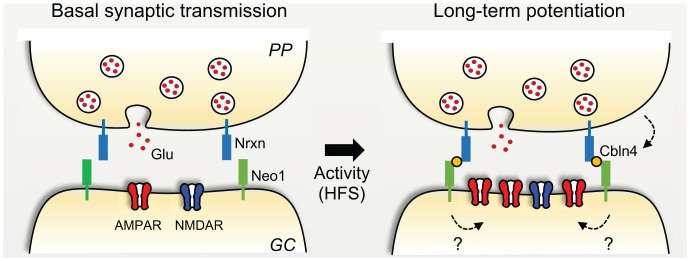
Long-term potentiation (LTP) of synaptic transmission, possibly one of the most studied cellular phenomena in neuroscience, refers to the long-lasting strengthening of a synaptic connection typically induced by brief repetitive activity. Much of the excitement about this phenomenon relies on the early idea that LTP could be a cellular correlate of some forms of learning and memory. Despite intense research on LTP since its discovery five decades ago (>17,000 publications listed in PubMed), important knowledge gaps remain regarding its molecular mechanisms and functional relevance. Growing evidence indicates that transsynaptic adhesion complexes known to play key roles in synapse development are also implicated in LTP at mature synapses, although the precise mechanism is poorly understood. A recent study published in PNAS has identified a transsynaptic protein–protein interaction that is essential for LTP but not for basal evoked synaptic transmission (1) (Fig. 1).
Fig. 1.
Transsynaptic signaling mediates LTP at the glutamatergic perforant path (PP) to GC synapse of the mouse dentate gyrus. High-frequency stimulation (HFS) of PP inputs releases the Nrxn–ligand Cbln4 which binds postsynaptic Neo1. The Nrxn–Cbln4–Neo1 transcellular complex enables LTP whose expression mechanism involves a postsynaptic enhancement of AMPARs but not NMDARs. The signaling mechanism downstream of Neo1 that mediates LTP is unknown.
LTP was first reported in the dentate gyrus, the main entry area of the hippocampus, at the excitatory synapse established between pyramidal neurons in the entorhinal cortex (EC) and granule cells (GC) (2), but it was later identified at several other synapses in vertebrates and invertebrates. LTP is not a unitary phenomenon. Several mechanistically distinct forms of long-term synaptic plasticity at both excitatory and inhibitory synapses have been reported, and it is unlikely that learning relies on a single form of activity-dependent synaptic plasticity. However, most studies, by far, have focused on a “classical” form of excitatory LTP (aka Hebbian LTP) like the one originally identified at EC–GC synapses. There, calcium influx via transient activation of N-methyl-D-aspartate receptors (NMDARs) by the neurotransmitter glutamate triggers a long-lasting synaptic strengthening that is most likely due to the postsynaptic recruitment of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs). LTP is accompanied by other changes such as an increase in synapse size and is regulated by numerous molecules. A recent addition to the long list of molecules implicated in LTP includes transsynaptic adhesion complexes. For example, neuroligin-1 and LRRTMs, two cell adhesion molecules which are postsynaptic receptors for presynaptic neurexins (Nrxn), are required for LTP induction at CA3 to CA1 pyramidal cell synapses (3), but whether these transsynaptic complexes are also required at other excitatory synapses remains unclear. Other postsynaptic receptors for presynaptic Nrxn, which have also been implicated in synaptic function, include the glutamate receptors GluD1 and GluD2. GluD1 is widely expressed in the brain and is required for normal AMPAR- and NMDAR-mediated synaptic transmission in the hippocampus (4, 5), whereas GluD2 is expressed in Purkinje cells and is required for long-term depression (LTD) in the cerebellum (6). Thus, consistent with the remarkable molecular diversity of synapses (7), transsynaptic complexes of distinct molecular composition can control basal synaptic transmission and long-term plasticity.
Cerebellins (Cbln) are secreted adaptor molecules that connect presynaptic neurexins to postsynaptic receptors, thereby forming transsynaptic Nrxn–Cbln–receptor complexes. Mammals contain four Cbln genes (Cbln1 to Cbln4 in mice) that are widely, albeit unevenly, expressed in the brain. Using RNA fluorescent in situ hybridization, Liakath-Ali et al. (1) find that the EC expresses high levels of Cbln4. Cbln4 is also expressed in hippocampal interneurons but not in principal cells (i.e., GCs and pyramidal cells), while neurexins (Nrxn1, Nrxn2, and Nrxn3) are highly expressed in the EC. The EC projects to GCs via the medial and lateral perforant paths—MPP and LPP, respectively. Remarkably, presynaptic deletion of Cbln4 using an acute conditional knockout (cKO) approach, that is, by injecting a Cre-viral vector in the EC, abolished LTP at both MPP–GC and LPP–GC synapses but had no effect on basal evoked synaptic transmission. This finding represents evidence for Cbln4 playing a role in synaptic plasticity.
Next, the authors sought to determine the Cbln4 target and identified neogenin 1 (Neo1), a multifunctional transmembrane receptor, as the postsynaptic receptor for Cbln4 (1). Neo1 is highly expressed in GC dendrites (8) and is required for LTP in the basolateral amygdala (9). Using a cell aggregation assay in HEK cells, they found that Neo1 binds Cbln4/Nrxn1 complexes, although the identity of the specific Nrxn isoform(s) involved in EC-GC LTP was not determined. In addition, postsynaptic deletion of Neo1 (again, using viral vectors in the mature brain), severely impaired EC–GC LTP but had no effect on basal synaptic transmission, indicating that postsynaptic deletion of Neo1 phenocopied presynaptic deletion of Cbln4. Together, these findings strongly suggest that secreted Cbln4 promotes the formation of transsynaptic complexes between presynaptic Nrxn and postsynaptic receptor Neo1, which renders EC–GC synapses competent for LTP.
The transsynaptic Nrxn–Cbln4–Neo1 complex requirement for LTP at MPP–GC and LPP–GC synapses resembles the transsynaptic interaction of presynaptic Nrxns with postsynaptic neuroligin-1 and LRRTMs which is required for LTP in the CA1 area of the hippocampus (3, 10). Given that Cbln4 is not detected in the CA1 area (1), another secreted Cbln is likely involved in this area. Unlike Neo1 cKO in the mature dentate gyrus (1), Neo1 conditional deletion in the amygdala from birth impairs both LTP and basal synaptic transmission (9), and transsynaptic complexes at other hippocampal synapses also contribute to basal synaptic plasticity (4, 5). The molecular basis underlying the selective role of Nrxn–Cbln4–Neo1 in EC–GC LTP without affecting basal transmission is unknown. Assuming Cbln4 and Neo1 deletions do not affect other properties, Cbln4 and Neo1 cKO mice, by selectively blocking EC–GC LTP, may offer a unique opportunity to test the role of this form of plasticity in learning and memory.
The mechanism downstream of Neo1 that enables LTP at EC–GC synapses remains to be determined. Cbln4 binding to Neo1 could regulate the function and nanoscale localization of numerous molecules that control postsynaptic AMPAR levels and are essential to the core mechanisms of EC–GC LTP. Further work is required to identify this signaling cascade. Transsynaptic complexes may allow bidirectional signaling, and, therefore, the Nrxn–Cbln4–Neo1 complex at EC–GC synapses could also signal in a retrograde manner and contribute to potential structural (11) and functional (12) presynaptic changes associated with a postsynaptic form of plasticity, such as EC–GC LTP. Regardless of the precise signaling mechanism, the findings by Liakath-Ali et al. (1) not only support the synapse specificity of transsynaptic complexes but also highlight the functional relevance and diversity of this form of signaling at the synapse. Lastly, the findings also emphasize how molecules involved in synapse formation or partner specificity during development can be repurposed for synaptic plasticity in the mature brain.
As is often the case with intriguing results, these trigger more questions. Is the signaling mediated by transsynaptic adhesion complexes a general requirement for postsynaptic forms of LTP and LTD? Are these complexes also important for plasticity at other synapses not involving principal cells (e.g., inhibitory interneurons)? Are Cbln4 and other Cblns secreted in an activity-dependent manner? As mentioned above, how exactly do these transsynaptic adhesion complexes enable plasticity? While classical LTP seems to rely on a common core mechanism, a diverse transsynaptic regulatory plasticity mechanism may provide a way to manipulate synaptic plasticity, neuronal circuits, and behaviors in a synapse type-specific manner (13).
Acknowledgments
P.E.C.’s research is supported by NIH Grants R01-NS113600, R01-MH125772, R01-NS115543, and R01-MH116673. Thanks go to Dr. Peri Kurshan for critical reading of the manuscript.
Footnotes
The author declares no conflict of interest.
See companion article, “Transsynaptic cerebellin 4–neogenin 1 signaling mediates LTP in the mouse dentate gyrus,” 10.1073/pnas.2123421119.
References
- 1.Liakath-Ali K., Polepalli J. S., Lee S.-J., Cloutier J.-F., Südhof T. C., Transsynaptic cerebellin 4–neogenin 1 signaling mediates LTP in the mouse dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 119, e2123421119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss T. V., Lomo T., Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiol. 232, 331–356 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang M., et al. , Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol. Psychiatry 22, 375–383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai J., Patzke C., Liakath-Ali K., Seigneur E., Südhof T. C., GluD1 is a signal transduction device disguised as an ionotropic receptor. Nature 595, 261–265 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tao W., Díaz-Alonso J., Sheng N., Nicoll R. A., Postsynaptic δ1 glutamate receptor assembles and maintains hippocampal synapses via Cbln2 and neurexin. Proc. Natl. Acad. Sci. U.S.A. 115, E5373–E5381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuzaki M., Aricescu A. R., A GluD coming-of-age atory. Trends Neurosci. 40, 138–150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Rourke N. A., Weiler N. C., Micheva K. D., Smith S. J., Deep molecular diversity of mammalian synapses: Why it matters and how to measure it. Nat. Rev. Neurosci. 13, 365–379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D., et al. , Neogenin, a regulator of adult hippocampal neurogenesis, prevents depressive-like behavior. Cell Death Dis. 9, 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X. D., et al. , Neogenin in amygdala for neuronal activity and information processing. J. Neurosci. 38, 9600–9613 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu X., et al. , Neuroligin-1 signaling controls LTP and NMDA receptors by distinct molecular pathways. Neuron 102, 621–635.e3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourne J. N., Harris K. M., Nanoscale analysis of structural synaptic plasticity. Curr. Opin. Neurobiol. 22, 372–382 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Min M. Y., Asztely F., Kokaia M., Kullmann D. M., Long-term potentiation and dual-component quantal signaling in the dentate gyrus. Proc. Natl. Acad. Sci. U.S.A. 95, 4702–4707 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki K., et al. , A synthetic synaptic organizer protein restores glutamatergic neuronal circuits. Science 369, eabb4853 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]