Summary
miR-10b is silenced in normal neuroglial cells of the brain but commonly activated in glioma, where it assumes an essential tumor-promoting role. We demonstrate that the entire miR-10b-hosting HOXD locus is activated in glioma via the cis-acting mechanism involving 3D chromatin reorganization and CTCF-cohesin-mediated looping. This mechanism requires two interacting lncRNAs, HOXD-AS2 and LINC01116, one associated with HOXD3/HOXD4/miR-10b promoter and another with the remote enhancer. Knockdown of either lncRNA in glioma cells alters CTCF and cohesin binding, abolishes chromatin looping, inhibits expression of all genes within HOXD locus, and leads to glioma cell death. Conversely, in cortical astrocytes, enhancer activation is sufficient for HOXD/miR-10b locus reorganization, gene de-repression, and neoplastic cell transformation. LINC01116 RNA is essential for this process. Our results demonstrate the interplay of two lncRNAs in the chromatin folding and concordant regulation of miR-10b and multiple HOXD genes normally silenced in astrocytes and triggering the neoplastic glial transformation.
eTOC blurb
Deforzh et al. investigated a common mechanism of HOXD/miR-10b genes’ derepression in glioblastoma and revealed the coordinated activity of two lncRNAs, HOXD-embedded HOXD-AS2 and distant enhancer-associated LINC01116, on CTCF/cohesin binding, chromatin topology, and astrocyte transformation. The work shed light on the molecular mechanisms of gliomagenesis.
Graphic Abstract
A model of the cooperative function of HOXD-AS2 and LINC01116 RNAs required for HOXD/miR-10b expression. The model proposes a requirement for specific CTCF/Rad21 binding, chromatin looping, and pRNA/eRNA stabilizing long-range chromatin interactions for transcription initiation.
Graphic Abstract

Introduction
Malignant gliomas are primary brain tumors that affect both adult and pediatric populations. Despite major basic and clinical research efforts, survival of patients with glioblastoma (GBM, or astrocytoma grade IV), the most common and aggressive among gliomas, has only marginally improved over the past 25 years and is only around fifteen months (Reardon et al., 2011; Tanaka et al., 2013; Van Meir et al., 2010). Adult GBM may arise de novo and present as primary GBM, or progress from lower-grade glioma (LGG) to so-called secondary GBM. GBM is a highly heterogeneous and multifactorial disease characterized by the wide landscape of mutations and signaling alterations (Ceccarelli et al., 2016; Goffart et al., 2013). Therefore, the tasks of understanding its etiology and devising effective therapeutic strategies are both very challenging.
Our work on GBM led us to focus on a unique regulatory molecule microRNA-10b (miR-10b) that is prominent in gliomas due to its expression pattern: while virtually undetectable in normal neural cells of the brain cortex (neurons, astrocytes, neuroprogenitors, and microglia), it becomes abundant in more than 90% of high-grade gliomas (Gabriely et al., 2011; Teplyuk et al., 2016). MiR-10b regulates many important targets controlling glioma cell cycle and cell death pathways and appears essential for the viability of glioma cells. In fact, GBM exhibits addiction to this miRNA; its genetic ablation or inhibition by antisense oligonucleotides leads to glioma cell death (El Fatimy et al., 2017; Gabriely et al., 2011; Guessous et al., 2013; Teplyuk et al., 2016). Therefore, therapeutic miR-10b inhibitors, including an investigational oligonucleotide drug (RGLS5799) presented by Regulus Therapeutics are under development (Regulus Therapeutics Inc., 2018). miR-10b also has a role in other cancers such as lung and breast, where it is associated with invasion and metastasis (Ma et al., 2007; Sheedy and Medarova, 2018). Normal functions of miR-10b, which is expressed in many extracranial tissues, are practically unknown and likely dispensable since miR-10b KO mice exhibit normal development, growth, fertility, and survival (Kim et al., 2016).
miR-10b silencing in the brain and its robust derepression in glioma, along with glioma dependence on miR-10b expression, pose a principal question: what is the mechanism of derepression? MiR-10b is encoded in the HOXD locus composed of ten homeobox transcription factor-coding genes, two antisense lncRNAs (HOXD-AS2 and HAGLR/HOXD-AS1) and miR-10b, all spread through the 110-Kb genomic region. This locus at chromosome 2q31 is regulated epigenetically during the development and silenced in the developed brain cortex (Goncalves et al., 2020). However, little work has been carried out to investigate its status in glioma thus far. Several studies suggested that some HOXD proteins (e.g., HOXD9), as well as associated ncRNAs (HOXD-AS1, HOXD-AS2, and LINC01116) may regulate glioma properties (Brodie et al., 2017; Chen et al., 2018; Qi et al., 2018; Tabuse et al., 2011; Wang et al., 2020; Ye et al., 2020; Zhang et al., 2019; Zhou et al., 2019). Here we demonstrate that not only miR-10b and its neighboring HOXD3 and HOXD4, but also most other HOXD genes become commonly transcribed in diverse types of gliomas despite the lack of the locus amplification or common mutations in gliomas. To investigate the mechanism underlying miR-10b/HOXD upregulation in gliomagenesis and gliomas, we performed the detailed epigenetic analysis of the locus in normal and cancer cells and tissues, including normal cortical astrocytes, neuroprogenitors (NPCs), and glioma cells. We report the identification of a remote enhancer of HOXD/miR-10b expression in glioma, and a new mechanism involving CTCF-cohesin dependent topologic reorganization of the chromatin caused cooperatively by two lncRNAs, the enhancer and promoter associated RNAs (eRNA LINC01116 and pRNA HOXD-AS2, respectively). Furthermore, we demonstrate that transcriptional activation of the eRNA is sufficient for the derepression of a multigene HOXD/miR-10b cluster that enhances the proliferation and transformation of astroglial cells. Conversely, depletion of the eRNA in glioma cells leads to the repression of the HOXD/miR-10b locus and reduces glioma viability.
Results
Characterization of HOXD genes expression and epigenetics in astrocytes and glioma
The analysis of miR-10b genetic locus suggested that miR-10b can be produced from a common HOXD3/D4/miR-10b transcriptional precursor (Figure 1A). The longest 32-Kb HOXD3 transcript (Ensembl Transcript: ENST00000432796) covers both miR-10b and HOXD4 genes, suggesting their possible co-transcription as a single transcription unit. Supporting this idea, Polymerase II (Pol II) ChlP-Seq analysis demonstrated one of the major peaks in the locus matching the promoter of the putative common miR-10b/HOXD3/D4 transcript (Figure 1A, GBM track from ENCODE database). This bidirectional promoter also produces an antisense lncHOXD-AS2 (promoter-associated asRNA, or pRNA) with its longest annotated variant (LNCpedia, version 5.2) overlapping HOXD8 and HOXD9 genes.
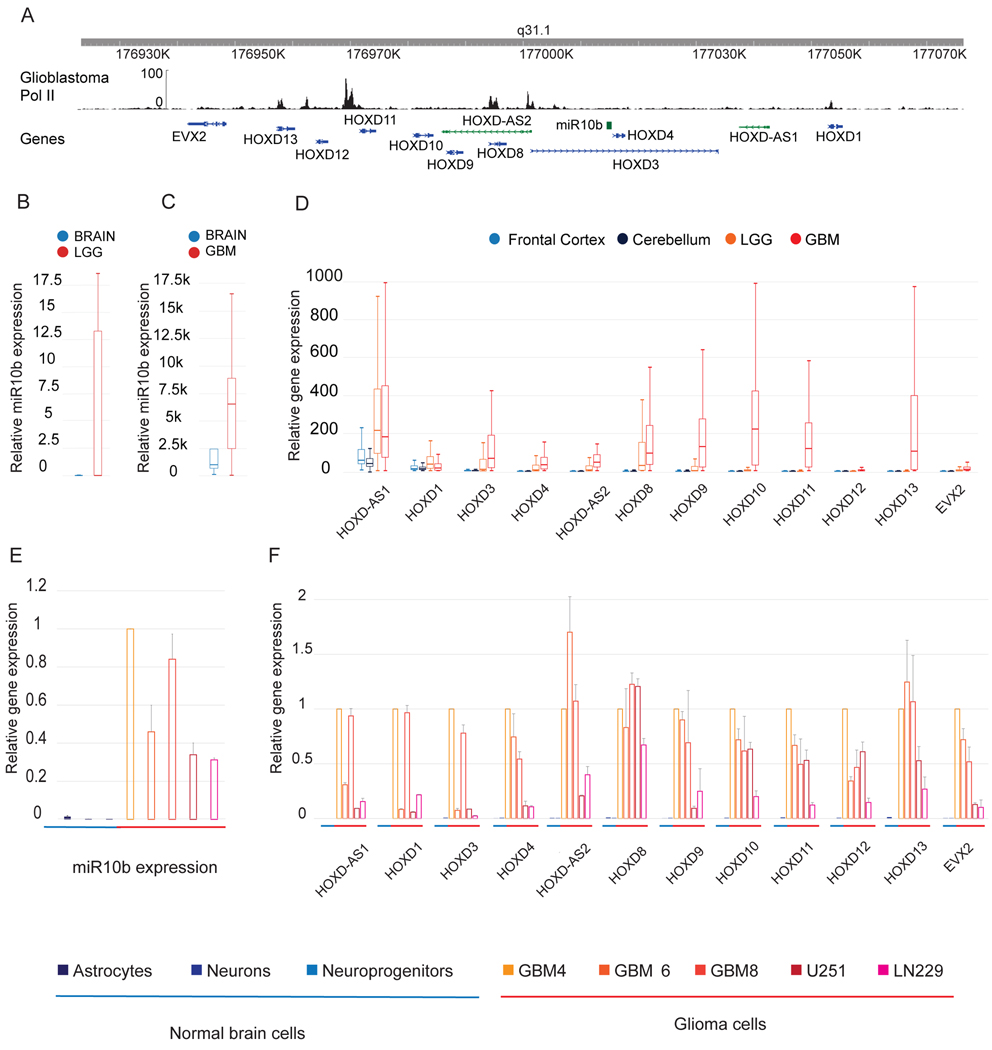
Figure 1. Gene expression in the HOXD/miR-10b locus is repressed in the human brain and activated in glioma cells and tumors.
A. Pol II ChIP-seq in HOXD locus, based on GBM track from ENCODE database (GSM808768). Gencode V19 was used for gene annotation.
B. Relative miR-10b expression in the brain and LGG. Illumina HiSeq data for the normal brain (n=5) and LGG (n=500) were retrieved from TCGA and visualized as boxplots. Unit: log2 (FPKM-UQ+1).
C. Relative miR-10b expression in the brain and GBM. Boxplot visualization of Illumina Human v2 MicroRNA expression BeadChip dataset (GEO number: GSE25632) for the normal brain (n=5) and GBM (n=103).
D. Expression of HOXD genes in human brain cortex, cerebellum, LGG, and GBM. TCGA TARGET GTEx dataset for cortex (n=300), cerebellum (n=300), LGG (n=500), and GBM (n=170) was visualized as boxplots. Unit: RSEM norm_count+1.
E. Relative miR-10b expression in normal neural cells, GSCs, and glioma lines was monitored by qRT-PCR. Each group included 3 biological replicates (mean+SD).
F. Relative expression of HOXD genes in normal neural and glioma cells was monitored by qRT-PCR (mean+SD, n=3).
To investigate expression in the locus in human glioma and compare it to the normal brain, miR-10b levels in 500 LGG and 95 GBM were analyzed using TCGA and other large datasets (Figure 1B, C). In parallel, HOXD mRNA levels in 500 LGG, 170 GBM tumors of various subtypes, and normal brain cortex and cerebellum were retrieved from TCGA (Figure 1D). The RNA levels were also measured in tumorigenic glioma cell lines grown in monolayer cultures (U251, LN229) and low-passage glioma-initiating stem-like cells (GSC) grown as spheroids (GBM4, GBM6, GBM8), and compared to those observed in normal human primary and low-passage brain-derived neural cell cultures, including astrocytes, neuron-enriched neuroglia, and neuroprogenitor cells (NPC) (Figure 1E, F). All tested transcripts were nearly undetectable in the normal neural cells and brain specimens; however, expression of the entire cluster was commonly and concordantly activated in the glioma cells and GBM tumors. Furthermore, miR-10b, HOXD3-D8 mRNAs, HOXD-AS1 and HOXD-AS2 lncRNAs were upregulated in LGG as well (Figure 1B, D). Upregulation of HOXD-AS2 lncRNA transcribed from the bidirectional Pol II promoter driving HOXD3/D4/miR-10b expression (Suppl. Fig. 1A, B) suggested its involvement in the regulated expression of these genes. Of note, TCGA LGG and GBM analysis identified no common mutations or locus amplification, and only 13 tumors out of 653 carrying individual mutations in the HOXD region (9 LGGs and 4 GBMs) (Suppl. Fig. 1C).
Silencing and activation of the HOXD locus during the development are controlled by mechanisms that include histone modifications and higher-order chromatin organization (Rodríguez-Carballo et al., 2017; Yakushiji-Kaminatsui et al., 2018). Local chromatin organization is largely mediated by the CCCTC-binding factor (CTCF) and cohesin complex that are responsible for the formation of the Topologically Associating Domains (TADs) (Mirny et al., 2019), and mediating enhancer-promoter interactions (Cuartero et al., 2018). To investigate the involvement of epigenetic mechanisms in the common derepression of this large multi-gene locus in glioma and compare its 3D chromatin architecture to the normal brain, we examined available Hi-C data (Johnston et al., 2019; Wang et al., 2018). The differential Hi-C map highlighting structural alterations between glioma and astrocytes with 10 Kb resolution (Figure 2A and Suppl. Fig. 2A) allowed us to make several observations: 1) HOXD/miR-10b locus is located closely to the left border shared by 3 TADs of approximately 300, 400 and 500 KB (Figure 2A); 2) frequency of interactions within the TADs is significantly higher in glioma than in astrocytes, especially at the spots corresponding to the TAD borders. We further examined HOXD TADs at higher resolution (~ 250 bp) using locus-targeted 4C-Seq of genetically diverse glioma cell lines, GSCs, and in parallel, NPCs and normal cortical astrocytes (the cells of glioma origin), produced with the HOXD3/D4/miR-10b bait as a viewpoint. This analysis confirmed interaction peaks between HOXD3/D4/miR-10b locus and the three specific loci located 300, 400 and 500 KB downstream of HOXD/miR-10b region (Figure 2B). The latter peak was prominent in all glioma samples, whereas two others were more variable (Figure 2B and Suppl. Fig. 2B). These peaks of enhanced contacts were absent in astrocytes and NPCs, suggesting spatial alterations in the TAD’s organization between normal neural cells and glioma. Most notably, a strong peak located ~ 500 KB downstream of HOXD/miR-10b locus matched the annotated bidirectionally transcribed enhancer producing putative non-protein-coding LINC01116 and LINC01117 RNA transcripts (Suppl. Fig. 2C). TCGA analysis demonstrated expression of the enhancer-associated LINC01116, but not of LINC01117, in LGG. Robust levels of LINC01116 were observed in GBM and cultured glioma cells, whereas it was undetectable in the normal brain and neural cells (Figure 2C and Suppl. Fig. 2D).
Figure 2. Alterations in the chromatin architecture and histone marks of the HOXD/miR-10b locus in human glioma.
A. Chromatin interactions within the HoxD/miR-10b TADs. Differential Hi-C heatmap represents the alterations in normalized pairwise DNA contacts within the HOXD TADs in glioma relative to normal astrocytes. Puncture lines denote the bins with boundary-forming CTCFs. Regions of glioma-enriched interactions covering HOXD locus and consistent between 4C-Seq and Hi-C are marked with black horizontal lines. CTCF motif orientation is indicated in green (forward) and red (reverse).
B. 4C-Seq tracks depict the interactions between the HOXD3/HOXD4/miR-10b bait (marked by arrowhead) and other fragments of the locus in glioma and normal neural cells. The corresponding glioma-enriched 4C peaks are highlighted in grey.
C. Relative expression of LINC01116 and LINC01117 RNA in the 600 brains, 500 LGGs and 170 GBMs. TCGA TARGET GTEx dataset was visualized as boxplots. Units: RSEM norm_count+1.
D. Comparative epigenetics of the 500 KB TAD containing miR-10b/HOXD and LINC01116 loci in glioma cells and astrocytes. Active (H3K27Ac, blue) and silent (H3K27Me3, red) chromatin marks were detected by ChIP-Seq. HOXD3/HOXD4/miR10b promoter and enhancer regions are highlighted in green and yellow.
CTCF and cohesin establish local chromosome organization through the process of loop extrusion (Nuebler et al., 2018) where cohesin extrudes loops (Davidson and Peters, 2021) and CTCF occludes this extrusion leading to formation of transient yet positioned loops. We hypothesized that CTCF binding and cohesin-mediated extrusion account for the chromatin interactions enriched in glioma cells. The comparative analysis of the CTCF and Rad21 quantitative ChIP-seq (ChIP-Rx, (Orlando et al., 2014)) in glioma and astrocytes revealed several major glioma-enriched peaks at CTCF sites within the 500 KB TAD (Suppl. Fig. 3A). Three of them matched the Hi-C/4C peaks of the glioma-enriched chromatin interactions. Several additional CTCF peaks are located proximal of HOXD3/D4/miR-10b/HOXD-AS2, with one prominent site with both CTCF and Rad21 glioma-specific peaks located right next to the HOXD3/D4/miR-10b/HOXD-AS2 promoter region (Suppl. Fig. 3A). Of note, these HOXD-embedded glioma-enriched CTCF sites have convergent orientation with distal glioma-enriched CTCF sites, enabling cohesin-mediated looping between the HOXD and enhancer regions (Figure 2A). Interestingly, iCLIP demonstrated that both HOXD-AS2 and LINC01116 nascent transcripts were highly enriched in CTCF and Rad21 immunoprecipitates (Suppl. Fig. 3B).
To further characterize the epigenetic status of the HOXD TADs in general, and HOXD3/miR-10b and LINC01116 (eRNA) regions specifically, we investigated the marks of active (H3K27Ac, H3K4Me3) and silent (H3K27Me3) chromatin. We first compared the available ChIP-Seq datasets produced for various areas of the normal brain and GBM tumors (Suppl. Fig. 4A, B). We further performed comparative ChIP-Seq for cultured glioma cells and astrocytes. A very low ratio of active-to-silent chromatin marks was observed for the almost entire HOXD locus in different brain regions with the exception of HOXD1 and HOXD-AS1 genes (Suppl. Fig. 4A), which, however, were silent in astrocytes (Figure 2D). In contrast, significant increase in this ratio, indicative of a switch to the bivalent state, was observed in GBM (Suppl. Fig. 4B). Similarly, a high H3K27Ac/H3K27Me3 ratio was observed in glioma cells, as opposed to the astrocytes (Figure 2D). Specifically, the altered H3K27Ac/H3K27Me3 ratio was observed in the HOXD3/D4/miR-10b promoter (Figure 2D, green highlight) and enhancer regions, with the strong H3K27Ac peak corresponding to the LINC01116 TSS in glioma that was replaced by H3K27Me3 in astrocytes (Figure 2D, yellow highlight, matching the corresponding 4C glioma-enriched interactions). No other H3K27Ac peaks have been detected between the HOXD region and the enhancer. Therefore, the chromatin status of the locus correlated with active gene expression in glioma and its repressed state in the normal brain (Figure 1).
Inhibition of HOXD-AS2 and LINC01116 modulates spatial organization of HOXD/miR-10b locus and reduces gene expression
We further hypothesized that HOXD-AS2 pRNA and LINC01116 eRNA transcripts play active regulatory roles in the locus organization and expression of HOXD genes. This hypothesis was supported by significant correlation observed between the expression of these lncRNAs and HOXD genes in LGG and GBM TCGA datasets (Figure 3A, B). To investigate the role of the two lncRNAs in the regulation of HOXD genes, we inhibited them in glioma cells using either specific lentivirus-encoded shRNAs or the pools of synthetic targeting siRNAs and analyzed the expression of the HOXD genes by qRT-PCR. About 70–75% and 70–90% KD of HOXD-AS2 and LINC01116, respectively, was achieved in different glioma lines by the targeting shRNAs (Figure 3C and Suppl. Fig. 5A). The KD of HOXD-AS2 reduced expression of all HOXD mRNAs and miR-10b about 2-fold on average and the KD of LINC01116 had even more potent effects on most of the transcripts (Figure 3C). Of note, KD of HOXD-AS2 and LINC01116 had mutual effects, albeit LINC01116 KD led to the stronger 2.5-fold HOXD-AS2 inhibition. As it was anticipated based on the observed miR-10b inhibition, KD of either HOXD-AS2 or LINC01116 strongly reduced the growth of glioma cells and tumor-initiating GSC spheroids (Suppl. Fig. 5B, D). The effect of LINC01116 KD mimicked the well-established growth-inhibitory effect of miR-10b inhibition (Teplyuk et al., 2016). LINC01116 siRNAs have not impacted the growth of normal astrocytes (Suppl. Fig. 5C).
Figure 3. Knock-down of HOXD-AS2 or LINC01116 lncRNAs reduces gene expression, looping, and CTCF and Rad21 binding in the HOXD/miR-10b TAD in glioma.
A and B. Correlation heatmaps for individual HOXD RNAs expressed in LGG (A) and GBM (B). Pearson correlation coefficient for each pair of transcripts is indicated by color and value.
C. KD of either HOXD-AS2 or LINC01116 reduces the levels of miR-10b and HOXD mRNAs in glioma cells. The lncRNAs were inhibited with targeting shRNAs, and relative levels of indicated transcripts quantified by qRT-PCR in control (non-targeting shRNA, blue bars), LINC01116 KD (orange bars) and HOXD-AS2 KD (grey bars) groups 48 hours post-transfection (mean+SD, n=3).
D. KD of either HOXD-AS2 or LINC01116 in GBM8 reduces interactions between HOXD3/D4/miR-10b and LINC01116 regions, abolishing loop formation within TADs, and CTCF and Rad21 binding. Representative 4C-Seq and ChIP-Seq tracks are shown. The HOXD3/D4/miR10b bait used in 4C-Seq (arrowhead), and major glioma-enriched interactions and CTCF/Rad21 binding sites lost upon the KD are highlighted in green and yellow. CTCF motif orientation is indicated in green (forward) and red (reverse).
The strong effect of the HOXD-AS2 pRNA and LINC01116 eRNA on HOXD gene expression suggested that they may modulate chromatin 3D structure and, thereby, influence transcription in the region. Indeed, 4C-seq demonstrated that KD of either HOXD-AS2 or LINC01116 RNA in heterogeneous glioma models abolished the interactions between the HOXD3/miR-10b viewpoint and three downstream TAD boundaries, including the interaction with LINC01116 genomic enhancer (Figure 3D and Suppl. Fig. 6A). We next monitored the effects of LINC01116 and HOXD-AS2 KD on the CTCF and Rad21 binding in glioma cells. KD of either lncRNA erased the binding between HOXD3/D4/miR-10b promoter and enhancer region (Figure 3D). Specifically, the CTCF and Rad21 binding to the HOXD3/D4/miR-10b promoter and LINC01116 enhancer that differentiated gliomas from normal astrocytes was abolished in glioma cultures lacking either pRNA or eRNA (Figure 3D). The effect was region-specific, as no reduced CTCF or cohesin binding was observed across the genome (shown for HOXA regions in Suppl. Fig. 6B). We concluded that eRNA and pRNA transcripts stabilize the loops observed between the HOXD locus, and specifically HOXD3/D4/miR-10b region and LINC01116 enhancer, by recruiting CTCF and Rad21 or stabilizing their binding, and thereby they play important roles in the spatial chromatin organization and gene expression in the HOXD TADs.
Nascent HOXD-AS2 and LINC01116 transcripts are co-expressed and colocalized in the nuclei of glioma cell lines
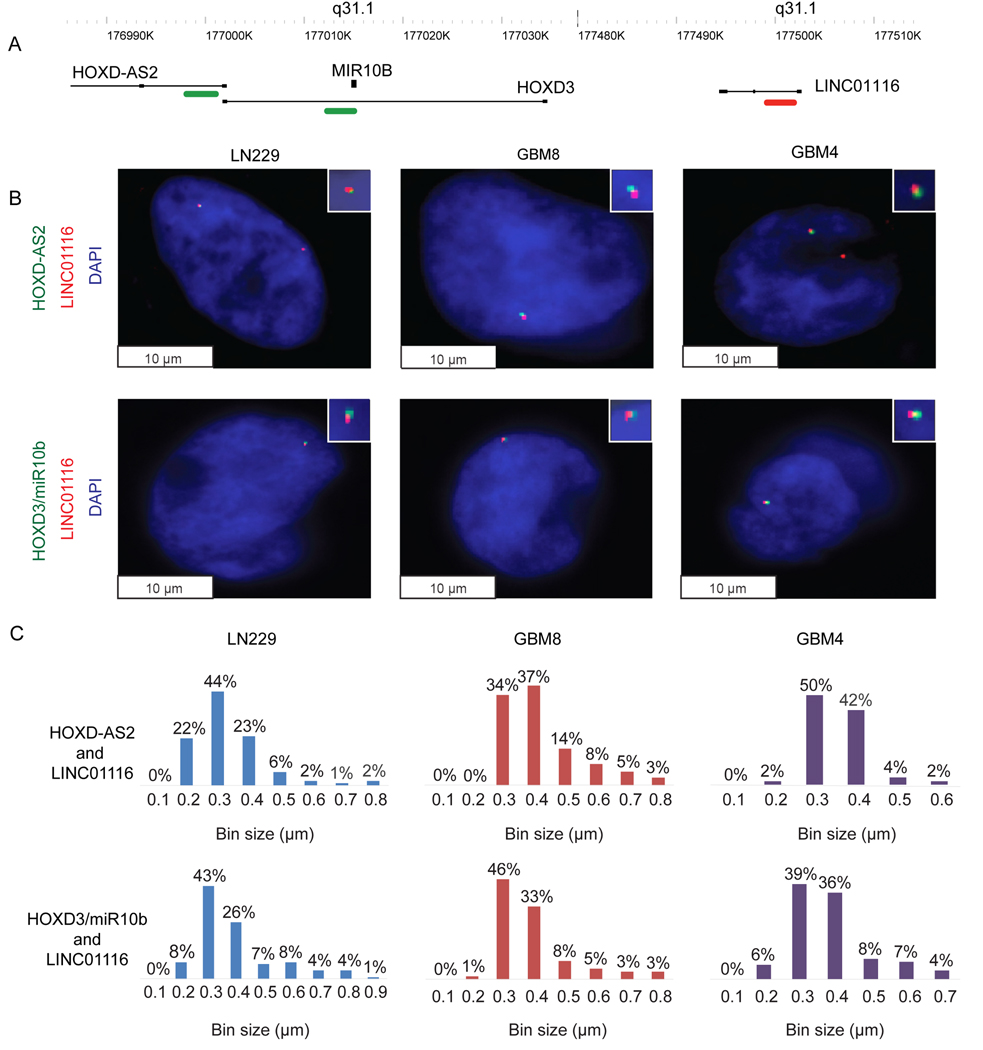
The remarkably similar effects of the promoter-associated HOXD-AS2 and enhancer LINC01116 RNA on the structural organization and gene expression in the HOXD/miR-10b locus suggested their functional and, possibly, physical interaction. To visualize the expression and localization of these transcripts at a single-cell and single-allele level, and investigate whether a chromatin looping could be regulated by HOXD-AS2 and LINC01116 transcripts, we carried out single-molecule RNA FISH (smFISH) with a set of Stellaris™ probes in glioma cells. Similar experiments were performed to detect the nascent HOXD3/D4/miR-10b transcript (Figures 1A and 4A). SmFISH enabled imaging of the HOXD-AS2, HOXD3/D4/miR-10b and LINC01116 transcripts accumulated at their transcription start sites (Figure 4B), and measuring the distances between the transcripts in individual cells (Figure 4C). This analysis demonstrated colocalization of HOXD-AS2 and LINC01116, as well as HOXD3/D4/miR-10b and LINC01116 signals in glioma cells, with most cells exhibiting the distance of 0.3–0.5 μm between them, which corresponds to the immediately adjacent transcripts (Rahman et al., 2017). shRNAs to the lncRNAs abolished smFISH signals, confirming the specificity of detection (Suppl. Fig. 7A). No colocalization was observed between LINC01116 and KIAA1715, the nearest nascent transcript outside the HOXD-containing TAD (Suppl. Fig. 7B, C). The observed colocalization of HOXD-AS2 and LINC01116 transcripts, associated with the remote promoter and enhancer, suggested their juxtaposition, likely via the loop formation, and supporting their common function in the derepression of the locus.
Figure 4. LINC01116 colocalizes with HOXD-AS2 and HOXD3/miR-10b transcripts in glioma cells.
A. A schematic diagram of specific probes utilized for smFISH imaging, depicted in green and red.
B. The transcripts’ colocalization in glioma cells was detected by smFISH, with LINC01116 visualized in red, and HOXD-AS2 or HOXD3/miR-10b visualized in green. Representative pictures are shown.
C. Frequency distribution histograms displaying the distances between LINC01116 and either HOXD-AS2 or HOXD3/miR-10b transcripts in glioma cells. Axis X represents bin intervals in μm. Percentage value for each bin is indicated. The experiment was repeated three times for each pair of transcripts and cell line, and the measurements were taken in 100 cells.
CRISPR-Cas9 editing of specific CTCF binding sites at HOXD3/miR-10b promoter and enhancer recapitulates the effects of LINC01116 KD in glioma
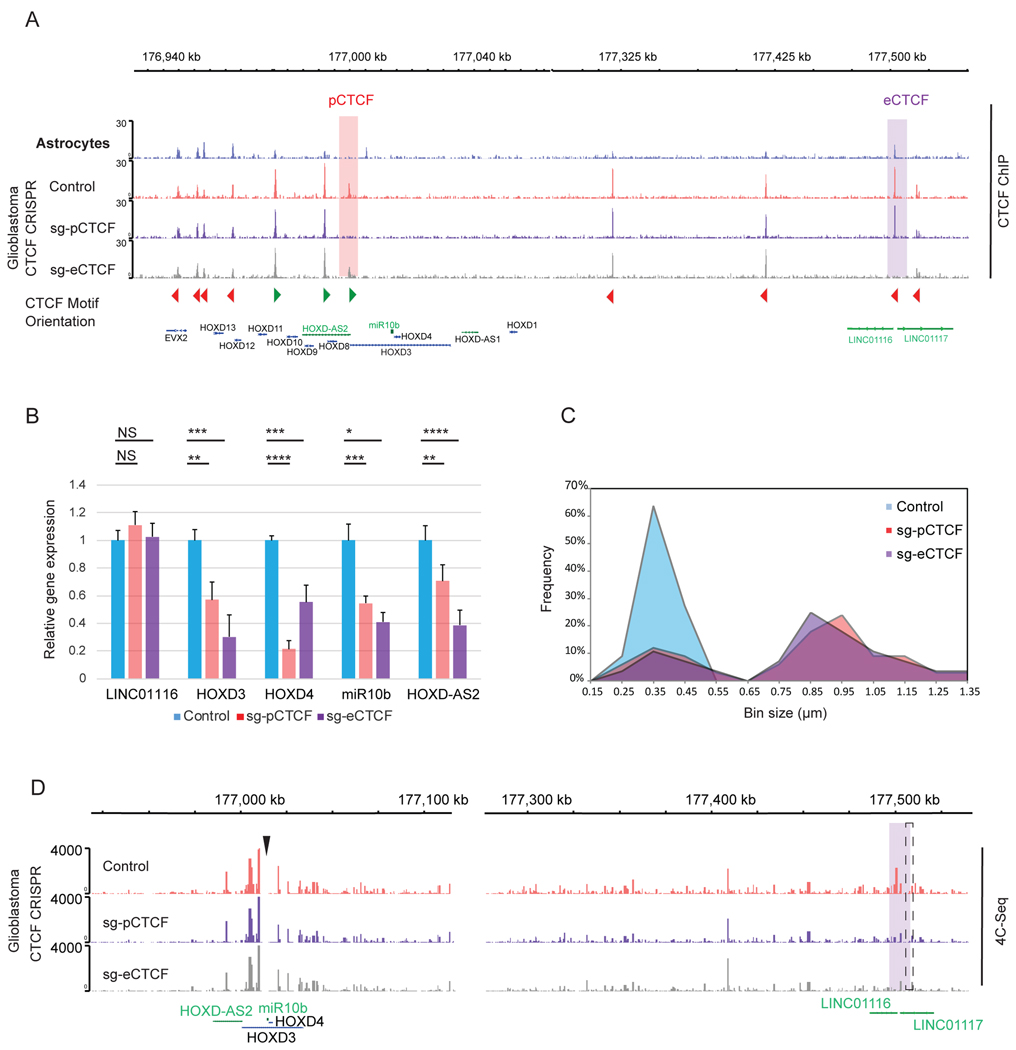
Since the KD of HOXD-AS2 or LINC01116 affected the locus conformation and HOXD/miR-10b gene expression (Figure 3), we hypothesized that these effects are mediated by cohesin that is stopped at CTCF sites near the promoter or enhancer. Specifically, CTCF binding and cohesin accumulation upstream of the HOXD3/miR-10b promoter, prominent in glioma but not in astrocytes (highlighted in Figure 5A and Suppl. Fig. 3A), may underlie reorganization of the region in glioma. Another CTCF binding site, just upstream of the LINC01116 (and not overlapping with its TSS or gene body), with more prominent ChIP-seq peak in glioma, might provide the paired CTCF binding required for the loop formation. CRISPR-Cas9 editing of either of these sites, called pCTCF and eCTCF (Figure 5A), reduced the corresponding CTCF binding in glioma and resulted in the downregulation of HOXD3, HOXD4 and miR-10b, without affecting the levels of LINC01116 (Figure 5B). Furthermore, smFISH-based experiments demonstrated that editing of either pCTCF or eCTCF sites was sufficient to increase the distance between the HOXD-AS2 and LINC01116 nascent transcripts, resulting in the nearly identical effects (Figure 5C). This editing also abolished 4C-seq interactions between the HOXD3/miR-10b viewpoint and LINC01116 (Figure 5D). Altogether, these data indicate that specific CTCF binding events play the key role in the lncRNA-mediated 3D organization and gene expression in the regions.
Figure 5. CRISPR-Cas9 editing of specific CTCF binding sites at the HOXD3/miR-10b promoter and enhancer recapitulates the effects of HOXD-AS2 and LINC01116 KD.
A. The CTCF binding sites in GBM and astrocytes, with representative visualization. Differential CTCF peaks in HOXD3/HOXD4/miR10b promoter (pCTCF) and LINC01116 enhancer (eCTCF) selected for editing, are highlighted in pink and purple, respectively. Two representative bottom tracks validate the loss of binding upon CRISPR-Cas9 editing of the corresponding sites (n=2).
B. CRISPR-Cas9 editing of pCTCF and eCTCF sites reduces expression of HOXD mRNAs and miR-10b in glioma cells. LN229 cells were treated with editing plasmids containing non-targeting Control sgRNA (blue), pCTCF (red) or eCTCF-targeting sgRNAs (purple), and gene expression was quantified by qRT-PCR (mean+SD, n=3).
C. CRISPR-Cas9 editing of pCTCF and eCTCF sites reduces the colocalization of HOXD-AS2 and LINC01116 transcripts. Frequency distribution of the distances between LINC01116 and HOXD-AS2 RNA are shown in control LN229 cells (Control sgRNA, blue), and cells edited with pCTCF sgRNA (red) or eCTCF sgRNA (purple). Axis X represents the bin intervals in μm, with indicated midpoints. The measurements were taken for 30 cells per condition.
D. CRISPR editing of either pCTCF or eCTCF reduces interactions between HOXD and LINC01116 enhancer. 4C-Seq was performed on LN229 with HOXD3/D4/miR10b bait (arrowhead). The major interaction, lost upon editing, is highlighted in purple. The representative track for each group is shown. Puncture line indicates the CRISPR-edited eCTCF region.
Activation of LINC01116 RNA leads to HOXD/miR-10b locus derepression in normal astrocytes and promotes their growth and transformation
Our results indicated that the enhancer LINC01116 is required for the expression of the HOXD/miR-10b locus. To further investigate whether LINC01116 activation is also sufficient for the locus derepression, we employed the lentivirus-encoded CRISPR-activation (CRISPRa) system targeted to the LINC01116 promoter in normal human astrocytes (NHA). The induced LINC01116 expression in astrocytes, the cells not normally exhibiting LINC01116 enhancer activity and transcription, led to the upregulation of HOXD-AS2, as well as miR-10b and most HOXD mRNAs (Figure 6A). The exceptions were HOXD12 and EVX2, two mRNAs also undetectable in GBM tumors (Figure 1D). Changes in the HOXD/miR-10b expression were accompanied by the increased peaks of H3K27Ac, CTCF and cohesin/Rad21 binding in the region, most notably in the HOXD3/miR-10b promoter and LINC01116 enhancer sites (Figure 6B). Furthermore, the 4C analysis demonstrated enhanced interactions within the region, with the consistently boosted peaks corresponding to the TAD borders characteristic for glioma cells. In these activated astrocytes, recruitment of CTCF and cohesin/Rad21, and the corresponding looping in the region have been fully abolished by LINC01116-targeting siRNA (Figure 6B, bottom purple tracks), indicating the essential role of this eRNA in the locus activation.
Figure 6. LINC01116 activation in human astrocytes by CRISPRa leads to reorganization of HOXD/miR-10b locus and induces gene expression.
A. LINC01116 activation leads to the induced expression of HOXD genes in normal human astrocytes. LINC01116 was activated by lentivirus-mediated CRISPRa and the gene expression measured by qRT-PCR in control cells transduced with non-targeting sgRNAs (Control, blue bars) or activating sgRNAs (orange bars).
B. LINC01116 activation increases chromatin interactions, H3K27Ac and CTCF/Rad21 binding in the HOXD-containing TAD, whereas siRNA-mediated KD of LINC01116 RNA abolishes these interactions in activated astrocytes. 4C-Seq with the HOXD3/D4/miR10b viewpoint marked by black arrow (top), H3K27Ac (middle) and CTCF/Rad21 ChIP-seq (bottom panels) were performed for conditions indicated in a color scheme (top right). Two biological replicates were analyzed per condition. Altered CTCF/Rad21 binding and corresponding enriched 4C interactions are highlighted in green and yellow. CTCF motif orientation is indicated in green (forward) and red (reverse).
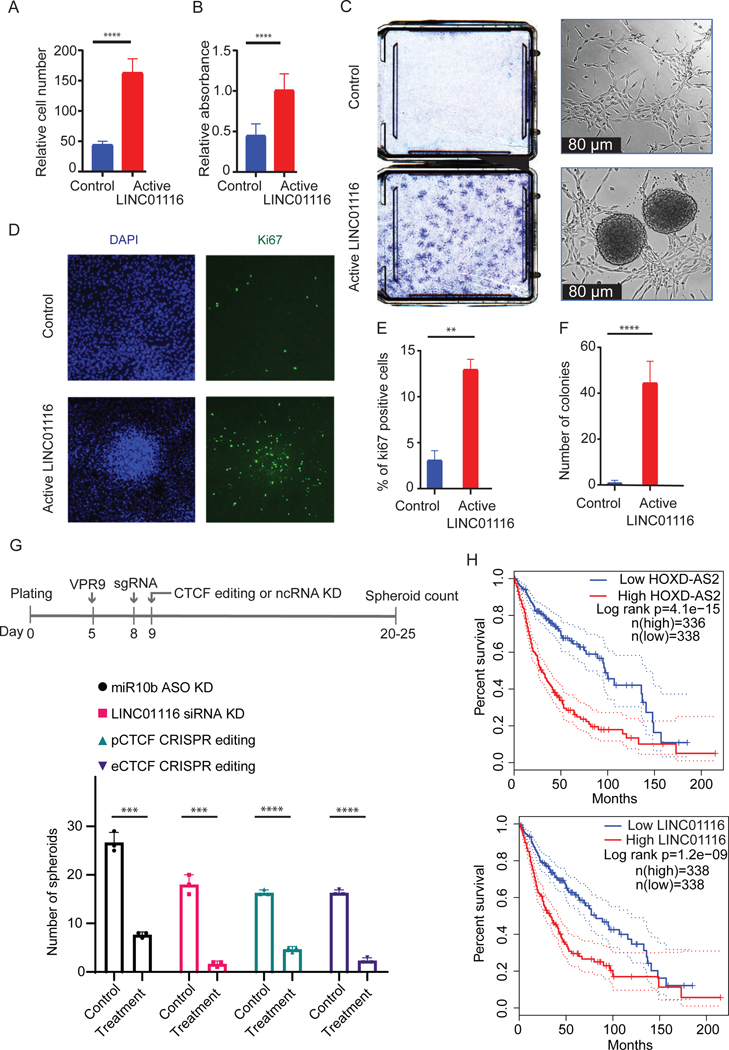
Finally, we investigated the effects of LINC01116 activation on the growth characteristics of astrocytes. The cell number and metabolic activity of astrocytes in low-passage cultures were strongly increased by LINC01116 (Figure 7A, B). Morphology of these cells also changed dramatically, with the typical spindle-like shape altered to a more round glioma-like phenotype. Furthermore, while the immortalized astrocytes formed monolayer cultures, LINC01116 activation triggered their neoplastic transformation, as evident by multiple highly proliferative Ki67-positive colonies and spheroids resembling glioma stem-like cells that grew in both attached and matrix-independent manner (Figure 7C–F). Such phenotype has been previously observed in astrocytes transformed with several potent oncogenes such as T-Ag, hTERT, and H-ras or constitutively active Akt/EGFRvIII (Rich et al., 2001; Sonoda et al., 2001). KD of either LINC01116 or miR-10b transcripts upon activation of the enhancer reduced the transformation (Figure 7G). Furthermore, either pCTCF or eCTCF editing of the activated cultures also strongly reduced the LINC01116-induced transformation (Figure 7G), indicating that it is mediated by the chromatin looping. In addition, significant association of LINC01116 and HOXD-AS2 with survival of glioma patients (Figure 7H) further supported the critical role of the regulatory RNAs and the locus’ reactivation in human tumors.
Figure 7. Activation of LINC01116 promotes proliferation and facilitates transformation of human astrocytes, and is associated with shortened GBM survival.
A. LINC01116 was activated in astrocytes by lentivirus-mediated CRISPRa and the cells were counted in control cultures transduced with non-targeting sgRNAs (Control, blue bars) or activating sgRNAs (red bars) two weeks later (n=4).
B. Metabolic activity of control and LINC01116-activated cultures has been monitored by WST1 assays (n=4).
C. LINC01116 activation promotes astrocyte transformation. The growth of control immortalized astrocytes transduced with non-targeting sgRNAs and upon LINC01116 activation has been monitored over three weeks and analyzed by Crystal blue staining (n=3, left). Spheroids formed in LINC01116 activated cultures, visualized by light microscopy, are shown at the right. There were no transformed colonies or spheroids detected in control cultures.
D. LINC01116 activation leads to increased proliferation, as indicated by Ki67 immunostaining of control cultures (top) and upon LINC01116 activation (bottom). The representative images of DAPI and Ki67 staining are shown.
E. Quantification of Ki67 positive cells in control cultures and upon LINC01116 activation (n=3).
F. Quantification of transformed colonies, shown in panel C, in control astrocytes cultures and upon LINC01116 activation (n=3).
G. LINC01116 activation in astrocytes followed by treatments of these cultures with either LINC01116 siRNAs, miR-10b-inhibiting ASO, pCTCF or eCTCF CRISPR system, or corresponding control oligonucleotides, and spheroid count.
H. HOXD-AS2 and LINC01116 are strongly associated with glioma survival. Kaplan-Meier analysis of 670 glioma patients (TCGA, including high- and low-grade gliomas datasets) demonstrates the association of each HOXD-AS2 and LINC01116 with shortened overall survival. Median score used as the threshold is shown by solid red (high expression group) and blue (low expression group) lines, and correspondent 95% confidence intervals are visualized as dotted lines. Overall survival analysis was performed with Log-rank test, a.k.a. the Mantel-Cox test, for hypothesis test with p-values indicated on the plots.
Discussion
Molecular heterogeneity of GBM is one of the key factors challenging the investigation of its etiology and hindering drug development. Here we demonstrate that derepression of a large genomic HOXD locus, comprised of the genes encoding multiple developmental transcription factors and miR-10b, which is normally silenced in the brain cortex, represents a common trait of GBM. Mutations in this region are rare, and its transcriptional activation is defined by spatial reorganization, with normal neuroglial cells and gliomas exhibiting contrasting chromatin marks and altered looping interactions. Two lncRNAs, one associated with HOXD3/D4/miR-10b promoter (pRNA) and another with the remote LINC01116 enhancer (eRNA), play a cooperative role in the concordant gene regulation in the locus. Remarkably, transcriptional activation of the eRNA in normal astrocytes not only triggers CTCF/cohesin recruitment, chromatin remodeling, spatial reorganization, and gene expression in the locus, mimicking the patterns observed in glioma, but also enhances cell proliferation and promotes their transformation, with a phenotypic switch toward glioma-like colonies.
Alterations in the genomic 3D structure can impact gene expression (Zheng and Xie, 2019). For example, an altered domain topology activates the oncogene PDGFRA that is normally insulated by domain boundaries and, thereby, contributes to gliomagenesis in IDH mutant gliomas (Flavahan et al., 2016). However, the impact of mechanisms controlling genome organization and topology on the gene expression in cancer generally, and in glioma specifically, has been so far only minimally investigated.
Our analysis of HOXD3/miR-10b TAD in glioma and normal glial cells using 4C and Hi-C reveals remarkable differences in the strength of chromatin looping in these closely related cell lineages. It, therefore, provides strong support for the role of local chromosome organization in gene regulation and identifies a distal enhancer located 500 KB downstream of the HOXD cluster and transcribed to the eRNA. While the function of enhancers activating remote genes is well established, roles of genome folding, and specifically cohesin-CTCF system, in this process are being actively investigated (Oudelaar et al., 2020). Interactions between distal genomic elements can emerge when cohesin-mediated loop extrusion is occluded by CTCF or other extrusion barriers, forming a transient loop (Gabriele et al., 2021). Enhancer-promoter interactions can be mediated by such looping. Both enhancer and promoter frequently have proximal CTCF sites, but what regulates CTCF binding there and, hence, looping is largely unknown. Here we demonstrate that expression of eRNA triggers CTCF binding at enhancer, promoter, and through the locus, leading to enhancer-promoter interactions and massive gene activation in the region.
Although most active enhancers and numerous active promoters produce sense and/or antisense ncRNAs in cancer and normal tissues (Mikhaylichenko et al., 2018; Tippens et al., 2018), with estimated 40,000–65,000 human eRNAs expressed (Andersson et al., 2014), only a few studies have demonstrated convincingly that an eRNA or pRNA itself is essential for gene regulation (Sartorelli and Lauberth, 2020). Here we show that RNA transcription can locally regulate genes through the induction of CTCF binding. Several elegant studies described regulatory activities of eRNAs that function by increasing the strength of enhancer-promoter looping interactions, at least partly in concert with CTCF and cohesin (Lai et al., 2013; Li et al., 2013; Melo et al., 2013; Saldaña-Meyer et al., 2019). There are several not mutually exclusive mechanisms through which eRNA expression can affect CTCF binding. The first mechanism relies on the direct interaction of RNA with CTCF’s RNA-binding domain. This domain was shown to modulate CTCF binding through the genome (Hansen et al., 2019; Saldaña-Meyer et al., 2019). The second mechanism involves interactions of eRNA with other chromatin regulators, which, through their activity, can trigger CTCF binding. For example, eRNA may affect the activity of the Polycomb complex (Mortimer et al., 2019) and thus derepress the locus. This is consistent with our observation that eRNA induction increases CTCF binding across the entire TAD and results in the stabilization of characteristic loops, leading to the upregulation of HOXD-AS2 pRNA and multiple genes transcribed from several independent promoters. The third mechanism may rely on the ability of a transcribed RNA to serve as an extrusion boundary (Banigan et al., 2022), stopping cohesin and inducing looping, and allowing CTCF binding through DNA demethylation or other epigenetic mechanism, akin to the mechanism underlying expression of protocadherin isoforms (Canzio and Maniatis, 2019). Such a mechanism can be rather general as it relies on the sole RNA transcription, consistent with local gene regulation by transcription of lncRNAs (Engreitz et al., 2016). Together these mechanisms can allow transcribed RNAs to locally affect gene expression through modulating looping interactions established by the CTCF/cohesin system.
Remarkably, HOXD3/miR-10b promoter-enhancer interactions involve two co-localized and co-functional lncRNAs transcribed from these remote regulatory elements. Of note, published data on spatial interactions obtained by two major techniques, chromosome conformation capture and FISH, can be discordant (Williamson et al., 2014). Our study, based on both methodologies, generated consistent results indicative of stable interaction between the two transcripts. Functionally, KD of either HOXD-AS2 or LINC01116 reduced expression of miR-10b and HOXD genes. Both pRNA and eRNA were required for the 3D reorganization and switching the chromatin status in the region, promoting its accessibility and assembly of the transcriptional machinery at the HOXD genes, and indicative of their cooperative function. Activation of the LINC01116 eRNA alone, however, was sufficient for triggering these changes, while leading to increased CTCF occupancy at both sites and the induction of HOXD-AS2. Conversely, the loss of interactions between the HOXD locus and the enhancer, or between the pRNA and eRNA, caused by the editing of specific loop-organizing CTCF sites or the KD of either lncRNA, led to the repression of HOXD/miR-10b genes and transformation capacity.
Our work demonstrates that activation of a sole LINC01116 eRNA is not only sufficient for induction of all HOXD genes but, phenotypically, it promotes proliferation and transformation of NHA that are largely post-mitotic, slowly-growing cells. Conversely, inhibition of either LINC01116 or HOXD-AS2 reduce glioma viability phenocopying miR-10b loss-of-function. Moreover, both lncRNAs are associated with survival of glioma patients. Interestingly, in addition to the reported here cis-function of HOXD-AS2 transcript, its trans-activity via K-homology splicing regulatory protein conferring temozolomide resistance has been recently proposed (Nie et al., 2021). miRNA-sponging functions have been also recently reported for LINC01116 (Jiang et al., 2021; Ye et al., 2020). Nevertheless, our data strongly implicated the chromatin-reorganizing activity of LINC01116 and HOXD-AS2 in gliomagenesis. Together with prior observations, it leads to the hypothesis that targeting the entire HOXD locus provides a promising strategy for a broad category of glioma patients. Thus, targeting either eRNA or pRNA may represent a new and powerful approach leading to the simultaneous effects on all HOXD genes, protein-coding and regulatory, for malignant glioma therapies. Of note, LINC01116 and HOXD-AS2 are also expressed in extracranial tissues and several types of cancer (Li et al., 2020; Xu et al., 2021). However, to what extent the epigenetic mechanism we discovered is operational and conserved in other non-neuroglial cells and tissues remains to be further investigated.
Finally, our work provides new insights to the question of glioma origin. The studies based on human GBM tumors and mouse models, including single-cell tumor analysis, suggest that different cell types within the brain, such as astrocytes, neuroprogenitors, oligodendrocyte progenitors, and potentially even neurons, have the capacity to transform to glioma-initiating cells (Alcantara Llaguno et al., 2009; Couturier et al., 2020; Friedmann-Morvinski et al., 2012; Kwon et al., 2008; Lee et al., 2018; Liu et al., 2011; Neftel et al., 2019; Patel et al., 2014); however, how this happens is unknown. Over the past two decades, dozens of GBM mouse models have been engineered to express transgenes bearing oncogenic mutations, such as EGFRvIII/InkA2/3-/PTEN- and P53-/NF1- (Zhu et al., 2009; Zhu et al., 2005). Neoplastic transformation of astrocytes has been also achieved by serial transduction of various oncogenes, such as SV40 large T antigen, HPV E6, E7 genes, H-ras, and TERT, or a combination of Oct4, Myc, Ras, and p53DD (Li et al., 2016; Rich et al., 2001; Sonoda et al., 2001). Astrocyte transformation by H-RasG12V/Ad-E1 or SV40 large T antigen led to the upregulation of miR-10b, suggestive of the derepression of the entire HOXD region as a step involved in the transformation, and genetic ablation of miR-10b inhibited the transformation (El Fatimy et al., 2017). Although various events promoting genomic instability may lead to astrocyte transformation, it is accepted that gliomagenesis is associated with accumulation of multiple driving mutations.
Here we report that activation of a single eRNA has genome-shaping effects that result in enhanced proliferation and transformation of astrocytes, including significant alterations in their morphology, loss of contact inhibition, and matrix-independent growth. Therefore, our study presents an additional, eRNA-mediated regulatory layer that may drive gliomagenesis. A triggered or sporadic activation of a single, potentially tissue-specific enhancer in the inherently plastic locus may alter folding of large genomic regions by interfering with cohesin/CTCF system, leading to simultaneous and massive alterations in gene expression, and promoting selection of cell populations that acquire highly proliferative properties. According to this hypothesis, a single, very rare regulatory event, such as activation of an enhancer, may trigger or facilitate the neoplastic growth (Graphical Abstract), an exciting possibility remaining to be tested in vivo. Identification of the specific eRNA and pRNA as essential players in this process implies the unique vulnerability of tumor-initiating cells and provides new strategies for glioma therapy.
LIMITATIONS OF THE STUDY
The main functional experiments of this study have been performed on genetically diverse glioma cell lines and low-passage GSCs. However, this may be insufficient for characterizing the epigenetic heterogeneity of GBM. Similarly, cultured astrocytes utilized in our study may not fully reflect the epigenetic landscape of diverse neuroglial cells that could serve as cells-of-origin for glioma. In-depth research of additional cell populations, and single-cell analysis, would be required to broaden our conclusions. Additionally, formaldehyde-based methods like 4C-Seq, Hi-C, ChIP-Seq, and smFISH provide a snapshot of the described interactions rather than a dynamic profile of epigenetic and transcriptional alterations that occur in our cell models and, potentially, during gliomagenesis.
STAR METHODS
Resource Availability
Lead Contact.
Further information and requests for resources and reagents should be directed to the lead contact, Anna M. Krichevsky (akrichevsky@bwh.harvard.edu).
Materials Availability.
This study did not generate new unique reagents.
Data and code availability
ChIP-Seq and 4C-Seq data generated in this study have been deposited at GEO with accession number GSE161258. Microscopy data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cells and Cell Lines
Human cells were used in accordance with the policies of institutional review boards at Brigham and Women’s Hospital. Human low-passage GBM stem-like cells (GBM4 and GBM8) were a generous gift from Dr. Hiroaki Wakimoto, MGH. The tumorigenic, genetic, and molecular properties of these cells have been previously described (Wakimoto et al., 2009; Wakimoto et al., 2012). Specifically, GBM4 is characterized by MYC amplification; GBM6 by EGFR and MDM4 amplification, and CDKN2A&B deletion; GBM8 by PDGFRA and MDM2 amplification, and CDKN2A&B deletion. U251 cells carry PTEN and P53 mutations and CDKN2A deletion; and LN229-P53 mutation and CDKN2A deletions (Ishii et al., 1999). The cells were maintained in serum-free Neurobasal media supplemented with N-2 and B-27 Plus Supplements (Gibco™), 3 mM of Gibco® GlutaMAX™ Supplement, 50 units/ml of penicillin and 50 units/ml of streptomycin (Gibco™), 2 μg/ml of heparin (Sigma-Aldrich), 20 ng/ml of FGF2 (Sigma-Aldrich), and 20 ng/ml of EGF (Sigma-Aldrich). The cells were passaged by dissociation using Neurocult Stem Cells chemical dissociation kit (Stem Cells Technologies). Human glioma cell lines U251 and LN229 were obtained from American Type Culture Collection (ATCC), cultured in DMEM/10% FBS (Gibco™), and passaged by trypsinization. For human primary and low-passage astrocyte cultures, and NPC cultures, fetal brain tissues were procured from Advanced Bioscience Resources (ABR), dissected, and astrocytes have been cultured in DMEM-F12/10% FBS media as previously described (Zeng et al., 2020). NPC cultures have been maintained in Neurobasal media supplemented with growth factors as previously described (Teplyuk et al., 2016). In addition, normal human astrocytes immortalized by E6/E7/hTERT (a generous gift from Dr. Yukihiko Sonoda (Sonoda et al., 2001)) were used for transformation assays. These cells were maintained in Astrocytes Medium (cat #1801, ScienCell). Cell cultures were periodically tested for mycoplasma and cell lines authenticated.
METHOD DETAILS
Transfections
Dharmacon Lincode SMARTpools (a mixture of 4 siRNAs, listed in Supplementary table 1, at 20 nM final concentration) targeting HOXD-AS2 and LINC01116 have been transfected to glioma cells using Lipofectamine RNAiMAX transfection reagent. Transfections of dissociated GBM4 and GBM8 spheroids with miR-10b ASO, si-LINC01116, and corresponding control oligonucleotides have been performed using magnetofection as previously described (Teplyuk et al., 2016). The images of neurospheres were taken at day 5 post-transfection and the analysis was performed using ImageJ software (Schneider et al., 2012).
Analysis and visualization of TCGA, GSE25632, and Hi-C datasets
TCGA TARGET GTEx and GDC TCGA Lower Grade Glioma (LGG) datasets have been downloaded from Xena Functional Genomics Explorer (Goldman et al., 2020). GSE25632 dataset has been downloaded from GEO. Relative expression is measured as log2(fpkm-uq+1) - Fragments Per Kilobase of transcript per Million mapped reads, upper quartile normalized plus pseudo count 1. Gene expression was visualized as boxplots using Highcharts (JavaScript charting library, version 8.1.0). The center line shows the median expression, box limits indicate the 25th and 75th percentiles, and whiskers extend to the minimum and maximum values.
Glioma (Tissue: Glioblastoma_G523, Type: Johnston_2019_KR, Resolution: 10 kb, Johnston et al., 2019) and Astrocytes of the cerebellum (Tissue: Astrocyte_cerebellum, Type: ENCODE3-iced, Resolution: 10 kb, ENCODE ID ENCSR011GNI) Hi-C data were processed, normalized using the pipeline by Yue lab and subtracted from one another (Wang et al., 2018) for Figure 2A. For Supplementary Figures 2A–B, Hi-C data for astrocytes of the cerebellum (ENCODE ID ENCSR011GNI) was downloaded from GEO (GSE105194), mapped with distiller-nf pipeline (https://doi.org/10.5281/zenodo.3350937) with default parameters and no_filter option; mapped Hi-C for glioma (G523, G567, G583) was downloaded at 10 Kb resolution from the supplementary materials of Johnston et al., 2019, and re-balanced with ICE (Imakaev et al., 2012). The maps were visualized at 10 Kb resolution in linear color scale with HiGlass (Kerpedjiev et al., 2018).
RNA isolation and analysis of gene expression by qRT-PCR
RNA was isolated with RNA Purification kit from Norgen Biotek Corporation. 100 ng of total RNA was used for reverse transcription (RT). The RT reactions for hsa-miR-10b-5p, hsa-miR-99a, hsa-miR-125–5p have been performed with miRCURY LNA RT Kit (Qiagen), followed by qPCR with miRCURY LNA miRNA PCR Assays with SYBR™ Green PCR Master Mix (ThermoFisher Scientific). The RT reactions for lncRNAs and mRNAs were carried out with High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems), followed by qPCR with Taqman Universal PCR Master Mix with TaqMan Gene Expression Assays. The sequences of primers used for PCR reactions are listed in Supplementary table 2. Fold-change in gene expression between conditions was calculated by 2^-ΔCt method. Gene expression was normalized to GAPDH for mRNAs and the geometric mean of miR-99a and miR-125a for miR-10b (mean+SD, n=3).
Quantitative ChIP-Seq (ChIP-Rx)
ChIP-Seq was performed with SimpleChIP® Enzymatic Chromatin IP Kit (Magnetic Beads) #9003. Briefly, the cells were collected in ice-cold PBS containing protease inhibitors, and centrifuged at 500×g for 5 min at 4 °C. Additional cell cultures harvested in parallel were used for cell count. Cell pellets were resuspended in ice-cold PBS to reach a concentration of 106 cells/ml and fixed with 1% formaldehyde for 15 min at room temperature with 30 rpm rotation. The fixation was quenched by addition of 125 mM glycine for 5 min at room temperature. Fixed cells were centrifuged at 500 g for 5 min at 4 °C and washed twice with 10 ml of ice-cold PBS containing protease inhibitors. The pellet was resuspended in 2 ml of RIPA buffer (BP-115X, Boston BioProducts) and fragmented to ~300 bp using MISONIX S-4000 Sonicator (amplitude 30%, 30 sec ON/30 sec OFF, over 40 min). Aliquots of sonicated chromatin were de-crosslinked and DNA concentrations measured by Qubit. 20 μg of chromatin was resuspended in 1ml IP dilution buffer (16.7 mM Tris-HCl pH 8, 0.01% SDS, 1% Triton X-100, 167 mM NaCl, 1.2 mM EDTA) per ChlP. Drosophila spike-in chromatin (Cat. 53083, Active Motif) and Drosophila-specific histone variant, H2Av (Cat.61686, Active Motif) were added to each sample for normalization prior to IP. 10 μg of the following antibodies were added per ChIP and incubated overnight with rotation at 4°C: CTCF (D31H2) XP Rabbit mAb (#3418), H3K27Ac (#4353), H3K27Me3 (#9733) (all from Cell Signaling), Rad21 (ab992, Abcam). Dynabeads™ Protein G (30 μl per sample) were added and incubated for additional 4 hours at 20 rpm at 4 °C. The samples were pelleted and washed twice for 5 min in a low salt buffer, followed by a wash in high salt buffer provided in the SimpleChIP® Enzymatic Chromatin IP Kit and, further, in TE buffer (1 mM EDTA, 10mM Tris-HCl pH 8.1). The antibody-protein complexes were eluted off the beads, crosslinking further reversed at 65 °C overnight with 6 μl 5M NaCl and 2 μl Proteinase K, and followed by DNA purification. ChIP-seq libraries have been prepared using NEBNext® Ultra™ II DNA Library Prep Kit for Illumina® (E7546S, NEB) and sequenced on the Illumina HiSeq 2500 platform configured for 50-bp single-end reads.
Quantitative ChIP-Seq (ChIP-Rx) data analysis
Samples with raw sequencing reads (fastqsanger files) were grouped by antibody and aligned to Drosophila genome (dm6) using Bowtie2 (Langmead and Salzberg, 2012) with default parameters. Scaling factors were generated as follows: the number of uniquely mapped Drosophila reads in a sample with the lowest unique Drosophila read number was divided by the number of uniquely mapped Drosophila reads in every sample. The resulting scaling factors were used to randomly subsample raw fastqsanger files with seqtk_sample tool (Li, 2013). Diluted fastqsanger files were aligned to the human reference genome (GRCh37/hg19) using Bowtie2 with default parameters. PCR duplicates were removed using samtools rmdup version 1.13 (Li et al., 2009). The resulting aligned Bam files were transformed to bigwig format without scaling or normalization using deeptools version 3.4.3 (Ramírez et al., 2016). IGV web application (Thorvaldsdottir et al., 2013) was used for visualization. The H3K27Ac/H3K27Me3 ChIP-Seq data shown in Figure 2 were not normalized or scaled. For differential CTCF and Rad21 binding analysis, the raw fastqsanger files were aligned to human GRCh37/hg19 with bowtie2, followed by the duplicates removal and peak calling with MACS version 2.0.10 (Zhang et al., 2008) with default parameters. Resulting bed (narrow peaks) and bam files were uploaded to Diffbind (Ross-Innes et al., 2012) (version 2.10.0) for differential binding analysis. Differential CTCF and RAD21 peaks between astrocytes and glioma cells located in HOXD TAD are indicated in Supplementary figure 2A. Full list of all differential binding sites for CTCF and Rad21 could be found in Supplementary tables 3 and 4.
Oriented CTCF binding sites were called by JASPAR UCSC tracks wrapper of PWMscan (Castro-Mondragon et al., 2022) accessed at https://github.com/wassermanlab/JASPAR-UCSC-tracks. Validated human CTCF motif MA0139.1 from JASPAR database was used as a reference. The calling was performed for hg38 genome, with a p-value threshold of 0.0001 and a relative score threshold of 0.8. Background GC content was set to match hg38 genome.
Circularized Chromatin Conformation Capture Sequencing (4C-Seq)
The 4C libraries have been prepared by the established protocol (Matelot and Noordermeer, 2016). Briefly, cells were trypsinized, counted, and fixed at 106 cells/ml with 1% formaldehyde (Sigma) in PBS for 15 min at RT. The fixation was quenched with cold glycine at a final concentration of 125 mM, the cells were washed with PBS and permeabilized on ice for 1 h with 10 mM Tris-HCl, pH 8, 100 mM NaCl, 0.1% NP-40, and protease inhibitors. Cell nuclei were resuspended in Nlalll restriction buffer to a concentration of 5×106 nuclei/ml, further permeabilized in 0.4% SDS at 37°C, followed by the SDS neutralization with 2.6% Triton-X100 at 37°C. The nuclei were digested overnight with 300 U Nlalll at 37°C, and the enzyme was inactivated by incubation at 65°C for 30 min, followed by two washes and resuspension in T4 DNA ligase buffer. In situ ligation was performed in 3.5 ml T4 DNA ligase buffer with 100 U of T4 DNA ligase overnight at 16°C. DNA was purified by reverse crosslinking with an overnight incubation at 65°C with proteinase K, followed by RNase A digestion for 30 min at 37°C, phenol/chloroform extraction, and isopropanol precipitation. The purified DNA was further digested with 5 U/μg of DpnII at 37°C overnight, then re-purified by phenol/chloroform extraction and isopropanol precipitation. The DNA was then circularized by ligation with 10 U/μg T4 DNA ligase under diluted conditions (5 ng/μl DNA), and purified by phenol/chloroform extraction and isopropanol precipitation. 100 ng aliquots of ligated DNA were used as a template for PCR with bait-specific primers containing Illumina adapter termini. The products of the PCR reactions were pooled, the primers removed by washing with 1.8× AMPure XP beads, and then sequenced by HiSeq 2500 SE50 (Illumina). All experiments were performed using two biological replicates per condition. The primers are listed in Supplementary Table 5.
4C-Seq data analysis
The first 20 nucleotides corresponding to the viewpoint-specific forward reading primer were trimmed from the raw sequencing reads, enabling mapping of the captured fragments. The reads were aligned with Bowtie2 (version 2.3.4.1) against the human reference genome (GRCh37/hg19). The resulting Bam files were processed with a variety of functions from Bioconductor packages: r3Cseq (Thongjuea et al., 2013) (Bioconductor version 1.34.0) and FourCSeq (Klein et al., 2015) (version 1.21.0). Briefly, the number of reads per restriction fragment was counted and normalized, cis-interactions were identified according to Thonqiuea S with r3Cseq package. The resulting bedgraph files were visualized using IGV web application (Thorvaldsdottir et al., 2013).
Crosslinking and immunoprecipitation (iCLIP)
To covalently crosslink the proteins to nucleic acids, glioma cells (2 × 107) were subjected to UV irradiation (200 mJ/cm2). The cells were then lysed with RIPA lysis buffer containing Protease Inhibition Cocktail and RNase Inhibitor. The immunoglobin-coated protein magnetic beads (Thermofisher scientific) were incubated with CTCF (D31H2) XP Rabbit mAb (#3418) and Rad21 (ab992, Abcam) antibodies to immunopurify the respective complexes. The complexes were washed with RIPA buffer, and the samples treated with DNAse I (Promega) for 30 min at 37°C followed by proteinase K (10% SDS and 10 mg/ml proteinase K in RIPA buffer) for 30 min at 37°C with shaking. RNA was further isolated using phenol:chloroform:isoamyl alcohol (25:24:1) solution, precipitated using isopropanol, and re-suspended in RNase-free water. qRT-PCR was performed for nascent HOXD-AS2 and LINC01116 transcripts. Primers are listed in Supplementary Table 2. Fold enrichment over IgG was calculated in two steps: 1) DDCt = (Ct IP) - (Ct IgG); 2) (2^-DDCt).
Fluorescence In Situ Hybridization (FISH), signal detection, and quantitative co-localization
Customized probes were designed using the Stellaris Probe Designer (https://www.biosearchtech.com/support/tools/design-software/stellaris-probe-designer) and synthesized by Biosearch Technologies (Coassin et al., 2014). These probes, each comprised of 48 labeled oligonucleotides designed to selectively bind to introns of HOXD-AS2, LINC01116, HOXD3/miR-10b, and KIAA1715 permit highly sensitive detection of a single RNA molecule and the probes’ sequences are indicated in Supplementary Table 6. The probes for HOXD-AS2 and HOXD3/miR-10b were labelled with Quasar 570 with Ex (nm)/Em (nm) = 548/566, and the probes for LINC01116 were labelled with Quasar 670 with Ex (nm)/Em (nm) = 548/566. The cells were seeded as a single-cell suspension on poly-L-lysine-coated glasses coverslips, and FISH carried out based on the Stellaris protocol for adherent cells. The range of minimal distances at which co-localizing signals could be resolved has been previously established and equals to 40–100 nm (Rahman et al., 2017). The images were acquired using a Leica DMi8 fluorescence microscope with a 63×NA 1.4 oil objective and analyzed as follows. The signals were detected in Z-planes axially spaced 0.2 μm apart, and the distances between the co-localized transcripts measured using the following algorithm: 1) determine signal intensities for all dots in a cell and split the dots to a high-intensity group representing a cluster of transcription start sites - associated nascent RNA transcripts, and a low-intensity group representing individual RNA molecules, and 2) measure the distance if the high-intensity signals for both lncRNAs are detected in the same z-plane.
CRISPR-Cas9 - mediated editing of CTCF binding sites
Plasmids coding human codon-optimized CRISPR associated protein 9 from Streptococcus pyogenes (SpCas9) with 3xFLAG tag and nuclear localization signal, and two sgRNAs targeting either pCTCF or eCTCF, were purchased from Vectorbuilder Inc. The sgRNA sequences are listed in Supplementary table 7. LN229 cells plated at 50,000 cells/well in 24-well plates have been transfected 24 hours later with the plasmids using Lipofectamine 2000. The cells were collected for FISH and chromatin analysis 48 hours post-transfection.
Preparation of CRISPRa and shRNA lentiviral constructs and lentivirus transduction
shRNA sequences targeting LINC01116 and HOXD-AS2 (listed in Supplementary table 1) were cloned in pU6GFP plasmid. The pU6GFP plasmid was made by ligating the EcoRI and T4 polymerase-filled Xhol cassette from pLKO1-TRC (Addgene 10878) into the pCDH-CMV-MCS-EF1-copGFP (System Biosciences) digested with EcoRI and T4 polymerase-filled SpeI.
A CRISPR activation system consisting of the deactivated Cas9 (dCas9) and two sequence-specific gRNAs was used for LINC01116 activation. Lentiviral plasmid for generation of stable dCas9-VPR nuclease-expressing cell populations, Edit-R CRISPRa Lentiviral sgRNA Non-targeting Control, and two custom Edit-R CRISPRa human LINC01116 lentiviral sgRNAs, from Dharmacon, have been employed. The sequences of sgRNAs are listed in Supplementary table 7. VSV-G pseudotyped lentiviral particles were produced by transfecting 293T cells grown in DMEM/10% FCS at 10% confluence with one ml DNA-calcium phosphate complex in HEBS buffer with 10 μg of each lentiviral construct along with 6.5 μg of pCMVR8.74 (Addgene 22036) and 6.5 μg of pMD2.G (Addgene 12259) in 100 mm dishes. The medium was replaced after 12 hours to Neurobasal medium without supplements. Lentiviral particles were harvested daily, starting from 48 hours after transfection, for five days, and 10 ml of fresh Neurobasal media was used for replacement. Cells and debris were removed from the 50 ml pooled conditioned media by filtration through a 0.45 μm syringe filter and the supernatants used directly to transduce the target cells for 8 hours, 3 times sequentially. The media was then replaced to the regular growth media, specific for each cell line.
Transformation assay
For astrocyte growth and transformation upon LINC01116 activation, 10,000 cells were plated in T-25 flasks or 24-well plates and transduced with lentiviral particles containing VPR at day 5, followed by transduction with sgRNA1+sgRNA2 lentiviral particles at day 8. SiRNAs, CRISPR sgRNAs/Cas9 pCTCF or eCTCF-editing plasmids, or corresponding controls were transfected with Lipofectamine 2000 on day 9. The cell growth and morphology were monitored using Leica DMi8 fluorescence microscopy and formed colonies or spheres counted two-three weeks later.
Cell viability assay and Ki67 immunofluorescence
Cell metabolism and viability were quantified by Cell Proliferation Reagent WST-1 (Sigma), according to manufacturer’s instructions. For immunostaining, the cells were fixed in 4% PFA in PBS for 10 minutes and permeabilized with Triton X-100 0.25% at room temperature. The samples were then blocked in 10% BSA/PBS for 30 min RT, incubated with Ki67 antibody (Abcam, ab 16667, rabbit monoclonal, 1:250) overnight at 4C, washed in PBS, and incubated with secondary antibody (Cy5 goat anti-rabbit IgG, A10523, 1:1000; ThermoFisher Scientific) for two hours at RT, further washed in PBS twice, incubated for 5 min with DAPI (DAPI 100x Cat No: TR-100-FJ, biosensis.com) and visualized with Leica DMi8 fluorescence microscopy.
Quantification and Statistical analysis
All statistical analyses were performed using Prism 8 GraphPad Software. Statistical significance of the differences between groups was measured with two-tailed unpaired t-test. In figure legends: *p<0.05, **p<0.01, ***p < 0.001, ****p<0.0001. Non-significant differences are marked with NS.
Supplementary Material
Supplemental table 1. siRNA and shRNA sequences used in HOXD-AS2 or LINC01116 KD experiments. Related to Figures 3 and 6.
Supplemental table 2. qPCR primers for HOXD genes and miRNAs detection. Related to Figures 1, 5, and 6.
Supplemental table 3. Differential CTCF binding between astrocytes and glioma cells. Related to Figure 5.
Supplemental table 4. Differential Rad21 binding between astrocytes and glioma cells. Related to Figures 3 and 5.
Supplemental table 5. 4C-Seq primers for HOXD3/HOXD4/miR10b viewpoint. Experiments werre performed with NlaIII-DpnII restriction enzyme pair. Related to Figures 2, 3, 5, and 6.
Supplemental table 6. Stellaris® RNA FISH Probes. Related to Figure 4.
Supplemental table 7. sgRNA sequences used for CRISPR editing and CRISPR activation assays. Related to Figures 5 and 7.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CTCF (D31H2) XP Rabbit mAb | Cell Signaling Technology | #3418, RRID:AB_2086791 |
| Rad21 | Abcam | Ab992, RRID:AB_2176601 |
| H3K27Ac | Cell Signaling Technology | #4353, |
| H3K27Me3 | Cell Signaling Technology | #9733, RRID:AB 2616029 |
| Drosophila-specific histone variant, H2Av | Active motif | Cat. 53083 |
| Ki67 rabbit monoclonal | Abcam | ab16667, RRID:AB 302459 |
| Critical commercial assays | ||
| NEBNext® Ultra™ II DNA Library Prep Kit for Illumina® | NEB | E7546S |
| SimpleChIP® Enzymatic Chromatin IP Kit | Cell Signaling | 9003 |
| Deposited data | ||
| Raw and analyzed data | GSE161258 | |
| Human reference genome NCBI build 37, GRCh37 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Experimental models: Cell lines | ||
| Human: GBM4 | Hiroaki Wakimoto laboratory | N/A |
| Human: GBM6 | Hiroaki Wakimoto laboratory | N/A |
| Human: GBM8 | Hiroaki Wakimoto laboratory | N/A |
| Human: U251 | ATCC | N/A |
| Human: LN229 | ATCC | CRL-2611; RRID: CVCL-0393 |
| Human: Fetal human astrocytes | Advanced Bioscience Resources (ABR) | N/A |
| Human: Neuroprogenitors (NPC) | Advanced Bioscience Resources (ABR) | N/A |
| Human: Normal human astrocytes | Dr. Yukihiko Sonoda laboratory | N/A |
| Oligonucleotides | ||
| siRNA and shRNA sequences used in HOXD-AS2 or LINC01116 KD experiments, see Supplementary table 1 | This paper | N/A |
| qPCR primers for HOXD genes and miRNAs < detection, see Supplementary table 2 | This paper | N/A |
| 4C-Seq primers for HOXD3/HOXD4/miR10b viewpoint, see Supplementary table 5 | This paper | N/A |
| Stellaris® RNA FISH Probes, see Supplementary table 6 | Biosearch Technologies | N/A |
| sgRNA sequences used for CRISPR editing and CRISPR activation assays, see Supplementary table 7 | Dharmacon and Vectorbuilder | N/A |
| Recombinant DNA | ||
| pLKO1-TRC | Addgene | 10878 |
| pCDH-CMV-MCS-EF 1-copGFP | Addgene | 99730 |
| pCMVR8.74 | Addgene | 22036 |
| pMD2.G | Addgene | 12259 |
| Software and algorithms | ||
| Bowtie2 (version 2.3.4.1) | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| ImageJ | Schneider et al., 2012 | https://imagej.nih.gov/ij/ |
| R 3.6.3 | R Core Team | https://www.r-project.org/ |
Highlights.
Chromatin topology of the HOXD locus is altered between the brain and glioblastoma
LncRNAs regulate CTCF/cohesin-dependent loop formation and HOXD gene expression
Activation of LINC01116 enhancer RNA leads to astrocyte transformation
The transformation depends on the loop formation and miR-10b derepression
ACKNOWLEDGEMENTS
We want to thank Dr. Yanhong Zhang for assistance with figure revision. We thank the NeuroTechnology Studio at Brigham and Women’s Hospital for providing instrumentation access and consultation on data acquisition and data analysis. This work was supported by the R21 NS098051, AG060019, R01 CA215072, and R01 NS113929 grants to AMK.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, and Parada LF (2009). Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56. 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Gebhard C, Miguel-Escalada I, Hoof I, Bornholdt J, Boyd M, Chen Y, Zhao X, Schmidl C, Suzuki T, et al. (2014). An atlas of active enhancers across human cell types and tissues. Nature 507, 455–461. 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banigan EJ, Tang W, van den Berg AA, Stocsits RR, Wutz G, Brandão HB, Busslinger GA, Peters J-M, and Mirny LA (2022). Transcription shapes 3D chromatin organization by interacting with loop-extruding cohesin complexes. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie S, Lee HK, Jiang W, Cazacu S, Xiang C, Poisson LM, Datta I, Kalkanis S, Ginsberg D, and Brodie C. (2017). The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget 8, 31785–31801. 10.18632/oncotarget.15991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzio D, and Maniatis T. (2019). The generation of a protocadherin cell-surface recognition code for neural circuit assembly. Current opinion in neurobiology 59, 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Mondragon JA, Riudavets-Puig R, Rauluseviciute I, Berhanu Lemma R, Turchi L, Blanc-Mathieu R, Lucas J, Boddie P, Khan A, and Manosalva Pérez N. (2022). JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic acids research 50, D165–D173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, et al. (2016). Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 164, 550–563. 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao F, Cui D, Jiang R, Chen J, Huang Q, and Shi J. (2018). H0XD-AS1/miR-130a sponge regulates glioma development by targeting E2F8. Int J Cancer 142, 2313–2322. 10.1002/ijc.31262. [DOI] [PubMed] [Google Scholar]
- Coassin SR, Orjalo AV Jr., Semaan SJ, and Johansson HE (2014). Simultaneous detection of nuclear and cytoplasmic RNA variants utilizing Stellaris(R) RNA fluorescence in situ hybridization in adherent cells. Methods Mol Biol 1211, 189–199. 10.1007/978-1-4939-1459-3_15. [DOI] [PubMed] [Google Scholar]
- Couturier CP, Ayyadhury S, Le PU, Nadaf J, Monlong J, Riva G, Allache R, Baig S, Yan X, and Bourgey M. (2020). Single-cell RNA-seq reveals that glioblastoma recapitulates a normal neurodevelopmental hierarchy. Nature communications 11, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuartero S, Weiss FD, Dharmalingam G, Guo Y, Ing-Simmons E, Masella S, Robles-Rebollo I, Xiao X, Wang YF, Barozzi I, et al. (2018). Control of inducible gene expression links cohesin to hematopoietic progenitor self-renewal and differentiation. Nat Immunol 19, 932–941. 10.1038/s41590-018-0184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson IF, and Peters J-M (2021). Genome folding through loop extrusion by SMC complexes. Nature Reviews Molecular Cell Biology 22, 445–464. [DOI] [PubMed] [Google Scholar]
- El Fatimy R, Subramanian S, Uhlmann EJ, and Krichevsky AM (2017). Genome Editing Reveals Glioblastoma Addiction to MicroRNA-10b. Mol Ther 25, 368–378. 10.1016/j.ymthe.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz JM, Haines JE, Perez EM, Munson G, Chen J, Kane M, McDonel PE, Guttman M, and Lander ES (2016). Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature 539, 452–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavahan WA, Drier Y, Liau BB, Gillespie SM, Venteicher AS, Stemmer-Rachamimov AO, Suvà ML, and Bernstein BE (2016). Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 529, 110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, and Verma IM (2012). Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 338, 1080–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele M, Brandão HB, Grosse-Holz S, Jha A, Dailey GM, Cattoglio C, Hsieh T-HS, Mirny L, Zechner C, and Hansen AS (2021). Dynamics of CTCF and cohesin mediated chromatin looping revealed by live-cell imaging. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely G, Yi M, Narayan RS, Niers JM, Wurdinger T, Imitola J, Ligon KL, Kesari S, Esau C, and Stephens RM (2011). Human glioma growth is controlled by microRNA-10b. Cancer research 71, 3563–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffart N, Kroonen J, and Rogister B. (2013). Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel) 5, 1049–1071. 10.3390/cancers5031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman MJ, Craft B, Hastie M, Repecka K, McDade F, Kamath A, Banerjee A, Luo Y, Rogers D, Brooks AN, et al. (2020). Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 38, 675–678. 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves CS, Le Boiteux E, Arnaud P, and Costa BM (2020). HOX gene cluster (de)regulation in brain: from neurodevelopment to malignant glial tumours. Cell Mol Life Sci 77, 3797–3821. 10.1007/s00018-020-03508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous F, Alvarado-Velez M, Marcinkiewicz L, Zhang Y, Kim J, Heister S, Kefas B, Godlewski J, Schiff D, and Purow B. (2013). Oncogenic effects of miR-10b in glioblastoma stem cells. Journal of neuro-oncology 112, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AS, Hsieh TS, Cattoglio C, Pustova I, Saldaña-Meyer R, Reinberg D, Darzacq X, and Tjian R. (2019). Distinct Classes of Chromatin Loops Revealed by Deletion of an RNA-Binding Region in CTCF. Mol Cell 76, 395–411 e313. 10.1016/j.molcel.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imakaev M, Fudenberg G, McCord RP, Naumova N, Goloborodko A, Lajoie BR, Dekker J, and Mirny LA (2012). Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nature methods 9, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, and Van Meir EG (1999). Frequent Co-Alterations of TP53, p16/CDKN2A, p14ARF, PTEN Tumor Suppressor Genes in Human Glioma Cell Lines. Brain pathology 9, 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Cheng C, Ji W, Wang H, Du Q, Dong X, Shao J, and Yu W. (2021). LINC01116 promotes the proliferation and invasion of glioma by regulating the microRNA-744–5p-MDM2-p53 axis. Molecular medicine reports 23, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MJ, Nikolic A, Ninkovic N, Guilhamon P, Cavalli FMG, Seaman S, Zemp FJ, Lee J, Abdelkareem A, Ellestad K, et al. (2019). High-resolution structural genomics reveals new therapeutic vulnerabilities in glioblastoma. Genome Res 29, 1211–1222. 10.1101/gr.246520.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerpedjiev P, Abdennur N, Lekschas F, McCallum C, Dinkla K, Strobelt H, Luber JM, Ouellette SB, Azhir A, and Kumar N. (2018). HiGlass: web-based visual exploration and analysis of genome interaction maps. Genome biology 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Siverly AN, Chen D, Wang M, Yuan Y, Wang Y, Lee H, Zhang J, Muller WJ, Liang H, et al. (2016). Ablation of miR-10b Suppresses Oncogene-Induced Mammary Tumorigenesis and Metastasis and Reactivates Tumor-Suppressive Pathways. Cancer Res 76, 6424–6435. 10.1158/0008-5472.CAN-16-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein FA, Pakozdi T, Anders S, Ghavi-Helm Y, Furlong EE, and Huber W. (2015). FourCSeq: analysis of 4C sequencing data. Bioinformatics 31, 3085–3091. 10.1093/bioinformatics/btv335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon CH, Zhao D, Chen J, Alcantara S, Li Y, Burns DK, Mason RP, Lee EY, Wu H, and Parada LF (2008). Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res 68, 3286–3294. 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, and Shiekhattar R. (2013). Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature 494, 497–501. 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, and Salzberg SL (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9, 357–359. 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK, Lee JK, Park J, et al. (2018). Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature 560, 243–247. 10.1038/s41586-018-0389-3. [DOI] [PubMed] [Google Scholar]
- Li F, Liu X, Sampson JH, Bigner DD, and Li C-Y (2016). Rapid reprogramming of primary human astrocytes into potent tumor-initiating cells with defined genetic factors. Cancer research 76, 5143–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. (2013). Seqtk: a fast and lightweight tool for processing FASTA or FASTQ sequences. Github. [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and Genome Project Data Processing, S. (2009). The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079. 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al. (2013). Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 498, 516–520. 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Huang S, Wei Z, and Yang B. (2020). A putative competing endogenous RNA network in cisplatin-resistant lung adenocarcinoma cells identifying potentially rewarding research targets. Oncology letters 19, 4040–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, and Zong H. (2011). Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell 146, 209–221. 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Teruya-Feldstein J, and Weinberg RA (2007). Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 449, 682–688. 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- Matelot M, and Noordermeer D. (2016). Determination of high-resolution 3D chromatin organization using circular chromosome conformation capture (4C-seq). In Polycomb Group Proteins, (Springer; ), pp. 223–241. [DOI] [PubMed] [Google Scholar]
- Melo CA, Leveille N, and Agami R. (2013). eRNAs reach the heart of transcription. Cell Res 23, 1151–1152. 10.1038/cr.2013.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylichenko O, Bondarenko V, Harnett D, Schor IE, Males M, Viales RR, and Furlong EE (2018). The degree of enhancer or promoter activity is reflected by the levels and directionality of eRNA transcription. Genes Dev 32, 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirny LA, Imakaev M, and Abdennur N. (2019). Two major mechanisms of chromosome organization. Curr Opin Cell Biol 58, 142–152. 10.1016/j.ceb.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer T, Wainwright EN, Patel H, Siow BM, Jaunmuktane Z, Brandner S, and Scaffidi P. (2019). Redistribution of EZH2 promotes malignant phenotypes by rewiring developmental programmes. EMBO Rep 20, e48155. 10.15252/embr.201948155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, Richman AR, Silverbush D, Shaw ML, Hebert CM, et al. (2019). An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell 178, 835–849 e821. 10.1016/j.cell.2019.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie E, Jin X, Miao F, Yu T, Zhi T, Shi Z, Wang Y, Zhang J, Xie M, and You Y. (2021). TGF-β1 modulates temozolomide resistance in glioblastoma via altered microRNA processing and elevated MGMT. Neuro-oncology 23, 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuebler J, Fudenberg G, Imakaev M, Abdennur N, and Mirny LA (2018). Chromatin organization by an interplay of loop extrusion and compartmental segregation. Proceedings of the National Academy of Sciences 115, E6697–E6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando DA, Chen MW, Brown VE, Solanki S, Choi YJ, Olson ER, Fritz CC, Bradner JE, and Guenther MG (2014). Quantitative ChIP-Seq normalization reveals global modulation of the epigenome. Cell reports 9, 1163–1170. [DOI] [PubMed] [Google Scholar]
- Oudelaar AM, Beagrie RA, Gosden M, de Ornellas S, Georgiades E, Kerry J, Hidalgo D, Carrelha J, Shivalingam A, and El-Sagheer AH (2020). Dynamics of the 4D genome during in vivo lineage specification and differentiation. Nature communications 11, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, et al. (2014). Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401. 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Y, Wang Z, Wu F, Yin B, Jiang T, Qiang B, Yuan J, Han W, and Peng X. (2018). Long noncoding RNA HOXD-AS2 regulates cell cycle to promote glioma progression. J Cell Biochem. 10.1002/jcb.28117. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zorca CE, Traboulsi T, Noutahi E, Krause MR, Mader S, and Zenklusen D. (2017). Single-cell profiling reveals that eRNA accumulation at enhancer-promoter loops is not required to sustain transcription. Nucleic acids research 45, 3017–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, Heyne S, Dundar F, and Manke T. (2016). deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic acids research 44, W160–W165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon DA, Perry JR, Brandes AA, Jalali R, and Wick W. (2011). Advances in malignant glioma drug discovery. Expert Opin Drug Discov 6, 739–753. 10.1517/17460441.2011.584530. [DOI] [PubMed] [Google Scholar]
- Regulus Therapeutics Inc. (2018). Regulus Presents Preclinical Survival Data Highlighting Potential of Novel Treatment for Glioblastoma Multiforme. http://ir.regulusrx.com/news-releases/news-release-details/regulus-presents-preclinical-survival-data-highlighting.
- Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, and Counter CM (2001). A genetically tractable model of human glioma formation. Cancer Res 61, 3556–3560. [PubMed] [Google Scholar]
- Rodríguez-Carballo E, Lopez-Delisle L, Zhan Y, Fabre PJ, Beccari L, El-Idrissi I, Huynh THN, Ozadam H, Dekker J, and Duboule D. (2017). The HoxD cluster is a dynamic and resilient TAD boundary controlling the segregation of antagonistic regulatory landscapes. Genes Dev 31, 2264–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, and Green AR (2012). Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481, 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldaña-Meyer R, Rodriguez-Hernaez J, Escobar T, Nishana M, Jácome-López K, Nora EP, Bruneau BG, Tsirigos A, Furlan-Magaril M, and Skok J. (2019). RNA interactions are essential for CTCF-mediated genome organization. Molecular cell 76, 412–422. e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorelli V, and Lauberth SM (2020). Enhancer RNAs are an important regulatory layer of the epigenome. Nat Struct Mol Biol 27, 521–528. 10.1038/s41594-020-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, and Eliceiri KW (2012). NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9, 671–675. 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy P, and Medarova Z. (2018). The fundamental role of miR-10b in metastatic cancer. Am J Cancer Res 8, 1674–1688. [PMC free article] [PubMed] [Google Scholar]
- Sonoda Y, Ozawa T, Hirose Y, Aldape KD, McMahon M, Berger MS, and Pieper RO (2001). Formation of intracranial tumors by genetically modified human astrocytes defines four pathways critical in the development of human anaplastic astrocytoma. Cancer Res 61, 4956–4960. [PubMed] [Google Scholar]
- Tabuse M, Ohta S, Ohashi Y, Fukaya R, Misawa A, Yoshida K, Kawase T, Saya H, Thirant C, Chneiweiss H, et al. (2011). Functional analysis of HOXD9 in human gliomas and glioma cancer stem cells. Mol Cancer 10, 60. 10.1186/1476-4598-10-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Louis DN, Curry WT, Batchelor TT, and Dietrich J. (2013). Diagnostic and therapeutic avenues for glioblastoma: no longer a dead end? Nature reviews Clinical oncology 10, 14–26. [DOI] [PubMed] [Google Scholar]
- Teplyuk NM, Uhlmann EJ, Gabriely G, Volfovsky N, Wang Y, Teng J, Karmali P, Marcusson E, Peter M, Mohan A, et al. (2016). Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: first steps toward the clinic. EMBO Mol Med 8, 268–287. 10.15252/emmm.201505495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongjuea S, Stadhouders R, Grosveld FG, Soler E, and Lenhard B. (2013). r3Cseq: an R/Bioconductor package for the discovery of long-range genomic interactions from chromosome conformation capture and next-generation sequencing data. Nucleic Acids Res 41, e132. 10.1093/nar/gkt373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorvaldsdottir H, Robinson JT, and Mesirov JP (2013). Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14, 178–192. 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippens ND, Vihervaara A, and Lis JT (2018). Enhancer transcription: what, where, when, and why? Genes Dev 32, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, and Olson JJ (2010). Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60, 166–193. 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto H, Kesari S, Farrell CJ, Curry WT Jr., Zaupa C, Aghi M, Kuroda T, Stemmer-Rachamimov A, Shah K, Liu TC, et al. (2009). Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res 69, 3472–3481. 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto H, Mohapatra G, Kanai R, Curry WT Jr., Yip S, Nitta M, Patel AP, Barnard ZR, Stemmer-Rachamimov AO, Louis DN, et al. (2012). Maintenance of primary tumor phenotype and genotype in glioblastoma stem cells. Neuro Oncol 14, 132–144. 10.1093/neuonc/nor195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cao L, Dong X, Wu F, De W, Huang L, and Wan Q. (2020). LINC01116 promotes tumor proliferation and neutrophil recruitment via DDX5-mediated regulation of IL-1β in glioma cell. Cell death disease 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Song F, Zhang B, Zhang L, Xu J, Kuang D, Li D, Choudhary MNK, Li Y, Hu M, et al. (2018). The 3D Genome Browser: a web-based browser for visualizing 3D genome organization and long-range chromatin interactions. Genome Biol 19, 151. 10.1186/s13059-018-1519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson I, Berlivet S, Eskeland R, Boyle S, Illingworth RS, Paquette D, Dostie J, and Bickmore WA (2014). Spatial genome organization: contrasting views from chromosome conformation capture and fluorescence in situ hybridization. Genes Dev 28, 2778–2791. 10.1101/gad.251694.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Yu X, Zhang M, Zheng Q, Sun Z, He Y, and Guo W. (2021). Promising Advances in LINC01116 Related to Cancer. Frontiers in Cell Developmental Biology, 2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushiji-Kaminatsui N, Lopez-Delisle L, Bolt CC, Andrey G, Beccari L, and Duboule D. (2018). Similarities and differences in the regulation of HoxD genes during chick and mouse limb development. PLoS Biol 16, e3000004. 10.1371/journal.pbio.3000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Zhu J, Chen H, Qian J, Zhang L, Wan Z, Chen F, Sun S, Li W, and Luo C. (2020). A novel lncRNA-LINC01116 regulates tumorigenesis of glioma by targeting VEGFA. Int J Cancer 146, 248–261. 10.1002/ijc.32483. [DOI] [PubMed] [Google Scholar]
- Zeng A, Wei Z, Rabinovsky R, Jun HJ, El Fatimy R, Deforzh E, Arora R, Yao Y, Yao S, Yan W, et al. (2020). Glioblastoma-Derived Extracellular Vesicles Facilitate Transformation of Astrocytes via Reprogramming Oncogenic Metabolism. iScience 23, 101420. 10.1016/j.isci.2020.101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Shuai K, Cheng J, Yang W, and Kan Z. (2019). LncRNA linc01116 prometes glioma cell migration and invasion by modulation of radixin targeted by miR-31. Int J Clin Exp Pathol 12, 1078–1086. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, and Liu XS (2008). Model-based analysis of ChIP-Seq (MACS). Genome Biol 9, R137. 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, and Xie W. (2019). The role of 3D genome organization in development and cell differentiation. Nat Rev Mol Cell Biol 20, 535–550. 10.1038/s41580-019-0132-4. [DOI] [PubMed] [Google Scholar]
- Zhou H, Ma Y, Zhong D, and Yang L. (2019). Knockdown of lncRNA HOXD-AS1 suppresses proliferation, migration and invasion and enhances cisplatin sensitivity of glioma cells by sponging miR-204. Biomed Pharmacother 112, 108633. 10.1016/j.biopha.2019.108633. [DOI] [PubMed] [Google Scholar]
- Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, Bronson RT, Chen JW, Weissleder R, Housman DE, and Charest A. (2009). Oncogenic EGFR signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A 106, 2712–2716. 10.1073/pnas.0813314106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Guignard F, Zhao D, Liu L, Burns DK, Mason RP, Messing A, and Parada LF (2005). Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell 8, 119–130. 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental table 1. siRNA and shRNA sequences used in HOXD-AS2 or LINC01116 KD experiments. Related to Figures 3 and 6.
Supplemental table 2. qPCR primers for HOXD genes and miRNAs detection. Related to Figures 1, 5, and 6.
Supplemental table 3. Differential CTCF binding between astrocytes and glioma cells. Related to Figure 5.
Supplemental table 4. Differential Rad21 binding between astrocytes and glioma cells. Related to Figures 3 and 5.
Supplemental table 5. 4C-Seq primers for HOXD3/HOXD4/miR10b viewpoint. Experiments werre performed with NlaIII-DpnII restriction enzyme pair. Related to Figures 2, 3, 5, and 6.
Supplemental table 6. Stellaris® RNA FISH Probes. Related to Figure 4.
Supplemental table 7. sgRNA sequences used for CRISPR editing and CRISPR activation assays. Related to Figures 5 and 7.
Data Availability Statement
ChIP-Seq and 4C-Seq data generated in this study have been deposited at GEO with accession number GSE161258. Microscopy data reported in this paper will be shared by the lead contact upon request.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.