Significance
Dozens of hypotheses have been proposed to explain the immense variation in species richness across the planet. Among those hypotheses, mechanisms involving variation in the rate of speciation and/or extinction have featured prominently. These include the Metabolic Theory of Ecology, a textbook theory conceptually linking climate and species richness. We reject these mechanisms for seed plants, a diverse (circa 332,000 species) and ecologically fundamental group of organisms. Together with an emerging body of evidence for animals, our results suggest that it may be time to retire those hypotheses that explain global species richness patterns with variation in diversification rate and focus on “time for speciation” or ecological processes instead.
Keywords: plant diversity drivers, biodiversity, macroecology, diversification, biogeography
Abstract
Species richness varies immensely around the world. Variation in the rate of diversification (speciation minus extinction) is often hypothesized to explain this pattern, while alternative explanations invoke time or ecological carrying capacities as drivers. Focusing on seed plants, the world’s most important engineers of terrestrial ecosystems, we investigated the role of diversification rate as a link between the environment and global species richness patterns. Applying structural equation modeling to a comprehensive distribution dataset and phylogenetic tree covering all circa 332,000 seed plant species and 99.9% of the world’s terrestrial surface (excluding Antarctica), we test five broad hypotheses postulating that diversification serves as a mechanistic link between species richness and climate, climatic stability, seasonality, environmental heterogeneity, or the distribution of biomes. Our results show that the global patterns of species richness and diversification rate are entirely independent. Diversification rates were not highest in warm and wet climates, running counter to the Metabolic Theory of Ecology, one of the dominant explanations for global gradients in species richness. Instead, diversification rates were highest in edaphically diverse, dry areas that have experienced climate change during the Neogene. Meanwhile, we confirmed climate and environmental heterogeneity as the main drivers of species richness, but these effects did not involve diversification rates as a mechanistic link, calling for alternative explanations. We conclude that high species richness is likely driven by the antiquity of wet tropical areas (supporting the “tropical conservatism hypothesis”) or the high ecological carrying capacity of warm, wet, and/or environmentally heterogeneous environments.
Species richness varies by several orders of magnitude from species-poor deserts to hyperdiverse tropical rainforests, but the mechanistic basis of this variation is hotly debated. Some explanations suggest that high species richness could be the result of more time to accumulate species (1, 2) or the ecological capacity to house many species (3, 4). There are, however, several prominent explanations that involve geographic variation in diversification rate, the net rate of speciation minus extinction (Table 1). To understand what drives species richness, we thus need empirical answers to the following questions. 1) What are the most important environmental correlates of species richness and diversification rate? 2) Do those correlations support the idea that diversification rate is the causal link between the environment and regional species richness?
Table 1.
Hypothesized mechanisms for environmental effects on species richness via the rate of diversification (speciation minus extinction)
| Hypotheses | Prediction |
|---|---|
| H1: Warm, wet climate causes low extinction rates due to high productivity and thus, larger/more abundant populations and high speciation rates due to high metabolic rates and thus, high mutation rates (Metabolic Theory of Ecology) |

|
| H2: High climatic stability causes low extinction rates due to stable niches requiring no adaptation or migration and high speciation rates due to populations being able to differentiate genetically without getting constantly mixed |

|
| H3: Strong climate seasonality causes low speciation rates due to the requirement of broad climatic niches, preventing ecological differentiation and allopatric speciation by climatic barriers |

|
| H4: Large environmental heterogeneity causes low extinction rates by buffering against climate change and high speciation rates due to greater opportunity for ecological specialization and geographic isolation |

|
| H5: Certain biomes, such as tropical rainforest, have low extinction rates and/or high speciation rates due to their historically large area and/or biotic habitat characteristics |

|
Black arrows indicate positive effects, and red arrows indicate negative effects.
The most biodiverse places on Earth are warm and wet (5–7). This is reflected in the latitudinal diversity gradient, the observation that species richness peaks near the equator and decreases toward the poles in many taxa (2, 8). Among dozens of hypotheses put forward to explain correlations of species richness with climate and latitude (7, 9), the Metabolic Theory of Ecology (10, 11) is one of the most popular ones, featuring prominently in biogeography textbooks (e.g., ref. 12). This theory postulates that climate affects species richness via two pathways involving the rates of speciation and extinction. First, high ambient energy (temperature) is thought to cause high metabolic rates, leading to high mutation rates and thus, high speciation rates. Second, the high net primary productivity of warm and wet environments is thought to facilitate larger population sizes, thus reducing extinction rates. Together, these mechanisms predict that climate (temperature, precipitation) influences species richness, not directly but via the net rate of diversification (H1 in Table 1).
Besides climate per se, climatic stability on timescales over millions of years has long been recognized as a potential driver of species richness in the tropics (13, 14). Correlations of climatic stability with species richness are increasingly being documented by modern studies (15). Well-known examples are the depauperate floras and faunas of regions that were severely affected by the ice ages of the Pleistocene (e.g., refs. 16 and 17). Pronounced climate change is usually thought to cause extinction (e.g., ref. 17), which in the case of global extinctions, should be reflected in diversification rates. Climate change has also been suggested to impede speciation by preventing newly separated populations from evolving reproductive isolation before getting reshuffled by climate change (18). Together, these mechanisms predict that climatic stability, measured as the difference in climate between some point in the past and the present, influences species richness, not directly but via the net rate of diversification (H2 in Table 1).
At much shorter timescales, seasonal variation in precipitation or temperature can impact species richness via niche specialization (19). According to this hypothesis, species living in seasonal climates require a broader climatic tolerance to survive both summer and winter or both wet and dry seasons. Conversely, less seasonal climates allow for a greater climatic specialization. This hypothesis famously predicts that “mountain passes are higher in the tropics” (20), where species have narrower climate niches than their temperate counterparts and thus, are less able to cross climatic barriers to dispersal. This should lead to higher degrees of population fragmentation, genetic divergence, and ultimately, allopatric speciation. Narrower niches may also simply allow more ecological speciation by means of adaptation to different climates. Together, these mechanisms predict that seasonality in temperature or precipitation influences species richness negatively, not directly but via the net rate of diversification (H3 in Table 1).
Spatial heterogeneity in environmental conditions, such as climate, soil, or land cover, is one of the most important and widely recognized drivers of species richness (21). A sampling unit that contains many different (micro-)climates, soils, vegetation types, etc. is expected to harbor more species than a unit with a more homogeneous environment for various reasons. At a large scale (i.e., sampling units of >100 km2), long climatic gradients (e.g., caused by mountains) or large numbers of different soil types should allow high rates of ecological speciation via adaptation to different climatic and/or edaphic niches (22, 23). At the same time, sampling units within which the different environments are patchily distributed (e.g., in topographically rugged areas) should experience high rates of population fragmentation, genetic divergence, and allopatric speciation (24, 25). We here follow Stein and Kreft (26) in using environmental heterogeneity as an umbrella term for both the variability (range or number of values) of environmental conditions in a region and their spatial configuration (“patchiness”). Together, these mechanisms predict that environmental heterogeneity influences species richness, not directly but via the net rate of diversification (H4 in Table 1).
Large parts of the literature on species richness and diversification rate are focused on biomes, broad units of vegetation with widely divergent histories and habitat characteristics (27–29). Biomes are commonly viewed as distinct “evolutionary arenas” (30, 31), and biomes that are particularly species rich [e.g., tropical rainforest (32)] or characterized by particularly rapid diversification [e.g., the alpine biome (33)] have received particular attention. Biomes may differ in diversification rate both due to their different area over time (31, 34) and due to differences in their vegetation structure, allowing different degrees of ecological speciation (2). Vegetation greatly modifies the environment by influencing local climatic and edaphic conditions and creating microhabitats, allowing for niche differentiation. For example, trees create a dynamic mosaic of light and shade (35) and form a complex substrate for epiphytes, which have recently been shown to contribute substantially to global plant diversity patterns (36). Although the distribution of biomes often mirrors the abiotic environment, such as climate and soils, this is not always the case (37). The idea of biomes as evolutionary arenas thus predicts that the presence of certain biomes influences species richness, not directly but via the net rate of diversification (H5 in Table 1).
We address these hypotheses using plants, which contribute the bulk of terrestrial biomass (38) and are often thought to drive species richness at higher trophic levels (7), yet their own species richness patterns remain incompletely documented and understood. Previous studies have been either restricted geographically or taxonomically (e.g., the studies reviewed by ref. 7) or based on a subsample of overall plant diversity (e.g., ref. 6). The most comprehensive study on plant species richness to date confirmed that species richness is highest in warm and wet places, highlighting the importance of climatic drivers, but it also identified environmental heterogeneity as a significant secondary driver (6). The role of speciation and extinction on species richness was not explicitly tested. Conversely, the most comprehensive study of geographic variation in plant speciation rates (39), which was based on circa 20% of all plant species, did not include any climatic or other environmental information. Thus, there is a need for a comprehensive study investigating the global relationships between environment, diversification rates, and species richness in plants.
Here, we present a global analysis of plant species richness and diversification rates based on a comprehensive distribution dataset and complete all-evidence phylogeny for seed plants (Spermatophyta). Using structural equation modeling, we explicitly test whether or not diversification rate serves as a link between environment and species richness, thus evaluating five central hypothesized drivers of species richness (Table 1).
Results
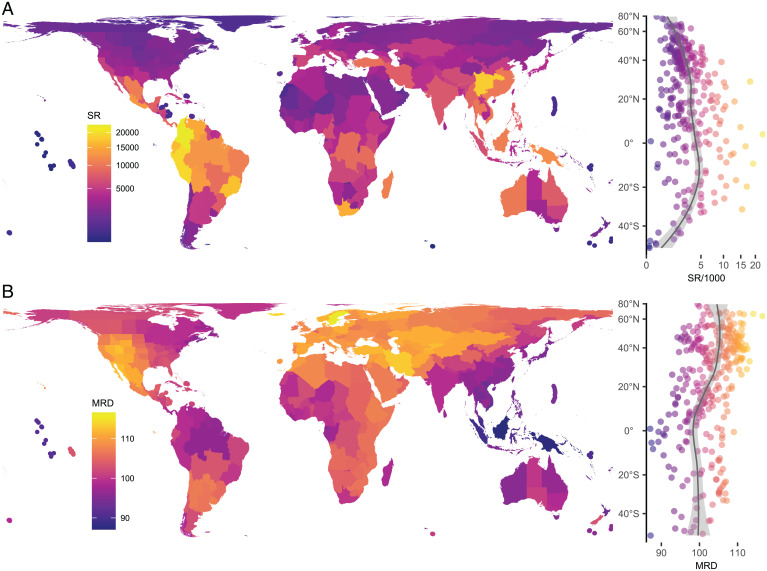
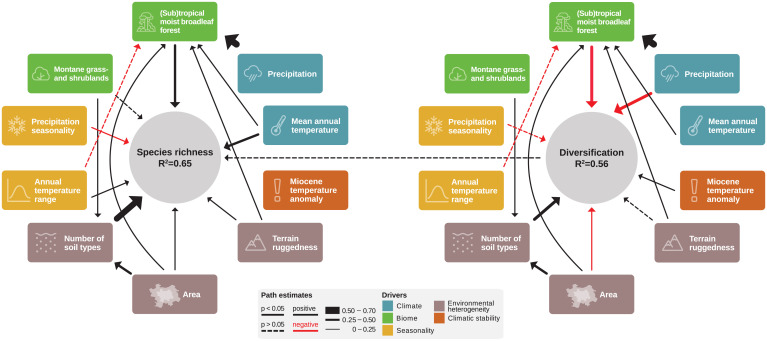
Relating seed plant species richness to diversification rate (quantified as mean root distance [MRD]) (Materials and Methods) across 310 botanical countries covering 99.9% of the world’s terrestrial surface (excluding Antarctica), we found no connection between the two variables. Species richness and diversification varied independently along latitude and showed no univariate correlation (Fig. 1 and SI Appendix, Fig. S1). While species richness increased toward equatorial regions (Fig. 1 and SI Appendix, Fig. S2), diversification was higher toward the poles (albeit only significantly so in the Northern Hemisphere). The latitudinal patterns varied slightly among continents (SI Appendix, Fig. S1), but the two variables were always unrelated. Our final structural equation model, which explained 65% of the global variation in species richness and 56% of the global variation in diversification (Fig. 2), also supported that the two variables were unrelated (a weak and nonsignificant effect of diversification on species richness). The model had an adequate fit (comparative fit index [CFI] = 0.979, root mean squared error of approximation [RMSEA] = 0.067, χ2 = 31, P = 0.009, degrees of freedom = 15), with a scaling correction factor of 1.31, and it was not substantially affected by spatial autocorrelation, scale dependencies, or the biogeographic particularities of remote oceanic islands (SI Appendix). Similar analyses with an alternative measure of diversification (40) yielded qualitatively identical results (SI Appendix). Taken together, these results firmly reject any hypotheses involving diversification as a mechanistic link between the environment and species richness.
Fig. 1.
Species richness and diversification rates in 310 botanical countries. (A) Species richness (SR) is the number of species in a botanical country. (B) Diversification rates are estimated as mean root distance (MRD), which is the average number of edges from tip to root in a phylogeny of all species occurring in a botanical country. A, Right and B, Right show scatterplots of the map data with local polynomial regression (gray lines) and 95% CIs (light gray areas) of SR and MRD to highlight latitudinal patterns. Botanical countries with areas smaller than 1,200 km2 (n = 12) are highlighted with thicker border lines. Maps are in Behrmann equal-area projection.
Fig. 2.
The structural equation model depicts direct and indirect drivers of species richness (SR) and diversification rates (MRD). The width of arrows is proportional to relative effect size (SI Appendix, Table S2). Black arrows represent positive effects, and red arrows represent negative effects; nonsignificant effects are shown as dashed lines. Drivers are color coded for the hypotheses they address.
Despite rejecting environmental effects on species richness via diversification, we were still able to test environmental effects on diversification (i.e., the first step in the causal chains predicted by H1 to H5 in Table 1) and direct environmental effects on species richness. Ten of the 32 initial environmental predictor variables (SI Appendix, Fig. S3 and Table S1) remained in our structural equation model after variable and model selection. Climate influenced both species richness and diversification, with a moderate negative effect of precipitation on diversification (counter to H1) and a moderate positive effect of both temperature and precipitation on species richness (Fig. 2 and SI Appendix, Table S2). Climate stability had no effect on species richness, but climate change since the Miocene significantly increased diversification (counter to H2). Seasonality had no effects on diversification (counter to H3) but contrasting weak influence on species richness through temperature and precipitation seasonality. Environmental heterogeneity influenced both diversification and species richness in a complex way. We found a moderate positive effect of the number of soil types on diversification, as predicted by H4. However, area, a “catchall” proxy for environmental heterogeneity, had a negative effect on diversification, running counter to H4. All measures of environmental heterogeneity that were present in the final model had a positive direct effect on species richness. Of note, the direct effect of the number of soil types on species richness was by far the strongest individual effect in the entire model (SI Appendix, Table S2). As predicted by H5, two biomes were represented in the final model: (sub-)tropical moist broadleaf forest (in the following, “tropical rainforest” for short) and montane grass- and shrublands. The presence of tropical rainforest had a moderate positive direct effect on species richness. As it was itself strongly determined by precipitation and weakly by temperature, area, and environmental heterogeneity, tropical rainforest acts as an intermediate variable (mechanistic link) in the model rather than an ultimate cause. Despite being included in the model, montane grass- and shrublands only played a minor role, mostly as a weak correlate of the number of soil types.
Discussion
Among the many hypotheses put forward to explain variation in species richness (7), those that involve variation in diversification rate (Table 1) are some of the most popular (9, 12, 32). None of these hypotheses stand up to the comprehensive empirical evidence we have gathered for seed plants. Although species richness in other groups, such as animals, fungi, or prokaryotes, may be controlled by different mechanisms, our results are in line with other studies on birds, mammals, and ants (40–43). Thus, there is a growing body of evidence casting substantial doubt on the importance of diversification rate for explaining species richness. Instead, other mechanisms, such as time for speciation (1, 2) or ecological carrying capacity (3, 4), may be more important.
Climate.
The mechanisms we reject include those predicted by the Metabolic Theory of Ecology (11, 44–46), a textbook theory explaining variation and species richness (H1 in Table 1). Not only was species richness unrelated to diversification, but we also rejected the hypothesized positive influence of temperature and precipitation on diversification rate. The expected effect of ambient energy (temperature) on metabolic rates and speciation either does not exist in plants or does not translate into diversification rates: for example, due to high extinction rates counteracting high speciation rates. Similarly, the expected lower extinction rates in productive, warm, and wet environments due to larger population sizes either do not exist in plants or are counteracted by low speciation rates. Either way, these mechanisms do not explain global variation in plant species richness.
Instead, we found a direct positive effect of temperature and precipitation on species richness. This finding fits the tropical conservatism hypothesis (47) that most lineages originated in tropical climates, which are relatively old and were widespread during much of the Cenozoic, providing more time and opportunity to accumulate high diversity (48). Interestingly, the effect of climate on species richness in our model is partly mediated by the presence of tropical rainforest, the biome to which the tropical conservatism hypothesis applies most neatly. Alternatively, the “more individuals” hypothesis (49) assumes that warmer and wetter environments, due to their higher primary productivity, can accommodate more individuals and thus, more species (all else being equal) than colder/drier environments. Further studies are required to distinguish between those two hypotheses.
Climatic Stability.
Despite ample evidence in the literature for climate change driving extinction (15, 17), neither diversification rate nor species richness were higher in more stable climates (H2 in Table 1). This could have several reasons. Extinctions may have been primarily local, such as the range contractions observed in many Northern Hemisphere plant species during the ice ages (50), thus not affecting diversification rate. However, a direct effect on species richness was also absent. This may partly be explained by the notorious difficulty of separating effects of past and present climate (51); for example, our measure of precipitation change since the Last Glacial Maximum never entered into model selection due to its strong correlation with current precipitation, which did affect species richness. Thus, effects of climate stability may be implicated in effects of current climate (the discussion on the tropical conservatism hypothesis above).
Instead, our results support the idea that Neogene climate change has promoted plant speciation (52). The origin and expansion of temperate climates are thought to have triggered adaptive diversification in several plant groups [e.g., Hypericum (53) and Carex (54)]. Similarly, increasing aridification from the Miocene (or the Oligocene) onward together with a drop in atmospheric CO2 levels exacerbating water stress is thought to have driven adaptive diversification in numerous plant groups (e.g., refs. 55 and 56). Spatial relationships between aridity and proxies of diversification rate have previously been found in lineages as different as conifers (51) and Zygophyllaceae (57). We think that these dynamics are reflected in the positive effect of temperature change since the late Miocene (5.6 Ma) and the negative effect of current precipitation on diversification rate recovered by our model (Fig. 2) (52). Our results are thus consistent with a parallel late Cenozoic boost in diversification across various temperate and/or dryland plant lineages against the backdrop of an ancestral, slower diversification regime characteristic of warm mesic climates.
Seasonality.
We did not find any evidence for a negative effect of seasonality on diversification, which would be expected if species in more seasonal climates have wider climatic niches (H3 in Table 1). Perhaps the climatic niches of plants are in fact not narrower in less seasonal climates; this interpretation is supported by evidence on species’ range sizes (58), which tend to be correlated with niche breadth (59). Alternatively, narrow niches may not translate into higher rates of ecological or allopatric speciation, or higher rates of speciation may be counteracted by higher rates of diversification. The latter is particularly plausible, considering that small-ranged, narrow-niched species are thought to be particularly extinction prone (17, 59, 60).
Instead of the expected effects via diversification, we found seasonality to be directly (albeit weakly) related to species richness. The weak negative effect of precipitation seasonality on species richness may suggest that stronger specialization and finer niche partitioning simply allow the coexistence of larger numbers of species (19). Negative effects of seasonal drought on plant diversity have been shown previously (61). Meanwhile, temperature seasonality had an unexpected positive effect on species richness. Closer investigation showed that the effect direction for both seasonality variables depended on temperature, showing that species richness decreased with seasonality in relatively warm regions but increased with seasonality in relatively cold regions (SI Appendix, Figs. S4 and S5). We conjecture that in cold regions, seasonality could be beneficial as it suggests the presence of a warmer, wetter growth season, which can promote species richness (62). In warm regions, however, seasonality (especially in precipitation) should be detrimental to species richness for the reasons outlined above. These relationships and their mechanistic basis require further study.
Environmental Heterogeneity.
There are strong reasons to expect that environmental heterogeneity promotes both diversification and species richness (21), and our model confirms these expectations. We found a moderate positive effect of soil diversity on diversification, supporting the idea that environmental heterogeneity increases diversification by fostering ecological speciation (H4 in Table 1). This effect, however, did not translate into higher species richness (counter to H4). Of note, we did not find significant evidence for higher diversification via allopatric speciation (25) in areas with more rugged topography. This may be due to the fact that we measured average diversification rate throughout the history of seed plants. While some of the most topographically rugged areas are renowned for spectacularly rapid radiations [e.g., the Andes (33, 63)], these are usually recent and may not be reflected in the average diversification rate (Fig. 1B). Also, we may in fact be underestimating the effect of topography, as some floras contain a mix of mountainous and lowland regions (e.g., Peru). To our surprise, we found a weak negative effect of area on diversification rates. This correlation may in fact be artificial, potentially due to the fact that our sampling units are partly defined by political boundaries.
Species richness was strongly and directly influenced by environmental heterogeneity. The effect of soil diversity on species richness was the strongest relationship in the entire model, highlighting the importance of environmental heterogeneity (21). Diversity of soil types indicates resource and nutrient diversity (64), increasing niche space and thus, allowing more species to coexist at a regional scale. The observed positive effect of area on species richness is consistent with the well-known species–area relationship (24, 65). The positive effect of terrain ruggedness on species richness in our model may be due to metacommunity dynamics (66), allowing larger numbers of species to coexist in spatially fragmented landscapes. However, it may also—at least partly—reflect a correlation between terrain ruggedness and elevational range (SI Appendix, Fig. S3), which implies a wider range of environmental conditions and hence, larger niche space.
Biomes as Evolutionary Arenas.
We included biomes in our model on the premise that they represent historical (age, area) and biotic (vegetation complexity) drivers of species richness that are not fully captured by our abiotic predictors (H5 in Table 1). This suspicion was confirmed by our results. Acknowledging that the occurrence of biomes is extensively [but not exclusively (37)] driven by the abiotic environment (27), we allowed the abiotic variables to affect biome distribution in our model. With this setup, we found that the presence of tropical rainforest played an important role in the effects of precipitation on both diversification rate and species richness. Running counter to the popular “cradle” and “museum” hypotheses (ref. 14; reviewed in ref. 32) but in line with accumulating evidence for lower diversification rates in the tropics (39, 67, 68), we found that diversification rates were low in tropical rainforest regions. It is thus becoming increasingly clear that tropical rainforests are species rich due to their age (13, 69) or area over time (34), not due to rapid speciation or little extinction.
The effect of precipitation on species richness was entirely mediated by tropical rainforest, indicating that the tropical rainforest habitat plays a major role in the “water” part of water–energy dynamics (7). This result agrees with the importance of biotic interactions as prerequisites for biodiversity accumulation, which has been postulated on geological timescales (70). Specifically, it seems likely that high precipitation (in warm areas) allows tropical rainforest to occur, which in turn, allows a variety of growth forms to coexist [including an astonishing diversity of epiphytes (36)]. This is not possible where rainforest cannot occur despite the right climate (e.g., for edaphic reasons [edaphic savannas, wetlands]). To test this mechanism in more detail, deconstructing biomes into their biotic habitat characteristics (71) would be worthwhile; here, we intentionally chose the most widely used biome classification to facilitate comparison with the existing literature.
Reliability and Limitations.
Although all macroecological analyses are fraught with uncertainty and data limitations, there are several reasons to believe that our findings are robust. Most importantly, we recovered latitudinal patterns of diversification rate and environment–species richness correlations that had previously been shown with independent datasets and different methods (6, 39). Although all studies may share some biases and limitations (discussed below), the overall congruence of results suggests that the recovered patterns are real.
While the strengths of our distribution dataset are its geographic breadth, covering nearly all of the world’s terrestrial surface that supports plants, and its taxonomic completeness, representing an exhaustive search of the taxonomic and floristic literature (72), its weakness is that the sampling units combine both political borders and biogeographical expert knowledge (82) and also, vary greatly in size (Fig. 1). We addressed this weakness in three ways. First, we included sampling unit area as a predictor of species richness [thus accounting for the well-known species–area relationship (24, 73)], diversification rate, and environmental factors that may be area dependent. Second, we conducted a supplementary analysis accounting for possible scale dependencies of environment–species richness relationships (74). Third, we conducted a supplementary analysis separating small oceanic islands, which are subject to highly specific biogeographic dynamics (75), from the rest of the data. The results suggest that our findings are robust to size variation among our sampling units. However, we cannot rule out that we have underestimated the effects of some environmental factors (e.g., topography; discussed above) that are hard to quantify for large heterogeneous countries. Conversely, we are unlikely to overestimate environmental effects with our data, and the ones that we have found (Fig. 2) should be reliable.
We acknowledge that our measure of diversification represents circa 350 My of evolution (76), while our environmental variables (except climatic stability) are snapshots of the environment at a specific point in time: the present. However, our findings should be robust to this mismatch. First, we repeated our analysis with an alternative measure that represents mostly recent diversification, reducing temporal mismatch (SI Appendix, Fig. S6 and Table S3). This analysis supported our main results. Second, current climate is often a reasonable proxy for past climate (e.g., ref. 51). The latitudinal position of most regions has changed little (77) since the beginning of the rise of angiosperms 120 Ma in the early Cretaceous (76). Even with the absolute global cooling since circa 55 Ma (78), the global latitudinal temperature gradient has remained mostly stable (79). Perhaps most importantly, climatic niches are highly conserved at broad scales in plants (80). Specifically, assuming tropical conservatism (47), most tropical lineages will have been tropical for their entire history, whereas the early diversification of temperate lineages would have taken place in the tropics. Accounting for this would only amplify our finding of higher diversification rates at higher latitudes. There is a somewhat higher risk of underestimating the effects of nonclimatic variables on diversification; these should be reevaluated once obtaining estimates of present diversification rates (e.g., ref. 81) becomes computationally feasible at the scale of our analysis.
A bias that may affect all studies, including our study, is the so-called “latitudinal taxonomy gradient” [i.e., an underestimation of true species richness in the tropics (82) due to cryptic species and falsely treating isolated populations as one species (the “allopatry problem”)]. If there was a high proportion of undetected plant species in the tropics, this could bias latitudinal patterns in both species richness and diversification rate. However, assuming that most undetected species are close relatives of described species (82), their absence only affects the diversification rates of one or a few species. Thus, we expect that diversification rate is a little more affected than species richness, and the relationship between those variables would remain largely unchanged. Given that undetected species are thought to occur where current species richness estimates are highest, we expect that addressing the latitudinal taxonomic bias would only make our findings for species richness more pronounced. Gauging how taxonomic bias might influence the relationship between diversification and environment is harder. Information on sampling effort could shed more light on the influence of such a taxonomic bias on observed macroecological patterns in the future.
Conclusions.
Mechanisms involving the rates of speciation and extinction are no longer in contention as drivers of global patterns in species richness. Species richness and diversification rates are simply not correlated geographically, ruling out a mechanistic relationship. Our study shows this decisively for seed plants, the engineers of the world’s terrestrial habitats. Viewing our results together with previous results on animals, we believe that our conclusion applies broadly across taxa. While diversification rates may still explain species richness in some systems at narrower spatial or phylogenetic scales, research on global patterns in biodiversity, including the latitudinal diversity gradient, may now have to look elsewhere for explanations. Both the time for speciation hypothesis and purely ecological explanations focusing on carrying capacities are still in the running. The way forward now requires a two-pronged approach. First, we should test if time is the main factor explaining species richness now that we have demonstrated that diversification rate is not. This will require a more explicit reconstruction of the accumulation of diversity in space and time. Second, more work is needed to fully unpick the unexpected relationships between diversification rate and environment that were revealed by our analysis. This will require a deep time perspective on environmental conditions to better match the timescale of diversification. Our results suggest that there are exciting times ahead for explaining the global diversity and diversification history of plants and other taxa. We may well see current textbook explanations, such as the Metabolic Theory of Ecology, replaced with new mechanisms that only become visible through the integration of new very large phylogenies with ever more powerful global distributional and environmental datasets.
Materials and Methods
All steps are summarized as the flowchart in SI Appendix, Fig. S7.
Geographical Data.
Species richness was calculated on the botanical countries level following the international standard of the World Geographical Scheme for Recording Plant Distributions (83). These geographical units mostly follow political countries, subdividing especially large countries (e.g., the United States) into smaller regions (83). Species presence data per botanical country were derived from the World Checklist of Vascular Plants (WCVP) (72) on 30 June 2021. We dismissed occurrences marked as introduced or extinct (regionally or globally) prior to calculations. Acknowledging the different sizes of botanical countries and the species–area relationship (24, 73), we decided to abstain from correcting potentially affected variables and instead, used area as a predictor variable in our analysis. Problems with standardization encompass the debated form of the species–area relationship (84) and the discrepancy between area and actually sampled area (85). Including area as a predictor variable avoids these pitfalls and allows for interaction with other potentially scale-dependent variables, like heterogeneity (86).
Phylogeny.
Phylogeny (87) tip labels were matched with WCVP species names to allocate diversification rates to botanical countries. Matching comprises several steps of string matching of taxonomic information; a detailed description is in SI Appendix. Tip labels with no matches were excluded. WCVP species missing in the phylogeny but accepted sensu WCVP (excluding ferns and fern allies) were added taxonomically to the most recent common ancestor node of the corresponding taxonomic group, preferring genus over family over order. Species that were not part of a genus, family, or order represented in the tree were dropped. Polytomies were resolved repeatedly (n = 1,000) to account for the random introduction of bifurcations. The average root distance for each species was used for MRD calculation.
Diversification Rates.
Diversification rates were calculated as MRD (88) based on a phylogenetic tree of all seed plant species (87). Root distance of a species is the number of nodes (speciation events with surviving descendants) separating the species from the root of the tree. This metric is expected to be high in clades that have undergone high rates of speciation and/or low rates of extinction, on average, over the course of their history (89). MRD for a botanical country is the average root distance of all the species it contains. We chose MRD as our main metric because it reflects not just recent diversification but the entire diversification history of the clade. For comparison, we also computed the DR statistic (42), which does emphasize recent diversification. We refer to the average DR statistic of the species in a botanical country as . Root distance was based on the “ALLMB” tree by Smith and Brown (87). was based on the average across 50 stochastic replicates of supplementing the “GBMB” tree with missing species using TACT (90).
Environmental Data.
We obtained data on climate, soil diversity, topography, and the proportional coverage with different biome types for each botanical country. Soil diversity was extracted as the number of soil types per botanical country. Temperature, precipitation, and potential evapotranspiration were calculated as mean values for annual temperature, annual temperature range, annual sum of potential evapotranspiration, annual sum of precipitation, and precipitation seasonality (coefficient of variation of precipitation). We also calculated the SD for all climate variables in each botanical country as a measure of spatial heterogeneity. Terrain ruggedness is estimated with the terrain ruggedness index (91) for each botanical country. The percentage of area covered by 11 different biome types (28) was calculated for each botanical country by overlaying the countries’ areas with the biome map. Each of these proportions was treated as a separate variable in the following steps. All environmental variables are listed with sources in detail in SI Appendix, Table S1.
Data Summary.
Our final dataset comprised species richness, diversification rates, and environmental variables for 310 botanical countries based on 331.939 species. From 369 initial botanical countries, 59 were excluded due to missing climate data. All analyses were done in R version 4.1.2 (92).
Variable Selection and Scaling.
Estimates of a variable’s influence on species richness and diversification were assessed using Pearson‘s product moment correlation coefficient (SI Appendix, Fig. S3) and generalized boosted regression models (GBMs). We ran 100 GBMs to account for stochasticity in the internal cross-validation procedure; results were summarized as relative variable position (i.e., most important, second important, etc.) in all GBMs (SI Appendix, Fig. S8). Environmental variables were transformed to approximate normal distribution when necessary. All variables were z transformed to account for different orders of magnitude between variables and avoid ill conditioning in the modeling process. Testing for multicollinearity between variables showed increased variance inflation factors for mean potential evapotranspiration and mean annual temperature as well as for Last Glacial Maximum precipitation anomaly and annual precipitation. Hence, we excluded mean potential evapotranspiration and Last Glacial Maximum precipitation anomaly from model selection.
Structural Equation Model.
We used structural equation modeling to allow for direct and indirect effects of variables. Structural equation models were fitted using the ML (maximum likelihood) estimator with robust SEs and the Satorra–Bentler scaled test statistic (93) to account for remaining nonnormality (94, 95). Model fit was evaluated focusing on CFI, RMSEA, and Akaike information criterion. The structural equation model structure was informed by variable correlation and influence estimates from GBMs and theoretical consideration. We assumed that 1) species richness is influenced by diversification and environmental variables, 2) diversification is influenced by environmental variables, 3) soil diversity and other potentially scale-dependent variables are influenced by area, and 4) tropical rainforest coverage is influenced by climate (SI Appendix, Fig. S9). While this structure and variables of interest were kept fixed, the set of predictor variables that provided the best model fit was found using randomized variable selection from a pool of 10 additional most important variables as identified by the GBMs (SI Appendix, Structural Equation Model Fitting). The number and composition of variables were unconstrained. Models with inherently acceptable fit (CFI > 0.9, RMSEA < 0.11) were further modified manually; the final model was chosen based on CFI and RMSEA, preferring models with more variables. To avoid underestimating SEs due to spatial autocorrelation (96), SE estimates were corrected using effective sample size based on the observed global Moran’s I of model residuals (97).
Sensitivity Analysis.
We performed several sensitivity analyses to address spatial scale–related interaction effects (74) and the biogeographic particularities of remote oceanic islands. First, we fitted our best model with additional area interaction effects for each environmental variable. Second, we separated botanical countries in mainland and oceanic islands of volcanic origin (98) and ran a multimodel structural equation model to identify significantly different path estimates between the groups. Third, we performed the complete variable and model selection using the average DR statistic () as an alternative diversification measure. Details are in SI Appendix.
Supplementary Material
Acknowledgments
We thank Nicholas Black and Robert Turner for providing various downloads of the WCVP and Miao Sun for help with matching the phylogeny to the distribution data and valuable thoughts on previous versions of the manuscript. Some of the computing for this project was performed on the GenomeDK cluster. A.A. acknowledges financial support from Swedish Research Council Grant 2019-05191, Swedish Foundation for Strategic Research Grant FFL15-0196, and the Royal Botanic Gardens, Kew. This research was supported by VILLUM FONDEN Grant 00025354 (to W.L.E.) and an Aarhus University Research Foundation research grant (to W.L.E.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2120662119/-/DCSupplemental.
Data Availability
Scripts and metrics for each botanical country have been deposited in Zenodo (DOI: 10.5281/zenodo.6350285 (99). We are using geographic distribution data (a presence matrix for all included plant species in botanical countries) for our work that have been collected by the authors for several years and will, therefore, be published as a separate data publication. The release/publication of these data is currently underway in the form of a special issue. We will publish all other data and code except for this data file. The files to repeat the final analysis steps (statistics, figures, etc.) are provided in the Zenodo repository as mentioned.
References
- 1.Wiens J. J., The causes of species richness patterns across space, time, and clades and the role of “ecological limits.” Q. Rev. Biol. 86, 75–96 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Baker H. G., Evolution in the tropics. Biotropica 2, 101 (1970). [Google Scholar]
- 3.Rabosky D. L., Ecological limits and diversification rate: Alternative paradigms to explain the variation in species richness among clades and regions. Ecol. Lett. 12, 735–743 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Rabosky D. L., Hurlbert A. H., Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Balslev H., Valencia R., Paz y Miño G., Christensen H., Nielsen I., “Species count of vascular plants in one hectare of humid lowland forest in Amazonian Ecuador” in Forest Biodiversity in North, Central and South America, and the Caribbean: Research and Monitoring, Dallmeier F., Comiskey J. A., Eds. (Parthenon Publishing Group, Camforth, United Kingdom, 1998), pp. 585–594. [Google Scholar]
- 6.Kreft H., Jetz W., Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. U.S.A. 104, 5925–5930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins B. A., et al. , Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003). [Google Scholar]
- 8.Fischer A. G., Latitudinal variations in organic diversity. Evolution 14, 64 (1960). [Google Scholar]
- 9.Mittelbach G. G., et al. , Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 10, 315–331 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Allen A. P., Brown J. H., Gillooly J. F., Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297, 1545–1548 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Allen A. P., Gillooly J. F., Savage V. M., Brown J. H., Kinetic effects of temperature on rates of genetic divergence and speciation. Proc. Natl. Acad. Sci. U.S.A. 103, 9130–9135 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomolino M. V., Riddle B. R., Whittaker R. J., Biogeography (Oxford University Press, 2017). [Google Scholar]
- 13.Wallace A. R., Tropical Nature, and Other Essays (Macmillan and Company, 1878). [Google Scholar]
- 14.Stebbins G. L., Flowering Plants: Evolution above the Species Level (Belknap Press of Harvard University Press, 1974). [Google Scholar]
- 15.Svenning J.-C., Eiserhardt W. L., Normand S., Ordonez A., Sandel B., The influence of paleoclimate on present-day patterns in biodiversity and ecosystems. Annu. Rev. Ecol. Evol. Syst. 46, 551–572 (2015). [Google Scholar]
- 16.Latham R. E., Ricklefs R. E., “Continental comparisons of temperate-zone tree species diversity” in Species Diversity in Ecological Communities: Historical and Geographical Perspectives, Ricklefs R. E., Schluter D., Eds. (University of Chicago Press, 1993), pp. 294–314. [Google Scholar]
- 17.Sandel B., et al. , The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Dynesius M., Jansson R., Evolutionary consequences of changes in species’ geographical distributions driven by Milankovitch climate oscillations. Proc. Natl. Acad. Sci. U.S.A. 97, 9115–9120 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens G. C., The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 133, 240–256 (1989). [Google Scholar]
- 20.Janzen D. H., Why mountain passes are higher in the tropics. Am. Nat. 101, 233–249 (1967). [Google Scholar]
- 21.Stein A., Gerstner K., Kreft H., Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Rundle H. D., Nosil P., Ecological speciation. Ecol. Lett. 8, 336–352 (2005). [Google Scholar]
- 23.Rahbek C., et al. , Building mountain biodiversity: Geological and evolutionary processes. Science 365, 1114–1119 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Rosenzweig M. L., Species Diversity in Space and Time (Cambridge University Press, 1995). [Google Scholar]
- 25.Barthlott W., Mutke J., Rafiqpoor D., Kier G., Kreft H., Global centers of vascular plant diversity. Nova Acta Leopoldina NF 92, 61–83 (2005). [Google Scholar]
- 26.Stein A., Kreft H., Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev. Camb. Philos. Soc. 90, 815–836 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Mucina L., Biome: Evolution of a crucial ecological and biogeographical concept. New Phytol. 222, 97–114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson D. M., et al. , Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 51, 933 (2001). [Google Scholar]
- 29.Willis K., McElwain J., The Evolution of Plants (OUP, Oxford, United Kingdom, 2014). [Google Scholar]
- 30.Ringelberg J. J., Zimmermann N. E., Weeks A., Lavin M., Hughes C. E., Biomes as evolutionary arenas: Convergence and conservatism in the trans‐continental succulent biome. Glob. Ecol. Biogeogr. 29, 1100–1113 (2020). [Google Scholar]
- 31.Jetz W., Fine P. V. A., Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS Biol. 10, e1001292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eiserhardt W. L., Couvreur T. L. P., Baker W. J., Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. New Phytol. 214, 1408–1422 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Hughes C. E., Atchison G. W., The ubiquity of alpine plant radiations: From the Andes to the Hengduan Mountains. New Phytol. 207, 275–282 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Fine P. V. A., Ree R. H., Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity. Am. Nat. 168, 796–804 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Denslow J. S., Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Syst. 18, 431–451 (1987). [Google Scholar]
- 36.Taylor A., et al. , Vascular epiphytes contribute disproportionately to global centres of plant diversity. Glob. Ecol. Biogeogr. 31, 62–74 (2022). [Google Scholar]
- 37.Bond W. J., What limits trees in C4 grasslands and savannas? Annu. Rev. Ecol. Evol. Syst. 39, 641–659 (2008). [Google Scholar]
- 38.Bar-On Y. M., Phillips R., Milo R., The biomass distribution on Earth. Proc. Natl. Acad. Sci. U.S.A. 115, 6506–6511 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igea J., Tanentzap A. J., Angiosperm speciation cools down in the tropics. Ecol. Lett. 23, 692–700 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Hawkins B. A., et al. , Different evolutionary histories underlie congruent species richness gradients of birds and mammals. J. Biogeogr. 39, 825–841 (2012). [Google Scholar]
- 42.Economo E. P., Narula N., Friedman N. R., Weiser M. D., Guénard B., Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 9, 1778 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soria-Carrasco V., Castresana J., Diversification rates and the latitudinal gradient of diversity in mammals. Proc. Biol. Sci. 279, 4148–4155 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tittensor D. P., Worm B., A neutral-metabolic theory of latitudinal biodiversity. Glob. Ecol. Biogeogr. 25, 630–641 (2016). [Google Scholar]
- 45.Brown J. H., Gillooly J. F., Allen A. P., Savage V. M., West G. B., Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004). [Google Scholar]
- 46.Stegen J. C., Enquist B. J., Ferriere R., Advancing the metabolic theory of biodiversity. Ecol. Lett. 12, 1001–1015 (2009). [DOI] [PubMed] [Google Scholar]
- 47.Wiens J. J., Donoghue M. J., Historical biogeography, ecology and species richness. Trends Ecol. Evol. 19, 639–644 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Stephens P. R., Wiens J. J., Explaining species richness from continents to communities: The time-for-speciation effect in emydid turtles. Am. Nat. 161, 112–128 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Gaston K. J., Global patterns in biodiversity. Nature 405, 220–227 (2000). [DOI] [PubMed] [Google Scholar]
- 50.Svenning J.-C., Skov F., Limited filling of the potential range in European tree species. Ecol. Lett. 7, 565–573 (2004). [Google Scholar]
- 51.Eiserhardt W. L., Borchsenius F., Sandel B., Kissling W. D., Svenning J.-C., Late Cenozoic climate and the phylogenetic structure of regional conifer floras world-wide. Glob. Ecol. Biogeogr. 24, 1136–1148 (2015). [Google Scholar]
- 52.Zhang L., Hay W. W., Wang C., Gu X., The evolution of latitudinal temperature gradients from the latest Cretaceous through the Present. Earth Sci. Rev. 189, 147–158 (2019). [Google Scholar]
- 53.Nürk N. M., Uribe-Convers S., Gehrke B., Tank D. C., Blattner F. R., Oligocene niche shift, Miocene diversification - cold tolerance and accelerated speciation rates in the St. John’s Worts (Hypericum, Hypericaceae). BMC Evol. Biol. 15, 80 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Escudero M., Hipp A. L., Waterway M. J., Valente L. M., Diversification rates and chromosome evolution in the most diverse angiosperm genus of the temperate zone (Carex, Cyperaceae). Mol. Phylogenet. Evol. 63, 650–655 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Arakaki M., et al. , Contemporaneous and recent radiations of the world’s major succulent plant lineages. Proc. Natl. Acad. Sci. U.S.A. 108, 8379–8384 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S.-D., et al. , Insights into the historical assembly of global dryland floras: The diversification of Zygophyllaceae. BMC Evol. Biol. 18, 166 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q., et al. , Niche conservatism and elevated diversification shape species diversity in drylands: Evidence from Zygophyllaceae. Proc. Biol. Sci. 285, 20181742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morueta-Holme N., et al. , Habitat area and climate stability determine geographical variation in plant species range sizes. Ecol. Lett. 16, 1446–1454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slatyer R. A., Hirst M., Sexton J. P., Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 16, 1104–1114 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Harnik P. G., Simpson C., Payne J. L., Long-term differences in extinction risk among the seven forms of rarity. Proc. Biol. Sci. 279, 4969–4976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wright S. J., Seasonal drought, soil fertility and the species density of tropical forest plant communities. Trends Ecol. Evol. 7, 260–263 (1992). [DOI] [PubMed] [Google Scholar]
- 62.Tonkin J. D., Bogan M. T., Bonada N., Rios-Touma B., Lytle D. A., Seasonality and predictability shape temporal species diversity. Ecology 98, 1201–1216 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Hughes C., Eastwood R., Island radiation on a continental scale: Exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. U.S.A. 103, 10334–10339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andersen K. M., Turner B. L., Dalling J. W., Soil-based habitat partitioning in understorey palms in lower montane tropical forests. J. Biogeogr. 37, 278–292 (2010). [Google Scholar]
- 65.Connor E. F., McCoy E. D., The statistics and biology of the species-area relationship. Am. Nat. 113, 791–833 (1979). [Google Scholar]
- 66.Wilson D. S., Complex interactions in metacommunities, with implications for biodiversity and higher levels of selection. Ecology 73, 1984–2000 (1992). [Google Scholar]
- 67.Weir J. T., Schluter D., The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 315, 1574–1576 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Rabosky D. L., et al. , An inverse latitudinal gradient in speciation rate for marine fishes. Nature 559, 392–395 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Couvreur T. L. P., Forest F., Baker W. J., Origin and global diversification patterns of tropical rain forests: Inferences from a complete genus-level phylogeny of palms. BMC Biol. 9, 44 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofmann R., Tietje M., Aberhan M., Diversity partitioning in Phanerozoic benthic marine communities. Proc. Natl. Acad. Sci. U.S.A. 116, 79–83 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Higgins S. I., Buitenwerf R., Moncrieff G. R., Defining functional biomes and monitoring their change globally. Glob. Change Biol. 22, 3583–3593 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Govaerts R., Nic Lughadha E., Black N., Turner R., Paton A., The World Checklist of Vascular Plants, a continuously updated resource for exploring global plant diversity. Sci. Data 8, 215 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lomolino M. V., Ecology’s most general, yet protean pattern: The species-area relationship. J. Biogeogr. 27, 17–26 (2000). [Google Scholar]
- 74.Keil P., Chase J. M., Global patterns and drivers of tree diversity integrated across a continuum of spatial grains. Nat. Ecol. Evol. 3, 390–399 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Fernández-Palacios J. M., et al. , Evolutionary winners are ecological losers among oceanic island plants. J. Biogeogr. 48, 2186–2198 (2021). [Google Scholar]
- 76.Condamine F. L., Silvestro D., Koppelhus E. B., Antonelli A., The rise of angiosperms pushed conifers to decline during global cooling. Proc. Natl. Acad. Sci. U.S.A. 117, 28867–28875 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scotese C. R., An atlas of Phanerozoic paleogeographic maps: The seas come in and the seas go out. Annu. Rev. Earth Planet. Sci. 49, 679–728 (2021). [Google Scholar]
- 78.Veizer J., Prokoph A., Temperatures and oxygen isotopic composition of Phanerozoic oceans. Earth Sci. Rev. 146, 92–104 (2015). [Google Scholar]
- 79.Tietje M., Rödel M.-O., Schobben M., The effect of geographic range and climate on extinction risk in the deep-time amphibian fossil record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 537, 109414 (2020). [Google Scholar]
- 80.Crisp M. D., et al. , Phylogenetic biome conservatism on a global scale. Nature 458, 754–756 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Rabosky D. L., Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS One 9, e89543 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Freeman B. G., Pennell M. W., The latitudinal taxonomy gradient. Trends Ecol. Evol. 36, 778–786 (2021). [DOI] [PubMed] [Google Scholar]
- 83.Brummitt R. K., Pando F., Hollis S., Brummitt N. A., “World geographical scheme for recording plant distributions” (Rep., International Working Group on Taxonomic Databases for Plant Sciences, York, UK, 2001).
- 84.Sizling A. L., Kunin W. E., Sizlingová E., Reif J., Storch D., Between geometry and biology: The problem of universality of the species-area relationship. Am. Nat. 178, 602–611 (2011). [DOI] [PubMed] [Google Scholar]
- 85.Gotelli N. J., Chao A., Measuring and Estimating Species Richness, Species Diversity, and Biotic Similarity from Sampling Data (Elsevier Ltd., 2013). [Google Scholar]
- 86.Allouche O., Kalyuzhny M., Moreno-Rueda G., Pizarro M., Kadmon R., Area-heterogeneity tradeoff and the diversity of ecological communities. Proc. Natl. Acad. Sci. U.S.A. 109, 17495–17500 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith S. A., Brown J. W., Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 105, 302–314 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Kerr J. T., Currie D. J., The relative importance of evolutionary and environmental controls on broad-scale patterns of species richness in North America. Ecoscience 6, 329–337 (1999). [Google Scholar]
- 89.Svenning J. C., Borchsenius F., Bjorholm S., Balslev H., High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. J. Biogeogr. 35, 394–406 (2008). [Google Scholar]
- 90.Chang J., Rabosky D. L., Alfaro M. E., Estimating diversification rates on incompletely sampled phylogenies: Theoretical concerns and practical solutions. Syst. Biol. 69, 602–611 (2020). [DOI] [PubMed] [Google Scholar]
- 91.Riley S. J., DeGloria S. D., Elliot R., Index that quantifies topographic heterogeneity. Intermt. J. Sci. 5, 23–27 (1999). [Google Scholar]
- 92.R Core Team, R: A language and environment for statistical computing (2021). R Foundation for Statistical Computing, Vienna, Austria.
- 93.Satorra A., Bentler P., “Scaling corrections for statistics in covariance structure analysis” (UCLA Department of Statistics Paper, University of California, Los Angeles, CA, 1988).
- 94.Hu L. T., Bentler P. M., Kano Y., Can test statistics in covariance structure analysis be trusted? Psychol. Bull. 112, 351–362 (1992). [DOI] [PubMed] [Google Scholar]
- 95.Curran P. J., West S. G., Finch J. F., The robustness of test statistics to nonnormality and specification error in confirmatory factor analysis. Psychol. Methods 1, 16–29 (1996). [Google Scholar]
- 96.Davis J. C., Sampson R. J., Statistics and Data Analysis in Geology (John Wiley and Sons, 1986). [Google Scholar]
- 97.Byrnes J., Data from “Correcting Standard Errors in SEMs fit with Covariance Matrices and ML using Moran’s I.” GitHub. https://github.com/jebyrnes/spatial_correction_lavaan. Accessed ▪▪▪.
- 98.Kissling W. D., et al. , Quaternary and pre-Quaternary historical legacies in the global distribution of a major tropical plant lineage. Glob. Ecol. Biogeogr. 21, 909–921 (2012). [Google Scholar]
- 99.M. Tietje, pebgroup/Global-plant-diversity: Revisions2_release (2022) 10.5281/zenodo.6543973. Accessed May 12, 2022. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Scripts and metrics for each botanical country have been deposited in Zenodo (DOI: 10.5281/zenodo.6350285 (99). We are using geographic distribution data (a presence matrix for all included plant species in botanical countries) for our work that have been collected by the authors for several years and will, therefore, be published as a separate data publication. The release/publication of these data is currently underway in the form of a special issue. We will publish all other data and code except for this data file. The files to repeat the final analysis steps (statistics, figures, etc.) are provided in the Zenodo repository as mentioned.