Abstract
Background
Connexin is a basic molecule that forms gap junctions and undergoes localization changes to the cytoplasm in association with carcinogenesis. We aimed to investigate and clarify the significance of cytoplasmic Cx26 expression in gastric cancer.
Methods
We included 87 patients with intestinal‐ and mix‐type gastric cancer and 111 patients with diffuse type gastric cancer who underwent surgery for gastric cancer between 1999 and 2006. Immunohistochemical staining for Cx26, β‐catenin, and Wnt3a was performed and analyses of the relationship to clinicopathological factors were conducted based on the Lauren classification. In an in vitro study, the gastric cancer cell lines MKN7, MKN74, and MKN45 were used to evaluate the proliferative capacity using the water‐soluble tetrazolium salt assay through forced expression of Cx26, and the relationship between Cx26 and β‐catenin was investigated using proximity ligation assay (PLA) and co‐immunoprecipitation. Additionally, functional analysis was performed by Cage analysis.
Results
In this study, high cytoplasmic Cx26 expression was associated with favorable prognosis in intestinal‐ and mix‐type gastric cancer and could be an independent prognostic factor for overall survival. In terms of the mechanism, in in vitro experiments changes in Cx26 localization to the cytoplasm were shown to suppress the change of localization of β‐catenin to the nucleus by binding to it in the cytoplasm.
Conclusions
Cytoplasmic Cx26 was found to be a prognostic factor in intestinal‐ and mix‐type gastric cancer. Regarding the mechanism, in vitro studies revealed that cytoplasmic Cx26 inhibits the translocation of β‐catenin to the nucleus.
Keywords: connexin 26, gap junctions, second messengers, β‐catenin
Cytoplasmic Cx26 was found to be a prognostic factor in intestinal‐ and mix‐type gastric cancer. We revealed that cytoplasmic Cx26 inhibits the translocation of β‐catenin to the nucleus.
1. INTRODUCTION
Gastric cancer is one of the most typical cancers. While the molecular biological understanding of gastric cancer has gradually deepened, the mechanism of onset and progression of gastric cancer has not been fully elucidated. A better understanding of the molecular biology of gastric cancer may contribute to better prognoses.
Gap junctions are structures located on the cell membrane that play a role in the diffuse transport of metabolites and second messengers, such as cyclic adenosine monophosphate (cAMP), inositol trisphosphate, and calcium (Ca) between cells. 1 Connexins (Cxs) are the basic molecules that form gap junctions and over 20 Cxs have been identified in humans to date. 2 Defective gap junctions are known to contribute to cancer progression in various carcinomas. In addition, aberrant changes in the expression and subcellular localization of Cxs have also been reported in a variety of cancers. 3 , 4 , 5 Cxs regulate cell proliferation, migration, and differentiation in cancer. 6 , 7
Connexin 26 (Cx26) is one of the most commonly reported isotypes of connexins in cancer and its function varies with the type of cancer. However, in all cancers the localization of Cx26 is seen to have changed from the cell membrane to the cytoplasm. For example, cytoplasmic Cx26 expression correlated with lymphatic invasion and poor prognosis in breast cancer. 8 In colorectal cancer, high cytoplasmic Cx26 expression was associated with venous invasion, lung metastases, and poor prognoses. 9 In lung cancer, cytoplasmic Cx26 has also been reported to promote tumor growth, epithelial‐mesenchymal transition (EMT), migration, and invasion via the phosphoinositide 3‐kinase (PI3K)/Akt pathway. 10 We reported that the abnormal expression of Cx26 correlated with the progression of esophageal squamous cell carcinoma. 11 In gastric cancer, Liu et al suggested that abnormal expression of cytoplasmic Cx26 is a prognostic factor independent of the role of tumor inhibition in intestinal‐type gastric cancer 12 ; however, little is known about the function of cytoplasmic Cx26.
The Wnt/β‐catenin pathway increases cytoplasmic β‐catenin. An increase in cytoplasmic β‐catenin leads to translocation of these to the nucleus and subsequent induction of expression of transcription factors that induce EMT. 13 Cx26 expression correlated positively with β‐catenin with colorectal cancer. 14 Furthermore, Cx26 inhibits both EMT and angiogenesis in both hepatocellular carcinoma and mammary carcinoma. 15 , 16 Few studies have reported the relationship Cx26 with β‐catenin or EMT with gastric cancer. Thus, there is also a need to clarify the roles and relationship between Cx26 and the Wnt/β‐catenin pathway in gastric cancer.
The aim of this study was to clarify the significance of cytoplasmic Cx26 expression in gastric cancer. The significance of changes in Cx26 localization to the cytoplasm in gastric cancer has not been investigated to date and our investigation is the first report.
2. MATERIALS AND METHODS
2.1. Patients and samples
In this study, 198 patients who underwent gastric cancer surgery at the Department of General Surgical Science, Gunma University Hospital between 1999 and 2006 were included. We used tissue microarray in this study. We excluded preoperative treated cases and collected cases with multiple blocks containing the invasion front region for tissue microarray. These cases did not include endoscopic submucosal dissection cases. The staging of gastric cancer was according to the Japanese Classification of Gastric Carcinoma: 3rd English edition. 17 This study was conducted with approval from the Institutional Review Board of Gunma University (approval no. HS2019‐127).
2.2. Immunohistochemistry
All specimens were cut into 4‐μm thick sections and mounted on glass slides. All sections were deparaffinized with xylene, rehydrated, and incubated in 0.3% hydrogen peroxide for 30 min at room temperature to block endogenous peroxidase activity. For Cx26 and β‐catenin, antigen activation was performed with Immunosaver (Nishin EM, Tokyo, Japan) at 98°C to 100°C for 45 min. For Wnt3a, a citric acid method was adopted as the antigen activation method, and the specimens were immersed in a hot bath at 98°C for 30 min and then in hot water at 75°C for 10 min using sodium citrate buffer (LSI Medience, Tokyo, Japan). Nonspecific binding sites were blocked by incubating with Protein Block Serum‐Free (DAKO, Burlingame, CA, USA) for 30 min. Specimens were incubated overnight at 4°C with a primary antibody (diluted by DAKO REAL antibody diluent). The Cx26 antibody used was Cx26 (Thermo Fisher Scientific, Waltham, MA, USA). Anti‐Connexin26 monoclonal antibody (1:100 dilution), the β‐catenin antibody was Non‐phospho (Active) β‐Catenin (Ser33/37/Thr41) (Cell Signaling Technology, Tokyo, Japan). Anti‐Non‐phospho β‐catenin monoclonal antibody (1:600 dilution), and the Wnt3a antibody was Wnt3a (Bioss Antibodies, MA, USA), Wnt3a Polyclonal Antibody (1:200 dilution). The Histofine Simple Stain MAX‐PO (Multi) Kit (Nichirei, Tokyo, Japan) was used for the secondary antibody. A 0.02% solution of chromogen 3,3‐diaminobenzidine tetrahydrochloride was formulated in 50 mmol/L ammonium acetate‐citrate buffer containing 0.005% hydrogen peroxide and used to develop color. Sections were lightly stained with hematoxylin and mounted with a cover glass.
Immunostainings were evaluated independently by two independent evaluators who were blinded to the patient data. We calculated and examined kappa statistics (κ) for the error between the evaluators and confirmed it for the evaluations of Cx26 (k = 0.79), wnt3a (κ = 0.78), and nuclear β‐catenin expressions (κ = 0.75). Cx26 was assessed using the Allred Score. 18 Proportions of stained cells were classified into six categories (0: completely negative; 1: <1% positive; 2:1%–10% positive; 3:11%–33% positive; 4:34%–66% positive; and 5:67%–100% positive). Staining intensity was classified into three categories (0: no staining; 1: weak positive staining; 2: moderate positive staining; 3: strong positive staining) and a score of 5 including both proportion and intensity scores was set as the cutoff, and a high‐expression group defined as a group with a score of 5 or higher. Wnt3a was assessed using the intensity score. Patients were assigned to the Wnt3a‐low (0,1+) or high expression (2+,3+) groups, according to their staining scores. Nuclear β‐catenin expression was judged to be positive if it was expressed in at least one cancer cell under 200‐fold magnification.
2.3. Cell culture
We used the MKN7, MKN74, and MKN45 lines. Cells were cultured in RPMI‐1640 medium (Wako, Osaka, Japan) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin‐streptomycin (Invitrogen, Carlsbad, CA, USA).
2.4. In situ proximity ligation assay (PLA)
Proximity ligation assay is a method to clarify the interrelationship between two different proteins. When the PLUS and MINUS pair of oligonucleotide probes (PLA probes) are in close proximity (within 40 nm), they are cyclized and can be visualized, detected, and quantified from the signal generated via rolling circle amplification. Duolink PLA (Sigma Aldrich, St. Louis, MO, USA) was used, and we followed the attached protocol. The MKN7 and MKN45 cell lines were used, and fixed by adding ice‐cold 100% methanol. Blocking was performed by incubating in Protein Block Serum‐Free (DAKO) for 30 min. Cx26 (Thermo Fisher Scientific) and β‐catenin (EPITMICS, Cambridge, UK) were combined as primary antibodies, after which 200 μL of a 100‐fold dilution of each in DAKO REAL antibody diluent was added and incubated at 4°C overnight. PLA MINUS and PLA PLUS probes were added at required amounts and diluted in antibody diluent to prepare PLA probe solutions. Thereafter, another wash was performed and the included ligase was added to cause ligation, and amplification was done by adding the included polymerase solution. The included detector stock solution (Brightfield) was added as a horseradish peroxidase (HRP)‐labeled probe and incubated at room temperature, after addition of the Duolink In Situ Mountain Medium with DAPI, and observation under a fluorescence microscope.
2.5. Co‐IP
2.5.1. Co‐immunoprecipitation and western blotting
MKN7, MKN45, and MKN 74 cells were lysed with 1× cell lysis buffer. A biotinylated β‐catenin antibody was prepared using a Pierce Antibody Biotinylation kit for immunoprecipitation (Thermo Fisher Scientific) and a β‐catenin antibody for immunoprecipitation (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Homogeneously loaded whole cell lysates were loaded into prepared biotinylated anti‐β catenin antibodies, incubated at 4°C overnight with rotation, and then incubated with streptavidin magnetic beads (Solulink Biosciences, San Diego, CA, USA) in the dark at room temperature using the Magnetic Separation product (Promega, Madison, WI, USA). The beads were washed with cold phosphate‐buffered saline (PBS) and then heated in Laemmli 2× buffer to elute proteins from the beads. Fractions of eluted proteins and input fractions were analyzed by western blotting using Cx26 (Abcam, Cambridge, UK) and anti‐β‐catenin antibodies. An equal amount of input proteins and an equal volume of concentrated eluted proteins were electrophoresed on 12.5% sodium dodecyl sulfate (SDS)‐polyacrylamide gel electrophoresis (PAGE) gels, blotted onto a nitrocellulose membrane, and probed with the designated antibody.
2.6. Overexpression
Lentivirus overexpressing Cx26 was purchased (VectorBuilder, Chicago, IL, USA). MKN7 and MKN74 cell lines were used. Viral particles and 1 μL of polybrene were dissolved in medium and the concentration was standardized to 10 multiplicity of infection. This medium was used to culture cells for 24 h and then replaced with normal medium. Green fluorescent protein expression was confirmed using a fluorescent cell imager and transduction efficiency was assessed. Cells were selected and cultured with medium supplemented with puromycin as appropriate.
2.7. Proliferation assay
Cell proliferation capacity was assessed using cell counting kit‐8 (Dojindo Laboratories, Kumamoto, Japan). Ten wells were seeded and cultured in 96‐well culture plates to ensure a density of 2000 cells per well. The first evaluation was performed (0 h) after cultures were incubated for 24 h and cell proliferation capacity was evaluated at 24, 48, and 72 h. The absorbance of each well was measured at 450 nm using a spectrophotometer (Bio‐Rad, Hercules, CA, USA) with the reference wavelength set at 650 nm.
2.8. Protein extraction and western blotting analysis
Using a nuclear/cytosolic fractionation kit (Cell Biolabs, San Diego, CA, USA), cytoplasmic fractionation and nuclear fractionation were performed in compliance with the manufacturer's protocol and proteins were extracted. For Cx26 and β‐actin, 12% Bis‐Tris gels were used, and for β‐catenin and proliferating cell nuclear antigen (PCNA) 10% Bis‐Tris gels were used, and a transfer to nitrocellulose membranes (Cell Signaling, Beverly, MA, USA) was performed. Membranes were blocked with 5% skim milk or 5% bovine serum albumin and incubated with the primary antibodies. The antibodies used were Cx26 (1:750; Thermo Fisher Scientific), active β‐catenin (1:1000; Cell Signaling), β‐actin (1:1000; Cell Signaling), and PCNA (1:1000; Cell Signaling). Membranes were then processed with horseradish peroxidase (HRP)‐conjugated secondary antibodies and then recorded and evaluated using an ECL Prime Western Blotting Detection Reagent and an ImageQuant LAS 4000 imager (GE Healthcare, Buckinghamshire, UK).
2.9. Cap analysis of gene expression (Cage analysis)
Pellets of both MKN74 cells with forced Cx26 expression and MKN74 cells as the negative control were mailed to a DNA firm (Yokohama, Japan) for analysis. Cage library preparation, sequencing, mapping, gene expression, and motif discovery analysis were performed. In the enrichment analysis, the focus was on downstream genes of Wnt/β‐catenin. Additionally, in the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, the focus was on the Wnt signaling pathway.
2.10. Statistical analysis
Continuous data are presented as means ± standard deviations (SDs). Statistical significance was analyzed using Mann–Whitney U tests for continuous variables and chi‐square tests for categorical variables. Survival was calculated using the Kaplan–Meier method and statistical significance was determined using Wilcoxon analyses. Univariate and multivariate analyses of overall survival (OS) were performed using the Cox proportional hazards model. Multivariate analysis was performed on the factors that were significant in the univariate analysis. Factors with a 95% confidence interval (CI) greater than 1 were judged to be independent prognostic factors. Probability values less than 0.05 were considered significant. All statistical analyses were performed using JMP Pro 12.0 software (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Evaluation of immunohistochemical staining of Cx26, activated β‐catenin, and Wnt3a expression in gastric cancer tissues
In noncancerous regions, Cx26 was stained on the cell membrane (Figure 1A). In the analysis of Cx26 expression in cancerous regions, representative low expression is shown in Figure 1B and high expression is shown in Figure 1C. With carcinogenesis, Cx26 expression was well stained in the cytoplasm. The results of immunohistochemical staining of active β‐catenin are shown in Figure 1D,E. Activated β‐catenin was well stained in the nucleus. The results of immunohistochemical staining of Wnt3a are shown in Figure 1F,G, and Wnt3a was well stained with both cancer cells and cancer stroma.
FIGURE 1.
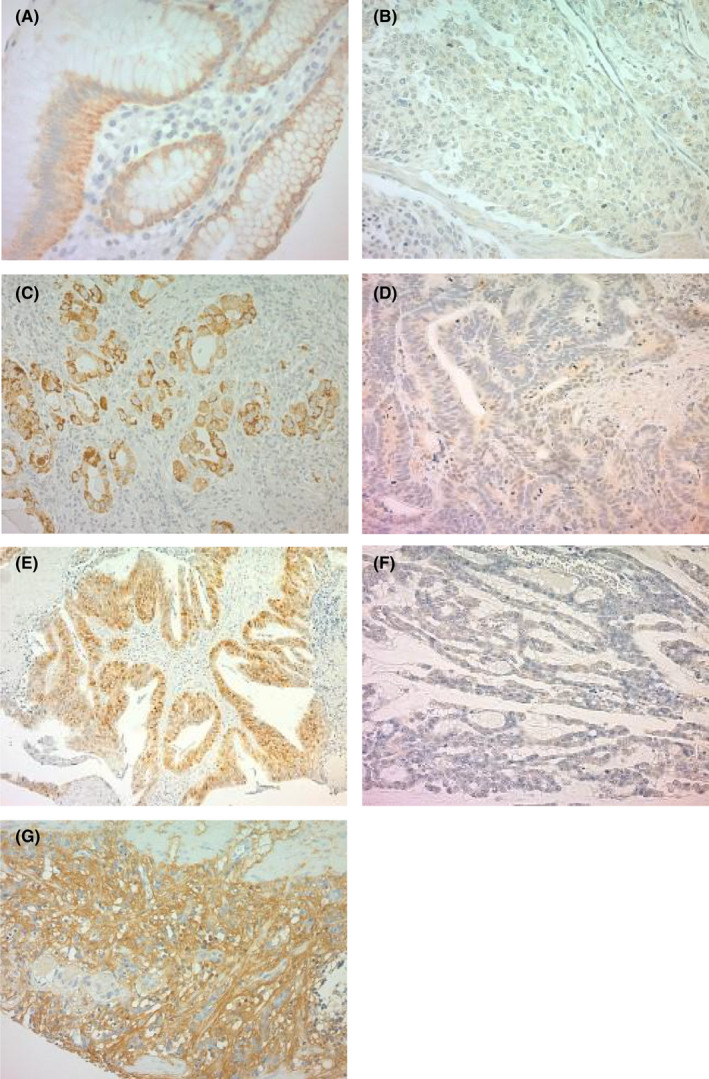
Representative images of immunohistochemical staining of Cx26, β‐catenin, and Wnt3a in gastric cancer. 200‐fold magnification. A: Cx26 expression in noncancerous regions; B: low cytoplasmic Cx26 expression in cancerous regions; C: high cytoplasmic Cx26 expression in cancerous regions; D: low nuclear β‐catenin expression in cancerous regions; E: high nuclear β‐catenin expression in cancerous regions; F: low nuclear Wnt3a expression in cancerous regions; G: high Wnt3a expression in cancerous regions
3.2. Evaluation of cytoplasmic Cx26 expression and clinicopathological factors according to the Lauren classification
The evaluation of Cx26 expression and clinicopathological factors in intestinal‐ and mix‐type gastric cancer are shown in Table 1. There was no significant correlation between Cx26 expression and age, sex, tumor diameter, depth of invasion, degree of differentiation, presence or absence of lymph node metastases, lymphatic or vascular invasion, stage, or human epidermal growth factor receptor 2 (HER2) expression. However, in those with high Cx26 expression there was less nuclear β‐catenin (P = .035) and significantly fewer relapses (P = .017). Cx26 expression was correlated with Wnt3a expression (P = .0020). A similar evaluation for diffuse type gastric cancer is also shown in Table 1. As with the intestinal‐ and mix‐type gastric cancer, there was no significant correlation between Cx26 expression and age, sex, tumor diameter, depth of invasion, degree of differentiation, presence or absence of lymph node metastases, lymphatic or vascular invasion, stage, HER2 expression, or relapses. Additionally, high expression of Cx26 was correlated with high expression of Wnt3a, but there was no significant correlation between nuclear β‐catenin and Cx26.
TABLE 1.
Evaluation of cytoplasmic Cx26 expression and clinicopathological factors in both intestinal‐ and mix‐type and diffuse type gastric cancer
| Lauren classification | Intestinal and mix type | Diffuse type | ||||
|---|---|---|---|---|---|---|
| Factors | Cx26 | Cx26 | ||||
| Low expression | High expression | P value | Low expression | High expression | P value | |
| n = 50 | n = 37 | n = 75 | n = 36 | |||
| Age | ||||||
| Years | 67 | 66 | .72 | 62 | 64 | .64 |
| Gender | ||||||
| Male | 41 | 27 | .32 | 47 | 23 | .90 |
| Female | 9 | 10 | 28 | 13 | ||
| Tumor size | ||||||
| mm | 58.3 ± 3.8 | 55.6 ± 4.4 | .64 | 72.6 ± 4.6 | 65.8 ± 6.6 | .40 |
| Depth | ||||||
| M,SM,MP | 13 | 15 | .15 | 14 | 8 | .66 |
| SS,SE,SI | 37 | 22 | 61 | 28 | ||
| Differentiation | ||||||
| tub1,tub2,pap | 39 | 35 | .41 | 0 | 1 | .13 |
| por,sig | 9 | 2 | 75 | 35 | ||
| Lymph node metastasis | ||||||
| Absent | 17 | 14 | .71 | 21 | 11 | .78 |
| Present | 33 | 23 | 54 | 25 | ||
| Lymphatic invasion | ||||||
| Absent | 7 | 4 | .66 | 5 | 3 | .75 |
| Present | 43 | 33 | 70 | 33 | ||
| Venous invasion | ||||||
| Absent | 35 | 26 | .98 | 56 | 27 | .78 |
| Present | 15 | 11 | 19 | 8 | ||
| Stage | ||||||
| I,II | 27 | 24 | .31 | 29 | 16 | .56 |
| III,IV | 23 | 13 | 46 | 20 | ||
| Postoperative chemotherapy | ||||||
| Absent | 32 | 21 | .49 | 41 | 19 | .85 |
| Present | 18 | 16 | 34 | 17 | ||
| HER2 score | ||||||
| 0 | 34 | 20 | .16 | 65 | 22 | .07 |
| 1,2,3 | 12 | 14 | 4 | 5 | ||
| Recurrence | ||||||
| Absent | 28 | 30 | .017* | 40 | 23 | .20 |
| Present | 17 | 5 | 33 | 11 | ||
| βcatenin (nuclear) | ||||||
| Negative | 34 | 33 | .035* | 51 | 27 | .45 |
| Positive | 14 | 4 | 24 | 9 | ||
| Wnt3a | ||||||
| Low expression | 30 | 10 | .0020* | 36 | 5 | .0003* |
| High expression | 20 | 27 | 39 | 31 | ||
* P < .05.
3.3. Evaluation of cytoplasmic Cx26 expression according to the Lauren classification and prognosis
The prognostic curve for OS based on Cx26 expression is shown in Figure 2. Although the prognosis was significantly better with high Cx26 expression in those with intestinal‐ and mix‐type gastric cancer (P = .0094), there was no correlation between Cx26 expression and prognosis in those with diffuse type gastric cancer (P = .83). Next, the prognostic curve based on Wnt3a expression is shown in Figure 1C,D. Although low Wnt3a expression did not correlate with Cx26 expression (P = .32), high Wnt3a expression with high Cx26 expression was associated with significantly better prognosis (P = .043). In other words, when Wnt3a is activated, cytoplasmic Cx26 expression may work more advantageously.
FIGURE 2.
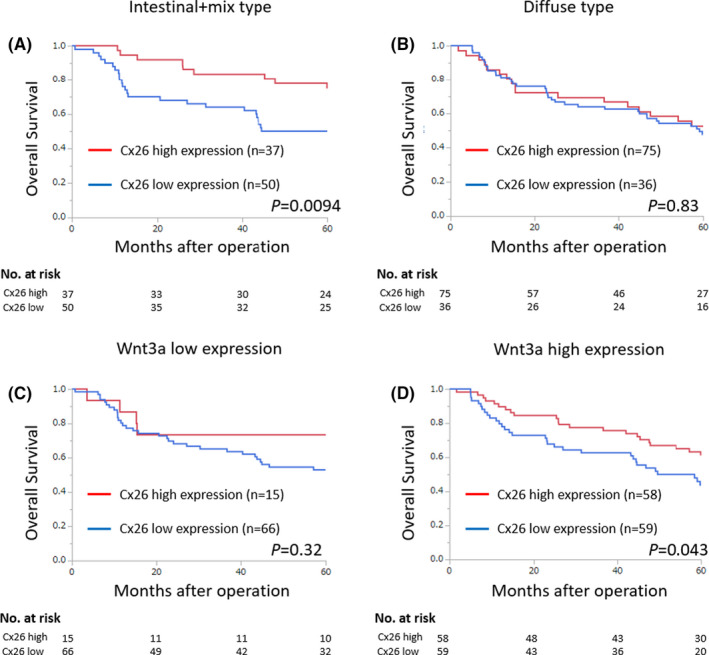
Kaplan–Meier analysis of 5‐y overall survival. (A) Prognosis based on cytoplasmic Cx26 expression in intestinal and mix‐type gastric cancer. (B) Prognosis based on cytoplasmic Cx26 expression in diffuse type gastric cancer. (C) Prognosis based on cytoplasmic Cx26 expression when Wnt3a expression is low. (D) Prognosis based on cytoplasmic Cx26 expression when Wnt3a expression is high
3.4. Univariate and multivariate analyses of intestinal‐ and mix‐type gastric cancer
As described above, Cx26 high expression was significantly associated with favorable prognosis in intestinal‐ and mix‐type gastric cancer. Thus, univariate and multivariate analyses were performed for OS in patients with intestinal‐ and mix‐type gastric cancer to investigate whether Cx26 expression could be an independent prognostic factor (Table 2). Univariate analyses showed that, in the presence of low Cx26 expression, patients with depth of invasion of subserosa (SS) or deeper, lymph node metastases, and vascular invasion were associated with poor prognosis. Multivariate analysis revealed that the presence of lymph node metastases (relative risk = 3.08, 95% CI = 1.28–8.80, P = .011) and low Cx26 expression (relative risk = 2.08, 95% CI = 1.04–4.44, P = .037) could be independent prognostic factors.
TABLE 2.
Univariate/multivariate analyses of overall survival in intestinal‐ and mix‐type gastric cancer
| Clinicopathological variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| RR | 95% Cl | P value | RR | 95% Cl | P value | |
| Age (<65 vs ≧65) | 1.48 | 0.75–3.14 | .27 | |||
| Gender (male vs female) | 1.64 | 0.73–4.36 | .25 | |||
| Depth (M,SM,MP vs SS,SE,SI) | 3.43 | 1.53–9.14 | .0019* | 2.07 | 0.87–5.73 | .10 |
| Lymph node metastasis (absent vs present) | 3.92 | 1.75–10.5 | .0005* | 3.08 | 1.28–8.80 | .011* |
| Vascular invasion (absent vs present) | 2.15 | 1.09–4.15 | .029* | 1.26 | 0.60–2.61 | .54 |
| Cx26 expression (high vs low) | 2.09 | 1.05–4.42 | .035* | 2.08 | 1.04–4.44 | .037* |
Abbreviations: Cl, confidence interval; RR, relative risk.
* P < .05.
3.5. Evaluation of relationship between cytoplasmic Cx26 and β‐catenin
The PLA results for the MKN7 cell line are shown in Figure 3A and the results for the MKN45 cell line are shown in Figure 3B. Red dots indicate findings of binding of Cx26 to β‐catenin. Both MKN7 and MKN45 showed binding of Cx26 to β‐catenin. Since co‐immunoprecipitation was also performed to prove the findings, the results are presented (Figure 3C). The binding of Cx26 to β‐catenin was demonstrated in the MKN7, MKN45, and MKN74 cell lines.
FIGURE 3.
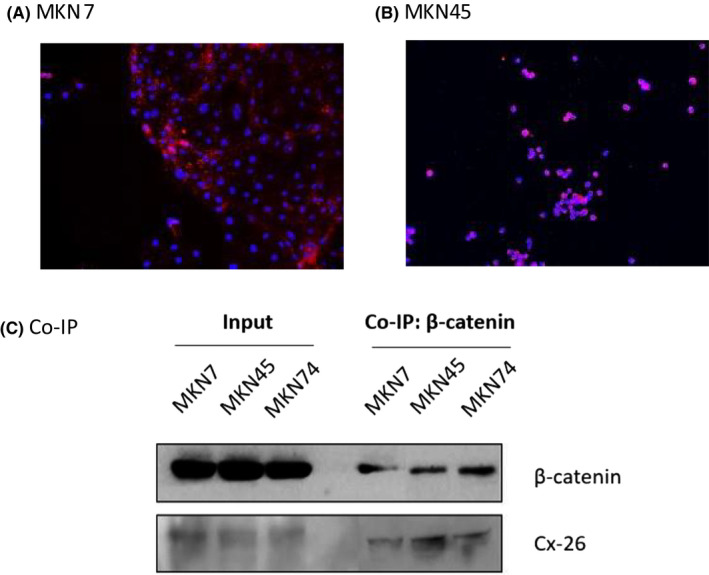
Investigation of the relationship between cytoplasmic Cx26 and β‐catenin (A,B) and PLA results for MKN7/MKN45. Red dots represent the binding of Cx26 to β‐catenin. (C) Co‐immunoprecipitation results for MKN7/MKN45/MKN74
3.6. Cell proliferation capacity upon forced expression of Cx26 and the relationship between cytoplasmic Cx26 and β‐catenin
The cell proliferation capacity results are shown in Figure 4. Results in MKN7 are shown in Figure 4A and results in MKN74 are shown in Figure 4B. For both results, the forced expression of Cx26 was associated with a significantly reduced cell proliferation capacity. Cytoplasmic Cx26 expression and nuclear β‐catenin expression were examined by western blotting. In both MKN7 and MKN74 (Figure 4C), cytoplasmic Cx26 overexpression was associated with reduced nuclear β‐catenin expression.
FIGURE 4.
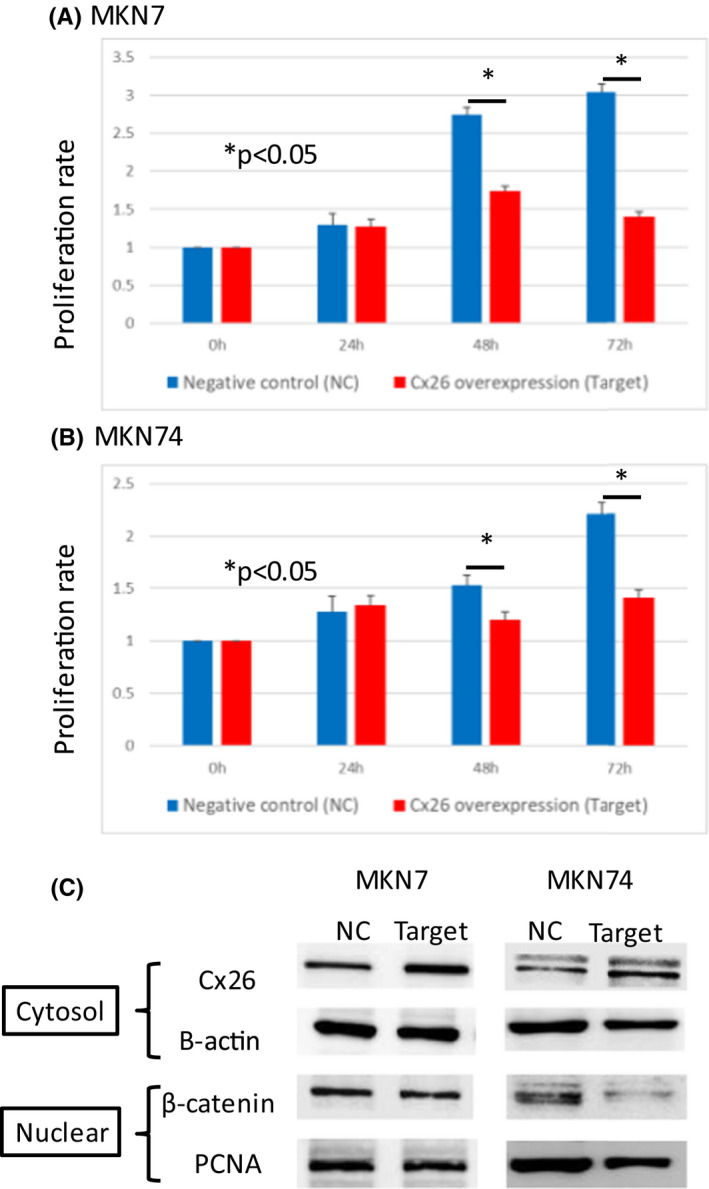
Changes in cell proliferation capacity upon forced expression of Cx26. (A) MKN7 (B) MKN74, cytoplasmic Cx26, and nuclear β‐catenin expressions examined by western blotting. (A) MKN7 (B) MKN74
3.7. Cage analyses
The differences in gene expression between the negative control and forced expression of Cx26 were evaluated in the MKN74 cell line. The results of the DAVID KEGG analysis are shown in Table S1. We examined the relationship between forced expression of Cx26 and the Wnt/β‐catenin pathway. The results of enrichment analyses are shown in Figure 5A. Focusing on downstream genes in the Wnt/β‐catenin pathway (BMP7, CCNB1, AXIN2, BMP4, CCNB2, APC2, CCND3, CCND1, CTNNB1), reductions in gene expression with forced expression of Cx26 is presented as log2 fold‐changes. For all downstream genes, gene expression decreased with Cx26 forced expression. In addition, there was also a focus on the Wnt signaling pathway in the KEGG pathway analysis, and the results are shown in Figure 5B.
FIGURE 5.
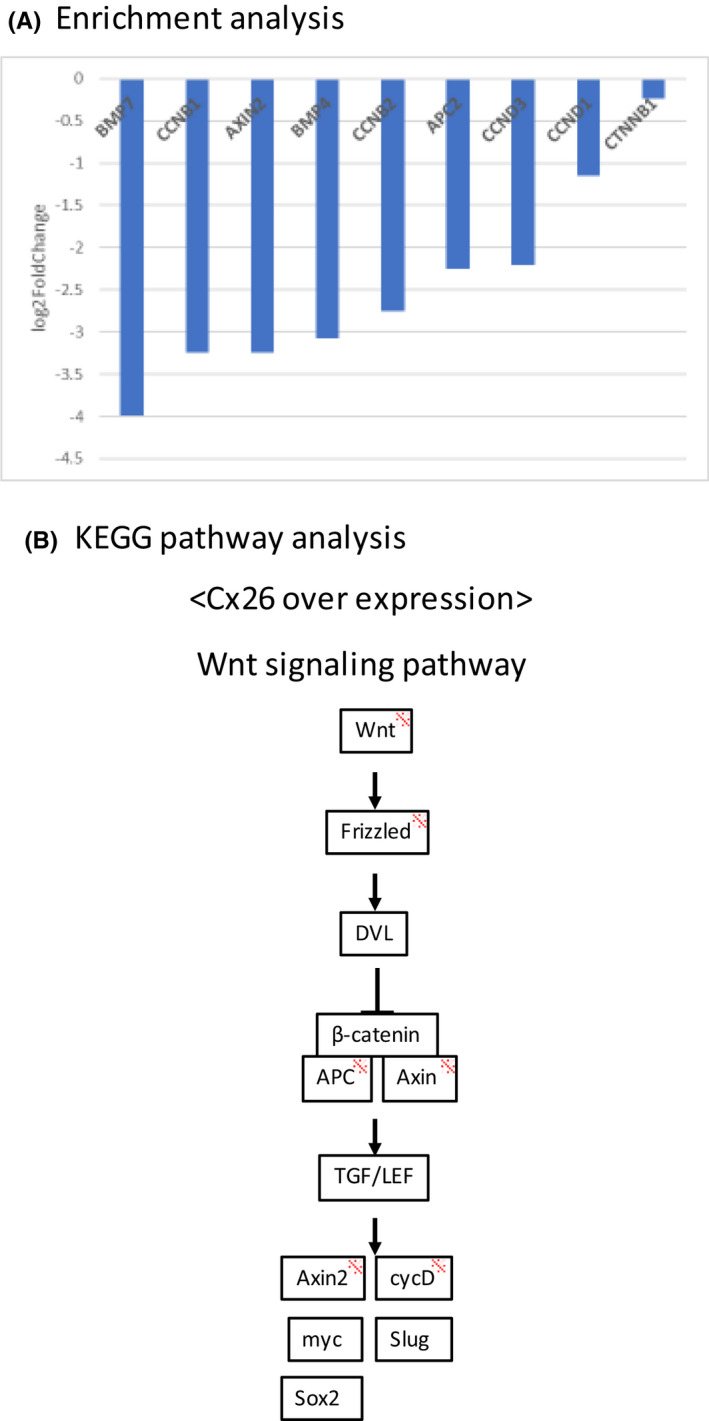
Cage analysis: changes in downstream genes and signaling pathways when expression of Cx26 is forced in the MKN74 cell line (A) Enrichment analysis. Changes in Wnt/β‐catenin downstream genes (B) KEGG pathway analysis focusing on the Wnt/β‐catenin pathway. *Reduced expression when Cx26 is highly expressed
4. DISCUSSION
Cytoplasmic Cx26 expression in gastric cancer has been reported to be associated with a favorable prognosis in patients with intestinal‐type gastric cancer. 12 Helicobacter pylori (H. pylori) produces and secretes various pathogenic factors, such as CagA and VacA (cavitating toxin), to deregulate the homeostasis of epithelial cells and immune cells in the gastric mucosa, resulting in persistent infection. 19 In gastric epithelial cells infected with H. pylori, E‐cadherin and β‐catenin are known to diffuse into the cytoplasm, and nuclear accumulation of β‐catenin occurs at a high frequency. 20 In the course of this loss of epithelial cell polarity, Cx26 that form gap junctions may have localized in the cytoplasm.
Next, why did cytoplasmic Cx26 expression affect the prognosis of patients with only intestinal‐ and mix‐type gastric cancer? Wnt signaling involves at least three distinct pathways: (1) the β‐catenin pathway that regulates gene expression via the β‐catenin pathway; (2) the PCP pathway that regulates planar cell polarity (PCP); and (3) the Ca2+ pathway that promotes intracellular Ca2+ mobilization. 21 Of these pathways, the PCP pathway and the Ca2+ pathway are considered β‐catenin‐independent and inhibit the β‐catenin pathway. 22 While Wnt3a activates the β‐catenin pathway, Wnt5a that activates the β‐catenin‐independent pathway is considered to suppress the β‐catenin pathway by binding to Fz2, a receptor that activates the β‐catenin pathway, in a competitive manner to Wnt3a. 23 Several reports have shown that Wnt/β‐catenin signaling was activated in intestinal‐type gastric cancer. 24 , 25 , 26 By contrast, Wnt5a was overexpressed in ~30% of patients with gastric cancer, and a significantly higher percentage of positive patients had high‐grade (diffuse type) scirrhous gastric carcinoma. 27 Furthermore, an in vitro study demonstrated that Wnt5a expression was significantly higher in diffuse type gastric cancer than in intestinal‐type gastric cancer. 28 As described above, the β‐catenin‐dependent pathway by Wnt3a is activated in intestinal‐type gastric cancer, while the β‐catenin‐independent pathway by Wnt5a is activated in diffuse type gastric cancer. This may be the reason for the difference in prognosis according to the Lauren classification.
An increase in cytoplasmic β‐catenin leads to translocation of these to the nucleus and subsequent induction of expression of transcription factors that induce EMT. 13 In intestinal‐type gastric cancer, Cx26 may play an important role in the induction of EMT. Our data showed that the low expression levels of cytoplasmic Cx26 might reflect EMT.
Although immune checkpoint inhibitors have recently been used in chemotherapy for gastric cancer and many have benefited from it, hot tumors such as those with more infiltration of cytotoxic T‐cell lymphocytes into tumors and high expression of programmed death ligand‐1 (PD‐L1) in cancer cells can be characteristic of patients who are likely to respond to immune checkpoint inhibitors. 29 Hot tumor was reported with the absence of Wnt/β‐catenin pathway activity. 30 Furthermore, the EMT induction in cancer tissues was associated with therapeutic resistance of immune checkpoint inhibitors. 31 Since cytoplasmic Cx26 was found to suppress the Wnt/β‐catenin pathway in this study, the hope is to further investigate the possibility that cytoplasmic Cx26 expression can be a biomarker to predict gastric cancer immune checkpoint inhibitor sensitivity, and the possibility that treatment targeting Cx26 may enhance immune checkpoint inhibitor sensitivity.
In conclusion, cytoplasmic Cx26 was found to be a prognostic factor in intestinal‐ and mix‐type gastric cancer. Here the mechanism by which cytoplasmic Cx26 inhibits the nuclear translocation of β‐catenin in vitro is reported.
DISCLOSURE
Conflict of interest: The authors declare no conflicts of interest for this article.
Human rights statement: The protocol for this research project has been approved by a suitably constituted Ethics Committee of the institution and it conforms to the provisions of the Declaration of Helsinki. The committee of the Institutional Review Board of Gunma University, Approval No. HS2019‐127.
Informed consent: All informed consent was obtained from the subjects or guardians.
Supporting information
Table S1
ACKNOWLEDGMENTS
This work was supported by JSPS KAKENHI Grant Number JP20K22831. In addition, we would like to express our gratitude for the research support provided by the Japanese Society for Gastroenterological Carcinogenesis. Moreover, we would also like to express our sincere gratitude to Dr. Kyoichi Ogata, Dr. Akiharu Kimura, Dr. Masaki Suzuki, Dr. Toru Yanoma, Dr. Norimichi Kogure, Dr. Seded Baatar, Dr. Bilguun Erkhem‐ochir, Dr. Yasunari Ubukata, Dr. Kengo Kuriyama, Ms. Mariko Nakamura, Ms. Kao Abe, and Ms. Harumi Kanai for their cooperation with this research.
Nakazawa N, Sohda M, Yokobori T, Gombodorj N, Sano A, Sakai M, et al. Cytoplasmic localization of connexin 26 suppresses transition of β‐catenin into the nucleus in intestinal‐ and mix‐type gastric cancer. Ann Gastroenterol Surg. 2022;6:505–514. doi: 10.1002/ags3.12552
REFERENCES
- 1. Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8. [DOI] [PubMed] [Google Scholar]
- 2. Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37. [DOI] [PubMed] [Google Scholar]
- 3. Uchida Y, Matsuda K, Sasahara K, Kawabata H, Nishioka M. Immunohistochemistry of gap junctions in normal and diseased gastric mucosa of humans. Gastroenterology. 1995;109:1492–6. [DOI] [PubMed] [Google Scholar]
- 4. Kanczuga‐Koda L, Sulkowski S, Koda M, Sulkowska M. Alterations in connexin26 expression during colorectal carcinogenesis. Oncology. 2005;68:217–22. [DOI] [PubMed] [Google Scholar]
- 5. Omori Y, Li Q, Nishikawa Y, Yoshioka T, Yoshida M, Nishimura T, et al. Pathological significance of intracytoplasmic connexin proteins: implication in tumor progression. J Membr Biol. 2007;218:73–7. [DOI] [PubMed] [Google Scholar]
- 6. Ito A, Koma Y‐I, Uchino K, Okada T, Ohbayashi C, Tsubota N, et al. Increased expression of connexin 26 in the invasive component of lung squamous cell carcinoma: significant correlation with poor prognosis. Cancer Lett. 2006;234:239–48. [DOI] [PubMed] [Google Scholar]
- 7. Kanczuga‐Koda L, Sulkowska M, Koda M, Rutkowski R, Sulkowski S. Increased expression of gap junction protein–connexin 32 in lymph node metastases of human ductal breast cancer. Folia Histochem Cytobiol. 2007;45:S175–80. [PubMed] [Google Scholar]
- 8. Naoi Y, Miyoshi Y, Taguchi T, Kim SJ, Arai T, Tamaki Y, et al. Connexin26 expression is associated with lymphatic vessel invasion and poor prognosis in human breast cancer. Breast Cancer Res Treat. 2007;106:11–7. [DOI] [PubMed] [Google Scholar]
- 9. Ezumi K, Yamamoto H, Murata K, Higashiyama M, Damdinsuren B, Nakamura Y, et al. Aberrant expression of connexin 26 is associated with lung metastasis of colorectal cancer. Clin Cancer Res. 2008;14:677–84. [DOI] [PubMed] [Google Scholar]
- 10. Yang J, Qin G, Luo M, Chen J, Zhang Q, Li L, et al. Reciprocal positive regulation between Cx26 and PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC cells via GJIC‐independent induction of EMT. Cell Death Dis. 2015;6:e1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Inose T, Kato H, Kimura H, Faried A, Tanaka N, Sakai M, et al. Correlation between connexin 26 expression and poor prognosis of esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:1704–10. [DOI] [PubMed] [Google Scholar]
- 12. Liu X, Furuya T, Li D, Xu J, Cao X, Li Q, et al. Connexin 26 expression correlates with less aggressive phenotype of intestinal type‐gastric carcinomas. Int J Mol Med. 2010;25:709–16. [DOI] [PubMed] [Google Scholar]
- 13. Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. [DOI] [PubMed] [Google Scholar]
- 14. Luiza KK, Andrzej W, Andrzej F, Tomasz A, Waldemar F, Marek B, et al. E‐cadherin and β‐catenin adhesion proteins correlate positively with connexins in colorectal cancer. Oncol Lett. 2014;7(6):1863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jie Y, Guihui Q, Junze C. Lentivirus‐mediated shRNA interference of Cx26 suppresses epithelial mesenchymal transition and invasion of highly invasive hepatocellular carcinoma cells in vitro. Nan Fang Yi Ke Da Xue Bao. 2014;34(12):1743–7. [PubMed] [Google Scholar]
- 16. Elizabeth ML, Qing S, Hong LW, Stephanie L, Dale WL. Connexins act as tumor suppressors in three‐dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66(20):9886–94. [DOI] [PubMed] [Google Scholar]
- 17. Gastric Cancer. Japanese gastric cancer association: Japanese classification of gastric carcinoma. 2011;14:101–12. [DOI] [PubMed] [Google Scholar]
- 18. Allred D, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–68. [PubMed] [Google Scholar]
- 19. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–86. [DOI] [PubMed] [Google Scholar]
- 20. Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, et al. Helicobacter pylori CagA phosphorylation‐independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. [DOI] [PubMed] [Google Scholar]
- 21. Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–71. [DOI] [PubMed] [Google Scholar]
- 22. Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta‐catenin‐independent Wnt signaling. Dev Cell. 2003;5:367–77. [DOI] [PubMed] [Google Scholar]
- 23. Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho YJ, Kim JH, Yoon J, Cho SJ, Ko YS, Park JW, et al. Constitutive activation of glycogen synthase kinase‐3beta correlates with better prognosis and cyclin‐dependent kinase inhibitors in human gastric cancer. BMC Gastroenterol. 2010;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. [DOI] [PubMed] [Google Scholar]
- 26. Oliveira LA, Oshima CT, Soffner PA, Silva MS, Lins RR, Malinverni ACM, et al. The canonical wnt pathway in gastric carcinoma. Arq Bras Cir Dig. 2019;32:e1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, et al. Expression of Wnt‐5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–48. [DOI] [PubMed] [Google Scholar]
- 28. Tanabe S, Aoyagi K, Yokozaki H, Sasaki H. Gene expression signatures for identifying diffuse‐type gastric cancer associated with epithelial‐mesenchymal transition. Int J Oncol. 2014;44:1955–70. [DOI] [PubMed] [Google Scholar]
- 29. Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T‐cell infiltration and PD‐L1. Cancer Res. 2015;75:2139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue G, Romano E, Massi D, Mandalà M. Wnt/β‐catenin signaling in melanoma: Preclinical rationale and novel therapeutic insights. Cancer Treat Rev. 2016;49:1–12. [DOI] [PubMed] [Google Scholar]
- 31. Wang L, Saci A, Szabo PM, Chasalow SD, Castillo‐Martin M, Domingo‐Domenech J, et al. EMT‐ and stroma‐related gene expression and resistance to PD‐1 blockade in urothelial cancer. Nat Commun. 2018;9(1):3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


