Abstract
Secretoglobin (SCGB) 3A2 was first identified in 2001 as a protein exhibiting similarities in amino acid sequence and gene structure to SCGB1A1, a multi-functional cytokine-like molecule highly expressed in airway epithelial Club cells that was the first identified and extensively studied member of the SCGB gene superfamily. SCGB3A2 is a small secretory protein of ~10 kDa that forms a dimer and a tetramer. SCGB3A2 is predominantly expressed in airway epithelial Club cells, and has anti-inflammatory, growth factor, anti-fibrotic, and anti-cancer activities that influence various lung diseases. This review summarizes the current understanding of SCGB3A2 biological functions and its role in human diseases with emphasis on its mechanisms of actions and signaling pathway.
Keywords: UGRP1, SCGB1A1, CCSP, CC10, SCGB3A1, anti-inflammatory, growth factor, anti-fibrotic, anti-cancer, lung diseases
1. Introduction
Secretoglobin 3A2 (secretoglobin (SCGB) family 3A member 2) was originally identified as a downstream target for the homeodomain transcription factor, NKX2-1 (NK2 Homeobox 1) in embryonic lungs of Nkx2-1-null vs. wild-type mice using a suppressive subtractive hybridization method (Niimi, et al., 2001). SCGB3A2 was initially named uteroglobin-related protein 1 (UGRP1) because it shared sequence similarity with SCGB1A1, also called uteroglobin, Club cell 10-kDa protein (CC10), Club cell 16-kDa protein (CC16), or Club cell secretory protein (CCSP) (Niimi, et al., 2001). SCGB3A2 has also been called HIN-2 (high in normal-2) (Bin, Nielson, Liu, Mason, & Shu, 2003) or LuLeu1 (Lung Leucine-rich 1) (Reynolds, Reynolds, Pryhuber, Finder, & Stripp, 2002). Both SCGB1A1 and SCGB3A2 have a signal peptide at their N-terminus and have a high degree of similarity in their amino acid sequences, particularly in alpha helix 3 of a secreted monomer from which antiflammin peptides are derived (amino acid residues 60–69 of mouse SCGB1A1 translation product) (Miele, Cordella-Miele, Facchiano, & Mukherjee, 1988; Niimi, et al., 2001), which is responsible for the phospholipase A2 inhibitory activity of SCGB1A1 (Chowdhury, et al., 2000; Mukherjee, Zhang, & Chilton, 2007). The sequence similarities and the subsequently revealed gene structures suggested that SCGB3A2 belongs to the same gene family as SCGB1A1 that has been extensively studied (Mukherjee, et al., 1999). There were many other individually described proteins with similar sequences and gene structures published under different names, which created much confusion (Klug, et al., 2000; Porter, Lahti-Domenici, Torres-Arzayus, Chin, & Polyak, 2002; Reynolds, et al., 2002). In 2000, an international conference on “Uteroglobin/Clara cell (the name previously used for “Club cell”) Protein Family” adopted a nomenclature of “secretoglobin” for this family of proteins with SCGB1A1 as the founding member, in which all have the common characteristics of being secreted, small size (~10 kDa), alpha-helical bundle monomeric structure, and dimeric or tetrameric secondary structure (Durairaj, Pageat, & Bienboire-Frosini, 2018; Klug, et al., 2000; Reynolds, et al., 2002). The Human Gene Nomenclature Committee (https://www.genenames.org) then officially approved the generic name “secretoglobin” and its symbol SCGB.
SCGB1A1 is a multifunctional cytokine-like molecule, highly expressed in airway Club cells having anti-inflammatory, anti-allergic, anti-tumorigenic, anti-fibrotic, and embryonic growth-stimulatory activities (Lee, Zhang, & Mukherjee, 2006; Mukherjee, et al., 1999; Mukherjee, et al., 2007). While the mechanisms for biological activities and their pathways remain elusive, its role was established in maintaining airway integrity and as a biomarker for healthy airway epithelial cells and their loss due to the progressive airway remodeling that characterizes chronic lung diseases such as asthma and chronic obstructive pulmonary disease (COPD) (Almuntashiri, et al., 2020; Broeckaert & Bernard, 2000; Wong, Keating, & Waddell, 2009; Zhai, et al., 2019).
Since its discovery in 2001, a number of studies demonstrated that SCGB3A2 exhibits many characteristics similar to those of SCGB1A1 including anti-inflammatory, growth stimulatory, anti-fibrotic, and anti-tumorigenesis activities; many reviews are available on various activities of SCGB1A1 (Agusti & Sin, 2014; Almuntashiri, et al., 2020; Laucho-Contreras, et al., 2016; Mootz, Jakwerth, Schmidt-Weber, & Zissler, 2021; Mukherjee, et al., 1999; Mukherjee, et al., 2007). In this review, we focus on SCGB3A2 by providing an overview of SCGB3A2 and a comparison with SCGB1A1 and SCGB3A1 wherever appropriate, summarizing the current understanding of its biological functions and their role in human diseases, the mechanisms of its actions, and the SCGB3A2 signaling pathway.
2. Fundamental characteristics of SCGB3A2
2-1. SCGB3A subfamily
SCGB3A2 together with another subfamily member SCGB3A1 (also called HIN-1 [high in normal-1 and UGRP2 [uteroglobin-related protein 2]) (Niimi, Copeland, et al., 2002) constitutes the SCGB3A subfamily within the SCGB gene superfamily (I. E. Krop, et al., 2001). Both genes consist of 3 exons characteristic of all SCGB family genes (Niimi, et al., 2001) (Figure 1). There are several variant transcripts derived from each of the Scgb3a1 and Scgb3a2 genes, in addition to the major form that arise through alternative splicing with retention of all or part of the introns, or the presence of additional exon (Niimi, Copeland, et al., 2002; Niimi, et al., 2001). Details on the spatiotemporal expression of these variants and what role they may play, remain elusive. The major forms of SCGB3A1 and SCGB3A2 are composed of 90–100 amino acids, of which the first ~20 residues constituting a signal peptide, producing a mature protein of ~10 kDa after its cleavage. Native human SCGB3A2 immunoreactivity was observed as a monomer (~6 kDa) and dimer (~12 kDa), along with other peptides that may represent alternate conformations (~10 kDa) and degradation products (<3 kDa) in tracheal aspirates (Cai, et al., 2014). Recombinant SCGB3A2 forms a homodimer and higher order multimers (Yokoyama, et al., 2018). Mouse SCGB3A1 and SCGB3A2 share ~30% amino acid identity (Niimi, et al., 2001).
Figure 1. Structure of the SCGB family genes.
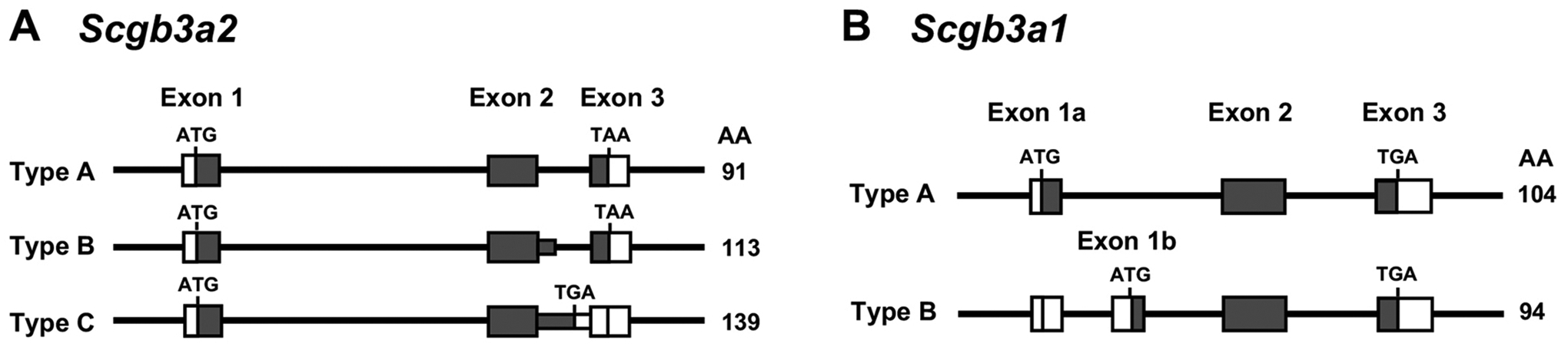
Most SCGB genes including those of mice and humans consist of 3 exons, typically within 3–4 kbp; the transcription start site is located within exon 1, while the termination codon is located in exon 3. The illustration shows Scgb3a2 (A) and Scgb3a1 (B) transcript variants (type B and C for Scgb3a2 and B for Scgb3a1) in addition to the major form (type A) that were identified in mouse adult lung by cDNA library screening. The number on the right in each form indicates the length of amino acid residues (AA). Empty and dark boxes indicate non-coding and coding sequences, respectively. Position and sequence of the initiation and the termination codons are shown.
Human SCGB3A1 and SCGB3A2, and mouse Scgb3a1 and Scgb3a2 genes are localized on human chromosome 5q35.3 and 5q32, and on mouse chromosome 11B1.2 and 18B3, which are the syntenic regions to each corresponding human gene, respectively (Niimi, Copeland, et al., 2002; Niimi, et al., 2001). The human SCGB3A2 gene resides within the 5q32 chromosomal loci known to be associated with asthma susceptibility and where many genes related to inflammation, including interleukin (IL)-3, −4, −5, −9, −13, and −17B, CD14, and colony stimulation factor 2 are located (Mathias, 2014; Niimi, Copeland, et al., 2002). This proximity led to the discovery of anti-inflammatory activity of SCGB3A2 as described below.
2-2. Unique spatiotemporal expression of SCGB3A2 in lung
Both SCGB3A1 and SCGB3A2 are predominantly expressed in the same airway Club cells as SCGB1A1 (Naizhen, Kido, Yokoyama, Linnoila, & Kimura, 2019; Niimi, Copeland, et al., 2002; Niimi, et al., 2001; Reynolds, et al., 2002). The expression of SCGB3A2 and SCGB1A1 were detected in both large (bronchus) and small (bronchioles) airways, while SCGB3A2 expression was observed at embryonic day (E) 14.5 during mouse gestation, which preceded SCGB1A1 expression detectable at E16.5. Historically, SCGB1A1 has been used as a lung epithelial Club cell marker (Broeckaert & Bernard, 2000). The two embryonic day difference in the detection of expression between SCGB1A1 (E16.5) and SCGB3A2 (E14.5) may be useful to distinguish the events occurring between E14.5 and 16.5 during lung development. The expression of SCGB3A2 peaked around birth, whereas that of SCGB1A1 continuously increased for at least 3 weeks after birth (Naizhen, et al., 2019; Reynolds, et al., 2002; Tomita, et al., 2008). As a downstream target of NKX2-1 that is expressed in thyroid, lung epithelium, and ventral forebrain during early embryogenesis, and in accordance with the fact that Nkx2-1-null mice lack the thyroid and pituitary (Kimura, et al., 1996), the expression of SCGB3A2 was also found in thyroid albeit at low levels (Niimi, et al., 2001) and pituitary (see section 3-8) and thus might contribute to the development/function of these organs (Miyano, et al., 2014).
The expression of SCGB3A1 was found only in large, but not small airways at E16.5, and was increased for at least 3 weeks after birth (Naizhen, et al., 2019; Reynolds, et al., 2002). Low level expression of Scgb3a1 was detected in many other adult and developing organs (Niimi, Copeland, et al., 2002; Porter, et al., 2002). SCGB3A1 expression levels are high in normal but low in cancerous tissues, thus accounting for the original nomenclature of high in normal-1, HIN-1 (I. E. Krop, et al., 2001). SCGB3A1 is known as a tumor suppressor in various cancers including those derived from mammary gland, prostate, lung, and pancreas, due to methylation of the SCGB3A1 gene promoter (I. Krop, et al., 2004; I. E. Krop, et al., 2001); however, little is known about SCGB3A1 expression and function beyond the tumor suppressor activity.
Scgb3a1- and Scgb3a2-null mice show no changes in the expression levels of Scgb3a2 and Scgb3a1, respectively, suggesting no compensatory mechanisms in the expression of these two SCGBs (Naizhen, et al., 2019). This is in contrast to the Ccsp (Scgb1a1)-null mice that have increased Scgb3a2 expression (Watson, et al., 2001) and Scgb3a1 (Reynolds, et al., 2002). How the functions of these three SCGBs expressed in airway Club cells relate to each other, and in particular whether there is any compensatory function shared among the three SCGBs remain to be understood.
2-3. Transcriptional regulation of Scgb3a2
In vitro promoter assays, electrophoretic mobility shift analysis, and chromatin immunoprecipitation analysis demonstrated that NKX2-1 binds to specific sites in the Scgb3a2 promoter synergistically with C/EBP (CCAAT/enhancer-binding protein) α and δ and activates transcription (Niimi, et al., 2001; Tomita, et al., 2008). NXK2–1 also regulates Scgb3a2 gene transcription through binding in the Scgb3a2 gene promoter in conjunction with RBPJ (recombination signal binding protein for immunoglobulin kappa J region; a transcriptional regulator of Notch signaling), which contributes to secretory cell fate determination in developing murine airways (Guha, et al., 2012). Further, in human lung epithelial cells, SCGB3A2 gene expression was induced by IL-10 in an NKX2-1-dependent manner at the transcriptional level (Srisodsai, et al., 2004).
2-4. SCGB3A2 receptors and binding proteins
The macrophage scavenger receptor with collagenous structure (MARCO) was initially identified using expression cloning techniques as a SCGB3A2 receptor expressed on the surface of alveolar macrophages (Bin, et al., 2003). The same study also demonstrated that SCGB3A2 binds to apolipoprotein A-1, a major protein component of high density lipoprotein, LPS (as described in 3-6 below), and Gram-positive, Gram-negative bacteria, and yeast (Bin, et al., 2003). MARCO also binds to Gram-positive and Gram-negative bacteria, and unopsonized environmental dusts, and is critically involved in their clearance (Arredouani, et al., 2005; Elomaa, et al., 1995). It was proposed that SCGB3A2 acts as an opsonin, and the SCGB3A2-MARCO ligand-receptor pair is likely involved in inflammation and pathogen clearance in lung (Bin, et al., 2003). Another study suggested the presence of another SCGB3A2 receptor different from MARCO on the surface of mesenchymal cells of fetal mouse lungs in primary culture (Kurotani, et al., 2008). Recently, syndecan-1 (SDC1), a transmembrane heparan sulfate (HS) proteoglycan and a member of the syndecan proteoglycan family, was identified as a receptor for SCGB3A2 using a protein-protein interaction array (Yokoyama, et al., 2018). Binding of SCGB3A2 to SDC1 was abrogated by the addition of heparin, suggesting the importance of heparan sulfate (HS) moiety of SDC1 for the binding of SCGB3A2 (see below 3-6) (Yokoyama, et al., 2018).
3. Biological properties of SCGB3A2 and their implication in human diseases/conditions
3-1. Anti-inflammatory activity
As mentioned above, the chromosomal localization of the human SCGB3A2 gene suggested the possibility that SCGB3A2 is involved in regulating inflammation. The initial experiments showed that reduced Scgb3a2 mRNA expression levels in allergen-exposed mouse lungs was reversed after dexamethasone treatment (Niimi, et al., 2001). Similarly, the levels of Scgb3a1 mRNA slightly reduced in allergen-exposed mouse lungs, also returned to normal after dexamethasone treatment (Niimi, et al., 2001). When mice were subjected to an ovalbumin (OVA)-induced airway inflammation model, their lungs had lower SCGB3A2 protein as well as mRNA expression, that was inversely correlated with the increased Il5 and Il9 mRNA levels in bronchoalveolar lavage fluid (BALF) (Chiba, Kusakabe, & Kimura, 2004; Chiba, Srisodsai, Supavilai, & Kimura, 2005). On the other hand, intranasal administration of IL-5 or IL-9 to naïve mice decreased Scgb3a2 expression in lungs (Chiba, et al., 2004; Chiba, et al., 2005). Further, IL-10, an anti-inflammatory cytokine, induced Scgb3a2 mRNA and/or protein expression in lung epithelial cells of naïve mouse when intranasally administered IL-10, and E15.5 embryonic mouse lungs in culture (Srisodsai, et al., 2004). When OVA-treated model mice were given an intranasally administered recombinant adenovirus expressing SCGB3A2, they presented reduced levels of pro-inflammatory TH2 cytokines such as IL-4, 5, and 13, and inflammatory cell numbers in BALF, and lung histopathology similar to control lungs (Chiba, et al., 2006) (Figure 2). Further, when Scgb3a2-null mice were subjected to either OVA- or house dust mite (HDM)-induced allergic airway inflammation models, they exhibited exacerbated inflammation as compared with wild-type mice (albeit the effect was small but significant), confirming the anti-inflammatory activity of SCGB3A2 (Kido, et al., 2014; Yoneda, et al., 2016). Since CCR4 (C-C chemokine receptor type 4) and CCL17 (chemokine (C-C) motif ligand 17), but not CCR3 or CCL11 mRNA expression were both elevated in lungs of HDM-challenged Scgb3a2-null mice compared to wild-type mice, SCGB3A2 appears to modulate the CCR4-CCL17 but not CCR3- CCL11 pathway (Yoneda, et al., 2016). The biological role of this pathway remains unknown.
Figure 2. Summary of the major biological functions of SCGB3A2 in lung.
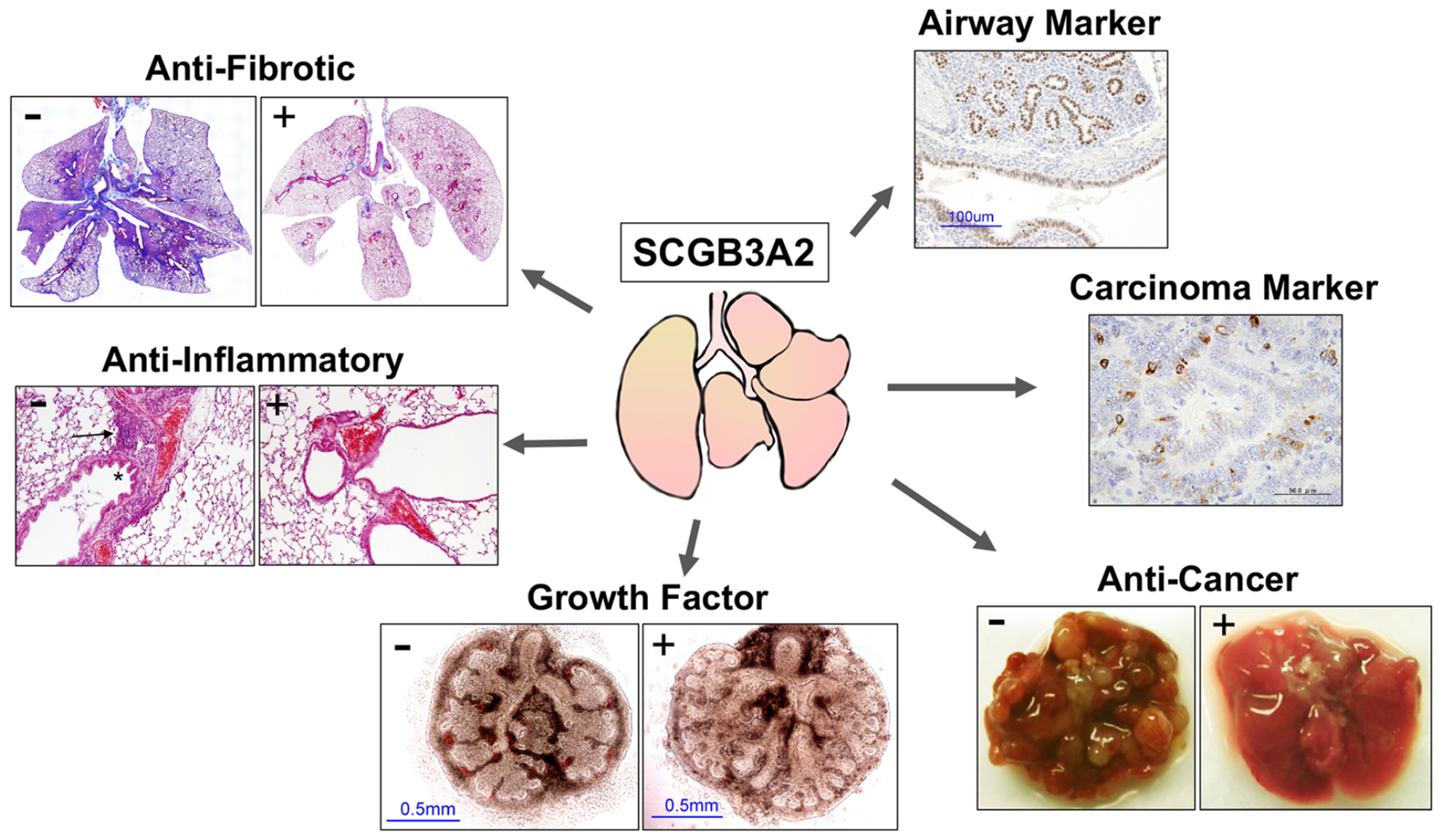
No (−) or addition/administration (+) of SCGB3A2 as indicated. Blue in anti-fibrotic activity is Masson trichrome staining to detect collagen fiber. Airway and carcinoma markers are immunohistochemistry results (brown) for SCGB3A2. Arrow and asterisk in anti-inflammatory activity are infiltrating immune cells and reactive hyperplasia of epithelial cells, respectively.
The amino acid residues 42–51 of mature (secreted form) murine SCGB3A2 has high similarity to the amino acid residues 39–48 of mature murine SCGB1A1, the latter referred to as an “antiflammin peptide” located in the alpha helix 3 region of SCGB1A1 (Niimi, et al., 2001). It is responsible for phospholipase A2 inhibitory activity and at least part of the anti-inflammatory activity of SCGB1A1 (Facchiano, Cordella-Miele, Miele, & Mukherjee, 1991; Mukherjee, et al., 1999). SCGB3A2 was also shown to exhibit phospholipase A2 inhibitory activity (Cai, et al., 2014). The mechanism for the anti-inflammatory activity of SCGB3A2, and whether and/or how SCGB3A2’s anti-inflammatory activity is related to its phospholipase A2 inhibitory activity, has not been elucidated.
Altered levels of SCGB3A2 were reported in inflammatory diseases; down-regulation of SCGB3A2 protein in the upper airway of chronic rhinosinusitis patients (Lu, et al., 2011), induced protein levels in the sputum of patients with asthma and rhinitis (de Burbure, et al., 2007), and lower plasma SCGB3A2 levels in asthmatics vs. mild/moderate asthmatic patients or controls (Inoue, et al., 2008). Pre-incubation of SCGB3A2 with BEAS-2B cells for 3 hours before addition of lipopolysaccharide (LPS) inhibited upregulation of tumor necrosis factor (TNF)-α and C-X-C motif chemokine ligand 8 (CXCL8) mRNA expression through the JNK and ERK pathways (Wang, et al., 2015). Thus, all available results suggest the possibility that administration of SCGB3A2 protein may alleviate the symptoms of allergic airway inflammation including asthma and rhinosinusitis/rhinitis.
3-2. SCGB3A2 as a biomarker for Club cells and airway integrity
Naphthalene exposure has been used as a model to study lung injury in mice. Naphthalene specifically ablates Club cells in the airways. Scgb3a2 mRNA was found co-expressed with SCGB1A1 protein in cells that were initially thought to be nascently regenerated Club cells at regenerating bronchiolar epithelium, thus indicating that SCGB3A2 is an early differentiation marker for Club cell lineage (Reynolds, et al., 2002). Several years after this paper was published, a minor population of Club cells located around the bronchioalveolar duct junction (BADJ) were characterized as bronchioalveolar stem cells (BASC) that are resistant to the naphthalene-induced damage and are involved in the repair and regeneration of lung epithelial cells (C. F. Kim, et al., 2005). These cells express SCGB1A1 and the alveolar type II cell marker surfactant protein C (SP-C). Thus, SCGB1A1+ Club cells located at BADJ have been a focus for understanding the role of these cells in the maintenance of lung homeostasis, repair, and carcinogenesis (Jones-Freeman & Starkey, 2020; Liu, et al., 2019) (C. F. Kim, et al., 2005; Rawlins, et al., 2009). It was suggested that SCGB3A2 may also be expressed in BASC (Reynolds, et al., 2002).
The earlier onset of SCGB3A2 expression was used as an early lung epithelial marker to facilitate the purification and characterization of human airway lineages during pluripotent stem cell differentiation (McCauley, et al., 2018) and to distinguish from Krt17(keratin 17)+ basal cell progenitors (Kiyokawa, et al., 2021). SCGB3A2 has been used as a marker, together with SCGB1A1, in identification of Club cells during development of human induced pluripotent stem cells (hiPSCs) (Tsuji, Yamada, Hirai, Asakura, & Kanda, 2021) and in studies on the role of Club cells in pathogenesis of idiopathic pulmonary fibrosis (Zuo, et al., 2020) (Figure 2).
A sensitive sandwich ELISA assay was developed to determine the level of SCGB3A2 in human biological fluids, including serum, urine, nasal lavage fluid, BALF, induced sputum, and amniotic fluid (Van De Velde, Courtens, & Bernard, 2010). The levels of SCGB3A2 present in serum and urine in healthy subjects were similar to those of SCGB1A1 (~5–10 μg/L (Van De Velde, et al., 2010)). This expression level was much higher than the plasma SCGB3A2 levels of ~0.12 μg/L reported by another study (Inoue, et al., 2008). SCGB3A2 was not detected in nasal lavage fluid while SCGB1A1 was present at similar levels to serum. SCGB1A1 was found at much higher level than SCGB3A2 in BALF (~710 vs. ~180 μg/L) and induced sputum (9,400 vs. ~370), while amniotic fluid showed lower levels of SCGB1A1 expression than SCGB3A2 (~50 vs. ~410). In a clinical trial of recombinant human (rh) SCGB1A1 in severely premature infants (24–29 weeks post-menstrual age), tracheal aspirate fluids (TAF) from placebo controls at 24 hours after delivery had SCGB3A2 concentrations that ranged from 395–804 μg/L while SCGB1A1 concentrations ranged from 85–1,669 μg/L (Cai, et al., 2014; Levine, et al., 2005). That both SCGB proteins are found in amniotic fluid and the TAF of premature infants is in agreement with the fact that both play a role in lung development (see section 3-4 for SCGB3A2) (Kurotani, et al., 2008; Mukherjee, et al., 2007). It should be noted that these studies used different ELISAs for SCGB3A2 and comparisons of concentrations studies may not be ideal. Nevertheless, SCGB3A2 in different fluids may serve as a surrogate marker that is less variable than SCGB1A1, depending on individual situation, to assess airway epithelium integrity and/or a risk factor in development of asthma/respiratory allergies (Van De Velde, et al., 2010).
3-3. Polymorphisms associated with human diseases
A polymorphism of G→A conversion located at −112 bp from the transcription start site of the human SCGB3A2 gene was initially identified in association with increased risk of developing asthma in Japanese populations (Niimi, Munakata, et al., 2002). The homozygous AA variant had reduced transcriptional activity of the SCGB3A2 gene (Niimi, Munakata, et al., 2002; Song, et al., 2009) and significantly decreased serum SCGB3A2 levels (Chistiakov, Voronova, Turakulov, & Savost’anov, 2011). The reduced plasma SCGB3A2 levels were associated with the G-112A polymorphism and the severity of asthma in Japanese populations (Inoue, et al., 2008). Studies supporting the association of the G-112A (rs1368408) polymorphism to asthma include those of Korean populations (S. K. Kim, et al., 2017) and meta-analysis in Asian populations but not Caucasian populations (Xie, et al., 2014). Other studies did not find an association to asthma, including studies of an Indian population (Batra, Niphadkar, Sharma, & Ghosh, 2005), Caucasian population (Heinzmann, Dietrich, & Deichmann, 2003), Sicilian population (Rigoli, et al., 2007), in childhood asthma in Japanese population (Jian, Nakayama, Noguchi, Shibasaki, & Arinami, 2003). It is possible that even though the G-112A polymorphism is associated with the susceptibility to asthma, the association may be weak and could be masked by other stronger association(s), depending on study design and/or sample cohorts. Detection of SCGB3A2-associated susceptibility to asthma may also depend on environmental and lifestyle factors such as exposure to pollutants, cleaning chemicals, and cigarette smoke in the targeted population. Thus, additional studies using increased sample numbers are warranted. SCGB3A2 is one of many genetic loci identified using candidate gene and/or genome-wide association approaches that are associated with the phenotypes of asthma (Mathias, 2014). Other single nucleotide polymorphisms in the SCGB3A2 gene were reported, such as G-659A (rs6882292) for increased risk of asthma (S. K. Kim, et al., 2017), and rs7726552 for allergic rhinitis without any association to asthma (Andiappan, et al., 2011).
Genome-wide analysis demonstrated a susceptibility locus for autoimmune thyroid disease on Chromosome 5q31-q33 (Sakai, et al., 2001) and Graves’ disease on 5q31 (Jin, et al., 2003), the region in which SCGB3A2 gene is located. An association of the G-112A polymorphism to the increased risk of developing thyroid Graves’ disease was demonstrated by genome-wide association studies, linkage analysis, and meta-analysis both in Chinese and Caucasian populations (Chistiakov, et al., 2011; Kus, et al., 2019; Simmonds, et al., 2010; Song, et al., 2009; Xue, Han, Pan, & Song, 2014). On the other hand, a few studies of Chinese populations demonstrated no association (Kus, et al., 2019; Yang, et al., 2005), however, these studies used much smaller sample sizes than those having the association, suggesting that the G-112A polymorphism may likely be one of the susceptible loci to thyroid Graves’ disease. Another study suggested that SCGB3A2 might be used as a marker to predict Graves’ disease patients who develop hypothyroidism (Zhou, et al., 2019).
3-4. Growth factor activity
Based on the fact that the lungs of Nkx2-1-null mouse embryos are severely hypoplastic, and SCGB3A2 is highly expressed in airway epithelial cells, it was hypothesized that the NKX2-1 downstream target SCGB3A2 may play a role in embryonic lung development (Kurotani, et al., 2008). Careful examination found low expression of SCGB3A2 by immunohistochemistry in the epithelial cells of embryonic day (E) 11.5 and around the growing tips of bronchi of E13.5 mouse fetal lungs, the latter particularly suggesting a possible role for SCGB3A2 in lung branching morphogenesis (Kurotani, et al., 2008). An ex vivo mouse lung organ culture study using E11.5 embryonic lungs demonstrated that the addition of recombinant mouse (rm) SCGB3A2 in the culture media promoted branching morphogenesis, while it was suppressed by the addition of anti-SCGB3A2 antibody or SCGB3A2 siRNA (Kurotani, et al., 2008) (Figure 2). Further, the embryonic lung development was promoted by the intravenous administration of rmSCGB3A2 to the pregnant female mice from E13.5 to E16.5, followed by examination of the embryos at E17.5 (1 day before natural birth) by lung histology and breathing scores of newborns (Kurotani, et al., 2008). Embryos administered SCGB3A2 and examined at E17.5 had the same breathing score as normal E18.5 newborns (initially labored breathing, followed by unlabored breathing once fluid was cleared from the lung). E17.5 embryos without SCGB3A2 administration exhibited no breathing or gasping, indicating that SCGB3A2 promoted lung development in vivo (Kurotani, et al., 2008). When Nkx2-1-null lungs were cultured ex vivo in the presence of rmSCGB3A2, the epithelia showed drastic morphological changes such as appearance of pleated and/or dentate epithelia and duct-like structures, and increased layers of mesenchyme. Further, increased Ki-67 and phosphorylated histone H3 positive signals as proliferation markers were observed (Kurotani, et al., 2008). Similarly, the lungs of Nkx2-1-null mice administered rmSCGB3A2 had increased numbers of cells positive for Ki-67 and phosphorylated histone H3 upon administration of rmSCGB3A2. Thus, SCGB3A2 is a growth factor accelerating embryonic lung development. Recombinant human (rh) SCGB3A2, when used in ex vivo and in vivo studies, promoted murine lung branching morphogenesis and lung development, respectively, to a similar degree to those obtained with rmSCGB3A2 protein (Cai, et al., 2014). The results suggest that rhSCGB3A2 may be used to treat severely premature babies with respiratory distress to stimulate lung development. The mechanism for the growth factor activity of SCGB3A2 remains to be understood.
3-5. Anti-fibrotic activity
A bleomycin (BLM)-induced pulmonary fibrosis model mouse was used to examine the effect of SCGB3A2 in the development of pulmonary fibrosis (Kurotani, Okumura, et al., 2011). Mice were intratracheally intubated with BLM and treated either with SCGB3A2 or PBS daily for a week starting 2 weeks after intubation, followed by lung examination on day 21 (Figure 2). Mice treated with SCGB3A2 presented no fibrosis in contrast to BLM+PBS group, which had grade 1 (fibrosis found at 0–25% of a whole lung) or 2 (25–50%) fibrosis with strong Masson trichrome staining that detects collagen fibers. Those treated with SCGB3A2 also exhibited very low macrophage and neutrophil numbers in contrast to massive increase of their numbers in bronchoalveolar lavage fluid (BALF) of BLM+PBS group of mice. Similar levels of anti-fibrotic activity were demonstrated using the bleomycin model with the administration of rhSCGB3A2 instead of rmSCGB3A2 (Cai, et al., 2014). These results suggest the possible use of SCGB3A2 as a therapeutic to treat pulmonary fibrosis.
In the murine BLM model, pulmonary fibrosis typically resolves over time without treatment and Scgb3a2-null mice exposed to BLM exhibited exacerbated pulmonary fibrosis (Cai & Kimura, 2015), while transgenic mice specifically overexpressing Scgb3a2 in the lungs exhibited accelerated resolution of pulmonary fibrosis (Cai, et al., 2015). The results confirmed the earlier findings that SCGB3A2 possesses anti-fibrotic activity.
The partial mechanism for the anti-fibrotic activity of SCGB3A2 was demonstrated by in vitro studies of culturing mouse primary lung fibroblasts in the presence of transforming growth factor (TGF)-β with and without SCGB3A2 (Kurotani, Okumura, et al., 2011) (Figure 3). TGF-β increased phosphorylation of SMAD2 and SMAD3, and the expression levels of α-smooth muscle actin protein and collagen genes as expected, however, these increases were all suppressed by the addition of SCGB3A2. At the same time, SCGB3A2 administration increased phosphorylation of STAT1 and expression of SMAD7, the inhibitory SMAD, thus suppressing TGF-β signaling. The addition of neutralizing antibody to the receptor for Interferon (IFN)-γ, a prototypical cytokine known to activate STAT1 phosphorylation, did not interfere SCGB3A2-induced STAT1 phosphorylation and SMAD7 elevation. Further, the addition of cycloheximide suppressed SCGB3A2-induced STAT1 phosphorylation, suggesting that SCGB3A2-induced STAT1 phosphorylation is unrelated to IFN-γ signaling, and that a cycloheximide-sensitive intermediate molecule is involved in SCGB3A2 signaling, leading to anti-fibrotic activity. However, the receptor to which SCGB3A2 binds and initiates the anti-fibrotic signaling was not determined in this study.
Figure 3. Mechanism for the anti-fibrotic activity of SCGB3A2.
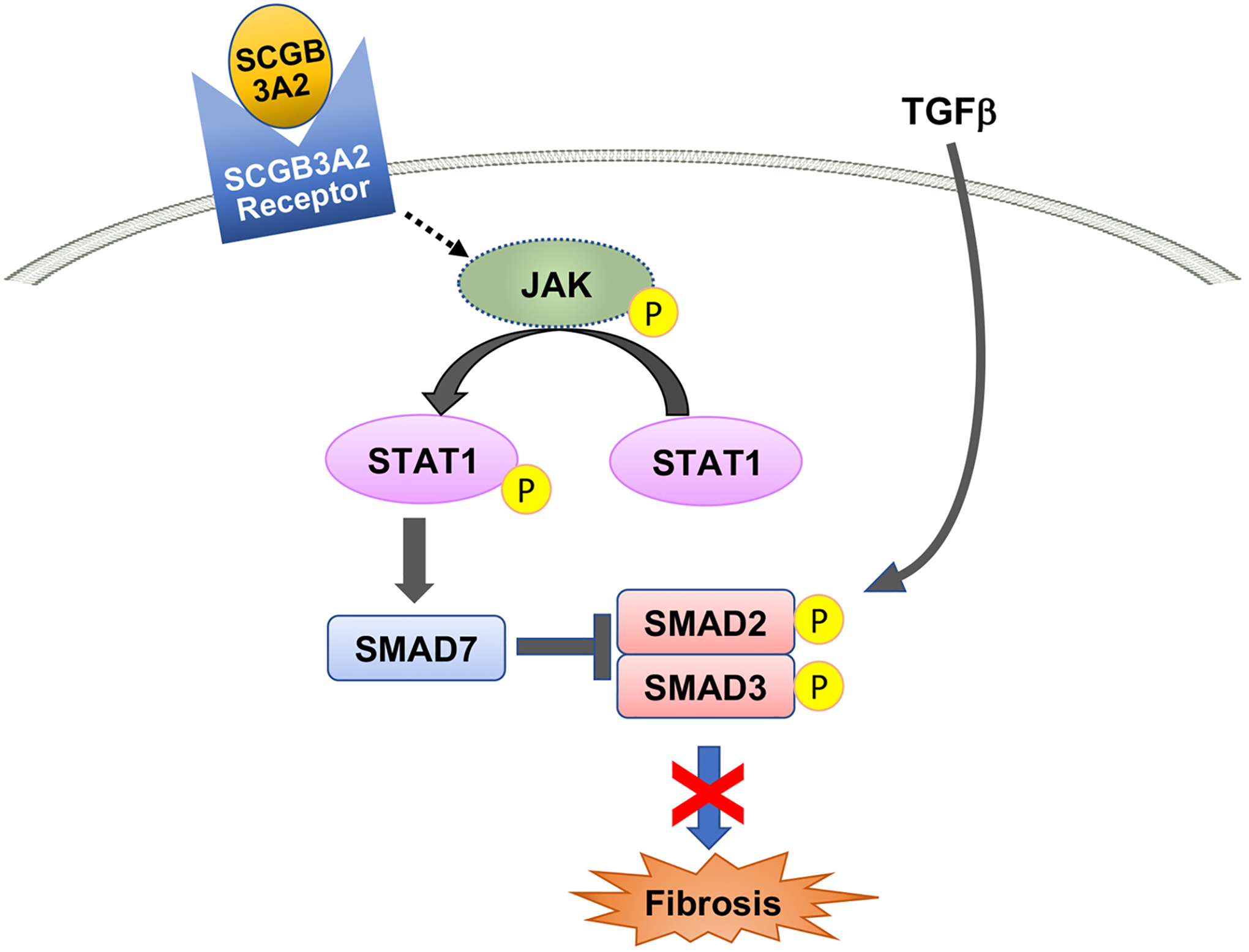
SCGB3A2 anti-fibrotic signaling starts upon binding of SCGB3A2 to a SCGB3A2 receptor, which activates STAT1 phosphorylation, increases SMAD7, and suppresses SMAD2/3 phosphorylation, resulting in the suppression of expression of genes involved in fibrosis such as collagens, leading to inhibition of fibrosis.
3-6. Anti-cancer activity
SCGB3A2 suppressed the growth of Lewis lung carcinoma (LLC) cells in vitro. In an in vivo model of LLC intravenous metastasis, Scgb3a2-null mice had increased number of lung tumors as compared with wild-type mice and the administration of rhSCGB3A2 suppressed development of lung tumors in comparison to mice without SCGB3A2 administration (Figure 2). When Scgb3a2-null mice were given rhSCGB3A2, the number of tumors was similar to those of wild-type controls (Yokoyama, et al., 2018). These results suggested that SCGB3A2 has anti-cancer activity and detailed studies were carried out to understand the mechanism for SCGB3A2 anti-cancer activity. It was observed that SCGB3A2 is a lipopolysaccharide (LPS) binding protein and that it chaperones LPS into cell cytosol through binding of an SCGB3A2-LPS complex to a cell surface receptor, syndecan-1 (SDC1). Internalized LPS binds to and activates caspase-11 (in mice) or caspase-4 (in human), which further activates the non-canonical NLRP3 (NOD-like receptor family, pyrin domain containing 3) inflammasome pathway, leading to pyroptosis, a type of inflammatory cell death (Yokoyama, et al., 2018) (Figure 4). Involvement of internalized LPS in the activation of the non-canonical inflammasome pathway was well documented (Rathinam, Zhao, & Shao, 2019; Skirecki & Cavaillon, 2019), however, how extracellular LPS enters cells remained elusive. This study clearly demonstrated a SCGB3A2-dependent route for LPS entry to cells through SDC1. Moreover, SDC1 is expressed on the surface of LLC cells as well as on macrophage-derived RAW264.7 cells. Both cells were susceptible to SCGB3A2-LPS-induced pyroptosis, suggesting this evolutionarily conserved mechanism in cancer cells that was initially evolved in immune cells to fight against infection (Yokoyama, et al., 2018). SCGB3A2-LPS-induced pyroptosis was inhibited in the presence of heparin, suggesting that the heparan sulfate (HS) moiety of SDC1 is critical for SCGB3A2-LPS-induced pyroptosis.
Figure 4. Mechanism for the anti-cancer activity of SCGB3A2 through activation of the non-canonical inflammasome pathway.
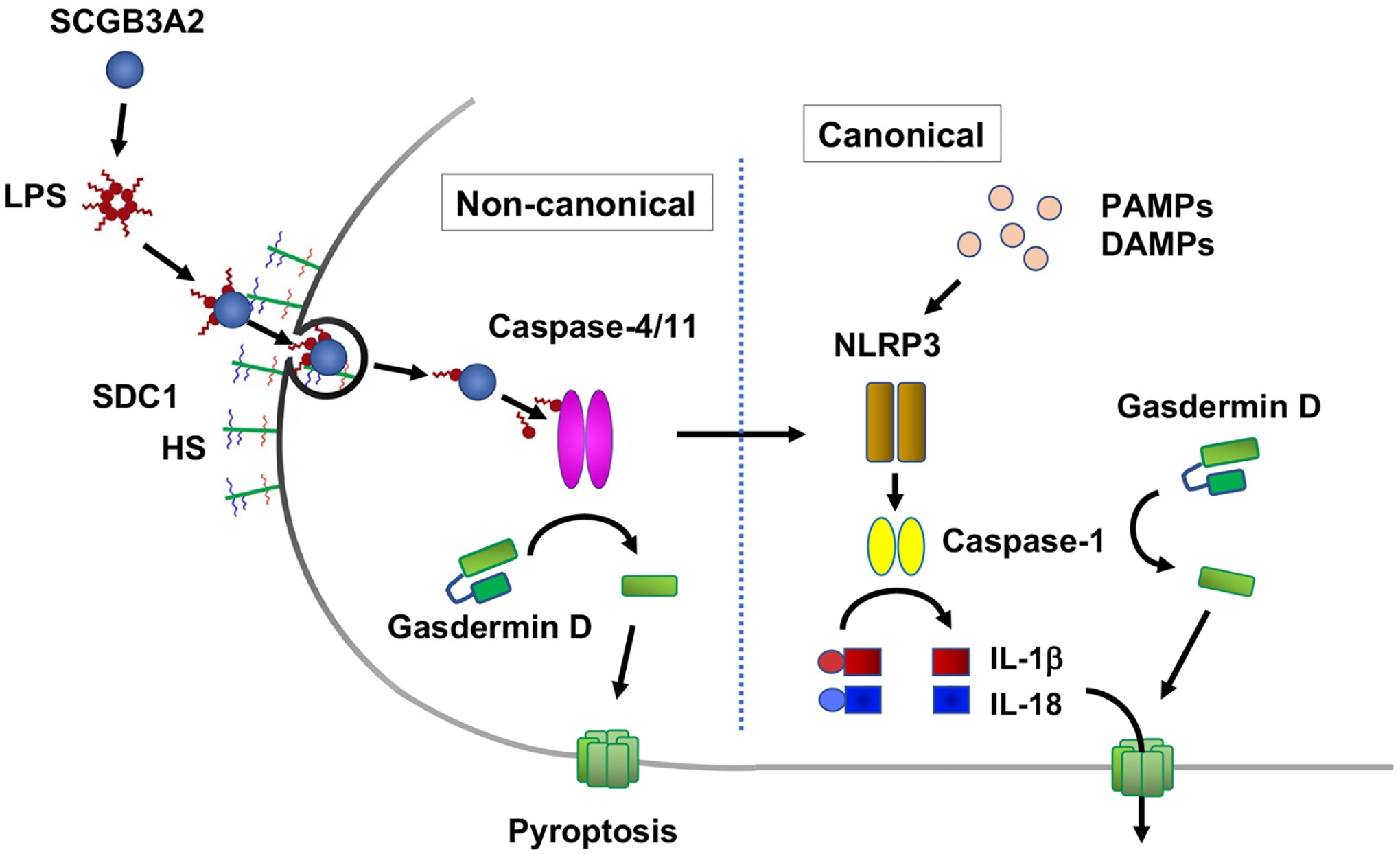
SCGB3A2 binds to LPS, and the SCGB3A2-LPS complex enters cell cytosol through binding to syndecan 1 (SDC1) followed by endocytosis. Internalized LPS binds and activates caspase-11 (in mice) and −4 (in human) that activates gasdermin D forming membrane pores, and eventually leading to pyroptosis. The non-canonical inflammasome cross-talks with canonical inflammasome, in which activated caspase-1 through the NLRP3 inflammasome releases IL-1β and IL-18 through gasdermin D pores. HS: heparan sulfate, PAMP: pathogen-associated molecular patterns such as double-strand RNA, cytosolic DNA, etc. derived from pathogens. DAMP: danger-associated molecular patterns such as host DNA, ATP, etc, NLRP3: NLR family pyrin domain containing 3. Upon entering cells, whether and/or how the SCGB3A2-LPS complex dissociates, and/or releases LPS remain unknown.
In order to determine whether pyroptosis can be utilized as a way to kill cancer cells, a panel of 20 human cancer-derived cell lines from different origins (17 lung, 2 colon, and 1 cervix) were analyzed for the susceptibility to SCGB3A2 (Yokoyama, Nakayama, Xu, Pilon, & Kimura, 2021). Five out of 11 NSCLC (non-small cell lung cancer) cells (H596, H358, H322, A549, and H157) showed SCGB3A2-induced growth inhibition in the presence of LPS in vitro and reduced tumor sizes in vivo in xenografts of SCGB3A2-treated mice. All susceptible NSCLC cells expressed SDC1 on their cell surface with abundant HS and caspase-4 (CASP4), the critical molecule in the non-canonical inflammasome pathway. In contrast, none of 6 SCLC cells (H1688, H146, H526, H417, H446, H82) examined showed growth inhibition, and none expressed SDC1 and/or CASP4 (Yokoyama, et al., 2021). Two epithelial-derived colorectal cancer cell lines (HCT116 and SW620), that abundantly expressed SDC1 on the cell surface and CASP4, also underwent SCGB3A2-induced pyroptosis. These results suggest that SCGB3A2 may serve as a novel therapeutic to treat cancers regardless of tissue types, as long as cells express SDC1 with abundant HS and CASP4 (Yokoyama, et al., 2021). Pyroptosis has been gaining attention in recent years as a tool to treat cancer (Fang, et al., 2020; Nagarajan, Soundarapandian, Thorne, Li, & Li, 2019).
3-7. SCGB3A2 as a cancer marker
The prevalence of expression of SCGB3A2 in lung cancer was investigated by immunohistochemistry using mice as well as human lung cancer tissues. In mouse studies, the expression of SCGB3A2, which is normally found in Club cells, was observed in alveolar Type II cell carcinomas and Club cell adenocarcinomas using 28 lung tumors from aging B6;129 mice and 9 lung adenocarcinoma from CC10 (SCGB1A1) transgenic mice that express SV40 large T antigen under the mouse Scgb1a1 gene promoter (Kurotani, Kumaki, et al., 2011) (Figure 2). No expression of SCGB3A2 was found in Type II cell hyperplasias or adenomas. Of interest was that SCGB3A2 expression in carcinomas was observed in the portion of the tumor where NKX2-1 expression was reduced or nearly absent, suggesting that Scgb3a2 gene expression is no longer under the regulation of NKX2-1. When 23 human non-small cell lung carcinoma (NSCLC) specimens were examined, SCGB3A2 expression was observed in papillary adenocarcinomas and atypical adenomatous hyperplasia, a premalignant lesion (Kurotani, Kumaki, et al., 2011). A similar correlation was also observed between high SCGB3A2 expression and low/no NKX2-1 expression in these specimens. Another human study using 156 primary human lung cancer specimens, demonstrated that SCGB3A2 is highly expressed in adenocarcinomas (87%), particularly papillary adenocarcinomas, however this study found no significant relationship between SCGB3A2 expression and tumor differentiation, pathological stage, or survival (Tachihara-Yoshikawa, et al., 2008). Another study showed that plasma levels of SCGB3A2 in patients increased in the order of pneumonia, stage I-II adenocarcinoma, and stage III-IV adenocarcinoma relative to normal controls (Li, et al., 2018). These results indicate a potential use of plasma SCGB3A2 and/or SCGB3A2 in lung cancer biopsies by immunohistochemistry as a specific marker for primary pulmonary adenocarcinomas both in mice and humans.
3-8. Other biological roles of SCGB3A2
Acrolein, an abundant component in cigarette smoke, acts on lung epithelial cells and reacts with macromolecules, leading to alteration of lung pathogenesis, cell survival, and cell signaling (Bein & Leikauf, 2011). Acrolein is thought to contribute to the development of various lung diseases including bronchitis and emphysema in chronic obstructive pulmonary disease. Acrolein increases reactive oxygen species (ROS) and phosphorylation of p53 (Ser18), resulting in increased apoptosis in mouse lung fibroblast derived MLg cells (Kurotani, et al., 2015). SCGB3A2 suppressed the acrolein-induced apoptosis through decreased phosphorylation of p53 without altering ROS levels. The results suggest that SCGB3A2 may play a role in the development of cigarette smoke-related emphysema.
SCGB3A2 immunopositive protein was found in the posterior as well as the anterior lobes of pituitary of the adult mouse pituitary gland (Miyano, et al., 2014). Because NKX2-1 is expressed only in the posterior lobe of the pituitary (Miyano, et al., 2014) and SCGB3A2 is a direct target of NKX2-1 (Niimi, et al., 2001; Tomita, et al., 2008), the involvement of transcription factors other than NKX2-1, such as C/EBPs was suggested in the regulation of the Scgb3a2 gene in the anterior pituitary (Miyano, et al., 2014). SCGB3A2 was co-localized with gonadotrophs expressing luteinizing hormone (LH) and follicular stimulating hormone (FSH) in adult mouse pituitary by immunohistochemistry (Miyano, et al., 2014). SCGB3A2 suppressed the mRNA levels of LH and FSH in rat primary anterior pituitary cells in culture (Miyano, et al., 2014). These results suggest that SCGB3A2 may regulate LH/FSH production in the anterior pituitary.
4. Conclusions and future prospects
Two decades have passed since SCGB3A2 was first identified (Niimi, et al., 2001), however, the function of this protein remains poorly understood. During this period, a number of studies have revealed multiple immunomodulatory functions of SCGB3A2, particularly in the lung, including anti-inflammatory, growth factor, anti-fibrotic, and anti-cancer activities (Figure 2). The fetal growth factor activity of SCGB3A2 could be applied to treat or prevent chronic lung disease and prevent arrested lung development in premature babies, as well as in vitro applications to culture artificial lungs for transplant. Inflammation and fibrosis are the two most prevalent conditions in the pathophysiology of most lung diseases, suggesting a wide range of applications for SCGB3A2 in the treatment and/or prevention of lung diseases. The association of the SCGB3A2 G-112A allele with Grave’s disease suggests a potential application for SCGB3A2 augmentation therapy in this disease. Finally, the anti-cancer activity of SCGB3A2 may be maximized perhaps in combination with immuno- and/or targeted therapies. Major advances will be expected in the next decade.
Acknowledgment
This work was supported by the National Cancer Institute Intramural Research Program (ZIA BC 010449).
Abbreviations
- BALF
bronchoalveolar lavage fluid
- BLM
bleomycin
- CASP
caspase
- CC10
Club cell 10-kDa protein (also known as CCSP, CC16, uteroglobin, urine protein-1)
- CCSP
Club cell secretory protein
- C/EBP
CCAAT/enhancer-binding protein
- HS
heparan sulfate
- IL
interleukin
- LLC
Lewis lung carcinoma
- LPS
lipopolysaccharide
- rh
recombinant human
- rm
recombinant mouse
- NKX2-1
homeodomain transcription factor NK2 homeobox 1
- NSCLC
non-small cell lung cancer
- SCGB
secretoglobin
- SCLC
small cell lung cancer
- SDC1
syndecan-1
- TAF
tracheal aspirate fluids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
ALP is an employee of APCBio Innovations, Inc. and has a >50% interest in APCBio Innovations, Inc., an affiliate of Trove Therapeutics, Inc., both of which have an interest in commercializing the rhSCGB3A2. No conflicts of interest, financial or otherwise, are declared by the other authors.
References
- Agusti A, & Sin DD (2014). Biomarkers in COPD. Clin Chest Med, 35, 131–141. [DOI] [PubMed] [Google Scholar]
- Almuntashiri S, Zhu Y, Han Y, Wang X, Somanath PR, & Zhang D (2020). Club Cell Secreted Protein CC16: Potential Applications in Prognosis and Therapy for Pulmonary Diseases. J Clin Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiappan AK, Yeo WS, Parate PN, Anantharaman R, Suri BK, Wang de Y, & Chew FT (2011). Variation in Uteroglobin-Related Protein 1 (UGRP1) gene is associated with allergic rhinitis in Singapore Chinese. BMC Med. Genet, 12, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredouani MS, Palecanda A, Koziel H, Huang YC, Imrich A, Sulahian TH, Ning YY, Yang Z, Pikkarainen T, Sankala M, Vargas SO, Takeya M, Tryggvason K, & Kobzik L (2005). MARCO is the major binding receptor for unopsonized particles and bacteria on human alveolar macrophages. J. Immunol, 175, 6058–6064. [DOI] [PubMed] [Google Scholar]
- Batra J, Niphadkar PV, Sharma SK, & Ghosh B (2005). Uteroglobin-related protein 1(UGRP1) gene polymorphisms and atopic asthma in the Indian population. Int. Arch. Allergy Immunol, 136, 1–6. [DOI] [PubMed] [Google Scholar]
- Bein K, & Leikauf GD (2011). Acrolein - a pulmonary hazard. Mol Nutr Food Res, 55, 1342–1360. [DOI] [PubMed] [Google Scholar]
- Bin LH, Nielson LD, Liu X, Mason RJ, & Shu HB (2003). Identification of uteroglobin-related protein 1 and macrophage scavenger receptor with collagenous structure as a lung-specific ligand-receptor pair. J. Immunol, 171, 924–930. [DOI] [PubMed] [Google Scholar]
- Broeckaert F, & Bernard A (2000). Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin. Exp. Allergy, 30, 469–475. [DOI] [PubMed] [Google Scholar]
- Cai Y, & Kimura S (2015). Secretoglobin 3A2 Exhibits Anti-Fibrotic Activity in Bleomycin-Induced Pulmonary Fibrosis Model Mice. PLoS One, 10, e0142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Winn ME, Zehmer JK, Gillette WK, Lubkowski JT, Pilon AL, & Kimura S (2014). Preclinical evaluation of human secretoglobin 3A2 in mouse models of lung development and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol, 306, L10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yoneda M, Tomita T, Kurotani R, Okamoto M, Kido T, Abe H, Mitzner W, Guha A, & Kimura S (2015). Transgenically-expressed secretoglobin 3A2 accelerates resolution of bleomycin-induced pulmonary fibrosis in mice. BMC Pulm Med, 15, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Kurotani R, Kusakabe T, Miura T, Link BW, Misawa M, & Kimura S (2006). Uteroglobin-related protein 1 expression suppresses allergic airway inflammation in mice. Am J Respir Crit Care Med, 173, 958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba Y, Kusakabe T, & Kimura S (2004). Decreased expression of uteroglobin-related protein 1 in inflamed mouse airways is mediated by IL-9. Am. J. Physiol. Lung Cell. Mol. Physiol, 287, L1193–1198. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Srisodsai A, Supavilai P, & Kimura S (2005). Interleukin-5 reduces the expression of uteroglobin-related protein (UGRP) 1 gene in allergic airway inflammation. Immunol. Lett, 97, 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Voronova NV, Turakulov RI, & Savost’anov KV (2011). The -112G>A polymorphism of the secretoglobin 3A2 (SCGB3A2) gene encoding uteroglobin-related protein 1 (UGRP1) increases risk for the development of Graves’ disease in subsets of patients with elevated levels of immunoglobulin E. J. Appl. Genet, 52, 201–207. [DOI] [PubMed] [Google Scholar]
- Chowdhury B, Mantile-Selvaggi G, Kundu GC, Miele L, Cordella-Miele E, Zhang Z, & Mukherjee AB (2000). Amino acid residues in alpha-helix-3 of human uteroglobin are critical for its phospholipase A2 inhibitory activity. Ann N Y Acad Sci, 923, 307–311. [DOI] [PubMed] [Google Scholar]
- de Burbure C, Pignatti P, Corradi M, Malerba M, Clippe A, Dumont X, Moscato G, Mutti A, & Bernard A (2007). Uteroglobin-related protein 1 and clara cell protein in induced sputum of patients with asthma and rhinitis. Chest, 131, 172–179. [DOI] [PubMed] [Google Scholar]
- Durairaj R, Pageat P, & Bienboire-Frosini C (2018). Another cat and mouse game: Deciphering the evolution of the SCGB superfamily and exploring the molecular similarity of major cat allergen Fel d 1 and mouse ABP using computational approaches. PLoS One, 13, e0197618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, & Tryggvason K (1995). Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell, 80, 603–609. [DOI] [PubMed] [Google Scholar]
- Facchiano A, Cordella-Miele E, Miele L, & Mukherjee AB (1991). Inhibition of pancreatic phospholipase A2 activity by uteroglobin and antiflammin peptides: possible mechanism of action. Life Sci, 48, 453–464. [DOI] [PubMed] [Google Scholar]
- Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, Yu T, Wu X, Shi Y, Ma P, & Shu Y (2020). Pyroptosis: A new frontier in cancer. Biomed Pharmacother, 121, 109595. [DOI] [PubMed] [Google Scholar]
- Guha A, Vasconcelos M, Cai Y, Yoneda M, Hinds A, Qian J, Li G, Dickel L, Johnson JE, Kimura S, Guo J, McMahon J, McMahon AP, & Cardoso WV (2012). Neuroepithelial body microenvironment is a niche for a distinct subset of Clara-like precursors in the developing airways. Proc. Natl. Acad. Sci. U.S.A, 109, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzmann A, Dietrich H, & Deichmann KA (2003). Association of uteroglobulin-related protein 1 with bronchial asthma. Int. Arch. Allergy Immunol, 131, 291–295. [DOI] [PubMed] [Google Scholar]
- Inoue K, Wang X, Saito J, Tanino Y, Ishida T, Iwaki D, Fujita T, Kimura S, & Munakata M (2008). Plasma UGRP1 levels associate with promoter G-112A polymorphism and the severity of asthma. Allergol. Int, 57, 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian Z, Nakayama J, Noguchi E, Shibasaki M, & Arinami T (2003). No evidence for association between the -112G/A polymorphism of UGRP1 and childhood atopic asthma. Clin. Exp. Allergy, 33, 902–904. [DOI] [PubMed] [Google Scholar]
- Jin Y, Teng W, Ben S, Xiong X, Zhang J, Xu S, Shugart YY, Jin L, Chen J, & Huang W (2003). Genome-wide scan of Graves’ disease: evidence for linkage on chromosome 5q31 in Chinese Han pedigrees. J Clin Endocrinol Metab, 88, 1798–1803. [DOI] [PubMed] [Google Scholar]
- Jones-Freeman B, & Starkey MR (2020). Bronchioalveolar stem cells in lung repair, regeneration and disease. J Pathol, 252, 219–226. [DOI] [PubMed] [Google Scholar]
- Kido T, Yoneda M, Cai Y, Matsubara T, Ward JM, & Kimura S (2014). Secretoglobin superfamily protein SCGB3A2 deficiency potentiates ovalbumin-induced allergic pulmonary inflammation. Mediators Inflamm, 2014, 216465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, & Jacks T (2005). Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell, 121, 823–835. [DOI] [PubMed] [Google Scholar]
- Kim SK, Seok H, Park HJ, Han K, Kang SW, Ban JY, Jung HJ, Kim KI, Lee BJ, Kim J, & Chung JH (2017). Association Between Secretoglobin Family 3A Member 2 (SCGB3A2) Gene Polymorphisms and Asthma in a Korean Population. Med. Sci. Monit, 23, 1880–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox CH, Ward JM, & Gonzalez FJ (1996). The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev, 10, 60–69. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H, Yamaoka A, Matsuoka C, Tokuhara T, Abe T, & Morimoto M (2021). Airway basal stem cells reutilize the embryonic proliferation regulator, Tgfβ-Id2 axis, for tissue regeneration. Dev Cell. [DOI] [PubMed] [Google Scholar]
- Klug J, Beier HM, Bernard A, Chilton BS, Fleming TP, Lehrer RI, Miele L, Pattabiraman N, & Singh G (2000). Uteroglobin/Clara cell 10-kDa family of proteins: nomenclature committee report. Ann N Y Acad Sci, 923, 348–354. [DOI] [PubMed] [Google Scholar]
- Krop I, Player A, Tablante A, Taylor-Parker M, Lahti-Domenici J, Fukuoka J, Batra SK, Papadopoulos N, Richards WG, Sugarbaker DJ, Wright RL, Shim J, Stamey TA, Sellers WR, Loda M, Meyerson M, Hruban R, Jen J, & Polyak K (2004). Frequent HIN-1 promoter methylation and lack of expression in multiple human tumor types. Mol. Cancer Res, 2, 489–494. [PubMed] [Google Scholar]
- Krop IE, Sgroi D, Porter DA, Lunetta KL, LeVangie R, Seth P, Kaelin CM, Rhei E, Bosenberg M, Schnitt S, Marks JR, Pagon Z, Belina D, Razumovic J, & Polyak K (2001). HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc. Natl. Acad. Sci. U.S.A, 98, 9796–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani R, Kumaki N, Naizhen X, Ward JM, Linnoila RI, & Kimura S (2011). Secretoglobin 3A2/uteroglobin-related protein 1 is a novel marker for pulmonary carcinoma in mice and humans. Lung Cancer, 71, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani R, Okumura S, Matsubara T, Yokoyama U, Buckley JR, Tomita T, Kezuka K, Nagano T, Esposito D, Taylor TE, Gillette WK, Ishikawa Y, Abe H, Ward JM, & Kimura S (2011). Secretoglobin 3A2 suppresses bleomycin-induced pulmonary fibrosis by transforming growth factor β signaling down-regulation. J. Biol. Chem, 286, 19682–19692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani R, Shima R, Miyano Y, Sakahara S, Matsumoto Y, Shibata Y, Abe H, & Kimura S (2015). SCGB3A2 Inhibits Acrolein-Induced Apoptosis through decreased p53 Phosphorylation. Acta Histochem Cytochem, 48, 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotani R, Tomita T, Yang Q, Carlson BA, Chen C, & Kimura S (2008). Role of secretoglobin 3A2 in lung development. Am. J. Respir. Crit. Care Med, 178, 389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kus A, Radziszewski M, Glina A, Szymanski K, Jurecka-Lubieniecka B, Pawlak-Adamska E, Kula D, Wawrusiewicz-Kurylonek N, Kus J, Miskiewicz P, Ploski R, Bolanowski M, Daroszewski J, Jarzab B, Bossowski A, & Bednarczuk T (2019). Paediatric-onset and adult-onset Graves’ disease share multiple genetic risk factors. Clin Endocrinol (Oxf), 90, 320–327. [DOI] [PubMed] [Google Scholar]
- Laucho-Contreras ME, Polverino F, Tesfaigzi Y, Pilon A, Celli BR, & Owen CA (2016). Club Cell Protein 16 (CC16) Augmentation: A Potential Disease-modifying Approach for Chronic Obstructive Pulmonary Disease (COPD). Expert Opin Ther Targets, 20, 869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YC, Zhang Z, & Mukherjee AB (2006). Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett, 580, 4515–4520. [DOI] [PubMed] [Google Scholar]
- Levine CR, Gewolb IH, Allen K, Welch RW, Melby JM, Pollack S, Shaffer T, Pilon AL, & Davis JM (2005). The safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. Pediatr Res, 58, 15–21. [DOI] [PubMed] [Google Scholar]
- Li W, Zheng H, Qin H, Liu G, Ke L, Li Y, Li N, & Zhong X (2018). Exploration of differentially expressed plasma proteins in patients with lung adenocarcinoma using iTRAQ-coupled 2D LC-MS/MS. Clin Respir J, 12, 2036–2045. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu K, Cui G, Huang X, Yao S, Guo W, Qin Z, Li Y, Yang R, Pu W, Zhang L, He L, Zhao H, Yu W, Tang M, Tian X, Cai D, Nie Y, Hu S, Ren T, Qiao Z, Huang H, Zeng YA, Jing N, Peng G, Ji H, & Zhou B (2019). Lung regeneration by multipotent stem cells residing at the bronchioalveolar-duct junction. Nat Genet, 51, 728–738. [DOI] [PubMed] [Google Scholar]
- Lu X, Wang N, Long XB, You XJ, Cui YH, & Liu Z (2011). The cytokine-driven regulation of secretoglobins in normal human upper airway and their expression, particularly that of uteroglobin-related protein 1, in chronic rhinosinusitis. Respir. Res, 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RA (2014). Introduction to genetics and genomics in asthma: genetics of asthma. Adv Exp Med Biol, 795, 125–155. [DOI] [PubMed] [Google Scholar]
- McCauley KB, Alysandratos KD, Jacob A, Hawkins F, Caballero IS, Vedaie M, Yang W, Slovik KJ, Morley M, Carraro G, Kook S, Guttentag SH, Stripp BR, Morrisey EE, & Kotton DN (2018). Single-Cell Transcriptomic Profiling of Pluripotent Stem Cell-Derived SCGB3A2+ Airway Epithelium. Stem Cell Reports, 10, 1579–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miele L, Cordella-Miele E, Facchiano A, & Mukherjee AB (1988). Novel anti-inflammatory peptides from the region of highest similarity between uteroglobin and lipocortin I. Nature, 335, 726–730. [DOI] [PubMed] [Google Scholar]
- Miyano Y, Tahara S, Sakata I, Sakai T, Abe H, Kimura S, & Kurotani R (2014). Regulation of LH/FSH expression by secretoglobin 3A2 in the mouse pituitary gland. Cell Tissue Res, 356, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootz M, Jakwerth CA, Schmidt-Weber CB, & Zissler UM (2021). Secretoglobins in the big picture of immunoregulation in airway diseases. Allergy. [DOI] [PubMed] [Google Scholar]
- Mukherjee AB, Kundu GC, Mantile-Selvaggi G, Yuan CJ, Mandal AK, Chattopadhyay S, Zheng F, Pattabiraman N, & Zhang Z (1999). Uteroglobin: a novel cytokine? Cell Mol Life Sci, 55, 771–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee AB, Zhang Z, & Chilton BS (2007). Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev, 28, 707–725. [DOI] [PubMed] [Google Scholar]
- Nagarajan K, Soundarapandian K, Thorne RF, Li D, & Li D (2019). Activation of Pyroptotic Cell Death Pathways in Cancer: An Alternative Therapeutic Approach. Transl Oncol, 12, 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naizhen X, Kido T, Yokoyama S, Linnoila RI, & Kimura S (2019). Spatiotemporal Expression of Three Secretoglobin Proteins, SCGB1A1, SCGB3A1, and SCGB3A2, in Mouse Airway Epithelia. J Histochem Cytochem, 67, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi T, Copeland NG, Gilbert DJ, Jenkins NA, Srisodsai A, Zimonjic DB, Keck-Waggoner CL, Popescu NC, & Kimura S (2002). Cloning, expression, and chromosomal localization of the mouse gene (Scgb3a1, alias Ugrp2) that encodes a member of the novel uteroglobin-related protein gene family. Cytogenet. Genome Res, 97, 120–127. [DOI] [PubMed] [Google Scholar]
- Niimi T, Keck-Waggoner CL, Popescu NC, Zhou Y, Levitt RC, & Kimura S (2001). UGRP1, a uteroglobin/Clara cell secretory protein-related protein, is a novel lung-enriched downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor. Mol. Endocrinol, 15, 2021–2036. [DOI] [PubMed] [Google Scholar]
- Niimi T, Munakata M, Keck-Waggoner CL, Popescu NC, Levitt RC, Hisada M, & Kimura S (2002). A polymorphism in the human UGRP1 gene promoter that regulates transcription is associated with an increased risk of asthma. Am. J. Hum. Genet, 70, 718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D, Lahti-Domenici J, Torres-Arzayus M, Chin L, & Polyak K (2002). Expression of high in normal-1 (HIN-1) and uteroglobin related protein-1 (UGRP-1) in adult and developing tissues. Mech. Dev, 114, 201–204. [DOI] [PubMed] [Google Scholar]
- Rathinam VAK, Zhao Y, & Shao F (2019). Innate immunity to intracellular LPS. Nat Immunol, 20, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, & Hogan BL (2009). The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell, 4, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Reynolds PR, Pryhuber GS, Finder JD, & Stripp BR (2002). Secretoglobins SCGB3A1 and SCGB3A2 define secretory cell subsets in mouse and human airways. Am. J. Respir. Crit. Care Med, 166, 1498–1509. [DOI] [PubMed] [Google Scholar]
- Rigoli L, Di Bella C, Procopio V, Finocchiaro G, Amorini M, Lo Giudice G, Cuppari C, & Salpietro CD (2007). Uteroglobin-related protein 1 gene -112G/A polymorphism and atopic asthma in Sicilian children. Allergy Asthma Proc, 28, 667–670. [DOI] [PubMed] [Google Scholar]
- Sakai K, Shirasawa S, Ishikawa N, Ito K, Tamai H, Kuma K, Akamizu T, Tanimura M, Furugaki K, Yamamoto K, & Sasazuki T (2001). Identification of susceptibility loci for autoimmune thyroid disease to 5q31-q33 and Hashimoto’s thyroiditis to 8q23-q24 by multipoint affected sib-pair linkage analysis in Japanese. Hum Mol Genet, 10, 1379–1386. [DOI] [PubMed] [Google Scholar]
- Simmonds MJ, Yesmin K, Newby PR, Brand OJ, Franklyn JA, & Gough SC (2010). Confirmation of association of chromosome 5q31–33 with United Kingdom Caucasian Graves’ disease. Thyroid, 20, 413–417. [DOI] [PubMed] [Google Scholar]
- Skirecki T, & Cavaillon JM (2019). Inner sensors of endotoxin - implications for sepsis research and therapy. FEMS Microbiol Rev, 43, 239–256. [DOI] [PubMed] [Google Scholar]
- Song HD, Liang J, Shi JY, Zhao SX, Liu Z, Zhao JJ, Peng YD, Gao GQ, Tao J, Pan CM, Shao L, Cheng F, Wang Y, Yuan GY, Xu C, Han B, Huang W, Chu X, Chen Y, Sheng Y, Li RY, Su Q, Gao L, Jia WP, Jin L, Chen MD, Chen SJ, Chen Z, & Chen JL (2009). Functional SNPs in the SCGB3A2 promoter are associated with susceptibility to Graves’ disease. Hum. Mol. Genet, 18, 1156–1170. [DOI] [PubMed] [Google Scholar]
- Srisodsai A, Kurotani R, Chiba Y, Sheikh F, Young HA, Donnelly RP, & Kimura S (2004). Interleukin-10 induces uteroglobin-related protein (UGRP) 1 gene expression in lung epithelial cells through homeodomain transcription factor T/EBP/NKX2.1. J. Biol. Chem, 279, 54358–54368. [DOI] [PubMed] [Google Scholar]
- Tachihara-Yoshikawa M, Ishida T, Watanabe K, Sugawara A, Kanazawa K, Kanno R, Suzuki T, Niimi T, Kimura S, & Munakata M (2008). Expression of secretoglobin3A2 (SCGB3A2) in primary pulmonary carcinomas. Fukushima J Med Sci, 54, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Kido T, Kurotani R, Iemura S, Sterneck E, Natsume T, Vinson C, & Kimura S (2008). CAATT/enhancer-binding proteins alpha and delta interact with NKX2-1 to synergistically activate mouse secretoglobin 3A2 gene expression. J. Biol. Chem, 283, 25617–25627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Yamada S, Hirai K, Asakura H, & Kanda Y (2021). Development of alveolar and airway cells from human iPS cells: toward SARS-CoV-2 research and drug toxicity testing. J Toxicol Sci, 46, 425–435. [DOI] [PubMed] [Google Scholar]
- Van De Velde V, Courtens W, & Bernard A (2010). Development of a new sensitive ELISA for the determination of uteroglobin-related protein 1, a new potential biomarker. Biomarkers, 15, 619–624. [DOI] [PubMed] [Google Scholar]
- Wang X, Tanino Y, Sato S, Nikaido T, Misa K, Fukuhara N, Fukuhara A, Saito J, Yokouchi H, Ishida T, Fujita T, & Munakata M (2015). Secretoglobin 3A2 attenuates lipopolysaccharide-induced inflammation through inhibition of ERK and JNK pathways in bronchial epithelial cells. Inflammation, 38, 828–834. [DOI] [PubMed] [Google Scholar]
- Watson TM, Reynolds SD, Mango GW, Boe IM, Lund J, & Stripp BR (2001). Altered lung gene expression in CCSP-null mice suggests immunoregulatory roles for Clara cells. Am. J. Physiol. Lung Cell. Mol. Physiol, 281, L1523–1530. [DOI] [PubMed] [Google Scholar]
- Wong AP, Keating A, & Waddell TK (2009). Airway regeneration: the role of the Clara cell secretory protein and the cells that express it. Cytotherapy, 11, 676–687. [DOI] [PubMed] [Google Scholar]
- Xie H, Wu M, Shen B, Niu Y, Huo Y, & Cheng Y (2014). Association between the -112G/A polymorphism of uteroglobulin-related protein 1 gene and asthma risk: A meta-analysis. Exp Ther Med, 7, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Han B, Pan C, & Song H (2014). The association of SCGB3A2 polymorphisms with the risk of Graves’ disease: a meta-analysis. Endocrine, 45, 365–369. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lingling S, Ying J, Yushu L, Zhongyan S, Wei H, & Weiping T (2005). Association study between the IL4, IL13, IRF1 and UGRP1 genes in chromosomal 5q31 region and Chinese Graves’ disease. J. Hum. Genet, 50, 574–582. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Cai Y, Murata M, Tomita T, Yoneda M, Xu L, Pilon AL, Cachau RE, & Kimura S (2018). A novel pathway of LPS uptake through syndecan-1 leading to pyroptotic cell death. eLife, 7, e37854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Nakayama S, Xu L, Pilon AL, & Kimura S (2021). Secretoglobin 3A2 eliminates human cancer cells through pyroptosis. Cell Death Discov, 7, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda M, Xu L, Kajiyama H, Kawabe S, Paiz J, Ward JM, & Kimura S (2016). Secretoglobin Superfamily Protein SCGB3A2 Alleviates House Dust Mite-Induced Allergic Airway Inflammation in Mice. Int. Arch. Allergy Immunol, 171, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Insel M, Addison KJ, Stern DA, Pederson W, Dy A, Rojas-Quintero J, Owen CA, Sherrill DL, Morgan W, Wright AL, Halonen M, Martinez FD, Kraft M, Guerra S, & Ledford JG (2019). Club Cell Secretory Protein Deficiency Leads to Altered Lung Function. Am J Respir Crit Care Med, 199, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zuo CL, Li XS, Ye XP, Zhang QY, Wang P, Zhang RX, Chen G, Yang JL, Chen Y, Ma QY, & Song HD (2019). Uterus globulin associated protein 1 (UGRP1) is a potential marker of progression of Graves’ disease into hypothyroidism. Mol Cell Endocrinol, 494, 110492. [DOI] [PubMed] [Google Scholar]
- Zuo WL, Rostami MR, LeBlanc M, Kaner RJ, O’Beirne SL, Mezey JG, Leopold PL, Quast K, Visvanathan S, Fine JS, Thomas MJ, & Crystal RG (2020). Dysregulation of club cell biology in idiopathic pulmonary fibrosis. PLoS One, 15, e0237529. [DOI] [PMC free article] [PubMed] [Google Scholar]


