Abstract
Immune checkpoint inhibitors (ICIs) have transformed the treatment paradigm for several malignancies. While the use of single-agent or combined ICIs has achieved acceptable disease control rates in a variety of solid tumors, such approaches have yet to show substantial therapeutic efficacy in select difficult-to-treat cancer types. Recently, select chemotherapy regimens are emerging as extensive modifiers of the tumor microenvironment, leading to the reprogramming of local immune responses. Accordingly, data is now emerging to suggest that certain anti-neoplastic agents modulate various immune cell processes, most notably the cross-presentation of tumor antigens, leukocyte trafficking, and cytokine biosynthesis. As such, the combination of ICIs and cytotoxic chemotherapy are beginning to show promise in many cancers that have long been considered poorly responsive to ICI-based immunotherapy. Here, we discuss past and present attempts to advance chemo-immunotherapy in these difficult-to-treat cancer histologies, mechanisms through which select chemotherapies modify tumor immunogenicity, as well as important considerations when designing such approaches to maximize efficacy and improve therapeutic response rates.
Keywords: Cancer, chemotherapy, immunology, immune checkpoint inhibitors, tumor microenvironment
1 –. INTRODUCTION
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy in the last decade and are now the preferred first-line treatment for several solid cancers. ICI-based immunotherapy consists of neutralizing antibodies against surface proteins that serve to negatively regulate immune function, most notably cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), programmed cell death protein 1 (PD-1), and PD-1 ligand 1 (PD-L1), thus impeding the ability of tumor cells to evade the cytotoxic immune program (Wei, Duffy, & Allison, 2018). These approaches have demonstrated substantial anti-tumor activity in most cancers (Borghaei, et al., 2015; Darvin, Toor, Sasidharan Nair, & Elkord, 2018; Garon, et al., 2015; Gibney, Weiner, & Atkins, 2016; Hodi, et al., 2010; Larkin, et al., 2015; Robert, et al., 2011), and have largely replaced chemotherapy as the preferred treatment for select cancers including melanoma, renal cell carcinoma, and others (Robert, 2020). However, despite significant advances in cancer immunotherapy, there are several cancer types in which ICIs have yet to show significant single-agent efficacy. Additionally, the development of ICI-refractory disease remains a pressing issue in clinical oncology, as many patients initially able to achieve adequate disease control with ICI-based immunotherapy may eventually progress on treatment and require additional line therapy (Barrueto, et al., 2020).
Contrasting the long-held belief that chemotherapy is immunosuppressive, mounting evidence suggests several cytotoxic chemotherapy regimens have various immunostimulatory effects, leading to extensive reprogramming of the tumor immune microenvironment and potentiating therapeutic responses to immunotherapy (Bracci, Schiavoni, Sistigu, & Belardelli, 2014; Emens, 2008; Opzoomer, Sosnowska, Anstee, Spicer, & Arnold, 2019; Wargo, Reuben, Cooper, Oh, & Sullivan, 2015). Accordingly, several chemo-immunotherapy regimens have now been approved by the FDA, with others showing early promise in clinical trials. In this review, we discuss past and present advances in chemo-immunotherapy, with a particular emphasis on difficult-to-treat cancer histologies. Additionally, we describe the many mechanisms through which chemotherapy can modify the tumor immune microenvironment and how this can be utilized in novel combinations to maximize treatment efficacy and improve therapeutic response rates for cancers in which ICIs have yet to show significant benefit.
2 –. EARLY RATIONALE FOR COMBINING CHEMOTHERAPY AND IMMUNOTHERAPY
The intersection between chemotherapy and immunotherapy has long been under clinical evaluation. Though initial reports showed no added benefit to combining Bacille Calmette-Guérin (BCG) and broad-spectrum chemotherapy (Jacquillat, Banzet, & Maral, 1982), several subsequent trials sought to exploit preclinical observations that select chemotherapy agents can deplete what would come to be known as regulatory T-lymphocytes (Tregs), thereby promoting local immune responses. Tregs comprise a specialized T-cell subpopulation that acts to inhibit sterilizing immune responses and promote peripheral tolerance (Kondelkova, et al., 2010), and several have hypothesized that a reduction in tumor-associated Tregs may augment responses to cancer immunotherapy. Much of the early research on chemotherapy-induced Treg-depletion focused on cyclophosphamide, an alkylating agent belonging to the nitrogen mustard family (Ahmed & Hombal, 1984; Hughes, et al., 2018).
Tregs are highly sensitive to cyclophosphamide, particularly when compared to cytotoxic T lymphocytes (CTLs) and helper T cells (Heylmann, et al., 2013). Thus, cyclophosphamide has long been suggested as a potential means of targeting Tregs to potentiate cancer immunotherapy (Figure 1). This concept has been under investigation for nearly 40 years, following the 1982 observation that cyclophosphamide enhances the efficacy of adoptive transfer in the Meth A fibrosarcoma mouse model (North, 1982). More recent studies have confirmed that cyclophosphamide-mediated Treg depletion can enhance the efficacy of immunotherapy in a rat model of implanted PROb colon cancer cells (Ghiringhelli, et al., 2004), potentiate non-myeloablative allogeneic stem cell transplantation through increased activation of autoreactive T-cells (Takeuchi, et al., 2012), and increase the frequency of active T-cell infiltration in tumor-bearing mice (P. Liu, Jaffar, Hellstrom, & Hellstrom, 2010).
Figure 1. Cyclophosphamide-mediated depletion of regulatory T-lymphocytes.
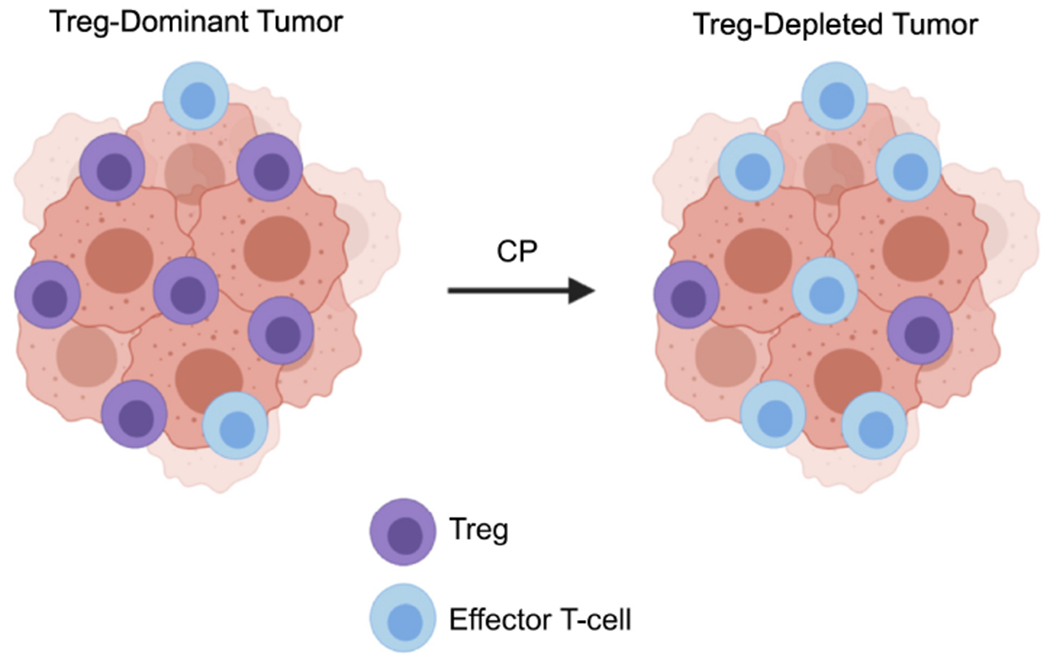
The immunogenic effects of cyclophosphamide (CP) are well documented, and much of the early rationale for combining chemotherapy and immunotherapy stemmed from observations that cyclophosphamide chemotherapy can deplete tumor-associated regulatory T-lymphocytes (Tregs). This specialized T-cell subpopulation acts to inhibit sterilizing immune responses and maintain peripheral tolerance. Tregs are highly sensitive to cyclophosphamide compared to effector T-cells, and cyclophosphamide has long been suggested as a potential means of targeting Tregs to potentiate cancer immunotherapy.
Though these and several other studies support cyclophosphamide as an immune modifier, translating these findings to clinical practice has been difficult. Very early reports indicated that treatment with cyclophosphamide can enhance the development of delayed-type hypersensitivity responses in otherwise unreactive patients with metastatic colorectal cancer or melanoma (Berd, Mastrangelo, Engstrom, Paul, & Maguire, 1982). Subsequent studies suggested that cyclophosphamide can enhance local immune cues and/or deplete suppressive CD4 responses (Berd, Maguire, & Mastrangelo, 1984a, 1984b; Berd & Mastrangelo, 1987, 1988). This led to trials using cyclophosphamide as an adjuvant to therapeutic vaccines, first in melanoma. In a 1986 trial, pre-treatment with cyclophosphamide followed by an autologous melanoma cell vaccine led to objective clinical responses in only 2/19 patients (Berd, Maguire, & Mastrangelo, 1986), despite observations that cyclophosphamide can impede suppressor cell responses (Hoon, Foshag, Nizze, Bohman, & Morton, 1990). Similarly, cyclophosphamide failed to meaningfully enhance immune responses to a melanoma antigen vaccine, inducing delayed-type hypersensitivity responses in 15/18 patients compared to the 16/22 receiving only the vaccine, with no difference in overall or disease-free survival between groups (Oratz, et al., 1991).
Subsequent trials have also shown mixed results. A larger trial of 64 melanoma patients reported that the combination of cyclophosphamide and a therapeutic vaccine led to clinical responses in 5/40 evaluable patients with a median duration of 10 months (Berd, Maguire, McCue, & Mastrangelo, 1990). In a cohort of 121 patients with stage III melanoma, adjuvant cyclophosphamide and vaccination with either a GM2 ganglioside or GM2 ganglioside plus BCG showed poor efficacy, failing to show a statistically significant improvement in disease-free or overall survival (Livingston, et al., 1994). Cyclophosphamide was next combined with a therapeutic lyophilized melanoma vaccine and interferon α (IFNα), and of the 39 evaluable patients, 10.2% showed a response with a median time to progression of 8 months (Vaishampayan, Abrams, Darrah, Jones, & Mitchell, 2002). Subsequent trials have explored cyclophosphamide in combination with several other immunotherapies, though overall therapeutic efficacy has been relatively modest (Alexandru, Van Horn, & Bota, 2010; Audia, et al., 2007; Dudley, et al., 2005; Dudley, et al., 2008; Emens, et al., 2009; Ghiringhelli, et al., 2007; Holtl, et al., 2005; Ladoire, et al., 2010; Laheru, et al., 2008; Nistico, et al., 2009). Hence, while cyclophosphamide is still being evaluated in combination with newer immunotherapy approaches, research has primarily shifted to explore the immunomodulatory effects of several other chemotherapy agents, as discussed in detail below.
3 –. CHEMOTHERAPY-INDUCED ANTIGEN PRESENTATION
As ICIs have become a cornerstone of cancer therapy, there is an ever-growing interest in identifying new ways to predict treatment responses. Recent evidence suggests that select cancers with a high tumor mutational burden (TMB-H) are likely to be sensitive to ICIs, particularly when PD-L1 positive (Chan, et al., 2019). Similarly, several tumors deficient in DNA mismatch repair (dMMR) with high microsatellite instability (MSI-H) are sensitive to anti-PD-1 therapy (Le, et al., 2017), leading to the tissue-agnostic FDA approval of the anti-PD-1 antibody Pembrolizumab for MSI-H/dMMR solid tumors in 2017. The sensitivity of MSI-H/dMMR tumors to ICIs is primarily due to the presumptive increase in mutational burden, leading to a high prevalence of abnormal peptides (Le, et al., 2017). These endogenous proteins can be subjected to proteasomal degradation into peptides, some of which can be further trimmed by cytosolic proteases. These peptides then translocate to the endoplasmic reticulum (ER) via Transporters associated with Antigen Processing (TAP). They can undergo additional processing by ER Aminopeptidase (ERAP), as either free peptides or after being loaded onto a class I Human Leukocyte Antigen (HLA-I) molecule (Major Histocompatibility Complex or MHC in mice). In brief, once loaded onto an HLA-I molecule, a complex consisting of the HLA-I heavy chain, a β2-microglobulin (β2m), and the antigenic peptide is exported to the cell surface where it is presented for recognition by primed CD8+ CTLs (Jhunjhunwala, Hammer, & Delamarre, 2021).
However, for the majority of tumors that are not MSI-H/dMMR, diminished antigen presentation can be a significant barrier to the therapeutic efficacy of ICI-based immunotherapy, particularly for those with a low mutational burden such as pancreatic ductal adenocarcinoma (PDAC) (Principe, Korc, Kamath, Munshi, & Rana, 2021). Several reports suggest that chemotherapy can enhance the antigen presentation capacity of tumor cells, as discussed below. This potentially allows for more efficient priming of CD8+ CTLs and improving therapeutic responses to ICI-based immunotherapy, particularly for poorly immunogenic cancers (Figure 2).
Figure 2. Chemotherapy-induced HLA Class I expression enhances anti-tumor immune responses.
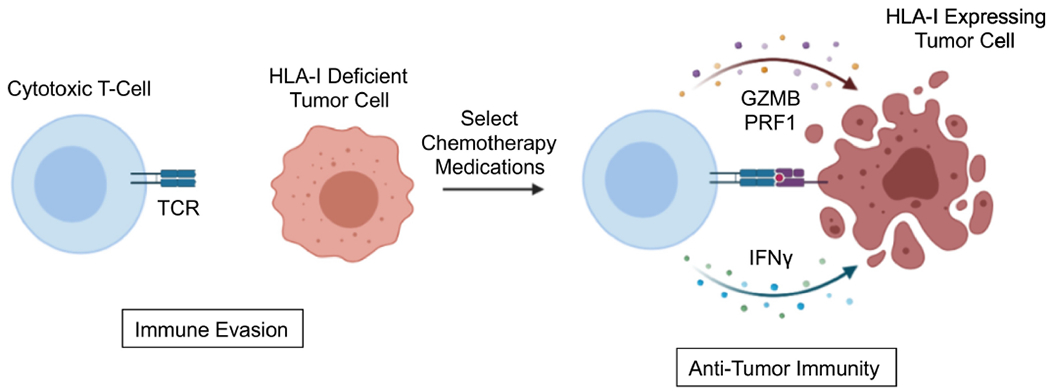
The loss of HLA Class I (HLA-I) is a widely utilized mechanism for immune evasion in cancer, impeding the ability of cytotoxic T-lymphocytes to recognize and destroy tumor cells. Several chemotherapy agents have been shown to augment tumor cell expression of HLA-I. While most studies to date have focused on platinum-based medications such as cisplatin and oxaliplatin, emerging data also supports a pro-antigen presentation role for non-platinum-based chemotherapy. Thus, by increasing the expression of HLA-I on the tumor cell surface, chemotherapy can potentially lead to the enhanced antigen presentation capacity of tumor cells, thereby allowing for more efficient priming of cytotoxic T-cells and improving therapeutic responses to ICI-based immunotherapy. Abbreviations: T-cell receptor (TCR); Granzyme B (GZMB), Perforin 1 (PRF1); Interferon γ (IFNγ).
3.1 –. Platinum-based Chemotherapy
Platinum-based chemotherapy such as cisplatin, carboplatin, and oxaliplatin are widely used to treat several cancers. These drugs exert their cytotoxic effects mainly by interacting with select guanine moieties of DNA, resulting in intrastrand crosslinks (Di Francesco, Ruggiero, & Riccardi, 2002), impeding strand replication and transcription (Woynarowski, Chapman, Napier, Herzig, & Juniewicz, 1998). However, in addition to their tumoricidal activity, these medications have extensive and well-studied effects on local immune responses, many of which can enhance antigen availability within the tumor microenvironment (TME) (Table 1) (Rebe, Demontoux, Pilot, & Ghiringhelli, 2019).
Table 1.
Select studies exploring the effects of platinum-based chemotherapy on tumor cell expression of antigen presenting molecules
| Chemotherapy | Cancer Type | Effect on Antigen Presentation | Notes | Reference |
|---|---|---|---|---|
| Cisplatin | Breast | Increased MHC class I | - | (Wan, et al., 2012) |
| Colon | Increased MHC class I | - | (Ohtsukasa, et al., 2003). | |
| Esophageal | Increased HLA-I | Combined with 5-FU | (Tsuchikawa, et al., 2012). | |
| Head & Neck | Increased MHC class I | - | (S. J. Park, et al., 2019). | |
| Head & Neck | Increased antigen presentation via MHC class I | - | (Tran, et al., 2017). | |
| Lung | Enhanced sensitivity to HLA-restricted cell death | Combined with Vinorelbine | (Gameiro, et al., 2012) | |
| Mesothelioma | Broadened the range of tumor antigens presented on MHC class I | - | (Jackaman, et al., 2012) | |
| Plasmacytoma | Increased MHC class I antigens | - | (Nio, et al., 2000). | |
|
| ||||
| Oxaliplatin | Colon | Increased mesothelin tumor antigens | (Galaine, et al., 2019) | |
| Colon | Increased antigen processing/presentation and MHC class I | - | (Y. Zhou, et al., 2021) | |
| Head & Neck | Increased MHC class I | - | (S. J. Park, et al., 2019). | |
| Liver | Increased HLA-I in tumors lacking an oncogenic RAS mutation | - | (Ledys, et al., 2018) | |
| Pancreas | Increased HLA-I | - | (Principe, et al., 2020) | |
| Prostate | Increased antigen processing/presentation and expression of MHC class I | - | (Y. Zhou, et al., 2021) | |
|
| ||||
| Carboplatin | Ovarian | Increased HLA-I | - | (Alagkiozidis, et al., 2011) |
The immuno-stimulatory effects of cisplatin are perhaps the best-studied among the platinum-based antineoplastics, directing various immune cell processes, including antigen presentation (de Biasi, Villena-Vargas, & Adusumilli, 2014). This increase in antigen presentation has been suggested as a potential means through which cisplatin can potentiate anti-tumor immune responses alone or when combined with immunotherapy (Spanos, et al., 2009; Tseng, et al., 2008). For example, early reports demonstrate that cisplatin enhances tumor expression of MHC class I in colon cancer cells, exceeding that induced by non-platinum chemotherapy agents 5-fluorouracil (5-FU) or the irinotecan metabolite SN-38 in vivo (Ohtsukasa, Okabe, Yamashita, Iwai, & Sugihara, 2003). Similarly, in BALB/c mice inoculated with MOPC 104E plasmacytoma cells, cisplatin enhanced anti-tumor immune responses, increasing the expression of MHC class I antigens, but not that of MHC class II antigens (Nio, et al., 2000).
In patients with esophageal squamous cell carcinoma, the combination of neoadjuvant cisplatin and 5-FU led to a substantial increase in tumor-infiltrating CD4+ and CD8+ T-cells, as well as an increase in HLA-I expression when compared to chemo-naïve patients (Tsuchikawa, et al., 2012). In lung cancer cells, sublethal exposure to cisplatin and vinorelbine enhanced sensitivity to HLA-restricted cell death induced by CTLs, consistent with the enhanced presentation of tumor antigen (Gameiro, Caballero, & Hodge, 2012). Cisplatin also enhanced MHC class I expression in breast cancer cells (Wan, et al., 2012) and broadened the range of tumor antigens presented to CD8+ CTLs in a model of murine mesothelioma, suggesting that cisplatin reveals weaker tumor antigens to the cytotoxic arm of the immune system and may cooperate with cancer immunotherapy (Jackaman, Majewski, Fox, Nowak, & Nelson, 2012). This hypothesis is supported by recent preclinical observations in head and neck squamous cell carcinoma (HNSCC), where cisplatin enhanced antigen presentation and T-cell killing in vitro and cooperated with anti-PD-L1/PD-1 in vivo (Tran, et al., 2017).
Oxaliplatin also appears to have immunomodulatory effects in tumor cells, increasing antigenicity and promoting adaptive immune responses (W. M. Liu, Fowler, Smith, & Dalgleish, 2010). Cisplatin and oxaliplatin have been suggested to induce similar immune alterations, also in preclinical models of HNSCC. Specifically, both increased surface expression of MHC class I and enhanced therapeutic responses to anti-PD-1 in vivo (S. J. Park, et al., 2019). In colon cancer cells, oxaliplatin resistance led to the differential expression of telomerase reverse transcriptase (TERT), cytochrome C oxidase assembly factor 1 (COA-1), and mesothelin tumor antigens, leading to tumor-cell targeting by antigen-specific CD4+ T-cells (Galaine, et al., 2019). This appears to be relevant clinically as high-risk rectal patients undergoing neoadjuvant oxaliplatin-based chemotherapy demonstrated systemic immune responses associated with improved overall survival (Kalanxhi, et al., 2018). Similarly, in patients with metastatic hepatocellular carcinoma (HCC), neoadjuvant oxaliplatin-based chemotherapy enhanced both HLA-I and PD-L1 expression in tumors lacking an oncogenic RAS mutation, and the degree of immune response was associated with improved survival (Ledys, et al., 2018).
A recent study has offered a potential mechanism for oxaliplatin-induced antigen presentation. The lysine acetyltransferases p300/CREB-binding protein (CBP) appear to control the expression of MHC class I, as well as direct antigen processing/presentation, thereby controlling the abundance of neoantigen in tumor cells. The authors further demonstrated that, through an NF-κB-dependent mechanism, oxaliplatin enhanced p300-mediated upregulation of MHC class I proteins, independent of IFNγ. Thus, oxaliplatin may enhance antigen presentation by overcoming epigenetic downregulation of MHC class I (Y. Zhou, et al., 2021).
Carboplatin may also enhance antigen presentation, though this is less established than with other platinum-based chemotherapies. Carboplatin appears to have immunogenic properties (Braly, et al., 2009) and increases HLA-I expression in ovarian cancer cells (Alagkiozidis, et al., 2011). However, in carboplatin-resistant ovarian cancer patients, increased HLA-I expression is associated with improved overall survival (Shehata, et al., 2009). Hence, the immunostimulatory effects of carboplatin warrant continued exploration, particularly in light of observations for structurally and functionally similar medications.
Beyond modulating antigen processing and presentation, platinum-based chemotherapy can also mobilize tumor-antigen by inducing immunogenic cell death (Figure 3). This process leads to increased damage-associated molecular patterns (DAMPs) within the TME, increasing the availability of tumor-antigen to professional antigen-presenting cells (APCs), thereby enhancing T-cell activation (Galluzzi, Buque, Kepp, Zitvogel, & Kroemer, 2017; Zhou, et al., 2019). The mechanisms through which cells undergo immunogenic cell death are diverse and highly complicated and have been linked to any number of cellular events, including exposure of calreticulin and other endoplasmic reticulum chaperones on the cell surface, autophagy and subsequent ATP release, Toll-like receptor 3 (TLR3) activation, as well as several cytokines and immune modulators including interleukin-1β (IL-1β), type I interferon (IFN), and CXCL10 (Galluzzi, et al., 2017; Zhou, et al., 2019).
Figure 3. Chemotherapy-induced immunogenic cell death mobilizes tumor-antigen.
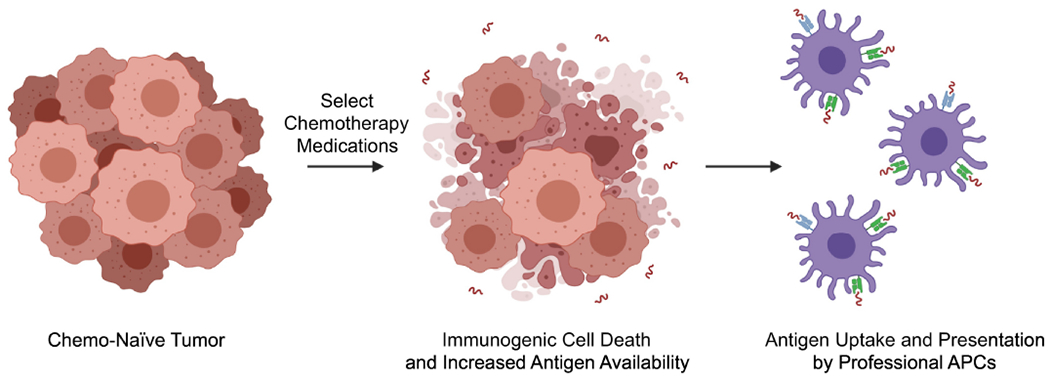
In addition to enhancing HLA Class I-dependent antigen presentation, several chemotherapy medications can also promote immunogenic cell death. This process improves the availability of damage-associated molecular patterns (DAMPs) within the TME, thereby increasing the availability of tumor-antigen to professional antigen-presenting cells (APCs) and augmenting anti-tumor T-cell responses. The mechanisms through which chemotherapy induces immunogenic cell death are complex and, like more conventional antigen presentation, are best studied for platinum-based medications.
In a seminal 2010 study, both oxaliplatin and cisplatin were shown to trigger the release of high-mobility group box 1 protein (HMGB1), an obligate step for immunogenic cell death (Tesniere, et al., 2010). However, only oxaliplatin stimulated the pre-apoptotic release of calreticulin. As both steps are required for immunogenic cell death, they determined that oxaliplatin was a more potent inducer of immunogenic cell death than cisplatin. In subcutaneous xenograft experiments, oxaliplatin-treated tumor cells induced a functional anti-cancer immune response that was mitigated by the silencing of calreticulin or HMGB1. Conversely, cisplatin failed to promote an anti-tumor immune response in vivo unless cells were supplemented with calreticulin. The authors further demonstrate that oxaliplatin-induced immunogenic cell death requires the HMGB1 receptor Toll-like receptor 4 (TLR4). Patients with a loss-of-function mutation to TLR4 display poorer progression-free and overall survival when treated with oxaliplatin-based chemotherapy (Tesniere, et al., 2010). In a subsequent study from this group, the authors expanded on the failure of cisplatin to induce immunogenic cell death, which they attributed to an inability to activate protein kinase-like ER kinase (PERK)-dependent phosphorylation of eukaryotic translation initiation factor 2α (eIF2α). Cisplatin similarly failed to stimulate the formation of stress granules and macroautophagy, though this was reversed by the addition of ER stress-inducers thapsigargin or tunicamycin (I. Martins, et al., 2011). Hence, this approach warrants continued exploration.
While the ability of cisplatin to induce immunogenic cell death is still emerging, oxaliplatin is a well-established and robust inducer of immunogenic cell death, the degree of which is closely linked to its therapeutic efficacy (Tesniere, et al., 2010). In addition to triggering calreticulin and HMGB1, oxaliplatin-induced immunogenic cell death also involves several other mechanisms, including autophagy. Autophagy is dispensable for chemotherapy-induced cell death but is required for chemotherapy-induced trafficking of T-lymphocytes and dendritic cells (Michaud, et al., 2011). Knockdown of autophagy-related genes severely impairs pre-apoptotic secretion of ATP by tumor cells undergoing immunogenic cell death, and only autophagy-proficient tumor cells can induce anti-cancer immune responses in vivo (I. Martins, et al., 2012). Consistent with these observations, while autophagy-deficient tumor cells exposed calreticulin, released HMGB1, and underwent apoptosis in response to oxaliplatin, they secreted less ATP than autophagy-proficient controls. As autophagic cell death is often disabled in tumor cells, this may impair chemotherapy-induced cell death, including that induced by oxaliplatin, and strategies to compensate for this diminished ATP release warrant additional exploration (Michaud, et al., 2011).
3.2 –. Non-Platinum-based Chemotherapy
Though platinum-based agents are perhaps the best-studied regarding chemotherapy-induced antigen presentation, several non-platinum medications have also been implicated in regulating the cross-presentation of tumor antigen (Table 2) and immunogenic cell death. For example, there is mounting evidence that the nucleoside analog gemcitabine (2′, 2′-difluoro 2′deoxycytidine or dFdC) has several effects on tumor cell antigenicity (Gravett, Trautwein, Stevanovic, Dalgleish, & Copier, 2018). Gemcitabine has been shown to enhance HLA-I expression in lung, breast, and colon cancer cells in vitro, as well as subcutaneous xenografts of colon cancer cells in vivo (W. M. Liu, et al., 2010). The effect of gemcitabine on HLA-I expression surpassed that of other medications in most cell lines, including cyclophosphamide and oxaliplatin (W. M. Liu, et al., 2010). Similarly, in B16 melanoma tumors, gemcitabine improved the cross-presentation efficiency of nuclear antigen in a dose-dependent manner (Anyaegbu, Lake, Heel, Robinson, & Fisher, 2014). The effects of gemcitabine on HLA-I have been confirmed in a similar study that determined that gemcitabine also altered the peptides eluted from HLA-I molecules (Gravett, et al., 2018). Though in vivo studies exploring are more limited, gemcitabine has also been shown to enhance HLA-I in various PDAC cell lines, and both xenograft and transgenic models of PDAC display enhanced MHC Class 1 expression following long-term treatment with gemcitabine (Principe, et al., 2020). Recent evidence suggests that gemcitabine treatment is also associated with a decrease in TAP, suggesting that gemcitabine may induce TAP-independent peptide loading of HLA-I (D. Li, et al., 2021). Accordingly, gemcitabine has been shown to overcome poorly immunogenic phenotypes and cooperate with immune checkpoint inhibition in vivo (Principe, et al., 2020; Salewski, et al., 2021).
Table 2.
Select studies exploring the effects of non-platinum-based chemotherapy on tumor cell expression of antigen presenting molecules
| Chemotherapy | Cancer Type | Effect on Antigen Presentation | Notes | Reference |
|---|---|---|---|---|
| Gemcitabine | Breast | Increased HLA-I | - | (W. M. Liu, et al., 2010). |
| Breast | Increased HLA-I, Altered peptides eluted from HLA-I molecules | - | (Gravett, et al., 2018). | |
| Colon | Increased HLA-I | - | (W. M. Liu, et al., 2010). | |
| Colon | Increased HLA-I, Altered peptides eluted from HLA-I molecules | - | (Gravett, et al., 2018). | |
| Lung | Increased HLA-I | - | (W. M. Liu, et al., 2010). | |
| Lung | Increased HLA-I, Altered peptides eluted from HLA-I molecules | - | (Gravett, et al., 2018). | |
| Melanoma | Improved the cross-presentation efficiency of nuclear antigen | - | (Anyaegbu, et al., 2014) | |
| Pancreas | Increased MHC Class I in murine and HLA-I in human tumor cells | - | (Principe, et al., 2020). | |
|
| ||||
| Paclitaxel | Breast | Increased MHC Class I | - | Wan, et al., 2012) |
| Pancreas | Increased HLA-I | - | (Principe, et al., 2020) | |
|
| ||||
| Mitoxantrone | Colon | Enhanced MHC class I | - | (Y. Zhou, et al., 2021) |
| Prostate | Enhanced MHC class I | - | (Y. Zhou, et al., 2021) | |
|
| ||||
| 5-FU | Pancreas | Increased HLA-I | - | (Principe, et al., 2020) |
|
| ||||
| Etoposide | Breast | Increased MHC Class I | - | (Wan, et al., 2012) |
|
| ||||
| Irinotecan | Pancreas | Increased HLA-I | - | (Principe, et al., 2020) |
|
| ||||
| Topotecan | Breast | Increased HLA-I | - | (Wan, et al., 2012) |
|
| ||||
| Vinblastine | Breast | Increased MHC Class I | - | (Wan, et al., 2012) |
Several other drugs also appear to enhance antigen presentation and HLA expression. These include but are not limited to the topoisomerase I inhibitor topotecan, which enhanced HLA-I expression in breast cancer cells (Wan, et al., 2012). Similar results were observed using the non-platinum agents etoposide, paclitaxel, and vinblastine (Wan, et al., 2012). Additionally, the topoisomerase inhibitor mitoxantrone was reported to enhance p300-mediated upregulation of MHC class I, independent of autocrine IFNγ signaling (Y. Zhou, et al., 2021). In pancreatic cancer cells, several non-platinum medications have been shown to enhance surface expression of HLA-I, including paclitaxel, 5-FU, irinotecan, some surpassing that induced by oxaliplatin (Principe, et al., 2020). Hence, while data regarding non-platinum-based agents and HLA-I are still emerging, several widely used medications may similarly enhance antigen presentation and warrant continued exploration.
Finally, a variety of non-platinum chemotherapies promote immunogenic cell death. Gemcitabine can lead to immunogenic cell death, increasing the exposure of calreticulin and HMGB1 in lung cancer cells (Zhang, et al., 2020). For example, mitoxantrone, as well as the related medication doxorubicin, have also been linked to immunogenic cell death (Casares, et al., 2005; Obeid, et al., 2007), which is not observed using other topoisomerase inhibitors including camptothecin and etoposide (Sukkurwala, et al., 2014). Similarly, several studies demonstrate that bortezomib, a selective inhibitor of the 26S proteasome, can also induce immunogenic cell death, in part via activation of the cyclic GMP-AMP Synthase (cGAS)/Stimulator of Interferon Genes (STING) pathway (Gulla, et al., 2021; Serrano-Del Valle, Anel, Naval, & Marzo, 2019; Spisek, et al., 2007). Pemetrexed, a folate pathway inhibitor widely used in lung cancer, also induces immunogenic cell death, augmenting systemic intratumor immune responses and cooperating with cancer immunotherapy (Lu, et al., 2020; Schaer, et al., 2019). Accordingly, these and other medications have been suggested as a potential means of overcoming poor antigenicity and restoring functional anti-tumor immune responses (Casares, et al., 2005; Dudek, Garg, Krysko, De Ruysscher, & Agostinis, 2013; Schaer, et al., 2019).
3.3 –. Chemotherapy and Dendritic Cell-Mediated Antigen Presentation
Though most studies have focused on chemotherapy-induced antigen presentation by tumor cells, additional evidence supports a role for chemotherapy in modulating the antigen function of professional antigen-presenting cells, mainly dendritic cells (DCs). Several chemotherapy agents have been shown to improve DC function at low doses, including cyclophosphamide, doxorubicin, methotrexate, mitomycin-C, paclitaxel, vinblastine, and vincristine (Kaneno, Shurin, Tourkova, & Shurin, 2009; Shurin, Tourkova, Kaneno, & Shurin, 2009). Several studies have offered mechanistic insight into the effects of chemotherapy on DC biology. For example, paclitaxel can directly affect DC maturation (John, et al., 2010; Pfannenstiel, Lam, Emens, Jaffee, & Armstrong, 2010). Further, paclitaxel has lipopolysaccharide-mimetic activity in mice, leading to the activation of TLR4 and enhancing DC activation and cytokine biosynthesis (Byrd-Leifer, Block, Takeda, Akira, & Ding, 2001; Kawasaki, et al., 2000).
Cyclophosphamide can increase circulating DCs during the recovery phase of drug-induced lymphodepletion, inducing their Flt3 ligand-dependent proliferation in the bone marrow prior to their expansion in the periphery (Salem, et al., 2010). These cyclophosphamide-induced DCs appear to have normal phagocytosis and antigen-presenting capacity (Salem, et al., 2009), though cyclophosphamide can enhance anti-tumor immunity by preferentially depleting CD8+ T-cell-resident DCs, leading to diminished Treg suppression and increased effector T-cell function (Nakahara, et al., 2010).
Several other drugs can alter DC biology. Conditioned media from gemcitabine-treated PDAC cells stimulates DC maturation, thereby potentiating tumor-specific cytotoxic T-cell responses (Pei, et al., 2014). Accordingly, gemcitabine treatment increases both monocytes and dendritic cells in patients with advanced PDAC (Soeda, et al., 2009), and gemcitabine has been successfully combined with a DC-based vaccine in murine PDAC (Bauer, et al., 2007). Additional evidence suggests that 5-FU and oxaliplatin decrease DC expression of immune checkpoints PD-L1 and PD-L2, promote DC maturation, and cooperate with therapeutic vaccination in tumor-bearing mice (X. Hong, et al., 2018). However, oxaliplatin can also promote PD-L1 expression on DCs and reduce the expression of the co-stimulatory molecules CD80/CD86, thereby decreasing T-cell responses (Tel, et al., 2012). Thus, the effects of these and other medications on DC function are likely complex, and additional factors, including drug dosing and duration, should be considered.
4 –. CHEMOTHERAPY-INDUCED EXPRESSION OF IMMUNE CHECKPOINTS
Though chemotherapy has been shown to modulate immune-stimulating processes such as antigen presentation, several chemotherapy agents can also enhance the expression of immune checkpoints, with most focusing on PD-L1 (Figure 4). For example, in addition to enhancing immunogenic cell death and HLA-I, both cisplatin and oxaliplatin enhance surface expression of PD-L1 in HNSCC cells (S. J. Park, et al., 2019). Accordingly, cisplatin enhances therapeutic responses to PD-1/PD-L1 inhibition in a syngeneic mouse model of HNSCC (Tran, et al., 2017). In esophageal squamous cell carcinoma (ESCC) cells, incubation with carboplatin and paclitaxel or 5-FU and cisplatin enhanced PD-L1 expression in an Epidermal Growth Factor Receptor (EGFR)/Extracellular signal-regulated kinase (ERK)-dependent mechanism (Ng, et al., 2018). This relationship has also been evaluated in colorectal cancer, where neoadjuvant, oxaliplatin-based chemotherapy enhanced tumor PD-L1 expression only for patients without an activating RAS mutation (Ledys, et al., 2018). In TNBC cells, carboplatin, doxorubicin, gemcitabine, and paclitaxel have all been shown to enhance PD-L1 expression (Samanta, et al., 2018).
Figure 4. Chemotherapy-induced PD-L1 expression as a means of immune escape.
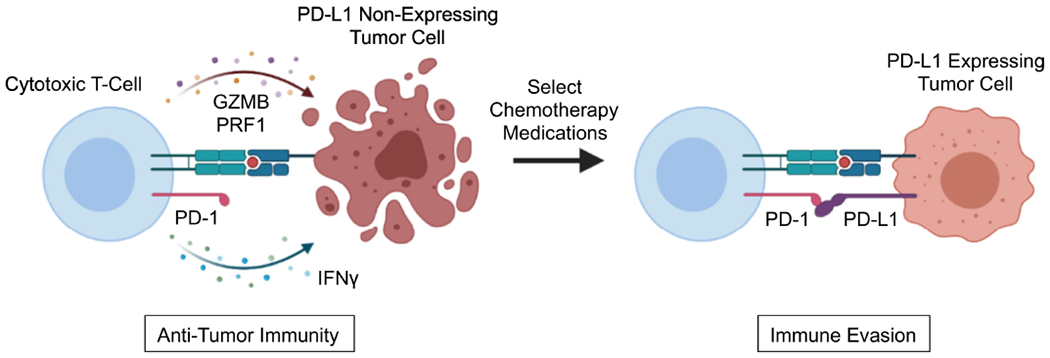
Though chemotherapy can enhance tumor cell immunogenicity and potentiate immune-stimulating processes, such as antigen presentation, several chemotherapy agents can also enhance the expression of negative immune checkpoints, importantly PD-L1. In this context, PD-L1 can associate with the PD-1 receptor on effector T-cells, blunting anti-tumor immune responses and facilitating immune escape. This ligand/receptor interaction is neutralized by antibodies against PD-1 (e.g., pembrolizumab and nivolumab) or PD-L1 (e.g., atezolizumab, avelumab, and durvalumab), and several combinations of chemotherapy and anti-PD-1/PD-L1 antibodies are either in clinical use or under clinical evaluation. Abbreviations: Granzyme B (GZMB), Perforin 1 (PRF1); Interferon γ (IFNγ).
In NSCLC, patients receiving platinum-based neoadjuvant chemotherapy similarly displayed increased PD-L1 expression (Guo, et al., 2019; Shin, et al., 2019). In breast cancer cells, several drugs, including doxorubicin, paclitaxel, and topotecan, increase PD-L1 expression, which the authors presumed was due to a cell-stress response (Gilad, et al., 2019). In ovarian cancer, cisplatin paradoxically enhances antigen presentation and immunogenic cell death, as well as increases the surface expression of PD-L1 (Grabosch, et al., 2019). In PDAC, several chemotherapy agents have been shown to enhance tumor expression of PD-L1, and in some cases, PD-L1 and CTLA-4. These include gemcitabine, paclitaxel, 5-FU, irinotecan, and oxaliplatin (Principe, et al., 2020). Gemcitabine-induced PD-L1 expression is further enhanced by the addition of a transforming growth factor β (TGFβ) inhibitor (D. Li, et al., 2021), consistent with prior observations that tumors with systemic ablation of TGFβ signals display increased PD-L1 expression (Principe, et al., 2019). Similar results have been reported in esophageal cancer in patients receiving neoadjuvant 5-FU and cisplatin, with enhanced PD-L1 expression localized to tumor-infiltrating immune cells (Fukuoka, et al., 2019). Though not an exhaustive summary, these and other related studies continue to provide evidence of chemotherapy-induced upregulation of immune checkpoints; the appropriately matched combination strategies warrant continued investigation (Bailly, Thuru, & Quesnel, 2020).
5 –. CHEMOTHERAPY-INDUCED ALTERATIONS IN CYTOKINE SYNTHESIS
Chemotherapy has several, often contradictory roles in directing the local cytokine milieu, enhancing the biosynthesis of both immune-stimulatory and immune-suppressive signaling molecules (Figure 5). Early clinical observations noted that serum levels of the cytokines Granulocyte colony-stimulating factor (G-CSF) and IL-6 fluctuated in response to chemotherapy (Y. M. Chen, et al., 1996). Similar results have been observed in vitro, where etoposide and mitomycin C enhanced the production of several inflammatory cytokines, notably CXCL8 and Tumor Necrosis Factor α (TNFα) (Darst, et al., 2004). A seminal report in melanoma demonstrated that dacarbazine, temozolomide, and cisplatin enhance the release of various T-cell-attracting chemokines in vitro. This included several CXCR3 ligands as well as CCL5, which cooperated to attract tumor-infiltrating effector T-cells (M. Hong, et al., 2011). Similarly, in HNSCC, low doses of 5-fluorouracil and cisplatin increased tumor cell release of IL-6 and G-CSF, as well as reduced IL-1β levels. In this study, primary tumor cells displayed chemotherapy-induced upregulation of Granulocyte-macrophage colony-stimulating factor (GM-CSF) and Tumor necrosis factor α (TNFα), though 5-fluorouracil and cisplatin led to a decline in GM-CSF and TNFα levels in metastatic tumor cells (Reers, et al., 2013).
Figure 5. Chemotherapy enhances local cytokine synthesis and can facilitate lymphocyte trafficking into the tumor microenvironment.
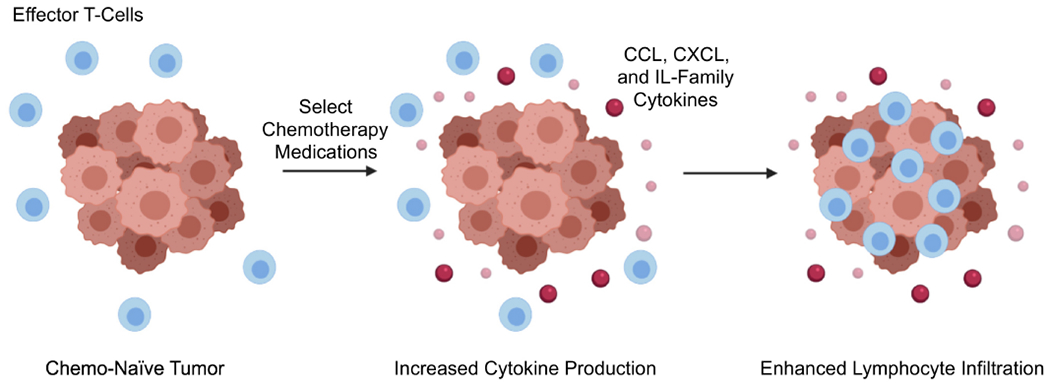
Several reports suggest that various chemotherapy medications can modulate tumor cytokine synthesis. For many cancers, chemotherapy can increase local levels of immune-stimulating cytokines/chemokines, thereby enhancing the recruitment of effector T-cells and promoting anti-tumor immunity. However, other reports suggest that chemotherapy often has contradictory roles regarding the local cytokine milieu and can also increase the release of several immune-suppressive signaling molecules. Hence, the success of future combination strategies may require the selective inhibition of chemotherapy-induced immune suppression in order to take full advantage of its immune-stimulating effects.
In PDAC, gemcitabine treatment can enhance the release of several T-cell attracting cytokines both in vitro and in vivo. These include several CCL, CXCL, and IL family members, as well as several components of the TGFβ pathway (Principe, et al., 2020). In breast cancer cells, paclitaxel and docetaxel induced TNFα biosynthesis in a toll-like receptor 4 (TLR4)-dependent mechanism (Sprowl, et al., 2012). Similar results have been observed in other cell lines, and the authors concluded that taxane-induced inflammatory cytokine production is dependent on the duration of exposure and is mechanistically distinct from LPS-induced cytokine production (Edwardson, et al., 2017). A similar study explored the effects of cyclophosphamide in tumor cells and showed that cyclophosphamide treatment led to an acute secretory activating phenotype characterized by increased release of CCL4, IL8, VEGF, and TNFα. These paracrine factors enhanced macrophage infiltration and phagocytic activity, suggesting that chemotherapy can increase the tumoricidal effects of the innate immune system by altering local cytokine levels (Pallasch, et al., 2014). Cyclophosphamide has also been shown to enhance the expression of GM-CSF, IL-1β, IL-7, IL-15, IL-2, IL-21, and IFNγ, thereby potentiating anti-tumor immune responses (Bracci, et al., 2007). Also related to IFN signals, a recent study has demonstrated that anthracycline-based chemotherapy stimulates the rapid production of type I IFNs following activation of TLR3. This leads to enhanced levels of CXCL10 within the breast tumor microenvironment, enhancing local immune responses. The authors concluded that chemotherapy could mimic sterilizing immune responses, which may constitute a hallmark of successful chemotherapy (Sistigu, et al., 2014). Additional research suggests that this response can be potentiated through STAT3 inhibition, which may also be of clinical utility (Yang, et al., 2015).
It is important to note that ovarian tumor cell debris induced by cisplatin or paclitaxel stimulated macrophage release of pro-inflammatory cytokines and bioactive lipids, thereby enhancing tumorigenesis. This was abrogated through pharmacologic inhibition of cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) pathways, suggesting that the effect of chemotherapy on local immune cues is both complex and context-specific (Gartung, et al., 2019). Additionally, chemotherapy-induced cytokine production has been implicated in chemotherapy-associated cognitive impairment (Cheung, et al., 2015; Ren, St Clair, & Butterfield, 2017). Hence, while chemotherapy-induced alterations to the tumor secretome may offer potential avenues for combination therapy, these and other adverse effects should be considered.
6 –. LYMPHOCYTE TRAFFICKING, DIFFERENTIATION, AND EFFECTOR FUNCTION
Consistent with observations that select chemotherapies can alter tumor cytokine production, several studies have also identified a relationship between chemotherapy and tumor-infiltrating immune cell populations. For example, an early report in breast cancer determined that patients receiving neoadjuvant paclitaxel had enhanced tumor-infiltrating lymphocytes, the degree of which correlated with therapeutic responses (Demaria, et al., 2001). Several studies have also suggested that chemotherapy-induced immune responses are predictive of favorable outcomes, with most focusing on breast cancer. For example, breast cancer patients with a high CD8+ and low FoxP3 infiltrates have markedly improved overall survival following neoadjuvant treatment (Ladoire, et al., 2011). This has been confirmed through other studies, all suggesting that local immune responses may be a cornerstone of the anti-tumoricidal effects of chemotherapy (Asano, et al., 2017; Denkert, et al., 2010; H. Lee, Lee, Seo, Gong, & Lee, 2020; Ono, et al., 2012; Sasada, et al., 2020; K. Wang, Xu, Zhang, & Xue, 2016; N. R. West, et al., 2011). Comparable results have been observed in other disease histologies, including but not limited to colon, esophageal, ovarian, and pancreatic cancers (Cha, Park, Baik, Lee, & Kang, 2019; Fukuoka, et al., 2019; Lo, et al., 2017; Morris, Platell, & Iacopetta, 2008; Nejati, et al., 2017; Shibutani, et al., 2018).
Consistent with these observations, beyond modulating the immunogenicity of tumor cells, several chemotherapy agents directly affect leukocytes, most notably T-cells. As discussed, much of the early rationale for chemo-immunotherapy stemmed from observations that cyclophosphamide can deplete tumor-associated Tregs and improve responses to immunotherapy by favoring effector T-cell responses. In addition to cyclophosphamide, many other chemotherapy agents also selectively target Tregs, favoring effector CD4+ T-cell responses (Roselli, et al., 2013). For example, docetaxel treatment increased the ratio of either CD4+ or CD8+ T-cells to T-regs in patients with metastatic breast cancer, with similar results observed in NSCLC patients treated with cisplatin and vinorelbine (Roselli, et al., 2013). Accordingly, lung cancer patients who received four cycles of docetaxel-based chemotherapy showed fewer peripheral Tregs than present at baseline (J. Y. Li, et al., 2014). In metastatic colon cancer, the multidrug regimens FOLFOX (5-FU, leucovorin, and oxaliplatin) and FOLFIRI (5-FU, leucovorin, and irinotecan) also significantly reduced peripheral blood Tregs (Maeda, et al., 2011). Gemcitabine has also been shown to deplete T-cells in both humans and mice, as well as enhance the effector function of vaccine-specific CD8+ T-cells (Rettig, et al., 2011), with similar results observed in lung cancer patients (C. Chen, et al., 2015).
In addition to shifting the balance between Tregs and effector T-cells, several chemotherapy agents appear to directly alter T-cell function. For example, oxaliplatin has been shown to reduce spleen size and cellularity in BALB/c mice yet increased the relative frequency of pan-CD4+ and CD8+ T-cells, Tregs, and increased levels of TNFα. The authors presumed this was due to selective depletion of B-cells, thereby allowing for T-cell dominance (Stojanovska, et al., 2019). Further, the FOLFOX regimen (5-FU, leucovorin, and oxaliplatin) has been shown to depend on CD8+ T-cell responses to control tumor growth in vivo. The authors identified that FOLFOX-enabled tumor-infiltrating lymphocytes have a functional differentiation state characterized by lower levels of immune checkpoints PD-1 and TIM-3, and that T-cells from FOLFOX-treated tumors have improved effector function. They concluded that FOLFOX promotes a functional shift from an exhausted to functional T-cell phenotype (Guan, et al., 2020).
Similar results have been observed with cisplatin, as tumor-bearing mice treated with cisplatin demonstrated increased tumor CD8+ T-cell infiltration (Wakita, et al., 2019). Accordingly, cisplatin cooperated with immune checkpoint inhibition (Wakita, et al., 2019) and has similarly been shown to enhance CD8+ T-cell responses induced by DNA vaccination (Tseng, et al., 2008). These and other studies have suggested that the therapeutic efficacy of cisplatin is dependent on CD8+ T-cell activation and sustained by CD80/86-mediated co-stimulation (Beyranvand Nejad, et al., 2016; Wakita, et al., 2019).
Several other medications also alter T-cell responses. Though paclitaxel is known to increase the T-cell-activating ability of ovarian cancer cells (Tsuda, et al., 2007), additional evidence supports more direct mechanisms through which paclitaxel can enhance T-cell function, particularly in combination platinum-based agents. In NSCLC, paclitaxel, carboplatin, and bevacizumab directly enhanced CD8+ T-cell proliferation, notably for effector and memory subsets (de Goeje, et al., 2019). In cervical cancer, neoadjuvant paclitaxel and cisplatin reduced the tumor infiltration of T-regs and increased the accumulation of active CD8+ cells, though no change was observed with cisplatin alone (Heeren, et al., 2019). In metastatic melanoma patients, paclitaxel and carboplatin were able to overcome clinical resistance to anti-PD-1 therapy, associated with an increase in a subset of tumor-reactive CD8+ effector T-cells. Subsequent in vitro experiments determined that incubation with chemotherapy potentiated the cytotoxic function of these T-cells, supporting a direct stimulatory role for these agents in isolated T-cells (Yan, Dronca, Liu, Markovic, & Dong, 2017). Accordingly, low dose paclitaxel has been shown to support therapeutic vaccination with melanoma antigens in mice (Sevko, et al., 2012).
Gemcitabine also appears to have direct effects on T-cell biology, though our understanding of these actions is still emerging. Following a transient reduction in absolute lymphocytes, gemcitabine may decrease the presence of memory T-cells while also promoting naive T-cell activation in PDAC (Plate, Plate, Shott, Bograd, & Harris, 2005). However, subsequent studies suggest that gemcitabine may also impede T-cell activation, suppressing proliferation and inducing apoptosis in a T-cell subtype and dose-dependent manner (Glenn, Xue, & Whartenby, 2018; Smith, Yogaratnam, Samad, Kasow, & Dalgleish, 2021). While these and other chemotherapy agents have been shown to contextually promote T-cell function, it is important to note that chemotherapy has long been known to cause lymphopenia over time (Aldarouish, et al., 2019; Grossman, et al., 2015; Lissoni, Fumagalli, Paolorossi, & Mandala, 1999; Menetrier-Caux, Ray-Coquard, Blay, & Caux, 2019; Verma, et al., 2016). Hence, the proper dose and duration of these and other immune-stimulating chemotherapies warrants continued investigation.
7 –. CHEMOTHERAPY AND OTHER IMMUNE CELL POPULATIONS
While T-cells have become the primary focus in cancer immunotherapy due to their capacity to kill tumor cells (Coulie, Van den Eynde, van der Bruggen, & Boon, 2014; Galon, et al., 2006), several additional leukocyte subtypes are critical for therapeutic responses. Though the effects of chemotherapy on these immune cell subsets are less studied than the more classic mediators of sterilizing immunity, emerging data suggests that several chemotherapy agents modulate the effects of several additional leukocytes. Much of this data pertains to macrophages and natural killer (NK) cells, both of which are briefly discussed below.
7.1 –. Macrophage Recruitment and Polarization
Macrophages are central to therapeutic responses to immune checkpoint inhibition (DeNardo & Ruffell, 2019), and several recent studies now suggest that several aspects of macrophage biology are impacted by cytotoxic chemotherapy. Several studies have explored the effects of docetaxel on macrophages and myeloid-derived suppressor cells (MDSCs). Very early reports demonstrated that cyclophosphamide, doxorubicin, and paclitaxel enhance the anti-tumor immune response of GM-CSF-secreting whole-cell vaccines in murine models of breast cancer, in part through potentiating Th1 responses (Machiels, et al., 2001) that can enhance the tumoricidal effects of macrophages (T. Li, Wu, Yang, Zhang, & Jin, 2020). More recently, vincristine, cyclophosphamide, and doxorubicin have been shown to cooperate with a CD40-agonist and cytosine-phosphate-guanosine-containing oligodeoxynucleotide 1826 (CpG-ODN) immunotherapy regimen through the repolarization of tumor-associated macrophages (Buhtoiarov, et al., 2011). Similarly, incubation with cyclophosphamide metabolites enhanced the production of pro-inflammatory cytokines IL-6 and IL-12 and down-regulated the suppressive cytokines IL-10 and TGFβ in mouse peritoneal macrophages (Bryniarski, Szczepanik, Ptak, Zemelka, & Ptak, 2009), and cyclophosphamide-educated peritoneal macrophages have been shown to protect effector T-cells from suppression (Majewska-Szczepanik, et al., 2018).
The effects of other chemotherapy agents on macrophage biology are highly varied and context-dependent. Docetaxel has several effects on monocyte-derived cell populations. Incubation with docetaxel re-polarized MDSCs toward an M1-like phenotype (Kodumudi, et al., 2010). This is consistent with other observations suggesting that docetaxel promotes the generation of anti-tumorigenic human macrophages, promotes the differentiation of immature monocytes into M1 macrophages, and increases the antigen presentation capacity of myeloid cells. (Millrud, Mehmeti, & Leandersson, 2018). Cisplatin also appears to prime peritoneal macrophages for enhanced expression of several inflammatory cytokines and transcription factors upon co-culture with murine fibroblasts (Chauhan, Sodhi, & Shrivastava, 2009).
However, taxane and non-taxane neoadjuvant chemotherapy have been shown to enhance the recruitment of Tie2+ macrophages in breast cancer, facilitating the entry of tumor cells into circulation and promoting metastasis (Karagiannis, et al., 2017). Human peripheral blood monocytes co-cultured with esophageal SCC cells treated with 5-FU and cisplatin shifted macrophages toward a more immune suppressive, CD163+ phenotype in an IL-34-dependent mechanism (Nakajima, et al., 2021). Similarly, ovarian tumor cells treated with platinum-based agents also induced an immune-suppressive M2 phenotype in adjacent macrophages, which was reversed by either COX or IL-6 inhibition (Dijkgraaf, et al., 2013). Though gemcitabine can deplete MDSCs (Eriksson, Wenthe, Irenaeus, Loskog, & Ullenhag, 2016), conditioned media from PDAC cells treated with gemcitabine shifts macrophages toward an M2 phenotype, characterized by increased expression of arginase-1 and TGFβ1 (Deshmukh, et al., 2018). Thus, the effects of chemotherapy on macrophage function are varied, with these and other studies suggesting both immune-stimulating and immune-suppressive effects. Given the roles of macrophages and MDSCs in cancer immunotherapy, this is an important area that warrants further study.
7.2 –. Natural Killer Cells
Though cytotoxic T-cells are considered the main effector cells in most cancer immunotherapies, the importance of NK cells is now recognized (Shimasaki, Jain, & Campana, 2020). Several chemotherapy agents have been shown to modulate NK cell function, and like macrophages, the results are often contradictory and context-dependent. For example, gemcitabine enhances NK cell-mediated cytotoxicity (Okita, et al., 2015), and increases NK cell proliferation (Dammeijer, et al., 2021). Accordingly, low-dose gemcitabine treatment enhances NK cell-mediated anti-tumor immunity in lung cancer (Zhang, et al., 2020), and the combination of gemcitabine and cytokine-activated NK cells has shown preclinical promise in HCC (Morisaki, et al., 2011). Also supporting an activating role for chemotherapy, metronomic dosing with cyclophosphamide enhances NK cell effector function in end-stage cancer patients (Ghiringhelli, et al., 2007), and cisplatin appears to enhance NK cell function by enhancing tumor cell expression of MHC class I chain-related molecule A and B (Okita, et al., 2016).
However, several studies have reported that chemotherapy can suppress NK cell function. Notable examples include the observation that paclitaxel inhibits the binding of NK cells to their targets through the down-regulation of adhesion molecules (Loubani & Hoskin, 2005). Similarly, paclitaxel and vinblastine downregulate CD11a and CD54 expression in P815 mastocytoma cells, conferring resistance to killing by non-specific killer lymphocytes (Zhao, Morgan, Haeryfar, Blay, & Hoskin, 2003). Hence, this area also warrants additional exploration, particularly as NK cell-centric therapies advance in the clinic.
8 –. CLINICAL ADVANCES IN CHEMO-IMMUNOTHERAPY
In light of the mounting preclinical evidence described above, several recent clinical trials have explored novel combinations of chemotherapy and ICI-based immunotherapy. Although a comprehensive list of these trials is beyond the scope of this review, several are showing considerable promise. Here we discuss select major trials in this rapidly evolving field, with a particular emphasis on those that have been evaluated in phase 3 trials and/or approved by the FDA.
8.1 –. Lung Cancer
The anti-PD-1 antibody pembrolizumab has revolutionized the treatment landscape for NSCLC, both as a single agent for patients with high PD-L1 expression and in combination with chemotherapy (Reck, et al., 2016). Recent evidence has solidified the concept that pembrolizumab may synergize with chemotherapy in NSCLC. Based on encouraging phase 2 data (Langer, et al., 2016), a large phase 3 trial has recently evaluated the anti-PD-1 antibody pembrolizumab as a monotherapy or in combination with pemetrexed and platinum-based chemotherapy in untreated patients with metastatic, non-squamous NSCLC without sensitizing EGFR or ALK mutations. After one year, the median overall survival for the combination group was 69.2% compared to 49.4% for the monotherapy group. The survival benefit of the combination treatment was observed across all patients independent of PD-L1 status, with clinically meaningful improvements in median overall survival for patients in the combination arm (Gandhi, et al., 2018). In squamous NSCLC, the combination of pembrolizumab, carboplatin, and paclitaxel or nab-paclitaxel was also effective, independent of PD-L1 status. Combination immunotherapy led to a median overall survival of 15.9 months compared to 11.3 months for chemotherapy alone, extending median progression-free survival to 6.4 months compared to 4.8 months for the chemotherapy group (Paz-Ares, et al., 2018). Based on these and related studies, pembrolizumab, in combination with pemetrexed and platinum-based chemotherapy, was approved in 2018 as a first-line treatment for metastatic, non-squamous NSCLC lacking EGFR or ALK mutations.
Similar regimens are also under evaluation, notably the combination of carboplatin, pemetrexed, and the anti-PD-1 antibody camrelizumab, show promising results based on interim analysis in a phase 3 trial (C. Zhou, et al., 2021). Similar results have been observed in extensive-stage small-cell lung cancer (SCLC), where the addition of atezolizumab to carboplatin and etoposide resulted in significantly longer overall survival and progression-free survival than chemotherapy alone (Horn, et al., 2018). The combination of the anti-PD-1 antibody durvalumab and platinum and etoposide chemotherapy has also been evaluated as a first-line treatment for extensive-stage SCLC. This approach showed improved overall survival compared to chemotherapy alone, extending median overall survival from 10.3 to 13 months (Paz-Ares, et al., 2019).
In addition to showing efficacy as a first-line therapy (Table 3), ICI-based immunotherapy is showing promise as a second-line treatment in NSCLC. For example, the anti-PD-1 antibody nivolumab has been combined with platinum-based doublet chemotherapy (cisplatin or carboplatin plus either gemcitabine, pemetrexed, or paclitaxel) with encouraging results (Rizvi, et al., 2016). The anti-PD-L1 antibody avelumab showed substantial activity in patients with progressive or platinum-resistant metastatic or recurrent disease. Of the 184 patients enrolled, 22 demonstrated objective clinical responses and 70 had stable disease, for an overall disease control rate of 50% (Gulley, et al., 2017). Though early phase 2 data suggested that subsequent-line avelumab may be more efficacious than docetaxel (Fehrenbacher, et al., 2016), more recent phase 3 data suggest that avelumab does not improve survival beyond docetaxel but has a favorable toxicity profile (Barlesi, et al., 2018) . After two years of follow-up, the authors concluded that avelumab did not significantly prolong overall survival compared to docetaxel in patients with platinum-treated, PD-L1-expressing NSCLC (K. Park, et al., 2021).
Table 3.
Landmark clinical trials supporting chemo-immunotherapy as a first-line treatment for lung cancer patients
| Cancer Type | Chemotherapy | Immunotherapy | Phase | Notes | Ref |
|---|---|---|---|---|---|
| NSCLC | Pemetrexed and Platinum-based | Pembrolizumab | 3 | Metastatic, non-squamous, non EGFR- or ALK-Mutated | (Gandhi, et al., 2018) |
| Carboplatin, and Paclitaxel/nab-Paclitaxel | Pembrolizumab | 3 | Squamous NSCLC only | (Paz-Ares, et al., 2018) | |
| Carboplatin and Pemetrexed | Camrelizumab | 3 | - | (C. Zhou, et al., 2021) | |
| Carboplatin and nab-Paclitaxel | Atezolizumab | 3 | - | (H. West, et al., 2019) | |
|
| |||||
| SCLC | Carboplatin and Etoposide | Atezolizumab | 3 | Extensive-stage disease | (Horn, et al., 2018) |
| Etoposide and Platinum-Based | Durvalumab | 3 | Metastatic, non EGFR- or ALK-mutated | (Paz-Ares, et al., 2019) | |
| Carboplatin and nab-Paclitaxel | Atezolizumab | 3 | Included the VEGF-inhibiting antibody Bevacizumab | (Socinski, et al., 2018) | |
Abbreviations: Non-Small Cell Lung Cancer (NSCLC); Small Cell Lung Cancer (SCLC); Epidermal Growth Factor Receptor (EGFR); Anaplastic Lymphoma Kinase (ALK); Vascular Endothelial Growth Factor (VEGF).
The anti-PD-L1 antibody atezolizumab has also been explored in previously treated NSCLC, extending survival beyond that observed with second-line docetaxel (Rittmeyer, et al., 2017). The combination of atezolizumab, carboplatin, and nab-paclitaxel has since been evaluated in NSCLC as a first-line treatment, particularly for those with stage IV disease and no ALK or EGFR mutations. This combination showed substantial therapeutic efficacy, extending median overall survival to 18.6 months compared to 13.9 months with chemotherapy alone (H. West, et al., 2019). The combination of atezolizumab, carboplatin, and nab-paclitaxel has also been combined with the VEGF-inhibiting antibody bevacizumab. As a first-line treatment, this combination regimen led to a median overall survival of 19.2 months, compared to 14.7 months for bevacizumab and chemotherapy only (Socinski, et al., 2018). In patients with baseline liver metastases, this combined regimen was similarly effective, extending median overall survival to 13.3 months compared to 9.4 months for patients receiving bevacizumab and chemotherapy only (Reck, et al., 2019). Several other combination strategies are also being evaluated in clinical trials for NSCLC, as well as for other forms of lung cancer.
8.2 –. Head & Neck Cancer
Chemo-immunotherapy has been evaluated extensively in HNSCC. For example, pembrolizumab plus platinum-based chemotherapy and 5-FU has been highly effective in patients with untreated locally incurable recurrent or metastatic FINSCC. A recent phase 3 study demonstrated that pembrolizumab and platinum plus 5-FU chemotherapy is superior to either single-agent pembrolizumab or cetuximab and chemotherapy. Importantly, this approach was non-inferior for all patients regardless of PD-L1 expression. Based on these data, this approach is now FDA approved and is recommended as an appropriate first-line treatment for PD-L1+ recurrent or metastatic HNSCC (Burtness, et al., 2019).
Both pembrolizumab and nivolumab have also been evaluated as a second-line treatment option for platinum-refractory HNSCC. Pembrolizumab was explored in a single-arm phase 2 study for patients with platinum- and cetuximab-refractory HNSCC, showing clinically meaningful anti-tumor activity and an acceptable safety profile (Bauml, et al., 2017). In a larger phase 3 study, pembrolizumab was also evaluated for HNSCC patients who progressed during or after treatment with platinum chemotherapy for recurrent or metastatic disease, demonstrating significant anti-tumor activity reflected by meaningful improvements in overall survival (Cohen, et al., 2019). Consequently, the FDA has approved pembrolizumab for use in this indication for all patients.
Similar results have been observed using nivolumab, which has also been evaluated in patients with recurrent or metastatic HNSCC who had experienced disease progression within 6 months of receiving platinum-based chemotherapy. This approach has now been evaluated in a phase 3 trial, where nivolumab extended median overall survival to 7.5 months compared to 5.1 months with chemotherapy alone (Ferris, et al., 2016). Subsequent evaluation has confirmed that nivolumab has superior therapeutic efficacy than the previous standard of care chemotherapy, which was unrelated to prior cetuximab exposure (Ferris, et al., 2019). Based on these data, nivolumab has also been approved by the FDA for this indication.
Though these and other studies have shown considerable promise, others have posted negative results. For example, in locally advanced HNSCC, the combination of avelumab, cisplatin, and radiation therapy did not meet the primary objective of prolonging progression-free survival (N. Y. Lee, et al., 2021). Similarly, durvalumab with or without tremelimumab failed to improve clinical outcomes beyond cetuximab and chemotherapy (Ferris, et al., 2020).
8.3 –. Gastro-Esophageal Cancer
Combined chemo-immunotherapy has shown rapid progress for gastro-esophageal cancers in recent years, with several approaches earning FDA approval. In advanced, HER2 negative gastric, gastro-esophageal junction (GEJ), or esophageal adenocarcinoma, nivolumab and FOLFOX showed superior overall survival compared to FOLFOX alone. The combination arm demonstrated a median overall survival of 13.1 months compared to 11.1 months for chemotherapy alone. As nivolumab and chemotherapy showed superior overall and disease-specific survival (Y. Y. Janjigian, et al., 2021), this combined regimen has now been FDA approved for initial treatment of advanced/metastatic gastric, GEJ, and esophageal adenocarcinomas, irrespective of PD-L1 expression.
Pembrolizumab has also been evaluated extensively in gastro-esophageal cancers, including in combination with chemotherapy. A recent phase 3 trial compared single-agent pembrolizumab with pembrolizumab and cisplatin plus 5-FU or capecitabine for patients with untreated, advanced gastric/GEJ cancers and found that pembrolizumab was non-inferior to chemotherapy. This study determined that the combination of pembrolizumab and chemotherapy is not superior to pembrolizumab alone (Shitara, et al., 2020). However, a more recent phase 3 study determined that the combination of pembrolizumab, cisplatin, and 5-fluorouracil was highly effective as a first-line treatment. This approach was superior to chemotherapy alone, extending median overall survival from 8.8 months to 13.9 months for PD-L1+ patients and from 9.8 months to 12.4 months for all randomized patients (Sun, et al., 2021). Based on these data, this approach has also been approved by the FDA.
Additional trials have explored similar combinations for HER2+ gastro-esophageal cancer. Importantly pembrolizumab is under investigation in combination with trastuzumab and platinum and fluoropyrimidine chemotherapy for patients with locally advanced or metastatic gastric or gastro-esophageal junction cancer that is not amenable to surgical resection or definitive chemo-radiation. Though only interim results for the first 264 patients have been presented, the addition of pembrolizumab improved the response rate from 52 to 74%, with evidence of durable anti-tumor efficacy (Yelena Y. Janjigian, et al., 2021). This led to the accelerated FDA approval of this regimen in 2021.
Several immunotherapy approaches have been evaluated following progression on chemotherapy. Early data suggested that pembrolizumab has significant anti-tumor activity in metastatic esophageal cancer patients who have progressed on at least two prior lines of therapy (Doi, et al., 2018). Similar results were observed in a phase 2 trial (Shah, et al., 2019) , as well as in a subsequent study for previously treated advanced gastric or GEJ cancers (Fuchs, et al., 2018). This was confirmed in a larger phase 3 study, where pembrolizumab was superior to paclitaxel, docetaxel, or irinotecan as a second-line treatment for patients with advanced/metastatic esophageal cancer (Kojima, et al., 2020).
Nivolumab has shown similar efficacy in treatment-refractory esophageal cancer (Kudo, et al., 2017), as well as for advanced, treatment-refractory gastric or gastro-esophageal cancer (Y.-K. Kang, et al., 2017). In a phase 3 trial, nivolumab was again highly effective in advanced gastric or GEJ cancers refractory to at least two previous chemotherapy regimens, increasing 12-month overall survival from 10.9% with placebo to 26.2% (Y. K. Kang, et al., 2017). The combination of nivolumab and ipilimumab has also been evaluated as a second-line treatment in advanced gastric or GEJ cancers. This approach led to improved responses compared to nivolumab monotherapy, though phase 3 studies have yet to share results (Janjigian, et al., 2018). The anti-PD-1 antibody camrelizumab has also shown promise as second-line therapy for Chinese patients with metastatic esophageal cancer. This phase 3 trial only included patients with squamous histology, and camrelizumab led to significant improvements in overall survival compared to second-line chemotherapy (Huang, et al., 2020). Avelumab has been explored in chemotherapy-treated advanced gastric or GEJ cancers, particularly as first-line switch-maintenance therapy or second-line treatment. However, the clinical benefit achieved with avelumab has been marginal, and the approved treatment options are preferred (Chung, et al., 2019).
8.4 –. Urothelial Cancer
In urothelial cancer, pembrolizumab is now FDA approved as a first-line treatment for PD-L1+ patients not eligible for platinum-based chemotherapy (Balar, Castellano, et al., 2017). Similarly, pembrolizumab has shown promise for platinum-refractory advanced urothelial carcinoma, with improved survival and fewer treatment-related adverse events than chemotherapy with paclitaxel, docetaxel, or vinflunine (Bellmunt, et al., 2017). Nivolumab has shown similar efficacy as a second-line treatment irrespective of PD-L1 status (Sharma, et al., 2017). Atezolizumab was granted accelerated approval by the FDA for platinum-refractory urothelial carcinoma based on single-arm phase 2 data (Balar, Galsky, et al., 2017). However, subsequent phase 3 data has since demonstrated that atezolizumab was not associated with significantly longer overall survival than chemotherapy in this indication (Powles, et al., 2018). Based on these observations, the FDA has withdrawn approval for atezolizumab in patients with advanced, platinum-refractory urothelial carcinoma. Other approaches have also been explored in clinical trials, including avelumab for metastatic, platinum-refractory urothelial carcinoma. In this study, avelumab showed superior progression-free survival compared to chemotherapy alone (Patel, et al., 2018). This led to the FDA approval for avelumab, though it is important to note that these conclusions were drawn from expansion cohorts of phase 1 trials and that phase 3 studies are still pending.
Despite these observations, progress has been difficult for chemo-immunotherapy in urothelial cancer. The combination of atezolizumab and platinum-based chemotherapy is under investigation and has shown superior progression-free survival compared to chemotherapy alone (Galsky, et al., 2020). However, the addition of the anti-PD-1 antibody pembrolizumab to first-line platinum-based chemotherapy (gemcitabine and either cisplatin or carboplatin) did not significantly improve clinical outcomes, and the authors suggested that this approach should not be adopted for the treatment of advanced urothelial carcinoma (Powles, et al., 2021). Hence, this area warrants continued exploration in urothelial carcinoma.
8.5 –. Breast and Ovarian Cancers
Several trials are also evaluating chemo-immunotherapy in breast and ovarian cancers. A recent multicenter trial has explored the efficacy of atezolizumab and nab-paclitaxel in patients with unresectable, locally advanced, or metastatic triple-negative breast cancer (TNBC). In patients with advanced disease, this combination extended median progression-free survival to 7.2 months compared to 5.5 months in the nab-paclitaxel group (Schmid, et al., 2018). In the second analysis, the addition of atezolizumab to nab-paclitaxel marginally improved median overall survival from 18.7 to 21 months, though for PD-L1+ tumors, median overall survival was 25 months compared to 18 months with placebo (Schmid, Rugo, et al., 2020) . Patient-reported outcomes suggest that this approach was reasonably well tolerated, without compromising the patients’ health-related quality of life (Adams, et al., 2020). However, a similar trial explored the combination of neoadjuvant atezolizumab with anthracycline, cyclophosphamide, and taxane-based chemotherapy in early-stage TNBC. In this group, 58% of patients in the combination arm had pathologic complete responses to treatment compared to 41% for patients with chemotherapy alone. For PD-L1+ tumors, 69% of patients in the combination arm had pathologic complete responses, as did 49% for patients receiving chemotherapy alone (Mittendorf, et al., 2020).
Importantly, pembrolizumab has been evaluated in combination with neoadjuvant paclitaxel and carboplatin for early-stage TNBC. At the first interim analysis, 64.8% of patients in the combination arm demonstrated pathologic complete responses compared to 51.2% with chemotherapy and placebo. After 15.5 months, 7.4% of patients in the pembrolizumab, paclitaxel, and carboplatin group and 11.8% in the control group experienced disease progression precluding definitive surgery, demonstrated clinical recurrence, or died from any cause (Schmid, Cortes, et al., 2020). This led to the FDA approval of pembrolizumab in combination with neoadjuvant chemotherapy for early-stage TNBC in 2021. In a related study, pembrolizumab was administered to patients with metastatic TNBC in combination with nab-paclitaxel, paclitaxel, or gemcitabine and carboplatin. This study also considered PD-L1 expression, and the combination of pembrolizumab and chemotherapy showed a significant improvement in progression-free survival compared to the control group, particularly for PD-L1 expressing tumors (Cortes, et al., 2020). This approach was initially given accelerated approval by the FDA in 2020, but converted to full approval in 2021.
Though these and other important trials are encouraging, progress for immunotherapy in ovarian cancer has been difficult. For instance, avelumab failed to improve either progression-free or overall survival in platinum-resistant or platinum-refractory ovarian cancer, either as a monotherapy or combined with pegylated liposomal doxorubicin (Pujade-Lauraine, et al., 2021). Additionally, avelumab failed to show significant efficacy as front-line therapy in ovarian cancer, even when combined with carboplatin and paclitaxel (Monk, et al., 2021) . Pembrolizumab has shown only modest activity in monotherapy for patients with advanced, recurrent ovarian cancer, with higher response rates in patients with PD-L1+ disease (Matulonis, et al., 2019). Pembrolizumab has also been studied in combination with bevacizumab and oral metronomic cyclophosphamide, also in recurrent ovarian cancer, though early data suggests an objective response rate of 47.5% with a median progression-free survival of 10.0 months (Zsiros, et al., 2021).
8.6 –. Pancreatic Cancer
Several combinations of chemo- and immunotherapy have been explored in PDAC, in large part attributed to poor disease control rates using either approach alone (Principe, et al., 2021). For example, the combination of gemcitabine and the anti-CTLA-4 antibody tremelimumab showed an overall response rate of 10.5%, with 2/19 patients achieving a partial response and a median overall survival of 7.4 months (Aglietta, et al., 2014). Similarly, gemcitabine and the anti-CTLA-4 antibody ipilimumab led to a disease control rate was 43%, though median progression-free survival was 2.5 months, and median overall survival 8.5 months (Kalyan, et al., 2016). This approach has been explored in a different patient cohort, with similar results of a median overall survival of 6.9 months, progression-free survival of 2.8 months, and an overall response rate was 14%. Of the three responding patients, one showed a complete response, with a median response duration of 11 months (Kamath, et al., 2019).
Similarly, a phase 1 trial explored the combination of the anti-PD-1 antibody nivolumab with chemotherapy. This study consisted of two arms: patients who had received one prior chemotherapy regimen and treatment-naïve patients. Previously treated patients were administered nivolumab and nab-paclitaxel, and treatment-naïve patients nivolumab, nab-paclitaxel, and gemcitabine. In previously treated patients, 2/9 had a partial response and 4/9 had stable disease, for a disease control rate of 66.6%. For the treatment-naïve patients, 3/6 had a partial response and 3/6 had stable disease (Wainberg, et al., 2017). In an extended Phase 1 study of a larger cohort of treatment-naïve patients, the combination of nivolumab, nab-paclitaxel, and gemcitabine led to 1/50 complete response, 8/50 partial responses, and 23/50 patients with stable disease. Median progression-free survival in this group was 5.5 months, with a median overall survival of 9.9 months. However, this combination was poorly tolerated, with 48/50 patients had at least 1 grade 3/4 treatment-related adverse event (Wainberg, et al., 2019).
Pembrolizumab has also been explored in combination with chemotherapy in PDAC. In a seminal study, 2/11 PDAC patients showed a partial response, with a median overall survival of 8.0 months (Weiss, et al., 2017). A phase 2 study from the same group evaluated the combination of pembrolizumab, gemcitabine, and nab-paclitaxel in patients with metastatic PDAC as a first-line treatment. The authors observed a disease control rate of 100%, with 3/11 patients showing a partial response and the remaining 8/11 showing stable disease. Overall survival was 15.0 months and progression-free survival 9.1 months, though the primary endpoint of a 15% complete response rate was not met. (Weiss, et al., 2018).
Other combinations have also been evaluated, including cisplatin, gemcitabine, nab-paclitaxel, and pembrolizumab. Of the 25 metastatic PDAC patients in this study, 15/24 patients had a partial response and 4/24 had stable disease, with a median overall survival of 16.5 months. (Jameson, et al., 2017). The combination of gemcitabine, nab-paclitaxel, durvalumab, and tremelimumab is also showing promise, with preliminary findings from 11 patients reporting a disease control rate of 100%, with 8/11 patients demonstrating a partial response. (Renouf, et al., 2018). The combination of durvalumab and the TGFβ receptor inhibitor galunisertib has also been evaluated in a phase 1b trial, specifically for patients with metastatic PDAC that have progressed on two prior lines of chemotherapy. This led to an overall disease control rate of 25%, and the authors suggested that this approach may have improved efficacy either as an earlier line of therapy or for patients with predictive biomarkers associated with TGFβ signaling (Melisi, et al., 2021). These and similar combinations continue to show promise for PDAC and other difficult to treat cancer types, and pending additional study, may either improve outcomes beyond the current standard-of-care or offer an effective treatment strategy in the second or third-line setting.
9 –. SUMMARY AND FUTURE DIRECTION
Though long considered immunosuppressive, there is mounting evidence to support select immune-stimulating properties of cytotoxic chemotherapy. Accordingly, several chemo-immunotherapy regimens are now regularly used in cancer therapy, with many others showing promise in clinical trials. Though these approaches have shown rapid progress for select cancers, for others, progress has been difficult. As the field has reached consensus regarding the promise of cancer immunotherapy for difficult-to-treat cancer histologies, the immunomodulatory effects of chemotherapy warrant continued investigation to design the most effective combination strategies.
Based on studies described in this review, it appears as though the immune-stimulating effects of several chemotherapy agents are often predictable, consistently directing many of the same immune cell processes across a wide range of tumor types. Should certain chemotherapy medications indeed have predictable patterns of immune remodeling, this opens the possibility that these can be exploited for therapy through tailored combination strategies. Such approaches have already shown early promise in preclinical experiments, taking advantage of chemotherapy-enhanced antigen presentation and incorporating selective inhibition of local chemotherapy-induced immune suppression within the tumor microenvironment (Principe, et al., 2020). Recent evidence also supports that such combinations of chemo- and immunotherapy may further benefit from refinement of drug delivery and timing, including sequential administration with different waves, spatial delivery, or co-delivery strategies (Luo, Zhang, Luo, & Jiang, 2019). Hence, this area warrants continued investigation.
However, it important to note that select chemo-immunotherapy approaches will require caution when translated to patient care. Bone marrow suppression is one of the most important dose-limiting toxicities associated with chemotherapy (Y. Wang, Probin, & Zhou, 2006), and the primary reason chemotherapy had previously been considered universally immune suppressive. Hence, as combination chemo-immunotherapy regimens advance in the clinic, it will be important to carefully balance the immune-stimulating effects of chemotherapy with the long-term negative effects on hematopoiesis, and alterations to dose and schedule may be indicated. Additionally, the adverse effects of immune checkpoint inhibitors can be severe, and in some cases, life-threatening (F. Martins, et al., 2019). As immune-mediated adverse events are often predictive of anti-tumor immune responses (Das & Johnson, 2019), the improved efficacy of combined chemo-immunotherapy may increase the risk of autoimmune toxicity. However, given the promise for these combination strategies, mechanisms of chemotherapy-induced immune remodeling warrant continued exploration in hopes of maximizing efficacy and improving therapeutic response rates, particularly in cancers for which there is currently no effective treatment.
ACKNOWLEDGEMENTS
This article is dedicated to the memory of our good friend Daniel J. Takala who recently passed away after a courageous battle with glioblastoma. This work was supported by NIH R01CA07059 to M. Korc, by NIH F30CA236031 and UIC Award for Graduate Research to D. R. Principe, and by NIH R01CA217907 and R21CA255291 and the Veterans Affairs Merit Award I01BX002922 to H.G. Munshi.
ABBREVIAITONS
- ALK
Anaplastic Lymphoma Kinase
- APCs
Antigen-presenting cells
- BCG
Bacille Calmette-Guérin
- CBP
CREB-binding protein
- cGAS
Cyclic GMP-AMP Synthase
- COX
Cyclooxygenase
- COA-1
Cytochrome C oxidase assembly factor 1
- CTLA-4
Cytotoxic T-lymphocyte-associated protein 4
- CTLs
Cytotoxic T lymphocytes
- CpG-ODN
Cytosine-phosphate-guanosine-containing oligodeoxynucleotide 1826
- DAMPs
Damage-associated molecular patterns
- DCs
Dendritic cells
- dFdC
2′, 2′-difluoro 2′deoxycytidine
- dMMR
DNA mismatch repair
- eIF2α
Eukaryotic translation initiation factor 2α
- EGFR
Epidermal Growth Factor Receptor
- ER
Endoplasmic reticulum
- ERAP
ER Aminopeptidase
- ERK
Extracellular signal-regulated kinase
- ESCC
Esophageal squamous cell carcinoma
- FOLFOX
5-FU, leucovorin, and oxaliplatin
- FOLFIRI
5-FU, leucovorin, and irinotecan
- GEJ
Gastro-esophageal junction
- G-CSF
Granulocyte colony-stimulating factor
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- GZMB
Granzyme B
- HCC
Hepatocellular carcinoma
- HLA-I
Class I Human Leukocyte Antigen
- HNSCC
Head and neck squamous cell carcinoma
- HMGB1
High-mobility group box 1 protein
- ICIs
Immune checkpoint inhibitors
- IFN
Interferon
- IL
Interleukin
- MHC
Major Histocompatibility Complex
- MDSCs
Myeloid-derived suppressor cells
- NK
Natural killer
- NSCLC
Non small-cell lung cancer
- MSI-H
High microsatellite instability
- PDAC
Pancreatic ductal adenocarcinoma
- PRF1
Perforin 1
- PD-1
Programmed cell death protein 1
- PD-L1
PD-1 ligand 1
- PERK
Protein kinase-like ER kinase
- SCLC
Small-cell lung cancer
- sEH
Soluble epoxide hydrolase
- STING
Stimulator of Interferon Genes
- TAP
Transporters associated with Antigen Processing
- TCR
T-cell receptor
- TERT
Telomerase reverse transcriptase
- TGFβ
Transforming Growth Factor β
- TMB-H
Tumor mutational burden
- TME
Tumor microenvironment
- TLR
Toll-like receptor
- TNBC
Triple-negative breast cancer
- Tregs
Regulatory T-lymphocytes
- TNFα
Tumor necrosis factor α
- VEGF
Vascular Endothelial Growth Factor
- 5-FU
5-fluorouracil
- β2m
β2-microglobulin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure: The authors have no conflicts to disclose.
DECLARATION OF COMPETING INTEREST
The authors have no conflicts to disclose.
REFERENCES
- Adams S, Dieras V, Barrios CH, Winer EP, Schneeweiss A, Iwata H, Loi S, Patel S, Henschel V, Chui SY, Rugo HS, Emens LA, & Schmid P (2020). Patient-reported outcomes from the phase III IMpassion130 trial of atezolizumab plus nab-paclitaxel in metastatic triple-negative breast cancer. Ann Oncol, 31, 582–589. [DOI] [PubMed] [Google Scholar]
- Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH Jr., Bagala C, Colombi F, Cagnazzo C, Gioeni L, Wang E, Huang B, Fly KD, & Leone F (2014). A phase I dose escalation trial of tremelimumab (CP-675,206) in combination with gemcitabine in chemotherapy-naive patients with metastatic pancreatic cancer. Ann Oncol, 25, 1750–1755. [DOI] [PubMed] [Google Scholar]
- Ahmed AR, & Hombal SM (1984). Cyclophosphamide (Cytoxan). A review on relevant pharmacology and clinical uses. J Am Acad Dermatol, 11, 1115–1126. [DOI] [PubMed] [Google Scholar]
- Alagkiozidis I, Facciabene A, Tsiatas M, Carpenito C, Benencia F, Adams S, Jonak Z, June CH, Powell DJ Jr., & Coukos G (2011). Time-dependent cytotoxic drugs selectively cooperate with IL-18 for cancer chemo-immunotherapy. J Transl Med, 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldarouish M, Su X, Qiao J, Gao C, Chen Y, Dai A, Zhang T, Shu Y, & Wang C (2019). Immunomodulatory effects of chemotherapy on blood lymphocytes and survival of patients with advanced non-small cell lung cancer. Int J Immunopathol Pharmacol, 33, 2058738419839592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandru D, Van Horn DK, & Bota DA (2010). Secondary fibrosarcoma of the brain stem treated with cyclophosphamide and Imatinib. J Neurooncol, 99, 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyaegbu CC, Lake RA, Heel K, Robinson BW, & Fisher SA (2014). Chemotherapy enhances cross-presentation of nuclear tumor antigens. PLoS One, 9, e107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T, Noda S, Takashima T, Onoda N, Tomita S, Motomura H, Ohsawa M, Hirakawa K, & Ohira M (2017). Prediction of survival after neoadjuvant chemotherapy for breast cancer by evaluation of tumor-infiltrating lymphocytes and residual cancer burden. BMC Cancer, 17, 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audia S, Nicolas A, Cathelin D, Larmonier N, Ferrand C, Foucher P, Fanton A, Bergoin E, Maynadie M, Arnould L, Bateman A, Lorcerie B, Solary E, Chauffert B, & Bonnotte B (2007). Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: a Phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clin Exp Immunol, 150, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C, Thuru X, & Quesnel B (2020). Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR Cancer, 2, zcaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balar AV, Castellano D, O’Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D , & Bellmunt J (2017). First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol, 18, 1483–1492. [DOI] [PubMed] [Google Scholar]
- Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Duran I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thastrom A, Abidoye OO, Fine GD, Bajorin DF, & Group IMS (2017). Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet, 389, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Ozguroglu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabro L, Aren Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, & Park K (2018). Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol, 19, 1468–1479. [DOI] [PubMed] [Google Scholar]
- Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, & Deshmukh RR (2020). Resistance to Checkpoint Inhibition in Cancer Immunotherapy. Transl Oncol, 13, 100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, Endres S, Eigler A, & Dauer M (2007). Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut, 56, 1275–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, Kang H, Gibson MK, Massarelli E, Powell S, Meister A, Shu X, Cheng JD, & Haddad R (2017). Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results From a Single-Arm, Phase II Study. J Clin Oncol, 35, 1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF, & K.-. Investigators (2017). Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med, 376, 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berd D, Maguire HC Jr., & Mastrangelo MJ (1984a). Impairment of concanavalin A-inducible suppressor activity following administration of cyclophosphamide to patients with advanced cancer. Cancer Res, 44, 1275–1280. [PubMed] [Google Scholar]
- Berd D, Maguire HC Jr., & Mastrangelo MJ (1984b). Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res, 44, 5439–5443. [PubMed] [Google Scholar]
- Berd D, Maguire HC Jr., & Mastrangelo MJ (1986). Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res, 46, 2572–2577. [PubMed] [Google Scholar]
- Berd D, Maguire HC Jr., McCue P, & Mastrangelo MJ (1990). Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol, 8, 1858–1867. [DOI] [PubMed] [Google Scholar]
- Berd D, & Mastrangelo MJ (1987). Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res, 47, 3317–3321. [PubMed] [Google Scholar]
- Berd D, & Mastrangelo MJ (1988). Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res, 48, 1671–1675. [PubMed] [Google Scholar]
- Berd D, Mastrangelo MJ, Engstrom PF, Paul A, & Maguire H (1982). Augmentation of the human immune response by cyclophosphamide. Cancer Res, 42, 4862–4866. [PubMed] [Google Scholar]
- Beyranvand Nejad E, van der Sluis TC, van Duikeren S, Yagita H, Janssen GM, van Veelen PA, Melief CJ, van der Burg SH, & Arens R (2016). Tumor Eradication by Cisplatin Is Sustained by CD80/86-Mediated Costimulation of CD8+ T Cells. Cancer Res, 76, 6017–6029. [DOI] [PubMed] [Google Scholar]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr., Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, & Brahmer JR (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med, 373, 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracci L, Moschella F, Sestili P, La Sorsa V, Valentini M, Canini I, Baccarini S, Maccari S, Ramoni C, Belardelli F, & Proietti E (2007). Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res, 13, 644–653. [DOI] [PubMed] [Google Scholar]
- Bracci L, Schiavoni G, Sistigu A, & Belardelli F (2014). Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ, 21, 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, Schoonmaker C, Whiteside T, Smith LM, & Method M (2009). The Immune adjuvant properties of front-line carboplatin-paclitaxel: a randomized phase 2 study of alternative schedules of intravenous oregovomab chemoimmunotherapy in advanced ovarian cancer. J Immunother, 32, 54–65. [DOI] [PubMed] [Google Scholar]
- Bryniarski K, Szczepanik M, Ptak M, Zemelka M, & Ptak W (2009). Influence of cyclophosphamide and its metabolic products on the activity of peritoneal macrophages in mice. Pharmacol Rep, 61, 550–557. [DOI] [PubMed] [Google Scholar]
- Buhtoiarov IN, Sondel PM, Wigginton JM, Buhtoiarova TN, Yanke EM, Mahvi DA, & Rakhmilevich AL (2011). Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology, 132, 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G Jr., Psyrri A, Baste N, Neupane P, Bratland A, Fuereder T, Hughes BGM, Mesia R, Ngamphaiboon N, Rordorf T, Wan Ishak WZ, Hong RL, Gonzalez Mendoza R, Roy A, Zhang Y, Gumuscu B, Cheng JD, Jin F, Rischin D, & K.-. Investigators (2019). Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYnOtE-048): a randomised, open-label, phase 3 study. Lancet, 394, 1915–1928. [DOI] [PubMed] [Google Scholar]
- Byrd-Leifer CA, Block EF, Takeda K, Akira S, & Ding A (2001). The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol, 31, 2448–2457. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E , Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Metivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, & Kroemer G (2005). Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med, 202, 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YJ, Park EJ, Baik SH, Lee KY, & Kang J (2019). Clinical significance of tumor-infiltrating lymphocytes and neutrophil-to-lymphocyte ratio in patients with stage III colon cancer who underwent surgery followed by FOLFOX chemotherapy. Sci Rep, 9, 11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, & Peters S (2019). Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol, 30, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P, Sodhi A, & Shrivastava A (2009). Cisplatin primes murine peritoneal macrophages for enhanced expression of nitric oxide, proinflammatory cytokines, TLRs, transcription factors and activation of MAP kinases upon co-incubation with L929 cells. Immunobiology, 214, 197–209. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen Z, Chen D, Zhang B, Wang Z, & Le H (2015). Suppressive effects of gemcitabine plus cisplatin chemotherapy on regulatory T cells in nonsmall-cell lung cancer. J Int Med Res, 43, 180–187. [DOI] [PubMed] [Google Scholar]
- Chen YM, Whang-Peng J, Liu JM, Kuo BI, Wang SY, Tsai CM, & Perng RP (1996). Serum cytokine level fluctuations in chemotherapy-induced myelosuppression. Jpn J Clin Oncol, 26, 18–23. [DOI] [PubMed] [Google Scholar]
- Cheung YT, Ng T, Shwe M, Ho HK, Foo KM, Cham MT, Lee JA, Fan G, Tan YP, Yong WS, Madhukumar P, Loo SK, Ang SF, Wong M, Chay WY, Ooi WS, Dent RA, Yap YS, Ng R, & Chan A (2015). Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: a multi-centered, prospective, cohort study. Ann Oncol, 26, 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HC, Arkenau HT, Lee J, Rha SY, Oh DY, Wyrwicz L, Kang YK, Lee KW, Infante JR, Lee SS, Kemeny M, Keilholz U, Melichar B, Mita A, Plummer R, Smith D, Gelb AB, Xiong H, Hong J, Chand V, & Safran H (2019). Avelumab (anti-PD-L1) as first-line switch-maintenance or second-line therapy in patients with advanced gastric or gastroesophageal junction cancer: phase 1b results from the JAVELIN Solid Tumor trial. J Immunother Cancer, 7, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ, & K.-. investigators (2019). Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet, 393, 156–167. [DOI] [PubMed] [Google Scholar]
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P, & K.-. Investigators (2020). Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet, 396, 1817–1828. [DOI] [PubMed] [Google Scholar]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, & Boon T (2014). Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer, 14, 135–146. [DOI] [PubMed] [Google Scholar]
- Dammeijer F, De Gooijer CJ, van Gulijk M, Lukkes M, Klaase L, Lievense LA, Waasdorp C, Jebbink M, Bootsma GP, Stigt JA, Biesma B, Kaijen-Lambers MEH, Mankor J, Vroman H, Cornelissen R, Baas P, Van der Noort V, Burgers JA, & Aerts JG (2021). Immune monitoring in mesothelioma patients identifies novel immune-modulatory functions of gemcitabine associating with clinical response. EBioMedicine, 64, 103160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darst M, Al-Hassani M, Li T, Yi Q, Travers JM, Lewis DA, & Travers JB (2004). Augmentation of chemotherapy-induced cytokine production by expression of the platelet-activating factor receptor in a human epithelial carcinoma cell line. J Immunol, 172, 6330–6335. [DOI] [PubMed] [Google Scholar]
- Darvin P, Toor SM, Sasidharan Nair V, & Elkord E (2018). Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med, 50, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, & Johnson DB (2019). Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer, 7, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Biasi AR, Villena-Vargas J, & Adusumilli PS (2014). Cisplatin-induced antitumor immunomodulation: a review of preclinical and clinical evidence. Clin Cancer Res, 20, 5384–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goeje PL, Poncin M, Bezemer K, Kaijen-Lambers MEH, Groen HJM, Smit EF, Dingemans AC, Kunert A, Hendriks RW, & Aerts J (2019). Induction of Peripheral Effector CD8 T-cell Proliferation by Combination of Paclitaxel, Carboplatin, and Bevacizumab in Non-small Cell Lung Cancer Patients. Clin Cancer Res, 25, 2219–2227. [DOI] [PubMed] [Google Scholar]
- Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, & Symmans WF (2001). Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res, 7, 3025–3030. [PubMed] [Google Scholar]
- DeNardo DG, & Ruffell B (2019). Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol, 19, 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Torne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, & von Minckwitz G (2010). Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol, 28, 105–113. [DOI] [PubMed] [Google Scholar]
- Deshmukh SK, Tyagi N, Khan MA, Srivastava SK, Al-Ghadhban A, Dugger K, Carter JE, Singh S, & Singh AP (2018). Gemcitabine treatment promotes immunosuppressive microenvironment in pancreatic tumors by supporting the infiltration, growth, and polarization of macrophages. Sci Rep, 8, 12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco AM, Ruggiero A, & Riccardi R (2002). Cellular and molecular aspects of drugs of the future: oxaliplatin. Cell Mol Life Sci, 59, 1914–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkgraaf EM, Heusinkveld M, Tummers B, Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR, & van der Burg SH (2013). Chemotherapy alters monocyte differentiation to favor generation of cancer-supporting M2 macrophages in the tumor microenvironment. Cancer Res, 73, 2480–2492. [DOI] [PubMed] [Google Scholar]
- Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, & Bennouna J (2018). Safety and Antitumor Activity of the Anti-Programmed Death-1 Antibody Pembrolizumab in Patients With Advanced Esophageal Carcinoma. J Clin Oncol, 36, 61–67. [DOI] [PubMed] [Google Scholar]
- Dudek AM, Garg AD, Krysko DV, De Ruysscher D, & Agostinis P (2013). Inducers of immunogenic cancer cell death. Cytokine Growth Factor Rev, 24, 319–333. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, & Rosenberg SA (2005). Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol, 23, 2346–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF , Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, & Rosenberg SA (2008). Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol, 26, 5233–5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardson DW, Boudreau J, Mapletoft J, Lanner C, Kovala AT, & Parissenti AM (2017). Inflammatory cytokine production in tumor cells upon chemotherapy drug exposure or upon selection for drug resistance. PLoS One, 12, e0183662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA (2008). Chemotherapy and tumor immunity: an unexpected collaboration. Front Biosci, 13, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, & Jaffee EM (2009). Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol, 27, 5911–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson E, Wenthe J, Irenaeus S, Loskog A, & Ullenhag G (2016). Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med, 14, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, Braiteh F, Waterkamp D, He P, Zou W, Chen DS, Yi J, Sandler A, Rittmeyer A, & Group PS (2016). Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet, 387, 1837–1846. [DOI] [PubMed] [Google Scholar]
- Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, & Gillison ML (2016). Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med, 375, 1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, Clement PM, Mesia R, Kutukova S, Zholudeva L, Daste A, Caballero-Daroqui J, Keam B, Vynnychenko I, Lafond C, Shetty J, Mann H, Fan J, Wildsmith S, Morsli N, Fayette J, & Licitra L (2020). Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol, 31, 942–950. [DOI] [PubMed] [Google Scholar]
- Ferris RL, Licitra L, Fayette J, Even C, Blumenschein G Jr., Harrington KJ, Guigay J, Vokes EE, Saba NF, Haddad R, Ramkumar S, Russell J, Brossart P, Tahara M, Colevas AD, Concha-Benavente F, Lynch M, Li L, & Gillison ML (2019). Nivolumab in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck: Efficacy and Safety in CheckMate 141 by Prior Cetuximab Use. Clin Cancer Res, 25, 5221–5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, Garrido M, Golan T, Mandala M, Wainberg ZA, Catenacci DV, Ohtsu A, Shitara K, Geva R, Bleeker J, Ko AH, Ku G, Philip P, Enzinger PC, Bang YJ, Levitan D, Wang J, Rosales M, Dalal RP, & Yoon HH (2018). Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol, 4, e180013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka E, Yamashita K, Tanaka T, Sawada R, Sugita Y, Arimoto A, Fujita M, Takiguchi G, Matsuda T, Oshikiri T, Nakamura T, Suzuki S, & Kakeji Y (2019). Neoadjuvant Chemotherapy Increases PD-L1 Expression and CD8(+) Tumor-infiltrating Lymphocytes in Esophageal Squamous Cell Carcinoma. Anticancer Res, 39, 4539–4548. [DOI] [PubMed] [Google Scholar]
- Galaine J, Turco C, Vauchy C, Royer B, Mercier-Letondal P, Queiroz L, Loyon R, Mouget V, Boidot R, Laheurte C, Lakkis Z, Jary M, Adotevi O, Borg C, & Godet Y (2019). CD4 T cells target colorectal cancer antigens upregulated by oxaliplatin. Int J Cancer, 145, 3112–3125. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Buque A, Kepp O, Zitvogel L, & Kroemer G (2017). Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol, 17, 97–111. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, & Pages F (2006). Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science, 313, 1960–1964. [DOI] [PubMed] [Google Scholar]
- Galsky MD, Arija JAA, Bamias A, Davis ID, De Santis M, Kikuchi E, Garcia-Del-Muro X, De Giorgi U, Mencinger M, Izumi K, Panni S, Gumus M, Ozguroglu M, Kalebasty AR, Park SH, Alekseev B, Schutz FA, Li JR, Ye D, Vogelzang NJ, Bernhard S, Tayama D, Mariathasan S, Mecke A, Thastrom A, Grande E, & Group IMS (2020). Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet, 395, 1547–1557. [DOI] [PubMed] [Google Scholar]
- Gameiro SR, Caballero JA, & Hodge JW (2012). Defining the molecular signature of chemotherapy-mediated lung tumor phenotype modulation and increased susceptibility to T-cell killing. Cancer Biother Radiopharm, 27, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M , Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N , Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, & K.-. Investigators (2018). Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med, 378, 2078–2092. [DOI] [PubMed] [Google Scholar]
- Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L, & K.-. Investigators (2015). Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med, 372, 2018–2028. [DOI] [PubMed] [Google Scholar]
- Gartung A, Yang J, Sukhatme VP, Bielenberg DR, Fernandes D, Chang J, Schmidt BA, Hwang SH, Zurakowski D, Huang S, Kieran MW, Hammock BD, & Panigrahy D (2019). Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor. Proc Natl Acad Sci U S A, 116, 1698–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, & Martin F (2004). CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol, 34, 336–344. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, & Chauffert B (2007). Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother, 56, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney GT, Weiner LM, & Atkins MB (2016). Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol, 17, e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad Y, Eliaz Y, Yu Y, Han SJ, O’Malley BW, & Lonard DM (2019). Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci Rep, 9, 15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn JD, Xue P, & Whartenby KA (2018). Gemcitabine directly inhibits effector CD4 T cell activation and prevents experimental autoimmune encephalomyelitis. J Neuroimmunol, 316, 7–16. [DOI] [PubMed] [Google Scholar]
- Grabosch S, Bulatovic M, Zeng F, Ma T, Zhang L, Ross M, Brozick J, Fang Y, Tseng G, Kim E, Gambotto A, Elishaev E, R PE, & Vlad AM. (2019). Cisplatin-induced immune modulation in ovarian cancer mouse models with distinct inflammation profiles. Oncogene, 38, 2380–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravett AM, Trautwein N, Stevanovic S, Dalgleish AG, & Copier J (2018). Gemcitabine alters the proteasome composition and immunopeptidome of tumour cells. Oncoimmunology, 7, e1438107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman SA, Ellsworth S, Campian J, Wild AT, Herman JM, Laheru D, Brock M, Balmanoukian A, & Ye X (2015). Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. J Natl Compr Canc Netw, 13, 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Kraus SG, Quaney MJ, Daniels MA, Mitchem JB, & Teixeiro E (2020). FOLFOX Chemotherapy Ameliorates CD8 T Lymphocyte Exhaustion and Enhances Checkpoint Blockade Efficacy in Colorectal Cancer. Front Oncol, 10, 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulla A, Morelli E, Samur MK, Botta C, Hideshima T, Bianchi G, Fulciniti M, Malvestiti S, Prabhala RH, Talluri S, Wen K, Tai YT, Richardson PG, Chauhan D, Sewastianik T, Carrasco RD, Munshi NC, & Anderson KC (2021). Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation. Blood Cancer Discov, 2, 468–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, Heydebreck AV, Chin K, Cuillerot JM, & Kelly K (2017). Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol, 18, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Song P, Xue X, Guo C, Han L, Fang Q, Ying J, Gao S, & Li W (2019). Variation of Programmed Death Ligand 1 Expression After Platinum-based Neoadjuvant Chemotherapy in Lung Cancer. J Immunother, 42, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren AM, van Luijk IF, Lakeman J, Pocorni N, Kole J, de Menezes RX, Kenter GG, Bosse T, de Kroon CD, & Jordanova ES (2019). Neoadjuvant cisplatin and paclitaxel modulate tumor-infiltrating T cells in patients with cervical cancer. Cancer Immunol Immunother, 68, 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heylmann D, Bauer M, Becker H, van Gool S, Bacher N, Steinbrink K, & Kaina B (2013). Human CD4+CD25+ regulatory T cells are sensitive to low dose cyclophosphamide: implications for the immune response. PLoS One, 8, e83384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, & Urba WJ (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med, 363, 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, & Thurnher M (2005). Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother, 54, 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, Kato M, Prevost-Blondel A, Avril MF, Nardin A, & Abastado JP (2011). Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res, 71, 6997–7009. [DOI] [PubMed] [Google Scholar]
- Hong X, Dong T, Yi T, Hu J, Zhang Z, Lin S, & Niu W (2018). Impact of 5-Fu/oxaliplatin on mouse dendritic cells and synergetic effect with a colon cancer vaccine. Chin J Cancer Res, 30, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon DS, Foshag LJ, Nizze AS, Bohman R, & Morton DL (1990). Suppressor cell activity in a randomized trial of patients receiving active specific immunotherapy with melanoma cell vaccine and low dosages of cyclophosphamide. Cancer Res, 50, 5358–5364. [PubMed] [Google Scholar]
- Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F , Losonczy G, Johnson ML, Nishio M, Reck M, Mok T, Lam S, Shames DS, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Sandler A, Liu SV, & Group IMS (2018). First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med, 379, 2220–2229. [DOI] [PubMed] [Google Scholar]
- Huang J, Xu J, Chen Y, Zhuang W, Zhang Y, Chen Z, Chen J, Zhang H, Niu Z, Fan Q, Lin L, Gu K, Liu Y, Ba Y, Miao Z, Jiang X, Zeng M, Chen J, Fu Z, Gan L, Wang J, Zhan X, Liu T, Li Z, Shen L, Shu Y, Zhang T, Yang Q, Zou J, & Group ES (2020). Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol, 21, 832–842. [DOI] [PubMed] [Google Scholar]
- Hughes E, Scurr M, Campbell E, Jones E, Godkin A, & Gallimore A (2018). T-cell modulation by cyclophosphamide for tumour therapy. Immunology, 154, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, Majewski D, Fox SA, Nowak AK, & Nelson DJ (2012). Chemotherapy broadens the range of tumor antigens seen by cytotoxic CD8(+) T cells in vivo. Cancer Immunol Immunother, 61, 2343–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquillat C, Banzet P, & Maral J (1982). Clinical trials of chemotherapy and chemoimmunotherapy in primary malignant melanoma. Recent Results Cancer Res, 80, 254–258. [DOI] [PubMed] [Google Scholar]
- Jameson GS, Borazanci EH, Babiker HM, Poplin E, Niewiarowska AA, Gordon MS, Barrett MT, Ansaldo K, Lebron L, Stoll AC, Rosenthal A, Shemanski LR, Korn RL, Ramanathan RK, & Hoff DDV (2017). A phase Ib/II pilot trial with nab-paclitaxel plus gemcitabine plus cisplatin in patients (pts) with stage IV pancreatic cancer. Journal of Clinical Oncology, 35, 341–341. [Google Scholar]
- Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, Ott PA, Peltola K, Jaeger D, Evans J, de Braud F, Chau I, Harbison CT, Dorange C, Tschaika M, & Le DT (2018). CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol, 36, 2836–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjigian YY, Kawazoe A, Yanez PE, Luo S, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz L, Shitara K, Qin S, Cutsem EV, Tabernero J , Li L, Shih C-S, Bhagia P, Chung HCC, & o. b. o. t. K.-. investigators, (2021). Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. Journal of Clinical Oncology, 39, 4013–4013. [Google Scholar]
- Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K , Li M, & Ajani JA (2021). First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet, 398, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhunjhunwala S, Hammer C, & Delamarre L (2021). Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer, 21, 298–312. [DOI] [PubMed] [Google Scholar]
- John J, Ismail M, Riley C, Askham J, Morgan R, Melcher A, & Pandha H (2010). Differential effects of Paclitaxel on dendritic cell function. BMC Immunol, 11, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalanxhi E, Meltzer S, Schou JV, Larsen FO, Dueland S, Flatmark K, Jensen BV, Hole KH, Seierstad T, Redalen KR, Nielsen DL, & Ree AH (2018). Systemic immune response induced by oxaliplatin-based neoadjuvant therapy favours survival without metastatic progression in high-risk rectal cancer. Br J Cancer, 118, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyan A, Kircher SM, Mohindra NA, Nimeiri HS, Maurer V, Rademaker A, Benson AB, & Mulcahy MF (2016). Ipilimumab and gemcitabine for advanced pancreas cancer: A phase Ib study. Journal of Clinical Oncology, 34, e15747–e15747. [Google Scholar]
- Kamath SD, Kalyan A, Kircher S, Nimeiri H, Fought AJ, Benson A 3rd, & Mulcahy M (2019). Ipilimumab and Gemcitabine for Advanced Pancreatic Cancer: A Phase Ib Study. Oncologist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneno R, Shurin GV, Tourkova IL, & Shurin MR (2009). Chemomodulation of human dendritic cell function by antineoplastic agents in low noncytotoxic concentrations. J Transl Med, 7, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y-K, Satoh T, Ryu M-H, Chao Y, Kato K, Chung HC, Chen J-S, Muro K, Kang WK, Yoshikawa T, Oh SC, Tamura T, Lee K-W, Boku N, & Chen L-T (2017). Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): A double-blinded, randomized, phase III trial. Journal of Clinical Oncology, 35, 2–2. [Google Scholar]
- Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, & Chen LT (2017). Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet, 390, 2461–2471. [DOI] [PubMed] [Google Scholar]
- Karagiannis GS, Pastoriza JM, Wang Y, Harney AS, Entenberg D, Pignatelli J, Sharma VP, Xue EA, Cheng E, D’Alfonso TM, Jones JG, Anampa J, Rohan TE, Sparano JA, Condeelis JS, & Oktay MH (2017). Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci Transl Med, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki K, Akashi S, Shimazu R, Yoshida T, Miyake K, & Nishijima M (2000). Mouse toll-like receptor 4.MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by Taxol. J Biol Chem, 275, 2251–2254. [DOI] [PubMed] [Google Scholar]
- Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, & Djeu JY (2010). A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res, 16, 4583–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP, & K.-. Investigators (2020). Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol, 38, 4138–4148. [DOI] [PubMed] [Google Scholar]
- Kondelkova K, Vokurkova D, Krejsek J, Borska L, Fiala Z, & Ctirad A (2010). Regulatory T cells (TREG) and their roles in immune system with respect to immunopathological disorders. Acta Medica (Hradec Kralove), 53, 73–77. [DOI] [PubMed] [Google Scholar]
- Kudo T, Hamamoto Y, Kato K, Ura T, Kojima T, Tsushima T, Hironaka S, Hara H, Satoh T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Doki Y, & Kitagawa Y (2017). Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol, 18, 631–639. [DOI] [PubMed] [Google Scholar]
- Ladoire S, Eymard JC, Zanetta S, Mignot G, Martin E, Kermarrec I, Mourey E, Michel F, Cormier L, & Ghiringhelli F (2010). Metronomic oral cyclophosphamide prednisolone chemotherapy is an effective treatment for metastatic hormone-refractory prostate cancer after docetaxel failure. Anticancer Res, 30, 4317–4323. [PubMed] [Google Scholar]
- Ladoire S, Mignot G, Dabakuyo S, Arnould L, Apetoh L, Rebe C, Coudert B, Martin F, Bizollon MH, Vanoli A, Coutant C, Fumoleau P, Bonnetain F, & Ghiringhelli F (2011). In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol, 224, 389–400. [DOI] [PubMed] [Google Scholar]
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, & Jaffee E (2008). Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res, 14, 1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H , Gandhi L, & K.-. Investigators (2016). Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol, 17, 1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, McArthur GA, Ascierto PA, Long GV, Callahan MK, Postow MA, Grossmann K, Sznol M, Dreno B, Bastholt L, Yang A, Rollin LM, Horak C, Hodi FS, & Wolchok JD (2015). Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med, 373, 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, Wong F, Azad NS, Rucki AA, Laheru D, Donehower R, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Greten TF, Duffy AG, Ciombor KK, Eyring AD, Lam BH, Joe A, Kang SP, Holdhoff M, Danilova L, Cope L, Meyer C, Zhou S, Goldberg RM, Armstrong DK, Bever KM, Fader AN, Taube J, Housseau F, Spetzler D, Xiao N, Pardoll DM, Papadopoulos N, Kinzler KW, Eshleman JR, Vogelstein B, Anders RA, & Diaz LA Jr. (2017). Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science, 357, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledys F, Klopfenstein Q, Truntzer C, Arnould L, Vincent J, Bengrine L, Remark R, Boidot R, Ladoire S, Ghiringhelli F, & Derangere V (2018). RAS status and neoadjuvant chemotherapy impact CD8+ cells and tumor HLA class I expression in liver metastatic colorectal cancer. J Immunother Cancer, 6, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee M, Seo JH, Gong G, & Lee HJ (2020). Changes in Tumor-infiltrating Lymphocytes After Neoadjuvant Chemotherapy and Clinical Significance in Triple Negative Breast Cancer. Anticancer Res, 40, 1883–1890. [DOI] [PubMed] [Google Scholar]
- Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PM, Lin JC, Razaq MA, Teixeira MM, Lovey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, De Beukelaer S, Pavlov D, Thurm H, & Cohen E (2021). Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol, 22, 450–462. [DOI] [PubMed] [Google Scholar]
- Li D, Schaub N, Guerin TM, Bapiro TE, Richards FM, Chen V, Talsania K, Kumar P, Gilbert DJ, Schlomer JJ, Kim SJ, Sorber R, Teper Y, Bautista W, Palena C, Ock CY, Jodrell DI, Pate N, Mehta M, Zhao Y, Kozlov S, & Rudloff U (2021). T Cell-Mediated Antitumor Immunity Cooperatively Induced By TGFbetaR1 Antagonism and Gemcitabine Counteracts Reformation of the Stromal Barrier in Pancreatic Cancer. Mol Cancer Ther. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Duan XF, Wang LP, Xu YJ, Huang L, Zhang TF, Liu JY, Li F, Zhang Z, Yue DL, Wang F, Zhang B, & Zhang Y (2014). Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J Immunol Res, 2014, 286170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wu B, Yang T, Zhang L, & Jin K (2020). The outstanding antitumor capacity of CD4(+) T helper lymphocytes. Biochim Biophys Acta Rev Cancer, 1874, 188439. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Fumagalli L, Paolorossi F, & Mandala M (1999). Changes in lymphocyte number during cancer chemotherapy and their relation to clinical response. Int J Biol Markers, 14, 115–117. [PubMed] [Google Scholar]
- Liu P, Jaffar J, Hellstrom I, & Hellstrom KE (2010). Administration of cyclophosphamide changes the immune profile of tumor-bearing mice. J Immunother, 33, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WM, Fowler DW, Smith P, & Dalgleish AG (2010). Pre-treatment with chemotherapy can enhance the antigenicity and immunogenicity of tumours by promoting adaptive immune responses. Br J Cancer, 102, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PO, Wong GY, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves MJ, Helling F, Ritter G, & et al. (1994). Improved survival in stage III melanoma patients with GM2 antibodies: a randomized trial of adjuvant vaccination with GM2 ganglioside. J Clin Oncol, 12, 1036–1044. [DOI] [PubMed] [Google Scholar]
- Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA, & Nelson BH (2017). Neoadjuvant Chemotherapy of Ovarian Cancer Results in Three Patterns of Tumor-Infiltrating Lymphocyte Response with Distinct Implications for Immunotherapy. Clin Cancer Res, 23, 925–934. [DOI] [PubMed] [Google Scholar]
- Loubani O, & Hoskin DW (2005). Paclitaxel inhibits natural killer cell binding to target cells by down-regulating adhesion molecule expression. Anticancer Res, 25, 735–741. [PubMed] [Google Scholar]
- Lu CS, Lin CW, Chang YH, Chen HY, Chung WC, Lai WY, Ho CC, Wang TH, Chen CY, Yeh CL, Wu S, Wang SP, & Yang PC (2020). Antimetabolite pemetrexed primes a favorable tumor microenvironment for immune checkpoint blockade therapy. J Immunother Cancer, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Zhang L, Luo C, & Jiang M (2019). Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett, 454, 191–203. [DOI] [PubMed] [Google Scholar]
- Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, & Jaffee EM (2001). Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res, 61, 3689–3697. [PubMed] [Google Scholar]
- Maeda K, Hazama S, Tokuno K, Kan S, Maeda Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, Yoshino S, & Oka M (2011). Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res, 31, 4569–4574. [PubMed] [Google Scholar]
- Majewska-Szczepanik M, Kowalczyk P, Biala D, Marcinska K, Strzepa A, Wozniak D, Sura P, Pearson J, Wen L, & Szczepanik M (2018). Cyclophosphamide-modified murine peritoneal macrophages induce CD4(+) T contrasuppressor cells that protect contact sensitivity T effector cells from suppression. Pharmacol Rep, 70, 796–803. [DOI] [PubMed] [Google Scholar]
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G , Spertini F, Thompson JA, & Obeid M (2019). Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol, 16, 563–580. [DOI] [PubMed] [Google Scholar]
- Martins I, Kepp O, Schlemmer F, Adjemian S, Tailler M, Shen S, Michaud M, Menger L, Gdoura A, Tajeddine N, Tesniere A, Zitvogel L, & Kroemer G (2011). Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene, 30, 1147–1158. [DOI] [PubMed] [Google Scholar]
- Martins I, Michaud M, Sukkurwala AQ, Adjemian S, Ma Y, Shen S, Kepp O, Menger L, Vacchelli E, Galluzzi L, Zitvogel L, & Kroemer G (2012). Premortem autophagy determines the immunogenicity of chemotherapy-induced cancer cell death. Autophagy, 8, 413–415. [DOI] [PubMed] [Google Scholar]
- Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, Sehouli J, Colombo N, Gonzalez-Martin A, Oaknin A, Ottevanger PB, Rudaitis V, Katchar K, Wu H, Keefe S, Ruman J, & Ledermann JA (2019). Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol, 30, 1080–1087. [DOI] [PubMed] [Google Scholar]
- Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, Macarulla T, Merz V, Zecchetto C, Zhao Y, Gueorguieva I, Man M, Gandhi L, Estrem ST, Benhadji KA, Lanasa MC, Avsar E, Guba SC, & Garcia-Carbonero R (2021). Safety and activity of the TGFbeta receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetrier-Caux C, Ray-Coquard I, Blay JY, & Caux C (2019). Lymphopenia in Cancer Patients and its Effects on Response to Immunotherapy: an opportunity for combination with Cytokines? J Immunother Cancer, 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, & Kroemer G (2011). Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science, 334, 1573–1577. [DOI] [PubMed] [Google Scholar]
- Millrud CR, Mehmeti M, & Leandersson K (2018). Docetaxel promotes the generation of anti-tumorigenic human macrophages. Exp Cell Res, 362, 525–531. [DOI] [PubMed] [Google Scholar]
- Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, & Harbeck N (2020). Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet, 396, 1090–1100. [DOI] [PubMed] [Google Scholar]
- Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, Poddubskaya EV, Scambia G, Shparyk YV, Lim MC, Bhoola SM, Sohn J, Yonemori K, Stewart RA, Zhang X, Perkins Smith J, Linn C, & Ledermann JA (2021). Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol. [DOI] [PubMed] [Google Scholar]
- Morisaki T, Onishi H, Koya N, Kiyota A, Tanaka H, Umebayashi M, Ogino T, Nagamatsu I, & Katano M (2011). Combinatorial cytotoxicity of gemcitabine and cytokine-activated killer cells in hepatocellular carcinoma via the NKG2D-MICA/B system. Anticancer Res, 31, 2505–2510. [PubMed] [Google Scholar]
- Morris M, Platell C, & Iacopetta B (2008). Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res, 14, 1413–1417. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Uchi H, Lesokhin AM, Avogadri F, Rizzuto GA, Hirschhorn-Cymerman D, Panageas KS, Merghoub T, Wolchok JD, & Houghton AN (2010). Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood, 115, 4384–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Mimura K, Saito K, Thar Min AK, Endo E, Yamada L, Kase K, Yamauchi N, Matsumoto T, Nakano H, Kanke Y, Okayama H, Saito M, Neupane P, Saze Z, Watanabe Y, Hanayama H, Hayase S, Kaneta A, Momma T, Ohki S, Ohira H, & Kono K (2021). Neoadjuvant Chemotherapy Induces IL34 Signaling and Promotes Chemoresistance via Tumor-Associated Macrophage Polarization in Esophageal Squamous Cell Carcinoma. Mol Cancer Res, 19, 1085–1095. [DOI] [PubMed] [Google Scholar]
- Nejati R, Goldstein JB, Halperin DM, Wang H, Hejazi N, Rashid A, Katz MH, Lee JE, Fleming JB, Rodriguez-Canales J, Blando J, Wistuba II, Maitra A, Wolff RA, Varadhachary GR, & Wang H (2017). Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Chemotherapy. Pancreas, 46, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HY, Li J, Tao L, Lam AK, Chan KW, Ko JMY, Yu VZ, Wong M, Li B, & Lung ML (2018). Chemotherapeutic Treatments Increase PD-L1 Expression in Esophageal Squamous Cell Carcinoma through EGFR/ERK Activation. Transl Oncol, 11, 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nio Y, Hirahara N, Minari Y, Iguchi C, Yamasawa K, Toga T, & Tamura K (2000). Induction of tumor-specific antitumor immunity after chemotherapy with cisplatin in mice bearing MOPC-104E plasmacytoma by modulation of MHC expression on tumor surface. Anticancer Res, 20, 3293–3299. [PubMed] [Google Scholar]
- Nistico P, Capone I, Palermo B, Del Bello D, Ferraresi V, Moschella F, Arico E, Valentini M, Bracci L, Cognetti F, Ciccarese M, Vercillo G, Roselli M, Fossile E, Tosti ME, Wang E, Marincola F, Imberti L, Catricala C, Natali PG, Belardelli F, & Proietti E (2009). Chemotherapy enhances vaccine-induced antitumor immunity in melanoma patients. Int J Cancer, 124, 130–139. [DOI] [PubMed] [Google Scholar]
- North RJ (1982). Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med, 155, 1063–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Metivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, & Kroemer G (2007). Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med, 13, 54–61. [DOI] [PubMed] [Google Scholar]
- Ohtsukasa S, Okabe S, Yamashita H, Iwai T, & Sugihara K (2003). Increased expression of CEA and MHC class I in colorectal cancer cell lines exposed to chemotherapy drugs. J Cancer Res Clin Oncol, 129, 719–726. [DOI] [PubMed] [Google Scholar]
- Okita R, Wolf D, Yasuda K, Maeda A, Yukawa T, Saisho S, Shimizu K, Yamaguchi Y, Oka M, Nakayama E, Lundqvist A, Kiessling R, Seliger B, & Nakata M (2015). Contrasting Effects of the Cytotoxic Anticancer Drug Gemcitabine and the EGFR Tyrosine Kinase Inhibitor Gefitinib on NK Cell-Mediated Cytotoxicity via Regulation of NKG2D Ligand in Non-Small-Cell Lung Cancer Cells. PLoS One, 10, e0139809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita R, Yukawa T, Nojima Y, Maeda A, Saisho S, Shimizu K, & Nakata M (2016). MHC class I chain-related molecule A and B expression is upregulated by cisplatin and associated with good prognosis in patients with non-small cell lung cancer. Cancer Immunol Immunother, 65, 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M, Tsuda H, Shimizu C, Yamamoto S, Shibata T, Yamamoto H, Hirata T, Yonemori K, Ando M, Tamura K, Katsumata N, Kinoshita T, Takiguchi Y, Tanzawa H, & Fujiwara Y (2012). Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat, 132, 793–805. [DOI] [PubMed] [Google Scholar]
- Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, & Arnold JN (2019). Cytotoxic Chemotherapy as an Immune Stimulus: A Molecular Perspective on Turning Up the Immunological Heat on Cancer. Front Immunol, 10, 1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oratz R, Dugan M, Roses DF, Harris MN, Speyer JL, Hochster H, Weissman J, Henn M, & Bystryn JC (1991). Lack of effect of cyclophosphamide on the immunogenicity of a melanoma antigen vaccine. Cancer Res, 51, 3643–3647. [PubMed] [Google Scholar]
- Pallasch CP, Leskov I, Braun CJ, Vorholt D, Drake A, Soto-Feliciano YM, Bent EH, Schwamb J, Iliopoulou B, Kutsch N, van Rooijen N, Frenzel LP, Wendtner CM, Heukamp L, Kreuzer KA, Hallek M, Chen J, & Hemann MT (2014). Sensitizing protective tumor microenvironments to antibody-mediated therapy. Cell, 156, 590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Ozguroglu M, Vansteenkiste J, Spigel D, Yang JCH, Ishii H, Garassino M, de Marinis F, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabro L, Aren Frontera O, Xiong H, Bajars M, Ruisi M, & Barlesi F (2021). Avelumab Versus Docetaxel in Patients With Platinum-Treated Advanced NSCLC: 2-Year Follow-Up From the JAVELIN Lung 200 Phase 3 Trial. J Thorac Oncol, 16, 1369–1378. [DOI] [PubMed] [Google Scholar]
- Park SJ, Ye W, Xiao R, Silvin C, Padget M, Hodge JW, Van Waes C, & Schmitt NC (2019). Cisplatin and oxaliplatin induce similar immunogenic changes in preclinical models of head and neck cancer. Oral Oncol, 95, 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel MR, Ellerton J, Infante JR, Agrawal M, Gordon M, Aljumaily R, Britten CD, Dirix L, Lee KW, Taylor M, Schoffski P, Wang D, Ravaud A, Gelb AB, Xiong J, Rosen G, Gulley JL, & Apolo AB (2018). Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol, 19, 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Ozguroglu M, Ji JH, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Kazarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Shire N, Jiang H, Goldman JW, & investigators C (2019). Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet, 394, 1929–1939. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM, & K.-. Investigators (2018). Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med, 379, 2040–2051. [DOI] [PubMed] [Google Scholar]
- Pei Q, Pan J, Zhu H, Ding X, Liu W, Lv Y, Zou X, & Luo H (2014). Gemcitabine-treated pancreatic cancer cell medium induces the specific CTL antitumor activity by stimulating the maturation of dendritic cells. Int Immunopharmacol, 19, 10–16. [DOI] [PubMed] [Google Scholar]
- Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, & Armstrong TD (2010). Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cell Immunol, 263, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate JM, Plate AE, Shott S, Bograd S, & Harris JE (2005). Effect of gemcitabine on immune cells in subjects with adenocarcinoma of the pancreas. Cancer Immunol Immunother, 54, 915–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T, Csoszi T, Ozguroglu M, Matsubara N, Geczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Flechon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A, & K.-. Investigators (2021). Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol, 22, 931–945. [DOI] [PubMed] [Google Scholar]
- Powles T, Duran I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, Flechon A, Gravis G, Hussain S, Takano T, Leng N, Kadel EE 3rd, Banchereau R, Hegde PS, Mariathasan S, Cui N, Shen X, Derleth CL, Green MC, & Ravaud A (2018). Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet, 391, 748–757. [DOI] [PubMed] [Google Scholar]
- Principe DR, Korc M, Kamath SD, Munshi HG, & Rana A (2021). Trials and tribulations of pancreatic cancer immunotherapy. Cancer Lett, 504, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe DR, Narbutis M, Kumar S, Park A, Viswakarma N, Dorman MJ, Kamath SD, Grippo PJ, Fishel ML, Hwang RF, Thummuri D, Underwood PW, Munshi HG, Trevino JG, & Rana A (2020). Long-Term Gemcitabine Treatment Reshapes the Pancreatic Tumor Microenvironment and Sensitizes Murine Carcinoma to Combination Immunotherapy. Cancer Res, 80, 3101–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe DR, Park A, Dorman MJ, Kumar S, Viswakarma N, Rubin J, Torres C, McKinney R, Munshi HG, Grippo PJ, & Rana A (2019). TGFbeta Blockade Augments PD-1 Inhibition to Promote T-Cell-Mediated Regression of Pancreatic Cancer. Mol Cancer Ther, 18, 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, Richardson GE, Sessa C, Yonemori K, Banerjee S, Leary A, Tinker AV, Jung KH, Madry R, Park SY, Anderson CK, Zohren F, Stewart RA, Wei C, Dychter SS, & Monk BJ (2021). Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol, 22, 1034–1046. [DOI] [PubMed] [Google Scholar]
- Rebe C, Demontoux L, Pilot T, & Ghiringhelli F (2019). Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA, & Group IMS (2019). Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med, 7, 387–401. [DOI] [PubMed] [Google Scholar]
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, & K.-. Investigators (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med, 375, 1823–1833. [DOI] [PubMed] [Google Scholar]
- Reers S, Pfannerstill AC, Rades D, Maushagen R, Andratschke M, Pries R, & Wollenberg B (2013). Cytokine changes in response to radio-/chemotherapeutic treatment in head and neck cancer. Anticancer Res, 33, 2481–2489. [PubMed] [Google Scholar]
- Ren X, St Clair DK, & Butterfield DA (2017). Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol Res, 117, 267–273. [DOI] [PubMed] [Google Scholar]
- Renouf DJ, Dhani NC, Kavan P, Jonker DJ, Wei A. C.-c., Hsu T, Tang PA, Graham B, Gallinaro L, Hasan T, Li W, Hart K, Tu D, & O’Callaghan CJ (2018). The Canadian Cancer Trials Group PA.7 trial: Results from the safety run in of a randomized phase II study of gemcitabine (GEM) and nab-paclitaxel (Nab-P) versus GEM, nab-P, durvalumab (D), and tremelimumab (T) as first-line therapy in metastatic pancreatic ductal adenocarcinoma (mPDAC). Journal of Clinical Oncology, 36, 349–349. [Google Scholar]
- Rettig L, Seidenberg S, Parvanova I, Samaras P, Curioni A, Knuth A, & Pascolo S (2011). Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer, 129, 832–838. [DOI] [PubMed] [Google Scholar]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR, & Group OAKS (2017). Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet, 389, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie SA, Goldman JW, Shepherd FA, Chen AC, Shen Y, Nathan FE, Harbison CT, & Antonia S (2016). Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer. J Clin Oncol, 34, 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C (2020). A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun, 11, 3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, Davidson N, Richards J, Maio M, Hauschild A, Miller WH Jr., Gascon P, Lotem M, Harmankaya K, Ibrahim R, Francis S, Chen TT, Humphrey R, Hoos A, & Wolchok JD (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med, 364, 2517–2526. [DOI] [PubMed] [Google Scholar]
- Roselli M, Cereda V, di Bari MG, Formica V, Spila A, Jochems C, Farsaci B, Donahue R, Gulley JL, Schlom J, & Guadagni F (2013). Effects of conventional therapeutic interventions on the number and function of regulatory T cells. Oncoimmunology, 2, e27025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML, Al-Khami AA, El-Naggar SA, Diaz-Montero CM, Chen Y, & Cole DJ (2010). Cyclophosphamide induces dynamic alterations in the host microenvironments resulting in a Flt3 ligand-dependent expansion of dendritic cells. J Immunol, 184, 1737–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem ML, Diaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, & Cole DJ (2009). Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C). J Immunol, 182, 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salewski I, Henne J, Engster L, Schneider B, Lemcke H, Skorska A, Berlin P, Henze L, Junghanss C, & Maletzki C (2021). Combined Gemcitabine and Immune-Checkpoint Inhibition Conquers Anti-PD-L1 Resistance in Low-Immunogenic Mismatch Repair-Deficient Tumors. Int J Mol Sci, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta D, Park Y, Ni X, Li H, Zahnow CA, Gabrielson E, Pan F, & Semenza GL (2018). Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A, 115, E1239–E1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada S, Kimura Y, Emi A, Masumoto N, Kadoya T, Arihiro K, & Okada M (2020). Tumor-infiltrating Lymphocyte Score Based on FDG PET/CT for Predicting the Effect of Neoadjuvant Chemotherapy in Breast Cancer. Anticancer Res, 40, 3395–3400. [DOI] [PubMed] [Google Scholar]
- Schaer DA, Geeganage S, Amaladas N, Lu ZH, Rasmussen ER, Sonyi A, Chin D, Capen A, Li Y, Meyer CM, Jones BD, Huang X, Luo S, Carpenito C, Roth KD, Nikolayev A, Tan B, Brahmachary M, Chodavarapu K, Dorsey FC, Manro JR, Doman TN, Donoho GP, Surguladze D, Hall GE, Kalos M, & Novosiadly RD (2019). The Folate Pathway Inhibitor Pemetrexed Pleiotropically Enhances Effects of Cancer Immunotherapy. Clin Cancer Res, 25, 7175–7188. [DOI] [PubMed] [Google Scholar]
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, & I. M. T. Investigators (2018). Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med, 379, 2108–2121. [DOI] [PubMed] [Google Scholar]
- Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J, & K.-. Investigators (2020). Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med, 382, 810–821. [DOI] [PubMed] [Google Scholar]
- Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA, & Investigators IM (2020). Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol, 21, 44–59. [DOI] [PubMed] [Google Scholar]
- Serrano-Del Valle A, Anel A, Naval J, & Marzo I (2019). Immunogenic Cell Death and Immunotherapy of Multiple Myeloma. Front Cell Dev Biol, 7, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevko A, Kremer V, Falk C, Umansky L, Shurin MR, Shurin GV, & Umansky V (2012). Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice. J Immunotoxicol, 9, 275–281. [DOI] [PubMed] [Google Scholar]
- Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT, Lockhart AC, Arkenau HT, El-Hajbi F, Gupta M, Pfeiffer P, Liu Q, Lunceford J, Kang SP, Bhagia P, & Kato K (2019). Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol, 5, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Retz M, Siefker-Radtke A, Baron A, Necchi A, Bedke J, Plimack ER, Vaena D, Grimm MO, Bracarda S, Arranz JA, Pal S, Ohyama C, Saci A, Qu X, Lambert A, Krishnan S, Azrilevich A, & Galsky MD (2017). Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol, 18, 312–322. [DOI] [PubMed] [Google Scholar]
- Shehata M, Mukherjee A, Deen S, Al-Attar A, Durrant LG, & Chan S (2009). Human leukocyte antigen class I expression is an independent prognostic factor in advanced ovarian cancer resistant to first-line platinum chemotherapy. Br J Cancer, 101, 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibutani M, Maeda K, Nagahara H, Fukuoka T, Iseki Y, Matsutani S, Kashiwagi S, Tanaka H, Hirakawa K, & Ohira M (2018). Tumor-infiltrating Lymphocytes Predict the Chemotherapeutic Outcomes in Patients with Stage IV Colorectal Cancer. In Vivo, 32, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimasaki N, Jain A, & Campana D (2020). NK cells for cancer immunotherapy. Nat Rev Drug Discov, 19, 200–218. [DOI] [PubMed] [Google Scholar]
- Shin J, Chung JH, Kim SH, Lee KS, Suh KJ, Lee JY, Kim JW, Lee JO, Kim JW, Kim YJ, Lee KW, Kim JH, Bang SM, & Lee JS (2019). Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res Treat, 51, 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, & Tabernero J (2020). Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol, 6, 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin GV, Tourkova IL, Kaneno R, & Shurin MR (2009). Chemotherapeutic agents in noncytotoxic concentrations increase antigen presentation by dendritic cells via an IL-12-dependent mechanism. J Immunol, 183, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, Vitale I, Goubar A, Baracco EE, Remedios C, Fend L, Hannani D, Aymeric L, Ma Y, Niso-Santano M, Kepp O, Schultze JL, Tuting T, Belardelli F, Bracci L, La Sorsa V, Ziccheddu G, Sestili P, Urbani F, Delorenzi M, Lacroix-Triki M, Quidville V, Conforti R, Spano JP, Pusztai L, Poirier-Colame V, Delaloge S, Penault-Llorca F, Ladoire S, Arnould L, Cyrta J, Dessoliers MC, Eggermont A, Bianchi ME, Pittet M, Engblom C, Pfirschke C, Preville X, Uze G, Schreiber RD, Chow MT, Smyth MJ, Proietti E, Andre F, Kroemer G, & Zitvogel L (2014). Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med, 20, 1301–1309. [DOI] [PubMed] [Google Scholar]
- Smith PL, Yogaratnam Y, Samad M, Kasow S, & Dalgleish AG (2021). Effect of Gemcitabine based chemotherapy on the immunogenicity of pancreatic tumour cells and T-cells. Clin Transl Oncol, 23, 110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, & Group IMS (2018). Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med, 378, 2288–2301. [DOI] [PubMed] [Google Scholar]
- Soeda A, Morita-Hoshi Y, Makiyama H, Morizane C, Ueno H, Ikeda M, Okusaka T, Yamagata S, Takahashi N, Hyodo I, Takaue Y, & Heike Y (2009). Regular dose of gemcitabine induces an increase in CD14+ monocytes and CD11c+ dendritic cells in patients with advanced pancreatic cancer. Jpn J Clin Oncol, 39, 797–806. [DOI] [PubMed] [Google Scholar]
- Spanos WC, Nowicki P, Lee DW, Hoover A, Hostager B, Gupta A, Anderson ME, & Lee JH (2009). Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg, 135, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, & Dhodapkar MV (2007). Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood, 109, 4839–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprowl JA, Reed K, Armstrong SR, Lanner C, Guo B, Kalatskaya I, Stein L, Hembruff SL, Tam A, & Parissenti AM (2012). Alterations in tumor necrosis factor signaling pathways are associated with cytotoxicity and resistance to taxanes: a study in isogenic resistant tumor cells. Breast Cancer Res, 14, R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovska V, Prakash M, McQuade R, Fraser S, Apostolopoulos V, Sakkal S, & Nurgali K (2019). Oxaliplatin Treatment Alters Systemic Immune Responses. Biomed Res Int, 2019, 4650695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukkurwala AQ, Adjemian S, Senovilla L, Michaud M, Spaggiari S, Vacchelli E, Baracco EE, Galluzzi L, Zitvogel L, Kepp O, & Kroemer G (2014). Screening of novel immunogenic cell death inducers within the NCI Mechanistic Diversity Set. Oncoimmunology, 3, e28473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K, & K.-. Investigators (2021). Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet, 398, 759–771. [DOI] [PubMed] [Google Scholar]
- Takeuchi A, Eto M, Yamada H, Tatsugami K, Naito S, & Yoshikai Y (2012). A reduction of recipient regulatory T cells by cyclophosphamide contributes to an anti-tumor effect of nonmyeloablative allogeneic stem cell transplantation in mice. Int J Cancer, 130, 365–376. [DOI] [PubMed] [Google Scholar]
- Tel J, Hato SV, Torensma R, Buschow SI, Figdor CG, Lesterhuis WJ, & de Vries IJ (2012). The chemotherapeutic drug oxaliplatin differentially affects blood DC function dependent on environmental cues. Cancer Immunol Immunother, 61, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, Mendiboure J, Pignon JP, Jooste V, van Endert P, Ducreux M, Zitvogel L, Piard F, & Kroemer G (2010). Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene, 29, 482–491. [DOI] [PubMed] [Google Scholar]
- Tran L, Allen CT, Xiao R, Moore E, Davis R, Park SJ, Spielbauer K, Van Waes C, & Schmitt NC (2017). Cisplatin Alters Antitumor Immunity and Synergizes with PD-1/PD-L1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer Immunol Res, 5, 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, Monie A, & Wu TC (2008). Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res, 14, 3185–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchikawa T, Miyamoto M, Yamamura Y, Shichinohe T, Hirano S, & Kondo S (2012). The immunological impact of neoadjuvant chemotherapy on the tumor microenvironment of esophageal squamous cell carcinoma. Ann Surg Oncol, 19, 1713–1719. [DOI] [PubMed] [Google Scholar]
- Tsuda N, Chang DZ, Mine T, Efferson C, Garcia-Sastre A, Wang X, Ferrone S, & Ioannides CG (2007). Taxol increases the amount and T cell activating ability of self-immune stimulatory multimolecular complexes found in ovarian cancer cells. Cancer Res, 67, 8378–8387. [DOI] [PubMed] [Google Scholar]
- Vaishampayan U, Abrams J, Darrah D, Jones V, & Mitchell MS (2002). Active immunotherapy of metastatic melanoma with allogeneic melanoma lysates and interferon alpha. Clin Cancer Res, 8, 3696–3701. [PubMed] [Google Scholar]
- Verma R, Foster RE, Horgan K, Mounsey K, Nixon H, Smalle N, Hughes TA, & Carter CR (2016). Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res, 18, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg ZA, Hochster HS, George B, Gutierrez M, Johns ME, Chiorean EG, Kwak EL, Kalyan A, Manax V, Ye M, Chen T, Trunova N, & O’Dwyer PJ (2017). Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: Interim results from the pancreatic cancer (PC) cohorts. Journal of Clinical Oncology, 35, 412–412.27937096 [Google Scholar]
- Wainberg ZA, Hochster HS, Kim EJ-H, George B, Kalyan A, Chiorean EG, Waterhouse DM, Gutierrez M, Parikh AR, Jain R, Carrizosa DR, Soliman HH, Bhore R, Banerjee S, Lyons L, Louis CU, Ong TJ, & O’Dwyer PJ (2019). Phase I study of nivolumab (Nivo) + nab-paclitaxel (nab-P) + gemcitabine (Gem) in advanced pancreatic cancer (APC). Journal of Clinical Oncology, 37, 298–298. [DOI] [PubMed] [Google Scholar]
- Wakita D, Iwai T, Harada S, Suzuki M, Yamamoto K, & Sugimoto M (2019). Cisplatin Augments Antitumor T-Cell Responses Leading to a Potent Therapeutic Effect in Combination With PD-L1 Blockade. Anticancer Res, 39, 1749–1760. [DOI] [PubMed] [Google Scholar]
- Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, & Liu LF (2012). Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One, 7, e32542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Xu J, Zhang T, & Xue D (2016). Tumor-infiltrating lymphocytes in breast cancer predict the response to chemotherapy and survival outcome: A meta-analysis. Oncotarget, 7, 44288–44298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Probin V, & Zhou D (2006). Cancer therapy-induced residual bone marrow injury-Mechanisms of induction and implication for therapy. Curr Cancer Ther Rev, 2, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo JA, Reuben A, Cooper ZA, Oh KS, & Sullivan RJ (2015). Immune Effects of Chemotherapy, Radiation, and Targeted Therapy and Opportunities for Combination With Immunotherapy. Semin Oncol, 42, 601–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SC, Duffy CR, & Allison JP (2018). Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov, 8, 1069–1086. [DOI] [PubMed] [Google Scholar]
- Weiss GJ, Blaydorn L, Beck J, Bornemann-Kolatzki K, Urnovitz H, Schutz E, & Khemka V (2018). Phase Ib/II study of gemcitabine, nab-paclitaxel, and pembrolizumab in metastatic pancreatic adenocarcinoma. Invest New Drugs, 36, 96–102. [DOI] [PubMed] [Google Scholar]
- Weiss GJ, Waypa J, Blaydorn L, Coats J, McGahey K, Sangal A, Niu J, Lynch CA, Farley JH, & Khemka V (2017). A phase Ib study of pembrolizumab plus chemotherapy in patients with advanced cancer (PembroPlus). Br J Cancer, 117, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, & Cappuzzo F (2019). Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 20, 924–937. [DOI] [PubMed] [Google Scholar]
- West NR, Milne K, Truong PT, Macpherson N, Nelson BH, & Watson PH (2011). Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res, 13, R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woynarowski JM, Chapman WG, Napier C, Herzig MC, & Juniewicz P (1998). Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol Pharmacol, 54, 770–777. [DOI] [PubMed] [Google Scholar]
- Yan Y, Dronca RS, Liu X, Markovic S, & Dong H (2017). Effect of paclitaxel and carboplatin on tumor-reactive T cells and the efficacy of PD-1 blockade. Journal of Clinical Oncology, 35, 65–65. [Google Scholar]
- Yang H, Yamazaki T, Pietrocola F, Zhou H, Zitvogel L, Ma Y, & Kroemer G (2015). STAT3 Inhibition Enhances the Therapeutic Efficacy of Immunogenic Chemotherapy by Stimulating Type 1 Interferon Production by Cancer Cells. Cancer Res, 75, 3812–3822. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong J, Zheng X, & Xu L (2020). Low-Dose Gemcitabine Treatment Enhances Immunogenicity and Natural Killer Cell-Driven Tumor Immunity in Lung Cancer. Front Immunol, 11, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Morgan M, Haeryfar SM, Blay J, & Hoskin DW (2003). Exposure to paclitaxel or vinblastine down-regulates CD11a and CD54 expression by P815 mastocytoma cells and renders the tumor cells resistant to killing by nonspecific cytotoxic T lymphocytes induced with anti-CD3 antibody. Cancer Immunol Immunother, 52, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, Wang Z, Shu Y, Shi J, Hu Y, Wang Q, Cheng Y, Wu F, Chen J, Lin X, Wang Y, Huang J, Cui J, Cao L, Liu Y, Zhang Y, Pan Y, Zhao J, Wang L, Chang J, Chen Q, Ren X, Zhang W, Fan Y, He Z, Fang J, Gu K, Dong X, Zhang T, Shi W, Zou J, & Came LSG (2021). Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med, 9, 305–314. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang G, Chen Y, Wang H, Hua Y, & Cai Z (2019). Immunogenic cell death in cancer therapy: Present and emerging inducers. J Cell Mol Med, 23, 4854–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bastian IN, Long MD, Dow M, Li W, Liu T, Ngu RK, Antonucci L, Huang JY, Phung QT, Zhao XH, Banerjee S, Lin XJ, Wang H, Dang B, Choi S, Karin D, Su H, Ellisman MH, Jamieson C, Bosenberg M, Cheng Z, Haybaeck J, Kenner L, Fisch KM, Bourgon R, Hernandez G, Lill JR, Liu S, Carter H, Mellman I, Karin M, & Shalapour S (2021). Activation of NF-kappaB and p300/CBP potentiates cancer chemoimmunotherapy through induction of MHC-I antigen presentation. Proc Natl Acad Sci U S A, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsiros E, Lynam S, Attwood KM, Wang C, Chilakapati S, Gomez EC, Liu S, Akers S, Lele S, Frederick PJ, & Odunsi K (2021). Efficacy and Safety of Pembrolizumab in Combination With Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol, 7, 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


