Abstract
Loss of bone and muscle mass is a frequent aging condition and has become a growing public health problem. The term “osteosarcopenia” denotes close links between bone and muscle. Mechanical exercise was once thought to be the only mechanism of crosstalk between muscle and bone. Sclerostin is an important player in the process of unloading-induced bone loss and plays an important role in mechanotransduction in the bone. Furthermore, bones and muscles are categorized as endocrine organs because they produce hormone-like substances, resulting in “bone-muscle crosstalk.” Sclerostin, an inhibitor of bone development, has recently been shown to play a role in myogenesis. This review discusses the importance of sclerostin in bone-muscle crosstalk.
Keywords: Aging, SOST protein, Bone, Muscles
INTRODUCTION
In recent decades, the aging population has dramatically increased. Loss of bone and muscle mass are common side effects of aging and have become a growing public health problem. Osteoporosis is a skeletal illness that affects bone microarchitecture and strength.1) Sarcopenia, in contrast, is defined as a loss of muscle mass, strength, and/or functional capacity.2) While sarcopenia and osteoporosis frequently co-occur, whether one condition occurs before the other and how the two are connected are unclear.3) Kirk et al.4) defined osteosarcopenia as a subpopulation of older adults with both osteoporosis and sarcopenia. Osteosarcopenia is a syndrome characterized by poor bone density (osteopenia/osteoporosis) and decreased muscle mass, strength, and/or functional capacity (sarcopenia).4) Because loss of bone mass is frequently coupled with a loss of muscle mass, this condition leads to a new paradigm in which bone may affect muscle and vice versa, necessitating additional research into the mechanisms of bone and muscle interaction. Muscle and bone are physically and physiologically linked and interact as a functional unit and both are influenced by aging.5) They share common mechanical and molecular mechanisms.6,7) The discovery of both bone and muscle as endocrine organs has recently demonstrated that muscle and bone can produce, express, and release cytokines and other peptides with paracrine, autocrine, or endocrine effects.7) The discovery of myokines, including myostatin, which are secreted by muscle, as well as the molecular biology revolution in the previous decade, has prompted in-depth investigations of muscle-bone interactions. Sclerostin is a cysteine knot protein released into the environment. It is part of the gene aberrative in neuroblastoma (DAN) family of proteins, which are proteins that stop bone morphogenetic protein (BMP) and Wingless-related integration site (Wnt) signaling.8) Produced by the sclerostin gene (SOST),8) sclerostin is a powerful inhibitor of bone growth that is produced mostly by osteocytes. Sclerostin was recently discovered in muscle cells in vitro and in muscles from variably aged mice with diverse metabolic and load-bearing capabilities.9) This finding could be the first step toward establishing the role of sclerostin in not only bone formation but also in myogenesis. Although a new paradigm has discovered the significance of sclerostin in bone-muscle crosstalk, the role of sclerostin remains controversial and the mechanisms are unknown. We discuss the role of sclerostin in bone-muscle crosstalk, as well as potential common pathways in the following sections. This new knowledge is critical for the development of therapeutics for pathophysiological illnesses such as aging-related osteosarcopenia. In addition to targeting pathways that control bone and muscle at a systemic level, manipulating pathways that allow communication between bone and muscle is also important and could lead to new therapeutic targets that prevent, mitigate, or restore bone and muscle degradation.
BONE-MUSCLE CROSSTALK BEGINS WITH EARLY DEVELOPMENT AND DIFFERENTIATION
The human musculoskeletal system is a complex organ system composed of bones, muscles, tendons, ligaments, cartilage, joints, and other connective tissues that work together to perform coordinated motor activity.10) Bone and muscle cells are produced from a common mesenchymal precursor during intrauterine development and undergo organogenesis through carefully regulated gene activation and inactivation so that bone and muscle develop simultaneously.10) Both tissues attain their maximum mass around the same period and begin to lose mass at around the same age.10) Numerous studies have reported pleiotropic genes that regulate both bone and muscle, including methyltransferase 21C, AARS1 Lysine (METTL21C), a member of the methyltransferase superfamily, and myocyte enhancer factor 2C (MEF2C).11) The deletion of both genes in rats affected both bone and muscle densities, indicating that bone and muscle share a similar signaling mechanism.11)
SCLEROSTIN
Molecular Aspects and Expression
Sclerostin was discovered by researchers of rare hereditary diseases associated with sclerostin. Sclerosteosis is an autosomal recessive illness that manifests in early childhood as increased bone mass, thickening of the bone cortex, gigantism, and facial and head malformations.12) With increasing age, these clinical signs become more prominent. This disorder is caused by a mutation in SOST, which encodes the protein sclerostin and is located on the long arm of chromosome 17 (17q12-q21).12) Sclerosteosis carriers have a normal phenotype, lower sclerostin levels, higher bone mineral density (BMD), and higher levels of bone production biomarkers.12)
Sclerostin is a 190-residue glycoprotein member of the DAN/Cerberus protein family that is thought to contain a cysteine knot and a glycoprotein that are secreted mostly by the bone matrix, and cartilage matrix.13) Sclerostin has two conserved N-linked glycosylation sites and several positively charged lysine and arginine residues, giving it a projected isoelectric point (pI) of 9.5.14) The structure of sclerostin is characterized by disordered N- and C-terminal arms in the free solution state, as well as three loops formed around the cysteine knot motif.15) The cysteine knot is at the base of Loops 1 and 3; their tips are linked by a disulfide link, giving the protein a structured core.15) The opposing side of the cysteine knot, Loop 2, is highly mobile in solution and is a functionally important portion of the protein that binds low-density lipoprotein receptor-related protein (LRP) 5/6 (Fig. 1).15)
Fig. 1.
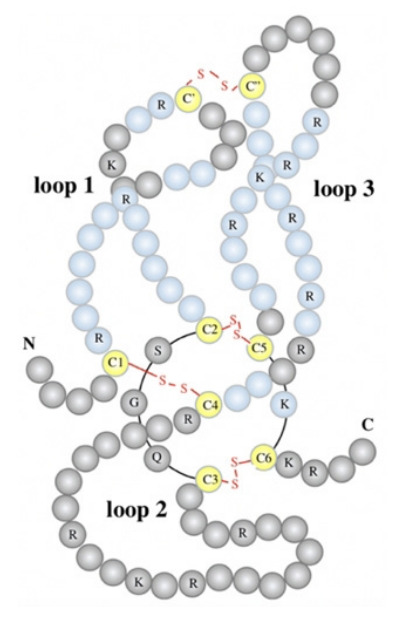
The molecular structure of sclerostin showing the presence of disulfide bonds, resulting in a 3-loop structure.11)
SCLEROSTIN AS A MEDIATOR OF BONE-MUSCLE CROSSTALK VIA MECHANICAL LOADING AND ACTIVITY
Mechanical interaction was once thought to be the only method of muscle-bone communication.11) For an organism to move, it relies on the movement of its bones, which are related to skeletal muscles.11) Muscle forces are the source of mechanical stress that results in bone strain.11) Loss of skeletal muscle mass induces bone loss due to bone unloading, which supports the mechanical link between bone and muscle.11) Osteosarcopenia is another disorder that demonstrates the close link between bone and muscle.1,16) The “mechanostat” theory describes the mechanical interaction between muscle and bone,16) in which the muscles put mechanical stress on bones and the balance of bone turnover shifts away from bone resorption and toward bone formation.16)
Skeleton unloading, which is frequently induced by immobilization, greatly reduces bone growth and mass.17) Understanding of the process of mechanotransduction has advanced at a breakneck pace. However, for many years, the mechanism by which osteocytes convert loading information into biochemical signals that direct bone formation has remained a mystery.17) Sclerostin expression may be regulated by mechanical loading in a way that affects osteocyte function.17) Sclerostin is a crucial regulator of unloading-induced bone loss and plays an important function in mechanotransduction in bone.18) Sclerostin levels were higher in human studies of bed rest.19) Some studies reported that increasing mechanical loading reduces osteocyte sclerostin expression, whereas decreased loading, such as from disuse or aging, is linked to increased sclerostin production and bone loss.17) Sclerostin is a protein that inhibits bone growth.13,17,20) Osteoclast resorption shifts the balance of bone remodeling in favor of osteoclast resorption when there is a lack of mechanical stimulus or disuse.20) During this mechanotransduction process, the lysosome rapidly degrades sclerostin, allowing new bone production by osteoblasts to occur.20) In contrast, the molecular processes by which osteocytes sense and transduce loading-related signals into changes in sclerostin expression remain unclear.20)
Immobilization, Aging, and Defective Autophagy
Several aging-related conditions, including aging itself, trigger alterations in autophagy, which could affect bone and muscle.21) Autophagy is a lysosome-dependent system that regulates cytoplasmic turnover in cells and is an important part of cellular proteostasis.21,22) Autophagosomes remove damaged, broken, or unnecessary proteins and organelles by fusion with lysosomes. This maintains a balance between protein formation and breakdown. Autophagy ability decreases with age, in part because lysosomal acidity decreases and fewer autophagy-related proteins are formed.21-23) The load-dependent breakdown of sclerostin is controlled by autophagy in bone, which is stimulated by mechanical load.20) Defects in lysosome function in older adults can affect bone health by preventing the removal of sclerostin, an inhibitor of bone formation.20)
Muscle, like bone, alters its mass in response to reduced load situations such as bed rest, immobilization, and inactivity.24) To meet the lower mechanical demand, the myofibrillar content and levels of supporting proteins are reduced, resulting in smaller myofibers and, consequently, muscle mass.24) Mechanical demand also influences skeletal muscle mass and performance. Outside of the normal homeostatic setpoint, high-intensity resistance training boosts the number of myofibrils and other muscle fiber contractile control mechanisms in the body.24) When these organelles and proteins are recruited, the cross-sectional area of muscle fibers increases, resulting in greater muscular hypertrophy (morphology) (function).24) The link between muscle loading and sclerostin expression is not as obvious as in the case of bone-induced mechanical loading. Sclerostin is a mediator that regulates bone-muscle crosstalk.5,11)
At the molecular level, osteocytes are believed to use at least two important chemicals, sclerostin and receptor activator of nuclear factor β ligand (RANKL), to control bone response to mechanical loading.25,26) Sclerostin, which is encoded by SOST, is only produced postnatally by mature osteocytes.27) Sclerostin reduces bone formation in vitro and in vivo by inhibiting osteoblast proliferation and differentiation through the canonical Wnt signaling pathway.27) Sclerostin likely suppresses Wnt-β-catenin signaling by binding to low-density LRP5/6.15,28) Furthermore, because its expression increases with mechanical unloading and decreases with loading,29) sclerostin may be crucial in bone response to mechanical loading.27,29) Lin et al.8) proved that unloading decreased Wnt/β-catenin signaling in WT mice, indicating that it is physiologically responsive to mechanical unloading. Sclerostin and Dkk1 were both responsive to mechanical stimulation, among several Wnt antagonists.29) Unloading had little effect on Wnt/β-catenin signaling in the absence of sclerostin, suggesting the major role of sclerostin in antagonizing Wnt/β-catenin signaling in the context of mechanotransduction.8) The increased sclerostin released by osteocytes in response to unloading suppresses Wnt/β-catenin signaling, causing apoptosis and reducing osteoblast activity, resulting in decreased bone production and lower bone mass.8)
Muscle was initially defined as a secretory organ by Pedersen6) who used the term “myokines” to describe the substances produced by muscles. Along with the discovery of myokines, the bone can also function as a secretory endocrine organ, producing many substances that regulate both bone and muscle. Muscle metabolites have recently been discovered to modulate bone mass.6) Sclerostin, which will be addressed further in the section below, is one of several myokines that affect bones.
ROLE OF SCLEROSTIN AND WNT SIGNALING PATHWAY IN BONE-MUSCLE CROSSTALK AS A PARACRINE ORGAN
Skeletal muscles and bones were long thought to be mechanically linked in the sense that muscles attach and put stress on bones.6,7,30) In the last decade, however, studies demonstrated that these two tissues work together at a higher level through crosstalk signaling processes that are important for the functioning of both paracrine and endocrine tissues.7,30) Muscle and bone create factors (myokines and osteokines, respectively) that circulate and can operate as endocrine molecules that target distant organs.30) This field of study is fairly new; future studies are needed to learn more about how this hypothesized paracrine or endocrine crosstalk and biochemical interactions work.
Sclerostin Expression in Bone and Muscle
Sclerostin is a glycoprotein produced primarily by the bone and cartilage matrixes.8,31) Animal tests have shown that sclerostin can inhibit the formation of new bone.14,31) Sclerostin overexpression causes reduced bone mass, bone growth, and bone strength in mice.31) Sclerostin inhibits osteoblastogenesis and preosteocyte differentiation in osteoblasts, and also causes osteocytes to release RANKL to induce osteoclastogenesis and rapid bone resorption.31) Bone remodeling refers to two processes that are constantly involved in bone construction and reconstruction throughout a person’s life. Osteoclasts and osteoblasts are responsible for bone resorption and production, respectively.32) Both of these cells are firmly connected in a basic multicellular unit (BMU) component, and bone resorption always occurs before bone formation.32) Sclerostin expression by nascent osteocytes at the start of osteoid mineralization serves as a negative feedback signal to osteoblasts, preventing excessive BMU.32) Sclerostin maintains quiescence in the cells surrounding the bone during bone remodeling to avoid osteoblast activation and bone growth without first resorbing the bone.32) Sclerostin inhibits osteoblast proliferation and differentiation during the early and late stages of osteoblast proliferation and differentiation, which is how it inhibits bone production.32) Sclerostin also promotes osteoblast death and osteocyte RANKL release, both of which are important for osteoclast activity and development.32) A higher BMD score is associated with more osteocytes.32,33) Moreover, sclerostin signaling to osteoblasts as target cells is more paracrine than endocrine (Fig. 2).33)
Fig. 2.
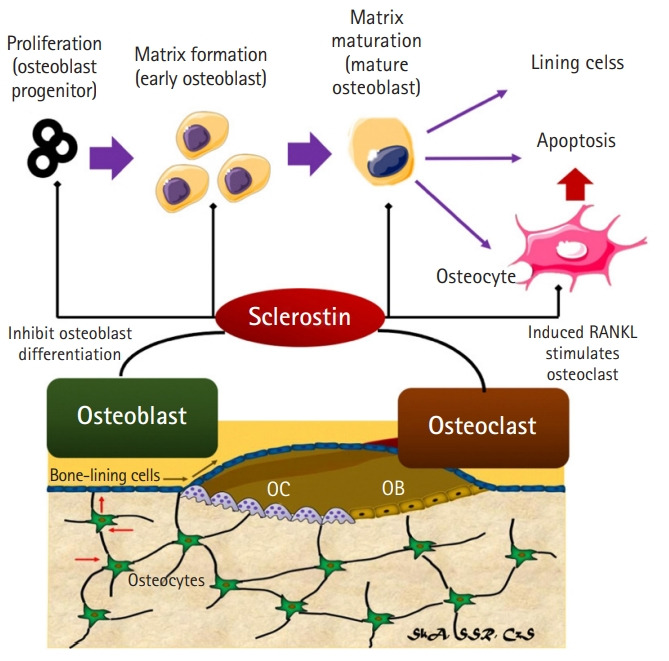
Sclerostin inhibits osteoblast proliferation and differentiation in both the early and late stages, limiting bone formation. Sclerostin also promotes osteoblast apoptosis and RANKL release, both of which are necessary for osteoclast activity and differentiation.26-29)
The action and control of sclerostin in bone are better understood than those in muscles. A few recent studies have suggested the role of sclerostin in muscle mass modulation. The Wnt signaling system is also important in muscle stem cell formation.34) Kim et al.35) reported increased lean body mass with age in SOST -/- mice (p=0.06). However, sclerostin overexpression via an adeno-associated virus resulted in the opposite body composition phenotype in SOST -/- mice, with increased body fat mass and a small but significant decrease in lean body mass. Humans with low muscle mass had higher circulating sclerostin levels than healthy controls.36) These findings have led to the concept that sclerostin also facilitates endocrine communication between bone and adipose tissue and influences adipose tissue accumulation.37) Sclerostin may influence adipocyte metabolism by indirectly modulating bone morphogenetic protein (Bmp) signaling.35) Early studies of sclerostin activity suggested that it might serve as a Bmp antagonist35,38); however, genetic and pharmacological evidence suggests that it predominantly operates as a Wnt signaling inhibitor.39) Wnt signaling markers were enhanced in the white adipose tissue of SOST -/- mice, as expected, whereas Bmp signaling was reduced.35) Consistent with this discovery, the expression levels of Bmp4 and its receptor Bmpr1a were inversely linked to the levels of Wnt signaling in adipocytes both in vitro and in vivo. Moreover, the Bmp antagonist noggin prevented the effects of recombinant sclerostin on adipocyte metabolism in vitro.35) These findings are consistent with recognized crosstalk between the Wnt and Bmp pathways, Bmp signaling’s adipogenic effects, and recent findings that Bmp4 enhances adipocyte hypertrophy by increasing fatty acid production while reducing oxidation.40)
The Wnt/β-catenin pathway is a prominent therapeutic target for osteoporosis. Since sclerostin, a bone-forming inhibitor, is inhibited, many studies have investigated whether muscle cells may also produce this protein. SOST mRNA expression levels were also reported in different tissues, including skeletal muscle, despite being regarded as an osteocyte-specific protein41); however, its synthesis in muscle has not been described in detail. Magaro et al.9) studied bone-muscle crosstalk in vitro in osteogenic (2T3) and myogenic (C2C12) cell lines to demonstrate the role of sclerostin. Conditioned media derived from differentiating C2C12 cells considerably affected the functional maturation of osteoblasts.9) They also examined SOST/sclerostin protein expression and secretion in C2C12 and C57 muscle cells and found that it is produced at all three levels.9) A transient gain-of-function experiment in young mice with growing bones assessed the similarity of muscle sclerostin and osseous sclerostin.9) The skeletal system was also negatively affected by muscle-released sclerostin in this study.9) The co-administration of muscle and osseous sclerostin showed a similar effect. These findings suggest that muscle sclerostin may function in tandem with bone sclerostin to prevent osteogenesis and subsequent bone growth.9)
Molecular Pathway of Sclerostin in Bone
The Wnt/β-catenin signaling system plays a key role in osteoblast cell proliferation and development.9,32,41) Wnt is a glycoprotein that binds to the LRP5/6 receptor and Frizzled co-receptor to trigger signaling pathways. Through suppression of glycogen synthase kinase 3 (GSK3) activity using protein complexes with Disheveled (Dsh), Axin, and adenomatous polyposis coli (APC), this receptor complex blocked the phosphorylation of β-catenin.9,32) β-catenin accumulates in the cytoplasm and translocates to the nucleus as a result of this protein complex interaction.32) β-catenin is a transcriptional coactivator that collaborates with other transcription factors to kick-start gene expression, which leads to cell proliferation and differentiation.42) Mutations in the N-terminal chain of the human LRP5 protein decrease its affinity for sclerostin, resulting in an abnormal condition defined by increased bone mass, indicating that sclerostin is involved in bone mass control.32,33) Meanwhile, β-catenin activity is necessary for the development of mesenchymal stem cells from osteoblasts. Wnt pathway activation increases family transcription factor 2 (Runx2) expression in mice, whereas β-catenin inactivation results in a decrease in components needed for osteoblast development, such as Runx2 and Osx, and poor osteoblast differentiation. β-catenin also stimulates osteoblastogenesis while inhibiting chondrogenesis.43)
Structural studies of sclerostin and LRP5/6 also provided information on how these two proteins interact. Sclerostin has a flexible loop domain surrounded by two cysteine-containing fixed-structure finger domains.13,32) Recently, the flexible loop domain was demonstrated to be responsible for its interaction with the LRP6 receptor.14,15) Additionally, a sclerostin monoclonal antibody can bind to this location, preventing sclerostin-LRP interaction.15) In contrast, LRP5/6 is a transmembrane protein. In its extracellular domain, LRP5/6 has four β-propeller domains separated by four epidermal growth factor (EGF) domains: low-density lipoprotein receptor domain class A (LDLA), a transmembrane domain, and a cytoplasmic domain.15) The cytoplasmic domain is required for the recruitment of the GSK3/Dsh/axin/APC complex, whereas the extracellular domain is antagonistic and is in charge of binding to Wnt protein.15) LRP6’s first two propellers (E1E2) are also involved in binding to Wnt and sclerostin ligands, suggesting that sclerostin and Wnt are competitors for binding to LRP6.15) Sclerostin inhibition is caused by a conformational shift in the LRP6 domain (Fig. 3).15)
Fig. 3.
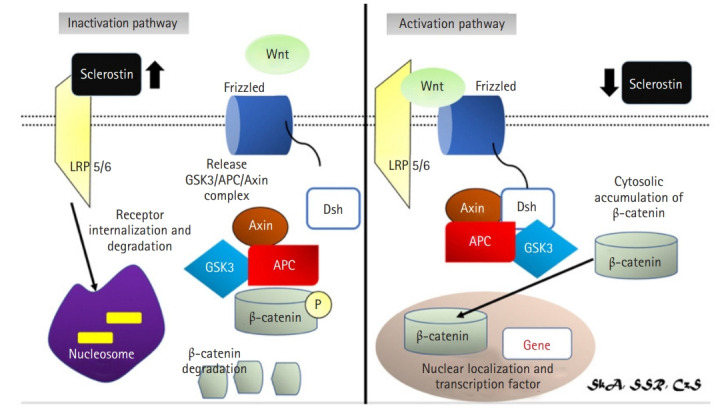
Activation (right): Wnt protein binds to the LRP5/6 receptor and the Frizzled co-receptor to activate signalling pathways. With the help of protein complexes with Disheveled (Dsh), Axin, and Adenomatous Polyposis coli, this receptor complex inhibits the phosphorylation of β-catenin (APC) causing β-catenin accumulation in the cytoplasm and translocated to the nucleus. Inactivation pathway (left): sclerostin will inhibit Wnt signalling pathway causing dissociation of LRP 5/6 complex and frizzled receptor.14,29,33,34) LRP, lipoprotein receptor-related protein; GSK3, glycogen synthase kinase 3; APC, adenomatous polyposis coli; Dsh, disheveled.
Molecular Pathway of Sclerostin in Muscle
Although the mechanism by which sclerostin modulates muscle mass is not fully understood, the Wnt signaling system, which is important for mediating sclerostin in bone, is also thought to function in muscles. Wnt signaling is required for embryonic muscle development and adult skeletal muscle homeostasis.44) Muscle tissue is a highly flexible and complicated structure. Myogenesis, or muscle development, is the consequence of a well-coordinated network of transcriptional cascades and signaling channels.44) Wnt signaling influences the expression of myogenic regulatory factors, which are important transcriptional regulators of myogenic lineage development and differentiation.34) Wnt signaling is also critical for the development of the dermomyotome.34) Mice lacking both Wnt1 and Wnt3a do not form the medial compartment of the dermomyotome, which is associated with decreased myogenic factor 5 (Myf5) expression via a β-catenin-dependent process.34) Additionally, Wnt6-catenin-dependent signaling from the dorsal ectoderm is necessary for somite epithelial structure and dermal myotome development.34)
The Wnt family of human proteins consists of 19 members: Wnt1, Wnt2, Wnt2b (Wnt13), Wnt3, Wnt3a, Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, Wnt7b, Wnt8a, Wnt8b, Wnt9a (Wnt14), Wnt9b (Wnt14b), Wnt10a, Wnt10b, Wnt11, and Wnt16.45) These genes encode cysteine-rich secreted glycoproteins.45) Wnts can connect with important autocrine regulators and/or participate in paracrine modification by adhering to nearby cell membrane receptors.45) Wnt3a and Wnt4 are hypothesized to be greatly involved in bone-muscle interaction.46) These two proteins can also be separated into two categories based on their functions: Wnt3a is involved in the canonical signaling pathway, whereas Wnt4 activates both canonical and noncanonical signaling pathways.46) A strict balance between signaling pathways and their precise activation is required for successful skeletal muscle regeneration, with a temporal switch from Notch to canonical Wnt signaling required for proper differentiation.46) Notch signaling is blocked by canonical Wnt signaling, allowing myogenic commitment and differentiation to proceed (Fig. 4).46)
Fig. 4.
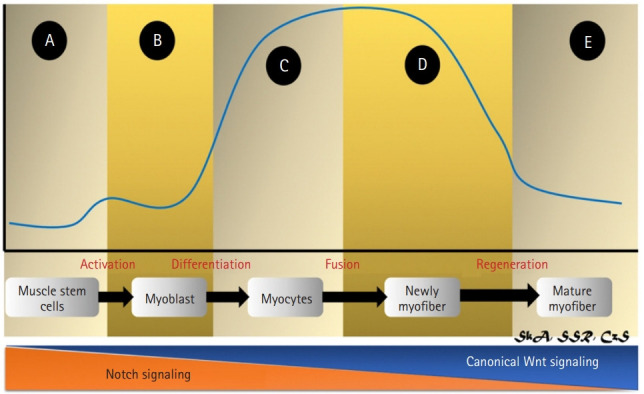
Activation of Wnt signaling during muscle regeneration. (A) In quiescent satellite cells, Wnt signaling (blue line) is inactive; (B, C) Canonical Wnt signaling suppresses the Notch pathway, leading the satellite cell to switch from proliferation to differentiation. (D) Wnt signaling induces myogenic gene expression via Barx2 activation, stimulates Follistatin, inhibits myostatin, and translocates the negative regulator Setdb1 into the cytoplasm, promoting myoblast development and fusion. (E) Finally, Wnt activity returns to low levels. Overall, Wnt/β-catenin signaling must be regulated in a timely manner for muscle regeneration to occur.30,35,37-39)
Sclerostin as inhibitor of Wnt3a signaling in muscle
Wnt3a, which is found in the extracellular matrix of various cell types, is expressed in C2C12 myoblast cells and 2T3 osteoblasts and occurs at relatively high levels in both human (16.9±2.4 ng/mL) and mouse (0.225–3.74 ng/mL) serum, suggesting a potential role of Wnt3a in bone-muscle crosstalk.45) Sclerostin inhibited Wnt3a-mediated crosstalk between MLO-Y4 osteocytes and muscle cells (C2C12) by modulating the Wnt/β-catenin pathway.5) Sclerostin, a negative regulator of the Wnt/β-catenin signaling pathway, was also used to assess if the effect of MLO-Y4 conditioned media (CM) and Wnt3a on C2C12 cell differentiation promotion was attributable to the Wnt/β-catenin signaling pathway.5) Sclerostin inhibited the capacity of 10% MLO-Y4 CM and 10 ng/mL WNT3a to differentiate C2C12 cells.5) The combination of CM and WNT3a, did not slow the acceleration of C2C12 cell differentiation by sclerostin.5) These findings indicate that the Wnt/β-catenin signaling pathway is involved in the MLO-Y4 CM and WNT3a promotion of C2C12 cell development.5) When WNT3a and sclerostin competed for binding to LRP5 and LRP6, they diminished sclerostin’s inhibitory effect on the Wnt/β-catenin signaling pathway.5)
Canonical Wnt signaling causes satellite cell development in adult skeletal muscles, mostly through the Wnt3a ligand. Rudolf et al.,47) reported that the disruption or stimulation of β-catenin in adult satellite cells affected regeneration, highlighting the importance of balanced canonical Wnt signaling for skeletal muscle regeneration. Wnt3a also activated MyoD and myogenin, which are essential myogenic regulatory proteins (Fig. 5).48)
Fig. 5.
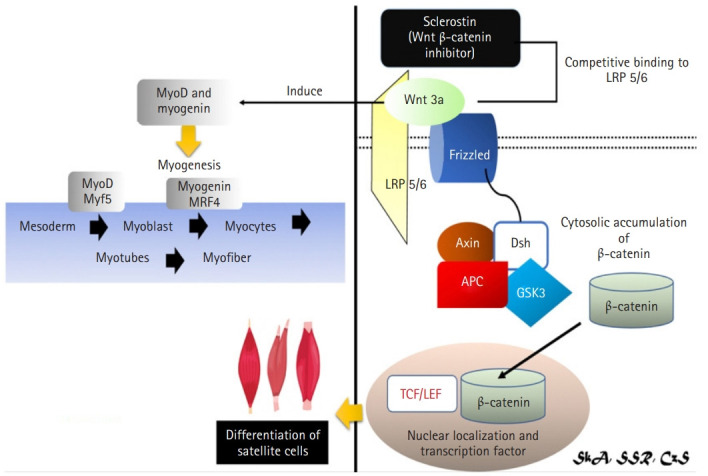
Mechanism of sclerostin as inhibitor of Wnt3a signaling in muscle via Wnt/β-catenin signaling pathway.4,29,38,40,41) Wnt3a may have a role in bone-muscle crosstalk via the canonical pathway (Wnt/β-catenin signaling). Sclerostin was revealed to inhibit Wnt3a-mediated crosstalk, resulting in reduction of the Wnt/β-catenin signaling pathway and inhibition of satellite cell development. Wnt3a also induces MyoD and Myogenin, both of them are required for myogenesis. Myf5, myogenic factor 5; LRP, lipoprotein receptor-related protein; GSK3, glycogen synthase kinase 3; APC, adenomatous polyposis coli; Dsh, disheveled; TCF/LEF, T-cell factor/lymphoid enhancer factor.
Sclerostin as inhibitor of the Wnt4 canonical signaling pathway in muscle
The Wnt4 pathway is also involved in the action of sclerostin in muscle.49) Myoblast differentiation consists of two primary steps: irreversible cell cycle exit and subsequent myogenic factor expression and activation.49) Myf5 and MyoD are expressed early in myogenesis. Myogenin and MRF4 are activated as cells proceed toward a differentiated phenotype and work together to form an irreversible commitment to final differentiation.49) Myf5 levels are elevated in the G0 phase of cell differentiation.49) Borello et al.50) previously demonstrated that the canonical Wnt/β-catenin signaling pathway regulates Myf5 expression directly during somitogenesis. Wnt4 overexpression induced Myf5 expression, while Wnt4 silencing suppressed Myf5 expression during myoblast and satellite cell differentiation, implying a role for the canonical pathway in Wnt4’s myogenic characteristics.49) While Wnt4 was initially described as a noncanonical Wnt, it is also involved in the activation of the canonical Wnt/β-catenin signaling pathway.49) Wnt4 hypertrophic activity in C2C12 and satellite cells was linked to Tcf/LEF promoter activation, demonstrating the involvement of Wnt/β-catenin signaling in myogenesis regulation.49)
Myostatin may also be involved in the Wnt4 pathway.51) Myostatin acts via Smad2 and Smad3 receptor-associated proteins.51) Phosphorylated Smad2 and Smad3 form a heterodimeric complex with the common mediator Smad4. These activated Smad proteins act as the primary intracellular mediators of myostatin signaling by translocating into the nucleus and activating target gene transcription via interactions with DNA and other nuclear factors.51) Takata et al.51) reported that Wnt4 had no effect on Smad2 phosphorylation in differentiated C2C12 myoblasts but suppressed myostatin-induced Smad2 phosphorylation. Wnt4 induced myogenesis by inhibiting myostatin.51) Wnt4-mediated suppression of the myostatin pathway may be related to decreased myostatin expression and/or decreased myostatin/Smad transduction.51) However, myostatin did not decrease Tcf/LEF activity caused by Wnt4 overexpression in proliferating and differentiated C2C12 myoblasts. Thus, myostatin did not affect the Wnt4/β-catenin signaling pathway (Fig. 6).51)
Fig. 6.
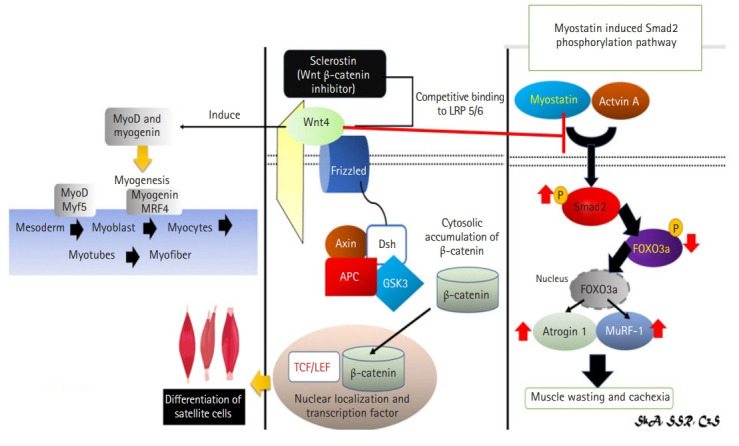
Mechanism of sclerostin as inhibitor of Wnt4 signaling in muscle via Wnt/β-catenin signaling pathway and Wnt4’s effect to myostatin.4,29,41,42,44,45) (Left and center) Wnt4 bone and muscle crosstalk occurs via the Wnt/β-catenin signaling pathway, which inhibit by sclerostin. Wnt4 overexpression increased Myf5 expression, which is necessary for myogenesis. (Right) Myostatin is also thought to be involved in the Wnt4 pathway. Wnt4 induces myogenesis by inhibiting myostatin thus preventing muscle wasting and cachexia. Myf5, myogenic factor 5; LRP, lipoprotein receptor-related protein; GSK3, glycogen synthase kinase 3; APC, adenomatous polyposis coli; Dsh, disheveled; TCF/LEF, T-cell factor/lymphoid enhancer factor.
CONCLUSION
Numerous investigations have confirmed the existence of bone-muscle communication. Mechanical and endocrine relationships explain how these two organs communicate. Sclerostin is a novel myokine and osteokine that increases bone metabolism via mechanical loading. Physical activity and mechanical loading reduce sclerostin levels, which prevent future bone and muscle loss. Aging causes changes in autophagy, which could affect bones and muscles.
According to a new paradigm, muscle and bone can function as paracrine organs, secreting chemicals to modulate their action. Sclerostin also plays a significant role in bone and muscle crosstalk by activating the Wnt/β-catenin signaling pathway, which acts as a powerful inhibitor. Wnt3a and Wnt4 also play a critical role in myogenesis in muscles. Some studies have shown that Wnt4 interacts with the myostatin pathway to regulate muscle hypertrophy. Further research is required to fully elucidate the processes underlying sclerostin regulation of bone-muscle communication to develop innovative methods to prevent the development of osteoporosis and sarcopenia (Fig. 7).
Fig. 7.
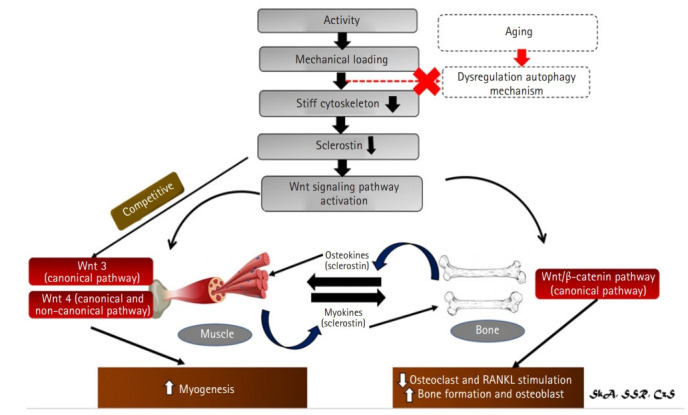
Summary of sclerostin role in bone and muscle crosstalk.46) Crosstalk between bones and muscles is mediated not just by mechanical loads and activity, but also as a paracrine organ. Sclerostin is a key regulator of unloading-induced bone loss and is involved in bone mechanotransduction. Sclerostin levels will be reduced as a result of increased activity and mechanical loading. Sclerostin is a Wnt antagonist that responds to mechanical stimulation. Mechanical loading reduces sclerostin levels, which activates Wnt/β signaling. A meanwaging will result in alterations in autophagy, which will have an impact on bone and muscle. Meanwhile, a new paradigm has emerged, claiming that bone and muscle can function as an endocrine organ, secreting sclerostin, which can function as both myokines and osteokine. Reduced sclerostin levels in the bone lower osteoclast and RANKL stimulation, resulting in increased bone growth and osteoblast. On the other hand, two key wnt families play important roles in muscle: Wnt3 which acts via the canonical pathway and Wnt4 which acts via both the canonical and non-canonical pathways. Wnt3 and Wnt4 activation leads to myogenesis. Sclerostin is an effective inhibitor of both.
Footnotes
CONFLICT OF INTEREST
The researchers claim no conflicts of interest.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization, IGPSA, SSR; CHS; Data curation, IGPSA, SSR, CHS; Investigation, IGPSA, SSR, CHS; Methodology, IGPSA, SSR, CHS; Supervision, IGPSA, CHS; Writing-original draft, IGPSA, SSR, CHS; Writing-review & editing. IGPSA, SSR, CHS.
REFERENCES
- 1.Sozen T, Ozisik L, Basaran NC. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020;11:609–18. doi: 10.1002/jcsm.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reginster JY, Beaudart C, Buckinx F, Bruyere O. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care. 2016;19:31–6. doi: 10.1097/MCO.0000000000000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirk B, Al Saedi A, Duque G. Osteosarcopenia: a case of geroscience. Aging Med (Milton) 2019;2:147–56. doi: 10.1002/agm2.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, et al. Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/β-catenin pathway. JBMR Plus. 2017;1:86–100. doi: 10.1002/jbm4.10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337–46. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 9.Magaro MS, Bertacchini J, Florio F, Zavatti M, Poti F, Cavani F, et al. Identification of sclerostin as a putative new myokine involved in the muscle-to-bone crosstalk. Biomedicines. 2021;9:71. doi: 10.3390/biomedicines9010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Land C, Schoenau E. Fetal and postnatal bone development: reviewing the role of mechanical stimuli and nutrition. Best Pract Res Clin Endocrinol Metab. 2008;22:107–18. doi: 10.1016/j.beem.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Bonewald L. Use it or lose it to age: a review of bone and muscle communication. Bone. 2019;120:212–8. doi: 10.1016/j.bone.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mare A, D'Haese PC, Verhulst A. The role of sclerostin in bone and ectopic calcification. Int J Mol Sci. 2020;21:3199. doi: 10.3390/ijms21093199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado-Calle J, Sato AY, Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdsworth G, Roberts SJ, Ke HZ. Novel actions of sclerostin on bone. J Mol Endocrinol. 2019;62:R167–85. doi: 10.1530/JME-18-0176. [DOI] [PubMed] [Google Scholar]
- 15.Holdsworth G, Slocombe P, Doyle C, Sweeney B, Veverka V, Le Riche K, et al. Characterization of the interaction of sclerostin with the low density lipoprotein receptor-related protein (LRP) family of Wnt co-receptors. J Biol Chem. 2012;287:26464–77. doi: 10.1074/jbc.M112.350108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clynes MA, Gregson CL, Bruyere O, Cooper C, Dennison EM. Osteosarcopenia: where osteoporosis and sarcopenia collide. Rheumatology (Oxford) 2021;60:529–37. doi: 10.1093/rheumatology/keaa755. [DOI] [PubMed] [Google Scholar]
- 17.Galea GL, Lanyon LE, Price JS. Sclerostin's role in bone's adaptive response to mechanical loading. Bone. 2017;96:38–44. doi: 10.1016/j.bone.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morse A, McDonald MM, Kelly NH, Melville KM, Schindeler A, Kramer I, et al. Mechanical load increases in bone formation via a sclerostin-independent pathway. J Bone Miner Res. 2014;29:2456–67. doi: 10.1002/jbmr.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickering ME, Simon M, Sornay-Rendu E, Chikh K, Carlier MC, Raby AL, et al. Serum sclerostin increases after acute physical activity. Calcif Tissue Int. 2017;101:170–3. doi: 10.1007/s00223-017-0272-5. [DOI] [PubMed] [Google Scholar]
- 20.Gould NR, Williams KM, Joca HC, Torre OM, Lyons JS, Leser JM, et al. Disparate bone anabolic cues activate bone formation by regulating the rapid lysosomal degradation of sclerostin protein. Elife. 2021;10:e64393. doi: 10.7554/eLife.64393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesce M, Ballerini P, Paolucci T, Puca I, Farzaei MH, Patruno A. Irisin and autophagy: first update. Int J Mol Sci. 2020;21:7587. doi: 10.3390/ijms21207587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nixon RA. The aging lysosome: an essential catalyst for late-onset neurodegenerative diseases. Biochim Biophys Acta Proteins Proteom. 2020;1868:140443. doi: 10.1016/j.bbapap.2020.140443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aguilar-Agon KW, Capel AJ, Martin N, Player DJ, Lewis MP. Mechanical loading stimulates hypertrophy in tissue-engineered skeletal muscle: molecular and phenotypic responses. J Cell Physiol. 2019;234:23547–58. doi: 10.1002/jcp.28923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O'Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17:1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 27.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, et al. The Wnt inhibitor sclerostin is up-regulated by mechanical unloading in osteocytes in vitro. J Biol Chem. 2015;290:16744–58. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 29.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–75. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 30.Brotto M, Johnson ML. Endocrine crosstalk between muscle and bone. Curr Osteoporos Rep. 2014;12:135–41. doi: 10.1007/s11914-014-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478–86. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manolagas SC. Wnt signaling and osteoporosis. Maturitas. 2014;78:233–7. doi: 10.1016/j.maturitas.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han Y, You X, Xing W, Zhang Z, Zou W. Paracrine and endocrine actions of bone-the functions of secretory proteins from osteoblasts, osteocytes, and osteoclasts. Bone Res. 2018;6:16. doi: 10.1038/s41413-018-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Maltzahn J, Chang NC, Bentzinger CF, Rudnicki MA. Wnt signaling in myogenesis. Trends Cell Biol. 2012;22:602–9. doi: 10.1016/j.tcb.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, et al. Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A. 2017;114:E11238–47. doi: 10.1073/pnas.1707876115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JA, Roh E, Hong SH, Lee YB, Kim NH, Yoo HJ, et al. Association of serum sclerostin levels with low skeletal muscle mass: the Korean Sarcopenic Obesity Study (KSOS) Bone. 2019;128:115053. doi: 10.1016/j.bone.2019.115053. [DOI] [PubMed] [Google Scholar]
- 37.Kim SP, Da H, Wang L, Taketo MM, Wan M, Riddle RC. Bone-derived sclerostin and Wnt/β-catenin signaling regulate PDGFRα+ adipoprogenitor cell differentiation. FASEB J. 2021;35:e21957. doi: 10.1096/fj.202100691R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003;22:6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgers TA, Williams BO. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone. 2013;54:244–9. doi: 10.1016/j.bone.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Modica S, Straub LG, Balaz M, Sun W, Varga L, Stefanicka P, et al. Bmp4 promotes a brown to white-like adipocyte shift. Cell Rep. 2016;16:2243–58. doi: 10.1016/j.celrep.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 41.Weivoda MM, Youssef SJ, Oursler MJ. Sclerostin expression and functions beyond the osteocyte. Bone. 2017;96:45–50. doi: 10.1016/j.bone.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan V, Bryant HU, Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest. 2006;116:1202–9. doi: 10.1172/JCI28551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–50. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Bryson-Richardson RJ, Currie PD. The genetics of vertebrate myogenesis. Nat Rev Genet. 2008;9:632–46. doi: 10.1038/nrg2369. [DOI] [PubMed] [Google Scholar]
- 45.He S, Lu Y, Liu X, Huang X, Keller ET, Qian CN, et al. Wnt3a: functions and implications in cancer. Chin J Cancer. 2015;34:554–62. doi: 10.1186/s40880-015-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmidt M, Schuler SC, Huttner SS, von Eyss B, von Maltzahn J. Adult stem cells at work: regenerating skeletal muscle. Cell Mol Life Sci. 2019;76:2559–70. doi: 10.1007/s00018-019-03093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudolf A, Schirwis E, Giordani L, Parisi A, Lepper C, Taketo MM, et al. β-Catenin activation in muscle progenitor cells regulates tissue repair. Cell Rep. 2016;15:1277–90. doi: 10.1016/j.celrep.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 48.Mo C, Zhao R, Vallejo J, Igwe O, Bonewald L, Wetmore L, et al. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle. 2015;14:1507–16. doi: 10.1080/15384101.2015.1026520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernardi H, Gay S, Fedon Y, Vernus B, Bonnieu A, Bacou F. Wnt4 activates the canonical β-catenin pathway and regulates negatively myostatin: functional implication in myogenesis. Am J Physiol Cell Physiol. 2011;300:C1122–38. doi: 10.1152/ajpcell.00214.2010. [DOI] [PubMed] [Google Scholar]
- 50.Borello U, Berarducci B, Murphy P, Bajard L, Buffa V, Piccolo S, et al. The Wnt/beta-catenin pathway regulates Gli-mediated Myf5 expression during somitogenesis. Development. 2006;133:3723–32. doi: 10.1242/dev.02517. [DOI] [PubMed] [Google Scholar]
- 51.Takata H, Terada K, Oka H, Sunada Y, Moriguchi T, Nohno T. Involvement of Wnt4 signaling during myogenic proliferation and differentiation of skeletal muscle. Dev Dyn. 2007;236:2800–7. doi: 10.1002/dvdy.21327. [DOI] [PubMed] [Google Scholar]
- 52.Fedon Y, Bonnieu A, Gay S, Vernus B, Bacou F, Bernardi H. In: Skeletal muscle from myogenesis to clinical relations. Cseri J, editor. London, UK: InTechOpen Limited; 2012. Role and function of Wnts in the regulation of myogenesis: when Wnt meets myostatin. [Google Scholar]


