Abstract
The genetic stability of selected epidemiologically linked strains of Campylobacter jejuni during outbreak situations was investigated by using subtyping techniques. Strains isolated from geographically related chicken flock outbreaks in 1998 and from a human outbreak in 1981 were investigated. There was little similarity in the strains obtained from the different chicken flock outbreaks; however, the strains from each of three chicken outbreaks, including strains isolated from various environments, were identical as determined by fla typing, amplified fragment length polymorphism (AFLP) analysis, and pulsed-field gel electrophoresis, which confirmed the genetic stability of these strains during the short time courses of chicken flock outbreaks. The human outbreak samples were compared with strain 81116, which originated from the same outbreak but has since undergone innumerable laboratory passages. Two main AFLP profiles were recognized from this outbreak, which confirmed the serotyping results obtained at the time of the outbreak. The major type isolated from this outbreak (serotype P6:L6) was exemplified by strain 81116. Despite the long existence of strain 81116 as a laboratory strain, the AFLP profile of this strain was identical to the profiles of all the other historical P6:L6 strains from the outbreak, indicating that the genotype has remained stable for almost 20 years. Interestingly, the AFLP profiles of the P6:L6 group of strains from the human outbreak and the strains from one of the recent chicken outbreaks were also identical. This similarity suggests that some clones of C. jejuni remain genetically stable in completely different environments over long periods of time and considerable geographical distances.
Campylobacter jejuni is a major cause of human acute bacterial enteritis worldwide. In England and Wales in 1998 there were just under 58,000 reported cases of campylobacter enteritis (3). The majority of cases are believed to be associated with the consumption of contaminated poultry meat; however, other sources of infection have been reported (21). Reliable epidemiological data are required to identify potential sources of human infection. A number of subtyping methods, both phenotypic and genotypic, have been applied to campylobacters, including serotyping, phage typing, pulsed-field gel electrophoresis (PFGE), PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of the flagellin locus (fla typing) (5), and a more recently developed technique, amplified fragment length polymorphism (AFLP) analysis (7, 12). AFLP analysis in particular is very sensitive and reflects the total genome of the organism. Data generated by such techniques have indicated that C. jejuni is a genotypically diverse species. There is also increasing evidence of instability in the genomes of campylobacters that has been shown to affect both fla typing (10, 11, 24) and PFGE (25). The mechanism of this instability is unknown, but it may be a consequence of both natural competence and genomic rearrangements (27). It may be that genetic instability is one way in which this organism maintains its diversity and ability to survive in a wide range of habitats (25), since the organism is ubiquitous in the environment and can be found in many different, sometimes hostile, habitats. Campylobacters must, therefore, readily adapt to the numerous stresses to which they are exposed in order to survive such diverse habitats. The possibility of genetic instability under naturally occurring conditions undermines the application of genetic subtyping. In this study, the occurrence of genetic instability under naturally occurring conditions was evaluated using epidemiologically linked strains.
Human outbreaks of campylobacteriosis are infrequent (21), but poultry flock outbreaks are common (14). Therefore, in this study the genetic stabilities of selected epidemiologically linked strains from poultry outbreak situations were investigated. In addition, strains from a human waterborne outbreak, first reported in 1983 at a boarding school in the United Kingdom (15), were investigated. This set of strains includes C. jejuni 81116, which has been extensively used as a standard strain worldwide and therefore has been subcultured frequently. Consequently, this strain has become laboratory adapted over time, as demonstrated by the fact that it has reduced colonization potential compared with fresh, wild-type isolates, which is increased only after passage through the chicken gut (6). The genomic stabilities of the strains examined were assessed by multiple techniques, including AFLP analysis, fla typing, and PFGE.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Poultry strains of C. jejuni were isolated from broiler flocks as part of an epidemiological survey conducted during 1997 and 1998 in and around the Exeter region in southwest England. Strains were isolated from cloacal swabs of birds, from bird feces, and from the environment in and around three broiler houses during production of one flock. The 21 poultry strains investigated in this study were selected on the basis of epidemiological linkage and subtype similarity by using fla typing data as the preliminary criteria. The nine human strains were isolated, as previously described (15), from a waterborne outbreak at a school in southeast England in 1981. C. jejuni 81116, now a routinely used laboratory strain, was originally isolated from this waterborne outbreak in 1982. All of the isolates used, their sources, and known epidemiological data are shown in Table 1. All of the strains have been stored at −80°C since isolation, and none of them was cloned (i.e., derived from single-colony cultures) prior to analysis; the whole bacterial population was analyzed in this study. Campylobacters were grown on blood agar plates supplemented with 10% sheep blood, Skirrow's antibiotics (Oxoid), and actidione (250 μg/ml) and were incubated for 24 h at 42°C under microaerobic conditions (85% [vol/vol] N2, 7.5% [vol/vol] CO2, 7.5% [vol/vol] O2).
TABLE 1.
Sources, times of isolation, subtypes, and PFGE types of all C. jejuni isolates investigated
| Outbreak | Isolate | Source | Time of isolation (day/mo/yr or mo/yr) | Subtypea | PFGE fingerprintb |
|---|---|---|---|---|---|
| Flock 1 | EX109 | Cow | 25/03/98–06/05/98 | fla: 3,14 | NDc |
| EX110 | Cow | 25/03/98–06/05/98 | fla: 3,14 | ND | |
| EX145 | Chicken | 25/03/98–06/05/98 | fla: 3,14 | ND | |
| EX146 | Chicken | 25/03/98–06/05/98 | fla: 3,14 | ND | |
| EX400 | Puddle | 25/03/98–06/05/98 | fla: 3,14 | ND | |
| Flock 2 | EX496 | Feces | 20/05/98–27/05/98 | fla: 2,5 | ND |
| EX497 | Feces | 20/05/98–27/05/98 | fla: 2,5 | S0, B1, K1 | |
| EX524 | Floor | 20/05/98–27/05/98 | fla: 2,5 | S0, B1, K1 | |
| EX537 | Support | 20/05/98–27/05/98 | fla: 2,5 | ND | |
| EX543 | Litter top | 20/05/98–27/05/98 | fla: 2,5 | ND | |
| EX602 | Feedhopper | 20/05/98–27/05/98 | fla: 2,5 | S0, B1, K1 | |
| Flock 3 | EX790 | Chicken | 13/07/98–21/07/98 | fla: 1,12 | ND |
| EX917 | Chicken | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| EX972 | Floor | 13/07/98–21/07/98 | fla: 1,12 | S2, B3, K3 | |
| EX983 | Drinker | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| EX1056 | Chicken | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| EX1178 | Drinker | 13/07/98–21/07/98 | fla: 1,12 | S2, B3, K3 | |
| EX1182 | Wall | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| EX1204 | Floor | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| EX1286 | Chicken | 13/07/98–21/07/98 | fla: 1,12 | S2, B3, K3 | |
| EX1401 | Floor | 13/07/98–21/07/98 | fla: 1,12 | ND | |
| Human | 82/55 | Human | 05/81–07/81 | ND | ND |
| 82/69 | Human | 05/81–07/81 | P6:L6 | S0, B1, K1 | |
| 82/71 | Water | 05/81–07/81 | P6:L6 | ND | |
| 82/72 | Water | 05/81–07/81 | P6:L6 | S0, B1, K1 | |
| 82/74 | Human | 05/81–07/81 | P58 | ND | |
| 82/77 | Human | 05/81–07/81 | P58 | ND | |
| 82/79 | Human | 05/81–07/81 | P6:L6 | ND | |
| 82/80 | Human | 05/81–07/81 | P6:L6 | S0, B1, K1 | |
| 82/86 | Human | 05/81–07/81 | P58 | S1, B2, K2 | |
| 82/87 | Human | 05/81–07/81 | P58 | S1, B2, K2 | |
| 81116 | Human | 05/81–07/81 | fla: 2,5 | S0, B1, K1 | |
| P6:L6 |
Each fla genotype designation comprises the HinfI and DdeI profile numbers, in that order. P and L refer to the Penner serotype and the Lior serotype, respectively.
Numbers were assigned arbitrarily to the PFGE fingerprints obtained after digestion with the following restriction enzymes: SmaI (S), BamHI (B), and KpnI (K). S0 indicates samples that were not digested with SmaI.
ND, not done.
Serotyping.
Serotyping was performed on selected strains (Table 1) (15) by using the method of Penner and Hennessy (18) based on heat-stable antigens, as well as the method of Lior et al. (13) based on heat-labile antigens.
Isolation of chromosomal DNA.
Campylobacter cultures were grown overnight on BASA plates at 42°C. Bacterial cells were scraped from the plates and washed with 1 ml of phosphate-buffered saline. Chromosomal DNA was extracted from all isolates by the hexadecyltrimethylammonium bromide method (4). Briefly, a bacterial pellet was resuspended in TE (10 mM Tris-Cl [pH 8.0], 1 mM EDTA [pH 8.0]), the cells were lysed with 10% (wt/vol) sodium dodecyl sulfate, and endonucleases were denatured during incubation with 20 mg of proteinase K per ml. Polysaccharides, cell wall debris, and denatured proteins were precipitated with 10% (wt/vol) hexadecyltrimethylammonium bromide–0.7 M NaCl and extracted with chloroform-isoamyl alcohol (24:1) and then with phenol-chlorofom-isoamyl alcohol (25:24:1). Chromosomal DNA was precipitated with isopropanol, and the pellet was washed with 70% (vol/vol) ethanol and resuspended in double-distilled H2O.
Flagellin PCR-RFLP analysis.
PCR-RFLP analysis of the flaA and flaB genes was carried out previously by using the technique of Ayling et al. (5), except that digestion was done with both DdeI and HinfI. Restriction enzymes were obtained from Promega, Madison, Wis. Briefly, the flaA and flaB genes were amplified in a standard PCR by using the following primers: P1 (5′-AAA GGA TCC GCG TAT TAA CAC AAA TGT TGC AGC-3′); P2 (5′-AAA GGA TCC GAG GAT AAA CAC CAA CAT CGG T-3′); and P3 (5′-GAT TTG TTA TAG CAG TTT CTG CTA TAT CC-3′). P1 and P2 bind to the 5′ region of flaA and flaB, respectively, and P3 binds to the 3′ region of both flaA and flaB. The approximately 1.5-kb product observed (representing both flaA and flaB) was digested with HinfI and DdeI separately. The resultant fragments were then separated on 2% agarose gels, the resultant profile was analyzed by using GelMatch software, version 1.2 (Ultraviolet Products, Cambridge, United Kingdom), and the fla type was determined by comparison to a database consisting of all profiles.
AFLP analysis.
AFLP analysis was performed by using an adaptation of the AFLP microbial fingerprinting protocol of PE Applied Biosystems (Perkin-Elmer, Norwalk, Conn.) as previously described (7) and restriction enzymes HindIII and HhaI. Briefly, chromosomal DNA was digested with HindIII and HhaI and simultaneously ligated to restriction site-specific adapters. A preselective PCR with an aliquot of the restriction-ligation mixture was carried out by using adapter-specific primers for the HindIII (5′-GAC TGC GTA CCA GCT T) and HhaI (5′-GAT GAG TCC TGA TCG C) adapters. Following amplification, an aliquot of the preselective PCR mixture was subjected to selective PCR by using a fluorescently labeled HindIII primer that contained an additional A at the 3′ end (5′-GAC TGC GTA CCA GCT TA) and an HhaI primer with an A extension (5′-GAT GAG TCC TGA TCG CA). The final products were electrophoresed on a 7.3% denaturing acrylamide sequencing gel by using an ABI 373A automated DNA sequencer. GelCompar v4.1 software was used to analyze the data, and the unweighted pair group method using average linkage was used to cluster the patterns. The results are presented as a dendrogram reflecting the genetic homologies between isolates as percentages.
PFGE.
The PFGE method used was based on that of Gibson et al. (9), with the following modifications. The bacterial suspension was adjusted to an optical density at 550 nm of 0.5 in phosphate-buffered saline prior to preparation of the plugs. The cells were lysed during two consecutive 24-h incubations in lysis buffer and proteinase K at 55°C. Prior to digestion of the genomic DNA with the enzymes SmaI, KpnI, and BamHI, the plugs were allowed to soak for 48 h in the enzyme reaction buffer. For PFGE a BioRad Chef-DR 111 system was used, and the DNA fragments were separated with a ramped pulse of 10 to 35 s for 21 h at 200 V and 14°C.
RESULTS
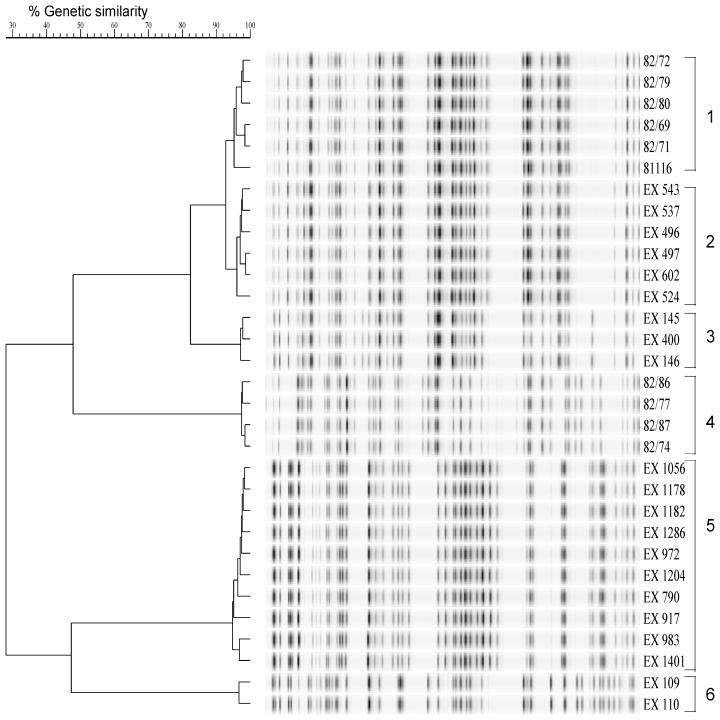
The five isolates from flock 1 were divided into two distinct groups on the basis of AFLP profiles (Fig. 1, clusters 3 and 6). EX109 and EX110, isolated from cows, formed one group (cluster 6) with a level of homology of 96%. The two chicken isolates, EX145 and EX146, and a puddle isolate, EX400, formed the second cluster (cluster 3) with a level of homology of more than 95%. The two groups were not related to each other, with a level of homology of only 30%. For flocks 2 and 3 one AFLP profile was obtained for all isolates obtained from the same broiler house, and the levels of homology were 96 and 95%, respectively (Fig. 1, clusters 2 and 5, respectively). The fla typing data (Table 1) confirmed the AFLP data, and both sets of data indicated that the poultry outbreaks were due to one subtype of C. jejuni.
FIG. 1.
Dendrogram based on fluorescently labelled AFLP patterns of 31 C. jejuni strains. Each cluster of isolates is numbered. Strains were isolated from three broiler flock outbreaks from chickens and the environment in and around broiler houses (clusters 2, 3, 5, and 6) and from a human waterborne outbreak (clusters 1 and 4). Strain 81116 is a laboratory-adapted strain which was originally isolated from the human outbreak in 1981. For the sources of isolation see Table 1.
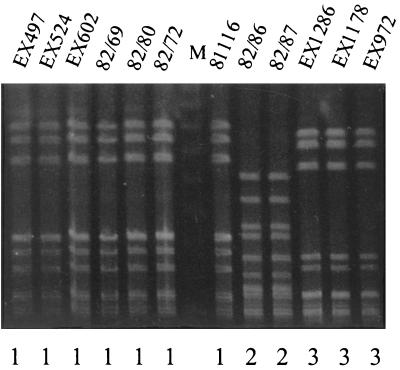
For the human outbreak studied, the AFLP profiles fell into two distinct clusters (Fig. 1, clusters 1 and 4). Five of the isolates, including two water isolates, formed cluster 1 with a level of homology of 96%. Four other strains formed a second cluster, cluster 4, with a level of homology of 98%. At the time of the outbreak a selection of isolates from both patients and the infected water source were serotyped (19). These isolates also fell into two main groups according to their serotypes. Isolates of cluster 1 (in this study) were serotype P6:L6, while isolates of cluster 4 were serotype P58. A widely used, laboratory-adapted strain of C. jejuni, strain 81116, was first isolated from a case of campylobacteriosis associated with this human waterborne outbreak and was serotype P6:L6. The original isolate was no longer available. Instead, the strain was included after it had been passaged on many occasions in the laboratory over the last 18 years. As Fig. 1 shows, the AFLP profile of this strain has remained virtually unchanged, and strain 81116 is still highly homologous with the other members of the P6:L6 group of isolates. Unexpectedly, we noted that the AFLP profiles of the isolates from flock 2 (cluster 2) were also highly homologous to the AFLP profiles of the P6:L6 group of isolates from the human waterborne outbreak. The level of homology was more than 90%, which indicated genetic relatedness of the strains. To determine whether this level of homology was detectable with another genotyping method, selected isolates were also tested by PFGE. To maximize sensitivity, the PFGE analysis was performed with SmaI, BamHI, and KpnI. With all of these enzymes the PFGE profiles of the poultry isolates from flock 2 and the cluster 1 human isolates were identical (Table 1). The KpnI PFGE patterns obtained are shown in Fig. 2. Moreover, the fla type of strain 81116 was 2,5, like that of the poultry strains from flock 2.
FIG. 2.
PFGE of KpnI-digested chromosomal DNA of C. jejuni isolates from flock 2 (EX497, EX524, EX602), flock 3 (EX1286, EX1178, EX972), and a human outbreak (82/69, 82/80, 82/72, 82/86, 82/87) and laboratory-attenuated strain 81116. The PFGE types are indicated below the lanes. Lane M contained a molecular mass marker.
DISCUSSION
The genetic stability of epidemiologically linked strains of C. jejuni, selected on the basis of fla type, was investigated by using the highly discriminatory AFLP technique in combination with PFGE. Initially, isolates were obtained from three separate broiler flocks over periods ranging from several days to 1 month from chickens and various points in and around broiler houses. It can be assumed that the bacterial population represented by these isolates was subjected to various environmental stresses during these periods.
Initially, it seemed that the flock 1 isolates were all of clonal origin and that the cows on the farm were a potential source of the broiler house infection. However, AFLP analysis showed that the bovine isolates were not related to the other isolates in this outbreak. This observation confirms the need for a multilayer strategy when the epidemiological relatedness between strains is investigated (26). In this case the similarity between the puddle and poultry isolates suggests either that the two habitats were infected from the same source or that infection of one led to contamination of the other.
All the poultry isolates and the isolates from associated environments (with the exception mentioned above) for each individual flock were identical as determined by fla typing, AFLP analysis, and PFGE. These observations support the previous report that the majority of United Kingdom broiler house outbreaks are due to just one or two different subtypes (5). Moreover, the short-term genetic stability of isolates is apparently maintained even though the campylobacters, which are isolated from various environments and are spread by the fecal-oral route, are likely to have been exposed to a number of different stresses.
Longer-term genetic stability was also demonstrable in this study. Isolates collected over a 2-month period from various patients and water during a human outbreak in 1981 were clustered by AFLP analysis. Within each cluster the level of similarity of AFLP profiles was identical to the level of reproducibility expected for duplicate samples (Duim, unpublished data). Interestingly, the laboratory-adapted strain, strain 81116, which was originally isolated from this outbreak, was closely related (similarity, >95%) to the other members of one cluster from this outbreak. Remarkably, this strain has remained stable for 18 years despite having been subcultured on many occasions in the laboratory, while the other isolates were minimally cultured. Interestingly, although strain 81116 has reduced chicken colonization potential, it retains the capacity to maximally colonize following in vivo passage, indicating that its full genetic complement is present (6).
A fortuitous observation indicated that human outbreak cluster 1 isolates had an AFLP profile identical to that of isolates from flock 2. The homology was confirmed by PFGE and fla typing. This appears to be an example of both temporal stability and spatial stability in a C. jejuni strain within avian and human hosts and their environments.
Previous reports documented genomic instability in campylobacters detectable by fla typing (11) and PFGE (10, 25). Such instability may result from insertions, deletions, point mutations at restriction sites, acquisition of foreign DNA through natural transformation, and random or programmed recombination (27). As yet the triggers and reasons why these genomic rearrangements occur are unclear. There is a paucity of regulatory genes in the genome of C. jejuni (16), and genome reorganization is a potential regulatory mechanism (22). It may also be a mechanism for antigenic variation that enables immune avoidance. This may be particularly relevant to recombinations observed in the campylobacter flagellin locus as a result of DNA exchange between flaA and flaB within the same genome (1, 24) or between the flaAB genes of different strains (1, 24) or even species (2, 23). One explanation is that strain 81116 has adapted to a universal niche so that genomic organization is no longer required, but this seems unlikely as the occurrence of strains of serotype P6:L6 (8, 17, 20) and fla type 2,5 is relatively uncommon. Alternatively, this strain might have lost the ability to reorganize its genome. This is also unlikely, as strain 81116 has been used extensively for genetic manipulation, including natural transformation (23, 24). Our observations suggest that genome shuffling may not be as essential for campylobacter stress adaptation as previously thought. The frequency and mechanisms of genetic instability of campylobacters require further investigation. However, our findings suggest that the genetic stability of campylobacters is sufficient for genotyping methods to be useful, at least for short-term epidemiological investigations.
ACKNOWLEDGMENTS
This work was funded by a European Commission project (CAMPYNET).
We thank Alan Rigter for his technical skill and help with the AFLP analysis and the Food Microbiology Group, Exeter Public Health Laboratory, for flock sampling.
REFERENCES
- 1.Alm R A, Guerry P, Power M E, Trust T J. Variation in antigenicity and molecular weight of Campylobacter coli VC167 flagellin in different genetic backgrounds. J Bacteriol. 1992;174:4230–4238. doi: 10.1128/jb.174.13.4230-4238.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Guerry P, Trust T J. Significance of duplicated flagellin genes in Campylobacter. J Mol Biol. 1993;230:359–363. doi: 10.1006/jmbi.1993.1151. [DOI] [PubMed] [Google Scholar]
- 3.Anonymous. Surveillance of Campylobacter jejuni. Commun Dis Rep. 1999;9:177. [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, Albright L M, Coen D M, Varki A, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1994. [Google Scholar]
- 5.Ayling R D, Woodward M J, Evans S, Newell D G. Restriction fragment length polymorphism of polymerase chain reaction products applied to the differentiation of poultry campylobacters for epidemiological investigations. Res Vet Sci. 1996;60:168–172. doi: 10.1016/s0034-5288(96)90013-2. [DOI] [PubMed] [Google Scholar]
- 6.Cawthraw S A, Wassenaar T M, Ayling R, Newell D G. Increased colonization potential of Campylobacter jejuni strain 81116 after passage through chickens and its implication on the rate of transmission within flocks. Epidemiol Infect. 1996;117:213–215. doi: 10.1017/s0950268800001333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duim B, Wassenaar T M, Rigter A, Wagenaar J. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl Environ Microbiol. 1999;65:2369–2375. doi: 10.1128/aem.65.6.2369-2375.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fricker C R, Park R W A. A two-year study of the distribution of ‘thermophilic’ campylobacters in human, environmental and food samples from the Reading area with particular reference to toxin production and heat-stable serotype. J Appl Bacteriol. 1989;66:477–490. doi: 10.1111/j.1365-2672.1989.tb04568.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibson J R, Fitzgerald C, Owen R J. Comparison of PFGE, ribotyping and phage-typing in the epidemiological analysis of Campylobacter jejuni serotype HS2 infections. Epidemiol Infect. 1995;115:215–225. doi: 10.1017/s0950268800058349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanninen M-L, Hakkinen M, Rautelin H. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl Environ Microbiol. 1999;65:2272–2277. doi: 10.1128/aem.65.5.2272-2275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrington C S, Thomson-Carter F M, Carter P E. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35:2386–2392. doi: 10.1128/jcm.35.9.2386-2392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kokotovic B, On S L W. High-resolution genomic fingerprinting of Campylobacter jejuni and Campylobacter coli by analysis of amplified fragment length polymorphisms. FEMS Microbiol Lett. 1999;173:77–84. doi: 10.1111/j.1574-6968.1999.tb13487.x. [DOI] [PubMed] [Google Scholar]
- 13.Lior H, Woodward D L, Edgar J A, Laroche L J, Gill P. Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors. J Clin Microbiol. 1982;15:761–768. doi: 10.1128/jcm.15.5.761-768.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newell D G, Wagenaar J A. Poultry infections and their control at the farm level. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: American Society for Microbiology; 2000. pp. 497–509. [Google Scholar]
- 15.Palmer S R, Gully P R, White J M, Pearson A D, Suckling W G, Jones D M, Rawes J C, Penner J L. Water-borne outbreak of campylobacter gastroenteritis. Lancet. 1983;i:287–290. doi: 10.1016/s0140-6736(83)91698-7. [DOI] [PubMed] [Google Scholar]
- 16.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 17.Patton C M, Nicholson M A, Ostroff S M, Ries A A, Wachsmuth I K, Tauxe R V. Common somatic O and heat-labile serotypes among Campylobacter strains from sporadic infections in the United States. J Clin Microbiol. 1993;31:1525–1530. doi: 10.1128/jcm.31.6.1525-1530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Penner J L, Hennessy J N. Passive hemagglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens. J Clin Microbiol. 1980;12:732–737. doi: 10.1128/jcm.12.6.732-737.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penner J L, Pearson A D, Hennessey J N. Investigation of a waterborne outbreak of Campylobacter jejuni enteritis with a serotyping scheme based on thermostable antigens. J Clin Microbiol. 1983;18:1362–1365. doi: 10.1128/jcm.18.6.1362-1365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjogren E, Alestig K, Kaijser B. Campylobacter strains from Swedish patients with diarrhoea. Distribution of serotypes over a five year period. APMIS. 1989;97:221–226. [PubMed] [Google Scholar]
- 21.Tauxe R. Epidemiology of Campylobacter infections in the United States and other industrialized nations. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni. Current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 9–19. [Google Scholar]
- 22.Taylor D E. Genetic analysis of Campylobacter spp. In: Nachamkin I, Blaser M J, Tompkins L S, editors. Campylobacter jejuni. Current status and future trends. Washington, D.C.: American Society for Microbiology; 1992. pp. 255–266. [Google Scholar]
- 23.Wassenaar T M, Fry B N, van der Zeijst B A. Genetic manipulation of Campylobacter: evaluation of natural transformation and electro-transformation. Gene. 1993;132:131–135. doi: 10.1016/0378-1119(93)90525-8. [DOI] [PubMed] [Google Scholar]
- 24.Wassenaar T M, Fry B N, van der Zeijst B A. Variation of the flagellin gene locus of Campylobacter jejuni by recombination and horizontal gene transfer. Microbiology. 1995;141:95–101. doi: 10.1099/00221287-141-1-95. [DOI] [PubMed] [Google Scholar]
- 25.Wassenaar T M, Geilhausen B, Newell D G. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl Environ Microbiol. 1998;64:1816–1821. doi: 10.1128/aem.64.5.1816-1821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassenaar T M, Newell D G. Genotyping of Campylobacter spp. Appl Environ Microbiol. 2000;66:1–9. doi: 10.1128/aem.66.1.1-9.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassenaar T M, On S L W, Meinersmann R J. Genotyping and the consequences of genetic instability. In: Nachamkin I, Blaser M J, editors. Campylobacter. 2nd ed. Washington, D.C.: American Society for Microbiology; 2000. pp. 369–380. [Google Scholar]