Abstract
Background
older HIV-positive adults experience a significant burden of geriatric conditions. However, little is known about the association between geriatric conditions and healthcare utilisation in this population.
Setting
outpatient safety-net HIV clinic in San Francisco.
Methods
in 2013, HIV-positive adults ≥50 years of age underwent geriatric assessment including functional impairment, fall(s)in past year, cognitive impairment (MOCA <26) and low social support (Lubben social network scale ≤12). We reviewed medical records from 2013 through 2017 to capture healthcare utilisation (emergency room (ER) visits and hospitalisations) and used Poisson models to examine the association between geriatric conditions and utilisation events over 4 years.
Results
among 192 participants, 81% were male, 51% were white, the median age was 56 (range 50–74), and the median CD4 count was 508 (IQR 338–688) cells/mm3. Sixteen percent of participants had ≥1 activities of daily living (ADL) dependency, 58% had ≥1 instrumental activities of daily living IADL dependency, 43% reported ≥1 falls, 31% had cognitive impairment, and 58% had low social support. Over 4 years, 90 participants (46%) had ≥1 ER visit (total of 289 ER visits), 39 (20%) had ≥1 hospitalisation (total of 68 hospitalisations), and 15 (8%) died. In unadjusted and adjusted analyses, IADL dependency and falls were associated with healthcare utilisation (adjusted incidence rate ratios IADL (95%CI): 1.73 (1.33–2.25); falls: 1.51 (1.21–1.87)).
Conclusion
IADL dependency and history of falls were associated with healthcare utilisation among older HIV-positive adults. Although our results are limited by sample size, improved understanding of the association between geriatric conditions and healthcare utilisation could build support for geriatric HIV care models.
Keywords: Geriatric assessment, health services, functional performance, older people
Key Points
Geriatric conditions are common among older HIV+ adults, but less is known about association with outcomes like utilisation
In this study, falls and dependence with ≥1 instrumental activities of daily living (IADLs) were independently associated with healthcare utilisation
As the HIV population ages, addressing geriatric conditions needs to be considered in HIV care models
Introduction
The number of older adults with human immunodeficiency virus (HIV) is increasing, with >50% of all people with HIV (PWH) in the US over the age of 50 [1]. Worldwide, with expansion of antiretroviral therapy, over 20% of the 39 million PWH will be over age 50 [2]. Despite these successes, older adults with HIV face new challenges including an increased risk of comorbidities such as cardiovascular disease, chronic kidney disease and osteoporosis [3–5], leading to higher rates of multimorbidity and polypharmacy than HIV-negative peers [6–9]. They also experience geriatric conditions such as frailty, functional impairment and falls at relatively younger ages than the general population, supporting the use of age 50 as ‘older’ for HIV-positive adults [10, 11]. Not only are geriatric conditions occurring at relatively younger ages in PWH, they are occurring frequently with fall rates of at least 25% in several studies and difficulty with instrumental activities of daily living, as common as 46% in one study [10, 12, 13]. Adding to these increased medical needs, many older HIV-positive adults are dealing with mental health conditions and psychosocial issues such as substance use, loneliness and or lack of social support [14, 15]. The ageing of PWH is important as current HIV care paradigms will need to adapt and incorporate geriatric principles to better address care needs.
In addition to adapting care models, concerns about increasing healthcare utilisation and costs for ageing PWH have been raised [16]. Although utilisation for acquired immunodeficiency syndrome (AIDS) defining diagnoses has dramatically declined over time, PWH still have higher hospitalisation rates than the general population [17]. Despite the high prevalence of geriatric conditions such as functional and cognitive impairment, falls and other geriatric conditions among older adults with HIV, less is known about geriatric conditions and association with healthcare utilisation in this population. Limited data exist on this topic, although the few published studies suggest geriatric conditions may be associated with hospitalisation. One study of HIV-positive and negative injection drug users showed that frailty is associated with incident hospitalisation and another study demonstrated that a composite social isolation score predicted incident hospitalisation among HIV-positive and negative veterans [18, 19]. Given the limited data and the increasing age of PWH in the US, we sought to examine the relationship between geriatric conditions with acute healthcare utilisation, defined as emergency room (ER) and hospitalisations, among a cohort of older adults with HIV in San Francisco.
Methods
Participants and data collection
This study utilises baseline geriatric condition data collected from the Silver Project, a demonstration project designed to enhance care in HIV primary care settings for HIV-positive adults aged ≥50 years [20]. All participants in this study were patients at the ‘Ward 86’ HIV Clinic, which is a safety-net outpatient clinic serving publicly insured and uninsured HIV-positive adults and adolescents on the San Francisco General Hospital campus. English speaking adults ages 50 years or older who received primary care at Ward 86 were eligible to participate. Between January 2013 and January 2014, participants were recruited and consecutively enrolled via flyers. Participants received $20 gift cards for participation. This study and the Silver Project were approved by the UCSF Committee on Human Research IRB numbers 17–23,067 and 12–08879. Baseline data and Silver Project procedures: The Silver Project involved a one-time visit where the same geriatric assessment was conducted for all participants involving surveys to capture self-reported items of physical function, falls and social support along with short cognitive assessments administered by medical assistants between January 2013 and January 2014 [20]. Data collected included demographic information (e.g. age, sex, race and ethnicity as defined by US Census categories [21]), CD4 count (cells/mm3) and viral load (copies/mL), and the Veterans Aging Cohort Study (VACS) index. The VACS index is a prognostic index for PWH which includes age, sex, race, CD4 count and HIV viral load, and indicators of comorbidity including haemoglobin, liver fibrosis and Hepatitis C and renal function [22]. The VACS index predicts all-cause mortality and hospitalisation and is associated with markers of inflammation and has also been proposed as a marker of frailty [23–28]. The following geriatric conditions were assessed: (i) self-report of fall(s) in the past year (yes to report of any fall) using the Prevention of Falls Network Europe definition of ‘an unexpected event in which a person comes to rest on the ground or lower level’, (ii) functional impairment as measured by self-report of dependency (needing help with) in daily activities using Activities of Daily Living (ADLs) e.g. bathing, dressing and Instrumental Activities of Daily Living (IADLs) e.g. cooking, shopping derived from Katz and Lawton scales, (iii) cognitive impairment measured using Montreal Cognitive Assessment (MOCA) (with cut-off of <26 used to indicate possible cognitive impairment), (iv) social support measured by the Lubben Social Network Scale (LSNS) [29–32]. The 6-item LSNS is a measure of physical social support, assessing the number of support network members, with a cut-point of ≤12 used in previous studies to indicate low social support [20, 33]. The MOCA is validated for HIV-Associated Neurocognitive Disorder using a cut-point of 26 to indicate cognitive impairment [34, 35]. Using the electronic medical record (EMR), we abstracted co-morbidities at baseline using the Charlson Comorbidity Index, adjusted for HIV/AIDS populations [36]. Substance use including alcohol use disorders as well as mental health conditions (depression, anxiety and other serious mental illness) were also abstracted from the EMR using International Classification of Diseases codes. We were unable to reliably capture past insurance data (only present-day insurance information was available) or living situation from the medical record.
Follow-up data and outcome measures
Four years of outcome data were collected for this study between 2013 and 2017 from the review of EMR using standardised chart abstraction forms. Healthcare utilisation was defined as ER visits and inpatient hospitalisations. We included both visit types as ER visits also reflect a degree of medical or social urgency (such as access issues) and are associated with high costs in the US. When patients were admitted to the hospital through the ER, these instances were only counted once as hospitalisation. Visits were classified into broad categories based on the Agency for Healthcare Research Quality classification system as has been done in other studies [18, 37]. The EMR primarily captured acute care utilisation within the San Francisco General Hospital system network, but some outside network acute care utilisation events were also captured. Deaths were abstracted from the EMR and we also captured when participants moved or transferred care outside of Ward 86 or otherwise were lost to follow-up (i.e. no additional documentation available in medical record and no confirmed death).
Statistical analysis
Baseline geriatric conditions were dichotomised as the following clinically relevant categories: (a) Presence or absence of any falls, (b) abnormal MOCA score of <26 (reflecting possible cognitive impairment) versus MOCA score of ≥26 [34], (c) low social support, using LSNS ≤12 versus >13 normal or high social support [20], (d) presence or absence of ≥1 ADL dependency and (e) presence of ≥1 IADL dependency. We created a composite outcome variable of ER visits and hospitalisations to capture overall utilisation rates. We used Poisson regression models to estimate overall utilisation rates per person-year as well as unadjusted/adjusted incidence-rate ratios (IRRs) with 95% confidence intervals for each geriatric condition. In these models we used the logarithm of follow-up time as the offset term to account for censoring due to death or lost to follow-up. Based on the literature review of healthcare utilisation among PWH, including the few specific studies of healthcare utilisation and geriatric conditions in PWH, we adjusted for age, gender, race, ethnicity, comorbidities, mental health and substance use [17, 18]. Given our smaller sample size, the VACS index was useful, as not only is it a prognostic measure associated with hospitalisation, but it combines multiple possible covariates (age, race, gender, CD4 count, viral load, comorbidities) into one variable. Other studies of healthcare utilisation in PWH have adjusted for VACS index [38]. As the VACS index does not include a formal measure of ethnicity and Latinx/Hispanic ethnicity is associated with utilisation, we conducted a sensitivity analysis adding ethnicity to the model. In addition, as a sensitivity analysis, instead of using the VACS index which includes multiple covariates, we conducted multivariate models adjusting for each component individually (e.g. age, gender, race, CD4 count, viral load) and used the Charlson Comorbidity index as a measure of comorbidity. We used listwise deletion methods in each model to handle missing data for each variable. Analyses were conducted using statistical software SAS 9.4 (SAS Institute Inc.) and STATA 15.1 (Stata Corp), and all tests of statistical significance were two-sided.
Results
197 participants had baseline visit data from the Silver Project but five participants had no follow-up visits or further documentation and were excluded from the analyses. Among the 192, participants were primarily male (n = 156, 81%), 51% were white/Caucasian and the median age was 56 (interquartile range (IQR) 53–60). The median CD4 T-cell count was 508 (IQR 338–688), and 79% had an undetectable viral load. Table 1 shows the participant characteristics overall and by healthcare utilisation. Supplementary Table 1 shows the participant characteristics by age.
Table 1.
Participant Characteristics and Healthcare Utilisationa
| Participant Characteristics | N (%) or Mean(SD)/Median(IQR) | ||
|---|---|---|---|
| OverallN = 192 | No Healthcare Utilisation (N = 92) | Healthcare Utilisation (N = 100) | |
| Age | |||
| 50–54 | 61 (31.8) | 26 (28.3) | 35 (35.0) |
| 55–59 | 78 (40.6) | 40 (43.5) | 38 (38.0) |
| ≥60 | 53 (27.6) | 26 (28.2) | 27 (27.0) |
| Education | |||
| High school education or less | 73 (38.0) | 28 (30.4) | 45 (45.0) |
| Some college | 77 (40.1) | 38 (41.3) | 39 (39.0) |
| College/bachelor’s degree | 20 (10.4) | 10 (10.9) | 10 (10.0) |
| Some graduate school or graduate degree | 22 (11.5) | 16 (17.4) | 6 (6.0) |
| Gender | |||
| Male | 156 (81.3) | 79 (85.9) | 77 (77.0) |
| Female | 36 (18.7) | 13 (14.1) | 23 (23.0) |
| Latinx ethnicity | 28 (14.6) | 12 (13.0) | 16 (16.0) |
| Race b | |||
| Asian | 8 (4.1) | 5 (5.4) | 3 (3.0) |
| African American | 69 (35.9) | 27 (29.4) | 42 (42.0) |
| Native American | 7 (3.6) | 2 (2.2) | 5 (5.0) |
| Native Hawaiian/Pacific Islander | 4 (2.1) | 1 (1.1) | 3 (3.0) |
| Caucasian | 97 (50.5) | 52 (56.5) | 45 (45.0) |
| Other | 19 (9.9) | 9 (9.8) | 10 (10.0) |
| Annual income * | |||
| < $10,000 | 68 (37.6) | 32 (37.2) | 36 (37.9) |
| $10,001–$20,000 | 79 (43.6) | 36 (41.9) | 43 (45.3) |
| >$20,001 | 34 (18.8) | 18 (20.9) | 16 (16.8) |
| Mental health *, c | |||
| Depression | 90 (46.9) | 42 (45.7) | 48 (48.0) |
| Anxiety | 15 (7.8) | 3 (3.3) | 12 (12.0) |
| Other | 23 (12.0) | 11 (12.0) | 12 (12.0) |
| Substance use disorderd | 19 (20.7) | 37 (37.0) | |
| Charlson Comorbidity Index e | |||
| 0 | 55 (28.6) | 30 (32.6) | 25 (25.0) |
| 1–2 | 105 (54.7) | 52 (56.5) | 53 (53.0) |
| 3+ | 32 (16.7) | 10 (10.9) | 22 (22.0) |
| VACS Index scoref,* | 28 (20–45) | 28 (18–40) | 29 (22–46) |
| CD4 T-cell count (cells/mm3)* | 508 (338–688) | 516 (361–685) | 500 (319–717) |
| Undetectable viral load (<40 copies/mL) | 152 (79.2) | 15 (16.3) | 25 (25.0) |
a N = 192 (5 participants did not have any follow-up data so were excluded from original 197) except where indicated by
*Income:11 respondents refused to answer; *Mental health: 13 had missing data 13 had missing data; *VACS index: 11 were missing 1 or more lab values to complete full index, *CD4 count: 4 did not have a CD4 count within one year of baseline);
bParticipants could report more than one race, will not add to 100%.
cCould have more than one mental health condition, obtained from medical record diagnoses;
dSubstance use included alcohol use disorder, stimulant and opiate use disorder, obtained from medical record;
eWe utilised the Charlson Comorbidity Index score adjusted for HIV/AIDS, categories correspond to mild (0–1) and moderate/severe (3–4 and 5+) comorbidity;
fVACS Index which includes age, race, gender, CD4 count, HIV viral load, and markers of comorbidities—hepatitis C status, haemoglobin, liver and renal function.
At baseline, 82 (42.7%) had at least one fall in the past year, 30 (15.6%) were dependent in ≥1ADL, almost 60% (n = 112, 58.3%) were dependent in ≥ IADL, one-third (n = 60, 31.2%) had an abnormal MOCA score (<26) and almost 60% (n = 111, 57.8%) had a low social network scale score (Figure 1).
Figure 1.
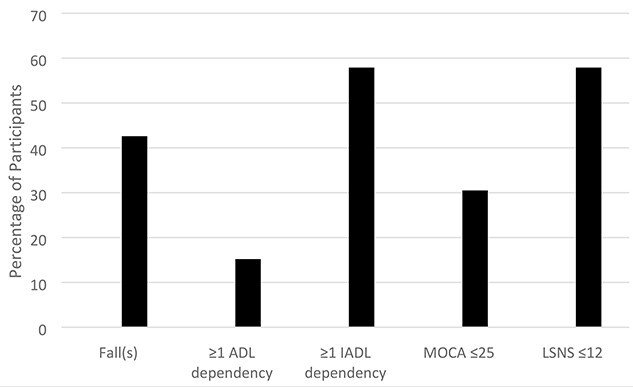
Percentage of participants with geriatric conditions. Footnote. n = 192 Fall(s) assessed as self-report fall(s) in past year; MoCA = Montreal Cognitive Assessment; LSNS = Lubben Social Network Scale.
The median length of follow-up time was 4 years. Over the 4-year follow-up period, 15 participants died and 31 participants were lost to follow-up, primarily for transferring care outside Ward 86. A relatively lower CD4 count (415 (212–554) vs. 552 (358–717) cells/mm3P = 0.01) and identifying as Caucasian (P = 0.05) were the only statistically significant differences between those lost to follow-up and those retained (Supplementary Table 2).
Ninety-seven participants had ≥1 healthcare utilisation events with 39 having ≥1 hospitalisation and 90 having ≥1 ER visit. ER visits were more frequent than hospitalisations. The most common categories for ER visits were infectious (n = 55 (19.1%); cellulitis most common), gastrointestinal (52 (18.1%); abdominal pain most common), musculoskeletal (37 (12.9%); non-traumatic joint pain most common), injuries (36 (12.5%); trauma) and cardiovascular (34 (11.8%); chest pain most common). Common reasons for hospitalisation were infectious (n = 18 (26.5%), cellulitis most common), gastrointestinal (11 (16.2%), complications cirrhosis most common), pulmonary (8 (11.8%), chronic obstructive pulmonary disease (COPD) most common), cardiovascular (7 (10.3%), acute coronary syndrome most common) and endocrine/metabolic (7 (10.3%); renal failure most common). Only one hospitalisation was for an AIDS defining condition, specifically pneumocystis pneumonia.
In unadjusted analysis, dependent in ≥1 IADLs, and having a fall in the past year were each associated with a statistically significant increased risk of healthcare utilisation events with rates of hospitalisation/ER visits per person-year of 0.69 (0.61, 0.77) for IADL dependency and 0.69 (0.59, 0.79) for falls (RR, 95% (CI) 1.82 (1.44, 2.29) and 1.49 (1.21, 1.83), respectively). Dependency in ADLs, cognitive impairment and low social support did not have a statistically significant association with healthcare utilisation. Female gender, non-white race, having a mental health condition, having a substance use disorder, increasing VACS index and Charlson comorbidity scores and having detectable viremia were also associated with increased risk of healthcare utilisation events. Latinx ethnicity, having a college or graduate degree level of education and increasing age were associated with decreased risk of having utilisation events. Table 2 depicts the univariate results.
Table 2.
Unadjusted Associations with Geriatric Conditions and Participant Characteristics with Healthcare Utilisationa
| Baseline Characteristics | Unadjusted Rates of Hospitalisation and ER Visits Per Person-year (95% Confidence Interval)a | Incidence-Rate Ratio (95% Confidence Interval)a,b |
|---|---|---|
| Dependent in ≥ 1 IADL | ||
| No | 0.38 (0.31, 0.45) | 1.00 (Ref) |
| Yes | 0.69 (0.61, 0.77) | 1.82 (1.44, 2.29) |
| Dependent in ≥ 1 ADL | ||
| No | 0.55 (0.48, 0.61) | 1.00 (Ref) |
| Yes | 0.67 (0.51, 0.83) | 1.22 (0.94, 1.60) |
| Fall(s) in past year | ||
| No | 0.46 (0.39, 0.53) | 1.00 (Ref) |
| Yes | 0.69 (0.59, 0.79) | 1.49 (1.21, 1.83) |
| Cognitive impairment (MoCA <26 c ) | ||
| No | 0.59 (0.51, 0.66) | 1.00 (Ref) |
| Yes | 0.52 (0.43, 0.62) | 0.89 (0.71, 1.12) |
| Low social support, LSNS ≤ 12 d | ||
| No | 0.61 (0.51, 0.70) | 1.00 (Ref) |
| Yes | 0.53 (0.46, 0.61) | 0.88 (0.71, 1.08) |
| Age, in years (continuous) | 0.95 (0.93, 0.97) | |
| Age, in years | ||
| 50–54 | 0.82 (0.70, 0.95) | 1.00 (Ref) |
| 55–59 | 0.43 (0.35, 0.50) | 0.52 (0.41, 0.66) |
| ≥60 | 0.47 (0.37, 0.58) | 0.58 (0.44, 0.75) |
| Gender | ||
| Male | 0.47 (0.41, 0.53) | 1.00 (Ref) |
| Female | 0.97 (0.80, 1.15) | 2.07 (1.66, 2.58) |
| Ethnicity | ||
| Non-Latinx/Hispanic | 0.59 (0.53, 0.66) | 1.00 (Ref) |
| Latinx/Hispanic | 0.40 (0.27, 0.53) | 0.68 (0.48–0.95) |
| Race | ||
| White | 0.39 (0.33, 0.46) | 1.00 (Ref) |
| Non-white | 0.73 (0.63, 0.82) | 1.84 (1.48–2.28) |
| Education level | ||
| Less than high school | 0.64 (0.54, 0.74) | 1.00 (Ref) |
| High school | 0.59 (0.50, 0.69) | 0.93 (0.75–1.16) |
| College | 0.40 (0.24, 0.55) | 0.62 (0.41–0.94) |
| Graduate degree | 0.31 (0.17, 0.45) | 0.49 (0.30–0.79) |
| Annual income | ||
| ≤ $10,000 | 0.46 (0.37, 0.54) | 1.00 (Ref) |
| $10,001–$20,000 | 0.69 (0.59, 0.79) | 1.52 (1.20, 1.93) |
| ≥ $20,001 | 0.48 (0.35, 0.60) | 1.04 (0.75, 1.45) |
| Mental health diagnosis | ||
| None | 0.42 (0.33, 0.50) | 1.00 (Ref) |
| Present | 0.66 (0.58, 0.74) | 1.59 (1.26–2.00) |
| Substance use disorder | ||
| None | 0.40 (0.34, 0.46) | 1.00 (Ref) |
| Present | 0.95 (0.82, 1.09) | 2.40 (1.95–2.95) |
| VACS index (continuous) e | 1.01 (1.00, 1.01) | |
| Charlson Comorbidity Index (continuous) f | 1.30 (1.21, 1.40) | |
| Charlson categories | ||
| 0 | 0.33 (0.25, 0.42) | 1.00 (Ref) |
| 1–2 | 0.60 (0.52, 0.68) | 1.82 (1.37, 2.41) |
| 3+ | 0.85 (0.67–1.02) | 2.54 (1.83, 3.53) |
| CD4 T-cell count continuous (cells/mm 3 ) g | 1.05 (1.01, 1.09) | |
| CD4 count <200 | 0.42 (0.25, 0.59) | 0.73 (0.48, 1.11) |
| CD4 count ≥200 | 0.57 (0.51, 0.64) | 1.00 (Ref) |
| Viral suppression | ||
| Undetectable viral load (<40 copies/mL) | 0.52 (0.46, 0.58) | 1.00 (Ref) |
| Detectable viral load (>40 copies/mL) | 0.74 (0.60, 0.89) | 1.43 (1.14–1.81) |
* N = 192 except 11 participants had missing annual income; 13 participants had missing mental health data, 11 participants were missing VACS index; 4 participants had missing CD4 count aHealthcare Utilisation defined as ER visits and hospitalisations; Univariate Poisson regression models were used to estimate rates of hospitalisation and ER visits per person-year and incidence-rate ratios with 95% confidence intervals. In each model, follow-up time was used as an exposure to account for censoring due to lost to follow-up or death; bText in bold indicates statistically significant results;
cMoCA = Montreal Cognitive Assessment; dLSNS = Lubben Social Network Scale;
eVACS Index which includes age, race, gender, CD4 count, HIV viral load and markers of comorbidity—hepatitis C status, haemoglobin, liver and renal function, reported per 1 unit increase in score;
fWe utilised the Charlson Comorbidity Index score adjusted for HIV/AIDS, continuous reported per 1 unit increase in score, categories correspond to mild (0–1) and moderate/severe (3–4 and 5+) comorbidity;
gReported per 100 unit increase in CD4 count.
In adjusted analyses, after adjusting for VACS index, mental health and substance use disorders, the association with IADL dependency and falls remained, with IRR (95% CI) of 1.73 (1.33–2.25) and 1.51 (1.21–1.87), respectively (Table 3). Adding Latinx/Hispanic ethnicity to the models did not change the adjusted IRR for IADL or falls (IRR 1.73 (1.33–2.25) and IRR 1.44 (1.16–1.79), respectively), and in both models, Latinx ethnicity remained statistically significant (IRR 0.58 (0.41–0.83) and 0.63 (0.44–0.90), respectively). In the sensitivity analysis where we adjusted for the individual variables instead of the VACS index, the IRRs were similar for IADL dependency and falls (IADL 1.68 (1.28–2.21), falls 1.54 (1.23–1.93)) (Supplementary Table 3).
Table 3.
Adjusted Associations with IADL Dependency, Falls and Healthcare Utilisation*
| Geriatric Condition | Unadjusted Rates of Hospitalisation and ER Visits Per Person-Year (95% Confidence Interval)a | Unadjusted Incidence-rate Ratio (95% Confidence Interval)a,b | Adjusted Incidence-rate Ratio (95% Confidence Interval)b,c,d |
|---|---|---|---|
| Dependent in 1 or more IADL | |||
| No | 0.38 (0.31,0.45) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 0.69 (0.61, 0.77) | 1.82 (1.44, 2.29) | 1.73 (1.33, 2.25) |
| VACS index c | 1.01 (1.00, 1.01) | 1.00 (0.99, 1.00) | |
| Substance use disorder | 0.95 (0.82, 1.09) | 2.40 (1.95–2.95) | 2.39 (1.92, 2.97) |
| Mental health condition | 0.66 (0.58–0.74) | 1.59 (1.26–2.00) | 1.27 (1.00, 1.62) |
| Fall in past year | |||
| No | 0.46 (0.39, 0.53) | 1.00 (Ref) | 1.00 (Ref) |
| Yes | 0.69 (0.59, 0.79) | 1.49 (1.21, 1.83) | 1.51 (1.21, 1.87) |
| VACS index c | 1.01 (1.00, 1.01) | 1.00 (0.99, 1.01) | |
| Substance use disorder | 0.95 (0.82, 1.09) | 2.40 (1.95–2.95) | 2.56 (2.06, 3.20) |
| Mental health condition | 0.66 (0.58–0.74) | 1.59 (1.26–2.00) | 1.40 (1.10, 1.78) |
*Overall N = 192 with 13 participants missing mental health data, 11 missing VACS index;
aUnivariate Poisson regression models were used to estimate rates of hospitalisation and ER visits per person-year and incidence-rate ratios with 95% confidence intervals. In each model, follow-up time was used as an exposure to account for censoring due to lost to follow-up or death; bText in bold indicates statistically significant results;
cAdjusted models include adjustment for VACS index, substance use and mental health conditions.
dVACS index includes age, gender, race, CD4 count, viral load, markers of comorbidities.
Discussion
As the number of older adults with HIV continues to increase worldwide, HIV care models will need to adapt to meet the needs of an ageing population. As healthcare systems adapt care models, considerations of cost and utilisation will often be prioritised, yet little is known about geriatric conditions and healthcare utilisation in this population. We set out to examine the association between geriatric syndromes and healthcare utilisation among a clinic-based population of older HIV-positive adults. We found that dependency in ≥1 IADLs and a history of falls were independently associated with an increased risk of ER use and hospitalisations after adjustment for other risk factors, including the VACS index, an established HIV prognostic index.
Our study adds to the few prior studies supporting that geriatric conditions, such as frailty, can predict healthcare utilisation in older adults with HIV. A previous study in the AIDS Linked to the Intravenous Experience cohort showed that, among a group of 1,300 current and former injection drug users, with mean age of 48 and 30% HIV-positive, frailty was associated with all-cause incident hospitalisation [18]. Our study adds support that other geriatric conditions, including functional impairment (dependency in one or more IADLs) and falls, were independently associated with healthcare utilisation. Although objective measures of physical performance, such as the Short Physical Performance Battery, are associated with mortality in HIV-positive adults, we are unaware of other studies showing associations with utilisation for self-reported functional deficits in PWH [39]. Given our small sample size, all negative findings, including for ADLs, cognitive impairment and low social support, may be explained by low power.
With regards to falls, our prevalence of 43% is consistent with other US-based HIV cohorts reporting similar rates of 43% and 41% [12, 13]. Reasons PWH may be at risk for falls include peripheral neuropathy, polypharmacy and balance disorders [40]. This is important as older PWH are at increased risk of osteoporosis and fractures, and a fall could be a sentinel health event. In a study of non-infectious comorbidities in PWH, those with osteoporosis or pathological fracture were 1.7 times more likely to be hospitalised than HIV-negative controls with osteoporosis [41].
Our results also add to the literature on healthcare utilisation in the current era of antiretroviral treatment for PWH. Reasons for hospitalisation are no longer focused on AIDS defining conditions but for other diseases. Non-AIDS defining infections, gastrointestinal, pulmonary and cardiovascular diagnoses were the most common reasons for hospitalisation in our study, consistent with other studies [18, 42]. In unadjusted models, female gender, non-white race, mental health diagnosis, substance use disorders, comorbidities, detectable viremia and the VACS index were associated with increased healthcare utilisation, whereas a higher level of education, increasing age and identifying as Latinx were all associated with decreased risk healthcare utilisation. In adjusted models, substance use disorder, mental health conditions and Latinx ethnicity remained significantly associated with healthcare utilisation. These findings are consistent with prior studies of PWH of all ages showing mental health conditions and substance use disorders are an important predictor of increased utilisation and that in the general population, Latinx/Hispanic ethnicity may be associated with decreased healthcare utilisation [17, 18, 38, 43]. Our finding of age associated with lower utilisation rates was unexpected but might be explained by a survivorship bias if older participants have lived with HIV the longest. As suggested in prior work, those diagnosed in the pre combination antiretroviral therapy who survived may have other protective factors or behaviours that not only helped them survive, but support their overall health [10]. Our study now adds support that geriatric conditions are an important predictor of utilisation, even after adjusting for these other risk factors.
Although the finding of geriatric conditions associated with utilisation may not be particularly surprising to geriatricians, it is important to demonstrate as potential costs often drive change in healthcare systems, and routine assessment of geriatric conditions is uncommon in HIV care settings. Our results suggest that identifying and addressing falls and functional impairment could potentially decrease utilisation. As examples of integrated geriatric HIV services are being developed around the world including in the US and UK, this study and larger future studies could help justify support for these programs [44, 45]. For clinicians encountering an older PWH, the overall approach may not necessarily be different than for someone without HIV, but requires an understanding of unique contributors from HIV and its treatment—such as for falls an increased risk of neuropathy or drug interactions with antiretrovirals and antihypertensives. In addition, for geriatricians, it is also important to recognise that in this relatively younger population of PWH (median age 56), geriatric conditions are common and warrant screening and consultations at earlier ages.
Although this is one of the first studies to examine geriatric conditions and healthcare utilisation among PWH, our study does have limitations. In general, given our small sample size, results, especially negative findings, should be interpreted with caution. In addition, we did not have an HIV-negative comparison group. We report findings from only a single clinic site and our population was predominately male, although this does reflect demographics of PWH in San Francisco [46]. Given the number of participants who died or were lost to follow-up, it is possible that bias was introduced, although the demographics of these groups were overall similar. With regards to measurements, the diagnostic codes used to capture reasons for ER visits or hospitalisations do not adequately capture severity, so it is possible that some of the ER visits could be for more minor issues conflating the composite outcome. However, the ER visits in our study were for similar reasons as hospitalisations, suggesting they were for more urgent medical issues. We were unable to capture housing status or if a participant lives alone in the EMR, which are to be associated with healthcare utilisation. We also were unable to capture baseline insurance status, although all patients in the San Francisco Health Network safety-net system have either public insurance (Medicare, Medicaid) or limited coverage within city boundaries through the municipal health plan. Our findings may not be applicable to other non-safety-net populations, although Medicaid is a large payor for HIV services in the US [47]. Our measures of mental health and substance use disorders were captured from the EMR, which has limitations. The prevalence of depression without significant overlap anxiety suggests recording errors were present—specifically the clinic had protocols for depression but not anxiety screening and measures such as anxiety may be under captured. In addition, our data only allowed for examination of ‘dependency with’ ADLs and IADLs, instead of ‘difficulty with’ which is likely more common in an outpatient-based clinic population [10]. Finally, our baseline data are older collected from 2013 to 2014; however, we do not expect that the association with geriatric conditions and utilisation would change over time.
In summary, we found that, among older PWH, being dependent with ≥1 IADLs and a history of falls were associated with ER use and hospitalisations, even after adjusting for other known contributors to healthcare utilisation. Given the known prevalence of geriatric conditions among older adults with HIV, these findings could have significant implications on future health care costs. Our findings are important to help build on the relevance of geriatric conditions for this population and the need to better integrate geriatric assessment into HIV care practices.
Supplementary Material
Acknowledgements
An abstract based on this paper was accepted as a poster presentation for the 2020 National American Geriatrics Society Meeting.
Declaration of Conflicts of Interest
Dr Greene receives grant support from Gilead and receives royalties from Wolters Kluwer Health for writing a chapter in UptoDate.
Declaration of Sources of Funding
National Institute on Aging at the National Institutes of Health (R03AG056341 to M.G.) and Hellman Fellows Fund; and (K24AG054415 to R.S).
References
- 1. HIV Surveillance Report; Vol 29 . Center for Disease Control and Prevention (online). 2017. Available from: https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (cited 4 February 2019).
- 2. Autenrieth CS, Beck EJ, Stelzle Det al. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000-2020. PLoS One 2018; 13: e0207005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CC, Kuller LHet al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173: 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yin MT, Brown TT. HIV and bone complications: understudied populations and new management strategies. Curr HIV/AIDS Rep 2016; 13: 349–58. [DOI] [PubMed] [Google Scholar]
- 5. Wyatt CM. Kidney disease and HIV infection. Top Antivir Med 2017; 25: 13–6. [PMC free article] [PubMed] [Google Scholar]
- 6. Guaraldi G, Malagoli A, Calcagno Aet al. The increasing burden and complexity of multi-morbidity and polypharmacy in geriatric HIV patients: a cross sectional study of people aged 65-74 years and more than 75 years. BMC Geriatr 2018; 18: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halloran MO, Boyle C, Kehoe Bet al. Polypharmacy and drug-drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther 2019; 24: 193–201. [DOI] [PubMed] [Google Scholar]
- 8. Moore HN, Mao L, Oramasionwu CU. Factors associated with polypharmacy and the prescription of multiple medications among persons living with HIV (PLWH) compared to non-PLWH. AIDS Care 2015; 27: 1443–8. [DOI] [PubMed] [Google Scholar]
- 9. Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis 2011; 53: 1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greene M, Covinsky KE, Valcour Vet al. Geriatric syndromes in older HIV-infected adults. J Acquir Immune Defic Syndr 2015; 69: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Althoff KN, Jacobson LP, Cranston RDet al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69: 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erlandson KM, Zhang L, Ng DKet al. Risk factors for falls, falls with injury, and falls with fracture among older men with or at risk of HIV infection. J Acquir Immune Defic Syndr 2019; 81: e117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharma A, Hoover DR, Shi Qet al. Longitudinal study of falls among HIV-infected and uninfected women: the role of cognition. Antivir Ther 2018; 23: 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greene M, Hessol NA, Perissinotto Cet al. Loneliness in older adults living with HIV. AIDS Behav 2018; 22: 1475–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Green TC, Kershaw T, Lin Het al. Patterns of drug use and abuse among aging adults with and without HIV: a latent class analysis of a US veteran cohort. Drug Alcohol Depend 2010; 110: 208–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krentz HB, Vu Q, Gill MJ. Updated direct costs of medical care for HIV-infected patients within a regional population from 2006 to 2017. HIV Med 2020; 21: 289–98. [DOI] [PubMed] [Google Scholar]
- 17. Navon L. Hospitalization trends and comorbidities among people with HIV/AIDS compared with the overall hospitalized population, Illinois, 2008-2014. Public Health Rep 2018; 133: 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piggott DA, Muzaale AD, Varadhan Ret al. Frailty and cause-specific hospitalization among persons aging with HIV infection and injection drug use. J Gerontol A Biol Sci Med Sci 2017; 72: 389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greysen SR, Horwitz LI, Covinsky KEet al. Does social isolation predict hospitalization and mortality among HIV+ and uninfected older veterans? J Am Geriatr Soc 2013; 61: 1456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. John MD, Greene M, Hessol NAet al. Geriatric assessments and association with VACS index among HIV-infected older adults in San Francisco. J Acquir Immune Defic Syndr 2016; 72: 534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The United States Census Bureau . 2020 Census Frequently Asked Questions About Race and Ethnicity. 2021. [Updated August 12, 2021]. Available from: https://www.census.gov/programs-surveys/decennial-census/decade/2020/planning-management/release/faqs-race-ethnicity.html (cited 7 January 2022).
- 22. Justice AC, McGinnis KA, Skanderson Met al. Towards a combined prognostic index for survival in HIV infection: the role of 'non-HIV' biomarkers. HIV Med 2010; 11: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reddon H, Grant C, Nosova Eet al. The veterans aging cohort study (VACS) index predicts mortality in a community-recruited cohort of HIV-positive people who use illicit drugs. Clin Infect Dis 2020; 73: 538–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Womack JA, Goulet JL, Gibert Cet al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 2013; 56: 1498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Akgün KM, Gordon K, Pisani Met al. Risk factors for hospitalization and medical intensive care unit (MICU) admission among HIV-infected veterans. J Acquir Immune Defic Syndr 2013; 62: 52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Akgün KM, Tate JP, Crothers Ket al. An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr 2014; 67: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Justice AC, Tate JP. Strengths and limitations of the veterans aging cohort study index as a measure of physiologic frailty. AIDS Res Hum Retroviruses 2019; 35: 1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Escota GV, Patel P, Brooks JTet al. The veterans aging cohort study index is an effective tool to assess baseline frailty status in a contemporary cohort of HIV-infected persons. AIDS Res Hum Retroviruses 2015; 31: 313–7. [DOI] [PubMed] [Google Scholar]
- 29. Hauer K, Lamb SE, Jorstad EC, Todd C, Becker C. Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age Ageing 2006; 35: 5–10. [DOI] [PubMed] [Google Scholar]
- 30. Lamb SE, Jørstad-Stein EC, Hauer Ket al. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc 2005; 53: 1618–22. [DOI] [PubMed] [Google Scholar]
- 31. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged: the index of ADL: a standardized measure of biological and psychosocial function. JAMA 1963; 185: 914–9. [DOI] [PubMed] [Google Scholar]
- 32. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–86. [PubMed] [Google Scholar]
- 33. Lubben JE. Assessing social networks among elderly populations. Fam Community Health 1988; 11: 42–52. [Google Scholar]
- 34. Milanini B, Wendelken LA, Esmaeili-Firidouni Pet al. The Montreal cognitive assessment to screen for cognitive impairment in HIV patients older than 60 years. J Acquir Immune Defic Syndr 2014; 67: 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Agarwal R, Aujla RS, Gupta A, Kumar M. Determining the neurocognitive status and the functional ability of patients to screen for HIV-associated neurocognitive disorder (HAND). Dement Neurocogn Disord 2020; 19: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zavascki AP, Fuchs SC. The need for reappraisal of AIDS score weight of Charlson comorbidity index. J Clin Epidemiol 2007; 60: 867–8. [DOI] [PubMed] [Google Scholar]
- 37. Healthcare Cost and Utilization Project (HCUP) . Agency for Healthcare Research and Quality (online). Available from: https://www.ahrq.gov/data/hcup/index.html (cited 4 February 2019). [PubMed]
- 38. Jiao JM, So E, Jebakumar Jet al. Chronic pain disorders in HIV primary care: clinical characteristics and association with healthcare utilization. Pain 2016; 157: 931–7. [DOI] [PubMed] [Google Scholar]
- 39. Greene M, Covinsky K, Astemborski Jet al. The relationship of physical performance with HIV disease and mortality. AIDS 2014; 28: 2711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Erlandson KM, Plankey MW, Springer Get al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med 2016; 17: 740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gallant J, Hsue P, Budd D, Meyer N. Healthcare utilization and direct costs of non-infectious comorbidities in HIV-infected patients in the USA. Curr Med Res Opin 2018; 34: 13–23. [DOI] [PubMed] [Google Scholar]
- 42. Karpiak SE, Shippy RA, Cantor MH. Research on older adults with HIV. New York: AIDS Community Research Initiative of America (online); 2006. Available from: https://www.health.ny.gov/diseases/aids/providers/conferences/docs/roah_final_report.pdf (cited 4 February 2019). [Google Scholar]
- 43. Weinick RM, Jacobs EA, Stone LC, Ortega AN, Burstin H. Hispanic healthcare disparities: challenging the myth of a monolithic Hispanic population. Med Care 2004; 42: 313–20. [DOI] [PubMed] [Google Scholar]
- 44. Davis AJ, Greene M, Siegler Eet al. Strengths and challenges of various models of geriatric consultation for older adults living with HIV. Clin Infect Dis 2021; 74: 1101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siegler EL, Burchett CO, Glesby MJ. Older people with HIV are an essential part of the continuum of HIV care. J Int AIDS Soc 2018; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. San Francisco Department of Public Health . HIV Epidemiology Annual Report. San Francisco: San Francisco Department of Public Health, 2020. [Google Scholar]
- 47. Kaiser Family Foundation . Medicaid and HIV. 2019. Available from:https://www.kff.org/hivaids/fact-sheet/medicaid-and-hiv/ (cited 7 January 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


