivity and specificity of 72.94% and 81.97%, respectively, with an area under the curve (AUC) value of 0.826, which were higher than those of CEA (sensitivity: 43.96%; specificity: 96.72%; AUC: 0.789) and CA19-9 (sensitivity: 14.99%; specificity: 96.61%; AUC: 0.590). The combination of mSEPT9, CEA, and CA19-9 further improved sensitivity, specificity, and AUC value (sensitivity: 78.43%; specificity: 86.07%; AUC: 0.878), respectively. Notably, the mSEPT9 positivity rate was significantly associated with TNM stage, T stage, N stage, tumor size, vascular invasion, and nerve invasion among patients with CRC. A 100% correlation was observed between the positive results of the mSEPT9 test and recurrence or metastasis in patients after therapeutic intervention.
Conclusion
Our findings suggest that mSEPT9 may represent a potential biomarker for the diagnosis and prognosis of CRC compared with CEA and CA19-9. Postoperative mSEPT9 status may represent the first noninvasive marker of CRC recurrence or metastasis.
1. Introduction
Colorectal cancer (CRC) is the third-most common malignancy worldwide, with more than 1,800,000 diagnosed CRC cases and 881,000 deaths reported in 2018 [1]. In China, the incidence and mortality rates of CRC both rank fifth among those of all malignant cancers [2]; the incidence and mortality rates of CRC are predicted to increase along with the development of economy and the Westernization of lifestyle. Therefore, early diagnosis of CRC is crucial to improve patient outcomes. Currently, several conventional methods, such as the fecal occult blood test, colonoscopy, and computed tomography (CT) tests, are available to diagnose CRC; however, these approaches contain several limitations, such as low sensitivity, low specificity, invasiveness, or high cost, which restrict their clinical application [3–6]. In contrast, surgical approach represents the best treatment option for patients with CRC; however, recurrence or persistence after resection is associated with severe prognosis [7]. Approximately 30–50% of patients who undergo curative resection of CRC show CRC recurrence or metastasis [8]. An optimal surveillance protocol for CRC includes CT scans, colonoscopy, and serum carcinoembryonic antigen (CEA) level measurement, which has low sensitivity and specificity. Therefore, the development of patient-friendly and less invasive approaches with high sensitivity and specificity is imperative to improve patient outcomes.
Septin 9 DNA which encodes GTP-binding proteins plays an important role in the occurrence and progression of CRC [9]. Methylated septin 9 DNA (mSEPT9) has been detected in almost all CRC tissues [10]. Recently, studies have shown that mSEPT9 represents a promising biomarker for CRC detection. Studies have shown that the rate of SEPT9 methylation in peripheral blood of patients with CRC is related to clinicopathological features; for example, SEPT9 methylation is positively correlated with the malignancy of CRC [11–13]. After radical resection of colorectal cancer, the level of mSEPT9 in peripheral blood decreases; however, the level increases after recurrence, suggesting that mSEPT9 in peripheral blood can be used for evaluating the pathological stages of CRC and may represent a molecular biological indicator for evaluating prognosis, recurrence, and metastasis [14, 15]. However, the reported sensitivity and specificity values of plasma mSEPT9 are highly variable across studies, with the sensitivity ranging from 50.9–93.1% and specificity ranging from 62.2–93.8% [16, 17]. This may be attributed to the relatively small sample size. For example, the patient sample sizes in the studies were all less than 300, which may be insufficient for accurately assessing the prognostic value of mSEPT9 in CRC.
In this study, we measured the cycle threshold (Ct) value of mSEPT9 in 616 patients with CRC to analyze the value of mSEPT9 in the diagnosis of CRC compared with CEA and carbohydrate antigen 19-9 (CA19-9). Second, we evaluated whether mSEPT9 may play a potential role as a prognostic biomarker in CRC by evaluating the association between the positivity rate of mSEPT9 and clinicopathological characteristics among patients with CRC. Furthermore, we analyzed the association between mSEPT9 status and recurrence or metastasis in CRC. This study provides valuable information for the screening, diagnosis, and monitoring of CRC, especially in those patients who are reluctant to undergo colonoscopy or in cases where it is difficult to obtain biopsy specimens.
2. Material and Methods
2.1. Study Subjects and Samples
All samples were collected from Fujian Medical University Union Hospital, Fuzhou, China. Subjects were recruited between October 1, 2019, and May 31, 2021. The main inclusion criteria were adults (age, >18 years) with complete clinicopathological information and confirmed diagnosis of CRC (for the patient cohort) or no evidence of disease (NED, for the control group) based on imaging examination (including ultrasound, endoscopy, CT, and magnetic resonance imaging (MRI)) and/or subsequent pathological examination. Information on patient sex, age, and tumor/lymph node/metastasis (TNM) staging according to the American Joint Committee on Cancer TNM classification guidelines [18], primary tumor size, tumor location, cancer differentiation, vascular invasion, and nerve invasion were collected. The main exclusion criteria were history of any cancer, pregnancy, and incomplete information. Only subjects who underwent simultaneous evaluation of mSEPT9, CEA, and CA19-9 before any intervention were enrolled. Ultimately, 738 subjects were included in this study, including 616 patients with CRC (397 men and 219 women, median age (interquartile range, IQR): 61 (52–69) years) and 122 NED cases (69 men and 53 women, median age (IQR): 61 (52–69) years). This study was approved by the Research Ethics Committee at Fujian Medical University Union Hospital (Clinical trial registration number: 2021KJCX013). The participants provided informed consent for the collection of samples and clinicopathological information.
To evaluate the potential of mSEPT9 to monitor recurrence/metastasis in CRC, we enrolled 18 patients who were either recently diagnosed and underwent initial treatment or were monitored for CRC recurrence/metastasis. Follow-up information, including the date of surgery, adjuvant treatment strategy, and recurrence status, were collected. Recurrences or metastases were determined based on diagnostic tests (colonoscopy, CT scans, MRI, or positron emission tomography scans) and confirmed via tissue pathology when available [19].
2.2. Plasma Preparation and Storage
Blood samples (10 mL) of each patient were collected in K2EDTA tubes (BD biosciences, Franklin Lake, NJ, USA) and processed immediately (<1 h) via double centrifugation at 1,400 × g for 12 min. The plasma obtained was transferred to a new tube and directly stored at -80°C for subsequent testing.
2.3. Analysis of the Methylation Status of Circulating SEPT9 DNA in Plasma
DNA was extracted from the plasma samples using a plasma processing kit (BioChain Science and Technology Inc., Beijing, China). The DNA sample was then incubated with bisulfite, during which unmethylated cytosine was converted to uracil, whereas methylated cytosine was not. Subsequently, the methylated target sequences in the bisulfite-converted DNA template were amplified via real-time polymerase chain reaction (PCR). PCR-blocking oligonucleotides and methylation-specific probes were used to distinguish between methylated and unmethylated DNA. PCR was performed in a 60 μL reaction system. The thermocycling program was as follows: activation at 94°C for 20 min; 45 cycles at 62°C for 5 s, 55.5°C for 35 s, and 93°C for 30 s; and cooling at 40°C for 5 s. The methylation of SEPT9 in plasma was measured using an ABI7500 fluorescent PCR instrument (Thermo Fisher Scientific, Waltham, MA, USA). Quantitative PCR was performed in duplicate, and the average Ct value was calculated. We recorded PCR data and then analyzed the mSEPT9 and β-actin gene (ACTB) Ct within 45 cycles of amplification [7]. Results were considered valid when the ACTB Ct was ≤32.1, and the external negative and positive controls met the validity criteria specified by the manufacturer. An mSEPT9 Ct value of ≤41 cycles was considered a positive result, while Ct > 41 or an undetermined Ct was considered a negative result (Table 1).
Table 1.
Criteria for the validity of the system according to manufacturer's instructions.
| Septin 9 result | mSEPT9 | ACTB |
|---|---|---|
| Positive | Ct ≤ 41.0 | Ct ≤ 32.1 |
| Negative | Undetermined or Ct > 41.0 | Ct ≤ 32.1 |
| Invalid | Any case | Ct>32.1 |
mSEPT9: methylated septin 9 DNA; ACTB: β-actin; Ct: threshold amplification cycle.
2.4. CEA and CA19-9 Levels
A total of 3–5 mL of venous blood was collected. Serum was isolated via centrifugation at 3,000 rpm for 10 min. The serum levels of CEA and CA19-9 were determined using a Cobas6000 Analyzer (Roche Diagnostics, Mannheim, Germany). The cut-off value for normal CEA was <5 ng/mL and that for normal CA19-9 was <37 U/mL, according to the manufacturer's instructions.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS version 21.0 software (IBM, Armonk, NY, USA) or GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). The diagnostic values of mSEPT9, CEA, and CA19-9 were estimated using receiver operating characteristic (ROC) curves. The Youden index was used to determine the optimal cut-off value to differentiate between healthy controls and patients with CRC. Combination analysis was performed using binary logistic regression. The relationship between mSEPT9 and clinicopathological characteristics was assessed via a chi-square test. All tests were two-tailed, and a p value < 0.05 was considered significant.
3. Results
3.1. Performance of the mSEPT9 Assay for Detecting CRC
To evaluate the performance of the blood mSEPT9 assay in the diagnosis of CRC, we plotted ROC curves for cancer against NED compared with CEA and CA19-9, which are the most commonly used blood-based tumor markers in the diagnosis of CRC. The area under the ROC curve (AUC) for mSEPT9, CEA, and CA19-9 as parameters in the diagnosis of CRC was 0.826, 0.789, and 0.590, respectively (Figure 1 and Table 2). At the cut-off value of 41.0 for mSEPT9, we distinguished patients with CRC from healthy controls with a sensitivity of 72.94% and a specificity of 81.97% (Table 3). Notably, the diagnostic performance of CEA and CA19-9 could be improved when mSEPT9, CEA, and CA19-9 were combined for detection (Table 2). The AUCs for mSEPT9 + CEA and mSEPT9 + CA19 − 9 were 0.877 and 0.836, respectively (Figure 1 and Table 2), which were significantly higher than that for CEA + CA19 − 9 (AUC: 0.788). Moreover, the sensitivity of mSEPT9 + CEA was significantly higher than that of mSEPT9 + CA19 − 9 and CEA + CA19 − 9. When the three markers were combined, the AUC, sensitivity, and specificity of mSEPT9 + CEA + CA19 − 9 were 0.878, 78.43%, and 86.07%, respectively. Taken together, these results suggested that circulating mSEPT9 may represent a promising biomarker for CRC. The detection of mSEPT9, CEA, and CA19-9 may provide better diagnostic performance in discriminating patients with CRC from healthy individuals, with higher sensitivity and specificity.
Figure 1.
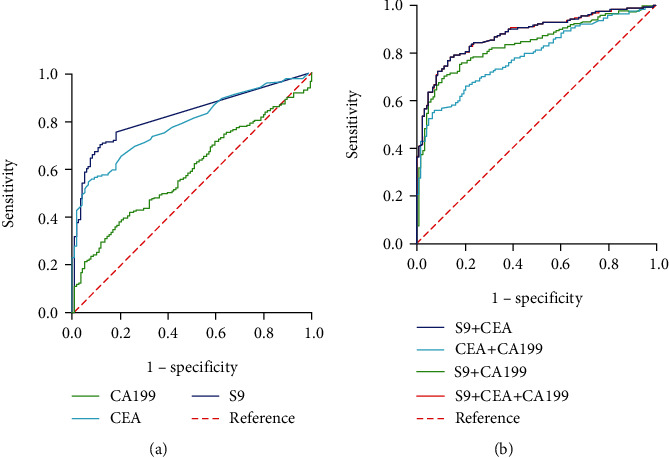
ROC curves of single mSEPT9 (S9), CEA, CA19-9, and their combination in discriminating patients with CRC from healthy participants. (a) ROC curves of single S9, CEA, and CA19-9 in discriminating patients with CRC from healthy participants. (b) ROC curves of S9 + CEA, S9 + CA19 − 9, CEA + CA19 − 9, and S9 + CEA + CA19 − 9 in discriminating patients with CRC from healthy participants. ROC: receiver operating characteristic curve; mSEPT9: methylated septin 9; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen-19-9.
Table 2.
The values of S9, CEA, and CA19-9 alone and in combination for differential diagnosis of health donors and patients with CRC.
| Variable | AUC | Cut-off | Sensitivity | Specificity | 95% confidence interval | |
|---|---|---|---|---|---|---|
| Upper limit | Lower limit | |||||
| S9 | 0.826 | 41 | 72.94% | 81.97% | 79.15% | 86.00% |
| CEA | 0.789 | 5 | 43.96% | 96.72% | 75.13% | 82.59% |
| CA19-9 | 0.590 | 37 | 14.99% | 96.61% | 53.89% | 64.05% |
| S9 + CEA | 0.877 | 78.43% | 85.25% | 84.88% | 90.61% | |
| S9 + CA19 − 9 | 0.836 | 66.91% | 91.80% | 80.27% | 87.01% | |
| CEA + CA19 − 9 | 0.788 | 55.76% | 92.62% | 75.04% | 82.53% | |
| S9 + CEA + CA19 − 9 | 0.878 | 78.43% | 86.07% | 84.89% | 90.62% | |
S9: methylated septin 9 DNA; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen-19-9; CRC: colorectal cancer.
Table 3.
Relationship between mSEPT9 and pathological characteristics of patients with CRC.
| Variables | Total | S9-positive cases | S9-negative cases | p value |
|---|---|---|---|---|
| CRC cases | 616 | 440 (71.4%) | 176 (28.6%) | |
| Gender | 0.079 | |||
| Male | 397 | 293 (73.8%) | 104 (26.2%) | |
| Female | 219 | 147 (67.1%) | 72 (32.9%) | |
| Age | 0.137 | |||
| <60 | 272 | 186 (68.4%) | 86 (31.6%) | |
| ≥60 | 344 | 254 (73.8%) | 90 (26.2) | |
| Location | 0.664 | |||
| Colon | 303 | 221 (72.9%) | 82 (27.1%) | |
| Rectosigmoid transition | 12 | 9 (75%) | 3 (25%) | |
| Rectum | 301 | 210 (69.8%) | 91 (30.29%) | |
| TNM stage | 0.0001 | |||
| I | 91 | 45 (49.5%) | 46 (50.5%) | |
| II | 170 | 128 (75.3%) | 42 (24.7%) | |
| III | 267 | 195 (73.0%) | 72 (27.0%) | |
| IV | 88 | 72 (81.8%) | 16 (18.2%) | |
| T stage | 0.0001 | |||
| T1 | 38 | 15 (39.5%) | 23 (60.5%) | |
| T2 | 70 | 36 (51.4%) | 34 (48.6%) | |
| T3 | 350 | 267 (76.3%) | 83 (23.7%) | |
| T4 | 158 | 122 (77.2%) | 36 (22.8%) | |
| N stage | 0.004 | |||
| N0 | 264 | 177 (67.0%) | 87 (33.0%) | |
| N1 | 156 | 108 (67.9%) | 51 (32.1%) | |
| N2 | 193 | 155 (80.3%) | 38 (19.7%) | |
| M stage | 0.220 | |||
| M0 | 528 | 382 (72.3%) | 146 (27.7%) | |
| M1 | 88 | 58 (65.9%) | 30 (34.1%) | |
| Histological grade | 0.836 | |||
| Low | 42 | 30 (71.4%) | 12 (28.6%) | |
| Moderate | 560 | 401 (71.6%) | 159 (28.4%) | |
| High | 14 | 9 (64.3%) | 5 (35.7%) | |
| Vascular invasion | 0.007 | |||
| Absent | 89 | 59 (66.3%) | 30 (33.7%) | |
| Present | 410 | 309 (75.4%) | 101 (24.6%) | |
| Unknown | 117 | 72 (61.5%) | 45 (38.5%) | |
| Nerve invasion | 0.030 | |||
| Absent | 133 | 97 (72.9%) | 36 (27.1%) | |
| Present | 366 | 271 (74.0%) | 95 (26.0%) | |
| Unknown | 117 | 72 (61.5%) | 45 (38.5%) | |
| Tumor size (cm) | 0.0001 | |||
| <5 | 351 | 230 (65.5%) | 121 (34.5%) | |
| ≥5 | 265 | 210 (79.2%) | 55 (20.8%) | |
| CEA | 0.061 | |||
| <5 | 341 | 254 (74.5%) | 87 (25.5%) | |
| ≥5 | 275 | 186 (67.6%) | 89 (32.4%) | |
| CA19-9 | 0.576 | |||
| <37 | 519 | 373 (71.9%) | 146 (28.1%) | |
| ≥37 | 97 | 67 (69.1%) | 30 (30.9%) |
S9: methylated septin 9 DNA; CEA: carcinoembryonic antigen; CA19-9: carbohydrate antigen-19-9; CRC: colorectal cancer.
3.2. Association between Plasma mSEPT9 Status and Clinicopathological Characteristics of Patients with CRC
After determining the performance of the plasma mSEPT9 assay for evaluating CRC, we further explored the correlation between mSEPT9 status and clinicopathological characteristics. The positivity rate of mSEPT9 was significantly higher in patients with more advanced TNM stages (stage I: 49.5%, stage II: 75.3%, stage III: 73.0%, stage IV: 81.8%, p = 0.0001) than that in patients with less advanced stages. Further analysis showed that mSEPT9 positivity was also significantly greater in patients with a more advanced T stage (stage T1: 39.5%, stage T2: 51.4%, stage T3: 76.3%, stage T4: 77.2%, p = 0.0001) and N stage (stage N0: 67.0%, stage N1: 67.9%, stage N2: 80.3%, p = 0.004) than that in patients with less advanced stages, although there was no significant relationship between mSEPT9 status and M stage (p = 0.220). The positivity rate of mSEPT9 was also higher in CRC cases with vascular invasion (p = 0.007) and nerve invasion (p = 0.030) compared to those without (Table 3). Notably, patients with CRC containing a tumor size ≥ 5 cm showed a significantly higher positivity rate of mSEPT9 than those with a tumor size < 5 cm (79.2% versus 65.5%, p = 0.0001). However, no significant association was found between mSEPT9 positivity rate and gender, age, location, histological grade, CEA level, and CA19-9 level. Taken together, these data indicate that the methylation status of circulating SEPT9 correlates with more advanced clinicopathological status in patients with CRC.
3.3. Plasma mSEPT9 Status for Monitoring CRC Recurrence
After validating the clinical value of mSEPT9 for CRC diagnosis, we further investigated whether mSEPT9 can be used as an indicator for recurrence or metastasis. We analyzed 18 CRC cases that were either recently diagnosed and underwent initial treatment or had been monitored for CRC recurrence (Table 4). The median period from primary diagnosis and treatment to mSEPT9 measurement was 18 months, ranging from 6–28 months. In total, 6 of the 18 CRC cases showed recurrence or metastasis based on diagnosis, and all cases showed positive mSEPT9 around the time of recurrence diagnosis. Notably, case no. 11 showed positive mSEPT9 after curative surgery and chemotherapy (Table 4). In comparison, 4 of the 6 recurrent cases (66.7%) showed excessive CEA levels. No evidence of recurrence was found in the remaining 12 cases, which was consistent with the correspondingly negative mSEPT9 status. Notably, in 11 of the remaining 12 cases, mSEPT9 was positive before initial treatment, suggesting that besides monitoring CRC recurrence, mSEPT9 may also be used for evaluating therapeutic efficacy in CRC.
Table 4.
Detection of CRC recurrence based on plasma mSEPT9 during follow-up.
| No. | Gender | Age (years) | TNM staging | Treat | Period† (months) | S9 | CEA (ng/mL) | Recurrence status | |
|---|---|---|---|---|---|---|---|---|---|
| Pre | Pos | ||||||||
| 1 | Male | 60 | T3N0M0 | S | 26 | + | - | 3.1 | NER |
| 2 | Male | 56 | T4aN2M0 | S + C | 26 | + | + | 153.4 | Retroperitoneal lymph node metastases |
| 3 | Female | 59 | T4bN2M0 | S + C | 20 | + | + | 50.2 | Lung metastases |
| 4 | Male | 55 | T4aN2M0 | S + C + R | 18 | + | - | 1.8 | NER |
| 5 | Male | 61 | T4bN2M0 | S + C | 18 | + | - | 3.0 | NER |
| 6 | Male | 57 | T4aN2M0 | S + C | 15 | + | + | 4.6 | Liver metastases |
| 7 | Male | 38 | T4bN1M0 | S + C + R | 16 | - | - | 1.4 | NER |
| 8 | Female | 70 | T2N0M0 | S | 18 | + | - | 3.0 | NER |
| 9 | Female | 49 | T3N2M0 | S + R | 18 | + | - | 1.9 | NER |
| 10 | Female | 61 | T4bN1M0 | S + C + R | 18 | + | + | 34.4 | Liver metastases |
| 11 | Male | 50 | T4bN1M0 | S + C | 18 | - | + | 24.0 | Liver metastases |
| 12 | Male | 51 | T3N0M0 | S + C | 18 | + | - | 1.6 | NER |
| 13 | Female | 64 | T3N0M0 | S + C | 18 | + | - | 1.2 | NER |
| 14 | Female | 69 | T4N2M0 | S + C | 6 | + | - | 1.7 | NER |
| 15 | Female | 51 | T3N2M0 | S + C | 18 | + | - | 1.1 | NER |
| 16 | Male | 47 | T4N2M0 | S + C | 17 | + | - | 3.6 | NER |
| 17 | Male | 51 | T2N0M0 | S | 28 | + | - | 2.0 | NER |
| 18 | Female | 78 | T4N0M0 | S + C | 6 | + | + | 1.5 | Recurrent CRC |
S9: methylated septin 9 DNA; Treat: treatment; S: curatively intended surgery; C: chemotherapy; R: radiation therapy; NER: no evidence of recurrence; +: positive; −: negative; boldface in CEA column represents positive; †: period after treatment.
4. Discussion
Early screening of CRC and efficient monitoring of metastases are urgently needed to improve the treatment outcomes of patients with CRC and reduce mortality. In this study, we evaluated the diagnostic value of mSEPT9 in blood-based CRC detection in Chinese patients compared to that of two traditional blood-based tumor biomarkers (CEA and CA19-9). MSEPT9 showed better performance than that of CEA and CA19-9 for CRC diagnosis, in which patients with CRC were distinguished from healthy individuals with a sensitivity of 72.94%, specificity of 81.97%, and AUC of 0.826. The combination of mSEPT9 with CEA and CA19-9 further improved the sensitivity, specificity, and AUC value. Our statistical analysis also indicated that plasma mSEPT9 DNA levels in patients with CRC were correlated with TNM stage, T stage, N stage, tumor size, vascular invasion, and nerve invasion. Furthermore, our data demonstrated that plasma mSEPT9 may represent a reliable prognostic marker to predict recurrence or metastasis in patients with CRC, as well as in the evaluation of the therapeutic efficacy of multimodality therapy in CRC.
CRC represents a leading cause of cancer-related deaths worldwide, and patients with late-stage CRC have low five-year survival rates [2]. Blood-based screening strategies present the advantage of minimal invasiveness compared to that of endoscopy, and they are expected to have higher compliance rates than those for stool-based tests [20]. However, the sensitivity and specificity of current blood-based markers, CEA and CA19-9, have been demonstrated to be low, especially for stratifying early stages of CRC [21, 22], which was further confirmed in our study. Although invasive colonoscopy has the highest sensitivity and specificity for CRC, it has the lowest patient compliance rate due to the need of bowel preparation and discomfort during the test. Furthermore, certain patients with severe cardiopulmonary insufficiency, enterostenosis, or intestinal perforation cannot undergo invasive tests. Hence, novel markers are sought after to improve the sensitivity and specificity in screening for CRC in patients [23]. Our data showed that plasma mSEPT9 had an AUC of 0.826 (95% confidence interval: 79.15–86%) for CRC detection with high sensitivity and specificity, which is similar to that reported previously [17]. Our findings also showed that the combined detection of mSEPT9, CEA, and CA19-9 improved the diagnostic performance of CEA and CA19-9 in discriminating patients with CRC from healthy participants. Taken together, plasma mSEPT9 represents a potential blood-based biomarker for the diagnosis of CRC, and this blood-based test is noninvasive, patient friendly, and is expected to obtain high compliance.
To the best of our knowledge, few studies have investigated the association between mSEPT9 status and clinicopathological characteristics of patients with CRC. TNM stage, which is based on the extent of tumor growth (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M), is the most studied classification system for evaluating CRC. The positive rates of mSEPT9 were significantly associated with the TNM stage, including T and N stages, and these findings were consistent with those of Sun et al. [24]. However, a previous study by Fu et al. [17] on 98 CRC cases showed no significant association between mSEPT9 and TNM stage, which may be caused by a relatively smaller sample size. Previous studies showed that vascular invasion and lymph node metastases are negatively associated with prognosis and represent potential independent prognostic markers of CRC [25–27]. However, few accurate protein biomarkers of vascular invasion in CRC are available. Our results showed that the positive rate of mSEPT9 was significantly associated with vascular invasion and nerve invasion. Notably, our data demonstrated that the positive rate of mSEPT9 in CRC cases with a tumor size of ≥5 cm was significantly higher than that in cases with a tumor size of <5 cm, which was similar to the findings of Fu et al. [17]. We speculate that the status of mSEPT9 has a positive correlation with tumor size. These findings also suggested that plasma mSEPT9 has potential as an additional biomarker for prognostic evaluation of CRC.
It is important to detect CRC recurrence or metastasis in postoperative patients. To our knowledge, CEA is the only blood-based test applied for conventionally monitoring CRC recurrences; however, it has low sensitivity and specificity [19, 28]. Our data showed a good agreement between the mSEPT9 status and CRC recurrence (100% sensitivity), suggesting that the mSEPT9 test may represent a reliable marker in monitoring CRC recurrence or metastasis. Additionally, our results revealed for the first time that the persistent positivity of plasma mSEPT9 after multimodality therapy was highly correlated with impending recurrence or metastasis, whereas the conversion of mSEPT9 positivity to negativity indicated the end of recurrence. Based on this perspective, mSEPT9 may serve as a reliable biomarker for assessing therapeutic efficacy in patients with CRC whose preoperative mSEPT9 was positive. Since this is a pilot study, we will further verify the potential predictive ability of mSEPT9 for tumor recurrence/metastasis by extending follow-up time, mSEPT9 monitoring, and combining samples from multiple medical centers to expand the sample size.
Some limitations of this study need to be addressed. First, the short follow-up duration in this study with single-centered retrospective design may provide bias towards sample selection and analysis. Moreover, we failed to collect data on overall survival; therefore, the relationship of mSEPT9 status with overall survival in patients with CRC was not evaluated. Future studies should further confirm whether plasma mSEPT9 has the potential to provide clinically relevant lead times compared to that of conventional diagnostic modalities for recurrence or metastasis detection, as well as in assessing the therapeutic efficacy in CRC. Hence, further prospective multicenter studies are needed to validate the clinical significance of mSEPT9 in patients with CRC.
5. Conclusion
In summary, our results showed that plasma mSEPT9 represents a promising biomarker in CRC diagnosis. Notably, we revealed a significant association between mSEPT9 status and clinicopathological characteristics of patients with CRC. Postoperative mSEPT9 during follow-up served as a significant indicator for CRC recurrence or metastasis.
Acknowledgments
This work was supported by Fujian province Joint Funds for the Innovation of Science and Technology (2019Y9060 and 2020Y9088), Fujian provincial health technology project for Young and Middle-aged Core Talents (2021GGA015), and National Natural Science Foundation of Fujian Province (2019J01151 and 2021J01738).
Contributor Information
Yingping Cao, Email: caoyingping@aliyun.com.
Zhengyuan Huang, Email: huangzy@fjmu.edu.cn.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Pingxia Lu and Xianjin Zhu contributed equally to this work. Yingping Cao and Zhengyuan Huang, corresponding authors, contributed equally to this work.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W., Zheng R., Baade P. D., et al. Cancer statistics in China, 2015. CA: a Cancer Journal for Clinicians . 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Uraoka T., Hosoe N., Yahagi N. Colonoscopy: is it as effective as an advanced diagnostic tool for colorectal cancer screening? Expert Review of Gastroenterology & Hepatology . 2015;9(2):129–132. doi: 10.1586/17474124.2015.960397. [DOI] [PubMed] [Google Scholar]
- 4.Morikawa T., Kato J., Yamaji Y., Wada R., Mitsushima T., Shiratori Y. A comparison of the immunochemical fecal occult blood test and total colonoscopy in the asymptomatic population. Gastroenterology . 2005;129(2):422–428. doi: 10.1016/j.gastro.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 5.Thomas D. S., Fourkala E. O., Apostolidou S., et al. Evaluation of serum CEA, CYFRA21-1 and CA125 for the early detection of colorectal cancer using longitudinal preclinical samples. British Journal of Cancer . 2015;113(2):268–274. doi: 10.1038/bjc.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dekker E., Rex D. K. Advances in CRC prevention: screening and surveillance. Gastroenterology . 2018;154(7):1970–1984. doi: 10.1053/j.gastro.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 7.Song L., Li Y. _SEPT9_ : a specific circulating biomarker for colorectal cancer. Advances in Clinical Chemistry . 2015;72:171–204. doi: 10.1016/bs.acc.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Young P. E., Womeldorph C. M., Johnson E. K., et al. Early detection of colorectal cancer recurrence in patients undergoing surgery with curative intent: current status and challenges. Journal of Cancer . 2014;5(4):262–271. doi: 10.7150/jca.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall P. A., Russell S. E. The pathobiology of the septin gene family. The Journal of Pathology. . 2004;204(4):489–505. doi: 10.1002/path.1654. [DOI] [PubMed] [Google Scholar]
- 10.Wasserkort R., Kalmar A., Valcz G., et al. Aberrant septin 9 DNA methylation in colorectal cancer is restricted to a single CpG island. BMC Cancer . 2013;13(1):p. 398. doi: 10.1186/1471-2407-13-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun J., Xu J., Sun C., et al. Screening and prognostic value of methylated septin9 and its association with clinicopathological and molecular characteristics in colorectal cancer. Frontiers in Molecular Biosciences . 2021;8:p. 568818. doi: 10.3389/fmolb.2021.568818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan S., Liu Z., Yu S., Bao Y. Diagnostic value of methylated septin9 for colorectal cancer screening: a meta-analysis. Medical Science Monitor . 2016;22:3409–3418. doi: 10.12659/MSM.900590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Church T. R., Wandell M., Lofton-Day C., et al. Prospective evaluation of methylatedSEPT9in plasma for detection of asymptomatic colorectal cancer. Gut . 2014;63(2):317–325. doi: 10.1136/gutjnl-2012-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon Arellano M., Garcia-Arranz M., Ruiz R., et al. A first step to a biomarker of curative surgery in colorectal cancer by liquid biopsy of methylated septin 9 gene. Disease Markers . 2020;2020:5. doi: 10.1155/2020/9761406.9761406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergheim J., Semaan A., Gevensleben H., et al. Potential of quantitative SEPT9 and SHOX2 methylation in plasmatic circulating cell-free DNA as auxiliary staging parameter in colorectal cancer: a prospective observational cohort study. British Journal of Cancer . 2018;118(9):1217–1228. doi: 10.1038/s41416-018-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie L., Jiang X., Li Q., et al. Diagnostic value of methylated septin9 for colorectal cancer detection. Frontiers in Oncology . 2018;8:p. 247. doi: 10.3389/fonc.2018.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu B., Yan P., Zhang S., et al. Cell-free circulating methylated SEPT9 for noninvasive diagnosis and monitoring of colorectal cancer. Disease Markers . 2018;2018:11. doi: 10.1155/2018/6437104.6437104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edge S. B., Compton C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology . 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Young G. P., Pedersen S. K., Mansfield S., et al. A cross-sectional study comparing a blood test for methylated BCAT1 and IKZF1 tumor-derived DNA with CEA for detection of recurrent colorectal cancer. Cancer Medicine . 2016;5(10):2763–2772. doi: 10.1002/cam4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orntoft M. B. Review of blood-based colorectal cancer screening: how far are circulating cell-free DNA methylation markers from clinical implementation? Clinical Colorectal Cancer . 2018;17(2):e415–e433. doi: 10.1016/j.clcc.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Song Y., Huang Z., Kang Y., et al. Clinical usefulness and prognostic value of red cell distribution width in colorectal cancer. BioMed Research International . 2018;2018:7. doi: 10.1155/2018/9858943.9858943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S. Y., Lin M., Zhang H. B. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. International Journal of Clinical and Experimental Pathology . 2015;8(8):9404–9409. [PMC free article] [PubMed] [Google Scholar]
- 23.Potter N. T., Hurban P., White M. N., et al. Validation of a real-time PCR-based qualitative assay for the detection of methylated SEPT9 DNA in human plasma. Clinical Chemistry . 2014;60(9):1183–1191. doi: 10.1373/clinchem.2013.221044. [DOI] [PubMed] [Google Scholar]
- 24.Sun J., Fei F., Zhang M., et al. The role of mSEPT9 in screening, diagnosis, and recurrence monitoring of colorectal cancer. BMC Cancer . 2019;19(1):p. 450. doi: 10.1186/s12885-019-5663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betge J., Langner C. Vascular invasion, perineural invasion, and tumour budding: predictors of outcome in colorectal cancer. Acta Gastro-Enterologica Belgica . 2011;74(4):516–529. [PubMed] [Google Scholar]
- 26.Naito Y., Goto K., Nagai K., et al. Vascular invasion is a strong prognostic factor after complete resection of node-negative non-small cell lung cancer. Chest . 2010;138(6):1411–1417. doi: 10.1378/chest.10-0185. [DOI] [PubMed] [Google Scholar]
- 27.Fujii T., Sutoh T., Morita H., et al. Vascular invasion, but not lymphatic invasion, of the primary tumor is a strong prognostic factor in patients with colorectal cancer. Anticancer Research . 2014;34(6):3147–3151. [PubMed] [Google Scholar]
- 28.Duffy M. J., Lamerz R., Haglund C., et al. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. International Journal of Cancer . 2014;134(11):2513–2522. doi: 10.1002/ijc.28384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


