Abstract
Background:
The long-term outcomes of both pancreas and islet allotransplantation have been compromised by difficulties in the detection of early graft dysfunction at a time when a clinical intervention can prevent further deterioration and preserve allograft function. The lack of standardized strategies for monitoring pancreas and islet allograft function prompted an international survey established by an IPITA/EPITA working group.
Methods:
A global survey was administered to 24 pancreas and 18 islet programs using Redcap. The survey addressed protocolized and “for cause” immunologic and metabolic monitoring strategies following pancreas and islet allotransplantation. All invited programs completed the survey.
Results:
The survey identified that in both pancreas and islet allograft programs, protocolized clinical monitoring practices included assessing body weight, fasting glucose/C-peptide, HbA1c, and DSA. Protocolized monitoring in islet transplant programs relied on the addition of MMTT, CGM and autoantibody titers. In the setting of either suspicion for rejection or serially increasing HbA1c/fasting glucose levels post-pancreas transplant; doppler US, CT, autoantibody titers and pancreas graft biopsy were identified as adjunctive strategies to protocolized monitoring studies. No additional assays were identified in the setting of serially increasing HbA1c levels post islet transplantation.
Conclusions:
This international survey identifies common immunologic and metabolic monitoring strategies utilized for protocol and “for cause” following pancreas and islet transplantation. In the absence of any formal studies to assess the efficacy of immunologic and metabolic testing to detect early allograft dysfunction, it can serve as a guidance document for developing monitoring algorithms following beta cell replacement.
INTRODUCTION
Beta-cell replacement performed with whole organ pancreas allotransplantation or isolated islet allotransplantation are clinically attractive options for long-term diabetes management and prevention of secondary diabetic complications worldwide1. The long-term outcomes of both pancreas and islet transplantation have been compromised by difficulties in detection of early graft dysfunction, at a time when clinical intervention can prevent further deterioration and preserve allograft function2. Monitoring has been heavily dependent on noninvasive strategies with biomarkers and metabolic assessment. Although biopsy of the whole organ pancreas transplant can provide valuable information for potentially reversible causes of dysfunction, technical challenges limit the routine use of this modality3. Because isolated islet transplantation has been largely performed by infusion of the pancreatic islets into the portal circulation, where they spread throughout the liver, the islet graft is inaccessible for routine biopsies/surveillance. For this reason, monitoring islet function is completely dependent on noninvasive metabolic assessment and biomarkers.
The lack of metrics for defining the therapeutic efficacy of different strategies for beta cell replacements, and the absence of standardized strategies for monitoring allograft function were identified as significant barriers to progress in the field of pancreas and islet transplantation at The Transplantation Society Opinion Leaders Meeting on the Future of Beta-Cell Replacement during the International Pancreas and Islet Transplant Association (IPITA) meeting in 2015 4,5. In an effort to establish standardized strategies for monitoring pancreas allograft as well as alloislet function following transplantation, a consortium of global investigators from many of the well-established pancreas and islet transplant programs participated in a joint workshop of the IPITA and the European Pancreas and Islet Transplant Association (EPITA) held in Igls, Austria in January 2017. This workshop generated the Igls Criteria for defining clinically successful graft functional outcomes of beta-cell replacement therapies 6,7. As the success of beta-cell replacement therapy ultimately depends on the prevention of graft failure from technical complications, metabolic exhaustion, alloimmune rejection and, for individuals with type 1 diabetes, autoimmune recurrence, the Igls Workshop and a subsequent Pre-IPITA Congress Symposium held in Lyon, France in July 2019 also included sessions on “Outcomes measures of immunologic mechanisms” and “Immune monitoring of beta cell replacement”. Discussion in these sessions included preliminary surveys of clinical pancreas and islet transplant program’s metabolic and immune monitoring practice that formed the genesis of an IPITA Beta-Cell Replacement Therapy Monitoring Task Force and Follow-Up Survey, which focused on current worldwide practice patterns for the immunologic and metabolic monitoring of beta cell allografts. This report is a summary of the most common monitoring strategies reported by this international consortium of pancreas and pancreatic islet transplant programs responding to this Survey.
MATERIALS AND METHODS
Survey Construction and Administration
Following the 2019 IPITA Conference in Lyon, France, the lead authors of this study (P.S, J.O, M.R) were tasked with formulating a follow-up survey. This study was institutional review board exempt and no informed consent was needed as no patient data was involved. Survey construction and study data were collected and managed using REDCap electronic data capture tools hosted at University of California-San Francisco8. Questions were distinct for islet and pancreas transplantation; all centers were asked to respond to applicable clinical islet and pancreas transplant sections. Questions were written to include “Other” for all sections to allow for full description of alternative monitoring strategies and timing. Descriptive statistics were performed using Stata and GraphPad Prism. See supplementary data for complete surveys.
Definition of “Protocol” and “For Cause” monitoring and frequency of monitoring:
Protocol monitoring was defined as routine monitoring post-transplantation at defined intervals.
“For Cause” monitoring was defined as the clinical work-up obtained in two specific clinical scenarios:1) in the setting of serially increasing fasting glucose and/or hemoglobin A1c (HbA1c) levels for both islet and pancreas transplantation, and 2) in the setting of an acute increase in serum amylase or lipase levels applicable to pancreas allotransplantation only. As most organ transplant patients undergo weekly and/or monthly laboratory testing post-transplant, we chose to ask if the frequency of monitoring for each test is less than every 30 days, every one to three months, every 6 months, or annually to better ascertain the frequency of testing incorporated into specific monitoring protocols.
Definition of static and dynamic metabolic monitoring studies
We defined static metabolic studies as monitoring body weight, fasting glucose, fasting and random C-peptide, fasting insulin, and HbA1c. Dynamic metabolic tests included oral glucose tolerance test (OGTT), mixed meal tolerance test (MMTT), insulin clamp studies and continuous glucose monitoring (CGM) studies.
Consensus
Recommendations were made at the following levels of consensus: If respondents met level of 45-60% agreement on topic of interest, a “suggested” endorsement was given. At 60-75% consensus agreement a “recommended” endorsement was given, and at >75% a “strongly recommended” endorsement was given. If respondents failed to achieve a level of 45% agreement, then monitoring was “not recommended”9.
RESULTS:
Participating Respondents
All 24 pancreas transplant and 18 islet transplant programs invited to participate as part of the IPITA Beta-Cell Replacement Therapy Monitoring Task Force following the IPITA Congress held in 2019 completed the survey.
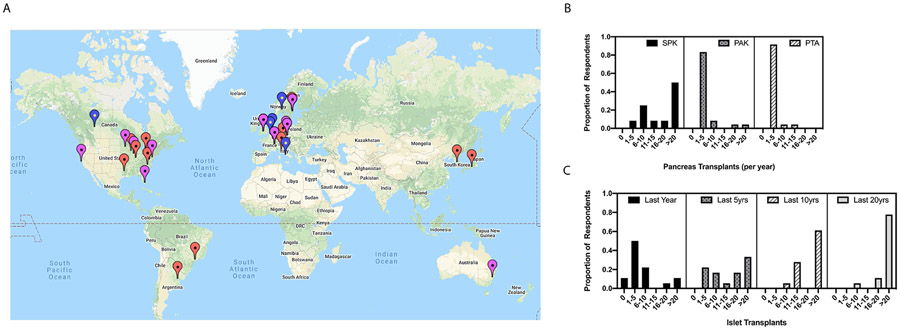
The majority of the pancreas transplant respondents were in North America (11, 45.8%), followed by Europe (8, 33.3%), South America (2, 8.3%), Asia (2, 8.3%) and Australia (1, 4.2%), respectively. This distribution was in comparison to islet transplant respondents which were predominantly residing in Europe (10, 55.6%), followed by North America (7, 38.9%) and Australia (1, 5.6%) (Figure 1A).
Figure 1:
Global location and transplant volume of pancreas and islet transplant center respondents. A. World map indicating geo-location of respondents with red arrows indicating pancreas transplant centers; blue, islet transplant centers and purple, combined programs. B. Pancreas transplant volumes per year stratified by type of solid organ pancreas transplantation. C. Cumulative islet transplant volumes stratified by number of transplants done in the last year, last 5 years, last 10 years and last 20 years, respectively.
Fifty percent of the surveyed respondents performed more than 20 simultaneous pancreas-kidney (SPK) transplants per year, making it the most common pancreas transplant procedure performed by survey respondents. This is congruent with trends seen in the International Pancreas Transplant Registry database for pancreas transplantation worldwide over the last decade10. Regarding pancreas after kidney (PAK) and pancreas transplant alone (PTA) transplants, 83.3% and 91.7% of respondents, respectively, reported performing on average 1-5 cases per year. Enteric drainage was performed by 87.5% respondents for all pancreas transplants with jejunal enteric drainage being the preferred location in 79.2% of respondents (16, 66.7% jejunum alone and 3, 12.5% jejunum with roux) (Figure 1B).
Regarding islet transplant volumes, there is stark heterogeneity among the survey respondents with European, Canadian and Australian respondents having significantly higher volumes in the past five years compared to the United States11. Globally, a minority (16.7%) of respondents have performed >15 islet transplants in the last year. Half of the surveyed respondents performed 1 to 5 islet transplants per year, congruent with global trends (Figure 1C). The most common anatomic site for islet transplantation remains the portal vein via either interventional radiologic percutaneous techniques or mini laparotomy. Less common approaches include omental, intra-peritoneal, intra-muscular, gastric submucosal, and intra-bone marrow placement (See Supplementary Table S1).
Pancreas Transplantation
Protocol Monitoring
Protocol monitoring was defined as routine testing post-transplantation at defined intervals (survey choices: not routinely monitored, less than 1 month, every 1-3 months, every 6 months or annually). The survey assessed four categories of protocol monitoring: static metabolic testing, dynamic metabolic testing, immunologic testing, and pancreas graft biopsy. Of note, since solid organ transplant recipients are routinely monitored with monthly pancreatic enzymes (serum amylase, lipase, or both), the timing of routine monitoring of these enzymes was not explicitly questioned in our survey.
Protocol Static and Dynamic Metabolic Testing:
All 24 pancreas transplant program respondents monitored body weight, fasting glucose and HbA1c levels and 92% of respondents monitored fasting C-peptide levels routinely post-pancreas transplant, which met criteria for strongly recommended. Only 6/24 (25%) respondents routinely monitored random C-peptide and 7/24 (29%) monitored fasting insulin levels, which met criteria for not recommended (Table 1). Within the first month, monitoring body weight and fasting glucose levels met criteria for suggested routinely. At an interval of 1-3 months post-transplant, monitoring fasting glucose, HbA1c and body weight met criteria for strongly recommended. Monitoring fasting C-peptide met criteria for recommended at 1–3-month intervals. (Table 1)
Table 1:
Consensus opinion recommendations for protocol monitoring of whole organ pancreas transplants.
| Static Metabolic Testing | ||||||
|---|---|---|---|---|---|---|
| Not monitored routinely |
≤ 1 month | Every 1-3 months |
Every 6 months |
Annually | Consensus Opinion | |
| Weight | 0 (0.0%) | 11 (45.8%) | 22 (91.7%) | 1 (4.2%) | 8 (33.3%) |
Suggested ≤ 1 month Strongly recommended: every 1-3 months |
| Fasting glucose | 0 (0.0%) | 14 (58.3%) | 23 (95.8%) | 2 (8.3%) | 2 (8.3%) |
Suggested ≤ 1 month Strongly recommended: every 1-3 months |
| HbA1c | 0 (0.0%) | 8 (33.3%) | 20 (83.3%) | 4 (16.7%) | 4 (16.6%) | Strongly recommended every 1-3 months |
| Fasting C-peptide | 2 (8.3%) | 10 (41.7%) | 15 (62.5%) | 3 (12.5%) | 5 (20.8%) | Recommended every 1-3 months |
| Random C-peptide | 18 (75%) | 3 (12.5%) | 3 (12.5%) | 1 (4.2%) | 3 (12.5%) | Not recommended routinely |
| Fasting insulin | 17 (70.8%) | 0 (0.0%) | 4 (16.7%) | 2 (8.3%) | 2 (8.3%) | Not recommended routinely |
| Dynamic Metabolic Testing | ||||||
| Oral Glucose Tolerance Test | 14 (58.3%) | 2 (8.3%) | 1 (4.2%) | 1 (4.2%) | 6 (25%) | Not recommended routinely |
| Mixed Meal/Stim Tests: (MMTT, AST, IVGTT) | 17 (70.8%) | 0 (0.0%) | 2 (8.3%) | 1 (4.2%) | 3 (12.5%) | Not recommended routinely |
| Clamp | 22 (91.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (4.2%) | Not recommended routinely |
| Continuous Glucose Monitoring | 18 (75%) | 1 (4.2%) | 0 (0.0%) | 0 (0.0%) | 2 (8.3%) | Not recommended routinely |
| Immunologic Testing | ||||||
| DSA's | 7 (29.2%) | 9 (37.5%) | 12 (50.0%) | 4 (16.7%) | 9 (37.5%) | Suggested every 1-3 months |
| Autoantibodies | 17 (70.8%) | 1 (4.2%) | 1 (4.2%) | 2 (8.3%) | 1 (4.2%) | Not recommended routinely |
| Pancreas Graft Protocol Biopsy | ||||||
| Type of pancreas transplant | Not monitored routinely | SPK | PAK | PTA | Consensus Opinion | |
| 19 (79.2%) | 2 (8.3%) | 5 (20.8%) | 5 (20.8%) | Not recommended routinely | ||
Neither OGTT, MMTT, insulin clamp studies nor CGM are routinely used at the majority of pancreas transplant programs. No dynamic metabolic test reached criteria for a recommendation of standard monitoring (Table 1).
Protocol Immunologic testing:
The most common form of immunologic monitoring across pancreas transplant respondents was measuring for anti-donor human leukocyte antigen antibody (donor specific antibody, DSA), which were monitored routinely in 70.8% of respondents. (Table 1). At an interval of 1-3 months post-transplant, monitoring for DSA met criteria for suggested. Monitoring of autoantibodies was not performed routinely and therefore met criteria for not recommended.
Protocol Pancreas Graft Biopsy:
Only 20.8% of respondents performed protocol pancreas graft biopsies, which met criteria for not recommended. Of the respondents that reported performing protocol pancreas transplant biopsies, the majority were performed in solitary pancreas recipients (Table 1).
“For cause” Monitoring
“For cause” monitoring of two clinical scenarios common in pancreas allotransplantation were assessed: 1) in the setting of serially increasing fasting glucose and/or HbA1c levels and 2) in the setting of suspicion for rejection (increasing serum amylase or lipase levels). The survey assessed the same five categories of monitoring in the “for cause” state as for protocol above: static and dynamic metabolic testing, immunologic testing, imaging, and graft biopsy.
In the setting of gradually increasing fasting glucose and/or HbA1c levels
Static and Dynamic Metabolic Testing:
In the setting of up-trending fasting glucose and/or HbA1c levels, survey respondents strongly recommended assessing body weight (95.8%), HbA1c (83.3%) and fasting C-peptide levels (95.8%). Additionally, monitoring fasting glucose levels (66.7%) met criteria for recommended. Of note, performing an OGTT (45.8%) met criteria for suggested. The remaining metabolic tests were not recommended routinely including random C-peptide and insulin levels, MMTT, clamp testing, and CGM (Figure 2A).
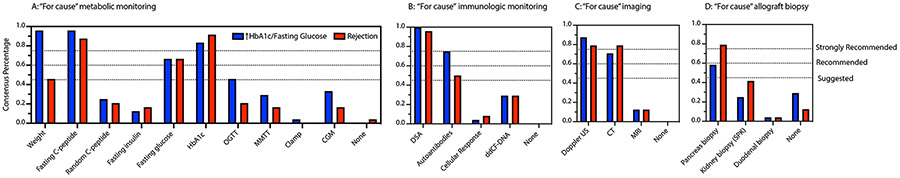
Figure 2:
Consensus opinion recommendations regarding “for cause” monitoring in the setting of increasing fasting glucose and/or HbA1c levels (blue bars) and suspicion of rejection (red bars) of pancreas allografts. Strongly recommended >75%; Recommended 60-75%; Suggested 45-60%. (A) Static and dynamic metabolic monitoring, (B) immunologic monitoring, (C) imaging modalities and (D) allograft biopsy.
Immunologic testing:
Regarding immunologic workup, respondents strongly recommended monitoring for DSA (100%) and autoantibodies (75.0%). Emerging assays and platforms looking at cellular response to autoantigen and donor derived cell-free deoxyribonucleic acid (dd-cfDNA) platforms did not meet criteria for routine monitoring and thus are not recommended at this time (Figure 2B).
Imaging:
All respondents selected imaging of at least one type in the setting of up-trending fasting glucose and/or HbA1c levels. The respondents strongly recommended Doppler ultrasound (US, 87.5%) and recommended cross-sectional imaging with computed tomography (CT) scans. 15/24 (62.5%) respondents selected both doppler US and CT scanning (Figure 2C).
Transplant allograft biopsy:
One of the more controversial topics regarding clinical management of increasing HbA1c levels in whole organ pancreas allografts relates to obtaining a graft biopsy of either pancreas/duodenum or kidney grafts in SPK transplantation. 17/24 (70.8%) respondents obtained a biopsy of at least one allograft: pancreas, kidney (in setting of SPK transplant) or duodenum. Pancreas graft biopsies are the most common form of biopsy [14/24 (58.3%)], meeting consensus criteria for a suggested recommendation. 3/24 (12.5%) respondents stated that they performed either kidney first followed by pancreas or simultaneous kidney and pancreas biopsies, and 2/24 (8.4%) respondents selected kidney biopsy alone (Figure 2D). 100% of respondents surveyed requested C4d staining on biopsy samples.
In the setting of suspicion for rejection (an acute increase in serum amylase or lipase levels)
Metabolic testing:
In the setting of suspicion for rejection in a whole organ pancreas transplant recipient, obtaining fasting C-peptide (87.5%) and HbA1c (91.7%) levels reached a strong recommendation. In addition, checking fasting glucose levels (66.7%) met the recommended criteria and monitoring body weight (45.8%) reached a suggested recommendation (Figure 3A). The remaining metabolic testing panel including random C-peptide levels, fasting insulin levels, OGTT, MMTT, clamp testing and CGM did not meet the threshold for any recommendation (Figure 2A).
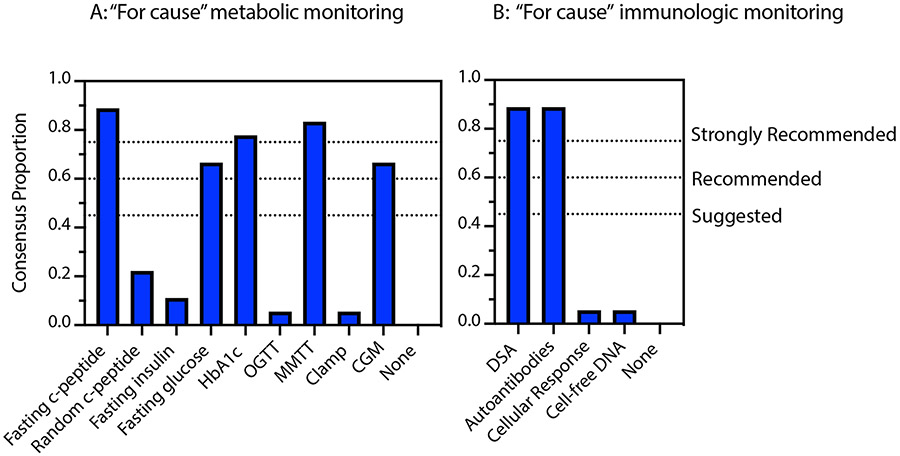
Figure 3:
Consensus opinion recommendations regarding “for cause” monitoring in the setting of increasing fasting glucose and/or HbA1c levels (blue bars) in islet allografts. Strongly recommended >75%; Recommended 60-75%; Suggested 45-60%. (A) Static and dynamic metabolic monitoring and (B) immunologic monitoring.
Immunologic tests:
Survey respondents strongly recommended monitoring for DSA (23/24, 95.8%) and suggested monitoring for autoantibodies (12/24, 50%). Cell-free DNA platforms (7/24, 29.2%) and cellular response to autoantigens (2, 8.3%) did not meet criteria for any recommendation (Figure 2B).
Imaging:
Survey respondents conferred a strong recommendation level consensus opinion for either Doppler US or cross-sectional imaging by CT scan (19/24, 79.2%). (Figure 2C). Most respondents selected both Doppler US and CT scanning (14/24, 58.3%).
Transplant graft biopsy:
In the setting of suspicion of rejection, 21/24 (87.5%) of respondents indicated they would perform a biopsy of either the pancreas, kidney (in the setting of SPK transplant) or duodenum. A majority of respondents performed a pancreas graft biopsy, 19/24 (79.2%), yielding a strong recommendation. 5/24 (20.8%) respondents indicated that they performed either a simultaneous kidney and pancreas graft biopsy or kidney biopsy followed by a pancreas graft biopsy in the setting of suspicion of pancreas rejection (Figure 2D). All respondents surveyed requested C4d staining on biopsy samples.
Islet Transplantation
Protocol Monitoring
Protocol Static and Dynamic Metabolic Testing
All, 18/18 (100%) respondents monitored fasting glucose and HbA1c levels and 17/18 (94.4%) monitored both body weight and fasting C-peptide levels routinely post islet transplant, thereby meeting criteria for a strong recommendation. Random C-peptide and fasting insulin levels were not recommended routinely.
At an interval of 1-3 months post-transplant, body weight monitoring (15/18, 83.3%) and monitoring fasting glucose levels (14/18, 77.8%), HbA1c levels (18/18, 100%) and fasting C-peptide levels (15/18, 83.3%) met criteria for a strong recommendation (Table 2).
Table 2:
Consensus opinion recommendations for protocol monitoring of islet allografts.
| Static Metabolic Testing | ||||||
|---|---|---|---|---|---|---|
| Not monitored routinely |
≤ 1 month | Every 1-3 months |
Every 6 months |
Annually | Consensus Opinion | |
| Weight | 1 (5.6%) | 5 (27.8%) | 15 (83.3%) | 1 (5.6%) | 7 (38.9%) | Strongly recommended every 1-3 months |
| Fasting glucose | 0 (0.0%) | 7 (38.9%) | 14 (77.8%) | 3 (16.7%) | 7 (38.9%) | Strongly recommended every 1-3 months |
| HbA1c | 0 (0.0%) | 4 (22.2%) | 18 (100%) | 2 (11.1%) | 7 (38.9%) | Strongly recommended every 1-3 months |
| Fasting C-peptide | 1 (5.6%) | 5 (27.8%) | 15 (83.3%) | 3 (16.7%) | 8 (44.4%) | Strongly recommended every 1-3 months |
| Random C-peptide | 11 (61.1%) | 2 (11.1%) | 5 (27.8%) | 1 (5.6%) | 4 (22.2%) | Not recommended routinely |
| Fasting insulin | 11 (61.1%) | 1 (5.6%) | 4 (22.2%) | 2 (11.1%) | 5 (27.8%) | Not recommended routinely |
| Dynamic Metabolic Testing | ||||||
| OGTT | 16 (88.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (11.1%) | Not recommended routinely |
| MMTT, AST, IVGTT | 2 (11.1%) | 3 (16.7%) | 9 (50.0%) | 3 (16.7%) | 13 (72.2%) |
Suggested every 1-3 months
Recommended annually |
| Clamp | 17 (94.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.6%) | Not recommended routinely |
| CGM | 4 (22.2%) | 2 (11.1%) | 6 (33.3%) | 0 (0.0%) | 6 (33.3%) | Suggested based on institution availability |
| Immunologic Testing | ||||||
| DSA's | 2 (11.1%) | 2 (11.1%) | 6 (33.3%) | 4 (22.2%) | 10 (55.6%) | Suggested annually |
| Autoantibodies | 1 (5.6%) | 2 (11.1%) | 8 (44.4%) | 1 (5.6%) | 10 (55.6%) | Suggested annually |
Regarding dynamic metabolic testing, MMTT is suggested at the 1–3-month interval (9/18, 50.0%) and recommended (13/18, 72.2%) annually after first year. CGM was used routinely by 14/18 (77.8%) respondents. Interestingly, there was no consensus opinion endorsed by respondents regarding timing of CGM with every 3 months and annually both only reaching 33% consensus, respectively (Table 2). There were multiple respondents who commented on their inconsistent use of CGM based on patient and insurance related factors. Thus, based on expert opinion, CGM use is suggested based on institution availability at this time.
Protocol Immunologic Testing
Both DSA (16/18, 88.9%) as well as autoantibodies (17/18, 94.4%) were strongly recommended as protocolized immunologic testing after islet transplantation. Of note, there was significant heterogeneity regarding the timing of protocol monitoring for both DSA and autoantibodies with both only reaching suggested criteria for monitoring these parameters annually (10/18, 55.6%) (Table 2).
“For Cause” Monitoring in the setting of gradually increasing fasting glucose and/or HbA1c levels
Metabolic testing
In the setting of up-trending fasting glucose and/or HbA1c levels, survey respondents strongly recommended obtaining fasting C-peptide levels (16/18, 88.9%), HbA1c levels (14/18, 77.8%) and MMTT (15/18, 83.3%). Additionally, fasting glucose levels (12/18, 66.7%) and CGM (12/18, 66.7%) received a recommended endorsement. The remaining tests including random C-peptide levels (4/18, 22.5%), fasting insulin levels (2/18, 11.1%), OGTT (1/18, 5.6%) and clamp testing (1/18, 5.6%) were not recommended routinely by respondents (Figure 3A).
Immunologic testing
Surveyed respondents strongly recommended assessing DSA (16/18, 88.9%) and autoantibodies (16/18, 88.9%) in the setting of increasing fasting glucose and/or HbA1c levels. Assessing cellular responses to auto/alloantigens (1/18, 5.6%) and/or dd-cfDNA platforms (2/18, 11.1%) are not recommended by our consensus panel at this time. (Figure 3B).
DISCUSSION
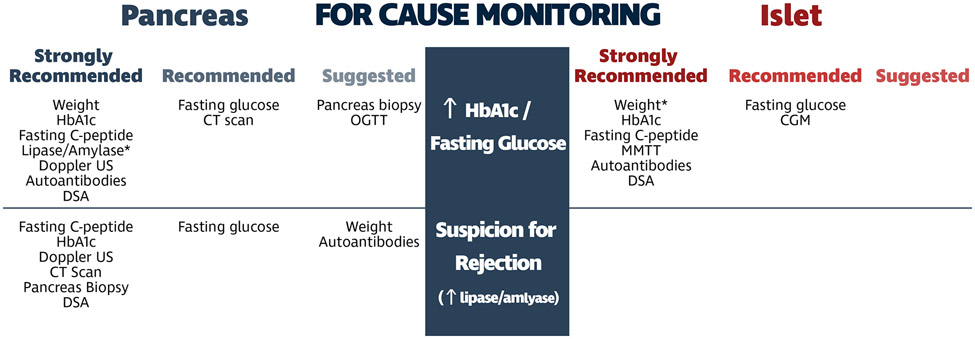
Despite the paucity of literature regarding standards for post-transplant monitoring following pancreas or islet transplantation, there were similar approaches identified by experienced programs globally. The commonalities identified by this global survey provide a strategy for protocolized and “for cause” monitoring following beta cell replacement (Figures 4 & 5).
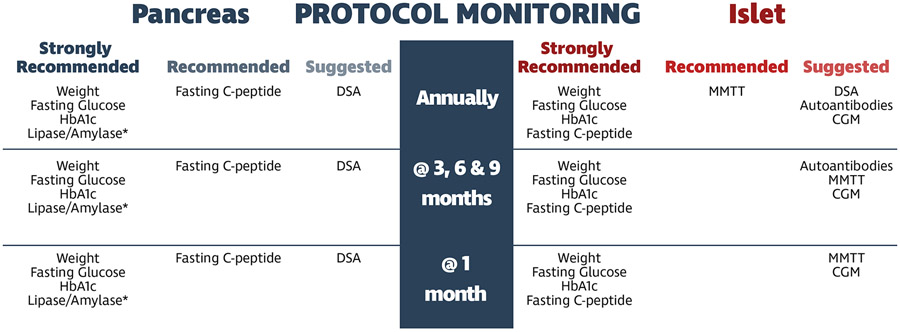
Figure 4:
Consensus summary and timeline for protocolized monitoring of pancreas and islet allografts. *lipase/amylase levels not formally assessed in consensus survey, added post-hoc.
Figure 5:
Consensus summary of “for cause” monitoring of pancreas and islet allografts in the setting of increasing HbA1c and in the setting of suspicion for rejection (acute increase in serum amylase or lipase levels). *lipase/amylase levels in pancreas transplantation and weight in islet transplantation not formally assessed in consensus survey, added post-hoc.
The algorithms recommended for protocolized and “for cause” metabolic and immunologic monitoring are significantly different between pancreas and islet transplantation, especially as related to imaging and tissue biopsy, two monitoring tools that are primarily specific to pancreas transplantation. Because islets are infused into the portal vein and embed in the sinusoids of the liver, they are not accessible for biopsy or any current clinically available imaging strategies. In addition, the vast majority of the cells of the pancreas (>95%) are involved with exocrine function, and inflammation can be reflected by elevations in serum amylase and lipase levels. For this reason, the cornerstone of monitoring allograft function following pancreas transplantation is measuring serum amylase and lipase values. Regarding an increase in amylase and/or lipase, prior studies have identified a correlation of 1.5 - 2-fold increase as a good independent variable to prompt “for cause” workup specifically in the setting of suspicion for rejection2. Conversely, the islet transplant field lacks a sensitive, non-invasive serial assay (“liquid biopsy”), which makes monitoring for rejection and beta cell functional mass profoundly difficult12.
Importantly, the source of increased pancreatic enzymes post-transplant is always an important diagnostic conundrum and relies heavily on the context of the patient’s clinical presentation. While the native pancreas can be a potential source, more commonly the pancreatic allograft is the source of pancreatic inflammation. Timely determination of the cause, targeted intervention and working towards achieving normalization of enzymes is an important determinant of the likelihood of long-term allograft function. Focusing on the pancreatic allograft, increased pancreatic enzymes or graft pancreatitis can represent post-surgical or anatomic abnormalities or ongoing immunological damage, most commonly alloimmune rejection. To focus in on the etiology, two tests are helpful in making a definitive diagnosis: cross-sectional imaging and graft biopsy. Together these two tests can rule out post-surgical causes (i.e., obstruction, enteric leak, abscess, pseudocyst, reduced perfusion, necrosis, dilated pancreatic duct, native pancreatic disease, etc.) and/or make a diagnosis of the type and severity of rejection stimulating appropriate interventions.
Pancreas Transplantation
In terms of protocol monitoring, outside of monthly pancreatic enzyme surveillance, recommendations for protocol metabolic monitoring were limited to static metabolic monitoring including body weight, fasting glucose/C-peptide and HbA1c levels. There were no recommendations for protocol dynamic metabolic monitoring. Of note, protocolized use of CGM has not been adopted by most pancreas transplant programs. This highlights the potential benefits of a multi-disciplinary team approach including both transplant surgeons and endocrinologists to incorporate evolving technology in the management of solid organ pancreas transplant recipients13. With increasing access to this technology, the “time in range” may prove to be a sensitive early marker of graft dysfunction that could be incorporated into routine protocol monitoring14-16.
The only protocol immunologic monitoring that was suggested following pancreas transplantation was DSA at baseline and every three months during the first year. Protocol biopsies were not recommended for SPK, PAK, or PTA. However, when protocol biopsies were performed, they were performed for solitary pancreas transplants only. The surprising lack of respondents performing protocol biopsies even following PTA may be problematic, as a recent study demonstrated rejection in 2/7 PTA recipients that was detected on protocol percutaneous pancreas biopsies performed at 3 months in the absence of any clinical signs17. In these two cases, early identification of rejection and treatment resulted in reversal of rejection (normalization of serum amylase/lipase levels) and long-term insulin independence.
The “for cause” monitoring strategies that were employed for gradually increasing fasting glucose and/or HbA1c levels or suspicion for rejection following pancreas transplantation were similar. Both scenarios included imaging with either Doppler US and/or CT imaging, followed by biopsy. Although a percutaneous biopsy of the pancreas is optimal in the setting of suspicion for rejection, the pancreas may not always be accessible. In these cases, a biopsy of the simultaneously transplanted kidney may provide information regarding concurrent rejection in SPK transplant recipients. A strategy of performing a biopsy on the simultaneously transplanted kidney was reported by several respondents as a potential monitoring tool in lieu of obtaining a biopsy of the transplanted pancreas. Others have reported the utilization of laparoscopic pancreatic biopsies when the transplanted pancreas graft is not accessible percutaneously18. Endoscopic biopsies of the donor duodenum have also been reported by centers who drain the pancreas graft via the native duodenum19. The challenges of biopsying grafts in high-risk recipients or highly suspicious cases has stimulated the need for DSA monitoring and interest in the utility of dd-cfDNA monitoring.
The recommended “for cause” immunologic monitoring included assaying for DSA20,21 and autoantibodies. It is important to recognize that the actual titer of autoantibodies is less relevant than the change in titer or the development of a new autoantibody not present prior to transplantation, and for that reason pre-transplant levels of autoantibodies should be part of protocolized immunologic monitoring strategies 22. Other immunologic monitoring assays, including determining the cellular responses to autoantigen22 have been largely restricted to experimental studies and have not yet been routinely adopted in the clinical setting. The use of dd-cfDNA assays did not meet the threshold for recommendation, although an increasing number of respondents are incorporating these assays into their monitoring algorithms. At the time of the survey, the commercial availability of these assays was limited but expanding at a rapid rate23.
The recommended “for cause” metabolic monitoring following pancreas transplantation was predominantly limited to static measurements of body weight, HbA1c, fasting glucose, and fasting C-peptide levels. Surprisingly, recommendations for dynamic metabolic testing were limited to OGTT with gradual increases in fasting glucose and/or HbA1C levels, but this only met the minimum consensus required for suggested testing. Most respondents infer insulin resistance in the setting of weight gain and gradual increases in fasting glucose and/or HbA1c levels in the absence of rejection. Dynamic testing that would provide additional evidence for the contribution of insulin resistance to increasing fasting glucose and/or HbA1c levels has been more commonly utilized in monitoring of islet transplant grafts. Elevated fasting insulin levels can be used in clinical practice to assess for insulin resistance; however, its use in pancreas transplantation is confounded by systemic venous drainage that bypasses first-pass hepatic extraction of insulin and creates peripheral hyperinsulinemia and transplantation of type 2 diabetic recipients.
Insulin resistance also leads to elevated levels of fasting C-peptide as beta cell function increases in response to the increased demand for insulin secretion. Fasting C-peptide levels require interpretation in the context of both concomitantly measured glucose (that stimulates secretion) and serum creatinine levels (as kidney function is responsible for C-peptide clearance). Nonetheless, routine monitoring of fasting C-peptide and glucose levels can help identify insulin resistance when the C-peptide level increases in the setting of weight gain and gradual increase in fasting glucose and/or HbA1c levels in the absence of rejection or a change in kidney function.
Islet Transplantation
Protocol monitoring and “for cause” metabolic and immunologic strategies reported by islet transplant respondents are more rigorous than solid organ pancreas transplant strategies based on the inability to image or biopsy the islets engrafted in the liver sinusoids. In addition to the same static metabolic monitoring adopted by pancreas transplant respondents, dynamic metabolic monitoring was further recommended. The preference for MMTT over OGTT in islet transplant recipients was potentially motivated by concerns of inducing beta cell stress following administration of a larger glucose load in the OGTT compared to MMTT24. CGM is also recommended every three months at a minimum as a more sensitive strategy to identify early changes in blood glucose stability that are amenable to therapeutic intervention.
Protocolized immunologic monitoring included serial measurements of DSA and autoantibodies. Similar to pancreas transplantation, the use of dd-cfDNA or beta cell cell-free DNA assays have not been widely incorporated into the monitoring regimens for islet transplantation. This likely reflects the cost, complexity, and current paucity of data demonstrating their sensitivity and specificity as well as that for beta cell specific gene expression profiling at the timing of this survey25. There is no question that the islet transplant field is lacking sensitive, non-invasive serial assays for detecting early rejection or autoimmune recurrence and ongoing loss of beta-cell functional mass. Evolving technology that could provide a “liquid biopsy” are eagerly desired and could markedly improve monitoring strategies that allow for early assessment and differentiation of allo- and autoimmune injury26.
Limitations
Although there is a global representation of both pancreas and islet transplant programs included in this study, there is a potential selection bias as the survey was sent to selected, experienced pancreas and islet transplant programs and not all centers worldwide. Additionally, the goal of this survey was to identify only “common practice” monitoring assays with the aim to help identify “best practices” or evidence-based recommendations in future randomized studies. Thus, no strict objective cutoffs were endorsed for any of the assays or “for cause” evaluation.
CONCLUSIONS
This comprehensive survey identifies common global strategies used to monitor allograft function following pancreas or islet transplantation. In the absence of formal studies to assess the efficacy of immunologic and metabolic testing to detect early allograft dysfunction, it can serve as a guidance document for developing local monitoring algorithms following beta cell replacement. Distinguishing immunologic from metabolic mechanisms for beta-cell graft dysfunction and/or failure is paramount to understanding and defining the efficacy of all strategies of beta cell replacement therapy. Of equal significance, early identification of immunologic causes for graft dysfunction can trigger aggressive treatment to reverse declining function. If graft dysfunction is related to increases in insulin resistance, interventions with appropriate antihyperglycemic agents along with weight reduction and possibly reductions in diabetogenic immunosuppression may provide an opportunity to reverse the gradual increases in fasting glucose and/or HbA1c levels.
In addition to immunologic and metabolic studies that were recommended for protocol and “for cause” monitoring as identified by majority consensus, there will likely be increased utilization of CGM in the monitoring of pancreas transplant recipients as this technology becomes increasingly available, and involvement of endocrinologists in the care of transplant recipients becomes standard of care. Similarly, non-invasive detection of beta cell injury with cell-free DNA assays and gene expression profiling will likely play an increasing role for detecting early damage at a time when an intervention can reverse ongoing allograft destruction and preserve beta cell functional mass. These non-invasive tests will have particular relevance for cellular replacement therapies that cannot be imaged or biopsied.
Supplementary Material
Funding:
This work was supported in part by educational grants received from The Transplantation Society and JDRF International to the International Pancreas and Islet Transplant Association in support of the 2017 Igls Workshop. M.R.R. is supported in part by U.S. Public Health Services research grant R01 DK091331. C.J.W is supported by the National Institutes of Health Grant Number T32AI125222.
Abbreviations:
- AST
Arginine stimulation test
- Cellular Response
cellular response to auto/alloantigen
- CGM
Continuous Glucose Monitoring
- CT
Computed tomography
- DNA
Deoxyribonucleic acid
- dd-cfDNA
donor derived cell-free DNA
- DSA
Donor Specific Antibody
- EPITA
European Pancreas and Islet Transplant Association
- HbA1c
Hemoglobin A1c
- IPITA
International Pancreas and Islet Transplant Association
- IR
Interventional radiology
- IVGTT
Intravenous glucose tolerance test
- MMTT
Mixed meal tolerance test
- MRI
Magnetic resonance imaging
- OGTT
Oral glucose tolerance test
- PAK
Pancreas After Kidney
- PTA
Pancreas Transplant Alone
- SPK
Simultaneous Pancreas-Kidney
- US
Ultrasound
Footnotes
All survey respondents are listed in the Supplementary Appendix.
Disclosure:
All the authors of this manuscript have no conflicts of interest to disclose as described by Transplantation. JSO is an investigator in multicenter trials supported by CareDx, Natera and Vertex. He is a principal investigator of a single center trial supported by Veloxis.
References:
- 1.Vantyghem MC, de Koning EJP, Pattou F, et al. Advances in beta-cell replacement therapy for the treatment of type 1 diabetes. Lancet. Oct 5 2019;394(10205):1274–1285. Doi: 10.1016/S0140-6736(19)31334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stock PG, Mannon RB, Armstrong B, et al. Challenges of calcineurin inhibitor withdrawal following combined pancreas and kidney transplantation: Results of a prospective, randomized clinical trial. Am J Transplant. 06 2020;20(6):1668–1678. Doi: 10.1111/ajt.15817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aziz F, Parajuli S, Uddin S, et al. How Should Pancreas Transplant Rejection Be Treated? Transplantation. 09 2019;103(9):1928–1934. Doi: 10.1097/TP.0000000000002694 [DOI] [PubMed] [Google Scholar]
- 4.Bartlett ST, Markmann JF, Johnson P, et al. Report from IPITA-TTS Opinion Leaders Meeting on the Future of beta-Cell Replacement. Transplantation. Feb 2016;100 Suppl 2:S1–44. Doi: 10.1097/TP.0000000000001055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markmann JF, Bartlett ST, Johnson P, et al. Executive Summary of the IPITA-TTS Opinion Leaders Meeting on the Future of Beta Cell Replacement. Transplantation. 2016 2016;100(7):e25–e31. Not in File. [DOI] [PubMed] [Google Scholar]
- 6.Rickels MR, Stock PG, de Koning EJP, et al. Defining Outcomes for beta-cell Replacement Therapy in the Treatment of Diabetes: A Consensus Report on the Igls Criteria From the IPITA/EPITA Opinion Leaders Workshop. Transplantation. Sep 2018;102(9):1479–1486. Doi: 10.1097/TP.0000000000002158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rickels MR, Stock PG, de Koning EJP, et al. Defining outcomes for beta-cell replacement therapy in the treatment of diabetes: a consensus report on the Igls criteria from the IPITA/EPITA opinion leaders workshop. Transpl Int. Apr 2018;31(4):343–352. Doi: 10.1111/tri.13138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 07 2019;95:103208. Doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederberger M, Spranger J. Delphi Technique in Health Sciences: A Map. Front Public Health. 2020;8:457. Doi: 10.3389/fpubh.2020.00457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kandaswamy R, Stock PG, Miller J, et al. OPTN/SRTR 2019 Annual Data Report: Pancreas. Am J Transplant. 02 2021;21 Suppl 2:138–207. Doi: 10.1111/ajt.16496 [DOI] [PubMed] [Google Scholar]
- 11.Witkowski P, Philipson LH, Kaufman DB, et al. The demise of islet allotransplantation in the United States: A call for an urgent regulatory update. Am J Transplant. 04 2021;21(4):1365–1375. Doi: 10.1111/ajt.16397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacotte S, Berney T, Shapiro AJ, et al. Immune monitoring of pancreatic islet graft: towards a better understanding, detection and treatment of harmful events. Expert Opin Biol Ther. Jan 2011;11(1):55–66. Doi: 10.1517/14712598.2011.536530 [DOI] [PubMed] [Google Scholar]
- 13.Rickels MR. A ROLE FOR TRANSPLANT ENDOCRINOLOGISTS--IT'S ABOUT TIME. Endocr Pract. Jun 2015;21(6):697–9. Doi: 10.4158/EP15665.CO [DOI] [PubMed] [Google Scholar]
- 14.Landstra CP, Andres A, Chetboun M, et al. Examination of the Igls Criteria for Defining Functional Outcomes of β-Cell Replacement Therapy: IPITA symposium report. J Clin Endocrinol Metab. Jun 2021;Doi: 10.1210/clinem/dgab386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadlani V, Kaur RJ, Stegall M, et al. Continuous glucose monitoring to assess glycemic control in the first 6 weeks after pancreas transplantation. Clin Transplant. 10 2019;33(10):e13719. Doi: 10.1111/ctr.13719 [DOI] [PubMed] [Google Scholar]
- 16.Mittal S, Franklin RH, Policola C, et al. Early postoperative continuous glucose monitoring in pancreas transplant recipients. Transpl Int. May 2015;28(5):604–9. Doi: 10.1111/tri.12541 [DOI] [PubMed] [Google Scholar]
- 17.Wisel SA, Gardner JM, Roll GR, et al. Pancreas-After-Islet Transplantation in Nonuremic Type 1 Diabetes: A Strategy for Restoring Durable Insulin Independence. Am J Transplant. Sep 2017;17(9):2444–2450. Doi: 10.1111/ajt.14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uva PD, Odorico JS, Giunippero A, et al. Laparoscopic Biopsies in Pancreas Transplantation. Am J Transplant. Aug 2017;17(8):2173–2177. Doi: 10.1111/ajt.14259 [DOI] [PubMed] [Google Scholar]
- 19.Perosa M, Noujaim H, Ianhez LE, et al. Experience with 53 portal-duodenal drained solitary pancreas transplants. Clin Transplant. Feb 2014;28(2):198–204. Doi: 10.1111/ctr.12297 [DOI] [PubMed] [Google Scholar]
- 20.Mittal S, Page SL, Friend PJ, et al. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. Jul 2014;14(7):1664–71. Doi: 10.1111/ajt.12750 [DOI] [PubMed] [Google Scholar]
- 21.Parajuli S, Alagusundaramoorthy S, Aziz F, et al. Outcomes of Pancreas Transplant Recipients With De Novo Donor-specific Antibodies. Transplantation. 02 2019;103(2):435–440. Doi: 10.1097/TP.0000000000002339 [DOI] [PubMed] [Google Scholar]
- 22.Burke GW, Vendrame F, Virdi SK, et al. Lessons From Pancreas Transplantation in Type 1 Diabetes: Recurrence of Islet Autoimmunity. Curr Diab Rep. Dec 2015;15(12):121. Doi: 10.1007/s11892-015-0691-5 [DOI] [PubMed] [Google Scholar]
- 23.Kataria A, Kumar D, Gupta G. Donor-derived Cell-free DNA in Solid-organ Transplant Diagnostics: Indications, Limitations, and Future Directions. Transplantation. 06 2021;105(6):1203–1211. Doi: 10.1097/TP.0000000000003651 [DOI] [PubMed] [Google Scholar]
- 24.Rickels MR. Metabolic and Endocrine Evaluation of Islet Transplant Function. In: Orlando GG RWG; Piemonti L; Stratta R; Ricordi C, ed. Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas. Elsevier Inc.; 2019. [Google Scholar]
- 25.Gala-Lopez BL, Neiman D, Kin T, et al. Beta Cell Death by Cell-free DNA and Outcome After Clinical Islet Transplantation. Transplantation. 06 2018;102(6):978–985. Doi: 10.1097/TP.0000000000002083 [DOI] [PubMed] [Google Scholar]
- 26.Korutla L, Rickels MR, Hu RW, et al. Noninvasive diagnosis of recurrent autoimmune type 1 diabetes after islet cell transplantation. Am J Transplant. Jun 2019;19(6):1852–1858. Doi: 10.1111/ajt.15322 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.