Abstract
The dibenzothiophene (DBT)-desulfurizing bacterium, Rhodococcus erythropolis D-1, removes sulfur from DBT to form 2-hydroxybiphenyl using four enzymes, DszC, DszA, DszB, and flavin reductase. In this study, we purified and characterized the flavin reductase from R. erythropolis D-1 grown in a medium containing DBT as the sole source of sulfur. It is conceivable that the enzyme is essential for two monooxygenase (DszC and DszA) reactions in vivo. The purified flavin reductase contains no chromogenic cofactors and was found to have a molecular mass of 86 kDa and four identical 22-kDa subunits. The enzyme catalyzed NADH-dependent reduction of flavin mononucleotide (FMN), and the Km values for NADH and FMN were 208 and 10.8 μM, respectively. Flavin adenine dinucleotide was a poor substrate, and NADPH was inert. The enzyme did not catalyze reduction of any nitroaromatic compound. The optimal temperature and optimal pH for enzyme activity were 35°C and 6.0, respectively, and the enzyme retained 30% of its activity after heat treatment at 80°C for 30 min. The N-terminal amino acid sequence of the purified flavin reductase was identical to that of DszD of R. erythropolis IGTS8 (K. A. Gray, O. S. Pogrebinsky, G. T. Mrachko, L. Xi, D. J. Monticello, and C. H. Squires, Nat. Biotechnol. 14:1705–1709, 1996). The flavin reductase gene was amplified with primers designed by using dszD of R. erythropolis IGTS8, and the enzyme was overexpressed in Escherichia coli. The specific activity in crude extracts of the overexpressed strain was about 275-fold that of the wild-type strain.
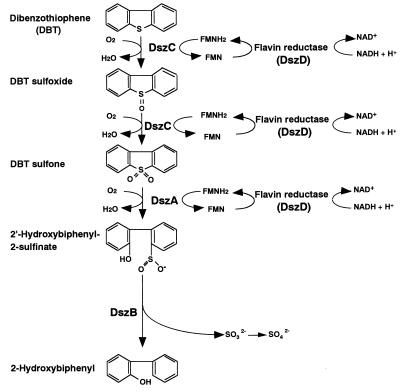
Organic sulfur compounds are found in fossil fuels, the combustion of which causes serious environmental problems, such as acid rain. At the refinery, hydrodesulfurization is currently performed to remove sulfur compounds from fossil fuels. This process is done at high temperatures and pressures by metal catalysis and is effective for removing inorganic sulfur and simple organic sulfur compounds. However, it is difficult to remove polycyclic sulfur compounds. As legislative limits on sulfur emissions have become tighter, the need to remove polycyclic sulfur compounds from fuel has become more pressing. Dibenzothiophene (DBT) is considered a model polycyclic sulfur compound contained in fossil fuels. It has been reported that some bacteria utilize DBT as a sole source of sulfur without breaking its carbon-carbon backbone. This sulfur-specific pathway has been extensively studied by using two Rhodococcus strains, Rhodococcus erythropolis IGTS8 (7, 11, 13) and R. erythropolis D-1 (10, 19, 20). The genes encoding enzymes involved in this pathway have been cloned and sequenced in R. erythropolis IGTS8 (2, 3, 25) and the thermophilic desulfurizing bacterium Paenibacillus sp. strain A11-2 (9). In this pathway, DBT is oxidized to DBT sulfone via DBT sulfoxide by DszC, DBT sulfone is converted to 2′-hydroxybiphenyl 2-sulfinic acid (HBPSi) by DszA, and HBPSi is desulfurized to 2-hydroxybiphenyl by DszB (Fig. 1). Flavin reductase is necessary for monooxygenase reactions by DszC and DszA. It has been reported that the flavin reductase from Vibrio harveyi complements the activities of purified DszA and DszC from R. erythropolis IGTS8 (31). Recently, coexpression of flavin reductase from V. harveyi and the enzymes encoded by the dsz operon, DszC, DszA, and DszB from R. erythropolis IGTS8, was investigated in Escherichia coli cells (26).
FIG. 1.
DBT desulfurization pathway of R. erythropolis D-1.
Two of the enzymes involved in microbial DBT desulfurization, DszC and DszA, have been purified to homogeneity from R. erythropolis D-1 and characterized (21, 22). Although all of the enzymes (DszC, DszA, DszB, and flavin reductase) have been purified from R. erythropolis IGTS8 (8, 17), detailed descriptions of their properties have not been published. In this study, we purified and characterized flavin reductase, which is essential for the activities of DszC and DszA, from R. erythropolis D-1 and overexpressed this enzyme in E. coli.
MATERIALS AND METHODS
Materials.
Q-Sepharose Fast Flow and Superdex 200 HR 10/30 were purchased from Amersham Pharmacia (Uppsala, Sweden). Butyl-Toyopearl 650M and Phenyl-Toyopearl 650M were obtained from Tohso (Tokyo, Japan). Flavin mononucleotide (FMN) agarose was obtained from Sigma (St. Louis, Mo.). Calibration proteins for gel chromatography and for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were obtained from Boehringer GmbH (Mannheim, Germany) and Amersham Pharmacia, respectively. The ultrafiltration apparatus (model 8200) and membranes (YM-10) were purchased from Millipore (Bedford, Mass.). NADH and FMN were obtained from Oriental Yeast (Tokyo, Japan) and Nacalai Tesque (Kyoto, Japan), respectively. Unless otherwise stated, all other chemicals used in this study were purchased from Wako Pure Chemicals (Osaka, Japan).
Medium and cultivation.
R. erythropolis D-1 was grown in medium A (10) supplemented with 50 mg of DBT per liter. Cultivation was done in 2-liter flasks containing 500 ml of medium with reciprocal shaking at 100 strokes/min at 30°C for 48 h. The cells were harvested by continuous centrifugation at 7,500 × g and stored at −20°C. Frozen cells (470 g, wet weight) were thawed, suspended in 940 ml of 50 mM Tris-HCl buffer (pH 8.0) containing 1 mM dithiothreitol (DTT) and 10% glycerol (basal buffer), and then disrupted with an ultraoscillator (Sonifier 450; Branson Instruments, Danbury, Conn.) at 20 kHz. The cell debris was removed by centrifugation at 7,500 × g for 30 min.
Purification of flavin reductase from R. erythropolis D-1.
All purification steps were performed at 4°C.
(i) Step 1: Q-Sepharose column chromatography.
Cell extracts were dialyzed against basal buffer containing 0.15 M KCl. The dialyzed cell extracts were applied to a Q-Sepharose Fast Flow column (5.6 by 50 cm) which had been equilibrated with the same buffer. The column was washed well with the same buffer, and the bound proteins were eluted with basal buffer containing 0.25 M KCl at a flow rate of 120 ml/h. The active fractions were combined and concentrated by ultrafiltration.
(ii) Step 2: Butyl-Toyopearl column chromatography.
The enzyme solution obtained in step 1 was dialyzed against basal buffer containing 0.95 M (NH4)2SO4. The dialyzed enzyme solution was applied to a Butyl-Toyopearl 650M column (3.6 by 30 cm) which had been equilibrated with the same buffer. The column was washed well with the same buffer, and the bound proteins were eluted with a linear 0.95 to 0.75 M (NH4)2SO4 gradient in basal buffer at a flow rate of 20 ml/h. The active fractions were dialyzed against basal buffer and then concentrated by ultrafiltration.
(iii) Step 3: Phenyl-Toyopearl column chromatography.
The enzyme solution obtained in step 2 was dialyzed against basal buffer containing 0.95 M (NH4)2SO4. The dialyzed enzyme solution was applied to a Phenyl-Toyopearl 650M column (3.6 by 30 cm) which had been equilibrated with the same buffer. The column was washed well with the same buffer, and the bound proteins were eluted with a linear 0.95 to 0.75 M (NH4)2SO4 gradient in basal buffer at a flow rate of 10 ml/h.
(iv) Step 4: membrane treatment.
In preliminary experiments, the flavin reductase was found to be partially adsorbed on a cellulose acetate membrane (YM-10; Millipore) used for ultrafiltration when the buffer dissolving the enzyme contained (NH4)2SO4 at a concentration of 0.3 M or higher and to be eluted with basal buffer. Therefore, the enzyme eluted from the Phenyl-Toyopearl 650M column was concentrated by ultrafiltration to a minimum volume, and the concentrated enzyme solution was removed. Then the membrane to which part of the enzyme was adsorbed was washed well with a small amount of basal buffer, and the enzyme released was recovered. The concentrated enzyme solution was diluted with basal buffer containing 0.3 M (NH4)2SO4 and ultrafiltered again as described above. This treatment was repeated three times, and the enzyme which was adsorbed to the membrane and released with basal buffer was collected.
(v) Step 5: FMN agarose chromatography.
The purified enzyme solution obtained in step 4 was dialyzed against 1.5 M potassium phosphate buffer (pH 8.0) containing 1 mM DTT and 10% glycerol. The dialyzed enzyme solution was applied to an FMN agarose column (1.0 by 5.0 cm) which had been equilibrated with the same buffer. The column was washed well with 1.2, 1.0, and 0.9 M potassium phosphate buffer (pH 8.0) containing 1 mM DTT and 10% glycerol, and the bound proteins were eluted with 0.8 M potassium phosphate buffer (pH 8.0) containing 1 mM DTT and 10% glycerol at a flow rate of 3 ml/h.
Enzyme assays.
Flavin reductase activity was determined at 35°C by using the decrease in absorbance at 340 nm due to oxidation of NADH. The reaction mixture contained 20 mM potassium phosphate buffer (pH 7.0), 0.4 mM NADH, 0.2 mM FMN, and the enzyme in a total volume of 0.5 ml. One unit of activity was defined as the amount of flavin reductase necessary to decrease 1 μmol of NADH per min. The coupling assay with DszC of R. erythropolis D-1 was done at 35°C, and the rate of conversion of DBT to DBT sulfone was measured by using the high-performance liquid chromatography system as described previously (22). Purified DszC of R. erythropolis D-1 was prepared as described previously (21). The reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0), 0.1 mM DBT, 3 mM NADH, 10 μM FMN, DszC, and the enzyme in a total volume of 0.25 ml.
Electrophoresis.
Purification of flavin reductase was monitored by SDS-PAGE by using the method of Laemmli (15). Slab gels (90 by 80 by 1 mm) with 12.5% polyacrylamide in the separating gel and 4% polyacrylamide in the stacking gels were used for electrophoresis, stained with 0.25% Coomassie brilliant blue G-250 dissolved in 50% methanol–10% acetic acid, and then destained with 30% methanol–10% acetic acid.
Recombinant DNA technique.
Plasmid DNA isolation, transformation, and restriction endonuclease digestion, as well as other recombinant DNA techniques, were performed as described by Sambrook et al. (27). DNA fragments were purified from an agarose gel by using a Sephaglas BandPrep kit (Amersham Pharmacia).
Amplification of flavin reductase gene.
The flavin reductase gene of R. erythropolis D-1 was placed under control of the tac and T7 promoters. The gene was amplified by PCR by using total DNA of R. erythropolis D-1 as the template, which was isolated by the method described by Denome et al. (2). PCR was performed with an Expand High Fidelity PCR system (Roche Diagnostics, Mannheim, Germany) under the buffer conditions recommended by the manufacturer by using a model 480 DNA thermal cycler (Perkin-Elmer, Norwalk, Conn.). The PCR mixture was heated at 95°C for 5 min and then subjected to 30 cycles of amplification (96°C for 45 s, 59°C for 45 s, and 72°C for 1 min) with primers YN-6 (5′-TTCCATATGTCTGACAAGCCGAATGCCGTT-3′ [the NdeI restriction site is underlined and the ATG initiation codon is indicated by boldface type]) and YN-3 (5′-CACAAGCTTCTATTGACCTAACGGAGTCGG-3′ [the HindIII restriction site is underlined and the CTA termination codon is indicated by boldface type]) for expression vector pET21-a (Novagen, Madison, Wis.) with the T7 promoter. Primers YN-5 (5′-GAGGAATTCATGTCTGACAAGCCGAATGCC-3′ [the EcoRI restriction site is underlined and the ATG initiation codon is indicated by boldface type]) and YN-3 were used for expression vector pKK223-3 (Amersham Pharmacia) with the tac promoter. Amplified DNA fragments were digested with NdeI and HindIII or with EcoRI and HindIII, separated by agarose gel electrophoresis, inserted into pET21-a or pKK223-3, and then used to transform E. coli JM109 cells. To overproduce flavin reductase by using the pET system, E. coli BL21 (DE3) was transformed with a constructed plasmid (pADT9) containing the complete flavin reductase gene region.
DNA sequencing.
DNA sequencing of the cloned PCR product was performed with double-stranded templates by using a DNA sequencing kit (Applied Biosystems, Inc., Foster City, Calif.) based on Taq DNA polymerase-initiated cycle sequencing reactions with fluorescence-labeled M13 primers using a model 373A DNA sequencer (Applied Biosystems, Inc.).
Purification of flavin reductase from the recombinant E. coli.
Flavin reductase from E. coli BL21 (DE3)(pADT9) was purified as described below. Cells of the E. coli transformant harboring overexpression plasmid pADT9 were grown at 37°C in 2-liter flasks containing 500 ml of Luria-Bertani medium supplemented with 100 mg of ampicillin per liter. After cultivation for 4 h, 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) was added to the medium, and cultivation was continued for an additional 6 h. The cells were harvested and suspended in 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM phenylmethanesulfonyl fluoride (PMSF); then they were sonicated and centrifuged at 7,500 × g for 30 min to remove cell debris.
(i) Step 1: ammonium sulfate fractionation.
Solid ammonium sulfate was added to the cell extract to 20% saturation, and the solution was stirred for 1 h. The resulting precipitate was removed by centrifugation at 7,500 × g for 30 min. Solid ammonium sulfate was added to the supernatant to 30% saturation, and the solution was stirred for 1 h. The resulting precipitate was collected by centrifugation at 7,500 × g for 30 min and dissolved in 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF.
(ii) Step 2: Q-Sepharose column chromatography.
The sample was dialyzed against 50 mM potassium phosphate buffer (pH 6.5) containing 0.1 M KCl, 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF. The dialyzed enzyme solution was applied to a Q-Sepharose Fast Flow column (2.5 by 14 cm) which had been equilibrated with the same buffer. The column was washed well with the same buffer, and the bound proteins were eluted with a linear 0.1 to 0.3 M KCl gradient in 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF at a flow rate of 25 ml/h.
(iii) Step 3: Butyl-Toyopearl column chromatography.
The enzyme solution obtained in step 2 was dialyzed against 50 mM potassium phosphate buffer (pH 6.5) containing 1.0 M (NH4)2SO4, 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF. The dialyzed enzyme solution was applied to a Butyl-Toyopearl 650M column (2.5 by 10 cm) which had been equilibrated with the same buffer. The column was washed well with the same buffer, and the bound proteins were eluted with a linear 1.0 to 0.7 M (NH4)2SO4 gradient in 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF at a flow rate of 20 ml/h. The active fractions were dialyzed against 50 mM potassium phosphate buffer (pH 6.5) containing 1 mM DTT, 10% glycerol, 1 mM EDTA, and 0.5 mM PMSF and then concentrated by ultrafiltration.
Other analytical methods.
The native molecular mass was determined by the AKTA system (Amersham Pharmacia) with a Superdex 200 HR 10/30 column at a flow rate of 0.25 ml/min by using Tris-HCl (pH 8.0) buffer containing 1 mM DTT and 0.15 M NaCl as the eluent. The calibration proteins used were aldolase (158 kDa), albumin (45 kDa), chymotrypsinogen A (25 kDa), and cytochrome c (12.5 kDa). The N-terminal amino acid sequence of the flavin reductase was determined with a PPSQ protein sequencer (Shimadzu, Kyoto, Japan). Protein concentrations were measured by the method of Bradford (1) by using bovine serum albumin as the standard.
Nucleotide sequence accession number.
The sequence of the flavin reductase gene amplified from R. erythropolis D-1 in this study has been deposited as the dszD sequence in the GenBank database under accession number AB051429.
RESULTS
Purification of flavin reductase from R. erythropolis D-1.
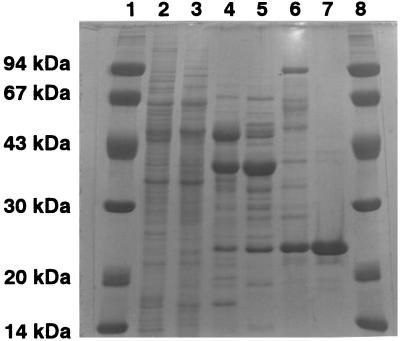
Flavin reductase was purified essentially to homogeneity (660-fold) from cell extracts of R. erythropolis D-1 (Table 1). In addition, when the coupling assay with DszC of R. erythropolis D-1 was done using the enzyme preparation at each purification step, the specific enzyme activity supporting DszC naturally increased with the progress of enzyme purification (data not shown). SDS-PAGE revealed that the molecular mass of the subunits of the enzyme was 22 kDa (Fig. 2). The native molecular mass of the enzyme was found to be 86 kDa by gel filtration. Therefore, flavin reductase was presumed to be a homotetramer. The N-terminal amino acid sequence of flavin reductase was found to be Ser-Asp-Lys-Pro-Asn-Ala-Val-Ser-Ser, which matches the sequence of DszD of R. erythropolis IGTS8 (8). The purified flavin reductase did not contain any chromophore like flavin because it had no absorption peak between 200 and 800 nm.
TABLE 1.
Purification of flavin reductase from R. erythropolis D-1
| Purification step | Protein (mg) | Total activity (U) | Sp act (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 15,400 | 2,850 | 0.2 | 1 | 100 |
| Q-Sepharose | 2,310 | 2,350 | 1.1 | 5.9 | 88.7 |
| Butyl-Toyopearl | 152 | 1,080 | 7.1 | 38.5 | 37.9 |
| Phenyl-Toyopearl | 41.0 | 590 | 14.4 | 77.8 | 20.7 |
| Membrane treatment | 5.0 | 353 | 70.2 | 379 | 12.4 |
| FMN agarose | 1.7 | 215 | 122 | 660 | 7.5 |
FIG. 2.
SDS-PAGE of flavin reductase from R. erythropolis D-1. Lanes 1 and 8, marker proteins; lane 2, cell extract of R. erythropolis D-1; lane 3, pooled fractions containing DszD after Q-Sepharose chromatography; lane 4, pooled fractions containing DszD after Butyl-Toyopearl chromatography; lane 5, pooled fractions containing DszD after Phenyl-Toyopearl chromatography; lane 6, fractions after membrane treatment; lane 7, purified DszD enzyme after FMN agarose chromatography.
Effects of pH and temperature on the activity and stability of flavin reductase.
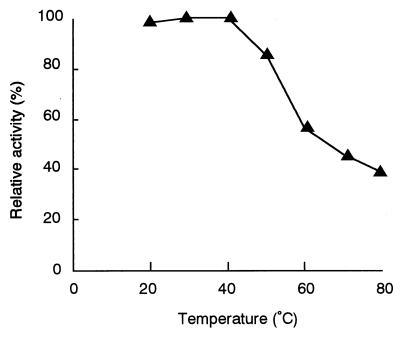
When the enzyme activity was measured at various pHs and temperatures, the optimum pH was found to be around 6.0 and the optimum temperature was found to be about 35°C. The stability of the enzyme was examined next. The enzyme activity was stable at temperatures below 50°C and at pH 6 to 8.5. After being incubated at 80°C for 30 min, the enzyme retained more than 30% of its activity (Fig. 3).
FIG. 3.
Effects of temperature on flavin reductase stability. After purified enzyme was preincubated at different temperatures for 30 min, enzyme reactions were carried out as described in Materials and Methods. The total reaction volume was 0.5 ml, and 0.55 μg of purified enzyme from the wild-type strain was added to each reaction mixture. The enzyme activity was determined by measuring the decrease in absorbance at 340 nm.
Effects of various compounds.
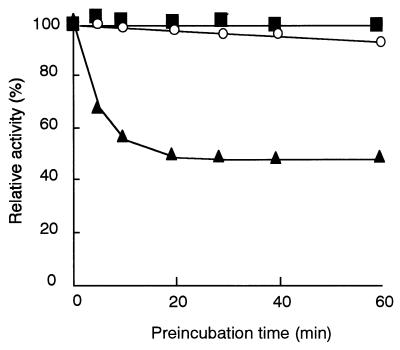
We examined the effects of various compounds on flavin reductase activity. No compound activated the enzyme, and the enzyme activity was strongly inhibited by heavy-metal compounds, such as Ag+, Cu2+, and Hg+ compounds, or the SH inhibitor p-chloromercuribenzoic acid. Although it was reported that FRase I, a well-studied flavin reductase from Vibrio fischeri, was inhibited by dicoumarol (14), the enzyme from R. erythropolis D-1 was not inhibited by dicoumarol. One of the coumarin derivatives tested, 7-hydroxycoumarin, inhibited flavin reductase activity. A competitive inhibitor of FMN, 7-hydroxycoumarin, was calculated to have a Ki of 3.38 μM by using recombinant enzyme (see below for a description of the recombinant enzyme). N-Ethylmaleimide (NEM) did not inhibit the enzyme when it was added to the reaction mixture simultaneously with the substrates NADH and FMN. However, NEM acted as an inhibitor when the enzyme was preincubated with NEM and NADH in the absence of FMN. The time course of inhibition by NEM is shown in Fig. 4. About one-half of the activity was inhibited after preincubation of the enzyme with NEM and NADH for 20 min. This result was consistent with the report about the flavin reductase from V. fischeri (29). NADH reduced a disulfide bond in the enzyme to form a sulfhydryl group, which NEM attacked. In the presence of FMN, this inhibition might not take place because the transfer of a proton from a sulfhydryl group to FMN is faster than the attack by NEM.
FIG. 4.
Effect of NEM on flavin reductase activity. Purified enzyme (5.5 μg) from the wild-type strain was preincubated in a total volume of 0.25 ml at 0°C with 1 mM NEM (■), 1 mM NEM and 200 μM FMN (○), or 1 mM NEM and 500 μM NADH (▴). At different times, 25-μl aliquots were withdrawn, and enzyme reactions were carried out by using 0.4 mM NADH and 0.2 mM FMN as described in Materials and Methods. The enzyme activity was determined by measuring the decrease in absorbance at 340 nm.
Substrate specificity.
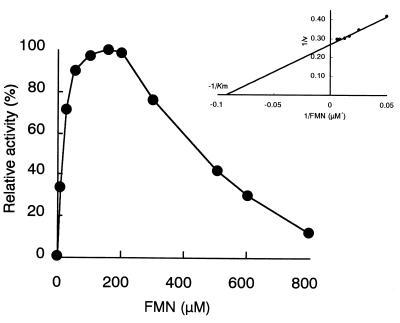
Flavin reductase activity was measured by using various electron acceptors instead of FMN. The substrate specificity was narrow, and flavin adenine dinucleotide (FAD) and 4-nitrophenol were approximately 10% as effective as FMN. Riboflavin, lumiflavin, various artificial electron acceptors, and cytochrome c were inert. The Km values for NADH and FMN were 218 and 10.8 μM, respectively. No activity was observed for NADPH. In the coupling assay with DszC, only the combination of FMN and NADH was effective (data not shown). Maximal enzyme activity was obtained at an FMN concentration around 150 μM under the experimental conditions used, whereas a concentration higher than 200 μM led to marked inhibition of the activity (Fig. 5).
FIG. 5.
Effect of FMN concentration on enzyme activity. Enzyme reactions were carried out as described in Materials and Methods by using reaction mixtures containing 20 mM potassium phosphate buffer (pH 7.0), 0.4 mM NADH, 0.55 μg of purified enzyme from the wild-type strain, and different concentrations of FMN in a total volume of 0.5 ml. The enzyme activity was determined by measuring the decrease in absorbance at 340 nm.
Amplification of the dszD gene by PCR and overexpression in E. coli.
We determined the N-terminal amino acid sequence of flavin reductase from R. erythropolis D-1, and it was identical to that of DszD from R. erythropolis IGTS8. Moreover, the molecular weights were also similar. Therefore, we designed primers based on the DNA sequence of dszD from R. erythropolis IGTS8 (GenBank accession no. AF048979) and amplified the flavin reductase gene of R. erythropolis D-1 by PCR. The PCR product was inserted into pKK223-3, and expression of the flavin reductase gene was examined; however, no expression was observed. The initiation codon was TTG in dszD of R. erythropolis IGTS8, and the experiment was done with primer YN-5, which contained an ATG sequence as the initiation codon. As a result, flavin reductase was expressed at 20 times the level in the wild-type strain, R. erythropolis D-1 (data not shown). To increase the level of flavin reductase expression in E. coli, the flavin reductase gene was inserted into pET21-a with the T7 promoter, and the resulting plasmid, pADT9, was introduced into E. coli BL21 (DE3). Cells of E. coli BL21 (DE3)(pADT9) induced by IPTG hyperexpressed the 22-kDa polypeptides, and the cell extracts of E. coli BL21 (DE3)(pADT9) were coupled with DszC of R. erythropolis D-1 to convert DBT to DBT sulfone. The level of expression of flavin reductase by the recombinant strain (50.9 U/mg) was 275-fold that by the wild-type strain, R. erythropolis D-1 (0.185 U/mg). The 579-bp PCR product was sequenced. Although the product differed at 14 bp from dszD of R. erythropolis IGTS8, the deduced amino acid sequence was identical to that of DszD from R. erythropolis IGTS8 (data not shown). The recombinant flavin reductase was purified from E. coli BL21 (DE3)(pADT9) (Table 2). At first, two faint minor protein bands just below the main bands were detected by SDS-PAGE after three purification steps, ammonium sulfate fractionation, Q-Sepharose Fast Flow column chromatography, and Butyl-Toyopearl 650M column chromatography. These minor bands were observed when the enzyme was purified from R. erythropolis D-1 (Fig. 2). Use of the proteolytic inhibitors EDTA and PMSF throughout purification was effective for obtaining the purified enzyme without the minor bands on SDS-PAGE gels. Similar results were reported in the case of ActVB, a flavin:NADH oxidoreductase involved in biosynthesis of the antibiotic actinorhodin (12). The properties of the purified enzyme were almost identical to those of the wild-type enzyme. The enzyme supported the activities of DszC and DszA, and formation of DBT sulfone and HBPSi was observed.
TABLE 2.
Purification of flavin reductase from recombinant E. coli
| Purification step | Protein (mg) | Total activity (U) | Sp act (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 791 | 40,300 | 50.9 | 1 | 100 |
| Ammonium sulfate fractionation | 389 | 29,400 | 75.6 | 1.5 | 73.0 |
| Q-Sepharose | 132 | 19,600 | 148 | 2.9 | 48.6 |
| Butyl-Toyopearl | 73.3 | 9,500 | 130 | 2.6 | 23.6 |
DISCUSSION
We describe the first detailed enzymological characterization of flavin reductase from a DBT-desulfurizing bacterium, R. erythropolis D-1. This enzyme is essential for desulfurization of DBT. It supports the activities of the DBT monooxygenase, DszC, and the DBT sulfone monooxygenase, DszA. Although the flavin reductase of R. erythropolis D-1 was expressed continuously in nutrient broth, we purified the enzyme from cells grown in a synthetic medium containing DBT as the sole source of sulfur in which DszC and DszA were produced, because it was thought that flavin reductase produced in this medium was involved in desulfurization of DBT. The specific activity of the purified enzyme (122 U/mg) and the N-terminal amino acid sequence were similar or identical to those of DszD from R. erythropolis IGTS8 (8). The entire amino acid sequence deduced from the amplified flavin reductase gene of R. erythropolis D-1 was also identical to that of R. erythropolis IGTS8. These facts indicated that we obtained substantially the same enzyme preparation as the enzyme preparation obtained from R. erythropolis IGTS8. dszD of R. erythropolis IGTS8, the DNA sequence of which has been deposited in the GenBank database under accession no. AF048979, is not encoded by the dsz operon, and it was reported that DBT desulfurization activity was lost completely in R. erythropolis IGTS8 when dszD was destroyed by an insertional inactivation (23). Although we have not tried to inactivate the dszD gene in R. erythropolis D-1, some small peaks having flavin reductase activity were observed during the column chromatography steps used for enzyme purification (data not shown). Furthermore, it was reported that some strains of E. coli and Bacillus subtilis had more than one enzyme showing flavin reductase activity (32, 34). We cannot exclude the possibility that R. erythropolis D-1 contained two or more kinds of proteins showing flavin reductase activity, and enzymes other than the enzyme purified here had little activity in the cells.
As shown in Fig. 3, even at 80°C 30% of the activity remained after a preparation was heated for 30 min. The heat stability of the flavin reductase from R. erythropolis D-1 was greater than that of each of the coupling enzymes, DszC and DszA, which lost all activity after heating for 30 min at 45 and 60°C, respectively (21, 22).
During the purification procedure, we found that the enzyme was adsorbed to the cellulose membrane used for ultrafiltration in the presence of (NH4)2SO4 at a concentration of 0.3 M or higher. Thibaut et al. made this observation and thought that it was impossible to concentrate the enzyme by ultrafiltration (28). However, we exploited this property and enhanced the purity to a large degree by membrane treatment, as shown in Table 1. Since it was not easy to purify the flavin reductase from wild-type strain R. erythropolis D-1 due to the small amounts of the enzymes in the cells, this step was very effective for enzyme purification.
The following two major classes of flavin reductases have been characterized to date (18): class I enzymes, which do not contain a flavin prosthetic group (4–6, 12, 24); and class II enzymes, which are flavoproteins (16, 28, 33, 34). The purified flavin reductase from R. erythropolis D-1 did not have an absorption spectrum typical of flavin-containing enzymes, suggesting that the enzyme did not contain any flavin cofactor. We confirmed that the deduced amino acid sequence of the flavin reductase from R. erythropolis D-1 is identical to the amino acid sequence of DszD from R. erythropolis IGTS8. The amino acid sequence of DszD showed 35, 31, 30, 29, and 27% identity to the amino acid sequences of the class I enzymes ActVB, VlmR, HpaC, Fre, and SsuE, respectively, which are flavin reductases without any flavin groups. On the other hand, DszD showed 40, 29, 28, and 26% identity to the class II enzymes SnaC, Frp, NfsB, and NfsA, respectively. There seems to be no relationship between the flavin reductase group described above and sequence homology. There are some differences in substrate specificity and other properties among class I flavin reductases. Fre (5) and VlmR (24) acted on riboflavin and FAD better than on FMN. SsuE utilized NADPH more than NADH (4). For ActVB (12), HpaC (6), and the flavin reductase from R. erythropolis D-1, NADH was the best substrate as the electron donor. HpaC is the coupling protein that enhanced the activity of 4-hydroxyphenylacetate-3-monooxygenase of E. coli, the activity of which was NADH and FAD dependent; FMN could not replace FAD, so this flavin specificity is different from that of the flavin reductase of R. erythropolis D-1. ActVB is the flavin reductase involved in oxidative dimerization and hydroxylation during biosynthesis of the antibiotic actinorhodin by Streptomyces coeliolor and has been reported to exist in rapid equilibrium between monomer and dimer states. The flavin reductase from R. erythropolis D-1, in contrast, consists of four identical subunits. It has been reported that some nitroreductases also have flavin reductase activities (30, 33, 34), all of which have flavin cofactors. The flavin reductase from R. erythropolis D-1 had little nitroreductase activity. There has been no information about such activity in flavin reductases without flavin cofactors. It might be of interest to investigate the relationship between a bound flavin and nitroreductase activity.
Excess amounts of FMN inhibited the flavin reductase activity of R. erythropolis D-1 (Fig. 5). A similar pattern of inhibition was observed in the case of SsuE from E. coli (4). We previously found that the flavin coenzyme was involved in desulfurization of DBT in experiments in which we used the crude enzyme of R. erythropolis D-1, and excess amounts of FMN or FAD inhibited DBT degradation (20). The results shown in Fig. 5 coincided with our previous observation. The inhibition experiments revealed that the flavin reductase activity of R. erythropolis D-1 was inhibited by 7-hydroxycoumarin but not by other coumarin derivatives, including dicoumarol, which inhibited FRase I activity and was used for analysis of its crystal structure (14). FRase I was a flavoprotein possessing FMN as a prosthetic group, and the flavin reductase of R. erythropolis D-1 had no flavin cofactor. These facts imply that there is a difference between the active sites of these two enzymes.
In the overexpression experiments, no expression of flavin reductase was observed when the PCR was performed with the primer containing the TTG sequence as the initiation codon and the resultant fragment was introduced into expression vector pKK223-3. This might be because the TTG codon is poorly recognized in E. coli. When the start codon was changed to ATG, flavin reductase was produced efficiently by E. coli cells under regulation of the T7 promoter. Judging from the specific activity in the cell extracts (50.9 U/mg), the recombinant E. coli cells expressed as much as 40% of the total soluble protein. We are currently trying to overexpress all the enzymes involved in desulfurization of DBT and will examine the coupling efficiency between monooxygenases and the flavin reductase.
ACKNOWLEDGMENTS
Part of this work was conducted with the support of the Petroleum Energy Center (PEC), subsidized by the Ministry of International Trade and Industry (presently the Ministry of Economy, Trade, and Industry).
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 2.Denome A S, Olson E S, Young K D. Identification and cloning of genes involved in specific desulfurization of dibenzothiophene by Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1993;59:2837–2843. doi: 10.1128/aem.59.9.2837-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denome A S, Oldfield C, Nash L J, Young K D. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J Bacteriol. 1994;176:6707–6716. doi: 10.1128/jb.176.21.6707-6716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eichhorn E, van der Ploeg J, Leisinger T. Characterization of a two-component alkanesulfonate monooxygenase from Escherichia coli. J Biol Chem. 1999;274:26639–26646. doi: 10.1074/jbc.274.38.26639. [DOI] [PubMed] [Google Scholar]
- 5.Fontecave M, Eliason R, Reichard P. NAD(P)H:flavin oxidoreductase of Escherichia coli. J Biol Chem. 1987;262:12325–12331. [PubMed] [Google Scholar]
- 6.Galán B, Díaz E, Prieto M A, García J L. Functional analysis of the small component of the 4-hydroxyphenylacetate 3-monooxygenase of Escherichia coli W: a prototype of a new flavin:NAD(P)H reductase subfamily. J Bacteriol. 2000;182:627–636. doi: 10.1128/jb.182.3.627-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher J R, Olson S E, Stanley C D. Microbial desulfurization of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiol Lett. 1993;107:31–36. doi: 10.1016/0378-1097(93)90349-7. [DOI] [PubMed] [Google Scholar]
- 8.Gray A K, Pogrebinsky O S, Mrachko G T, Xi L, Monticello D J, Squires C H. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- 9.Ishii Y, Konishi J, Okada H, Hirasawa K, Onaka T, Suzuki M. Operon structure and functional analysis of the genes encoding thermophilic desulfurizing enzymes of Paenibacillus sp. A11–2. Biochem Biophys Res Commun. 2000;270:81–88. doi: 10.1006/bbrc.2000.2370. [DOI] [PubMed] [Google Scholar]
- 10.Izumi Y, Ohshiro T, Ogino H, Hine Y, Shimao M. Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol. 1994;60:223–226. doi: 10.1128/aem.60.1.223-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kayser K J, Bielaga-Jones B B, Jackowski K, Oduson O, Kilbane J J. Utilization of organosulphur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. J Gen Microbiol. 1993;139:3123–3129. [Google Scholar]
- 12.Kendrew G S, Harding S E, Hopwood D A, Marsh E N G. Identification of a flavin:NADH oxidoreductase involved in the biosynthesis of actinorhodin. J Biol Chem. 1995;270:17339–17343. doi: 10.1074/jbc.270.29.17339. [DOI] [PubMed] [Google Scholar]
- 13.Kilbane J J, II, Jackowski K. Biodesulfurization of water-soluble coal-derived materials by Rhodococcus rhodochrous IGTS8. Biotechnol Bioeng. 1992;40:1107–1114. doi: 10.1002/bit.260400915. [DOI] [PubMed] [Google Scholar]
- 14.Koike H, Sasaki H, Kobori T, Zenno S, Saigo K, Murphy M E P, Adman E T, Tanokura M. 1.8 Å crystal structure of the major NAD(P)H:FMN oxidoreductase of a bioluminescent bacterium, Vibrio fischeri: overall structure, cofactor and substrate-analog binding, and comparison with related flavoproteins. J Mol Biol. 1998;280:259–273. doi: 10.1006/jmbi.1998.1871. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Lei B, Liu M, Huang S, Tu S-C. Vibrio harveyi NADPH-flavin oxidoreductase: cloning, sequencing and overexpression of the gene, and purification and characterization of the cloned enzyme. J Bacteriol. 1994;176:3552–3558. doi: 10.1128/jb.176.12.3552-3558.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei B, Tu S-C. Gene overexpression, purification, and identification of a desulfurization enzyme from Rhodococcus sp. strain IGTS8 as a sulfide/sulfoxide monooxygenase. J Bacteriol. 1996;178:5699–5705. doi: 10.1128/jb.178.19.5699-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niviére V, Fieschi F, Décout J-L, Fontecave M. The NAD(P)H:flavin oxidoreductase from Escherichia coli. J Biol Chem. 1999;274:18252–18260. doi: 10.1074/jbc.274.26.18252. [DOI] [PubMed] [Google Scholar]
- 19.Ohshiro T, Hine Y, Izumi Y. Enzymatic desulfurization of dibenzothiophene by a cell-free system of Rhodococcus erythropolis D-1. FEMS Microbiol Lett. 1994;118:341–344. doi: 10.1128/aem.60.1.223-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohshiro T, Kanbayashi Y, Hine Y, Izumi Y. Involvement of flavin coenzyme in dibenzothiophene degrading enzyme system from Rhodococcus erythropolis D-1. Biosci Biotechnol Biochem. 1995;59:1349–1351. [Google Scholar]
- 21.Ohshiro T, Suzuki K, Izumi Y. Dibenzothiophene (DBT) degrading enzyme responsible for the first step of DBT desulfurization by Rhodococcus erythropolis D-1: purification and characterization. J Ferment Bioeng. 1997;83:233–237. [Google Scholar]
- 22.Ohshiro T, Kojima T, Torii K, Kawasoe H, Izumi Y. Purification and characterization of dibenzothiophene (DBT) sulfone monooxygenase, an enzyme involved in DBT desulfurization, from Rhodococcus erythropolis D-1. J Biosci Bioeng. 1999;88:610–616. doi: 10.1016/s1389-1723(00)87088-7. [DOI] [PubMed] [Google Scholar]
- 23.Oldfield C, Wood N T, Gilbert S C, Murray F D, Faure F R. Desulfurization of benzothiophene and dibenzothiophene by actinomycete organisms belonging to the genus Rhodococcus, and related taxa. Antonie Leeuwenhoek. 1998;74:119–132. doi: 10.1023/a:1001724516342. [DOI] [PubMed] [Google Scholar]
- 24.Parry J R, Li W. An NADPH:FAD oxidoreductase from Valanimycin producer, Streptomyces viridifaciens. J Biol Chem. 1997;272:23303–23311. doi: 10.1074/jbc.272.37.23303. [DOI] [PubMed] [Google Scholar]
- 25.Piddington S A, Kovacevich B R, Rambosek J. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reichmuth S D, Hittle J L, Blanch H W, Keasling J D. Biodesulfurization of dibenzothiophene in Escherichia coli is enhanced by expression of a Vibrio harveyi oxidoreductase gene. Biotechnol Bioeng. 2000;67:72–79. [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Thibaut D, Ratet N, Bisch D, Faucher D, Debussche L, Blanche F. Purification of the two-enzyme system catalyzing the oxidation of the d-proline residue of pristinamycin IIB during the last step of pristinamycin IIA biosynthesis. J Bacteriol. 1995;177:5199–5205. doi: 10.1128/jb.177.18.5199-5205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu S-C, Becvar J E, Hastings J W. Kinetic studies on the mechanism of bacterial NAD(P)H:flavin oxidoreductase. Arch Biochem Biophys. 1979;193:110–116. doi: 10.1016/0003-9861(79)90013-4. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Nishino T, Takio K, Sofuni T, Nohmi T. Purification and characterization of wild-type and mutant “classical” nitroreductase of Salmonella typhimurium. J Biol Chem. 1998;273:23922–23928. doi: 10.1074/jbc.273.37.23922. [DOI] [PubMed] [Google Scholar]
- 31.Xi L, Squires C H, Monticello D J, Childs J D. A flavin reductase stimulates DszA and DszC proteins of Rhodococcus erythropolis IGTS8 in vitro. Biochem Biophys Res Commun. 1997;230:73–75. doi: 10.1006/bbrc.1996.5878. [DOI] [PubMed] [Google Scholar]
- 32.Zenno S, Kobori T, Tanokura M, Saigo K. Purification and characterization of NfrA1, a Bacillus subtilis nitro/flavin reductase capable of interacting with the bacterial luciferase. Biosci Biotechnol Biochem. 1998;62:1978–1987. doi: 10.1271/bbb.62.1978. [DOI] [PubMed] [Google Scholar]
- 33.Zenno S, Koike H, Kumar A N, Jayaraman R, Tanokura M, Saigo K. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J Bacteriol. 1996;178:4508–4514. doi: 10.1128/jb.178.15.4508-4514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zenno S, Koike H, Tanokura M, Saigo K. Gene cloning, purification, and characterization of NfsB, a minor oxygen-insensitive nitroreductase from Escherichia coli, similar in biochemical properties to FRaseI, the major flavin reductase in Vibrio fischeri. J Biochem. 1996;120:736–744. doi: 10.1093/oxfordjournals.jbchem.a021473. [DOI] [PubMed] [Google Scholar]