Abstract
Context
Selective androgen receptor modulators (SARMs), because of their preferential muscle vs prostate selectivity, are being developed for muscle-wasting conditions. Oral SARMs suppress high-density lipoprotein cholesterol (HDL-C) but their effects on functional capacity and atherogenic potential of HDL particles are unknown.
Objective
To determine the effects of an oral SARM (OPK-88004) on cholesterol efflux capacity, HDL particle number and size, apolipoprotein particle number and size and HDL subspecies
Methods
We measured cholesterol efflux capacity (CEC); HDL particle number and size; APOB; APOA1; and protein-defined HDL subspecies associated with coronary heart disease (CHD) risk in men, who had undergone prostatectomy for low-grade prostate cancer during 12-week treatment with placebo or 1, 5, or 15 mg of an oral SARM (OPK-88004).
Results
SARM significantly suppressed HDL-C (P < .001) but HDL particle size did not change significantly. SARM had minimal effect on CEC of HDL particles (change + 0.016, –0.036, +0.070, and –0.048%/µmol-HDL/L–1 at 0, 1, 5, and 15 mg SARM, P = .045). SARM treatment suppressed APOAI (P < .001) but not APOB (P = .077), and reduced APOA1 in HDL subspecies associated with increased (subspecies containing α2-macroglobulin, complement C3, or plasminogen) as well as decreased (subspecies containing APOC1 or APOE) CHD risk; relative proportions of APOA1 in these HDL subspecies did not change. SARM increased hepatic triacylglycerol lipase (HTGL) (P < .001).
Conclusion
SARM treatment suppressed HDL-C but had minimal effect on its size or cholesterol efflux function. SARM reduced APOA1 in HDL subspecies associated with increased as well as decreased CHD risk. SARM-induced increase in HTGL could contribute to HDL-C suppression. These data do not support the simplistic notion that SARM-associated suppression of HDL-C is necessarily proatherogenic; randomized trials are needed to determine SARM’s effects on cardiovascular events.
Keywords: effect of SARM on reverse cholesterol transport, protein-defined HDL subspecies, HDL proteome, cholesterol efflux, nonsteroidal SARM, androgen, apolipoprotein A-1, high-density lipoprotein cholesterol, hepatic lipase
Selective androgen receptor modulators (SARMs) are ligands that bind to androgen receptor (AR) and induce tissue-specific activation of AR-dependent signaling [1, 2]. Because of their relative muscle vs prostate selectivity and oral route of administration, several nonsteroidal SARMs are in development for a variety of anabolic indications [1-7]. The mechanisms of tissue-specific transcriptional activation and selectivity of SARMs remain incompletely understood; several mechanisms have been proposed to explain the tissue selectivity, including the differential recruitment of coregulator proteins, induction of specific conformational changes in the AR protein, which modulates subsequent protein-protein interactions between AR and other coregulators, and the inability of the nonsteroidal SARMs do no undergo 5 alpha reduction. In phase 1, 2, and 3 trials, several SARMs have been shown to increase lean body mass, maximal voluntary muscle strength, and stair-climbing power, and decrease adipose tissue mass [1, 4-9].
Orally administered nonsteroidal SARMs, similar to steroidal androgens, lower circulating high-density lipoprotein cholesterol (HDL-C) levels [3-8, 10, 11]; the physiologic significance of the HDL-C suppression induced by oral SARM treatment remains unclear. Endogenous HDL-C levels have been inversely associated with coronary heart disease (CHD) risk in some but not all epidemiologic studies; however, recent observations have shown that the HDL hypothesis is overly simplistic and that HDL-C is an imperfect marker of CHD risk. Raising of HDL-C by the inhibitors of cholesterol ester transfer protein (CETP) [12] or niacin [13] has not been associated with a reduction in cardiovascular events [14, 15]. Circulating HDL-C levels are limited in their ability to predict CHD risk because they do not necessarily reflect the functionality of the HDL particles in facilitating the efflux of cholesterol from the tissues to the liver for excretion or their anti-inflammatory, antioxidative, antiapoptotic, or antithrombotic functions [16-18]. The cholesterol efflux capacity (CEC) of serum HDL is a more robust predictor of CHD risk that is independent of circulating HDL-C level [16-18]. An analysis of the Dallas Heart Study cohort revealed that, after adjustment for traditional risk factors, HDL-C is no longer a predictor of cardiovascular events [16]. In contrast, the CEC remains a strong predictor of cardiovascular events even after adjustment for the traditional risk factors and HDL-C levels [16, 18]. Meta-analyses [19, 20] have confirmed that CEC is an independent marker of CHD risk. CETP inhibitors may interfere with the many physiologic functions of HDL, including reverse cholesterol transport [21]. It is unknown whether oral SARMs alter the CEC of circulating HDL particles; an important aim of this study was to determine the effects of an oral SARM on the CEC of circulating HDL particles.
Circulating HDL represents a heterogeneous collection of protein-lipid complexes containing APOA1, cholesterol, and other lipids, and a large number of lipophilic proteins that mediate the diverse functions of HDL particles [22-24]. The apoprotein composition of the HDL particles is more robustly associated with CHD risk than total HDL-C. Furtado et al [22, 25] characterized 16 novel protein-defined HDL subspecies; several HDL subspecies characterized by the presence of APOC3, α2 macroglobulin, complement C3, or plasminogen are more strongly associated with increased CHD risk than HDL subspecies that did not contain the defining protein. In contrast, HDL subspecies that contained APOC1 or APOE are associated with reduced CHD risk [22]. A subsequent analyses of 2 randomized trials of CETP inhibitors [15] revealed that CETP inhibitors increase APOA1 in dysfunctional HDL subspecies and that this shift in HDL subspecies may have contributed to the observed lack of beneficial effect of CETP inhibitors on cardiovascular events. Therefore, a second aim of this study was to determine the effect of oral SARM treatment on APOA1 in HDL subspecies that are associated with altered CHD risk.
Testosterone, the prototypical androgen, is known to suppress APOA1-containing particles in hypogonadal men, and stimulate hepatic triacylglycerol lipase (HTGL) activity [26-28], but testosterone does not affect CETP activity [29, 30]. Therefore, we assessed whether oral SARM also stimulates HTGL and whether the suppression of HDL-C is associated with increased HTGL.
We hypothesized that the measurement of CEC an detailed characterization of the proteome of HDL particles containing apoAI, and HDL particle concentration and size would provide a better assessment of the atherogenicity of HDL particles than HDL cholesterol alone. Accordingly, we characterized the effects of 12-week treatment with an oral SARM, OPK-88004 (OPKO Health), on functional CEC of APOB-depleted serum. We also characterized HDL particle number and size, and protein-defined subspecies that have been associated with CHD risk. We measured APOA1 in HDL subspecies containing APOC3, α2 macroglobulin, complement C3, plasminogen, APOC1 or APOE that are associated with CHD risk [15, 22]. The samples for these analyses were derived from the “A Selective Androgen Receptor Modulator for Symptom Management in Prostate Cancer Survivors (SARM-PC) Trial”, a placebo-controlled, randomized trial of OPK-88004 in men with organ-confined, low-grade prostate cancer, the primary findings of which have been published [5].
Materials and Methods
Study Design
The SARM-PC Trial (clinicaltrials.gov registration No.: NCT02499497) was a phase 2, randomized, placebo-controlled, parallel group, double-blind trial of an oral SARM (OPK-88004) in prostate cancer survivors. The study design and the primary findings of the SARM-PC trial have been published [5]. The prespecified primary aim of this substudy was to determine whether SARM treatment affects the functionality of the circulating HDL particles by characterizing the serum CEC of APOB-depleted serum and performing a targeted analysis of protein-defined HDL subspecies. An additional prespecified aim was to determine the effect of SARM treatment on apolipoproteins APOA1 (the main apolipoprotein in HDL) and APOB. Additional characterization of HDL particle concentration and size, and of APOA1 in HDL subspecies containing apoC3, α2 macroglobulin, complement C3, plasminogen, APOC1, or APOE that are associated with CHD risk [15, 22] was performed post hoc.
The trial was funded by the National Institute of Nursing Research (No. 1R01NR014502). Transition Therapeutics, a subsidiary of OPKO Health, provided funding to investigate the effects of the SARM on serum CEC and atherogenic potential of HDL. The funding agencies played no role in study design, analysis of data, writing of the manuscript, or in the decision to publish.
Study Participants
The eligibility criteria have been published [5]. Briefly, the participants were men, age 19 years or older, with confirmed diagnosis of prostate cancer, who had undergone radical prostatectomy for low-grade (Gleason score 6 or 7 [3 + 4]), organ-localized prostate cancer, and undetectable prostate-specific antigen (PSA) levels (< 0.1 ng/mL using a sensitive PSA assay) for 2 or more years after radical prostatectomy. The participants were required to have a fasting morning total testosterone level, measured using liquid chromatography tandem mass spectrometry, of less than 300 ng/dL and/or free testosterone measured by the equilibrium dialysis method less than or equal to 70 pg/mL, and one or more of the following: sexual dysfunction (low sexual desire [DeRogatis Inventory of Sexual Function for Men—II score ≤ 20] in the sexual desire domain); erectile dysfunction (International Index of Erectile Function) erectile function domain score less than 25; fatigue (The Functional Assessment of Chronic Illness Therapy—Fatigue scale score < 30); or physical dysfunction indicated by self-reported difficulty in walking a one-quarter mile or climbing 2 flights of stairs, and Short Physical Performance Battery score of 4-9 points).
We excluded men who had received radiation therapy or androgen-deprivation therapy, or had a hematocrit level greater than 50%; creatinine greater than 2.5 mg/dL; alanine transaminase or aspartate transaminase above the normal limits; hemoglobin A1c greater than 7.5% or diabetes requiring insulin therapy; body mass index greater than 40; myocardial infarction or stroke within 3 months of screening; untreated severe sleep apnea; or a major untreated psychiatric condition.
Intervention
The study intervention included 1 of 3 doses of OPKO SARM (1, 5, or 15 mg daily) taken orally. The comparator group received matching placebo tablets daily.
Concealed Randomized Allocation of Participants
As described previously [5], to minimize risk to the participants, initially, the participants were randomly assigned in a 1:1:1 ratio to placebo, 1 mg OPK-88004, or 5 mg OPK-88004 using permuted block with varying block sizes, stratified by age (19-50 and > 50 years) and phosphodiesterase 5 inhibitor use. Because in a prespecified blinded safety analysis none of the first 50 randomly assigned participants experienced a biochemical PSA recurrence or clinical disease recurrence, the 1 mg dose, which in phase 1 studies was found to be at the low end of the dose response curve, was discontinued and a 15 mg dose was added with the approval of the trial’s data and safety monitoring board and the institutional review board [5]. Subsequently, eligible individuals were randomly assigned in a 1:1:3 ratio to placebo, 5 mg OPK-88004 SARM, or 15 mg OPK-88004 SARM. In total, 114 participants were enrolled: 36 in the placebo arm, 28 in the 1 mg OPK-88004, 36 in the 5 mg OPK-88004, and 14 in the 15 mg OPK-88004 groups.
Blinding
The intervention assignment was masked from the participants, care provider, and the study investigators.
Outcomes
The primary outcome of this substudy was CEC of HDL particles in APOB-depleted serum. Secondary outcomes included HDL particle concentrations and size; the concentrations of APOA1 in HDL subspecies containing APOC3, α2 macroglobulin, complement C3, plasminogen, APOC1 or APOE; APOA1 and APOB levels; and HTGL levels.
Measurements
All measurements were conducted on fasting samples collected in the morning after an overnight fast before 11 am. Serum lipids, APOA-I, CEC, and HTGL were measured at baseline and during weeks 6 and 12. The measurements of APOB, HDL subspecies, and characterization of HDL particle numbers and size were performed at baseline and week 12.
Cholesterol Efflux Capacity
The CEC of APOB-depleted serum was measured using isotopically labeled cholesterol and THP-1–derived macrophage foam cells, as described previously [31]. Human monocyte cell line THP-1 was maintained at a cell density at or below 0.5 × 106 cells/mL in a T-75 flask in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% fetal bovine serum. The cells were collected by centrifugation at 400g × 5 minutes and resuspended in growth medium with added phorbol 12-myristate 13-acetate (100 nM) and seeded in a 24-well plate at 0.25 × 106 cells/mL/well. After 72 hours, cells were washed and incubated for 24 hours with medium containing 1 μCi/ml 3H-cholesterol (Perkin Elmer, No. NET139001MC) and 2 µg/mL of an acyl-CoA cholesterol acyltransferase (ACAT) inhibitor Sandoz 58-035 (Sigma-Aldrich, No. S9318). After washing with phosphate-buffered saline (PBS), cells were incubated with serum-free RPMI containing the ACAT inhibitor for 16 hours. Cells were washed and incubated for 2.5 hours with APOB-depleted serum, prepared by Demacker’s method [32]. The amount of isotope released and that which remained in the cells was measured by beta scintillation counting. CEC was calculated as the ratio of the 3H-cholesterol released into the medium within the first 2.5 hours and the sum of released and cellular 3H-cholesterol. The coefficients of variation of the CEC assay ranged from 4% to 7% in the female and male quality control pools, respectively.
Hepatic Triacylglycerol Lipase
The HTGL is the primary enzyme that hydrolyzes triacylglycerols and phospholipids in HDL particles [33, 34]. Serum concentration of HTGL protein was measured using a validated human HTGL assay (https://www.ibl-japan.co.jp/en/search/product/detail/id=4835; No. 27180); the HTGL levels measured using this assay are highly correlated with postheparin plasma activity [35].
Lipids and Apolipoproteins
Serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride levels were measured by the Quest diagnostic laboratories (https://www.questdiagnostics.com/); those data have been reported previously [5]. Serum APOA1 levels were measured by validated enzyme-linked immunosorbent assay (ELISA) methods (Abcam Inc, https://www.abcam.com/human-apolipoprotein-ai-elisa-kit-apoa1-ab108803.html). Serum APOB was measured by a clinically validated nephelometric method on a Beckman analyzer at the University of Washington Department of Laboratory Medicine and Pathology.
High-Density Lipoprotein Particle Concentration and Size Measurement
Concentration of HDL particles was quantified by calibrated differential ion mobility analysis on a differential mobility analyzer (DMA) (TSI Inc) as described previously [36]. Six HDL subspecies (extra small, ~7.6 nm; small, ~8.3 nm; medium, ~9.3 nm; medium-large, 9.7 nm; large, ~11.1 nm; and extra-large, ~12.4 nm) were fitted to the DMA raw data by unsupervised curve-fitting using individual Voigt functions within the Fityk program [37]. Measured peak areas under each curve fitted for each subspecies were quantified using a calibration curve constructed from a glucose oxidase standard whose concentration was assigned by amino acid analysis. For total HDL particle concentration, intraday and interday coefficients of variation (CVs) were less than 10%. The CV percentages were less than 10% for HDL subpopulations with the exception of extra-small HDL and small HDL (14.8% and 18.5%, respectively).
Analyses of Protein-defined High-Density Lipoprotein Subspecies
HDL subspecies were quantified by modified sandwich ELISA as the concentration of APOA1 (mg/dL) in that subspecies as described previously [21]. Briefly, samples were diluted in PBS and loaded into 6 prepared 96-well microplates, each one coated with a different antibody corresponding to the 6 subspecies-defining proteins. Following overnight incubation at 4 °C, the unbound fraction was removed. The plates were washed with PBS and the lipoprotein complexes bound to the plate were dissociated by incubating for 1 hour at 37 °C with Tween-containing diluent. The dissociated sample was transferred to a 96-well microplate coated with anti-APOA1 antibody, 1 per HDL subspecies, incubated for 1 hour at 37 °C and washed. Detection was performed with biotinylated anti-APOA1, streptavidin, and o-phenylenediamine substrate. The average CV of replicate samples was less than 15%.
Statistical Analysis
The baseline characteristics of the study participants were presented by treatment arm and overall. Distributional properties on endpoints were assessed graphically. The mixed-effect regression and analysis of covariance (ANCOVA) models were applied to analyze changes over time in cholesterol endpoints. The mixed-models included factors for visit, intervention arm, visit-by-intervention interaction, and were adjusted to baseline values and stratification factors (age and use of phosphodiesterase inhibitors). The unstructured covariance matrix was used to allow for within-subject correlation between week 6 and 12 values. Similarly, ANCOVA models included factors for treatment and were controlled for stratification variables and baseline values. All end points were expressed as change from baseline. Point estimates of each outcome’s change over 12-week follow-up, along with 95% CIs and P values, were extracted from the mixed-model or ANCOVA framework. The comparison between the 5 mg dose to placebo arm and test of any difference between groups was executed using treatment contrasts. Sensitivity analyses were performed on log-transformed data. Associations between change from baseline at week 12 between cholesterol outcomes in 3 OPK-88004 groups combined were analyzed using simple linear regression models. Magnitude of these relationships was assessed using R-squared metrics with corresponding P values. All tests were performed using 1-sided type I error α = .05. Statistical analyses were conducted using SAS 9.4 (SAS Institute) and R software version 3.2.5 (R Foundation).
Results
Flow of Participants Through the Trial
As described previously [5], among the 2729 men who underwent telephone screening, 488 were assessed in person, and 114 eligible participants were randomly assigned to 1 of 4 treatment groups to receive placebo (N = 36), 1 mg OPK-88004 (N = 28), 5 mg OPK-88004 (N = 36), or 15 mg OPK-88004 (N = 14) daily. A total of 103 participants who had sufficient serum available for the measurement of CEC were included in these analyses. Analyses of APOB, protein-defined HDL subspecies, HDL particle sizes, and numbers were performed on 52 individuals (13 from each treatment arm) randomly selected from study population.
Baseline Characteristics
The 4 treatment groups were similar in their baseline characteristics (Table 1). The mean ± SD age of the participants included in the analyses was 67.6 ± 7.6 years, body weight 89.3 ± 13.4 kg, and body mass index 29.1 ± 4.1.
Table 1.
Baseline characteristics of study participants by treatment arm and overall
| Variable | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | Overall |
|---|---|---|---|---|---|
| N = 22 | N = 36 | N = 13 | N = 32 | N = 103 | |
| Age, y | 66.9 ± 8.1 | 67.4 ± 8.6 | 69.8 ± 7.3 | 67.3 ± 6.4 | 67.6 ± 7.6 |
| Weight, kg | 94.3 ± 9.9 | 90.0 ± 15.9 | 88.3 ± 14.0 | 85.6 ± 11.5 | 89.3 ± 13.4 |
| BMI | 30.2 ± 3.9 | 29.1 ± 4.2 | 30.2 ± 5.3 | 27.9 ± 3.6 | 29.1 ± 4.1 |
| Diabetes mellitus | 4.6% (1) | 0% (0) | 7.7% (1) | 0% (0) | 1.9% (2) |
| Lipid panel | |||||
| Total cholesterol, mg/dL | 176.4 ± 32.6 | 173.4 ± 33.1 | 179.8 ± 31.8 | 176.6 ± 28.0 | 175.8 ± 30.9 |
| HDL cholesterol, mg/dL | 50.0 ± 11.4 | 51.3 ± 13.2 | 52.2 ± 15.0 | 55.5 ± 14.8 | 52.4 ± 13.6 |
| LDL cholesterol, mg/dL | 102.1 ± 30.8 | 100.7 ± 28.8 | 104.0 ± 25.2 | 100.9 ± 24.2 | 101.5 ± 27.1 |
| Triglycerides, mg/dL | 129.3 ± 53.9 | 107.9 ± 46.2 | 131.2 ± 83.7 | 100.8 ± 43.3 | 113.2 ± 53.6 |
| Cholesterol efflux, % | 3.73 ± 0.67 | 3.75 ± 0.86 | 3.16 ± 0.71 | 3.73 ± 0.75 | 3.67 ± 0.78 |
| Hepatic lipase, ng/dL | 38.8 ± 21.1 | 41.7 ± 23.0 | 42.5 ± 14.7 | 40.6 ± 21.4 | 40.9 ± 21.0 |
| ApoA1, mg/dL | 147.5 ± 28.8 | 145.1 ± 36.6 | 151.8 ± 30.4 | 159.0 ± 32.9 | 150.8 ± 33.2 |
| 1 mg/d | 5 mg/d | 15 mg/d | Placebo | Overall | |
| N = 13 | N = 13 | N = 13 | N = 13 | N = 52 | |
| Cholesterol efflux/HDL particle No. ratio (% per μmol HDL/L) | 0.40 ± 0.13 | 0.47 ± 0.14 | 0.39 ± 0.12 | 0.39 ± 0.09 | 0.41 ± 0.12 |
| ApoB, mg/dL | 74.2 ± 19.5 | 73.6 ± 19.7 | 80.8 ± 17.4 | 71.0 ± 14.9 | 74.9 ± 17.8 |
| HDL particle No. | |||||
| Total HDL particle concentration, μmol/L | 9.5 ± 2.2 | 8.3 ± 1.8 | 8.9 ± 3.5 | 9.9 ± 2.3 | 9.2 ± 2.5 |
| Extra-small HDL subspecies, μmol/L | 0.69 ± 0.29 | 0.71 ± 0.13 | 0.74 ± 0.32 | 0.67 ± 0.27 | 0.70 ± 0.26 |
| Small HDL subspecies, μmol/L | 2.1 ± 1.1 | 2.1 ± 0.77 | 2.2 ± 0.65 | 2.4 ± 0.98 | 2.2 ± 0.87 |
| Medium HDL subspecies, μmol/L | 3.5 ± 1.2 | 3.0 ± 0.92 | 3.1 ± 1.2 | 3.6 ± 1.1 | 3.3 ± 1.1 |
| Medium-large subspecies, μmol/L | 0.80 ± 0.75 | 0.44 ± 0.33 | 0.35 ± 0.31 | 0.54 ± 0.51 | 0.53 ± 0.52 |
| Large HDL subspecies, μmol/L | 1.8 ± 1.2 | 1.6 ± 0.99 | 2.0 ± 1.7 | 2.0 ± 1.5 | 1.8 ± 1.3 |
| Extra-large HDL subspecies, μmol/L | 0.52 ± 0.24 | 0.55 ± 0.27 | 0.57 ± 0.50 | 0.63 ± 0.48 | 0.57 ± 0.38 |
| HDL Particle Size | |||||
| Extra-small HDL subspecies, nm | 7.5 ± 0.08 | 7.5 ± 0.08 | 7.5 ± 0.09 | 7.5 ± 0.09 | 7.5 ± 0.08 |
| Small HDL subspecies, nm | 8.1 ± 0.08 | 8.1 ± 0.06 | 8.2 ± 0.08 | 8.1 ± 0.08 | 8.1 ± 0.07 |
| Medium HDL subspecies, nm | 8.9 ± 0.11 | 9.0 ± 0.09 | 9.0 ± 0.11 | 9.0 ± 0.12 | 9.0 ± 0.11 |
| Medium-large HDL subspecies, nm | 9.7 ± 0.11 | 9.7 ± 0.08 | 9.8 ± 0.08 | 9.7 ± 0.11 | 9.7 ± 0.10 |
| Large HDL subspecies, nm | 10.6 ± 0.14 | 10.7 ± 0.11 | 10.6 ± 0.12 | 10.7 ± 0.14 | 10.7 ± 0.12 |
| Extra-large HDL subspecies, nm | 12.2 ± 0.27 | 12.4 ± 0.22 | 12.4 ± 0.26 | 12.4 ± 0.26 | 12.4 ± 0.26 |
| Protein-defined HDL subspecies | |||||
| Total plasma APOA1 concentration (mg/dL) | 200.8 ± 24.1 | 178.9 ± 34.6 | 193.7 ± 26.3 | 193.9 ± 28.5 | 191.8 ± 28.9 |
| APOA1 concentration (mg/dL) of HDL that contains α2-macroglobulin | 4.4 ± 1.5 | 4.0 ± 1.4 | 5.1 ± 0.94 | 4.0 ± 1.4 | 4.4 ± 1.3 |
| APOA1 concentration (mg/dL) of HDL that contains apoC1 | 29.0 ± 6.4 | 27.8 ± 8.4 | 33.5 ± 10.0 | 26.6 ± 8.2 | 29.2 ± 8.5 |
| APOA1 concentration (mg/dL) of HDL that contains apoC3 | 10.0 ± 2.7 | 9.6 ± 4.2 | 8.6 ± 2.8 | 9.6 ± 5.4 | 9.4 ± 3.8 |
| APOA1 concentration (mg/dL) of HDL that contains complement C3 | 4.6 ± 1.7 | 3.8 ± 1.6 | 4.6 ± 1.7 | 4.4 ± 2.3 | 4.3 ± 1.8 |
| APOA1 concentration (mg/dL) of HDL that contains apoE | 33.1 ± 11.0 | 23.7 ± 8.0 | 26.0 ± 8.5 | 23.4 ± 5.9 | 26.6 ± 9.2 |
| APOA1 concentration (mg/dL) of HDL that contains plasminogen | 8.1 ± 3.4 | 5.2 ± 1.7 | 6.1 ± 2.5 | 6.7 ± 3.8 | 6.5 ± 3.1 |
Legend: values are presented as mean ± SD and percentage and number of participants for categorical variable.
Abbreviations: BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Serum Lipids, APOA1, and APOB
As reported previously [5], SARM treatment was associated with a dose-related suppression of HDL-C over the 12-week intervention period. The differences in changes from baseline in total cholesterol, triglycerides, and LDL-C levels were not statistically significant [5]. Serum APOA1 levels at baseline were similar among the 4 groups and did not change significantly from baseline during the intervention period in men randomly assigned to the placebo arm. OPK-88004 treatment was associated with a marked suppression of APOA1 concentrations (P < .001) from baseline to week 12 that was dose related (Table 2). The changes from baseline to week 12 in serum HDL-C levels were positively associated with changes in APOA1 levels in the SARM groups (r2 = 0.26; P < .001). Serum APOB concentrations did not change significantly in any group, and changes in APOB levels from baseline to week 12 did not differ among groups (P = 0.077; see Table 2).
Table 2.
Estimates of change from baseline in plasma lipids, cholesterol efflux capacity, and APOA1 and APOB
| Variable | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect |
|---|---|---|---|---|---|---|
| N = 22 | N = 36 | N = 13 | N = 32 | |||
| Lipid panel | ||||||
| Total cholesterol, mg/dL | –16.1 (–25.2 to –6.9) | –16.0 (–23.3 to –8.8) | –13.1 (–24.6 to –1.52) | –7.6 (–15.4 to 0.3) | .10 | .34 |
| HDL, mg/dL | –5.2 (–7.6 to –2.7) | –13.4 (–15.4 to –11.5) | –18.3 (–21.4 to –15.3) | –2.2 (–4.3 to –0.1) | < .001 | < .001 |
| LDL, mg/dL | –8.1 (–15.9 to –0.3) | –0.2 (–6.3 to 5.9) | 7.6 (–2.2 to 17.4) | –5.6 (–12.2 to 1.1) | .22 | .055 |
| Triglycerides, mg/dL | –12.0 (–28.2 to 4.2) | –15.2 (–27.7 to –2.6) | –14.0 (–34.2 to 6.3) | 2.1 (–11.6 to 15.8) | .053 | .23 |
| Hepatic lipase, ng/mL | 6.7 (2.0 to 11.5) | 18.8 (15.1 to 22.5) | 18.3 (12.2 to 24.3) | 0.6 (–3.5 to 4.7) | < .001 | < .001 |
| APOA1, mg/dL | –15.8 (–24.8 to –6.8) | –22.9 (–30.0 to –15.8) | –39.7 (–50.9 to –28.5) | –0.65 (–8.3 to 7.0) | < .001 | < .001 |
| 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect | |
| N = 13 | N = 13 | N = 13 | N = 13 | |||
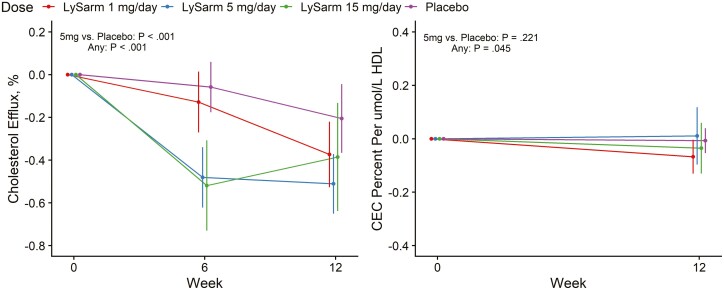
| Cholesterol efflux/HDL concentration ratio, % per μmol HDL/L–1 | –0.036 (–0.103 to 0.031) | 0.070 (0.005 to 0.135) | –0.048 (–0.111 to 0.014) | 0.016 (–0.049 to 0.080) | .221 | .045 |
| APOB, mg/dL | –6.9 (–14.6 to 0.838) | 4.0 (–3.5 to 11.5) | 3.5 (–4.0 to 11.0) | –5.2 (–12.8 to 2.4) | .074 | .077 |
Estimated changes from baseline, 95% CIs, and P values extracted from a mixed-model framework for variables with more than 2 visits, from ANCOVA for variables with 2 visits. P values are for comparison of the 5 mg/day arm with placebo group and for the test of any difference between groups in average change over 12-week follow-up.
Abbreviations: ANCOVA, analysis of covariance; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Cholesterol Efflux Capacity and High-Density Lipoprotein Particle Concentration
SARM treatment was associated with a decrease in CEC (P < .001); because suppression of CEC in serum could be due to a decrease in the number of circulating HDL particles without a change in the functional capacity of the circulating HDL particles, we measured the HDL particle concentration (Table 3) and normalized the changes in CEC by the directly measured serum concentration of HDL particles. The CEC of serum HDL particles, expressed as percentage CEC per micromole (μmol) HDL, did not show a consistent change from baseline to week 12: + 0.016, –0.036, +0.070, –0.048%/μmol HDL*L–1; P = .045; (see Table 2 and Fig. 1).
Table 3.
Estimates of change from baseline in high-density lipoprotein particle concentration
| Variable | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect |
|---|---|---|---|---|---|---|
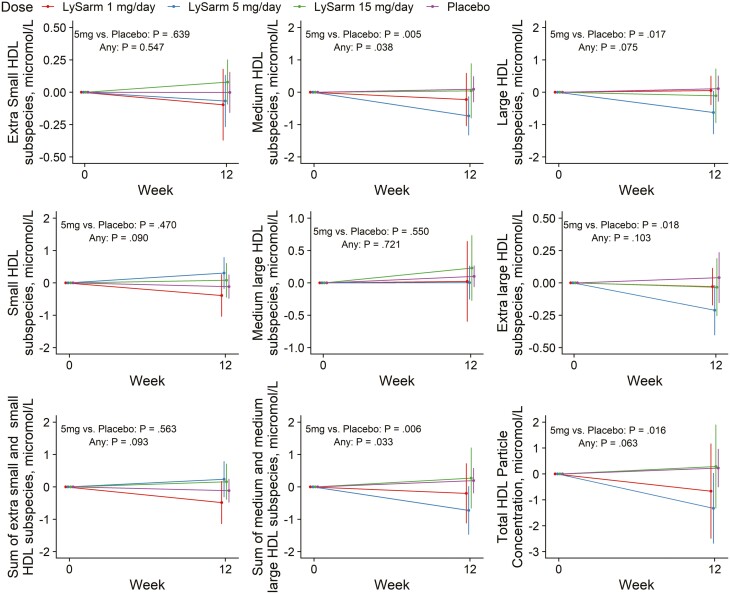
| Total HDL particle concentration, μmol/L | –0.993 (–2.31 to 0.326) | –1.99 (–3.30 to –0.688) | –0.055 (–1.32 to 1.21) | 0.210 (–1.09 to 1.51) | .0163 | .0627 |
| Extra-small HDL subspecies, μmol/L | –0.99 (–0.267 to 0.069) | –0.052 (–0.215 to 0.111) | 0.057 (–0.103 to 0.218) | 0.000 (–0.164 to 0.164) | .639 | .547 |
| Small HDL subspecies, μmol/L | –0.385 (–0.779 to 0.010) | 0.266 (–0.117 to 0.650) | 0.050 (–0.326 to 0.425) | 0.078 (–0.316 to 0.471) | .470 | .090 |
| Medium HDL subspecies, μmol/L | –0.247 (–0.850 to 0.355) | –0.966 (–1.562 to –0.370) | –0.173 (–0.755 to 0.410) | 0.215 (–0.378 to 0.807) | .005 | .038 |
| Medium-large subspecies, μmol/L | 0.012 (–0.380 to 0.404) | –0.184 (–0.555 to 0.187) | 0.101 (–0.264 to 0.465) | –0.035 (–0.405 to 0.335) | .550 | .721 |
| Large HDL subspecies, μmol/L | –0.083 (–0.635 to 0.469) | –0.832 (–1.37 to –0.289) | –0.112 (–0.637 to 0.413) | 0.060 (–0.479 to 0.599) | .017 | .075 |
| Extra-large HDL subspecies, μmol/L | –0.100 (–0.275 to 0.074) | –0.260 (–0.429 to –0.091) | –0.049 (–0.214 to 0.117) | 0.017 (–0.152 to 0.187) | .018 | .103 |
Estimated changes from baseline, 95% CIs, and P values extracted from ANCOVA framework. P values are for comparison of the 5 mg/day arm with placebo group and for the test of any difference between groups in average change over 12-week follow-up.
Abbreviations: ANCOVA, analysis of covariance; HDL, high-density lipoprotein.
Figure 1.
Changes from baseline to week 12 in serum cholesterol efflux capacity and cholesterol efflux capacity normalized to high-density lipoprotein particle number by treatment arm. Point estimates, 95% CIs, and P values extracted from a mixed-model framework. P values are for overall dose effect and comparison between the 5 mg daily dose group and placebo groups.
High-Density Lipoprotein (HDL) Particle Size and Distribution of HDL Among Various Categories of HDL Particles by Size
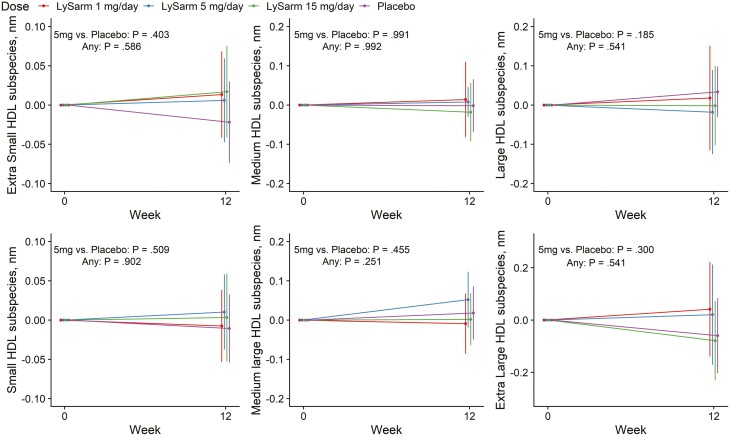
There was no statistically significant difference in change from baseline to week 12 for the average HDL particle size in any of the categories (see Fig. 2 and Table 4). SARM treatment was associated with a significant decrease in the concentration of medium (P = .038) and the sum of medium and medium-large HDL particles (P = .033) but only with a small change in total HDL particle number that was not statistically significant (P = .063) (Table 3 and Fig. 3). The changes from baseline to week 12 in the circulating concentrations of HDL particles in other size categories were not statistically significant and did not show a consistent relation to dose.
Figure 2.
Change from baseline in average particle size of various subcategories of high-density lipoprotein (HDL) particles classified by particle size. Point estimates, 95% CIs, and P values extracted from a mixed-model framework. P values are for overall dose effect and comparison between the 5 mg daily dose group and placebo groups.
Table 4.
Estimates of change from baseline in high-density lipoprotein particle size
| Variable | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect |
|---|---|---|---|---|---|---|
| Extra-small HDL subspecies, nm | 0.024 (–0.023 to 0.072) | 0.014 (–0.032 to 0.060) | 0.027 (–0.018 to 0.072) | –0.012 (–0.058 to 0.034) | .403 | .586 |
| Small HDL subspecies, nm | 0.002 (–0.043 to 0.046) | 0.019 (–0.025 to 0.062) | 0.012 (–0.031 to 0.054) | –0.001 (–0.044 to 0.043) | .509 | .902 |
| Medium HDL subspecies, nm | 0.006 (–0.060 to 0.071) | 0.004 (–0.059 to 0.067) | –0.007 (–0.068 to 0.055) | 0.005 (–0.059 to 0.068) | .991 | .992 |
| Medium-large HDL subspecies, nm | –0.006 (–0.055 to 0.439) | 0.060 (0.012 to 0.108) | 0.031 (–0.017 to 0.079) | 0.036 (–0.013 to 0.084) | .455 | .251 |
| Large HDL subspecies, nm | –0.019 (–0.091 to 0.539) | –0.028 (–0.098 to 0.042) | –0.016 (–0.085 to 0.053) | 0.035 (–0.035 to 0.106) | .185 | .541 |
| Extra-large HDL subspecies, nm | –0.046 (–0.151 to 0.059) | 0.050 (–0.049 to 0.150) | –0.005 (–0.103 to 0.094) | –0.019 (–0.119 to 0.081) | .300 | .541 |
Estimated changes from baseline, 95% CIs, and P values extracted from ANCOVA framework. P values are for comparison of the 5 mg/day arm with placebo group and for the test of any difference between groups in average change over 12-week follow-up.
Abbreviations: ANCOVA, analysis of covariance; HDL, high-density lipoprotein.
Figure 3.
Change from baseline in circulating total high-density lipoprotein (HDL) particle concentrations and concentrations of various subcategories of HDL particles classified by particle size. Point estimates, 95% CIs, and P values extracted from a mixed-model framework. P values are for overall dose effect and comparison between the 5 mg daily dose group and placebo groups.
Characterization of Protein-Defined High-Density Lipoprotein Subspecies
Changes in high-density lipoprotein subspecies with lipid metabolism function
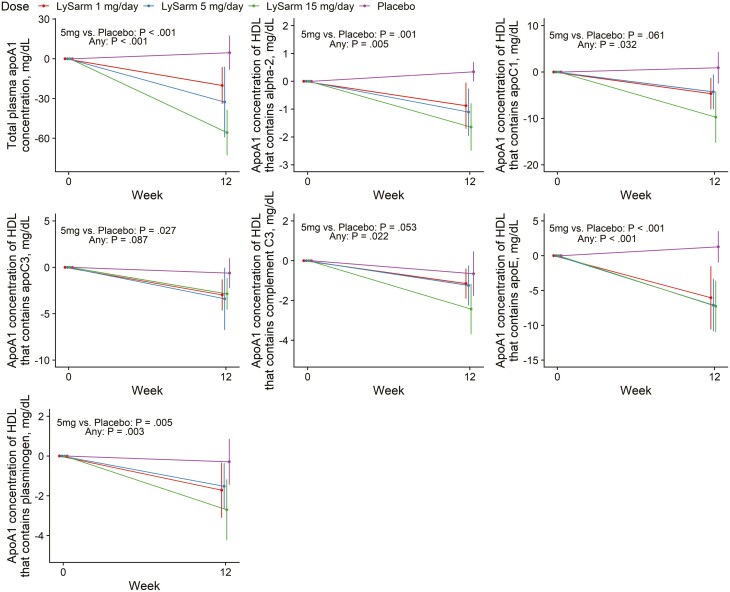
OPK-88004 treatment was associated with a significant suppression of total APOA1 concentration, compared with placebo, as well as a greater numerical decrease from baseline to week 12 in APOA1 concentrations in HDL subspecies containing APOC1 (P = .032) and APOE (P < .001) (Table 5 and Fig. 4) during OPK-88004 treatment. However, the relative proportion of apoA1 in these HDL subspecies in relation to the total APOA1 concentration did not change significantly from baseline (Table 6).
Table 5.
Estimates of change from baseline in protein-based high-density lipoprotein (HDL) subspecies associated with coronary heart disease risk. Panel A. Absolute change from baseline in HDL subspecies associated with coronary heart disease risk
| Variable | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect |
|---|---|---|---|---|---|---|
| Total plasma APOA1 concentration, mg/dL | –17.15 (–34.47 to 0.17) | –38.87 (–56.23 to –21.50) | –55.64 (–72.06 to –39.23) | 4.91 (–11.92 to 21.75) | < .001 | < .001 |
| APOA1 concentration (mg/dL) of HDL that contains α2-macroglobulin, mg/dL | –1.03 (–1.68 to –0.39) | –1.43 (–2.07 to –0.79) | –1.28 (–1.92 to –0.65) | 0.04 (–0.60 to 0.69) | .001 | .005 |
| APOA1 concentration (mg/dL) of HDL that contains APOC1 | –5.21 (–9.07 to –1.36) | –5.09 (–8.85 to –1.33) | –8.43 (–12.21 to –4.65) | –0.27 (–4.08 to 3.53) | .061 | .032 |
| APOA1 concentration (mg/dL) of HDL that contains APOC3 | –2.84 (–4.70 to –0.99) | –3.38 (–5.19 to –1.57) | –3.42 (–5.20 to –1.64) | –0.63 (–2.45 to 1.18) | .027 | .087 |
| APOA-I concentration (mg/dL) of HDL that contains complement C3 | –1.13 (–1.88 to –0.37) | –1.72 (–2.45 to –0.98) | –2.27 (–2.99 to –1.55) | –0.74 (–1.47 to –0.01) | .053 | .022 |
| APOA1 concentration (mg/dL) of HDL that contains APOE | –3.99 (–7.33 to –0.64) | –8.47 (–11.57 to –5.36) | –7.56 (–10.55 to –4.56) | –0.23 (–3.35 to 2.90) | < .001 | < .001 |
| APOA1 concentration (mg/dL) of HDL that contains plasminogen | –1.19 (–2.22 to –0.15) | –2.36 (–3.38 to –1.33) | –2.94 (–3.91 to –1.97) | –0.40 (–1.39 to 0.59) | .005 | .003 |
Abbreviation: HDL, high-density lipoprotein.
Figure 4.
Changes from baseline in total APOA1 concentration and APOA1 concentration in high-density lipoprotein (HDL) subspecies containing APOC1, APOC3, APOE, α2-macroglobulin, complement C3, and plasminogen. Point estimates, 95% CIs, and P values extracted from a mixed-model framework. P values are for overall dose effect and comparison between the 5 mg daily dose group and placebo groups.
Table 6.
Concentration of APOA1 in high-density lipoprotein subspecies expressed as a percentage of total APOA1 concentration by treatment arm at baseline and week 12 visits
| Variable | Visit | 1 mg/d | 5 mg/d | 15 mg/d | Placebo | P, 5 mg/d vs placebo | P, any effect |
|---|---|---|---|---|---|---|---|
| N = 13 | N = 13 | N = 13 | N = 13 | ||||
| APOA1 concentration of HDL that contains α2-macroglobulin/total plasma apoA1 concentration, % | Baseline | 2.16 ± 0.64 | 2.24 ± 0.65 | 2.65 ± 0.50 | 2.07 ± 0.55 | ||
| Week 12 | 1.93 ± 0.76 | 1.95 ± 0.61 | 2.51 ± 0.56 | 2.22 ± 0.57 | .085 | .095 | |
| APOA1 concentration of HDL that contains APOC1/total plasma apoA1 concentration, % | Baseline | 14.5 ± 2.94 | 15.8 ± 5.06 | 17.1 ± 3.54 | 13.5 ± 2.76 | ||
| Wk 12 | 13.5 ± 2.78 | 15.9 ± 3.76 | 17.1 ± 3.52 | 13.7 ± 2.49 | .376 | .104 | |
| APOA1 concentration of HDL that contains APOC3/total plasma APOA1 concentration, % | Baseline | 4.97 ± 1.22 | 5.42 ± 1.97 | 4.38 ± 1.13 | 4.78 ± 2.15 | ||
| Wk 12 | 3.87 ± 1.11 | 4.24 ± 1.74 | 4.04 ± 1.38 | 4.32 ± 2.10 | .498 | .720 | |
| APOA1 concentration of HDL that contains complement C3/total plasma APOA1 concentration, % | Baseline | 2.28 ± 0.83 | 2.09 ± 0.83 | 2.40 ± 0.87 | 2.23 ± 1.13 | ||
| Wk 12 | 1.90 ± 0.68 | 1.69 ± 0.61 | 1.60 ± 0.44 | 1.85 ± 0.86 | .659 | .646 | |
| APOA1 concentration of HDL that contains APOE/total plasma APOA1 concentration, % | Baseline | 16.6 ± 5.89 | 13.3 ± 3.81 | 13.7 ± 5.11 | 12.1 ± 2.57 | ||
| Wk 12 | 15.1 ± 6.39 | 11.5 ± 3.31 | 14.4 ± 4.94 | 12.3 ± 2.77 | .049 | .052 | |
| APOA1 concentration of HDL that contains plasminogen/total plasma APOA1 concentration, % | Baseline | 3.99 ± 1.46 | 2.88 ± 0.77 | 3.21 ± 1.32 | 3.33 ± 1.48 | ||
| Wk 12 | 3.51 ± 1.47 | 2.47 ± 0.66 | 2.55 ± 0.56 | 3.09 ± 1.04 | .180 | .241 |
Changes from baseline and 95% CI at baseline and week 12 by treatment arm. P values, extracted from an ANCOVA framework, are for comparison of the 5 mg/day arm with the placebo group and for the test of any difference between groups in average change over 12-week follow-up.
Abbreviations: ANCOVA, analysis of covariance; HDL, high-density lipoprotein.
Changes in high-density lipoprotein subspecies linked to functions other than lipid metabolism
Compared with placebo, SARM treatment was associated with a significantly greater suppression of APOA1 in HDL subspecies containing complement C3 (P = .022) (involved in inflammation and immunity), α2-macroglobulin (P = .005), and plasminogen (P = .003) (hemostasis) (see Fig. 4). The differences between arms in relative proportion of APOA1 in these HDL subspecies in relation to the total APOA1 concentration were small and did not show a dose-related pattern.
Effect of Treatment on Hepatic Triacylglycerol Lipase
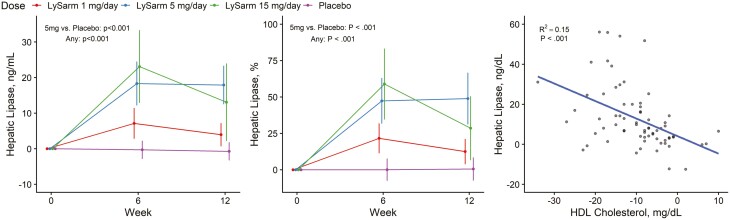
Serum HTGL levels were similar among the 4 groups at baseline and did not change appreciably from baseline to week 12 in the placebo arm. SARM treatment was associated with a significant increase in HTGL levels over the 12-week intervention duration (see Table 2 and Fig. 5). The changes in serum HDL-C from baseline to week 12 and changes in serum HTGL were negatively correlated (r2 = 0.15; P < .001) in men randomly assigned to the SARM groups.
Figure 5.
Absolute and percentage change from baseline in hepatic triacylglycerol lipase and the relation of change in hepatic triacylglycerol lipase with the change in high-density lipoprotein (HDL) cholesterol concentrations. Point estimates, 95% CIs, and P values extracted from a mixed-model framework. P values are for overall dose effect and comparison between the 5 mg daily dose group and placebo groups.
Discussion
The average CEC of circulating HDL particles, a measure of HDL’s functionality and an independent predictor of CHD risk, did not change appreciably during OPK-88004 treatment. The analyses of protein-defined HDL subspecies revealed that SARM treatment was associated with suppression of APOA1 in HDL subspecies containing APOC1 and APOE that are associated with reduced risk of CHD as well as in HDL subspecies containing α2-macroglobulin, complement C3, and plasminogen that are associated with increased CHD risk. However, the relative proportion of APOA1 in relation to the total APOA1 did not change after SARM treatment. Thus, in spite of the decrease in HDL-C levels during SARM treatment, a well-known class effect of oral androgens, OPK-88004 treatment was not associated with appreciable changes in HDL’s cholesterol efflux function, which is now accepted as a more important marker of cardiovascular disease (CVD) risk than HDL-C levels.
SARM treatment was associated with a significant decrease in the circulating concentrations of medium-sized HDL particles but the changes in total HDL particle concentration and large HDL particles were small and not statistically significant. Previous studies have reported a negative relation between the number of total and especially large HDL particles and CVD [38, 39]. Reduced mean HDL particle size (estimated by nuclear magnetic resonance) also is associated with CVD in large-scale clinical studies [39]. However, SARM treatment did not affect the size of any of the HDL particle subspecies measured directly.
The suppression of HDL-C levels during SARM treatment was associated with a significant, dose-related upregulation of HTGL levels. In the regression analysis, a substantial fraction of the variation in change in HDL-C levels from baseline could be explained by the increase of HTGL levels, suggesting that the suppression of HDL-C level during SARM administration is likely due to the increased HTGL.
Previous studies of testosterone and other steroidal androgens also have found an increase in serum HTGL activity [26, 28, 30, 40-44]. These studies have reported that testosterone-induced increase in HTGL is related to the testosterone dose [42] and that the effect on HTGL precedes the reduction in HDL-C [28, 43]. Furthermore, the genetic HTGL deficiency increases HDL-C levels and mitigates androgen-mediated suppression of serum HDL-C [45]. These data, taken together with our present studies of the effect of an oral SARM, implicate increased HTGL as an important target of SARM action, and a major contributor to increased HDL catabolism and reduced HDL-C and APOA1 levels during SARM treatment. The precise molecular mechanism by which SARMs regulate HTGL remains to be investigated.
There is some evidence that testosterone treatment suppresses APOA1, but not apoA2, synthesis [43]. The suppression of APOA1 may contribute to the SARM-induced reduction in HDL-C levels. However, we cannot exclude the possibility that the suppression of APOA1 is the consequence of reduced HDL-C levels due to the upregulation of HTGL by the SARM. In addition to HTGL, plasma lipoprotein lipase and endothelial lipase are also known to participate in serum lipoprotein catabolism [46-52]. Previous studies show that effect of testosterone and other steroidal androgens on lipoprotein lipase activity is minimal compared to its effect in elevating HTGL [26, 27, 40, 41, 43, 53].
Our study has several strengths and some limitations. The trial had attributes of good trial design—concealed randomization, double blinding, and parallel groups. The trial is also one of the largest randomized studies of the effects of a SARM on CEC. We also analyzed the HDL particle numbers and characterized the protein-defined HDL subspecies to evaluate SARM’s effects on HDL subspecies that are more robustly associated with CHD risk than HDL-C. We recognize that the observed changes in serum CEC, measured in an in vitro system, may not fully reflect the systemic effect of SARM treatment on CEC in vivo. For instance, some studies have reported that androgens upregulate scavenger receptor BI and stimulate cholesterol efflux from macrophages [54]. The measurement of in vivo and in vitro markers of the atherogenicity could potentially be useful in early-phase efficacy trials of SARMs as better markers of CVD risk than mere measurement of HDL-C alone. The administration of OPK-88004 was associated with a suppression of total testosterone levels [5], which could potentially affect HDL-C levels. However, this decrease in total testosterone was largely a reflection of the substantial dose-related suppression of SHBG levels; free testosterone levels did not significantly decrease during SARM treatment [5].
Conclusion
The administration of OPK-88004 increased HTGL and was associated with a dose-related reduction in serum HDL-C levels. However, SARM treatment did not appreciably change the total concentration of HDL particles or HDL particle size or its cholesterol efflux function. SARM treatment reduced total APOA1 and APOA1 in both dysfunctional HDL subspecies associated with increased CHD risk and in HDL subspecies associated with lower CHD risk. Our findings do not support the overly simplistic narrative that the decrease in HDL-C during oral SARM treatment will necessarily increase CHD risk. Undoubtedly, large, randomized trials of longer duration are needed to determine the effects of this and other SARMs on major adverse cardiovascular events.
Glossary
Abbreviations
- ACAT
cholesterol acyltransferase
- ANCOVA
analysis of covariance
- APO
apolipoprotein
- AR
androgen receptor
- CEC
cholesterol efflux capacity
- CETP
cholesterol ester transfer protein
- CHD
coronary heart disease
- CV
coefficient of variation
- CVD
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- HDL-C
high-density lipoprotein cholesterol
- HTGL
hepatic triacylglycerol lipase
- LDL-C
low-density lipoprotein cholesterol
- PBS
phosphate-buffered saline
- PSA
prostate-specific antigen
- SARM
selective androgen receptor modulator
Contributor Information
Wen Guo, Research Program in Men’s Health: Aging and Metabolism; Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA.
Karol M Pencina, Research Program in Men’s Health: Aging and Metabolism; Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA.
Jeremy D Furtado, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts 02115, USA.
Frank M Sacks, Department of Nutrition, Harvard TH Chan School of Public Health, Boston, Massachusetts 02115, USA.
Tomas Vaisar, Division of Metabolism, Endocrinology, and Nutrition, University of Washington, Seattle, Washington 98195, USA.
Ming Cheng, Research Program in Men’s Health: Aging and Metabolism; Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA.
Allan D Sniderman, Mike and Valeria Rosenbloom Centre for Cardiovascular Prevention, Department of Medicine, McGill University Health Centre, Montreal, Quebec QCH4A, Canada.
Stephanie T Page, Division of Metabolism, Endocrinology, and Nutrition, University of Washington, Seattle, Washington 98195, USA.
Shalender Bhasin, Email: sbhasin@bwh.harvard.edu, Research Program in Men’s Health: Aging and Metabolism; Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA.
Financial Support
The SARM-PC trial was funded primarily by a research grant from the National Institute of Nursing Research (No. R01NR014502). Transition Therapeutics, Inc, a subsidiary of OPKO Health, provided funding for this substudy to investigate the effects of the SARM on cholesterol efflux. The implementation of the trial at the Boston site was partly supported by the infrastructural resources of the Boston Claude D. Pepper Older Americans Independence Center (No. P30AG31679). HDL particle size analysis and APOB measurements were supported by the Quantitative and Functional Proteomics Core Diabetes Research Center at the University of Washington, Seattle, Washington (No. P30 DK017047) from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosures
S.B. reports receiving research grant support from the NIA, NINR, NICHD, AbbVie, Transition Therapeutics, FPT, and Metro International Biotechnology, Inc. These grants are managed by the Brigham and Women’s Hospital. He has received consulting fees from OPKO Pharmaceuticals and POXEL, Inc, and has equity interest in FPT and Xyone Therapeutics. The other authors have nothing to disclose.
Data Availability
Data described in this study are provided in the article. Additional requests for data should be submitted to Dr Karol Pencina (kpencina@bwh.harvard.edu).
Clinical Trial Information
Clinicaltrials.gov registration No: NCT02499497 (registered July 16, 2015).
References
- 1. Bhasin S, Calof OM, Storer TW, et al. Drug insight: testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat Clin Pract Endocrinol Metab. 2006;2(3):146-159. doi: 10.1038/ncpendmet0120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narayanan R, Coss CC, Dalton JT. Development of selective androgen receptor modulators (SARMs). Mol Cell Endocrinol. 2018;465:134-142. doi: 10.1016/j.mce.2017.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark RV, Walker AC, Andrews S, Turnbull P, Wald JA, Magee MH. Safety, pharmacokinetics and pharmacological effects of the selective androgen receptor modulator, GSK2881078, in healthy men and postmenopausal women. Br J Clin Pharmacol. 2017;83(10):2179-2194. doi: 10.1111/bcp.13316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dobs AS, Boccia RV, Croot CC, et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 2013;14(4):335-345. doi: 10.1016/S1470-2045(13)70055-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pencina KM, Burnett AL, Storer TW, et al. A selective androgen receptor modulator (OPK-88004) in prostate cancer survivors: a randomized trial. J Clin Endocrinol Metab. 2021;106(8):2171-2186. doi: 10.1210/clinem/dgab361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papanicolaou DA, Ather SN, Zhu H, et al. A phase IIA randomized, placebo-controlled clinical trial to study the efficacy and safety of the selective androgen receptor modulator (SARM), MK-0773 in female participants with sarcopenia. J Nutr Health Aging. 2013;17(6):533-543. doi: 10.1007/s12603-013-0335-x [DOI] [PubMed] [Google Scholar]
- 7. Basaria S, Collins L, Dillon EL, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68(1):87-95. doi: 10.1093/gerona/gls078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Neil D, Clark RV, Magee M, et al. GSK2881078, a SARM, produces dose-dependent increases in lean mass in healthy older men and women. J Clin Endocrinol Metab. 2018;103(9):3215-3224. doi: 10.1210/jc.2017-02644 [DOI] [PubMed] [Google Scholar]
- 9. Dalton JT, Barnette KG, Bohl CE, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2(3):153-161. doi: 10.1007/s13539-011-0034-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111(4):261-269. doi: 10.1016/s0002-9343(01)00833-6 [DOI] [PubMed] [Google Scholar]
- 11. Grunfeld C, Kotler DP, Dobs A, Glesby M, Bhasin S. Oxandrolone in the treatment of HIV-associated weight loss in men: a randomized, double-blind, placebo-controlled study. J Acquir Immune Defic Syndr. 2006;41(3):304-314. doi: 10.1097/01.qai.0000197546.56131.40 [DOI] [PubMed] [Google Scholar]
- 12. Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109-2122. doi: 10.1056/NEJMoa0706628 [DOI] [PubMed] [Google Scholar]
- 13. AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011;161(3):538-543. doi: 10.1016/j.ahj.2010.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riaz H, Khan SU, Rahman H, et al. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(5):533-543. doi: 10.1177/2047487318816495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furtado JD, Ruotolo G, Nicholls SJ, Dullea R, Carvajal-Gonzalez S, Sacks FM. Pharmacological inhibition of CETP (cholesteryl ester transfer protein) increases HDL (high-density lipoprotein) that contains ApoC3 and other HDL subspecies associated with higher risk of coronary heart disease. Arterioscler Thromb Vasc Biol. 2022;42(2):227-237. doi: 10.1161/ATVBAHA.121.317181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371(25):2383-2393. doi: 10.1056/NEJMoa1409065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364(2):127-135. doi: 10.1056/NEJMoa1001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Zhang Y, Ding D, et al. Cholesterol efflux capacity is an independent predictor of all-cause and cardiovascular mortality in patients with coronary artery disease: a prospective cohort study. Atherosclerosis. 2016;249:116-124. doi: 10.1016/j.atherosclerosis.2015.10.111 [DOI] [PubMed] [Google Scholar]
- 19. Lee JJ, Chi G, Fitzgerald C, et al. Cholesterol efflux capacity and its association with adverse cardiovascular events: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:774418. doi: 10.3389/fcvm.2021.774418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu C, Zhao X, Zhou Q, Zhang Z. High-density lipoprotein cholesterol efflux capacity is inversely associated with cardiovascular risk: a systematic review and meta-analysis. Lipids Health Dis. 2017;16(1):212. doi: 10.1186/s12944-017-0604-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaefer EJ. Effects of cholesteryl ester transfer protein inhibitors on human lipoprotein metabolism: why have they failed in lowering coronary heart disease risk? Curr Opin Lipidol. 2013;24(3):259-264. doi: 10.1097/MOL.0b013e3283612454 [DOI] [PubMed] [Google Scholar]
- 22. Furtado JD, Yamamoto R, Melchior JT, et al. Distinct proteomic signatures in 16 HDL (high-density lipoprotein) subspecies. Arterioscler Thromb Vasc Biol. 2018;38(12):2827-2842. doi: 10.1161/ATVBAHA.118.311607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sacks FM, Jensen MK. From high-density lipoprotein cholesterol to measurements of function: prospects for the development of tests for high-density lipoprotein functionality in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2018;38(3):487-499. doi: 10.1161/ATVBAHA.117.307025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Albers JJ, Aladjem F. Precipitation of 125 I-labeled lipoproteins with specific polypeptide antisera. Evidence for two populations with differing polypeptide compositions in human high density lipoproteins. Biochemistry. 1971;10(18):3436-3442. doi: 10.1021/bi00794a019 [DOI] [PubMed] [Google Scholar]
- 25. Sacks FM, Liang L, Furtado JD, et al. Protein-defined subspecies of HDLs (high-density lipoproteins) and differential risk of coronary heart disease in 4 prospective studies. Arterioscler Thromb Vasc Biol. 2020;40(11):2714-2727. doi: 10.1161/ATVBAHA.120.31460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herbst KL, Amory JK, Brunzell JD, Chansky HA, Bremner WJ. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am J Physiol Endocrinol Metab. 2003;284(6):E1112-E1118. doi: 10.1152/ajpendo.00524.2002 [DOI] [PubMed] [Google Scholar]
- 27. Sorva R, Kuusi T, Taskinen MR, Perheentupa J, Nikkilä EA. Testosterone substitution increases the activity of lipoprotein lipase and hepatic lipase in hypogonadal males. Atherosclerosis. 1988;69(2-3):191-197. doi: 10.1016/0021-9150(88)90014-7 [DOI] [PubMed] [Google Scholar]
- 28. Applebaum-Bowden D, Haffner SM, Hazzard WR. The dyslipoproteinemia of anabolic steroid therapy: increase in hepatic triglyceride lipase precedes the decrease in high density lipoprotein2 cholesterol. Metabolism. 1987;36(10):949-952. doi: 10.1016/0026-0495(87)90130-2 [DOI] [PubMed] [Google Scholar]
- 29. Tan KC, Shiu SW, Pang RW, Kung AW. Effects of testosterone replacement on HDL subfractions and apolipoprotein A-I containing lipoproteins. Clin Endocrinol (Oxf). 1998;48(2):187-194. [PubMed] [Google Scholar]
- 30. Rubinow KB, Vaisar T, Chao JH, Heinecke JW, Page ST. Sex steroids mediate discrete effects on HDL cholesterol efflux capacity and particle concentration in healthy men. J Clin Lipidol. 2018;12(4):1072-1082. doi: 10.1016/j.jacl.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Low H, Hoang A, Sviridov D. Cholesterol efflux assay. J Vis Exp. 2012(61):e3810. doi: 10.3791/3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demacker PN, Hessels M, Toenhake-Dijkstra H, Baadenhuijsen H. Precipitation methods for high-density lipoprotein cholesterol measurement compared, and final evaluation under routine operating conditions of a method with a low sample-to-reagent ratio. Clin Chem. 1997;43(4):663-668. [PubMed] [Google Scholar]
- 33. Shirai K, Barnhart RL, Jackson RL. Hydrolysis of human plasma high density lipoprotein 2- phospholipids and triglycerides by hepatic lipase. Biochem Biophys Res Commun. 1981;100(2):591-599. doi: 10.1016/s0006-291x(81)80217-3 [DOI] [PubMed] [Google Scholar]
- 34. Santamarina-Fojo S, González-Navarro H, Freeman L, Wagner E, Nong Z. Hepatic lipase, lipoprotein metabolism, and atherogenesis. Arterioscler Thromb Vasc Biol. 2004;24(10):1750-1754. doi: 10.1161/01.ATV.0000140818.00570.2d [DOI] [PubMed] [Google Scholar]
- 35. Miyashita K, Nakajima K, Fukamachi I, et al. A new enzyme-linked immunosorbent assay system for human serum hepatic triglyceride lipase. J Lipid Res. 2017;58(8):1591-1597. doi: 10.1194/jlr.M075432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hutchins PM, Ronsein GE, Monette JS, et al. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem. 2014;60(11):1393-1401. doi: 10.1373/clinchem.2014.228114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wojdyr M. Fityk: a general-purpose peak fitting program. J Appl Crystallogr. 2010;43(5-1):1126-1128. doi: 10.1107/S0021889810030499 [DOI] [Google Scholar]
- 38. Arsenault BJ, Lemieux I, Després JP, et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206(1):276-281. doi: 10.1016/j.atherosclerosis.2009.01.044 [DOI] [PubMed] [Google Scholar]
- 39. Kontush A. HDL particle number and size as predictors of cardiovascular disease. Front Pharmacol. 2015;6:218. doi: 10.3389/fphar.2015.00218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ehnholm C, Huttunen JK, Kinnunen PJ, Miettinen TA, Nikkilä EA. Effect of oxandrolone treatment on the activity of lipoprotein lipase, hepatic lipase and phospholipase A1 of human postheparin plasma. N Engl J Med. 1975;292(25):1314-1317. doi: 10.1056/NEJM197506192922503 [DOI] [PubMed] [Google Scholar]
- 41. Kantor MA, Bianchini A, Bernier D, Sady SP, Thompson PD. Androgens reduce HDL2-cholesterol and increase hepatic triglyceride lipase activity. Med Sci Sports Exerc. 1985;17(4):462-465. doi: 10.1249/00005768-198508000-00010 [DOI] [PubMed] [Google Scholar]
- 42. Colvin PL Jr, Auerbach BJ, Case LD, Hazzard WR, Applebaum-Bowden D. A dose-response relationship between sex hormone-induced change in hepatic triglyceride lipase and high-density lipoprotein cholesterol in postmenopausal women. Metabolism. 1991;40(10):1052-1056. doi: 10.1016/0026-0495(91)90129-k [DOI] [PubMed] [Google Scholar]
- 43. Haffner SM, Kushwaha RS, Foster DM, Applebaum-Bowden D, Hazzard WR. Studies on the metabolic mechanism of reduced high density lipoproteins during anabolic steroid therapy. Metabolism. 1983;32(4):413-420. doi: 10.1016/0026-0495(83)90052-5 [DOI] [PubMed] [Google Scholar]
- 44. Berg G, Schreier L, Geloso G, Otero P, Nagelberg A, Levalle O. Impact on lipoprotein profile after long-term testosterone replacement in hypogonadal men. Horm Metab Res. 2002;34(2):87-92. doi: 10.1055/s-2002-20521 [DOI] [PubMed] [Google Scholar]
- 45. Bausserman LL, Saritelli AL, Herbert PN. Effects of short-term stanozolol administration on serum lipoproteins in hepatic lipase deficiency. Metabolism. 1997;46(9):992-996. doi: 10.1016/s0026-0495(97)90267-5 [DOI] [PubMed] [Google Scholar]
- 46. DeSantis P, Coleman T, Schiekofer S, Nawroth PP, Schlimmer P, Schneider JG. Endothelial lipase: a key player in HDL metabolism modulates inflammation and atherosclerotic risk. Mini Rev Med Chem. 2008;8(6):619-627. doi: 10.2174/138955708784534427 [DOI] [PubMed] [Google Scholar]
- 47. Broedl UC, Jin W, Fuki IV, Millar JS, Rader DJ. Endothelial lipase is less effective at influencing HDL metabolism in vivo in mice expressing apoA-II. J Lipid Res. 2006;47(10):2191-2197. doi: 10.1194/jlr.M600036-JLR200 [DOI] [PubMed] [Google Scholar]
- 48. Jaye M, Krawiec J. Endothelial lipase and HDL metabolism. Curr Opin Lipidol. 2004;15(2):183-189. doi: 10.1097/00041433-200404000-00011 [DOI] [PubMed] [Google Scholar]
- 49. Cohen JC. Endothelial lipase: direct evidence for a role in HDL metabolism. J Clin Invest. 2003;111(3):318-321. doi: 10.1172/JCI17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jaye M, Lynch KJ, Krawiec J, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet. 1999;21(4):424-428. doi: 10.1038/7766 [DOI] [PubMed] [Google Scholar]
- 51. Kuusi T, Nikkilä EA, Taskinen MR, Tikkanen MJ. Role of hepatic endothelial lipase in the metabolism of plasma HDL. Atherosclerosis. 1982;44(2):237-240. doi: 10.1016/0021-9150(82)90117-4 [DOI] [PubMed] [Google Scholar]
- 52. Jin W, Marchadier D, Rader DJ. Lipases and HDL metabolism. Trends Endocrinol Metab. 2002;13(4):174-178. doi: 10.1016/s1043-2760(02)00589-1 [DOI] [PubMed] [Google Scholar]
- 53. Morikawa AT, Maranhão RC, Alves MJNN, Negrão CE, da Silva JL, Vinagre CGC. Effects of anabolic androgenic steroids on chylomicron metabolism. Steroids. 2012;77(13):1321-1326. doi: 10.1016/j.steroids.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 54. Langer C, Gansz B, Goepfert C, et al. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochem Biophys Res Commun. 2002;296(5):1051-1057. doi: 10.1016/s0006-291x(02)02038-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in this study are provided in the article. Additional requests for data should be submitted to Dr Karol Pencina (kpencina@bwh.harvard.edu).